Abstract
Nutritional intervention is a key strategy in the control and management of non-communicable diseases. Here, initially, we evaluated the effects of carrot juice (CJ) on some of the physical and biochemical parameters in rats fed with high-fructose diet, then in type 2 diabetic subjects. For the animal study, weanling male Wistar rats were given control (n = 6) or high fructose (HFr; n = 24) diet for 8 weeks. Then, the HFr group rats were subdivided into 4 groups (n = 6 in each) and continued either on HFr diet or shifted to control diet, with or without CJ (0.3 mg β-carotene) ingestion orally for 8 weeks. At the end, the ingestion of CJ reversed the HFr-induced adiposity (23 ± 1.6 vs 18 ± 1.1, P = .038), hypertriglyceridemia (182 ± 18.2 vs 90 ± 10.5 mg/dL, P<0.001), and hyperinsulinemia (81 ± 14.7 vs 40 ± 7.5 µU/mL, P = .014), while increased the retinol levels in liver (240 ± 38.4 vs 492 ± 61.2 µg/g, P = .002) and adipose tissue (1.8 ± 0.09 vs 2.5 ± 0.18 µg/g, P = .026). On the other hand, in the diabetic subjects (7 males and females each, n = 14) compared to their baseline, the daily consumption of 50 mL CJ (~2400 µg β-carotene) for 6 weeks significantly reduced the body weight (69.4 ± 4.13 vs 69.0 ± 4.09 kg, P = .014), BMI (27.4 ± 1.07 vs 27.2 ± 1.06 kg/m2, P = .007), and fat% (33.4 ± 1.87 vs 31.9 ± 2.13, P = .029) with an increase in plasma β-carotene levels (0.21 ± 0.045 vs 0.45 ± 0.089 µmol/L, P = .044). Although CJ increased the glucose (145 ± 10.4 vs 165 ± 11.4 mg/dL, P = .039), insulin, and glycated hemoglobin levels remained unaltered. In conclusion, the consumption of carrot juice reversed the HFr-induced metabolic abnormalities in a rat model and decreased body weight and BMI of diabetic subjects.
Keywords: Carotenoids, vegetables, metabolic syndrome, obesity, supplementation
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent (ranging <10%-⩾30%), asymptomatic and progressive disease, and it is a leading cause of liver-related deaths worldwide. 1 However, the disease progression from simple steatosis to hepatocellular carcinoma is highly complex and influenced by several endogenous (such as genotype and genetic makeup) and exogenous factors (eg, diet and physical activity). 2 Although it is a multi-factorial disease, the excessive intake of fructose, a key lipogenic sugar is known to be associated with a higher risk for the development of NAFLD and other co-morbidities, such as insulin resistance, obesity, hypertension, etc. 3 Further, obesity, diabetes, and metabolic syndrome contribute to the development of NAFLD and vice-a-versa. 4 The visceral adipose tissue plays a central role in the pathogenesis of fatty liver disease, as the adipose-derived several pro-inflammatory mediators and inflammatory adipocytokines are known to aggravate the primary metabolic insult and worsen the liver health. 5
Although the current understanding of the disease has identified several pathways, no specific treatment exists for NAFLD even now. Therefore, lifestyle modification is a viable option in common clinical practice, aimed not only at disease management and/or its progression, but also the associated complications, such as insulin resistance. 6 A meta-analysis study of Schwingshackl et al., 7 has found that the intake of fruits and vegetables influences the development of type 2 diabetes by preventing weight gain and adiposity. Previously, Poudyal et al., 8 have reported the attenuation and reversal of metabolic syndrome and its associated morbidities by purple carrot juice (rich in anthocyanins), while the administration of β-carotene alone had no reversal effect on some of the pathological conditions associated with high-carbohydrate, high fat diet feeding in rats. Further, the studies of Mahes et al.,9,10 have reported that the consumption of carrot juice (rich in β-carotene) along with high fructose did not prevent hepatic steatosis in weanling rats, however, decreased the pro-inflammatory cytokines. Initially, here, we tested in adult Wistar rats that (i) whether the carrot juice ingestion reveres the high fructose-mediated hepatic triglyceride accumulation and its associated complications; that is, excessive adiposity and insulin resistance and (ii) whether it can augment the reversal of hepatic steatosis and the associated metabolic aberrations when the diet was sans high fructose. Later, based on the findings in rats that showed beneficial effects on some of the insulin sensitivity-associated parameters, we conducted a pilot study to test whether these effects can be seen even in the subjects with type 2 diabetes.
Materials and Methods
Materials
All reagents, biochemicals and solvents were of analytical grade and/or ultra-pure quality. Triglyceride assay kit was purchased from BioSystems S.A., Barcelona, Spain. Colorimetric assay kits for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) from Biovision Inc., CA, USA and β-hydroxy butyrate from Sigma Aldrich Inc., MO, USA were purchased. ELISA kits for leptin, tumor necrosis factor α (TNFα), macrophage chemoattractant protein 1 (MCP1) (Life Technologies, Thermo Fischer Scientific Inc., MD, USA), high sensitive C-reactive protein (hsCRP) (CUSABIO, Hubei, China), insulin and adiponectin (Crystal Chem Inc., IL, USA), retinol binding protein 4 (RBP4) (Biovision Inc., CA, USA), and fibroblast growth factor 21 (FGF21) (R&D Systems, Minneapolis, USA) were used. Human insulin was measured, using a commercially available ELISA kit (Invitrogen, Thermo Fisher Scientific, MA, USA). Total RNA isolation kit was obtained from Qiagen GmbH, Hilden, Germany. Adipose tissue cytokines were analyzed using Milliplex Map Rat Adipocytokine Panel (Millipore, MA, USA). For quantitative real-time PCR analysis (qRT-PCR), first strand cDNA synthesis kit (New England Biolabs, MA, USA), pre-validated universal probe for rat (Roche diagnostics GmbH, Mannheim, Germany) and gene-specific primers (Integrated DNA Technologies Inc., Iowa, USA) were used. Primary antibodies were procured from Abcam Inc, MA, USA, [Stearoyl-CoA desaturase 1 (SCD1), carnitine palmitoyl transferase 1 (CPT1)], Novus Biologicals Inc. CO, USA [Perilipin and microsomal triglyceride transfer protein (MTTP)], and Sigma Aldrich Inc. MO, USA [Glycerol-3-phosphate acyltransferase 1 (GPAT1) and glyceraldehyde-3-phophate dehydrogenase (GAPDH)]. Recombinant human insulin, protease inhibitor cocktail, various standards including retinol, retinol acetate, β-carotene and β-apo-8′-carotenal were purchased from Sigma Aldrich Inc., MO, USA. T-PER tissue protein extraction reagent was purchased from Thermo Scientific, IL, USA. The polyvinylidine difluoride (PVDF) membrane and chemiluminescent reagents were from GE Healthcare Life Sciences GmbH, Munich, Germany.
Experimental design: Animal study
Male weanling Wistar rats, weighing an average of 41.0 g (n = 30) were taken from the National Centre for Laboratory Animal Sciences, the National Institute of Nutrition, Hyderabad, India, then divided into 2 groups initially and given either the control diet (Con; n = 6) or high fructose diet (HFr; n = 24) ad libitum for 8 weeks (Supplemental Table S1). After this initial phase, the rats belong to HFr diet group were randomly subdivided into 4 groups, consisting of 6 rats in each and continued either on the HFr diet or shifted to control diet, with or without carrot juice (CJ) administration (containing 0.3 mg of β-carotene) through oral route daily for 8 weeks. The groups were designated as control (Con), high fructose (HFr), high fructose diet with carrot juice (HFr + CJ), HFr shifted to control diet (HFr(s)Con) and HFr shifted to control diet with carrot juice (HFr(s)Con + CJ). The animals were housed individually (22.0 ± 1°C ambient temperature, 50%-60% relative humidity, 12/12 h light/ dark cycle) and given “humane care” in accordance with the principles of the Guide to the Care and Use of Experimental Animals. The Institutional Animal Ethics Committee (IAEC) of the National Institute of Nutrition, Hyderabad, India has approved the study (Ref. No. P08F/IAEC/NIN/6/2013/SMJ/WNIN M62). All the experimental procedures, including animal handling, oral administration of carrot juice were carried out by skilled personnel.
The carrot juice extraction and β-carotene quantification were carried out as described by Mahes et al.9,10 An average of 0.6 mL of carrot juice provided 0.3 mg of β-carotene. This was equivalent to 25% (i.e, 150 µg retinol equivalents; RE) of the recommended dietary allowance (RDA) of vitamin A (i.e, 600 µg RE) for humans from β-carotene, 11 considering the conversion efficiency of β-carotene to retinol in rats as 2:1. 12 The compositions of the experimental diets (isocaloric diet, 4 kcal/g) are given the Supplemental Table S2. Daily food intake and weekly body weight data were recorded. At the end of the experimental duration, blood was drawn from retro-orbital sinus in the EDTA-coated tubes from the overnight-fasted rats, killed humanely by cervical dislocation under anesthetized condition (Isoflurane—nasal inhalation) and various tissues were collected, weighed, and stored at −80°C for further analysis. The adiposity index was calculated as sum of adipose tissue weights divided by body weight and expressed as percent. 13
Oral glucose and intra-peritoneal insulin tolerance tests (OGTT and IPITT)
At the end of the 14th week, over-night-fasted animals were administered 2 g glucose through oral route or 0.5 U human recombinant insulin, by intra-peritoneal injection per kg body weight. The blood was drawn for glucose measurement using a glucometer (ACCU-CHEK, Roche) at various time intervals and the area under curve (AUC) was calculated, using the trapezoidal method. 9
Plasma and adipose tissue biochemistry
Quantification of various plasma biochemical parameter/analytes of both experimental and human samples were carried out using kits, according to the manufacturers’ instruction. Various cytokines were quantified in the RPWAT by milliplex assay kit, according to the manufacturer’s instruction. Triglyceride content of liver and adipose tissue, retinol levels in plasma (both experimental and human samples), liver and adipose tissues of experimental animals were quantified by HPLC method; however, for this purpose, the tissue samples (i.e, liver and adipose tissue) were subjected to saponification, and then retinol was extracted and quantified.10,14 Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated. 15
Hepatic gene expression by qRT-PCR and protein expression by immunoblotting
From the hepatic total RNA, reverse transcription reaction and then quantitative real time-PCR was performed (Roche LightCycler 480, Mannheim, Germany) using pre-validated probes and gene-specific primers for stearoyl-CoA desaturase 1 (SCD1), glycerol-3-phosphate acyltransferase 1(GPAT1) [Triglyceride synthesis pathway], microsomal triglyceride transfer protein (MTTP) [Triglyceride secretory pathway], and cartnitine palmitoyl transferase 1 (CPT1) [Fatty acid oxidation pathway]. Expression of acidic ribosomal phosphoprotein (ARPP) was used for normalization and relative quantification. The primer sequence details are provided in the Supplemental Table S3.
In brief, 100 to 250 mg of liver tissue was homogenized in the T-PER tissue protein extraction reagent added with 5% protease inhibitor and 1% phosphatase inhibitor cocktails, followed by centrifugation at 200 g for 1 min at 4°C to sediment the cell debris. From the supernatant of crude homogenate, a constant amount of protein (40 µg) was used for immunoblotting of SCD1, GPAT1, MTTP, and CPT1 proteins as described by Reddy et al. 16 The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control and the images of immunoblots were analyzed by the Image J 1.49 software (National Institute of Health, Bethesda, MD, USA).
Liver histology, immunohistochemistry, and fatty acid composition analysis by gas-liquid chromatography (GC)
Formalin-embedded liver sections were processed for histological examination by hematoxylin & eosin (H&E) staining and immunohistological examination of the lipid-droplet associated protein; perilipin, as reported by Mahes et al. 9
From the extracted total lipids from liver tissue, an aliquot was used to prepare the fatty acid methyl esters and analyzed by gas-liquid chromatography (GC) coupled with flame ionization detector (FID) (Agilent 6890 with autosampler, CA, USA). 13
Human study design
After the initial screening of 17 subjects with type 2 diabetes, 14 subjects aged between 30 and 60 years of both sexes (n = 7 from male and female each) found eligible for the pilot study, according to the inclusion criterion, that is, fasting blood glucose ⩾126 mg/dL and excluded the diabetic subjects with any other chronic diseases (such as cardiovascular diseases and cancers, etc.) or a history of infections, allergies, or thyroid deficiencies. The selected subjects were invited to take part in a meeting through phone calls, in which, the objectives and implementation methods of the study were described in detail. Further, before the commencement, written informed consent was obtained from each subject, after explaining the purpose of the study and its requirement. Freshly prepared 50 mL of carrot juice (~2400 μg β-carotene) was provided in a clean glass bottle to the each subject daily for 6 weeks period at their doorstep around noon time and instructed to consume immediately after lunch. According to the dietary guidelines for Indians, the daily recommended dietary allowance (RDA) of β-carotene is 4800 µg to meet 600 µg RE for vitamin A. Therefore, here we administered the carrot juice containing 2400 µg β-carotene to obtain 50% of the RDA for vitamin A (i.e, 300 µg RE), considering the conversion efficiency of β-carotene to retinol as 8:1 in humans. 11 Further, the participants were instructed not to change their routine/usual diet and physical activity or their medications. Utmost care and hygiene conditions were maintained during the extraction and delivery of carrot juice to the participants. Before and at the end of the study period (i.e, sixth week), blood pressure was measured, then 5.0 mL blood was drawn from the over-night fasted subjects (~12 hrs), in a potassium EDTA-coated vacuette tube. The study protocol (Study No. 07/I/2019) was approved by the Institutional Human Ethics Committee of the National Institute of Nutrition, Hyderabad, India and obtained before the commencement. Based on the data from pilot extraction and quantification of β-carotene in carrot juice, and our earlier studies, we fixed the volume as 50 mL to get ~2400 μg of β-carotene and initiated the study. However, the actual β-carotene content of the carrot juice was quantified at the end.
β-Carotene extraction, estimation, and total polyphenol content of carrot juice
From the local market, carrots were purchased daily, washed in running tap water, and the juice was extracted in a mechanical juicer (HR1861/00, Philips, India). The juice volume was measured every day (average juice volume is 488 mL/kg carrot), filtered through a nylon mesh and an aliquot of the juice was stored at −20°C and pooled randomly (6 samples/pooling), for estimating β-carotene content by HPLC method as described earlier.9,10 Finally, a total of 7 pooled samples of carrot juice were analyzed in duplicate and the average β-carotene content was found to be 48.6 µg/mL (ranging 40-64 µg/mL), which provided 2432 µg of β-carotene in 50 mL of raw carrot juice. In addition, the average total phenolic content was 0.293 mg gallic acid equivalents (GAE) (ranged between 0.148 and 0.548 mg) per mL of carrot juice, as measured by the method of Singleton et al. 17
Anthropometric, fat percent measurements, and food frequency questionnaire (FFQ)
From the study participants, the height and weight of the study participants were measured and calculated the body mass index (BMI). The body fat percent of each subject was obtained by bioelectrical impedance-based body composition analyzer; TANITA (BC-418, Tanita Corporation, Tokyo, Japan) as reported by Korrapati et al. 15
A semi-quantitative FFQ was developed (listing high β-carotene containing 20 food items, from different food groups) and categorized into 6, to assess the frequency of intake varying from “never to twice daily.” The frequency of consumption per week was assessed for 6 weeks and calculated the β-carotene intake from the consumed food items using the Indian Food Composition Tables (IFCT). 18
Human plasma clinical and biochemical parameters
Fasting blood glucose levels were measured by a glucometer and the glycated hemoglobin, HbA1c in the whole blood was measured using Alere Afinion AS100 analyzer (Alere Limited, Auckland, New Zealand). The β-carotene levels in the human plasma were quantified by reverse phase HPLC (Dionex-Ultimate 3000 series, CA, USA), according to the method of Bui. 19
Statistical analysis
Values are expressed as means ± standard error (SE). For the animal study, the data were subjected to One way ANOVA with post hoc least significant difference (post hoc LSD) test after analyzed them for normality distribution by Shapiro-Wilk test. For the human study, the data were analyzed by paired sample t-test either as pooled or segregated by sex. The mean difference was considered significant if the P-value is ⩽.05 level. Spearman’s rho non-parametric correlation analysis was also performed. The data were analyzed, using IBM SPSS statistics 21.0 software (IBM Corp., Armonk, NY, USA).
Results
Animal study
Impact of carrot juice on physical and biochemical parameters
Compared to the control diet, the feeding of HFr diet significantly increased the weight gain, which corroborated with increased visceral (retroperitoneal and epidydimal) and subcutaneous adipose depots weights. On the contrary, the administration of carrot juice to the rats consuming high fructose (HFr + CJ), significantly reduced the weights of visceral and subcutaneous adipose depots, thus adiposity index without affecting their food intake. Further, the groups shifted from HFr to control diet either with or without carrot juice ingestion (HFr(s)Con + CJ or HFr(s)Con) also exhibited similar changes, including a reduction of weight gain in the former group, when compared to that of HFr diet-fed rats, (Table 1).
Table 1.
Effect of carrot juice on physical and plasma biochemical parameters.
| Experimental groups | |||||
|---|---|---|---|---|---|
| Control | HFr | HFr + CJ | HFr(s)Con | HFr(s)Con + CJ | |
| Physical parameters | |||||
| Initial weight (g) | 260 ± 7.8 | 265 ± 14.2 | 261 ± 6.7 | 270 ± 6.3 | 272 ± 7.2 |
| Final weight (g) | 347 ± 11.6 | 373 ± 18.1 | 334 ± 10.7 | 348 ± 18.4 | 324 ± 25.3 |
| Weight gain (g) | 87 ± 7.0 | 108 ± 10.2*a | 73 ± 9.3a | 78 ± 136a | 52 ± 22.4b |
| Food intake (g) | 15 ± 1.8 | 20 ± 0.77* | 18 ± 0.59 | 19 ± 1.0 | 17 ± 1.2 |
| Liver (g) | 8.7 ± 0.79 | 11.9 ± 1.01*a | 9.6 ± 0.41ab | 9.8 ± 0.75ab | 9.2 ± 0.74b |
| Retroperitoneal WAT (g) | 9.0 ± 1.1 | 13.3 ± 0.67*a | 10.2 ± 0.73b | 10.0 ± 1.4b | 7.6 ± 1.1b |
| Epidydimal WAT (g) | 2.4 ± 0.16 | 3.6 ± 0.41*a | 2.7 ± 0.21b | 2.5 ± 0.2b | 2.0 ± 0.20b |
| Subcutaneous WAT (g) | 6.4 ± 1.3 | 9.0 ± 0.92*a | 6.8 ± 0.49a | 6.1 ± 0.66b | 4.4 ± 0.39b |
| Adiposity index | 16 ± 1.9 | 23 ± 1.6*a | 18 ± 1.1b | 17 ± 2.1b | 13 ± 1.5b |
| Plasma biochemical parameters | |||||
| Total cholesterol (mg/dL) | 112 ± 1.28 | 106 ± 7.0a | 98 ± 5.9ab | 109 ± 5.1a | 83 ± 5.6b |
| HDL-C (mg/dL) | 82 ± 1.2 | 81 ± 2.9a | 75 ± 3.6a | 79 ± 5.4a | 62 ± 3.8a |
| Total cholesterol/HDL-C | 1.4 ± 0.02 | 1.3 ± 0.04 | 1.3 ± 0.09 | 1.4 ± 0.10 | 1.4 ± 0.04 |
| Triglycerides (mg/dL) | 128 ± 14.1 | 182 ± 18.2*a | 90 ± 10.5b | 108 ± 18.3b | 107 ± 9.9b |
| ALT (mU/mL) | 30.6 ± 3.94 | 27.1 ± 4.60 | 27.6 ± 2.20 | 26.8 ± 151 | 28.4 ± 1.36 |
| AST (mU/mL) | 65.5 ± 1.61 | 69.6 ± 5.94 | 66.0 ± 3.93 | 69.1 ± 6.49 | 69.8 ± 4.37 |
| β-hydroxy butyrate (ng/mL) | 95 ± 5.8 | 95 ± 8.0 | 96 ± 10.8 | 104 ± 8.3 | 87 ± 7.9 |
| Retinol (µmol/L) | 2.9 ± 0.10 | 3.0 ± 0.13 | 2.6 ± 0.20 | 2.7 ± 0.08 | 2.8 ± 0.12 |
Abbreviations: ALT, Alanine aminotrasnsferase; AST-Aspartate aminotransferase; EP, Epidydimal; HFr, High fructose diet; HFr + CJ, High fructose diet with carrot juice; HFr(s)Con, High fructose shifted to control diet; HFr(s)Con + CJ, High fructose shifted to control diet with carrot juice; RP, Retroperitoneal; SC, Subcutaneous; WAT, White adipose tissue.
Values are expressed as means ± SE of 5 to 6 rats from each group. Data were analyzed by One way ANOVA with post hoc least significant difference tests (post hoc LSD) and P ⩽ .05 was considered significant.
Significant, when compared between control and HFr diets. The groups bearing common superscripts are not significantly different. Otherwise, the group bearing different superscripts is statistically different from other group(s) at P ⩽ .05 level.
Plasma total cholesterol (TC) and HDL-cholesterol (HDL-C) levels were comparable across the groups, except the group that received carrot juice, after shifted to control diet (HFr(s)Con + CJ ) displayed a significant reduction. However, the ratio of TC to HDL-C remained unaltered. Compared to the control diet, the feeding of HFr diet resulted in hypertriglyceridemia. On the contrary, the ingestion of carrot juice significantly brought down the hypertriglyceridemia (HFr + CJ or HFr(s)Con + CJ), as compared to the HFr diet-fed group and the withdrawal of the HFr diet also resulted in similar changes (HFr(s)Con). However, the circulatory levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), β-hydroxybutyrate (the fatty acid β-oxidation end product), and retinol remained comparable across the groups (Table 1) and no detectable levels of β-carotene in the plasma. The unaltered food intake and plasma ALT and AST levels suggest that the administration of carrot juice through oral gavage did not induce stress to experimental animals.
Impact of carrot juice on circulatory and tissue cytokines
In line with increased adiposity, leptin; the adipose-specific secretory cytokine levels showed a significant elevation both in plasma and visceral adipose depot; retroperitoneal white adipose tissue (RPWAT) of rats fed with high fructose diet, as compared to those of control diet-fed rats. On the other hand, the administration of carrot juice (HFr + CJ and HFr(s)Con + CJ) or withdrawal of high fructose from the diet (HFr(s)Con) brought down the elevated leptin levels. However, the adiponectin, fibroblast growth factor 21 (FGF21), and other inflammatory cytokines, such as tumor necrosis factor α (TNFα), high sensitive C-reactive protein (hsCRP), and macrophage chemoattractant protein 1 (MCP1) levels remained comparable among all the groups both in plasma and some of them in RPWAT, including total plasminogen activator inhibitor (tPAI). Further, the group that was shifted to control diet (HFr(s)Con) from high fructose regimen displayed a reduction of TNFα level in the RPWAT, when compared to the HFr diet-fed group (Table 2).
Table 2.
Effect of carrot juice on cytokines of plasma and adipose tissue; RPWAT.
| Adipocytokines | Experimental groups | ||||
|---|---|---|---|---|---|
| Control | HFr | HFr + CJ | HFr(s)Con | HFr(s)Con + CJ | |
| Plasma | |||||
| Leptin (ng/mL) | 8.8 ± 1.36 | 13.3 ± 0.85*a | 9.0 ± 0.86b | 6.2 ± 0.74b c | 5.3 ± 0.42 c |
| Adiponectin (µg/mL) | 6.4 ± 0.67 | 5.2 ± 0.75 | 6.0 ± 0.34 | 4.9 ± 0.74 | 5.3 ± 0.42 |
| TNFα (pg/mL) | 3.2 ± 0.22 | 2.9 ± 0.07 | 3.0 ± 0.10 | 3.0 ± 0.20 | 3.4 ± 0.29 |
| MCP1 (pg/mL) | 95 ± 8.0 | 101 ± 6.10 | 88 ± 14.7 | 109 ± 8.9 | 86 ± 8.8 |
| hsCRP (ng/mL) | 450 ± 126.7 | 467 ± 91.3 | 509 ± 204.7 | 250 ± 55.6 | 505 ± 71.9 |
| FGF21 (pg/mL) | 298 ± 42.8 | 207 ± 34.5 | 221 ± 32.2 | 228 ± 32.9 | 339 ± 79.2 |
| Adipose tissue; RPWAT | |||||
| Leptin (ng/mg protein) | 2.6 ± 0.41 | 4.4 ± 0.54*a | 3.0 ± 0.34b | 2.1 ± 0.39b | 1.7 ± 0.45b |
| Adiponectin (µg/mg protein) | 6.1 ± 0.80 | 5.3 ± 0.98 | 6.3 ± 0.56 | 4.8 ± 0.56 | 6.7 ± 0.76 |
| TNFα (ng/mg protein) | 0.17 ± 0.007 | 0.17 ± 0.007a | 0.17 ± 0.008a | 0.15 ± 0.005b | 0.17 ± 0.006ab |
| MCP1 (ng/mg protein) | 5.7 ± 0.82 | 7.2 ± 0.71 | 7.0 ± 0.80 | 5.8 ± 0.37 | 6.6 ± 0.85 |
| tPAI (ng/mg protein) | 0.42 ± 0.042 | 0.29 ± 0.024 | 0.32 ± 0.071 | 0.23 ± 0.075 | 0.16 ± 0.045 |
Abbreviations: FGF21, Fibroblast growth factor 21; HFr, High fructose diet; HFr + CJ, High fructose diet with carrot juice; HFr(s)Con, High fructose shifted to control diet; HFr(s)Con + CJ, High fructose shifted to control diet with carrot juice; hsCRP, High sensitive C-reactive protein; MCP1, Macrophage chemoattractant protein 1; RPWAT, Retroperitoneal white adipose tissue; tPAI, Total plasminogen activator inhibitor; TNFα, Tumor necrosis factor α.
Values are expressed as means ± SE of 5 to 6 rats from each group. Data were analyzed by One way ANOVA with post hoc least significant difference tests (post hoc LSD) and P ⩽ .05 was considered significant.
Significant, when compared between control and HFr diet. The groups bearing common superscripts are not significantly different. Otherwise, the group bearing different superscripts is statistically different from other group(s) at P ⩽ .05 level.
Impact of carrot juice on liver triglycerides, histology, lipid metabolic pathway, tissue retinol, and fatty acid composition
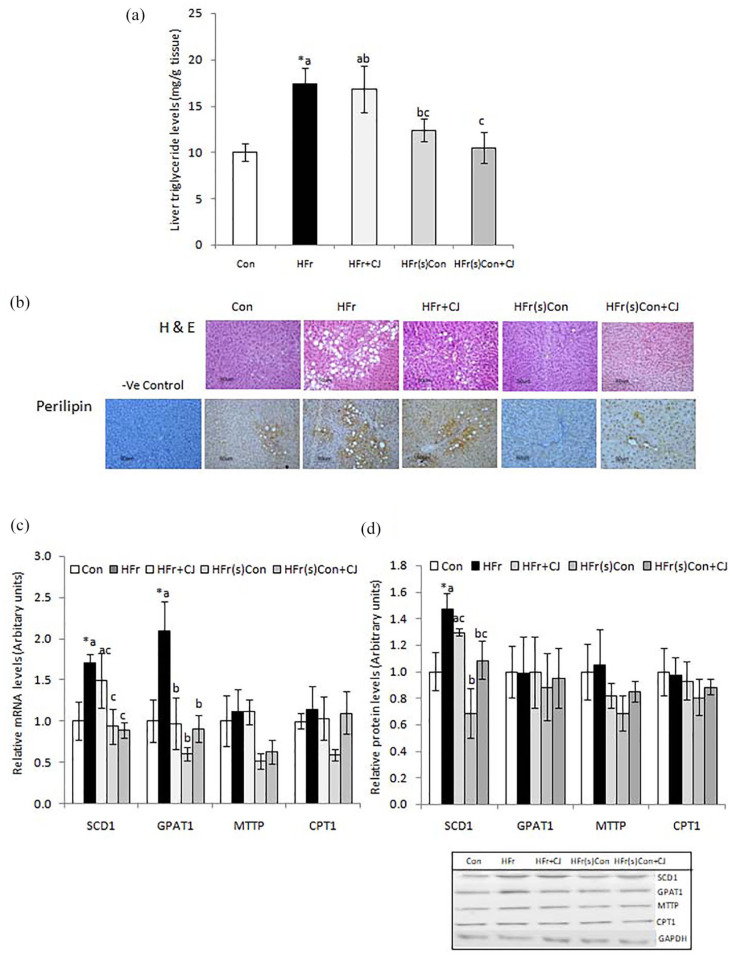
Compared to the control, the consumption of HFr diet significantly increased the triglycerides levels in the liver, whereas, the withdrawal of high fructose from the diet showed a reduction, which was evident from the group that got shifted from the HFr diet to control diet (HFr(s)Con). However, the ingestion of carrot juice neither attenuated the accumulation nor augmented the reduction of hepatic triglycerides (HFr + CJ and HFr(s)Con + CJ), when compared to the HFr diet-fed group (Figure 1a). In line with the biochemical data, the liver histological examination showed a higher hepatic lipid accumulation in the HFr diet-fed groups (HFr and HFr + CJ), and its reduction by the withdrawal of HFr in the diet (HFr(s)Con and HFr(s)Con + CJ). Further, all these observations corroborated with the lipid droplet-associated protein, perilipin of the liver, as evidenced by the immunohistochemistry (Figure 1b).
Figure 1.
Effect of carrot juice on tissue triglycerides, histology, and expression of lipid metabolic pathway genes and proteins. Values are expressed as means ± SE of 5 to 6 rats, except for gene and protein expression 4 rats from each group. (a) Liver triglycerides, (b) Representative photomicrographs of H and E stained liver sections and immunohistological staining of liver perilipin, a lipid droplet-associated protein (Top and Bottom, respectively) (20× magnification in Nikon-Eclipse E800 microscope), and (c and d) Hepatic lipid metabolic pathway mRNA and their protein levels with representative immunoblots. Data were analyzed by One way ANOVA with post hoc least significant difference test (post hoc LSD) and P ⩽ .05 level was considered significant. *Significant, when compared between control and HFr diet-fed groups. Bars bearing common superscripts are not different, otherwise, the bar bearing different superscripts is statistically different from others at P ⩽ .05 level.
Abbreviations: −ve, Negative; Con, Control diet; CPT1, Carnitine palmitoyl transferase 1; GPAT1, Glycerol-3-phosphate acyltransferase 1; HFr, High fructose diet; HFr + CJ, High fructose diet and carrot juice; HFr(s)Con, High fructose shifted to control diet; HFr(s)Con + CJ, High fructose shifted to control diet and carrot juice; MTTP, Microsomal triglyceride transfer protein; SCD1, Stearoyl-CoA desaturase 1.
Acidic ribosomal phosphoprotein (ARPP) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were used for normalization of mRNA and protein expression respectively.
The hepatic lipid metabolic pathway analysis showed that compared to the control, mRNA levels of stearoyl-CoA desaturase 1 (SCD1), and glycerol-3-phosphate acyltransferase 1 (GPAT) genes were significantly elevated in the HFr diet-fed rats. On the contrary, the expression was found suppressed in the groups that were shifted to either control diet alone or with the administration of carrot juice (HFr(s)Con or HFr(s)Con + CJ respectively) as compared to that of HFr diet-fed group. The intake of carrot juice along with the HFr diet (HFr + CJ) did not impact the hepatic SCD1 mRNA levels, however, it significantly decreased the mRNA levels of GPAT1. However, the expression of MTTP and CPT1 genes remained comparable among all the groups (Figure 1c). Further, to confirm the mRNA transcripts at protein levels, the immunoblotting analysis was carried out and found that the SCD1 protein expression corroborated with its mRNA levels, whereas the other proteins such as GPAT1, MTTP, and CPT1 showed no change (Figure 1d).
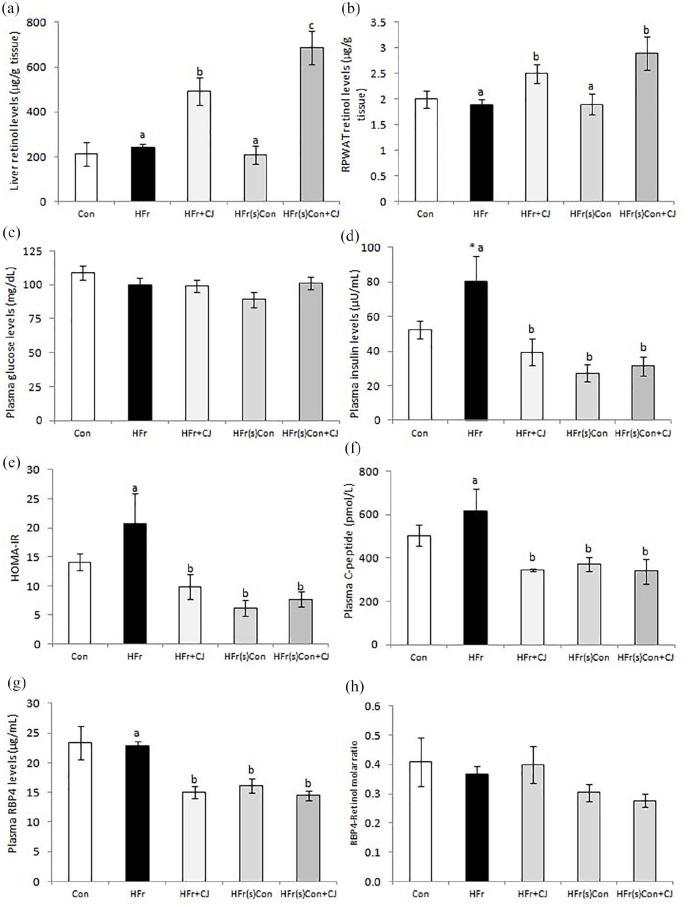
The retinol contents of the liver and RPWAT were comparable between the control and high fructose diet-fed rats and the ingestion of carrot juice (HFr + CJ and HFr(s)Con + CJ) resulted in significant accumulation of retinol in both tissues (Figure 2a and b, respectively).
Figure 2.
Effect of carrot juice on tissue retinols and indicators of insulin resistance. Values are expressed as means ± SE of 5 to 6 rats in each group. (a and b) Retinol levels of liver and RPWAT respectively. Plasma levels of (c) glucose, (d) insulin, (e) HOMA-IR index, (f) C-peptide, (g) RBP4, and (h) molar ratio of RBP4 to retinol. Data were analyzed by One way ANOVA with post hoc least significant difference test (post hoc LSD) and P ⩽ .05 level was considered significant. *Significant, when compared between control and HFr diet-fed groups. Bars bearing common superscripts are not different, otherwise, the bar bearing different superscripts is statistically different from others at P ⩽ .05 level.
Abbreviations: Con, Control diet; HFr, High fructose diet; HFr + CJ, High fructose diet and carrot juice; HFr(s)Con, High fructose shifted to control diet; HFr(s)Con + CJ, High fructose shifted to control diet and carrot juice; HOMA-IR, Homeostasis model assessment of insulin resistance; RBP4, Retinol binding protein 4; RPWAT, Retroperitoneal white adipose tissue.
Compared to the control group, feeding of HFr diet elevated the hepatic monounsaturated fatty acids (MUFA); palmitoleic (C16:1), oleic acid (C18:1), whereas polyunsaturated fatty acid (PUFA); linoleic (C18:2, n−6), and α-linolenic (C18:3, n−3) acid levels decreased significantly. However, the ingestion of carrot juice to the rats receiving the HFr diet (HFr + CJ) did not exhibit significant changes in any of the fatty acids of liver total lipids. On the other hand, the groups shifted to the control diet devoid of high fructose (either alone or with carrot juice, i.e, HFr(s)Con or HFr(s)Con + CJ respectively), reversed the fatty acid compositional changes in MUFA and PUFA (Supplemental Table S4).
Impact of carrot juice on indicators of insulin sensitivity
Compared to the control, the HFr diet feeding induced hyperinsulinemia but did not affect the fasting blood glucose levels. On the contrary, the ingestion of carrot juice to HFr diet-fed rats (HFr + CJ) significantly brought down the plasma insulin and its precursor; C-peptide, without affecting the glucose levels, as compared to those of fed with HFr diet, thereby resulting in improved HOMA-IR index. Similar effects were also seen in the animals that were shifted to control diet, without or with carrot juice administration (HFr(s)Con or HFr(s)Con + CJ respectively). Notably, one of the plasma insulin resistance markers; the RBP4 levels showed a significant reduction in the groups that received the carrot juice (HFr + CJ and HFr(s)Con + CJ), although the withdrawal of HFr diet also displayed similar changes (HFr(s)Con). However, the molar ratio of RBP4 to retinol remained comparable (Figure 2c-h). Further, the glucose clearance rate in response to either glucose load or insulin remained unchanged across the groups, which was evident from the area under curve (AUC) for glucose, calculated from both OGT and IPIT tests (Supplemental Figure S1).
Human study
Impact of carrot juice on physical, clinical, and biochemical parameters
The consumption of 50 mL freshly prepared raw carrot juice (containing ~2400 µg β-carotene) daily for 6 weeks significantly decreased body weight (P = .014) at the end, possibly due to reduction in body fat percent (P = .029), as evidenced by the body composition analysis and this was reflected in the reduction of BMI (P = .007) of the diabetic subjects. Further, it resulted in 2-fold increase (P = .044) of β-carotene levels in the plasma, compared to the baseline levels. However, the various other parameters, such as blood pressure (both systolic and diastolic), triglycerides, total cholesterol (TC), and HDL-C levels in the circulation remained unaltered. In addition, a significant increase in the fasting blood glucose levels (P = .039) was observed at the end of the carrot juice consumption period, compared to the initial levels, while the other diabetes-associated clinical parameters, namely glycated hemoglobin (HbA1c) and insulin levels remained unaltered. Notably, the systolic blood pressure and plasma total cholesterol levels of females at baseline were significantly higher (P = .026 and .028 respectively), whereas insulin level (P = .049) was lower than the males (Table 3).
Table 3.
Effect of carrot juice on physical, clinical, and biochemical parameters in type 2 diabetic subjects.
| Pooled (n = 14) | Male (n = 7) | Female (n = 7) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | P value | Baseline | Final | P value | Baseline | Final | P value | |
| Weight (kg) | 69.4 ± 4.13 | 69.0 ± 4.09* | .014 | 75.7 ± 5.32 | 75.1 ± 5.30 | .071 | 63.2 ± 5.70 | 62.9 ± 5.66 | .059 |
| BMI (kg/m2) | 27.4 ± 1.07 | 27.2 ± 1.06* | .007 | 27.7 ± 1.59 | 27.5 ± 1.56 | .057 | 27.1 ± 1.55 | 26.9 ± 1.52* | .047 |
| Fat % | 33.4 ± 1.87 | 31.9 ± 2.13* | .029 | 29.2 ± 1.89 | 28.2 ± 2.84 | .405 | 36.9 ± 2.28 | 35.0 ± 2.65* | .027 |
| Systolic blood pressure (mmHg) | 130 ± 4.7 | 128 ± 4.3 | .535 | 127 ± 4.7 | 128 ± 4.7 | .764 | 133 ± 8.5 $ | 128 ± 7.5 | .265 |
| Diastolic blood pressure (mmHg) | 85 ± 2.4 | 84 ± 2.5 | .560 | 85 ± 2.6 | 85 ± 3.2 | .880 | 85 ± 4.3 | 82 ± 4.0 | .391 |
| Triglycerides (mg/dL) | 175 ± 28.4 | 182 ± 23.6 | .774 | 202 ± 54.3 | 210 ± 41.0 | .844 | 148 ± 17.5 | 154 ± 21.8 | .851 |
| Total cholesterol (mg/dL) | 252 ± 17.2 | 226 ± 11.1 | .157 | 239 ± 33.1 | 213 ± 14.1 | .455 | 264 ± 11.5 $ | 240 ± 16.6 | .075 |
| HDL-cholesterol (mg/dL) | 37.5 ± 2.65 | 40.1 ± 2.40 | .173 | 32.5 ± 3.95 | 37.8 ± 3.68 | .130 | 42.5 ± 2.54 | 42.5 ± 3.07 | 0.993 |
| Blood glucose (mg/dL) | 145 ± 10.4 | 165 ± 11.4* | .039 | 143 ± 17.7 | 177 ± 20.7 | .057 | 146.6 ± 12.6 | 152.6 ± 9.50 | .440 |
| Insulin (µU/mL) | 9.6 ± 0.90 | 11.9 ± 1.31 | .233 | 9.8 ± 0.79 | 11.9 ± 2.11 | .415 | 9.3 ± 1.69 $ | 11.9 ± 1.72 | .430 |
| HbA1c (mmol/L) | 7.4 ± 0.22 | 7.3 ± 0.24 | .799 | 7.3 ± 0.37 | 7.5 ± 0.43 | .177 | 7.4 ± 0.28 | 7.1 ± 0.24 | .100 |
| β-carotene (µmol/L) | 0.21 ± 0.045 | 0.45 ± 0.089* | .044 | 0.20 ± 0.055 | 0.31 ± 0.90 | .382 | 0.22 ± 0.076 | 0.59 ± 0.140 | .081 |
| Retinol (µmol/L) | 2.3 ± 0.11 | 2.6 ± 0.19 | .223 | 2.2 ± 0.21 | 2.7 ± 0.37 | .294 | 2.3 ± 0.08 | 2.4 ± 0.15 | .620 |
Abbreviations: BMI, Body mass index; HbA1c, Glycated hemoglobin.
The values are means ± SE and the number of samples is given in the parentheses. The data were analyzed by paired sample t-test and the comparison between baseline and final values considered significant, if the P-value was ⩽.05 level.
Significant, when the baseline values were compared between male and female subjects, if the P-value was ⩽.05 level.
The sex-wise analysis showed no significant effects on these parameters in either of the sexes by the carrot juice consumption, except fat percent (P = .027) and BMI (P = .047) of females that showed a significant reduction at the end, compared to their baseline levels (Table 3). The data obtained from the FFQ indicated that the average intake of β-carotene (excluding the carrot juice) was around 4394 µg/week and found that the large proportion was from green leafy vegetables in these diabetic subjects (Supplemental Figure S2).
Discussion
Various studies have shown that the feeding of high fructose diet resulted in hepatic triglyceride accumulation by increasing the hepatic stearoyl-CoA desaturase 1 (SCD1)-mediated MUFA synthesis (i.e, palmitoleic (C16:1) and oleic (C18:1) acids), while the suppression of SCD1 and thereby MUFA synthesis reduced the triglyceride accumulation and improved the fatty liver.20-22 Similar observations were made even in the present study, with regard to the hepatic triglycerides contents, which go in line with the hepatic SCD1 expression and its MUFA levels. Importantly, the carrot juice (containing 0.3 mg β-carotene) ingestion neither attenuated the hepatic triglyceride accumulation nor augmented the reversal effect with the diet sans high fructose. These data are in line with the previous study of Poudyal et al., 8 who have reported that the supplementation of β-carotene (400 mg/kg diet) had no inhibitory effect on hepatic fat accumulation in high-carbohydrate, high-fat diet-fed rats, and a study of Mahes et al. 9 that has shown no protective effect of carrot juice against high fructose-induced hepatic steatosis. Unlike the anthocyanins-rich purple carrot juice that has shown attenuation of fat accumulation in the liver, 8 the β-carotene-rich carrot juice did not prevent hepatic steatosis in the present study. However, it could reverse hypertriglyceridemia. The circulatory triglycerides levels are majorly regulated by hepatic lipogenesis and secretion, fatty acid oxidation, and extra-hepatic clearance. 23 However, the expression data of these pathway proteins in the liver, such as GPAT1 (lipogenesis), MTTP (lipoprotein secretion) and PT1 (fatty acid β-oxidation), and the plasma β-hydroxy butyrate levels suggest the role of lipoprotein lipase (LPL)-mediated extra-hepatic tissue clearance of plasma triglyceride in regulating hypertriglyceridemia, as the lipoprotein lipase (LPL) present in the vascular bed of adipose tissue and muscle involves in the uptake of triglyceride from the triglyceride-rich lipoproteins by lipolytic action and thus regulates triglyceride levels in the circulation. 24
Interestingly, the rats that received carrot juice exhibited a reduction of adiposity and leptin content, which corroborated with elevated retinol concentration of the adipose tissue. In line with adiposity reduction, the ingestion of carrot juice or withdrawal of high fructose in the diet normalized hyperinsulinemia and C-peptide levels without impacting the plasma glucose levels, thus reflected in improved insulin sensitivity as indicated by the HOMA-IR index and plasma RBP4 levels; one of the markers of insulin sensitivity. 25 However, the molar ratio of RBP4 to retinol was comparable among all the groups. Previously, several studies have demonstrated vitamin A-mediated suppression of adiposity through multiple pathways, including adipocyte differentiation, apoptosis, and thermogenesis to name a few, thus obesity in various experimental models.26,27 Further, studies have shown that vitamin A and its metabolite; retinoic acid are the potent transcriptional suppressors of the leptin, which strongly associates with the degree of adiposity, which in turn with improved dyslipidemia.26-33 However, the study of Felipe et al., 34 has reported that the direct inhibitory effect of retinoic acid on leptin expression was independent of its anti-obesity effect.
Earlier, a study has reported that chronic vitamin A supplementation has resulted in improved adiposity, hyperinsulinemia, and insulin sensitivity in a genetic obese rat model (WNIN/Ob), 35 Previously, Amengual et al., 36 have reported that compared to the β-carotene 15,15′-oxygenase-1 gene knock-out mice (Bco1−/−), the β-carotene supplementation has markedly reduced adiposity, adipocyte size, and leptinemia in the wild type mice, thus underscored the role of β-carotene metabolizing enzymes; Bco1 and vitamin A in the adipose tissue development and function. All these evidences unambiguously support the involvement of vitamin A and/or its metabolites in the carrot juice-mediated adiposity reduction, improved hypertriglyceridemia, and hyperinsulinemia. However, the precise pathways or mechanisms involved in these processes cannot be speculated. Further, our data suggest that the high fructose-mediated changes are reversible and the carrot juice does not offer any additive or synergistic effects, upon withdrawal of high fructose.
On the other hand, in type 2 diabetic subjects, the carrot juice consumption reduced body weight, BMI, and body fat percent, accompanied by increased plasma β-carotene levels. The carotenoids, including β-carotene, have also been reported to decrease adiposity and improve adipose tissue functions. 37 In addition, the role of carotenoids and their cleavage products on adipose tissue biology and functions have been extensively reviewed recently. 38 Further, the intake of carotenoid-rich foods is shown to elevate the circulatory carotenoids, including β-carotene in humans. 39 The significant negative association between the plasma β-carotene and body weight in these diabetic subjects, as observed in Spearman’s rho correlation analysis (r = −.381 and P = .046) supports the speculation that the observed reduction in the body weight, possibly due to decreased body fat, was resulting out of increased β-carotene intake. However, despite increased fasting blood glucose levels, the other diabetes-associated clinical parameters, such as glycated hemoglobin (HbA1c) and insulin levels were not affected by the consumption of raw carrot juice. Some of these observations are quite contradictory to the present study, thus the role of various other factors, including species difference, genetics, dose used, and treatment duration cannot be ruled out. Though we have not measured the carbohydrate content of the carrot juice, according to the earlier estimate (i.e, 10% carbohydrate of which 6.6 to 7.7 g% soluble carbohydrate in the edible portion of carrots), 40 the carbohydrate intake would be less than 10 g/day. Hence, it appears that the increased blood glucose levels observed in these diabetic subjects may not be due to the carbohydrate content of the carrot juice. However, the phenomenon is quite unexplainable and needs further investigation.
A population-based cross-sectional study has found a significant association between low intake frequency of carrot intake and other carotenoid-rich fruits and vegetables with high HbA1c levels. 41 The data obtained from the FFQ indicated that the average intake of β-carotene (excluding from the carrot juice) was 4394 µg/week, which is far below the RDA of 4800 µg/day to meet the vitamin A requirement, 11 and the contribution of β-carotene was majorly from the green leafy vegetables. However, the plasma vitamin A levels were well within the normal range. Importantly, the β-carotene metabolism differs between humans and rodents and unlike humans, the intestinal conversion of β-carotene to retinol is rapid in rodents, due to the presence of highly active intestinal Bco1. 42 All these substantiate the observed elevation of tissue retinol concentration (liver and adipose) in rats and plasma β-carotene levels in the diabetic subjects upon ingestion of carrot juice. Importantly, a recent meta-analysis study, has found an inverse association between carotenoids and metabolic syndrome, and the strongest is one with β-carotene, however, no such association was observed with vitamin A. 43 Besides, a recent study has also demonstrated the inverse association of obesity, insulin resistance with carotenoid concentrations of serum, and adipose tissue in adults. 44
In the present study, we attribute the beneficial effects of carrot juice to the vitamin A and/or β-carotene, however, the role of other bioactive components of carrots, including phenolics (coumaric acid, caffeic acid, ferulic acid, chlorogenic acid, cinnamic acid, vanillic acid, pyrogallol, etc.), flavonoids (cirsimartin, didymin, kaempferol, quercetin etc.), and lignans (matairesinol, sesaminol, etc.)40,45 cannot be ruled out, as some of these phytochemicals from different plants and fruits have exhibited to offer beneficial health effects against various human diseases, including obesity, diabetes, cardiovascular disease, hypertension, and cancer. 46 Therefore, it is very hard to relate any particular compound(s)/active principle(s) for the beneficial effects rather should be viewed as a whole food.
Overall, here, we demonstrate the beneficial effects of carrot juice on some of the physical and clinical outcome parameters in a pre-clinical model and human diabetic subjects. However, the study owes a few limitations/flaws, such as (i) control group did not receive gavaged placebo (as some of the outcomes may be influenced by differences in the degree of stress due to oral gavage), (ii) the study did not address the possible underlying mechanisms/pathways involved in the regulation of adipose tissue, hypertriglyceridemia and hyperinsulinemia in the animal model, (iii) the human pilot trial was conducted with a less number of subjects for a shorter duration and the trial did not have a control/placebo group, and (iv) the study could not explain the observed increase in blood glucose concentration in type 2 diabetic subjects.
In conclusion, the carrot juice ingestion reversed the high fructose-induced adiposity, hypertriglyceridemia, and hyperinsulinemia, and did not attenuate the hepatic triglyceride accumulation/hepatic steatosis in adult male Wistar rats. Further, it did not exert any synergistic or additive effects on these outcome measures, on the diet sans high fructose. Furthermore, in type 2 diabetic subjects, the consumption of raw carrot juice also elicited a reduction of body weight, BMI, and body fat percent. Despite increased blood glucose levels, the plasma insulin and glycated hemoglobin, (HbA1c) levels remained unaltered in these subjects. Overall, carrot juice consumption indeed has a profound health effect on some of the abnormal metabolic changes and thus may be used as a potent functional food. Nevertheless, these data though preliminary, are of clinical significance, and thereby warrants a large-scale study, which may pave a way for carrot/carrot juice or other β-carotene-rich vegetables or fruits supplementation in the control and management of NAFLD-associated co-morbidities and other metabolic diseases, especially obesity and diabetes.
Supplemental Material
Supplemental material, sj-pdf-1-nmi-10.1177_11786388211014917 for Carrot Juice Consumption Reduces High Fructose-Induced Adiposity in Rats and Body Weight and BMI in Type 2 Diabetic Subjects by Malleswarapu Mahesh, Himanshi Pandey, Mooli Raja Gopal Reddy, Prashanti Prabhakaran Sobhana, Damayanti Korrapati, Putcha Uday Kumar, Ayyalasomayajula Vajreswari and Shanmugam Murugaiha Jeyakumar in Nutrition and Metabolic Insights
Acknowledgments
The authors thank and appreciate the participants for their support and co-operation, and thank Mr. E. Srinivas, the cook of the institute’s metabolic kitchen, for his help during the conduct of human study. Mrs. K. Sharada, is acknowledged for her support in preparing histology sections.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully acknowledge the Department of Biotechnology (DBT) for the financial support extended to the experimental study (Grant Ref no. BT/PR6145/FNS/20/566/2012) and the Director, ICMR-NIN, for the intramural grant towards the human study. Mr M. Raja Gopal Reddy duly acknowledges DST for the INSPIRE fellowship.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MM and RGR: Conduct of animal experiment and data acquisition. HP: Conduct of human study and data acquisition. PPS: Data acquisition of human study. PU: Histological examination. DK: Human experiment study design, study supervision, and data analysis. AV: Study design (Experimental and Human) and critical inputs and review. SMJ: Conception, study design (Experimental and Human), data analysis, manuscript drafting, reviewing, and over all supervision.
ORCID iD: Shanmugam Murugaiha Jeyakumar  https://orcid.org/0000-0002-4500-8431
https://orcid.org/0000-0002-4500-8431
Supplemental material: Supplemental material for this article is available online.
References
- 1. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672-2682. [DOI] [PubMed] [Google Scholar]
- 2. Bessone F, Razori MV, Roma MG. Molecular pathways of non-alcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76:99-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jegatheesan P, De Bandt JP. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients. 2017;9:E230.28273805 [Google Scholar]
- 4. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Divella R, Mazzocca A, Daniele A, Sabbà C, Paradiso A. Obesity, nonalcoholic fatty liver disease and adipocytokines network in promotion of cancer. Int J Biol Sci. 2019;15:610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchisello S, Di Pino A, Scicali R, Urbano F, Piro S, Purrello F, Rabuazzo AM. Pathophysio-logical, molecular and therapeutic issues of nonalcoholic fatty liver disease: an Overview. Int J Mol Sci. 2019;20:E1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PLoS One. 2015;10:e0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104:1322-1332. [DOI] [PubMed] [Google Scholar]
- 9. Mahesh M, Bharathi M, Reddy MR, Kumar MS, Putcha UK, Vajreswari A, Jeyakumar SM. Carrot juice administration decreases liver stearoyl-CoA desaturase 1 and improves docosahexaenoic acid levels, but not steatosis in high fructose diet-fed weanling wistar rats. Prev Nutr Food Sci. 2016;21:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahesh M, Bharathi M, Raja Gopal Reddy M, Pappu P, Putcha UK, Vajreswari A, Jeyakumar SM. Carrot juice ingestion attenuates high fructose-induced circulatory pro-inflammatory mediators in weanling Wistar rats. J Sci Food Agric. 2017;97:1582-1591. [DOI] [PubMed] [Google Scholar]
- 11. Indian Council of Medical Research. Nutrient requirements and recommended dietary allowances for Indians. A report of the expert group of the Indian Council of Medical Research, India; 2010. [Google Scholar]
- 12. Green AS, Fascetti AJ. Meeting the vitamin A requirement: the efficacy and importance of β-carotene in animal species. Sci World J. 2016;2016:7393620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeyakumar SM, Lopamudra P, Padmini S, Balakrishna N, Giridharan NV, Vajreswari A. Fatty acid desaturation index correlates with body mass and adiposity indices of obesity in Wistar NIN obese mutant rat strains WNIN/Ob and WNIN/GR-Ob. Nutr Metab (Lond). 2009;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeyakumar SM, Vajreswari A, Giridharan NV. Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity (Silver Spring). 2006;14:52-59. [DOI] [PubMed] [Google Scholar]
- 15. Korrapati D, Jeyakumar SM, Putcha UK, et al. Coconut oil consumption improves fat-free mass, plasma HDL-cholesterol and insulin sensitivity in healthy men with normal BMI compared to peanut oil. Clin Nutr. 2019;38:2889-2899. [DOI] [PubMed] [Google Scholar]
- 16. Reddy MRG, Asha GV, Manchiryala SK, Putcha UK, Vajreswari A, Jeyakumar SM. High-fat diet elevates liver docosahexaenoic acid possibly through over-expression of very long-chain fatty acid elongase 2 in C57BL/6J Mice. Int J Vitam Nutr Res. 2019;89:62-72. [DOI] [PubMed] [Google Scholar]
- 17. Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152-178. [Google Scholar]
- 18. Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian Food Composition Tables 2017. National Institute of Nutrition; 2017. [Google Scholar]
- 19. Bui MH. Simple determination of retinol, alpha-tocopherol and carotenoids (lutein, all-trans-lycopene, alpha- and beta-carotenes) in human plasma by isocratic liquid chromatography. J Chromatogr B Biomed Appl. 1994;654:129-133. [DOI] [PubMed] [Google Scholar]
- 20. Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164-25171. [DOI] [PubMed] [Google Scholar]
- 21. Mock K, Lateef S, Benedito VA, Tou JC. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem. 2017;39:32-39. [DOI] [PubMed] [Google Scholar]
- 22. Raja Gopal Reddy M, Pavan Kumar C, Mahesh M, et al. Vitamin A deficiency suppresses high fructose-induced triglyceride synthesis and elevates resolvin D1 levels. Biochim Biophys Acta. 2016;1861:156-165. [DOI] [PubMed] [Google Scholar]
- 23. Chait A, Subramanian S. Hypertriglyceridemia: pathophysiology, role of genetics, consequences, and treatment. [Updated 2019 April 23]. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK326743/ [Google Scholar]
- 24. Basu D, Bornfeldt KE. Hypertriglyceridemia and Atherosclerosis: using human research to guide mechanistic studies in animal models. Front Endocrinol (Lausanne). 2020;11:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fedders R, Muenzner M, Schupp M. Retinol binding protein 4 and its membrane receptors: a metabolic perspective. Horm Mol Biol Clin Investig. 2015;22:27-37. [DOI] [PubMed] [Google Scholar]
- 26. Brun PJ, Yang KJ, Lee SA, Yuen JJ, Blaner WS. Retinoids: potent regulators of metabolism. Biofactors. 2013;39:151-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jeyakumar SM, Vajreswari A. Vitamin A as a key regulator of obesity & its associated disorders: evidences from an obese rat model. Indian J Med Res. 2015;141:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonet ML, Oliver J, Picó C, Felipe F, Ribot J, Cinti S, Palou A. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J Endocrinol. 2000;166:511-517. [DOI] [PubMed] [Google Scholar]
- 29. Menendez C, Lage M, Peino R, Baldelli R, Concheiro P, Diéguez C, Casanueva FF. Retinoic acid and vitamin D(3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol. 2001;170:425-431. [DOI] [PubMed] [Google Scholar]
- 30. Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155-1161. [DOI] [PubMed] [Google Scholar]
- 31. Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754-764. [DOI] [PubMed] [Google Scholar]
- 32. Müller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussain Z, Khan JA. Food intake regulation by leptin: mechanisms mediating gluconeogenesis and energy expenditure. Asian Pac J Trop Med. 2017;10:940-944. [DOI] [PubMed] [Google Scholar]
- 34. Felipe F, Mercader J, Ribot J, Palou A, Bonet ML. Effects of retinoic acid administration and dietary vitamin A supplementation on leptin expression in mice: lack of correlation with changes of adipose tissue mass and food intake. Biochim Biophys Acta. 2005;1740:258-265. [DOI] [PubMed] [Google Scholar]
- 35. Jeyakumar SM, Vijaya Kumar P, Giridharan NV, Vajreswari A. Vitamin A improves insulin sensitivity by increasing insulin receptor phosphorylation through protein tyrosine phosphatase 1B regulation at early age in obese rats of WNIN/Ob strain. Diabetes Obes Metab. 2011;13:955-958. [DOI] [PubMed] [Google Scholar]
- 36. Amengual J, Gouranton E, van Helden YG, et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS One. 2011;6:e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys. 2015;572:112-125. [DOI] [PubMed] [Google Scholar]
- 38. Bonet ML, Ribot J, Galmés S, Serra F, Palou A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: pre-clinical and human studies. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158676. [DOI] [PubMed] [Google Scholar]
- 39. Esfahani A, Wong JM, Truan J, Villa CR, Mirrahimi A, Srichaikul K, Kendall CW. Health effects of mixed fruit and vegetable concentrates: a systematic review of the clinical interventions. J Am Coll Nutr. 2011;30:285-294. [DOI] [PubMed] [Google Scholar]
- 40. Sharma KD, Karki S, Thakur NS, Attri S. Chemical composition, functional properties and processing of carrot-a review. J Food Sci Technol. 2012;49:22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suzuki K, Ito Y, Nakamura S, Ochiai J, Aoki K. Relationship between serum carotenoids and hyperglycemia: a population-based cross-sectional study. J Epidemiol. 2002;12:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coronel J, Pinos I, Amengual J. β-carotene in obesity research: technical considerations and current status of the field. Nutrients. 2019;11:E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beydoun MA, Chen X, Jha K, Beydoun HA, Zonderman AB, Canas JA. Carotenoids, vitamin A, and their association with the metabolic syndrome: a systematic review and meta-analysis. Nutr Rev. 2019;77:32-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harari A, Coster ACF, Jenkins A, Xu A, Greenfield JR, Harats D, et al. Obesity and insulin resistance are inversely associated with serum and adipose tissue carotenoid concentrations in adults. J Nutr. 2020;150(1):38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Szczepańska J, Barba FJ, Skąpska S, Marszałek K. High pressure processing of carrot juice: effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020;307:125549. [DOI] [PubMed] [Google Scholar]
- 46. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33:2221-2243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-nmi-10.1177_11786388211014917 for Carrot Juice Consumption Reduces High Fructose-Induced Adiposity in Rats and Body Weight and BMI in Type 2 Diabetic Subjects by Malleswarapu Mahesh, Himanshi Pandey, Mooli Raja Gopal Reddy, Prashanti Prabhakaran Sobhana, Damayanti Korrapati, Putcha Uday Kumar, Ayyalasomayajula Vajreswari and Shanmugam Murugaiha Jeyakumar in Nutrition and Metabolic Insights