Abstract
Acute graft-versus-host disease (aGVHD) is one of the most common complications of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Janus kinase (JAK) inhibitors are considered as reliable and promising agents for patients with aGVHD. The prophylactic and therapeutic effects of SHR0302, a novel JAK inhibitor, were evaluated in aGVHD mouse models. The overall survival (OS), progression-free survival (PFS), bodyweight of mice, GVHD scores were observed and recorded. The bone marrow and spleen samples of diseased model mice or peripheral blood of patients were analyzed. SHR0302 could prevent and reverse aGVHD in mouse models with preserving graft-versus-tumor effect. Functionally, SHR0302 improved the OS and PFS, restored bodyweight, reduced GVHD scores, and reduced immune cells infiltrated in target tissues. SHR0302 treatment also enhanced the hematopoietic reconstruction compared to the control group. Mechanistically, our results suggested that SHR0302 could inhibit the activation of T cells and modulate the differentiation of helper T (Th) cells by reducing Th1 and increasing regulatory T (Treg) cells. In addition, SHR0302 decreased the expression of chemokine receptor CXCR3 on donor T cells and the secretion of cytokines or chemokines including interleukin (IL)-6, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), CXCL10, etc. thereby destroying the IFN-γ/CXCR3/CXCL10 axis which promotes the progression of GVHD. Besides, SHR0302 decreased the phosphorylation of JAK and its downstream STATs, AKT and ERK1/2, which ultimately regulated the activation, proliferation, and differentiation of lymphocytes. Experiments on primary cells from aGVHD patients also confirmed the results. In summary, our results indicated that JAK inhibitor SHR0302 might be used as a novel agent for patients with aGVHD.
Keywords: SHR0302, graft-versus-host-disease (GVHD), Janus kinase inhibitor, interferon-γ
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective modality to cure various benign and malignant hematological diseases and some genetic diseases 1 . With the development of unrelated donor (MUD) transplantation and mismatched related donor (MMRD) transplantation, graft-versus-host disease (GVHD) has become the most common complication of allo-HSCT 2 . Acute GVHD (aGVHD) affects 40–60% of patients after allo-HSCT, and turns into the main obstacle affecting the survival of patients 3,4 . In the past 40 years, corticosteroids remained to be the standard first-line agents for aGVHD, but only half of patients responded completely, while the rest of them developed to be steroid-resistant aGVHD, which increased the risk of non-relapse mortality (NRM) 5,6 . Although the biological mechanisms of aGVHD are still elusive, the understanding of the pathophysiological mechanisms of aGVHD is deepening. The occurrence of aGVHD initiates with the interaction between antigen presenting cell (APC) and donor T cells, which induces the differentiation of pathogenic helper T (Th) cells along Th1 rather than regulatory T (Treg) cells 4,7 . Activated immune cells and a large number of released cytokines and chemokines [e.g., interleukin(IL)-6, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α)] are cytotoxic to local tissues, thereby causing injuries to multiple GVHD target organs such as skin, liver, and small intestines 4 .
It has been confirmed that the Janus kinase (JAK) signals play a vital role in the pathophysiologic process of GVHD. JAK signaling pathway is essential to the activation, differentiation, and survival of T lymphocytes, the production of cytokines or chemokines, and multilayer immune response in aGVHD 8,9 . Previous researches have indicated that JAK inhibitors can successfully block the immune cell receptors such as IFN-γR and IL-6R 10,11 . These data suggested the potential effects of JAK inhibitors as medication for aGVHD. Several reports indicated the therapeutic effects of JAK1/2 inhibitor Ruxolitinib for patients with acute and chronic GVHD 12 –14 . The clinical trials of another JAK1/2 inhibitor Baricitinib for acute or chronic GVHD is ongoing (NCT04131738, NCT02759731) 15 . Recently, a JAK1 inhibitor Itacitinib was demonstrated to have a good response rate for acute GVHD in clinical trials 16,17 and its effects on chronic GVHD remain to be elucidated. SHR0302 is a novel JAK inhibitor with a good selectivity in JAK1 and JAK2, has been proved useful in autoimmune disease models such as rheumatoid arthritis 18 . Therefore, it would be a highly activea gent for GVHD.
Herein, we showed that SHR0302 not only prevented aGVHD but also reversed aGVHD in the mouse model. In this model, SHR0302 regulated T cell subsets (Th1/Treg), inhibited the production of inflammatory cytokines and chemokines, reduced chemokine receptor CXCR3 expression, and ultimately improved overall survival (OS) and progression-free survival (PFS) in mice. In conclusion, the results suggested the novel JAK inhibitor SHR0302 might be used as a novel preventive and therapeutic drug for aGVHD.
Materials and Methods
Mice
Male C57BL/6 (H-2Kb) and female Balb/c (H-2Kd) mice between 6 and 8 weeks of age were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd (China) (SCXK 2016-0006) and kept in a specific pathogen-free environment of Shanghai General Hospital Animal Experiment Center (ID: SYXK 2019-0028). The mice were maintained, handled and sacrificed under the guidance of Institutional Animal Care and Use Committee (IACUC) of Shanghai General Hospital (2019-A007-01).
Bone Marrow Transplantation
Bone marrow (BM) cells of donor mice were T-cell-depleted (TCD) with anti-CD3, -CD4 and -CD8 antibodies conjuncted with Streptavidin magnetic beads (StemCell Technologies, Vancouver, BC, Canada) in an EasySep™ Magnet Separator (StemCell). To establish the C57BL/6→Balb/c aGVHD murine model, each female Balb/c mouse were transplanted with 1 × 107 TCD bone marrow cells and 1.5 × 106 spleen cells from donor male C57BL/6 mice at day0 after lethally X-ray irradiation of 8 Gy at day-1 19 . The model mice developed aGVHD symptoms at 2 weeks after bone marrow transplantation (BMT) and peaked at about 3-4 weeks after BMT.
SHR0302 Prescription in Vivo
SHR0302 (C18H22N8O2S·H2SO4, 99% purity) was prescribed to aGVHD mice after BMT (SHR0302 group). SHR0302 was solubilized in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) and resuspended in drinking water. The final volume of oral medication given to each mouse was 100 µL and the final DMSO ratio was 1%. The mice in the aGVHD control group (Vehicle group) received the same amount of DMSO. In addition, we used the TCD-BMT mice(transplanted with TCD bone marrow cells only) as the negative control with no aGVHD symptoms.
Histology & Immunohistochemistry
In order to grade GVHD lesions histopathologically, model mice in each group were sacrificed on different time points after BMT, and skin, liver, small intestine and lung tissues were harvested. These tissues were fixed in 4% formalin and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) and classified with semi-quantitative scoring ranging from 0-4 by a pathologist who was blinded to animal experiments. The sections from mouse skin, liver, small intestine and lung were immunostained with anti-mouse CD3 (Cell Signal Technology, Danvers, MA, USA) and anti-myeloperoxidase (MPO, Cell Signal Technology) antibodies, and the fields with CD3+ cell or MPO+ cell infiltrations under the microscope were chosen to count these specific cell numbers by two trained technicians without prejudice.
Cell Preparations
Mouse spleen mononuclear cells were collected after erythrocyte lysis, and separated into CD3+CD4+ T cells, CD3+CD8+ T cells, CD19+ B cells, naïve CD4+T cells, CD4+CD25+regulatory T cells and NK cells respectively with corresponding mouse isolation kits (Miltenyi Biotic GmbH, Bergisch Gladbach, Germany). The separated T lymphocytes were activated and expanded by mouse T cell activation/expansion kit (Miltenyi). Mouse B-lymphoma cell line A20 was purchased from ATCC (Manassas, VA, USA).The basic culture medium is RPMI-1640 medium (Gibco, Grand Island, NY, USA), 10% fetal bovine serum (FBS, Gibco), 100 IU/mL penicillin and 100 μg/mL streptomycin (Gibco). Separated mouse T lymphocytes were cultured in the basic cell culture medium add with 2–10 ng/ml mouse Interleukin-2 (IL-2, PeproTech, Rocky Hill, NJ, USA) and anti- mouse CD3/CD28 microbeads (Miltenyi). Separated mouse B lymphocytes were cultured in the basic cell culture medium added with lipopolysaccharide (LPS, Sigma). Human peripheral blood mononuclear cells (PBMCs)were separated by lymphoprep (StemCell) and cultured in in the basic cell culture medium added with anti-human CD3/CD28 beads (Miltenyi). All cells were incubated in a humidified atmosphere at 37°C in 5% CO2. The collection of human peripheral blood samples was approved by Ethics Review Committee of Shanghai General Hospital ([2019]298). Informed consent was obtained from all subjects involved in the study.
Flow Cytometry Analysis
The spleen and bone marrow of the model mice and healthy recipient control mice were harvested at the 6th or 16th week after BMT. Single cell suspensions from spleens and bone marrows were labeled with anti-CD3 PE, -CD4 FITC, -CD8 AF700 (BD Pharmingen, San Diego, CA, USA) for T cells, anti-CD19 FITC (BD) for B cells, anti-CXCR3 BV421 and anti-CD80 FITC for immune molecular examination, anti-CD4 FITC (BD) and anti-Foxp3 PE (eBioscience, San Diego, CA, USA) for regulatory T cells (Treg), anti-IFN-γ PE and -IL-17A PE-cy7 (BD) for Th1/Th17 subsets, respectively. Before detecting the secretion of IFN-γ and IL-17A in T cells, cell suspensions were activated with anti-mouse CD3/CD28 microbeads for 3 hours. The spleen mononuclear cells of mice transplanted with A20 lymphoma cells were stained with anti-B220 PE and -H-2Kb BV421 (eBioscience) to examine the ratio of tumor cells. Activated human PBMCs with anti- human CD3/CD28 beads were stained with anti-human CD3 FITC and -CD69 PE for T cell activation analysis or with anti-human CD4 PE-cy7, -T-bet PE, -Foxp3 AF647, -GATA3 APC and -ROR?t PE (eBioscience) for T cell differentiation analysis after incubation with SHR0302 (1 μM) for 24 h. Fixable viability dye or DAPI (BD) were used to distinguish live cells from dead cells.
Luminex Cytokine Assay
The cell culture supernatant was respectively collected from separated CD4+ T, CD8+ T and CD19+B cells from spleens of normal recipient control mice incubated with different concentrations of SHR0302. Mouse plasma was collected from the GVHD model mice and normal recipient control mice. Cytokine levels in mouse plasma and cell culture supernatant were evaluated using Luminex assay including mouse CCL2, CCL4, CCL22, CXCL10, IL-6, IL-10, IL-12 p70, IL-17A, IFN-γ, TNF-α, MMP-3 and osteopontin (OPN).
Enzyme-Linked Immunosorbent Assay (ELISA)
Th1 cells were cultured from separated naïve CD4+T cells with cytokine IL-12 (Peprotech) stimulation and anti-IL-4 antibody (eBioscience) blockade. The cell culture supernatant was respectively collected from Th1, Treg and NK cells from spleens of normal recipient control mice incubated with different concentrations of SHR0302. The levels of IL-2, IL-10, IFN-γ and TNF-α protein in the cell culture supernatant were determined by using associated ELISA kit (IBL International GmbH, Hamburg, Germany) according to the manufacturer’s instructions.
Phosphorylated Protein Array Analysis
Fresh samples of PBMCs obtained from four aGVHD patients were incubated with SHR0302 or vehicle for 48 hours at 37°C in vitro. The collected cell pellets were stored at - 80 °C for analysis. Total proteins were extracted from cell samples and tested for multiple phosphorylated protein levels with RayBio® AAH-PPP-1-4 and AAH-PRTK-1-4 kit (RayBiotech, Norcross, GA, USA). The screening criteria of the difference between groups is fold Change =< 0.83 or >= 1.2.
Western Blotting Assay
Separated T cells and B cells incubated with different concentrations ofSHR0302 were lysed in RIPA lysis buffer (Cell Signaling Technology). Cytosolic proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene difluoride membranes, blocked in blocking solution (CST) for 1 h and incubated overnight at 4°C with the primary antibodies, including antibodies for total-JAK1, -JAK2, -STAT1, -STAT3, -STAT5, -extracellular regulated protein kinases (ERK)1/2, -AKT, phosphor-JAK1, -JAK2, -STAT1, -STAT3, -STAT5, -ERK1/2, -AKT and GAPDH (Cell Signaling Technology). Membranes were washed and incubated with the corresponding secondary antibodies for 1 h at room temperature. Protein signals were subsequently analyzed with the Amersham Imager 600 system (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Statistical Analysis
Survival analysis was performed according to the Kaplan–Meier method, and survival curves were compared using the log-rank testing. Data from GVHD patients were analyzed using the paired sample t test. Other data were analyzed using one-way ANOVA. Values of P < 0.05 were considered statistically significant.
Results
SHR0302 Prevented aGVHD and Enhanced Hematopoietic Reconstitution
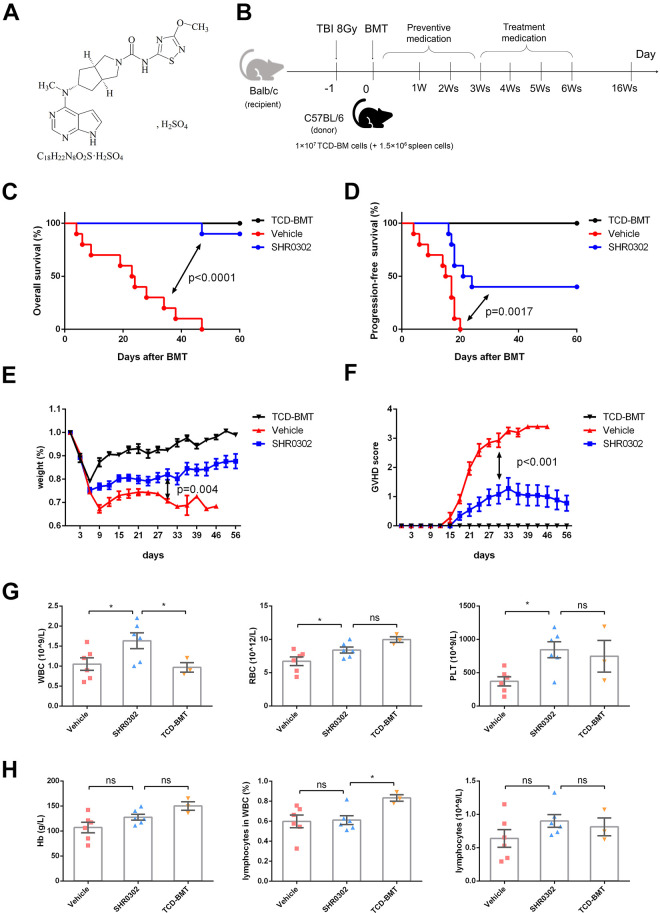
SHR0302 is a novel JAK inhibitor (Fig. 1A) with good selectivity especially on JAK1 and JAK2, and demonstrates superiority compared with other JAK inhibitors such as Ruxolitinib and Tofacitinib (Table 1). An aGVHD mouse model was established to study the effects of SHR0302 on aGVHD (Fig. 1B). Previous study showed that several dose levels of SHR0302 (2.5, 5.0, and 10.0 mg/kg bid) had a certain therapeutic effect on collagen-induced arthritis (CIA) in DBA/1 mice 18 . Therefore, the prophylactic dose in this experiment was set at 5 mg/kg twice a day for 3 weeks, and SHR0302 was given on the first day after BMT. The overall survival of mice in the SHR0302 group was significantly higher than that in the vehicle group (P < 0.0001) (Fig. 1C). The progression-free survival (PFS) also showed a significant difference (P = 0.0017) and 40% of mice in the SHR0302 group did not appear any aGVHD symptoms, while 100% of mice in the vehicle group presented with aGVHD at 30 days after BMT (Fig. 1D). The relative bodyweight of the mice in the SHR0302 group increased significantly as compared with the vehicle group (P = 0.004) at 30 days after BMT (Fig. 1E). At the same time, the aGVHD scores of mice in the SHR0302 group showed significantly lower than that in the vehicle group (P < 0.001) (Fig. 1F). In addition, compared with mice in the vehicle group, mice treated with SHR0302 showed faster recovery of blood cell counts, including white blood cells (WBCs), red blood cells (RBCs), and platelets (PLTs) (Fig. 1G). The total numbers of lymphocytes in the SHR0302 group were not significantly different from the TCD-BMT group (Fig. 1H).
Figure 1.
SHR0302 prevented aGVHD in vivo. (A) The chemical structure of SHR0302. (B) The model of murine aGVHD: female Balb/c mouse were transplanted with 1 × 107 TCD-BM cells and 1.5 × 106 spleen cells from male donor C57BL/6 mouse at day0 after total body irradiation of 8 Gy at day-1. (C) The overall survival of aGVHD mice in all groups including TCD-BMT (n = 5), vehicle (n = 10) and SHR0302 (n = 10) group. SHR0302 was administrated at dosage of 10 mg/kg twice a day from day 0 to day21 after BMT for preventive medication. (D) The progression-free survival (PFS) of aGVHD mice in all experimental groups as above. PFS is defined as the time from randomization to GVHD progression or death in mice. The worsening of GVHD-related symptoms in mice is considered to be the progression of GVHD. (E) The relative bodyweight of aGVHD mice in all experimental groups. (F) The GVHD scores of aGVHD mice in all experimental groups. (G) The white blood cell (WBC), red blood cell (RBC) and platelet (PLT) counts in the peripheral blood of aGVHD mice at day14 after BMT in all experimental groups. The counting was performed by Sysmex pocH-100iTM Automated Hematology Analyzer. *P < 0.05. (H) The hemaglobin (Hb), lymphocyte ratio and lymphocyte count in the peripheral blood of aGVHD mice at day14 after BMT in all experimental groups. The counting was analyzed by Sysmex pocH-100iTM Automated Hematology Analyzer. *P < 0.05.
Table 1.
The IC50 of SHR0302 and Other JAK Inhibitors.
| Janus kinase family | IC50(nM) | |||
|---|---|---|---|---|
| SHR0302 | Ruxolitinib | Tofacitinib | Baricitinib | |
| JAK1 | 0.1 | 3.3 | 112 | 5.9 |
| JAK2 | 0.9 | 2.8 | 20 | 5.7 |
| JAK3 | 7.7 | 428 | 1 | 560 |
| TYK2 | 42 | 19 | / | 53 |
SHR0302 Reversed aGVHD and Improved Survival in Vivo
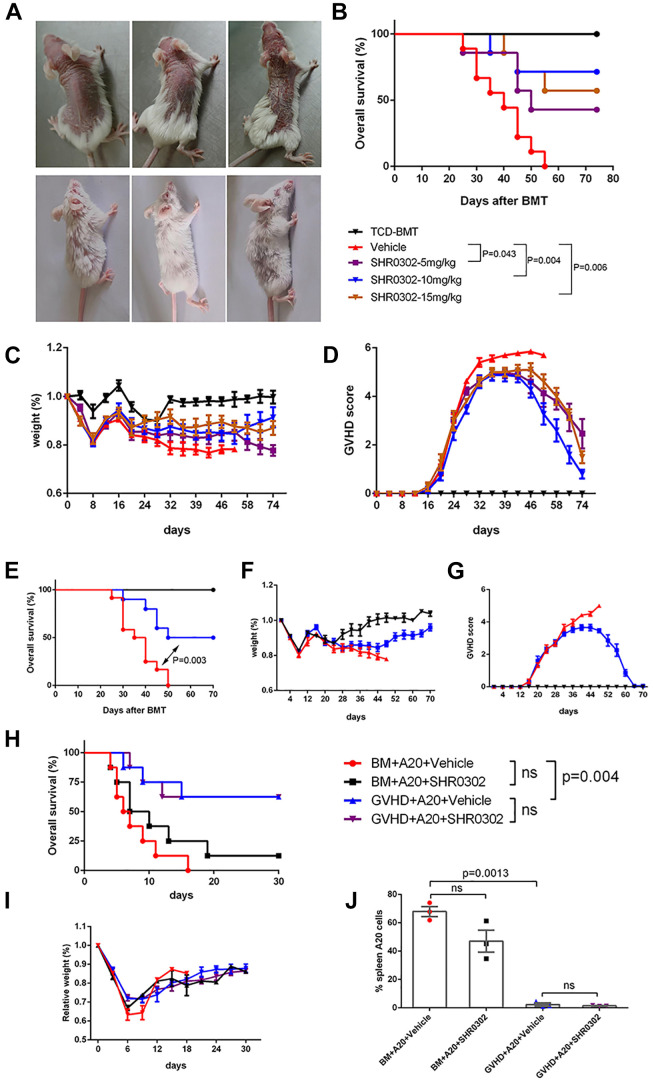
In order to study whether SHR0302 could reverse the aGVHD symptoms in mouse models, the first dose of SHR0302 was administrated at 21 days after BMT when they presented with severe GVHD symptoms. The administration doses were set at 5, 10, and 15 mg/kg twice a day for 3 weeks, respectively. The results showed that the skin lesions in the SHR0302 group gradually reduced and returned to normal between 6 weeks and 16 weeks after BMT (Fig. 2A). At 74 days after BMT, the survival of mice in the SHR0302 group was significantly higher than that in the vehicle group (5 mg/kg, P = 0.043; 10 mg/kg, P = 0.004; 15 mg/kg, P = 0.006), and the survival of mice in 5 mg/kg group was lower than that in 10 mg/kg and 15 mg/kg groups, but without a statistical difference (Fig. 2B). The relative bodyweight of the mice in SHR0302 10 mg/kg and 15 mg/kg groups completely recovered to normal after the weight loss caused by aGVHD and showed no statistical differences with that of mice in the TCD-BMT group (P = 0.685, P = 0.178 respectively). However, the relative bodyweight of mice in the SHR0302 5 mg/kg group recovered significantly slowly compared with that of mice in the TCD-BMT group (P = 0.012) (Fig. 2C). Correspondingly, the aGVHD scores in SHR0302 10 mg/kg and 15 mg/kg groups gradually reduced and showed no statistical differences with that in the TCD-BMT group (P = 0.270, P = 0.053 respectively), while not in SHR0302 5 mg/kg group (P = 0.009) (Fig. 2D).The comparison of the relative bodyweight and aGVHD score between the vehicle group and the SHR0302 group could not be completed due to the short survival of mice in the vehicle group. These data indicated that 5 mg/kg of SHR0302 couldn’t restore the weight of aGVHD mice to normal and had significantly lower survival compared with mice in 10 mg/kg dosage and 15 mg/kg dosage group, while 15 mg/kg dosage did not achieve a higher survival than 10 mg/kg dosage. Therefore, the dosage of SHR0302 10 mg/kg twice a day was chosen to carry out aGVHD animal experiments to re-evaluate the effects of SHR0302. The repeated experiments again proved that SHR0302 significantly improved the overall survival of aGVHD mice (P = 0.003) (Fig. 2E), restored bodyweight (Fig. 2F), and reduced aGVHD symptoms (Fig. 2G). These results suggested that SHR0302 could strongly reverse aGVHD progression.
Figure 2.
SHR0302 reversed aGVHD (Figure A–G) and maintained GVT effects (Figure H-K) in vivo. (A) The representative visual analysis of 3 mice in the vehicle group at 6th week after BMT and 3 mice in SHR0302 10 mg/kg treatment group at 16th week after BMT. SHR0302 was administrated at dosages of 5, 10, and 15 mg/kg twice a day respectively from day22 to day42 after BMT for treatment medication. (B) The overall survival of aGVHD mice in all groups including TCD-BMT (n = 5), vehicle (n = 9) and SHR0302 5 mg/kg (n = 7), SHR0302 10 mg/kg (n = 7) and SHR0302 15 mg/kg (n = 7) group. (C) The relative weight of aGVHD mice in all experimental groups. (D) The GVHD scores of aGVHD mice in all experimental groups. (E) The overall survival of aGVHD mice in another independent mouse experiment including TCD-BMT (n = 5), vehicle (n = 12) and SHR0302 10 mg/kg (n = 10) groups. SHR0302 was administrated at the dosage of 10 mg/kg twice a day from day22 to day42 after BMT. (F) The relative weight of aGVHD mice in all experimental groups. (G) The GVHD scores of aGVHD mice in all experimental groups. To evaluate the affecting of SHR0302 on GVT effects, lethally irradiated (8 Gy) BALB/c mice were transplanted with 1 × 107 TCD-BM cells from donor B6 mice plus 2 × 106 A20 cells with or without 2.5 × 106 spleen cells per mouse. SHR0302 was administrated at a dosage of 10 mg/kg twice a day from day 1to 14 after BMT. (H) The overall survival of mice in four groups including BM+A20+vehicle, BM+A20+SHR0302, GVHD+A20+vehicle and GVHD+A20+SHR0302 group (n = 8 in each group). (I) The relative weight of mice in BM+A20+vehicle, BM+A20+SHR0302, GVHD+A20+vehicle and GVHD+A20+SHR0302 group. (J) The percentages of H-2Kb-B220+ cells in spleens of mice on day 8 after BMT in BM+A20+vehicle, BM+A20+SHR0302, GVHD+A20+vehicle and GVHD+A20+SHR0302 group. Bars show the mean ± SEM. *P < 0.05.
SHR0302 Preserved Graft-Versus-Tumor Effects in vivo
Next, we evaluated whether SHR0302 could affect the graft-versus-tumor (GVT) effects. A20 cells were co-transplanted with TCD-BM cells (BM+A20) or TCD-BM plus spleen cells(GVHD+A20) into recipient mice for analyzing the effects of SHR0302 with a dosage of 10 mg/kg twice a day on GVT. Mice in BM+A20+Vehicle or SHR0302 groups died rapidly within 10 days after transplantation due to the lymphoma cell invasion (data not shown). However, about 60% of mice in GVHD+A20+Vehicle or SHR0302 groups survived over 30 days after BMT without GVHD symptoms. The survivals of mice with spleen cells were significantly higher than that without spleen cells (BM+A20+Vehicle vs GVHD+A20+vehicle, P = 0.004, or BM+A20+SHR0302 vs GVHD+A20+SHR0302, P = 0.046), whether SHR0302 was prescribed or not. The survivals of mice in GVHD groups were similar without any association with SHR0302 (GVHD+A20+vehicle vs GVHD+A20+SHR0302, P = 0.99) (Fig. 2H). No significant differences were observed in the weight trends of mice in each group (Fig. 2I). Then we tested the infiltration of A20 cells in the spleen of each group of mice. The percentages of A20 cells in mouse spleen were similar between groups with and without SHR0302 (BM+A20+vehicle vs BM+A20+SHR0302, P = 0.0971; GVHD+A20+vehicle vs GVHD+A20+SHR0302, P = 0.5239), while were significantly reduced in groups with spleen cells compared to without spleen cells (BM+A20+vehicle vs GVHD+A20+vehicle, 67.97 ± 3.552 vs 2.293 ± 1.150, P = 0.0013) (Fig. 2J). These results suggested that SHR0302 could not affect GVT effects.
SHR0302 Decreased the Damage of Target Tissues and Infiltration of Immune Cells Caused by aGVHD
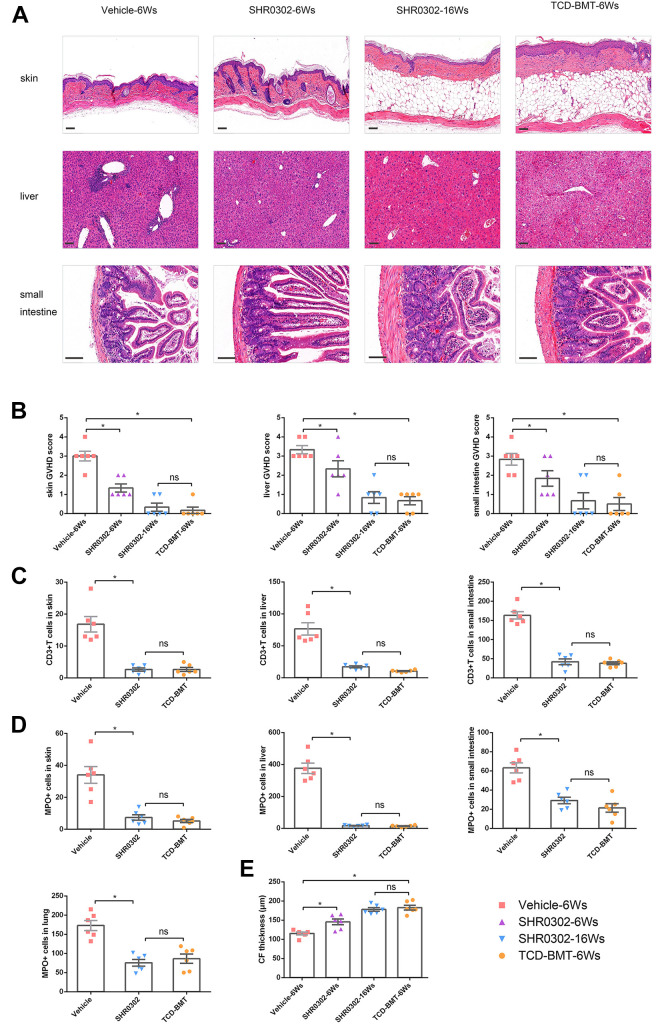
The therapeutic effects of SHR0302 on aGVHD were focused in our following experiments. Histological analysis showed that the tissue lesions of skin, liver, and small intestine were significantly reduced at the 6th week after BMT and returned to normal levels at the 16th week after BMT in aGVHD mice treated with SHR0302 compared with mice in the vehicle group (Fig. 3A). Compared to the vehicle group, the pathological scores of the skin (P < 0.001), liver (P < 0.001), and small intestine (P < 0.001) of mice in the SHR0302 group reduced significantly. On the other hand, at the 16th week after BMT, the skin, liver, and small intestine GVHD scores of mice in the SHR0302group showed no significant differences with that of mice in the TCD-BMT group (P = 0.588, P = 0.699, P = 0.754, respectively) (Fig. 3B). Immunohistochemical staining in these target tissues showed that SHR0302 prevented the recruitment of T cells to the target tissues of aGVHD mice (Supplemental Fig. S1A). Quantitative immunohistochemical analysis demonstrated that SHR0302 significantly reduced T cell infiltration in the skin (P < 0.001), liver (P = 0.004), and small intestine (P < 0.001) compared to that of mice in the vehicle group (Fig. 3C). The infiltration of neutrophils in various target tissues was more obvious than that of T cells, especially in the liver and lung (Supplemental Fig. S1B). As mentioned above, quantitative immunohistochemical analysis showed that the absolute numbers of neutrophils in the skin (P < 0.001), liver (P < 0.001), small intestine (P < 0.001), and lung (P < 0.001) decreased significantly after SHR0302 treatment (Fig. 3D). The skin collagen fiber (CF) thickness analysis showed that SHR0302 increased CF thickness compared with that in the vehicle group (P < 0.001) and retarded aGVHD progression in mouse models (Fig. 3E, Supplemental Fig S1C). In conclusion, SHR0302 could effectively decrease the pathological changes and reduce the infiltrating T cells and neutrophils in the target tissue of aGVHD mice.
Figure 3.
SHR0302 decreased the damage of target tissues and infiltration of immune cells caused by aGVHD. (A) H&E-staining of skin, liver and small intestine tissuesin4 groups of mice: vehicle-6Ws, SHR0302-6Ws, SHR0302-16Ws and TCD-BMT-6Ws (6Ws and 16Ws: at 6th and 16th weeks after transplantation) (n = 6 in each group). Representive figures of HE staining from one mouse were shown. Original magnification: skin and liver × 100, small intestine × 200. Scale bars: 100 μm. (B) The GVHD pathologic scores of skin, liver and small intestine in 4 groups of mice: vehicle-6Ws, SHR0302-6Ws, SHR0302-16Ws and TCD-BMT-6Ws (n = 6 in each group). (C) Blinded pathologic analysis of anti-CD3-stained skin, liver and small intestine tissues obtained from 3 groups of mice: vehicle, SHR0302, and TCD-BMT (n = 6 in each group). CD3+ T cells were calculated for each mouse. (D) Blinded pathologic analysis of anti-MPO-stained skin, liver and small intestine tissues from 3 groups of mice: vehicle, SHR0302, and TCD-BMT (n = 6 in each group). MPO+ neutrophils were calculated for each mouse. (E) Masson-stained skin preparations and average collagenous fiber thickness in 4 groups of mice: vehicle-6Ws, SHR0302-6Ws, SHR0302-16Ws and TCD-BMT-6Ws (n = 6 in each group). Data were pooled from two separate experiments. Bars show the mean ± SEM. *P < 0.05.
SHR0302 Increased Tregs and Reduced Th1 Cells
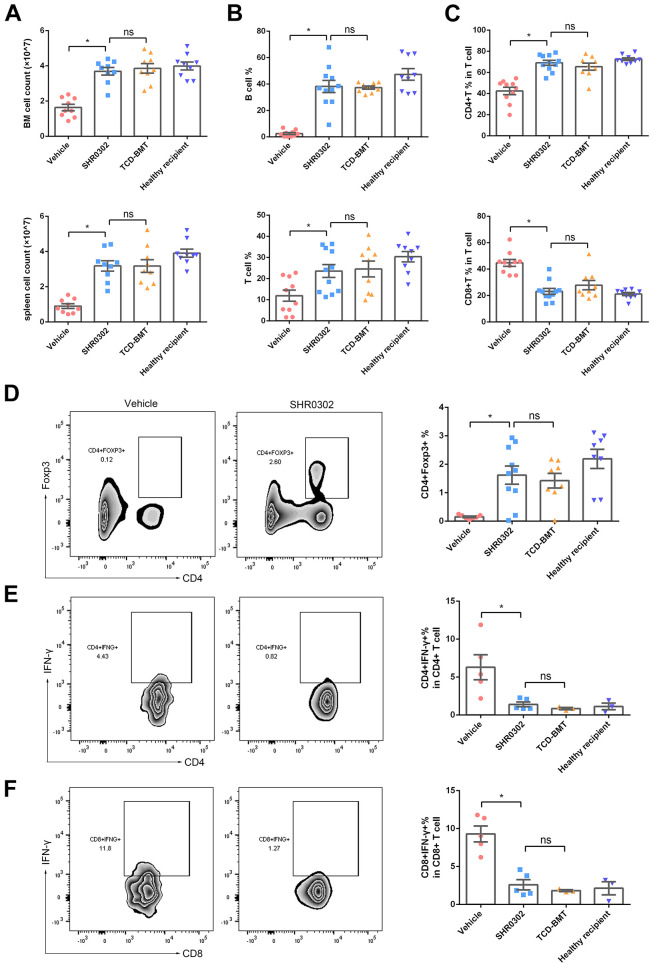
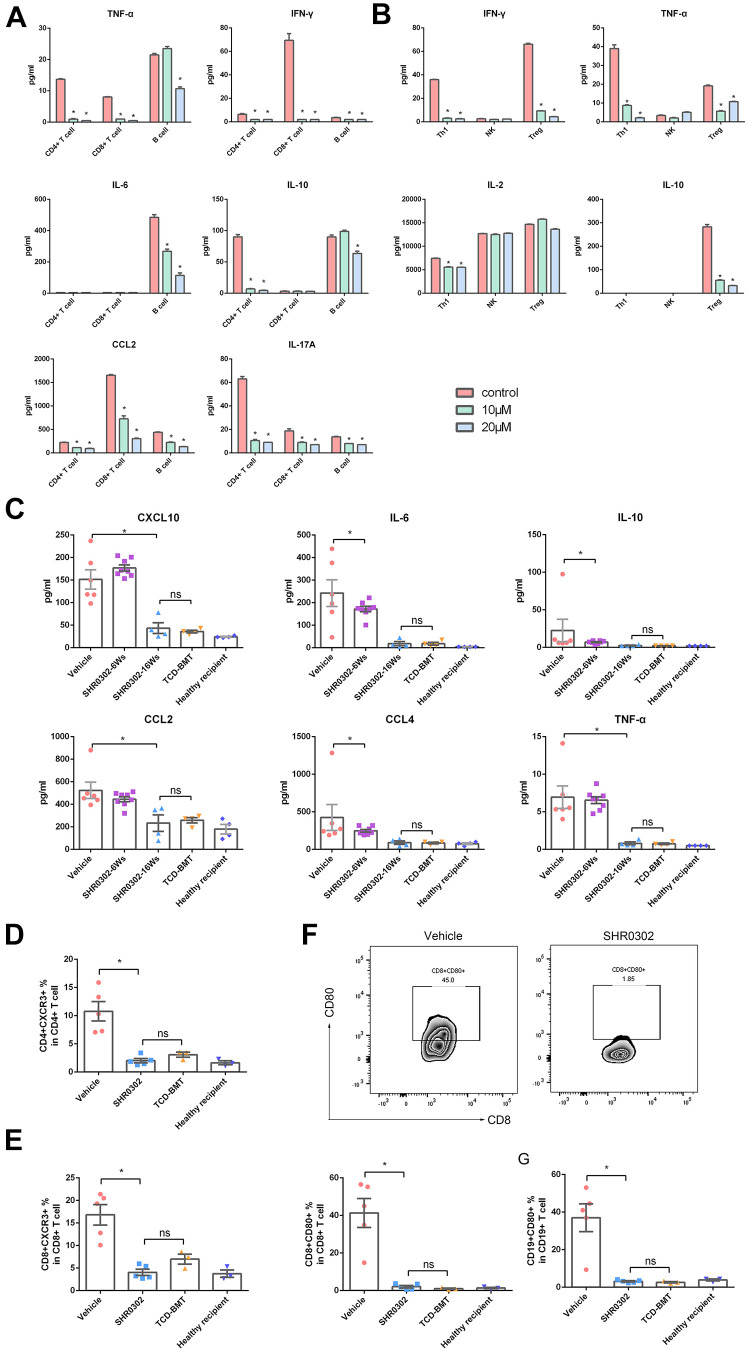
The total cell numbers in the spleen (P < 0.001) and bone marrow (P < 0.001) of mice in the vehicle group were much lower than that of the TCD-BMT group and SHR0302 group, indicating that the lower cell numbers in the spleen and bone marrow caused by aGVHD could be restored by SHR0302 treatment (Fig. 4A). The functional subgroups of immune cells in the GVHD environment need to be explored. We examined CD19+ B cells, CD4+ T cells, CD8+ T cells, CD4+IFN-γ+ cells, CD8+IFN-γ+ cells, and CD4+Foxp3+ cells by flow cytometer. The study showed that through the administration of SHR0302 in aGVHD mice, the percentages of B cells (P < 0.001) and T cells (P = 0.010) were increased (Fig. 4B), the inverted proportion of CD4+ T (P < 0.001)and CD8+ T cells (P < 0.001) returned to normal (Fig. 4C), the proportions of Treg cells increased dramatically (P = 0.001, Fig. 4D), while the proportions of CD4+IFN-γ+ cells (P = 0.040, Fig. 4E) and CD8+IFN-γ+ significantly decreased (P = 0.001, Fig. 4F) in total spleen cells. In the end, the total immune cell counts and percentages of T cell subgroups of the spleen in aGVHD mouse were restored after SHR0302 treatment and showed no statistical differences compared to that in the TCD-BMT mouse or the healthy recipient control mouse. The results demonstrated that SHR0302 could restore the proportion of T cell subsets to a normal state by reducing the pathological T cells in vivo.
Figure 4.
SHR0302 increased Tregs and reduced Th1 cells. (A) The cell counts of bone marrow (BM) and spleen in 4 group mice: vehicle-6Ws, SHR0302-16Ws, TCD-BMT-6Ws, and healthy recipient control (n = 9 for each group) (6Ws and 16Ws: at 6th and 16th weeks after transplantation, respectively). (B) The percentages of CD3+ T cells and CD19+ B cells in spleen cells in four groups: vehicle-6Ws (n = 10), SHR0302-16Ws (n = 11), TCD-BMT-6Ws (n = 9), and healthy recipient control (n = 9). (C) The percentages of CD4+ T cells and CD8+ T cells in all T cells of spleen in four groups: vehicle-6Ws (n = 10), SHR0302-16Ws (n = 11), TCD-BMT-6Ws (n = 9), and healthy recipient control group (n = 9). (D) The percentages of CD4+Foxp3+ cells of spleen in vehicle-6Ws (n = 7), SHR0302-16Ws (n = 11), TCD-BMT-6Ws (n = 9), and healthy recipient control groups (n = 9), respectively. (E) The percentages of CD4+IFN-γ+ cells in all CD4+ cells of spleen in four groups: vehicle-6Ws (n = 5), SHR0302-16Ws (n = 5), TCD-BMT-6Ws (n = 3), and healthy recipient control (n = 3). (F) The percentages of CD8+IFN-γ+ cells in all CD8+ cells of spleen in four groups: vehicle-6Ws (n = 5), SHR0302-16Ws (n = 5), TCD-BMT-6Ws (n = 3), and healthy recipient control mice (n = 3). Data were pooled from two separate experiments in Figure A-D. Bars show the mean ± SEM. *P < 0.05.
SHR0302 Reduced Expression ofTh2 Cytokine IL-6 and CXCR3/CXCL10
The mechanisms of SHR0302 on reducing aGVHD activity were associated with inhibiting T cell activation. The effects of SHR0302 on immune cell functions were first evaluated in vitro. The results showed that SHR0302 inhibited the cell expansion of CD4+T, CD8+T and B cells (Supplemental Fig. S2). Moreover, SHR0302 inhibited cell activation (CD69 as biomarker) and cell proliferation (Ki67 as biomarker) levels of both CD4+and CD8+ T cells in a dose-dependent manner (Supplemental Fig. S3). After incubation with 10 μM and 20 μM concentrations of SHR0302 for 48hours, the expression levels of multiple proinflammatory cytokines or chemokines associated with aGVHD were tested in isolated CD4+ T cells, CD8+ T cells, B cells as well as isolated Th1, Treg and NK cells in vitro. We found that IL-10 (mainly secreted by CD4+ T cells), IFN-γ, CCL2, CCL4 (mainly secreted by CD8+ T cells), TNF-α, and IL-6 were downregulated by SHR0302 (Fig. 5A). In addition, TNF-α, IFN-γ and IL-2 secretion were reduced in Th1 cells while TNF-α, IFN-γ and IL-10 but not IL-2 secretion was decreased in Treg cells by SHR0302. It was not found that SHR0302 affected the cytokine secretion of NK cells (Fig. 5B). The results showed that SHR0302 significantly suppressed the function of Th1 cells and partially inhibited the function of Treg cells without influencing NK cells. In the mouse model, the expression levels of these cytokines and chemokines in plasma of each group were also tested. The results in vivo demonstrated that Th2 cell-produced cytokines IL-6 and IL-10, and GVHD highly related chemokines CXCL10, CCL2, and CCL4 20 decreased after SHR0302 treatment in aGVHD mice. Moreover, at the 16th week after BMT, the expression levels of these cytokines and chemokines in the SHR0302 treated mice were similar to that in the TCD-BMT group and the healthy recipient control mice, while all the mice in the vehicle group died of disease progression (Fig. 5C). Flow cytometry analysis showed that CXCR3 (the receptor of CXCL10) expression on donor CD4+ T (P = 0.006) and CD8+ T cells (P = 0.004) decreased withSHR0302 treatment compared with mice in the vehicle group (Fig. 5D, E). On the other hand, the expression of CD80 in both allogeneic CD19+ APCs (P = 0.010) and CD8+ T cells (P = 0.007) was extremely downregulated and close to normal level after SHR0302 treatment in aGVHD mice (Fig. 5F, G). These findings indicated that the protective effect of SHR0302 on aGVHD is accomplished by inhibiting the activation and expansion of donor T cells mediated by IFN-γ/IL-6 and CXCR3/CXCL10.
Figure 5.
SHR0302 reduced Th2 cytokine IL-10 and CXCR3 expression. (A) The expression levels of TNF-α, IL-6, IL-10, CCL2, CCL4 and IFN-γ in the supernatant of separated mouse CD4+ T, CD8+T and B cells incubated with or without SHR0302 after 48 hours (n = 3 in each group). (B) The expression levels of TNF-α, IL-2, IL-10 and IFN-γ in the supernatant of separated mouse Th1, Treg and NK cells incubated with or without SHR0302 after 48 hours (n = 3 in each group). (C) The expression levels of TNF-α, IL-6, IL-10, CXCL10, CCL2 and CCL4 in plasma of 5 group mice: TCD-BMT-6Ws (n = 6), vehicle-6Ws (n = 8), SHR0302-6Ws (n = 4), SHR0302-16Ws (n = 4) and healthy recipient control mice (n = 4) (6Ws and 16Ws: at 6th and 16th weeks after transplantation, respectively). (D) The percentages of CD4+CXCR3+ cells in all CD4+ cells of spleen in four group mice: vehicle-6Ws (n = 5), SHR0302-16Ws (n = 5), TCD-BMT-6Ws (n = 3), and healthy recipient control (n = 3). (E) The percentages of CD8+CXCR3+ cells in all CD8+ cells of spleen of mice in four groups: vehicle-6Ws (n = 5), SHR0302-16Ws (n = 5), TCD-BMT-6Ws (n = 3), and healthy recipient control mice (n = 3). (F) The percentages of CD8+CD80+ cells in all CD8+ cells of spleen in four groups: vehicle-6Ws (n = 5), SHR0302-16Ws (n = 5), TCD-BMT-6Ws (n = 3), and healthy recipient control (n = 3). (G) The percentages of CD19+CD80+ cells in all CD19+ cells of spleen in four groups: vehicle-6Ws (n = 5), SHR0302-16Ws (n = 5), TCD-BMT-6Ws (n = 3), and healthy recipient control (n = 3). Bars show the mean ± SEM. *P < 0.05.
SHR0302 Regulated AKT and ERK1/2 Signal Pathways
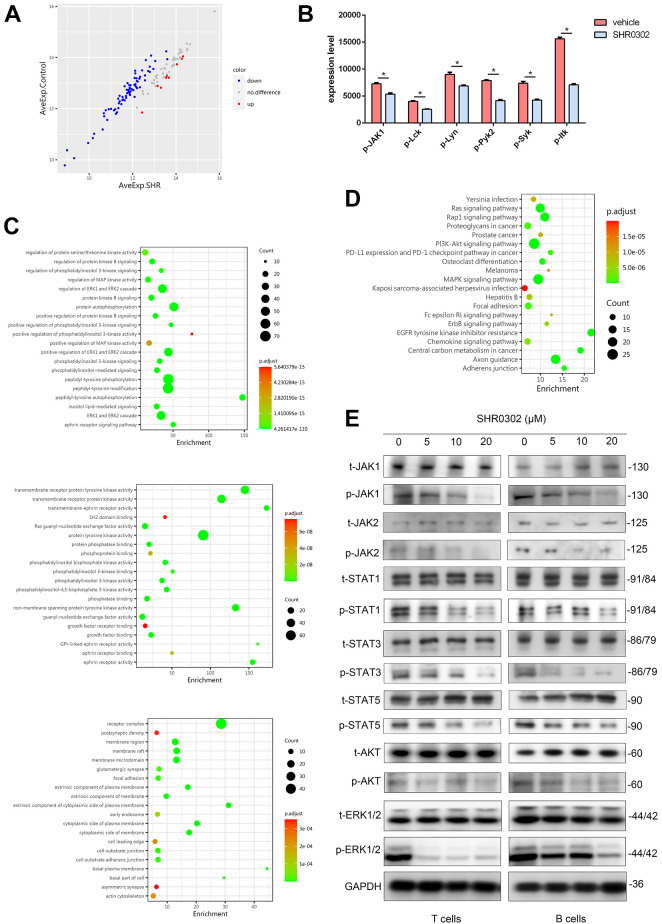
We are aware that SHR0302 is an inhibitor of JAK signals, but so far, the downstream molecules or pathways related with the effects of SHR0302 on aGVHD are still not clear. The results of the phosphorylated protein array experiments showed that SHR0302 inhibited the phosphorylation process of many important molecules that regulated various biological functions of lymphocytes (Fig. 6A, B). GO enrichment analysis on molecular function, biological process, and cellular component suggested that SHR0302 affected the receptor complex and membrane microdomain, which inhibited the activities of multiple protein kinases (Fig. 6C). KEGG signaling pathway analysis also showed that SHR0302 regulated pathways of phosphatidylinositol 3 kinases (PI3 K)/AKT, ERK1/2, osteoclast differentiation, and other signals (Fig. 6D). The western blotting assay showed that the expression levels of phosphorylated JAK1/2, phosphorylated STAT1/3/5, phosphorylated AKT, and phosphorylated ERK1/2were reduced by SHR0302 in a dose-dependent manner (Fig. 6E). Interestingly, the phosphorylated AKT was reduced mostly in B cells, while the ERK1/2 pathway was downregulated obviously in T cells. These results showed that SHR0302 controlled the progression of aGVHD by regulating AKT and ERK1/2 signaling pathways, which were the downstream molecules of JAK signals.
Figure 6.
SHR0302 regulated AKT and ERK1/2 pathways. The results of the phosphorylated protein array for human peripheral blood mononuclear cells (PBMCs) from 4 patients conditioned with vehicle or SHR0302 in vitro. After the original data is normalized by the software, fold change (FC) was used to screen the differential expressed proteins. GO enrichment analysis and KEGG pathway analysis were performed based on logFC and p value of these differential expressed proteins. (A) The horizontal and vertical coordinates represented the average expression (AveExp) levels of each group. Red represented the up-regulated protein, and blue indicated down-regulated protein, grey showed no significant difference. (B) The expression levels of phosphorylated proteins in the vehicle group and SHR0302 group: JAK1, Lck, Lyn, Pyk2, Syk, and Itk. (C) Gene Ontology (GO) can be divided into three parts: molecular function, biological process and cellular component. (D) The KEGG pathway with enrichment function obtained with phosphorylated protein array analysis. (E) Separated T cells and B cells were incubated with the different concentrations of SHR0302 (0, 5, 10 and 20 μM) for 48 h. Western blot analysis was performed to examine the protein levels of total-JAK1, phospho-JAK1, total-JAK2, phospho-JAK2, total-STAT1, phospho-STAT1, total-STAT3, phospho-STAT3, total-STAT5, phospho-STAT5, total-AKT, phospho-AKT, total-ERK1/2, phospho-ERK1/2 and GAPDH, respectively.
SHR0302 Blocked T Cell Activation in Patients with aGVHD
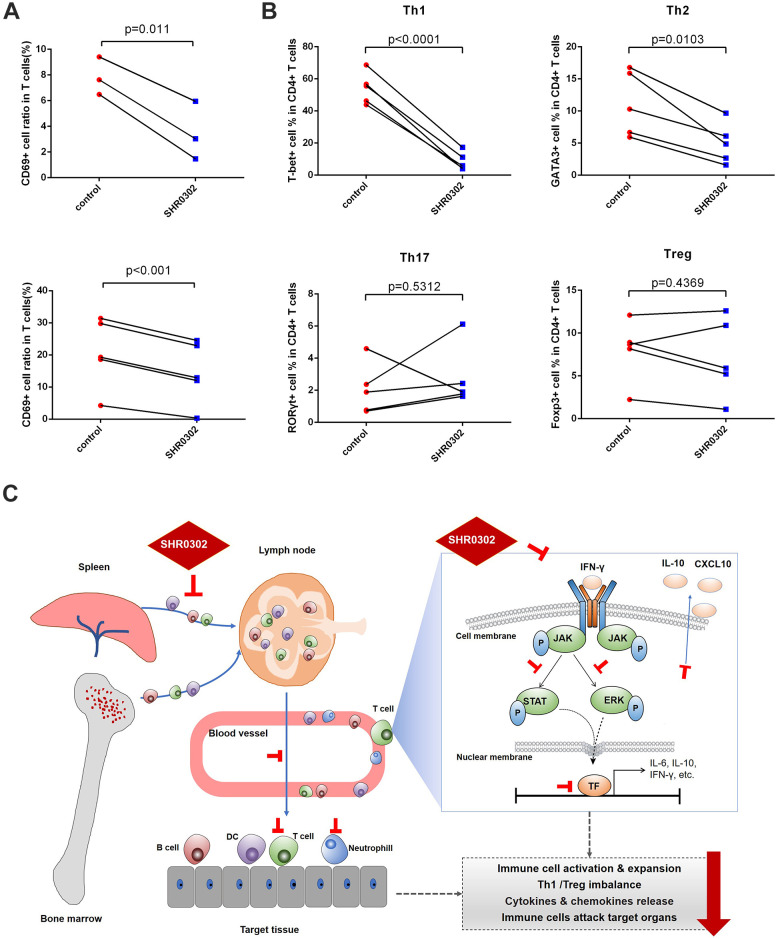
Our data demonstrated that the JAK pathway is essential for the progression of aGVHD, and SHR0302 could prevent and treat aGVHD in mouse models. To confirm that the therapeutic effects of SHR0302 are not limited to the mouse model, the functions of SHR0302 on T cells from patients suffered from severe or steroid-refractory aGVHD were tested. The results showed that after incubation of activated T lymphocytes (with anti-CD3/CD28 beads) with 1 μM SHR0302 for 24 hours, the CD69 surface marker was down-regulated in CD3+ T cells from both onset aGVHD patients (7.84% ± 0.69% vs 3.24% ± 1.07%, P = 0.011) and steroid-refractory aGVHD patients (20.67% ± 4.36% vs 14.52% ± 3.90%, P < 0.001) (Fig. 7A). These data showed that SHR0302 could block the immune receptor activation of human T cells from patients with aGVHD. We then explored the differentiation of T cells in human PBMCs, including Th1/Th2/Th17/Treg cells, marked by transcription factors T-bet/GATA3/RORγt/Foxp3, respectively. Flow cytometry analysis showed that SHR0302 significantly reduced the ratio of Th1 (54.24% ± 5.29% vs 8.56% ± 3.55%, P < 0.0001) and Th2 cells (11.26% ± 2.42% vs 6.14% ± 1.65%, P = 0.0103), but had no significant effects on Th17 and Treg cells (Fig. 7B). Overall, the novel JAK inhibitor SHR0302 could mitigate aGVHD through downregulating the phosphorylation of JAK1/2, inhibiting the activation and expansion of immune cells, regulating Th1/Treg cell differentiation, reducing the release of inflammatory factors of immune cells (Fig. 7C).
Figure 7.
SHR0302 blocked T cell activation from patients with aGVHD. PBMCs from patients with aGVHD were stimulated with anti-human CD3/CD28 beads and incubated with 1 μM SHR0302 or vehicle for 24 hours. (A) The CD69+ cell ratio among CD3+ T cells from onset aGVHD (up: n = 3) and steroid-refractory aGVHD patients (down: n = 5). (B) The cell ratio of Th1 (T-bet+), Th2 (GATA3+), Th17 (RORγt+) and Treg (Foxp3+) cells among CD4+ T cells. (C) Schematic diagram of mechanisms of SHR0302 mitigating aGVHD in mouse model.
Discussion
Our results also demonstrated that SHR0302 could successfully prevent and reverse aGVHD without affecting GVT effects. The possible mechanisms include regulating the differentiation of T cell subsets (especially Th1 and Treg cells), reducing the expression of CXCR3 on donor T cells, and inhibiting the secretion of inflammatory cytokines. Meanwhile, the results indicated that SHR0302 had no adverse effects on the multi-lineage hematopoietic reconstruction. Therefore, our preclinical data supported SHR0302 as a preventive or therapeutic drug for aGVHD without affecting GVT effects and the recovery of hematopoietic function.
Multiple studies have also shown that cytokines or chemokines 20 , including IL-6 21 , IL10 22 , IFN-γ 23 , TNF-α 24 , and CXCL10 25,26 , affected the activation, differentiation, and chemotaxis of effector T cells or regulatory T cells. JAK inhibitors could eliminate T lymphocytes and neutrophils accumulating in target organs and restore total cell numbers in bone marrow and spleen. Besides, aGVHD-related CD4+/CD8+T cell imbalance, Th1 cell expansion, Treg cell reduction in the spleen, and the upregulation of IL-6 and IL-10 can all be rectified by JAK inhibitors. It has been reported that IFN-γR signal transduction mediates alloreactive T cell trafficking and promotes the pathogenesis of aGVHD, while CXCR3, a downstream molecule of IFN-γR, induces the migration of donor CD4+ and CD8+ T cells to aGVHD target organs, which is related to total mortality 11,27 . On the other hand, CXCL10, the ligand of CXCR3, is also an important marker of GVHD 28,29 . TNF-α and IFN-γ secreted byTh1 cells of CD4+ T subsets are key factors that cause damages to target organs by stimulating the parenchymal cells to release CXCL10, which back to stimulate Th1 cells, thereby forming a positive feedback loop 30,31 . Since JAK inhibitors can successfully block the IFN-γR signaling pathway 10 , our experimental data also revealed that JAK inhibitors can break the IFN-γR/CXCL10/CXCR3 axis and re-modulate Th1 cell differentiation, CXCR3 expression, and CXCL10 secretion. In recent years, researchers have discovered that CD80 plays a very important role in the regulation of GVHD 32 . The interaction between IFN-γ-induced CD8+ T cells and PD-L1/CD80 can promote the expansion of donor CD8+ T cells 33 , while CD80 expression on APCs is also necessary to stimulate GVHD 34 . Some studies have indicated that the activation of AKT and ERK pathways regulate the activation of T lymphocytes in different ways 35 –37 , and they can also be inhibited by JAK inhibitors. We found that the expression level of phosphorylated ERK1/2 in T cells was more obviously inhibited by SHR0302 than JAK1 and JAK2 from western blot analysis. The reason might be that ERK1/2 is an important link in multiple molecular pathways and is influenced by other upstream molecules, which were demonstrated to be downregulated by SHR0302 according to the phosphorylated protein array analysis. In fact, JAK inhibitors do not directly kill pathogenic T cells, but inhibit their activation and expansion 12,38 , thereby reducing the infiltration of pathogenic T cells in target tissues and finally inhibit the progression of GVHD and restore it to a normal state. In our GVT model, the mice did not develop GVHD symptoms within 30 days after BMT in GVT mouse models and were sacrificed at 30 days after BMT, so the survivals of mice in “GVHD+A20+vehicle” and “GVHD+A20+SHR0302” groups were similar. It might be related with the large number of lymphoma cells in developing the GVT models. The onset of aGVHD in GVT mouse model might be delayed or did not occur, because the large amount of tumor cells could result in T cell exhaustion 39,40 .
Numerous researches have reported that JAK is an excellent target for the treatment of GVHD 8,10,11,14,16 , which influences the activation and expansion of donor T cells and their chemotaxis to target organs through multiple downstream pathways. A recent phase 3 study of JAK1 inhibitor Itacitinib on aGVHD showed the overall response rates in Itacitinib and placebo groups were 74% and 66% (P = 0.0782), respectively, and 6-month NRM also did not favor Itacitinib (NCT03139604), which suggested that balanced inhibition of JAK1/JAK2 is vital in treatment of GVHD 10,41 . The JAK1/2 inhibitor Ruxolitinib, the most common used JAK inhibitor in clinical practice, has been proven to be effective in the treatment of patients with aGVHD and has been approved by FDA 14,15 . We found that the novel JAK inhibitor SHR0302 has a lower half maximal inhibitory concentration (IC50) for JAK1 and JAK2 than Ruxolitinib, which suggested that SHR0302 might have stronger effects than Ruxolitinib on GVHD. Compared with Ruxolitinib, SHR0302 has similar therapeutic effects in treating aGVHD mice and showed similar mechanisms in regulating Th1/Treg differentiation and reducing cytokines production 12,13 , and even has a lower oral dosage in vivo. These encouraging experimental data supported the potential therapeutic effects of SHR0302 for patients with aGVHD. Moreover, although blood cell reduction is the most common side effect of JAK inhibitors in clinical applications, our research indicated that SHR0302 could accelerate the hematopoietic reconstruction as compared with the normal control group. The data from peripheral blood samples of patients with severe or steroid-refractory aGVHD also indicated that SHR0302 inhibited the activation of T lymphocytes and downregulated Th1 cells. In conclusion, our preclinical study suggested that the novel JAK inhibitor SHR0302 might provide a new opportunity for clinical steroid-refractory aGVHD patients by inhibiting JAK family and multiple downstream signals (including STAT1/3/5, ERK1/2 and AKT), suppressing the activation and proliferation of lymphocytes, regulating Th1/Treg cell differentiation, reducing the production of inflammatory cytokines and chemokines, blocking the receptor of IFN-γ and CXCR3 which resulting in breaking the IFN-γR/CXCL10/CXCR3 axis in aGVHD.
Supplemental Material
Supplemental Material, sj-pptx-1-cll-10.1177_09636897211033778 for Preventive and Therapeutic Effects of a Novel JAK Inhibitor SHR0302 in Acute Graft-Versus-Host Disease by Xi Sun, Qiaomei He, Jun Yang, Andi Wang, Fang Zhang, Huiying Qiu, Kun Zhou, Pengran Wang, Xiaodan Ding, Xiujie Yuan, Huajun Li, Yan Zhang and Xianmin Song in Cell Transplantation
Footnotes
Authorship note: Xi Sun, Qiaomei He and Jun Yang contributed equally to this work.
Ethical Approval: This study was approved by the Ethics Review Committee ([2019]298) and Institutional Animal Care and Use Committee (IACUC) (2019-A007-01) of Shanghai General Hospital, China.
Statement of Human and Animal Rights: The experiment procedures involving human subjects were conducted in accordance with the Ethics Review Committee guidance of Shanghai General Hospital. All animal experiments were completed under the of Institutional Animal Care guidance of Shanghai General Hospital.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Competing interests: The authors have declared that no competing interest exists.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by 3-year development project from Shanghai Shen Kang Hospital Development Center (SHDC2020CR1012B, 16CR1010A for Xianmin Song, SHDC12018X09 for Jun Yang); Clinical Research Innovation Plan of Shanghai General Hospital (CTCCR-2018BP03 for Jun Yang); Medical Guidance Project of Science and Technology Commission of Shanghai Municipality (18411968400 for Jun Yang); Clinical Research Special General Project of Shanghai Municipal Health and Family Planning Commission (201840043 for Jun Yang); National Natural Science Foundation of China (31801147 for Fang Zhang); Shanghai Sailing Program (18YF1410800 for Fang Zhang); and National Clinical Research Center for Hematologic Disease (2020ZKPC02 for Xianmin Song).
ORCID iDs: Fang Zhang  https://orcid.org/0000-0002-6808-9246
https://orcid.org/0000-0002-6808-9246
Xianmin Song  https://orcid.org/0000-0003-0637-5324
https://orcid.org/0000-0003-0637-5324
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Rafei H, Jenq RR. Microbiome-intestine cross talk during acute graft-versus-host disease. Blood. 2020;136(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lia G, Di Vito C, Cerrano M, Brunello L, Calcaterra F, Tapparo M, Giaccone L, Mavilio D, Bruno B. Extracellular vesicles after allogeneic hematopoietic cell transplantation: emerging role in post-transplant complications. Front Immunol. 2020;11:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Servais S, Beguin Y, Delens L, Ehx G, Fransolet G, Hannon M, Willems E, Humblet-Baron S, Belle L, Baron F. Novel approaches for preventing acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Expert Opin Inv Drug. 2016;25(8):957–972. [DOI] [PubMed] [Google Scholar]
- 4. Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NKC, Davies SM, Blazar BR. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–394. [DOI] [PubMed] [Google Scholar]
- 6. Hill L, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9(1):21–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mannina D, Kroger N. Janus kinase inhibition for graft-versus-host disease: current status and future prospects. Drugs. 2019;79(14):1499–1509. [DOI] [PubMed] [Google Scholar]
- 9. Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007; 282(28):20059–20063. [DOI] [PubMed] [Google Scholar]
- 10. Choi J, Cooper ML, Staser K, Ashami K, Vij KR, Wang B, Marsala L, Niswonger J, Ritchey J, Alahmari B, et al. Baricitinib-induced blockade of interferon gamma receptor and interleukin-6 receptor for the prevention and treatment of graft-versus-host disease. Leukemia. 2018;32(11):2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi J, Ziga ED, Ritchey J, Collins L, Prior JL, Cooper ML, Piwnica-Worms D, DiPersio JF. IFNγR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012; 120(19): 4093–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, Verbeek M, Fischer J, Otten V, Schmickl M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014; 123(24): 3832–3842. [DOI] [PubMed] [Google Scholar]
- 13. Carniti C, Gimondi S, Vendramin A, Recordati C, Confalonieri D, Bermema A, Corradini P, Mariotti J. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21(16):3740–3749. [DOI] [PubMed] [Google Scholar]
- 14. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10): 2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abboud R, Choi J, Ruminski P, Schroeder MA, Kim S, Abboud CN, DiPersio JF. Insights into the role of the JAK/STAT signaling pathway in graft-versus-host disease. Ther Adv Hematol. 2020;11:2040620720914489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schroeder MA, Khoury HJ, Jagasia M, Ali H, Schiller GJ, Arbushites M, Delaite P, Yan Y, Rhein K, Perales MA, Chen YB, et al. (ABSTRACT) A phase I trial of Janus kinase (JAK) inhibition with INCB039110 in acute graft-versus-host disease (aGVHD). Blood. 2016;128(22):390. [Google Scholar]
- 17. Schroeder MA, Khoury HJ, Jagasia M, Ali H, Schiller GJ, Staser K, Choi J, Gehrs L, Arbushites MC, Yan Y, et al. A phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease. Blood Adv. 2020;4(8):1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu H, Yan S, Chen J, Luo X, Li P, Jia X, Dai X, Wang C, Huang Q, Liu L, et al. JAK1-STAT3 blockade by JAK inhibitor SHR0302 attenuates inflammatory responses of adjuvant-induced arthritis rats and decreases Th17 and total B cells. Joint Bone Spine. 2016;83(5):525–532. [DOI] [PubMed] [Google Scholar]
- 19. Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4(3):318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loeffler J, Ok M, Morton OC, Mezger M, Einsele H. Genetic polymorphisms in the cytokine and chemokine system: their possible importance in allogeneic stem cell transplantation. Curr Top Microbiol Immunol. 2010;341:83–96. [DOI] [PubMed] [Google Scholar]
- 21. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. [DOI] [PubMed] [Google Scholar]
- 22. Wei X, Zhang J, Gu Q, Huang M, Zhang W, Guo J, Zhou X. Reciprocal expression of IL-35 and IL-10 defines two distinct effector treg subsets that are required for maintenance of immune tolerance. Cell Rep. 2017;21(7):1853–1869. [DOI] [PubMed] [Google Scholar]
- 23. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009; 30(5):646–655. [DOI] [PubMed] [Google Scholar]
- 24. Mehta AK, Gracias DT, Croft M. TNF activity and T cells. Cytokine. 2018;101:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. 2018;51:140–145. [DOI] [PubMed] [Google Scholar]
- 26. Romagnani S. Regulation of the T cell response. Clini Exp Allergy. 2006;36(11):1357–1366. [DOI] [PubMed] [Google Scholar]
- 27. Duffner U, Lu B, Hildebrandt GC, Teshima T, Williams DL, Reddy P, Ordemann R, Clouthier SG, Lowler K, Liu C, et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31(10):897–902. [DOI] [PubMed] [Google Scholar]
- 28. Mapara MY, Leng C, Kim YM, Bronson R, Lokshin A, Luster A, Sykes M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12(6):623–634. [DOI] [PubMed] [Google Scholar]
- 29. Paczesny S. Biomarkers for post transplantation outcomes. Blood. 2018;131(20):2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13(3):272–280. [DOI] [PubMed] [Google Scholar]
- 31. Lamarthee B, Malard F, Gamonet C, Bossard C, Couturier M, Renauld JC, Mohty M, Saas P, Gaugler B. Donor interleukin-22 and host type I interferon signaling pathway participate in intestinal graft-versus-host disease via STAT1 activation and CXCL10. Mucosal Immunol. 2016;9(2):309–321. [DOI] [PubMed] [Google Scholar]
- 32. Cassady K, Martin PJ, Zeng D. Regulation of GVHD and GVL activity via PD-L1 interaction With PD-1 and CD80. Front Immunol. 2018;9:3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ni X, Song Q, Martin PJ, Cassady K, Chen Y-Z, Deng R, Wang J, Jin H, Zeng D. PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Invest. 2017;127(5):1960–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105(5):2227–2234. [DOI] [PubMed] [Google Scholar]
- 35. Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185(2):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu S, Wang R, Zhang M, Liu K, Tao J, Tai Y, Zhou W, Wang Q, Wei W. BAFF promotes T cell activation through the BAFF-BAFF-R-PI3K-Akt signaling pathway. Biomed Pharmacother. 2019;114:108796. [DOI] [PubMed] [Google Scholar]
- 37. Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180(7):4476–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gerlach K, Lechner K, Popp V, Offensperger L, Zundler S, Wiendl M, Becker E, Atreya R, Rath T, Neurath MF, et al. The JAK1/3 inhibitor to Tofacitinib suppresses T cell homing and activation in chronic intestinal inflammation. J Crohns Colitis. 2021;15(2):244–257. [DOI] [PubMed] [Google Scholar]
- 39. Zhou M, Sacirbegovic F, Zhao K, Rosenberger S, Shlomchik WD. T cell exhaustion and a failure in antigen presentation drive resistance to the graft-versus-leukemia effect. Nat Commun. 2020;11(1):4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, Gunset G, Perna F, Kreines FM, Levy ER, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23(2):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ashami K, DiPersio JF, Choi J. Targeting IFNGR/IL6 R or downstream JAK1/JAK2 to control GvHD. Oncotarget. 2018;9(87):35721–35722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pptx-1-cll-10.1177_09636897211033778 for Preventive and Therapeutic Effects of a Novel JAK Inhibitor SHR0302 in Acute Graft-Versus-Host Disease by Xi Sun, Qiaomei He, Jun Yang, Andi Wang, Fang Zhang, Huiying Qiu, Kun Zhou, Pengran Wang, Xiaodan Ding, Xiujie Yuan, Huajun Li, Yan Zhang and Xianmin Song in Cell Transplantation