Abstract
Background:
Neuroelectrophysiological measures such as electroencephalograms (EEGs) in resting state and event-related potentials (ERPs) provide valuable information about the vulnerability and treatment-related changes in persons with alcoholism. This study examined the effectiveness of an Integrated Intervention Program for Alcoholism (IIPA) using electrophysiological measures.
Methods:
Fifty individuals with early onset of alcohol dependence participated. They were grouped randomly into two: the treatment as usual (TAU) group and the treatment group, matched on age (±1 year) and education (±1 year). eyes closed and resting state EEGs and ERPs on cognitive tasks (flanker task, alcohol Go/No-Go task, and single outcome gambling task) were recorded before and after treatment. The TAU group received pharmacotherapy, six days/week yoga sessions, and three sessions/week group therapy on relapse prevention while the treatment group received IIPA along with usual treatment (except yoga) for 18 days.
Results:
There was no significant difference between the groups pre-treatment. RM-ANOVA for pre- and post-treatment stages showed a significant difference between the two groups in the absolute power of alpha, beta, theta, and delta, during eye closure, in the resting-state EEGs. The treatment group showed significantly larger N200/N2 amplitude in congruent and incongruent conditions (flanker task), N200/N2 amplitude for alcohol No-Go, P300/P3 amplitude for neutral No-Go on alcohol Go/No-Go task, and outcome-related positivity (ORP) amplitude on single outcome gambling task.
Conclusion:
This exploratory study suggests that IIPA is effective for enhancing relaxation state and attentiveness, decreasing hyperarousal, and ameliorating neurocognitive dysfunctions of conflict-monitoring, response inhibition, and reward processing.
Keywords: EEG/ERPs, alcoholism, flanker, alcohol No-Go, SOG
Key Messages:
IIPA is an integrated intervention program. It comprises cognitive remediation with mind–body exercise (Qigong and Tai Chi). IIPA increases alpha power and reduces beta power in persons with alcoholism, indicating that it enhances relaxation and attentiveness and reduces hyperarousal. IIPA facilitates self-regulatory mechanism/cognitive control and improves conflict-monitoring, inhibitory control, and reward processing.
Abundant literature suggests neurocognitive deficits in persons with alcoholism.1,2 These deficits may persist even to the abstinence of one year or more.3 Neuroelectrophysiological measures such as resting-state electroencephalograms (EEGs), event-related potentials (ERPs), and outcome-related positivity (ORP) are very effective tools to understand the vulnerability for alcoholism, treatment-related changes, and the likelihood of relapse after treatment.4, 5
EEG, the frequency-dependent, spontaneous and continuous neural activity during a relaxed state in eyes open or eyes closed conditions or in any other specific mental state,6 is primarily analyzed in the frequency domain. The most common frequency bands are beta (13–30 Hz), alpha (8–12 Hz), theta (4–7 Hz), and delta (1–3 Hz).4 Persons with alcoholism display increased beta power,7–9 increased theta power,9–11 decreased/lower alpha power,10,12 and decreased delta power.13,14
Measures of time-locked neural responses embedded within the EEG, which are associated with the task-specific events such as cognitive tasks and are extracted from EEG trial epochs employing filtering and simple averaging techniques, are known as ERPs.15 The ERP waveforms can be described according to latency and amplitude. There are several ERP components or waveforms that are generated either from positive deflection/polarity (such as P300/P3, P200/P2, and P600/P6) or negative deflection/polarity (such as N200/N2, N300/N3, and N400/N4) within a specific time range after the stimulus onset.
Alcoholism is characterized by poor cognitive control, such as decreased inhibitory control for drinking behavior, increased attention towards alcohol-related cues, and deficits in error monitoring.16,17 An important requirement for cognitive control is the ability to monitor the information about the ongoing stimulus in the presence of conflict.18,19 Monitoring conflict is necessary for effective regulation and adaptation to problematic behaviors in everyday life, particularly in the presence of conflict situations.20 One of the most popular tasks used for response conflict is the “Eriksen flanker Task.”21 The N2 component of ERPs, typically seen at the frontocentral scalp location, is considered to be one of the indices of conflict-monitoring on flanker task.18,22 The N2 is more prominent in incongruent trials.22
The Go/No-Go task is most commonly used to assess inhibitory control. Two ERPs components have been considered to reflect inhibitory control in the No-Go condition.23 The first is N2. It is believed to be an index of cognitive control/top-down control, essential to inhibit automatic tendencies.24 The N2 is interpreted as an index of the early cognitive process required for inhibitory control. The second is P3. It is considered to be an index of the later stages of the inhibitory process.17 An abnormality in any one or both of these components (i.e., N2 and P3) may suggest a deficiency in inhibitory control in various clinical conditions.25
Similarly, studies have reported that alcoholism is associated with reward deficiency. They have demonstrated a disruption in the brain structures associated with the reward network.26,27 The electrophysiological task paradigm (gambling paradigm) has been used to investigate the reward processing during outcome processing.28,29 Individuals with alcoholism demonstrate significantly reduced amplitude of ORP or P3.30 However, studies have not examined changes from the pre- to post-treatment reward-process-related ORP amplitude.
The cognitive retraining program is known to be effective in ameliorating or enhancing executive functions in alcoholism.31,32 However, studies using cognitive retraining/remediation in alcoholism have been criticized due to methodological issues such as using the non-clinical population (e.g., college population) and using the same task for outcome assessment and training, and only a few studies had attempted to target the executive functions.33,34
Stress, emotion dysregulation, and cue-induced craving also play an essential role in substance use disorders, including alcoholism. Individuals use alcohol to get relief from stress, anxiety, and other negative affective states.35,36 Mind–body practices such as Tai Chi and Qigong, which originated from China,37 are a meditative form of exercise. Tai Chi has been found to enhance relaxation and reduce sympathetic nervous system activity.38,39 Tai Chi impacts autoregulatory signaling pathways (limbic reward and motivation circuitry).40 Similarly, Qigong impacts hypothalamic–pituitary–adrenal axis.41 Studies have shown Tai Chi and Qigong’s effectiveness in several clinical conditions such as anxiety, depression,37,42 and substance use disorders.43
We integrated cognitive remediation with mind–body exercise and developed an Integrated Intervention Program for Alcoholism (IIPA) to enhance executive control and affect regulation in persons with alcoholism. This study was exploratory, and we aimed to examine the effectiveness of IIPA in persons with alcoholism, using electrophysiological measures as outcome variables.
Materials and Methods
Participants and Procedure
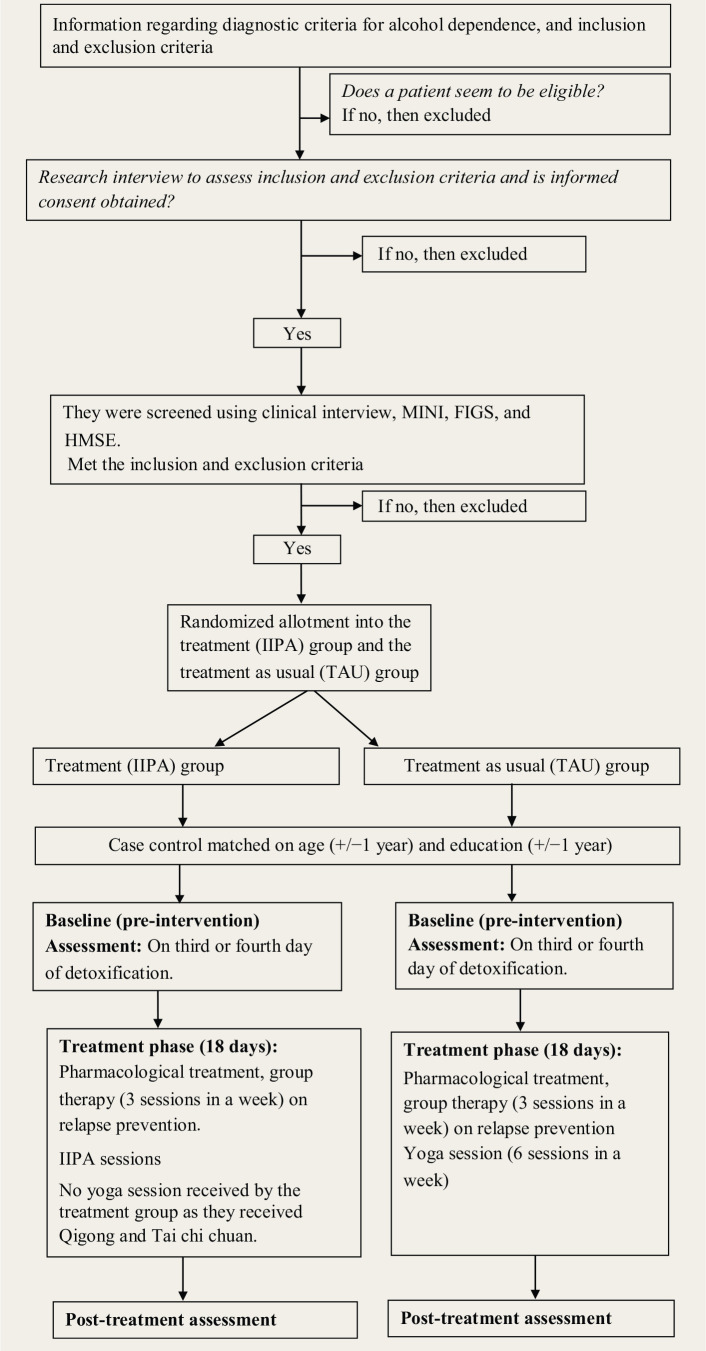
Fifty individuals, aged 18–45 years, diagnosed with early-onset (before 25 years of age) alcohol dependence, were recruited for the study. A1 depicts the design of the study. Participants were recruited from the inpatient ward at Centre for Addiction Medicine (CAM). The psychiatrist determined the alcohol dependence diagnosis as per ICD-10 criteria. Participants had to have one or more first-degree relatives with alcohol dependence. The exclusion criteria were dependence on other substance such as cannabis, barring nicotine; severe memory impairment (score < 24 on Hindi Mental State Examination); the presence of other psychiatric disorders such as schizophrenia and mood disorders in the participants or their first-degree family; mental retardation; receiving cognitive retraining, any form of meditation practices; or structured psychotherapy in the past one year.
Figure A1. Flow Diagram that Depicts the Design of the Study.
Written informed consent was obtained from the subjects. Ethical clearance was obtained. The study was registered retrospectively with Clinical Trial Registry—India (Ref. No.: CTRI/2017/08/009346). There was no monetary benefit for participation. Participants were categorized into the treatment (IIPA) group (n = 25) and the treatment as usual (TAU) group (n = 25) by the randomly matched method. They were matched on age (±1year) and education (±1year). The procedure of allotment was as follows: For example, if Mr A, a 25 year old with eight years of education, consented for the study, he was randomly categorized into either IIPA or TAU group. Suppose he is assigned to the IIPA group. Then, later, a participant who met the matched criteria of age (±1 year) and education (±1 year) with Mr A was allotted to the TAU group and vice versa. Recruitment and allotment are described in our previous publication.44
Both groups were assessed at the baseline, following 3–4 days of detoxification. After the baseline assessment, the TAU group received 18 days of pharmacological treatment, 6 days/week of yoga sessions, and 3 days/week of group therapy on relapse prevention, while the treatment group received IIPA for 18 days along with the usual treatment. Details about the IIPA can be seen in Kumar et al.44 Both groups were assessed on pre- and post-treatment electrophysiological outcome measures.
Behavioral Measures
Demographic details and clinical information related to alcohol dependence, such as the age of initiation and development of alcohol dependence, duration of alcohol use and dependence, etc., were recorded on a sociodemographic datasheet. Mini-international Neuropsychiatry Interview (MINI)45 was used as a screening tool for axis I psychiatry disorders. MINI has good reliability for most modules. Inter-rater reliability ranged from k = 0.88 to 1.0 and test–retest reliability ranged from 0.76 to 0.93. Alcohol dependence in the first-degree family members was assessed on Family Interview for Genetic Studies.46 Severe memory impairment was screened using Hindi Mental State Examination,47 which is a Hindi adaptation of the Mini-Mental State Examination. The Short Alcohol Dependence Data Questionnaire (SADDQ)48 was administered for assessing the severity of alcohol dependence. It is a suitable tool for patients seeking help with alcohol problems and has sensitivity across the full range of dependence. It is relatively free of sociocultural influences. Semi-Structured Assessment for Genetics of Alcoholism—version II (SSAGA-II)49 was used to assess externalizing spectrum disorders. The instrument has been translated and previously been used in several published studies from NIMHANS.
Electrophysiological Measures
The EEG was recorded in the eyes-closed relaxed state for 3 minutes and approximately 20 minutes of ERP measures on cognitive tasks paradigms (i.e., flanker, alcohol Go/No-Go and single outcome gambling [SOG] tasks). The cognitive task paradigms were designed in the “Stim2” software, Gentask application (Compumedics®, Neuroscan™). Practice trials were given to each participant before the actual task, to familiarize them.
Flanker Task
The flanker task21 has been used in several studies to measure conflict-monitoring18,50 and response conflict resolution.21 The flanker task used in this study had five equally sized and spaced white arrows, placed in a horizontal array. The arrows were presented with left or right orientation. The central arrow (target arrow) was flanked by either left ( < ) or right ( > ) direction. If all the flanking arrows pointed in the same direction as the central (target) arrow (e.g., < < < < < ), the trials are called congruent or compatible trials. On the other hand, if they were in a direction opposite to that of the central arrow (e.g., < < > < <), the trials are called incongruent or incompatible trials. We used 80 congruent trials (i.e., 40 with the target arrow pointing to the left and 40 to the right) and 160 incongruent trials (i.e., 80 in which the central arrow was pointing to the left, and the remaining 80, to the right). The number of incongruent trials was kept higher in order to increase the high conflict and obtain a larger N2.51,52 Trials were presented in random order, displayed on the computer screen for 150 milliseconds (ms). For all the trials, the response window was 1,000 ms and the inter-trial interval was 1,200 ms.
Alcohol Go/No-Go Task
Response inhibition was assessed on the alcohol Go/No-Go task paradigm developed for this study, using images of alcohol and non-alcohol (neutral) images. Approximately 400 alcohol-related pictures were shown and the patients were asked to rate the degree of craving induced by these images (on a 10-point scale) before finalization. The cue-induced craving is dependent on an individual’s preference for a particular type of alcohol (i.e., in a person who prefers “rum,” the images of “rum” would induce high intensity of craving, compared to images related to other types of alcohol). Therefore, various types of alcohol-related images were included to suit all patients. The order of No-Go trials was quasi-randomized. The size of the images was kept uniform.
This task has two parts (200 trials in each part). The first part consists of alcohol-related images as “go” trials (participants were instructed to respond to these trials by pressing a key) and neutral (object) images as “No-Go” trials (participants were instructed “do not press or withhold” on these trials). In the second part, the alcohol-related images were used as “No-Go” trials and the neutral (object) images, as “go” trials. The go trials were 150, and No-Go trials, 50. The image was displayed for 200 ms. The response window was kept as 500 ms for the “go” trials and 1,000 ms for the “No-Go” trials, and the inter-trial interval was kept as 1,200 ms. The Go and No-Go trials were counterbalanced across the participants, to remove the order effect.
The SOG Task
The SOG task53 was used to assess decision-making. A choice stimulus (CS) with two numbers, 10 and 50, with a monetary value in Indian rupee, were displayed for 800 ms. The participants were instructed to select one number by pressing the “l” button for “10” or the “3” for “50.” The outcome stimulus (OS) appeared either in a red box (indicative of loss) or a green box (indicative of gain) after the CS has disappeared, and lasted 800 ms. Thus, there were four possible outcomes: namely, gain 50 (+50), loss 50 (−50), gain 10 (+10), and loss 10 (−10). The participants had to respond by selecting either 10 or 50 within 1,500 ms of CS onset. Total trials were 172, and the inter-trial interval was 3,000 ms throughout the experiment. The task was presented in two blocks (i.e., 86 trials in one block). The participants were unaware of the probability of gain/loss or the sequence of gain/loss.
EEGs Data Acquisition and Analysis
The EEG was recorded using Compumedics/Neuroscan ACQUIRE program and SCAN 4.4 system (SynAmps bioamplifiers: SN1, SN2, SN3, and SN4), and NeuroScan (Neuroscan, Inc., El Paso, Tx, USA) with 9-Ag/AgCl electrodes at scalp sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4) fitted in a highly elastic cap (Quik-cap sintered electrodes of Neuroscan Inc, ECI, Electro Cap Int®, USA), positioned according to the international 10–20 system. Seven additional electrodes—two at left and right mastoids, two at the outer canthus of eyes (HEOG), two supra-orbitally and infra-orbitally near the left eye (VEOG), and one as a ground electrode at the forehead—were placed. The32-bit A-D converter was used to continuously digitize (1,024 Hz sampling rate), amplify (gain of 150), and filter (30 Hz low-pass filter and 0.1 Hz high-pass filter) the raw EEG signal in DC mode. The impedance values were kept ≤5 kΩ. Offline EEG data processing included removing artefacts and eye blink correction using the spatial filter. Response-locked epoch (200 ms prestimulus to 800 ms poststimulus) was created, and baseline correction (−200 to −100 ms) was applied. High amplitude artefacts were rejected using the artefact rejection criteria of ±75 microvolts.
Power Spectral Analysis
The EEG was recorded continuously for three minutes, with eyes closed, in a resting state, and 60–80 2 seconds’ artefact-free epochs were subjected to fast Fourier analysis. The power spectral analysis was done in the Neuroscan software toolbox. The epoched eyes-closed EEG data was subjected to spline fit and averaging in the frequency domain using the Hanning window. The wavebands defined for decomposition were theta (4–7 Hz), alpha (8–12 Hz), beta (12–22 Hz), and delta (1–3 Hz). Log-transformation was done (absolute power was log-transformed into the base of 10) to control the individual differences.
ERPs: Marking of N2/P3 and ORP
The ERP components of N200/N2 (the most negative peak potential occurring 200–350 ms after the stimulus), P300/P3 (the most positive peak potential occurring 250–600 ms after the stimulus), and ORP (the most positive peak potential occurring between 275 ms and 700 ms after the stimulus) were quantified in epoch EEG data obtained in time domain averaging.
Statistical Analysis
The total number of samples for analysis of electrophysiological measures was 34 (17 in each group). Electrophysiological data of 16 subjects were discarded due to artefacts (n = 8) in the EEG recording in any channel. If one subject’s electrophysiological data was rejected from the one group (IIPA or TAU), then the matched subject’s data from the second group was also excluded.
Statistical analysis was carried out using the Statistical Package for the Social Sciences-version 22 (IBM-SPSS-v22) for Windows. The Shapiro–Wilk test was used to test the normality of data, and it revealed that the data were normally distributed. Independent sample t-test was used for pre-treatment comparison of continuous variables and chi-square test, for comparison of categorical variables. Repeated measure analysis of variance (RM-ANOVA) was applied in time × region × electrodes as a within factor, and group (treatment vs TAU group) as a between factor for pre- to post-treatment changes. A pairwise comparison between the two groups was also done. Effect size in both groups was described employing partial eta squared (η2).
Results
Demographic and Clinical Characteristics
The mean age of the participants in the IIPA group was 33.88 years ± 5.05 (SD), and the TAU group, 33.47 years ± 5.35 (SD). The average education (in years) in the IIPA group was 11.76 years ± 2.63 (SD), and the TAU group, 11.88 years ± 2.55 (SD). Both groups were comparable in terms of socioeconomic status (χ2 = 0.144; P = 0.931), and the majority of participants were from middle socioeconomic status (59% in the IIPA group, 53% in the TAU group).
Similarly, both groups were comparable in terms of age of onset of alcohol use, age of onset of alcohol dependence, years of alcohol use and dependence, externalizing score, and severity of alcohol dependence (Table 1). Also, both groups were comparable in terms of pharmacotherapy. All the participants were detoxified with diazepam. Baclofen was received by 41% in the IIPA group and 47% in the TAU group. Similarly, 59% in the IIPA group and 53% in the TAU group received vitamin B complex injection (optineuron), and 32% in the IIPA group and 28% in the TAU group received vitamin supplements.
Table 1. Group Comparison on Clinical Variables at Baseline.
| Variables | Treatment group (n = 17)Mean ± SD | TAU group (n = 17)Mean ± SD | t value | P value |
| Age of onset (years) | 18.59 ± 2.50 | 19.65 ± 3.04 | −1.11 | 0.276 |
| Age of onset of dependence (years) | 23.65 ± 2.29 | 24.29 ± 2.26 | −0.83 | 0.413 |
| Total years of alcohol use | 14.71 ± 5.88 | 13.94 ± 6.05 | 0.37 | 0.711 |
| Total years of alcohol dependence | 9.76 ± 4.94 | 9.18 ± 5.02 | 0.34 | 0.733 |
| Externalizing traits | 13.59 ± 3.83 | 10.94 ± 4.34 | 1.89 | 0.068 |
| SADDQ scores | 21.71 ± 8.33 | 21.88±11.39 | −0.05 | 0.959 |
SADDQ: Short Alcohol Dependence Data Questionnaire, SSAGA-II: Semi-Structured Assessment for Genetics of Alcoholism—version II for externalizing trait.
Comparison Between Two Groups on the EEG Power Spectrum
There was no significant difference at baseline between the two groups in the absolute power of alpha (P = 0.216), beta (P = 0.328), theta (P = 0.444), or delta (P = 0.297) in resting-state eyes-closed EEGs (for mean and SD at baseline, see Table 2). Pre- to post-treatment RM-ANOVA results showed significantly increased alpha power and decreased beta power in the treatment group. The TAU group had significantly increased theta power and decreased delta power post intervention (Table 2).
Table 2. Pre- to Post-treatment Comparison of the Two Groups on Absolute Power for Eye Closed EEG in Relaxed State.
| Variables | Treatment group Mean ± SD | TAU group Mean ± SD | Time (T) | Group (G) | T × G | Within group pre to post | |
| Treatment group | TAU group | ||||||
| EEG alpha | Pre = 0.87 ± 0.29Post = 1.09 ± 0.36 | Pre = 0.73 ± 0.33Post = 0.92 ± 0.33 | F = 8.62P = 0.006** | F = 2.93P = 0.097 | F= 0.06P = 0.810 | P = 0.032*η2 = 0.14 L | P = 0.066η2 = 0.10 |
| EEG beta | Pre = 0.64 ± 0.17Post = 0.49 ± 0.24 | Pre = 0.56 ± 0.25Post = 0.61 ± 0.33 | F= 1.25P = 0.272 | F = 0.12P = 0.728 | F = 4.28P = 0.047* | P = 0.031*η2 = 0.14 L | P = 0.506η2 = 0.01 |
| EEG theta | Pre = 0.94 ± 0.20Post = 0.99 ± 0.18 | Pre = 0.88 ± 0.19Post = 1.03 ± 0.18 | F = 5.84P = 0.022* | F = 0.04P = 0.851 | F = 1.13P = 0.297 | P = 0.345η2 = 0.03 | P = 0.019*η2 = 0.16 |
| EEG delta | Pre = –0.10 ± 0.18Post = –0.03 ± 0.20 | Pre = –0.17 ± 0.18Post = –0.05 ± 0.22 | F = 5.56P = 0.025* | F = 0.61P = 0.440 | F = 0.36 P = 0.553 | P = 0.223η2 = 0.05 | P = 0.044* η2 = 0.12 |
*P < 0.05, **P < 0.01. L: large effect size, EEG: electroencephalogram, TAU: treatment as usual.
Comparison Between the Groups on ERP Measures
The amplitude of N2 was examined on the flanker task in the congruent trials as well as incongruent trials. There was no significant difference between the two groups in the congruent trials (P = 0.164) or the incongruent trials (P = 0.686) at baseline. Pre- to post-treatment RM-ANOVA results showed significantly increased N2 amplitude on flanker congruent as well as incongruent trials (Table 3). The grand-average ERP wave plot of a few electrodes can be seen in the appendix in Figures A2(a) and (b).
Table 3. Pre–Post Treatment Comparison of the Two Groups on ERPs Measures.
| Variable | Treatment groupMean ± SD | TAU groupMean ± SD | Time (T) | Group (G) | T × G | Within group pre to post | |
| Treatment group | TAU group | ||||||
| Flanker C N2 amplitude | Pre = −1.94 ± 1.25Post = −3.83 ± 2.05 | Pre = −1.03 ± 2.28Post = −1.17 ± 1.24 | F = 7.86P = 0.009** | F = 13.25P = 0.001 | F = 5.85P = 0.021* | P = 0.001***η2 = 0.30 L | P = 0.788η2 = 0.01 |
| Flanker IC N2 amplitude | Pre = −1.25 ± 1.73Post = −3.06 ± 1.96 | Pre = −0.95 ± 2.33Post = −1.04 ± 2.19 | F = 5.09P = 0.031* | F = 4.16P = 0.050 | F = 4.25P = 0.047* | P = 0.005**η2 = 0.23 L | P = 0.892η2 = 0.01 |
| Alcohol No-GoN2 amplitude | Pre = −2.02 ± 2.96Post = −3.54 ± 2.82 | Pre = −1.51± 3.53Post = −2.12 ± 1.85 | F = 4.54P = 0.041* | F = 1.33P = 0.258 | F = 0.82P = 0.373 | P = 0.040*η2 = 0.13 | P = 0.392η2 = 0.02 |
| Neutral No-Go P3 amplitude | Pre = 5.14 ± 2.22Post = 7.10 ± 3.83 | Pre = 4.68 ± 3.46Post = 6.01 ± 3.04 | F = 7.76P = 0.009** | F = 0.71P = 0.407 | F = 0.29P = 0.595 | P = 0.025*η2 = 0.15 L | P = 0.122η2 = 0.07 |
| Loss (50+10)ORP amplitude | Pre = 3.57 ± 2.27Post = 7.21 ± 5.11 | Pre = 4.21 ± 4.59Post = 5.31 ± 5.89 | F = 7.03P = 0.012** | F = 0.23P = 0.638 | F = 2.03P = 0.164 | P = 0.007**η2 = 0.21 L | P = 0.392η2 = 0.02 |
| Gain 50 ORP amplitude | Pre = 4.47 ± 3.38Post = 8.71 ± 4.37 | Pre = 6.42 ± 4.98Post = 6.00 ± 7.68 | F = 3.24P = 0.081 | F = 0.07P = 0.799 | F = 4.81P = 0.036* | P = 0.008**η2 = 0.20 L | P = 0.782η2 = 0.01 |
| Loss 50 ORP amplitude | Pre = 3.59 ± 2.69Post = 8.24 ± 4.92 | Pre = 3.93 ± 4.37Post = 5.77 ± 5.61 | F = 15.02P = 0.001*** | F = 0.66P = 0.423 | F = 2.82P = 0.103 | P = 0.001***η2 = 0.33 L | P = 0.130η2 = 0.07 |
*P < 0.05, **P < 0.01, ***P < 0.001. L: large effect size, C: congruent trials, IC: incongruent trials, ORP: outcome-related positivity.
On the alcohol Go/No-Go task, the amplitudes of N2 and P3 were examined in the alcohol No-Go and the neutral No-Go conditions. Both groups were comparable on alcohol No-Go, N2 amplitude (P = 0.650), and neutral No-Go P3 amplitude (P = 0.650). However, the treatment group showed significantly increased pre- and post-treatment N2 amplitude for alcohol No-Go (Table 3). Within-group, region-wise results showed significantly increased N2 amplitude in the frontal region only (Table 4). There were no significant differences in the TAU group. Similarly, the treatment group showed significantly increased pre- to post-treatment P3 amplitude for neutral No-Go (Table 3). Further, within group, region-wise results showed significantly increased P3 amplitudes in the frontal and central regions (Table 4). The grand-average ERP wave plot of a few electrodes can be seen in the appendix in Figures A3(a) and (b).
Table 4. Region-wise (Frontal [F3, FZ, F4], Central [C3, CZ, C4], and Parietal [P3, PZ, P4]) Within Group Pre-Post Treatment Comparison on ERP Measures.
| Regions | Treatment groupMean ± SD | Time Pre to Post | TAU groupMean ± SD | TimePre to Post |
| Flanker N2 amplitude in congruent trials | ||||
| Frontal | Pre = −1.04 ± 1.05Post = −2.49 ± 1.49 | P = 0.012**η2 = 0.18 L | Pre = −0.19 ± 2.41 Post = −0.38 ± 1.64 | P = 0.731η2 = 0.01 |
| Central | Pre = −1.48 ± 1.12Post = −3.44 ± 2.39 | P = 0.003**η2 = 0.24 L | Pre = −0.73 ± 2.47Post = −0.59 ± 1.31 | P = 0.826η2 = 0.01 |
| Parietal | Pre = −3.29 ± 2.27Post = −5.55 ± 3.58 | P = 0.008**η2 = 0.20 L | Pre = −2.17 ± 3.45Post = −2.53 ± 3.34 | P = 0.647η2 = 0.01 |
| Flanker N2 amplitude in incongruent trials | ||||
| Frontal | Pre = −0.28 ± 1.37Post = −1.87 ± 1.67 | P = 0.009**η2 = 0.19 L | Pre = −0.26 ± 1.98Post = 0.04 ± 1.97 | P = 0.599η2 = 0.01 |
| Central | Pre = −0.90 ± 1.82Post = −2.73 ± 2.03 | P = 0.012**η2 = 0.18 L | Pre = −0.36 ± 2.35Post = −0.71 ± 2.45 | P = 0.616η2 = 0.01 |
| Parietal | Pre = −2.54 ± 2.73Post = −4.59 ± 3.76 | P = 0.013**η2 = 0.18 L | Pre = −2.24 ± 3.45Post = −2.44 ± 3.50 | P = 0.796η2 = 0.01 |
| Alcohol No-Go, N2 amplitude | ||||
| Frontal | Pre = −1.01 ± 2.19Post = −3.37 ± 2.28 | P = 0.002**η2 = 0.26 L | Pre = −0.78 ± 4.30Post = −1.87 ± 3.02 | P = 0.129η2 = 0.07 |
| Central | Pre = −1.58 ± 3.09Post = −2.83 ± 2.62 | P = 0.103η2 = 0.08 | Pre = −1.29 ± 4.03Post = −1.78 ± 1.63 | P = 0.511η2 = 0.01 |
| Parietal | Pre = −3.47 ± 4.47Post = −4.43 ± 4.69 | P = 0.439η2 = 0.02 | Pre = −2.46 ± 5.35Post = −2.72 ± 4.85 | P = 0.832η2 = 0.01 |
| Neutral No-Go, P3 amplitude | ||||
| Frontal | Pre = 4.30 ± 2.21Post = 6.26 ± 3.21 | P = 0.050*η2 = 0.12 M | Pre = 4.05 ± 3.93Post = 5.77 ± 3.98 | P = 0.08η2 = 0.09 |
| Central | Pre = 4.85 ± 1.96Post = 6.74 ± 4.20 | P = 0.037*η2 = 0.13 M | Pre = 4.25 ± 3.37Post = 5.41 ± 3.30 | P = 0.190η2 = 0.05 |
| Parietal | Pre = 6.14 ± 3.39Post = 8.30 ± 4.74 | P = 0.085η2 = 0.09 | Pre = 5.74 ± 4.94Post = 6.85 ± 5.79 | P = 0.343η2 = 0.03 |
| SOG-loss (50+10), ORP amplitude | ||||
| Frontal | Pre = 3.65 ± 2.55Post = 7.38 ± 5.21 | P = 0.003**η2 = 0.24 L | Pre = 4.36 ± 4.32Post = 5.49 ± 4.99 | P = 0.339η2 = 0.03 |
| Central | Pre = 4.17 ± 2.30Post = 8.09 ± 5.63 | P = 0.005**η2 = 0.22 L | Pre = 4.88 ± 4.77Post = 5.84 ± 6.19 | P = 0.471η2 = 0.02 |
| Parietal | Pre = 2.88 ± 3.03Post = 6.17 ± 5.83 | P = 0.044*η2 = 0.12 | Pre = 3.40 ± 5.19Post = 4.61 ± 7.20 | P = 0.444η2 = 0.02 |
| SOG, ORP amplitude in gain 50 condition | ||||
| Frontal | Pre = 4.82 ± 2.55Post = 8.83 ± 4.44 | P = 0.009**η2 = 0.20 L | Pre = 6.88 ± 5.27Post = 6.05 ± 7.29 | P = 0.570η2 = 0.01 |
| Central | Pre = 5.35 ± 3.65Post = 10.07 ± 4.84 | P = 0.005**η2 = 0.22 L | Pre = 7.12 ± 5.15Post = 6.87 ± 8.34 | P = 0.874η2 = 0.01 |
| Parietal | Pre = 3.24 ± 5.38Post = 7.21 ± 5.60 | P = 0.028*η2= 0.14 L | Pre = 5.25 ± 5.20Post = 5.07 ± 8.30 | P = 0.918η2= 0.01 |
| SOG, ORP amplitude in loss 50 condition | ||||
| Frontal | Pre = 3.33 ± 2.83Post = 8.69 ± 5.67 | P = 0.001***η2 = 0.39 L | Pre = 4.15 ± 4.05Post = 6.06 ± 4.91 | P = 0.117η2 = 0.08 |
| Central | Pre = 4.28 ± 2.77Post = 9.27 ± 5.69 | P = 0.001***η2 = 0.32 L | Pre = 4.65 ± 4.54Post = 6.54 ± 6.08 | P = 0.150η2 = 0.06 |
| Parietal | Pre = 3.18 ± 3.45Post = 6.74 ± 4.95 | P = 0.017*η2 = 0.17 L | Pre = 3.01 ± 5.06Post = 4.71 ± 6.49 | P = 0.238η2 = 0.04 |
*significant at 0.05, **significant at 0.01, ***significant at 0.001. L: large effect size, M: medium effect size, SOG: single outcome gambling, ORP: outcome-related positivity, TAU: treatment as usual.
The amplitude of ORP/P3 was examined on the SOG task in different valence conditions (i.e., gain [50 + 10], loss [50 + 10], gain of 50, loss of 50, gain of 10, and loss of 10). Results showed that there was no significant difference between the two groups across different valences (p = 0.386 for gain [50 + 10]; P = 0.606 for loss [50 + 10]; P = 0.181 for gain of 50; and P = 0.787 for loss of 50) at the baseline. However, the pre- to post-treatment results showed significant ORP/P3 amplitude change (larger amplitude) for loss (50 + 10). The valence analysis showed that the treatment group showed significantly larger ORP amplitude for higher valances (i.e., gain 50 and loss 50; Table 3). No significant pre- to post-treatment OPR amplitude change was noted for lower valences (i.e., gain 10 or loss 10) in both groups. The grand-average ERP wave plot of a few electrodes can be seen in Figures A4(a)–(c).
Discussion
Neurocognitive theories have hypothesized dysfunction or hypofunctioning of the cognitive control system (the executive system which is generally associated with prefrontal cortex functioning) and heightened functioning of the impulsive systems (generally associated with limbic system) in addiction.54–56 Similarly, the roles of negative effect and stress in substance use disorders, including alcoholism, have been documented.36,57,58
We developed an IIPA to ameliorate cognitive deficits and enhance affect regulation in persons with alcoholism. We found its effectiveness in improving affect regulation and executive functions. Follow-up results showed a low relapse rate and prolonged abstinence in the treatment (IIPA) group as compared to the TAU group.44 This article aimed to report the effectiveness of IIPA on electrophysiological measures.
Changes in the Resting-State Eyes-Closed EEG Power
The treatment (IIPA) group has significantly increased pre- to post-treatment alpha power and decreased pre- to post-treatment beta power, indicating an improvement in relaxation state and attentiveness and decreased hyperarousal. The TAU group showed significantly increased theta power and decreased delta power, indicating a risk of relapse or increased craving for alcohol.
Poor alpha rhythm has been reported in persons with alcoholism.10,12 Persons with alcoholism, during relapse, demonstrate poor alpha power compared to those who maintain abstinence.13 Studies have shown that in normal individuals, increased alpha power indicates an improvement in cognitive functions.59 Alpha EEG is reported to play an important role in the integrative aspect, and a decreased alpha phase synchrony has been correlated with a decline in the cognitive functions.60 Studies have reported an increased beta power in persons with alcoholism.7–9 Relapsing individuals with alcoholism have demonstrated desynchronized beta activity than those who did not.8 Increased beta indicates neural excitability or central nervous system disinhibition, apparent in persons with alcoholism.61 Similarly, resting theta power is reported to be higher in persons with alcoholism.9–11 Increased resting theta power may reflect a deficiency in the information processing of the central nervous system.62 In the context of delta power, studies have reported decreased delta power in persons with substance use disorders, including alcoholism13,63
Transcendental meditation, such as mind–body exercise, facilitates neuronal coordination through enhancing alertness without cognitive activity.60 Tai Chi has been found to enhance relaxation and reduce sympathetic nervous system activity.38,39 It improves various aspects of mood, reduces stress, and promotes physical and psychological well-being.64,65 Tai Chi and Qigong practice helps in increasing alpha and decreasing theta power.66 Similarly, the cognitive remediation program has tasks to facilitate attention, concentration, and response inhibition. Hence, the treatment group showed increased alpha and decreased beta power.
The ERP Components Related to Cognitive Control
Alcoholism is characterized by poor cognitive control.16,17 An important requirement for cognitive control is the ability to monitor the information about the ongoing stimulus in the presence of conflict.18,19 Frontoparietal regions are involved during the performance on the flanker task.50, 67 We assessed the response conflict by the flanker task. Results showed increased N2 amplitude in the treatment group, indicating an improvement in attention, error monitoring, and conflict resolution.
Improvement in attentional control would be an important indicator of recovery. The attentional system in individuals with alcohol dependence can be highly sensitized to alcohol, and alcohol-related cues can be the focus of their attention. This may trigger cognitive, emotional, and behavioral responses that might be inconsistent with the individual’s effort to not drink. Due to habitual use and dependency, drinking behavior becomes automatic, and individuals may be unaware of the cognitive process that leads to drinking following an encounter with alcohol-related stimuli. For example, exposure to alcohol stimuli while watching television may lead the attentional resources to be disproportionately allocated to alcohol-related stimuli. Hence, increased attentional control due to the practice of IIPA may reflect a better control on behavior and the ability to direct the attention from irrelevant/distracting stimuli to goal-directed activities. This may help in diminishing the drinking behavior and reducing the attentional biases toward alcohol-related stimuli.
The IIPA has several cognitive remediation tasks that intend to enhance the attention and concentration (e.g., attention enhancement task), as well as error monitoring and conflict resolution, in the presence of competitive interference (e.g., encoding and error detection task). On the other hand, Qigong and Tai Chi Chuan are also known to enhance attention control, as these exercises demand focused attention on body movement and breathing. Mind–body exercise improves mental alertness, which is necessary to avoid mistakes.68 Tai Chi and Qigong can improve the attention in dual ways: by the eye focusing on the bodily movement and the mind focusing on the breathing and movement sequences.69 Hence, it can be presumed that IIPA, which has components of cognitive remediation and emotion regulation, enhanced selective attention, attentional control, and the ability for error monitoring in the treatment group.
Furthermore, better attentive control is reflected on the alcohol Go/No-Go task that assesses inhibitory control (response inhibition). Results showed that the treatment group demonstrated significantly increased N2 amplitude for the alcohol No-Go, particularly in the frontal region, and for the neutral No-Go, particularly in the frontal and central regions. The No-Go N2 reflects response inhibition.70,71 The N2 peak is predominantly seen at frontal sites, and the amplitude is larger upon successful No-Go responses.71,72 Similarly, reduced P3 on various task paradigms is consistently reported in individuals with alcoholism as well as at risk for alcoholism.5,23 Successful inhibition responses in individuals with alcoholism have been linked to increased control.73 Deficits in response inhibition are linked to frontal executive deficits.70
The IIPA has several cognitive remediation tasks intended to enhance the executive functions and exercise the inhibitory control or self-regulation. Similarly, Tai Chi and Qigong also improve self-regulatory capacity as these exercises require monitoring and regulating movement and breathing.44 Hence, it can be assumed that enhanced functioning of executive function and self-regulation would have led to the better response inhibition in the treatment group. Increased inhibitory control may help in better regulation of drinking behavior. Improved inhibitory control in alcohol No-Go may also reflect reduced attentional biases and implicit cognition and motivation towards alcohol-related stimuli. It is well demonstrated that in addictions such as alcoholism, the substance can produce neuro-adaptation in incentive motivation and reward network,74 making the network hypersensitive to both alcohol and alcohol-related stimuli. Over some time, due to repeated use, alcohol-related cues can be associated with alcohol consumption and can acquire incentive motivational and rewarding effects through conditioning. This may direct attention to alcohol-related stimuli when individuals encounter alcohol-related cues in the environment. Indeed, alcoholism is characterized by an increased salience of alcohol-related cues. Hence, increased inhibitory control and reduced attentional biases may help in better treatment recovery.
Alcoholism is associated with reward deficiency and disruption in the brain structures associated with the reward network.26,27 Studies have reported significantly reduced/lower amplitude of ORP/P3 in individuals with alcoholism, compared to healthy people.30 Our results indicate a significant improvement in the reward processing and the appreciation of reward valence in the decision-making ability in the treatment group. Hence, it can be presumed that IIPA is effective in ameliorating reward processing deficits or that it facilitates adaptive reward processing and decision-making. Kamarajan et al.53 reported that ORP might involve cognitive/evaluative as well as emotional/affective processing. ORP may reflect conscious awareness for the valance, and a reduced or lower ORP amplitude may reflect neurocognitive deficits related to the early detection of different outcomes and subsequent evaluation of quality (loss vs gain) and quantity (50 vs 10) of outcomes. Findings from this study also support this view, as there was a significant change for higher valence (i.e., gain or loss 50) than the lower valence (i.e., gain or loss 10). Increased ORP amplitude on the SOG task may reflect an enhanced functioning of interconnected regions of the prefrontal cortex, anterior cingulate cortex, and limbic structures.75,76 This circuitry has been reported to be involved in addictive-impulsive-compulsive spectrum disorders, including substance abuse.77,78
Limitations of the study are lack of further follow-up assessments and a small sample size (n = 17 in each group). A follow-up assessment at six weeks or three months would have provided more information about the changes in the electrophysiological markers related to abstinence/relapse. This was an exploratory study and used a combination of random allotment and matched method (i.e., matching subjects on variables that could affect the outcome variables). Further studies may use a random subject selection and assignment to increase generalizability. Future studies may also examine treatment-related electrophysiological changes in persons with addiction, including alcoholism, with more numbers of electrodes. This may provide better visualization (topographic map) of the brain regions and information related to the treatment effect.
Conclusion
The IIPA is a comprehensive intervention program, based on the neurocognitive theories of addiction, to address the self-regulatory mechanisms in persons with alcoholism. Electrophysiological findings suggest its effectiveness in improving relaxation state and attentiveness, decreasing hyperarousal, and facilitating self-regulatory mechanism/cognitive control as reflected on flanker, alcohol Go/No-Go, and SOG tasks, respectively.
Supplementary Material
Acknowledgments
We acknowledge the support from Mr Deepak Ullal, Senior Technician, Clinical Neuropsychology Lab, NIMHANS, in EEGs acquisition and analysis. Authors also acknowledge support from the online site “FreeDigitalPhotos.net” (http://www.freedigitalphotos.net/) for obtaining a few alcohol and neutral images.
Footnotes
Declaration of Conflicting Interests: The authors declare that they have no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was part of the doctoral research carried out at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India, and was funded by the Indian Council of Medical Research (ICMR), New Delhi (Ref. No.: 3/1/3JRF–2011/HRD–104). No financial interests, direct or indirect, exist for the individual contributors in connection with the content of this paper. The funding body (ICMR) had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Moselhy HF Georgiou G and Kahn A.. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol; (Oxford, Oxfordshire) 2001; 36(5): 357–368. [DOI] [PubMed] [Google Scholar]
- 2.Le Berre AP Fama R and Sullivan EV.. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res; 2017; 41(8): 1432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowe SF Cammisuli DM and Stranks EK.. Widespread cognitive deficits in alcoholism persistent following prolonged abstinence: an updated meta-analysis of studies that used standardised neuropsychological assessment tools. Arch Clin Neuropsychol; 2019; 35(1): 31-45. [DOI] [PubMed] [Google Scholar]
- 4.Rangaswamy M and Porjesz B.. Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol; 2014; 125: 383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamarajan C and Porjesz B.. Advances in electrophysiological research. Alcohol Res; 2015; 37(1): 53–87. [PMC free article] [PubMed] [Google Scholar]
- 6.Niedermeyer E and Lopes da Silva F.. Electroencephalography: basic principles, clinical applications, and related fields. Philadelphia, PA: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- 7.Rangaswamy M, Porjesz B, Chorlian DB. et al. Beta power in the EEG of alcoholics. Biol Psychiatry 2002; 52(8): 831–842. [DOI] [PubMed] [Google Scholar]
- 8.Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology; 2001; 25(3): 332–340. [DOI] [PubMed] [Google Scholar]
- 9.Fein G and Allen J.. EEG spectral changes in treatment-naive, actively drinking alcoholics. Alcohol Clin Exp Res; 2005; 29(4): 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Propping P Kruger J and Mark N.. Genetic disposition to alcoholism. An EEG study in alcoholics and their relatives. Hum Genet; 1981; 59(1): 51–59. [DOI] [PubMed] [Google Scholar]
- 11.Rangaswamy M, Porjesz B, Chorlian DB. et al. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res; 2003; 27(4): 607–615. [DOI] [PubMed] [Google Scholar]
- 12.Begleiter H and Platz A.. The effects of alcohol on the central nervous system in humans. In: Kissin B. and Begleiter H., eds. The biology of alcoholism. Vol. 2, Physiology and Behavior. New York: Plenum, 1972. [Google Scholar]
- 13.Saletu-Zyhlarz GM, Arnold O, Anderer P. et al. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol; (Oxford, Oxfordshire) 2004; 39(3): 233–240. [DOI] [PubMed] [Google Scholar]
- 14.Coutin-Churchman P, Moreno R, Anez Y. et al. Clinical correlates of quantitative EEG alterations in alcoholic patients. Clin Neurophysiol; 2006; 117(4): 740–751. [DOI] [PubMed] [Google Scholar]
- 15.Picton TW, Bentin S, Berg P. et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology; 2000; 37(2): 127–152. [PubMed] [Google Scholar]
- 16.Brion M, Dormal V, Lannoy S. et al. Imbalance between cognitive systems in alcohol-dependence and Korsakoff syndrome: an exploration using the alcohol flanker task. J Clin Exp Neuropsychol; 2018; 40(8): 820–831. [DOI] [PubMed] [Google Scholar]
- 17.Luijten M, Machielsen MW, Veltman DJ. et al. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci; 2014; 39(3): 149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botvinick MM, Braver TS, Barch DM. et al. Conflict monitoring and cognitive control. Psychol Rev; 2001; 108(3): 624–652. [DOI] [PubMed] [Google Scholar]
- 19.Yeung N Botvinick MM and Cohen JD.. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev; 2004; 111(4): 931–959. [DOI] [PubMed] [Google Scholar]
- 20.Dickter CL and Bartholow BD.. Ingroup categorization and response conflict: interactive effects of target race, flanker compatibility, and infrequency on N2 amplitude. Psychophysiology; 2010; 47(3): 596–601. [DOI] [PubMed] [Google Scholar]
- 21.Eriksen BA and Eriksen CW.. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys; 1974; 16(1): 143–149. [Google Scholar]
- 22.Van Veen V and Carter CS.. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci; 2002; 14(4): 593–602. [DOI] [PubMed] [Google Scholar]
- 23.Kamarajan C, Porjesz B, Jones KA. et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol; 2005; 69(3): 353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falkenstein M. Inhibition, conflict and the Nogo-N2. Clin Neurophysiol; 2006; 117(8): 1638–1640. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser S, Unger J, Kiefer M. et al. Executive control deficit in depression: event-related potentials in a Go/Nogo task. Psychiatry Res; 2003; 122(3): 169–184. [DOI] [PubMed] [Google Scholar]
- 26.Wrase J, Schlagenhauf F, Kienast T. et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage; 2007; 35(2): 787–794. [DOI] [PubMed] [Google Scholar]
- 27.de Greck M, Supady A, Thiemann R. et al. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics—a fMRI study. Hum Brain Mapp; 2009; 30(5): 1691–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mennes M, Wouters H, van den Bergh B. et al. ERP correlates of complex human decision making in a gambling paradigm: detection and resolution of conflict. Psychophysiology; 2008; 45(5): 714–720. [DOI] [PubMed] [Google Scholar]
- 29.Gehring WJ and Willoughby AR.. The medial frontal cortex and the rapid processing of monetary gains and losses. Science; (New York, NY) 2002; 295(5563): 2279–2282. [DOI] [PubMed] [Google Scholar]
- 30.Kamarajan C, Rangaswamy M, Tang Y. et al. Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J Psychiatr Res; 2010; 44(9): 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamito P, Oliveira J, Lopes P. et al. Executive functioning in alcoholics following an mHealth cognitive stimulation program: randomized controlled trial. J Med Internet Res. 2014; 16(4): e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupp CI, Kemmler G, Kurz M. et al. Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs; 2012; 73(4): 625–634. [DOI] [PubMed] [Google Scholar]
- 33.Horton L, Duffy T, Hollins Martin C. et al. Comprehensive assessment of alcohol-related brain damage (ARBD): gap or chasm in the evidence? J Psychiatr Ment Health Nurs; 2015; 22(1): 3–14. [DOI] [PubMed] [Google Scholar]
- 34.Svanberg J and Evans JJ.. Neuropsychological rehabilitation in alcohol-related brain damage: a systematic review. Alcohol Alcohol; (Oxford, Oxfordshire) 2013; 48(6): 704–711. [DOI] [PubMed] [Google Scholar]
- 35.Dawson DA Grant BF and Ruan WJ.. The association between stress and drinking: modifying effects of gender and vulnerability. Alcohol Alcohol; (Oxford, Oxfordshire) 2005; 40(5): 453–460. [DOI] [PubMed] [Google Scholar]
- 36.Koob G. F. and Kreek MJ.. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry; 2007;164(8):1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Man JKM, Lee EKO. et al. The effects of Qigong on anxiety, depression, and psychological well-being: a systematic review and meta-analysis. Evid Based Complement Alternat Med; 2013; 2013: 152738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motivala SJ Sollers J and Thayer J.. Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci; 2006; 61(11): 1177–1180. [DOI] [PubMed] [Google Scholar]
- 39.Irwin MR Olmstead R and Motivala SJ.. Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of Tai Chi Chih. Sleep; 2008; 31(7): 1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 40.Esch T, Duckstein J, Welke J. et al. Mind/body techniques for physiological and psychological stress reduction: stress management via Tai Chi training—a pilot study. Med Sci Monit; 2007; 13(11): CR488–497. [PubMed] [Google Scholar]
- 41.Lee MS, Kang CW, Lim HJ. et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health; 2004; 20(5): 243–248. [Google Scholar]
- 42.Wang F, Lee EK, Wu T. et al. The effects of tai chi on depression, anxiety, and psychological well-being: a systematic review and meta-analysis. Int J Behavior Med; 2014; 21(4): 605–617. [DOI] [PubMed] [Google Scholar]
- 43.Chen KW, Comerford A, Shinnick P. et al. Introducing qigong meditation into residential addiction treatment: a pilot study where gender makes a difference. J Altern Complement Med; (New York, NY) 2010; 16(8): 875–882. [DOI] [PubMed] [Google Scholar]
- 44.Kumar R, Kumar KJ, Benegal V. et al. Effectiveness of an Integrated Intervention Program for Alcoholism (IIPA) for enhancing self-regulation: preliminary evidence. Asian J Psychiatry; 2019; 43: 37–44. [DOI] [PubMed] [Google Scholar]
- 45.Sheehan DV, Lecrubier Y, Sheehan KH. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry; 1998; 59(suppl 20): 22–33; quiz 4–57. [PubMed] [Google Scholar]
- 46.Maxwell ME. Family interview for genetic studies: manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Programme, NIMH, 1992. [Google Scholar]
- 47.Ganguli M, Ratcliff G, Chandra V. et al. A Hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry; 1995; 10: 367–377. [Google Scholar]
- 48.Raistrick D Dunbar G and Davidson R.. Development of a questionnaire to measure alcohol dependence. Br J Addict; 1983; 78(1): 89–95. [DOI] [PubMed] [Google Scholar]
- 49.Bucholz KK, Cadoret R, Cloninger CR. et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol; 1994; 55(2): 149–158. [DOI] [PubMed] [Google Scholar]
- 50.Botvinick M, Nystrom LE, Fissell K. et al. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature; 1999; 402(6758): 179–181. [DOI] [PubMed] [Google Scholar]
- 51.Rueda MR, Posner MI, Rothbart MK. et al. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci; 2004; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santesso DL and Segalowitz SJ.. The error-related negativity is related to risk taking and empathy in young men. Psychophysiology; 2009; 46(1): 143–152. [DOI] [PubMed] [Google Scholar]
- 53.Kamarajan C, Porjesz B, Rangaswamy M. et al. Brain signatures of monetary loss and gain: outcome-related potentials in a SOG task. Behav Brain Res; 2009; 197(1): 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClure SM and Bickel WK.. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann N Y Acad Sci; 2014; 1327: 62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heatherton TF and Wagner DD.. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci; 2011; 15(3): 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verdejo-García A and Bechara A.. A somatic marker theory of addiction. Neuropharmacology; 2009; 56(suppl 1): 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheetham A, Allen NB, Yucel M. et al. The role of affective dysregulation in drug addiction. Clinical Psychol Rev; 2010; 30(6): 621–634. [DOI] [PubMed] [Google Scholar]
- 58.Sinha R, Fox HC, Hong KI. et al. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry; 2011; 68(9): 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanslmayr S, Sauseng P, Doppelmayr M. et al. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophysiol Biofeedback; 2005; 30(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 60.Hebert R, Lehmann D, Tan G. et al. Enhanced EEG alpha time-domain phase synchrony during transcendental meditation: implications for cortical integration theory. Signal Process; 2005; 85(11): 2213–2232. [Google Scholar]
- 61.Porjesz B, Rangaswamy M, Kamarajan C. et al. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol; 2005; 116(5): 993–1018. [DOI] [PubMed] [Google Scholar]
- 62.Klimesch W, Doppelmayr M, Wimmer H. et al. Theta band power changes in normal and dyslexic children. Clin Neurophysiol; 2001; 112(7): 1174–1185. [DOI] [PubMed] [Google Scholar]
- 63.Alper KR, Prichep LS, Kowalik S. et al. Persistent QEEG abnormality in crack cocaine users at 6 months of drug abstinence. Neuropsychopharmacology; 1998; 19(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 64.Li R, Jin L, Hong P. et al. The effect of Baduanjin on promoting the physical fitness and health of adults. Evid Based Complement Alternat Med; 2014; 2014: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M, Fang Q, Li J. et al. The effect of Chinese traditional exercise-Baduanjin on physical and psychological well-being of college students: a randomized controlled trial. PLoS One; 2015; 10(7): e0130544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, Mimura K, Wang L. et al. Psychological and physiological effects of 24-style taijiquan. Neuropsychobiology; 2005; 52(4): 212–218. [DOI] [PubMed] [Google Scholar]
- 67.Bunge SA, Hazeltine E, Scanlon MD. et al. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage; 2002; 17(3): 1562–1571. [DOI] [PubMed] [Google Scholar]
- 68.Kim THM Pascual-Leone J Johnson J. et al. The mental-attention Tai Chi effect with older adults. BMC Psychol; 2016; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wayne PM, Buring JE, Davis RB. et al. Tai Chi for osteopenic women: design and rationale of a pragmatic randomized controlled trial. BMC Musculoskelet Disord; 2010; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandey AK, Kamarajan C, Tang Y. et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol; 2012; 89(1): 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falkenstein M Hoormann J and Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol; 1999; 101(2–3): 267–291. [DOI] [PubMed] [Google Scholar]
- 72.Bokura H Yamaguchi S and Kobayashi S.. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol; 2001; 112(12): 2224–2232. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Caneda E, Cadaveira F, Crego A. et al. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction; (Abingdon, England) 2012; 107(10): 1796–808. [DOI] [PubMed] [Google Scholar]
- 74.Robinson TE and Berridge KC.. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction; (Abingdon, England) 2000; 95(suppl 2): S91–117. [DOI] [PubMed] [Google Scholar]
- 75.Liberzon I, Taylor SF, Fig LM. et al. Limbic activation and psychophysiologic responses to aversive visual stimuli: interaction with cognitive task. Neuropsychopharmacology; 2000; 23(5): 508–516. [DOI] [PubMed] [Google Scholar]
- 76.Elliott R Friston KJ and Dolan RJ.. Dissociable neural responses in human reward systems. J Neurosci; 2000; 20(16): 6159–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry; 2001; 6(3): 205–216. [DOI] [PubMed] [Google Scholar]
- 78.Cavedini P, Riboldi G, Keller R. et al. Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry; 2002; 51(4): 334–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.