Abstract
Rationale:
Pediatric COVID-19 studies have been mostly restricted to case reports and small case series, which have prevented the identification of specific pediatric lung disease patterns in COVID-19. The overarching goal of this systematic review and meta-analysis is to provide the first comprehensive summary of the findings of published studies thus far describing COVID-19 lung imaging data in the pediatric population.
Methods:
A systematic literature search of PubMed was performed to identify studies assessing lung-imaging features of COVID-19 pediatric patients (0–18 years). A single-arm meta-analysis was conducted to obtain the pooled prevalence and 95% confidence interval (95% CI).
Results:
A total of 29 articles (n = 1026 children) based on chest computerized tomography (CT) images were included. The main results of this comprehensive analysis are as follows: (1) Over a third of pediatric patients with COVID-19 (35.7%, 95% CI: 27.5%–44%) had normal chest CT scans and only 27.7% (95% CI: 19.9%–35.6%) had bilateral lesions. (2) The most typical pediatric chest CT findings of COVID-19 were ground-glass opacities (GGO) (37.2%, 95% CI: 29.3%–45%) and the presence of consolidations or pneumonic infiltrates (22.3%, 95% CI: 17.8%–26.9%). (3) The lung imaging findings in children with COVID-19 were overall less frequent and less severe than in adult patients. (4) Typical lung imaging features of viral respiratory infections in the pediatric population such as increased perihilar markings and hyperinflation were not reported in children with COVID-19.
Conclusion:
Chest CT manifestations in children with COVID-19 could potentially be used for early identification and prompt intervention in the pediatric population.
Keywords: lung CT scan, meta-analysis, pediatric COVID-19, SARS-CoV-2
1 |. INTRODUCTION
In early December of 2019, cases of a new lower respiratory “pneumonia-like” illness started to occur in Wuhan, Hubei Province, China. Early tests found that this illness was caused by a novel coronavirus (SARS-CoV-2). The Coronavirus Disease 2019 (COVID-19) rapidly spread to almost every single country in the world and in the matter of a few months was declared a global pandemic by the WHO.1–3 COVID-19 has become the most lethal pandemic of modern times, mostly due to the development of severe acute respiratory syndrome (SARS) in susceptible individuals.1–3 While published studies and case reports indicate that the individuals most affected during the COVID-19 pandemic are adults above 60 years of age,1–8 there have been a significant number of COVID-19 cases of infants, children, and young adults.4–6,8 Although SARS-CoV-2 respiratory infections are overall much less severe in children and younger individuals,4–6,8 SARS-CoV-2 can sometimes lead to serious and life-threatening illnesses in previously healthy children and adolescents.7
The fact that SARS-CoV-2 respiratory infections are more severe in adults than in children is highly atypical. Viral respiratory infections are usually more severe in children and globally are the top cause of sick visits, hospitalization, and mortality in the pediatric population.9,10 Thus the lung disease patterns in COVID-19 seem to be highly variable with age and likely distinct in infants, children, and adolescents. The lung manifestations of COVID-19 in the adult populations have been reported in detail in thousands of recent publications. In contrast, the lung disease patterns of COVID-19 in the pediatric population remain to be determined. Addressing this gap is of critical importance to (1) allow the early identification of potential SARS-CoV-2 infections in children; (2) distinguish SARS-CoV-2 infections from common viral respiratory illnesses in the pediatric population (e.g., viral bronchiolitis); (3) provide novel insights into the pathogenesis of SARS-CoV-2 in different age groups; and (4) define initial lung imaging framework for a novel scoring system using computer-assisted methods.
Lung imaging has become critically important for the early identification and treatment of individuals affected by COVID-19.11,12 To date, the most common lung imaging modalities utilized are chest radiographs (CXR) and chest computerized tomography (CT).11,12 Many studies and recent meta-analyses have already reported that ground-glass opacity (GGO) is the most common chest CT-based signature of SARS-CoV-2 in adults.13–16 In contrast, pediatric COVID-19 studies have been mostly restricted to case reports and small case series, which have prevented the identification of specific pediatric lung disease patterns in COVID-19. Accordingly, the overarching goal of this study is to provide the first comprehensive summary of the findings of published studies thus far describing lung imaging data in pediatric patients with COVID-19. For this purpose, we conducted a systematic review of the literature and a single-arm meta-analysis to evaluate the agreement and heterogeneity across different published studies in regard to (1) the proportion of children with normal lung CT results, and (2) the pooled prevalence of each of the lung imaging abnormalities reported in PCR-confirmed pediatric COVID-19 cases.
2 |. METHODS
2.1 |. Literature search and keywords
A systematic search of the literature was performed using PubMed from December 1, 2019 to July 11, 2020. The goal of the search was to identify studies in which lung imaging features of SARS-CoV-2 were described in children. We did not specify age criteria in our initial screening since some studies could include adults and children without specific title descriptions. The selection of studies was developed with the systematic review management platform COVIDENCE. The identified studies were first stored and checked for duplicates. This review was carried out following the “Preferred Reporting Items for Systematic reviews and Meta-Analyses, PRISMA.”17 The search was conducted for publications in the English language. The retrieval was a combination of subject words and free words, and the coded keywords were as follows: (“Coronavirus” OR “COVID-19” OR “2019 Novel Coronavirus” OR “2019 nCoV” OR “2019 novel coronavirus” OR “SARS-CoV-2” OR “COVID” OR “Wuhan Coronavirus”) AND (“imaging” OR “radiology” OR “radiography” OR “chest radiograph” OR “X-ray” OR “computed tomography” OR “CT” OR “HRCT” OR “ultrasound”).
2.2 |. Inclusion and exclusion criteria
Two independent investigators (GN and MGL) screened titles and abstracts of each retrieved article based on pre-determined inclusion and exclusion criteria. Any disagreements were resolved by discussion, and if necessary, a third reviewer was involved. Studies were included if (1) publications were peer-reviewed articles with full text; (2) patients had confirmed COVID-19 diagnosis by reverse transcription polymerase chain reaction (RT-PCR); (3) chest imaging features of COVID-19 were included; and (4) the age of patients was 0–18 years of age. For studies reporting adult and pediatric cases, we only included those in which pediatric cases were reported separately. Studies were excluded if they (1) were literature reviews, single case reports, editorial/comments, or clinical guidelines; (2) lacked full text; or (3) lacked clinical data or PCR-confirmation of SARS-CoV-2 infection. When studies reported findings from the same population, we selected only the most relevant study based on date, sample size, and reported analysis of data. The quality assessment was evaluated with the National Institutes of Health (NIH) Quality Assessment Tool for Case Series Studies,18 and the general quality rating was classified as poor, fair, or good with this tool.
2.3 |. Data extraction
From the included studies, we extracted descriptive data (author, year, imaging technique, and subject characteristics) and the key lung imaging features based on the Fleischner Society recommendations including (1) patterns of the lesion such as ground-glass opacifications (GGO), consolidation, pneumonic infiltrates, broncho-vascular bundle thickening, interstitial abnormalities including interlobular septal thickening, crazy paving pattern, bronchiectasis, hyperinflation, adjacent pleura thickening, pleural effusion, pericardial effusion, and lymphadenopathy; (2) lesion distribution (bilateral lung); and (3) lobe distribution (focal or multiple lobes involved).
2.4 |. Statistical analysis
We pooled data from the articles selected using a single-arm analysis design. Statistical heterogeneity between studies was evaluated with Cochran’s Q test and p < .01 was considered significant. The I2 value was used to evaluate the percentage of inter-study variation in the total variation and a value greater than 50% was considered significant for heterogeneity. The meta-analysis was conducted using the OpenMeta-Analyst software.19
3 |. RESULTS
3.1 |. Characteristics of studies and subjects included
The study selection process is presented in Figure 1. The electronic literature search yielded 3278 records in total. After removing 275 duplicates, we moved 2983 studies to the screening stage. A total of 2899 studies were excluded based on the title or abstract. Out of the 84 records selected for full-text analysis, we excluded 55 studies because they did not meet the inclusion criteria. There were 12 studies with the wrong patient population (e.g., adults or non-PCR-confirmed COVID-19 diagnosis), 11 did not provide lung imaging data, 10 articles were not available in English, 8 publications were case reports, and 7 records were either review studies or meta-analyses. A total of 29 studies were included for the final data extraction. The overall characteristics and primary lung imaging features of the 29 studies selected are presented in Table 1.
FIGURE 1.
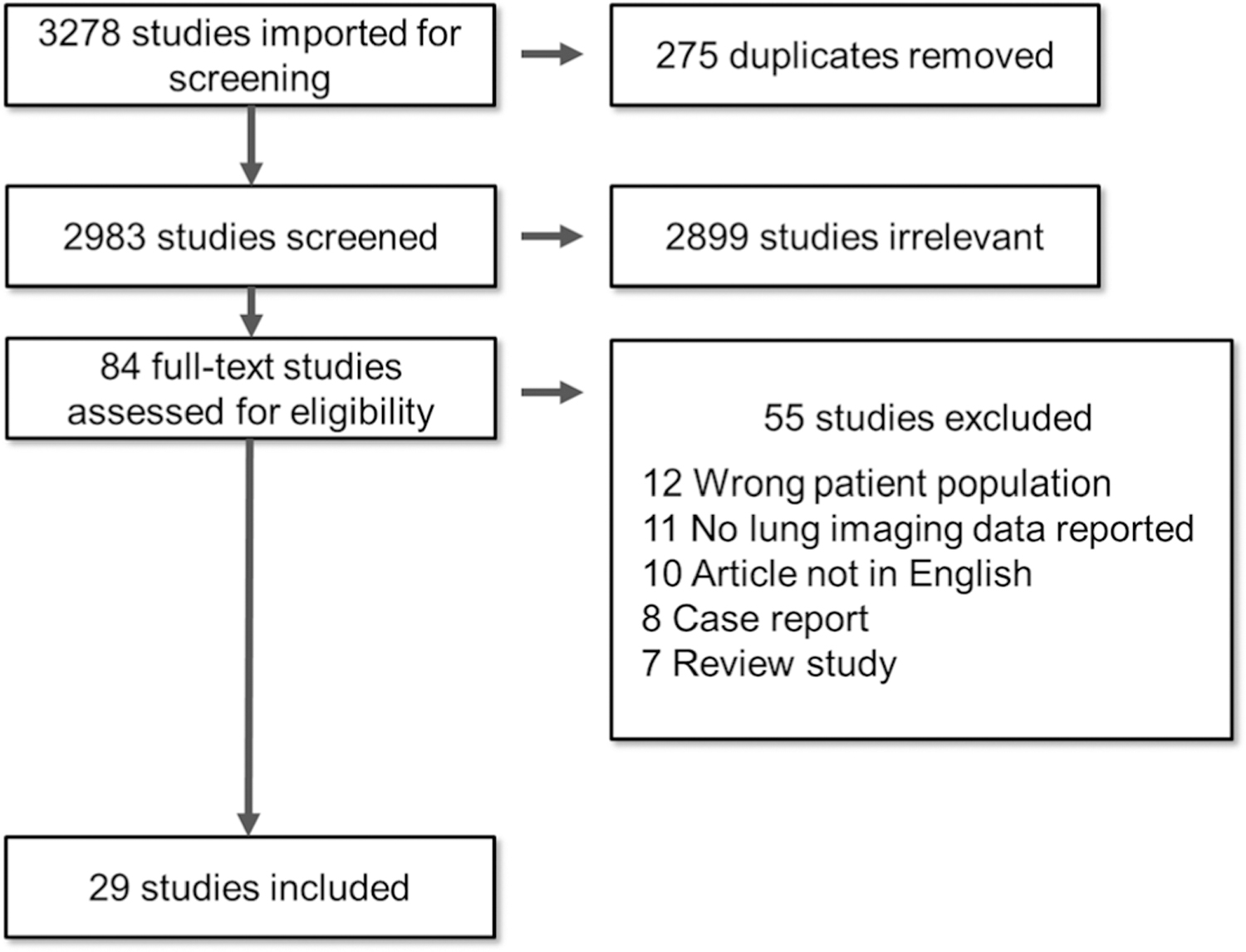
Diagram of document retrieval
TABLE 1.
Characteristics of the 29 pediatric COVID-19 lung imaging studies included in the meta-analysis
| Study | Month/year | Journal | Sample size | Mean or median age | Gender (male %) | Imaging manifestations |
|---|---|---|---|---|---|---|
| Sun et al.20 | Mar-20 | World J Pediatr | 8 | 7 | 75 | Bilateral lung, GGO, consolidation, infiltrates, pleural effusion |
| Chen et al.21 | Apr-20 | Genes Dis | 12 | 14.5 | 50 | GGO, consolidation, infiltrates |
| Liu et al.8 | Apr-20 | N Engl J Med | 6 | 3.5 | 33 | Bilateral lung, GGO, consolidation, infiltrates |
| Lu et al.22 | Apr-20 | N Engl J Med | 171 | 6.7 | 61 | Bilateral lung, GGO, interstitial pattern |
| Zheng et al.23 | Apr-20 | Curr Med Sci | 25 | 3 | 56 | GGO, consolidation, infiltrates |
| Lu et al.24 | Apr-20 | Clin Radiol | 9 | 7.8 | 56 | GGO, consolidation, infiltrates, bronchovascular bundle thickening, halo sign |
| Li et al.25 | May-20 | Indian Pediatr | 22 | 8 | 55 | Bilateral lung, GGO, consolidation, infiltrates, crazy paving, interstitial pattern |
| Li et al.26 | May-20 | Pediatr Radiol | 5 | 3.4 | 80 | Unilateral, focal GGO |
| Li et al.27 | May-20 | BMC Pediatr | 8 | 2.5 | 38 | Bilateral lung, GGO, consolidation, infiltrates, crazy paving, air bronchogram, bronchovascular bundle thickening, pleural thickening, halo sign |
| Liu et al.28 | May-20 | J Infect | 4 | 3.75 | 50 | GGO, consolidation, infiltrates, pleural effusion |
| Ma et al.29 | May-20 | BMC Med | 50 | 3 | 56 | Bilateral lung, GGO, consolidation, infiltrates, interstitial pattern |
| Steinberger et al.30 | May-20 | AJR Am J Roentgenol | 30 | 10 | 50 | Bilateral lung, GGO, consolidation, infiltrates, crazy paving, halo sign |
| Wang et al.31 | May-20 | Pediatr Infect Dis J | 43 | 6.5 | 62 | GGO, consolidation, infiltrates |
| Xia et al.32 | May-20 | Pediatr Pulmonol | 20 | 2 | 65 | Bilateral lung, GGO, consolidation, infiltrates, halo sign |
| Du et al.33 | Jun-20 | Infection | 14 | 6.3 | 43 | Bilateral lung, GGO, consolidation, infiltrates |
| Liu et al.34 | Jun-20 | J Comput Assist Tomogr | 5 | 5.8 | 80 | Bilateral lung, GGO, consolidation, infiltrates |
| Qiu et al.35 | Jun-20 | Lancet Infect Dis | 36 | 8.3 | 64 | GGO, consolidation, infiltrates |
| Shen et al.36 | Jun-20 | Pediatr Pulmonol | 9 | 7.55 | 33 | Unilateral, focal GGO |
| Song et al.37 | Jun-20 | J Clin Virol | 16 | 8.5 | 63 | Bilateral lung, GGO, consolidation, infiltrates, halo sign, lymphadenopathy |
| Tan et al.38 | Jun-20 | J Clin Virol | 10 | 7 | 30 | Bilateral lung, GGO, consolidation, infiltrates, air bronchogram, bronchovascular bundle thickening |
| Wu et al.39 | Jun-20 | JAMA Netw Open | 148 | 7 | 41 | Bilateral lung, GGO |
| Zhu et al.40 | Jun-20 | Pediatr Pulmonol | 10 | 9.1 | 50 | GGO, consolidation, infiltrates |
| Du et al.41 | Jun-20 | Allergy | 182 | 6 | 66 | Bilateral lung, GGO, consolidation, infiltrates, bronchovascular bundle thickening |
| Korkmaz et al.42 | Jun-20 | J Korean Med Sci. | 30 | 9.5 | 59 | GGO, consolidation, infiltrates |
| Li et al.43 | Jun-20 | Int J Infect Dis. | 57 | 1.5 | 61 | GGO, consolidation, infiltrates |
| Zhang et al.44 | Jun-20 | Plos Med | 34 | 2.9 | 41 | Bilateral lung, GGO, consolidation, infiltrates |
| Zhang et al.45 | Jun-20 | Front. Public Health | 33 | 9.6 | 50 | GGO, consolidation, infiltrates |
| Bai et al.46 | Jul-20 | Pediatr Infect Dis J | 25 | 11 | 56 | GGO, consolidation, infiltrates, bronchovascular bundle thickening |
| Lan et al.47 | Jul-20 | Korean J Radiol | 4 | 9 | 50 | Bilateral lung, GGO, consolidation, infiltrates |
Abbreviations: COVID-19, coronavirus disease 2019; GGO, ground-glass opacity.
Our data extraction only included chest CT scan features since chest X-ray descriptions were not consistently reported (only four studies mentioned chest radiographs). There were no studies with ultrasound or other radiologic data available. The 29 studies included in the meta-analysis had a pooled sample size of 1026 children. The mean/median age reported was 6.57 years (age range 1.5–14.5 years) and the pooled proportion of males was 54.2%. The general quality rating of the studies included was classified as fair (all 29 studies) with variable performance using the NIH Quality Assessment Tool for Case Series Studies (Table 2). The main quality issues identified were (1) small sample sizes in which subjects may not be comparable; (2) variability in the outcome measures reported (heterogeneity in lung imaging feature description); and (3) uncertainty about the length of follow-up.
TABLE 2.
Quality assessment of the included studies according to the National Institutes of Health Quality Assessment Tool for case series studies
| Study | Criteriaa |
Quality rating | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Sun et al.20 | Yes | Yes | Yes | CD | N/A | CD | Yes | Yes | Yes | Fair |
| Chen et al.21 | Yes | Yes | Yes | CD | N/A | CD | CD | Yes | Yes | Fair |
| Liu et al.8 | Yes | Yes | Yes | CD | N/A | Yes | Yes | NR | Yes | Fair |
| Lu et al.22 | Yes | Yes | Yes | Yes | N/A | CD | CD | Yes | Yes | Fair |
| Zheng et al.23 | Yes | Yes | Yes | CD | N/A | NR | NR | Yes | Yes | Fair |
| Lu et al.24 | Yes | Yes | Yes | CD | N/A | Yes | Yes | Yes | Yes | Fair |
| Li et al.25 | Yes | Yes | Yes | CD | N/A | Yes | CD | Yes | Yes | Fair |
| Li et al.26 | Yes | Yes | Yes | CD | N/A | Yes | CD | Yes | Yes | Fair |
| Li et al.27 | Yes | Yes | Yes | CD | N/A | Yes | CD | Yes | Yes | Fair |
| Liu et al.28 | Yes | Yes | Yes | CD | N/A | Yes | NR | Yes | Yes | Fair |
| Ma et al.29 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Fair |
| Steinberger et al.30 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Fair |
| Wang et al.31 | Yes | Yes | Yes | Yes | N/A | CD | CD | Yes | Yes | Fair |
| Xia et al.32 | Yes | Yes | Yes | CD | N/A | Yes | CD | Yes | Yes | Fair |
| Du et al.33 | Yes | Yes | Yes | CD | N/A | CD | CD | Yes | Yes | Fair |
| Liu et al.34 | Yes | Yes | Yes | CD | N/A | Yes | NR | Yes | Yes | Fair |
| Qiu et al.35 | Yes | Yes | Yes | Yes | N/A | CD | NR | Yes | Yes | Fair |
| Shen et al.36 | Yes | Yes | Yes | CD | N/A | Yes | CD | Yes | Yes | Fair |
| Song et al.37 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Fair |
| Tan et al.38 | Yes | Yes | Yes | CD | N/A | Yes | CD | Yes | Yes | Fair |
| Wu et al.39 | Yes | Yes | Yes | Yes | N/A | CD | NR | Yes | Yes | Fair |
| Zhu et al.40 | Yes | Yes | Yes | Yes | N/A | CD | NR | Yes | Yes | Fair |
| Du et al.41 | Yes | Yes | Yes | Yes | N/A | CD | Yes | Yes | Yes | Fair |
| Korkmaz et al.42 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Fair |
| Li et al.43 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Fair |
| Zhang et al.44 | Yes | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Fair |
| Zhang et al.45 | Yes | Yes | Yes | Yes | N/A | NR | CD | Yes | Yes | Fair |
| Bai et al.46 | Yes | Yes | Yes | Yes | N/A | CD | NR | Yes | Yes | Fair |
| Lan et al.47 | Yes | Yes | Yes | CD | N/A | Yes | Yes | Yes | Yes | Fair |
Abbreviations: CD, cannot determine; N/A, not applicable; NR, not reported.
The questions of the NIH quality assessment tool for case series studies are: (1) Was the study question or objective clearly stated? (2) Was the study population clearly and fully described, including a case definition? (3) Were the cases consecutive? (4) Were the subjects comparable? (5) Was the intervention clearly described? (6) Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? (7) Was the length of follow-up adequate? (8) Were the statistical methods well-described? (9) Were the results well described?
3.2 |. Lung imaging features in children with COVID-19
In this meta-analysis, we found that 35.7% (95% CI: 27.5%–44%) of children with COVID-19 had no lung CT imaging abnormalities. Although lobe distribution was not consistently reported, we found that 27.7% (95% CI: 19.9%–35.6%) of children with COVID-19 had bilateral lung compromise. Figure 2 presents the forest plots of the pediatric COVID-19 studies reporting normal lung imaging and bilateral compromise. The most common lung CT imaging finding for COVID-19 in pediatric patients was GGO. The prevalence of GGO was 37.2% (95% CI: 29.3%–45%). The second most common lung imaging finding was the presence of consolidations or pneumonic infiltrates, which occurred in 22.3% of cases (95% CI: 17.8%–26.9%). Figure 3 presents the forest plots of the studies reporting GGO abnormalities and consolidations or pneumonic infiltrates.
FIGURE 2.
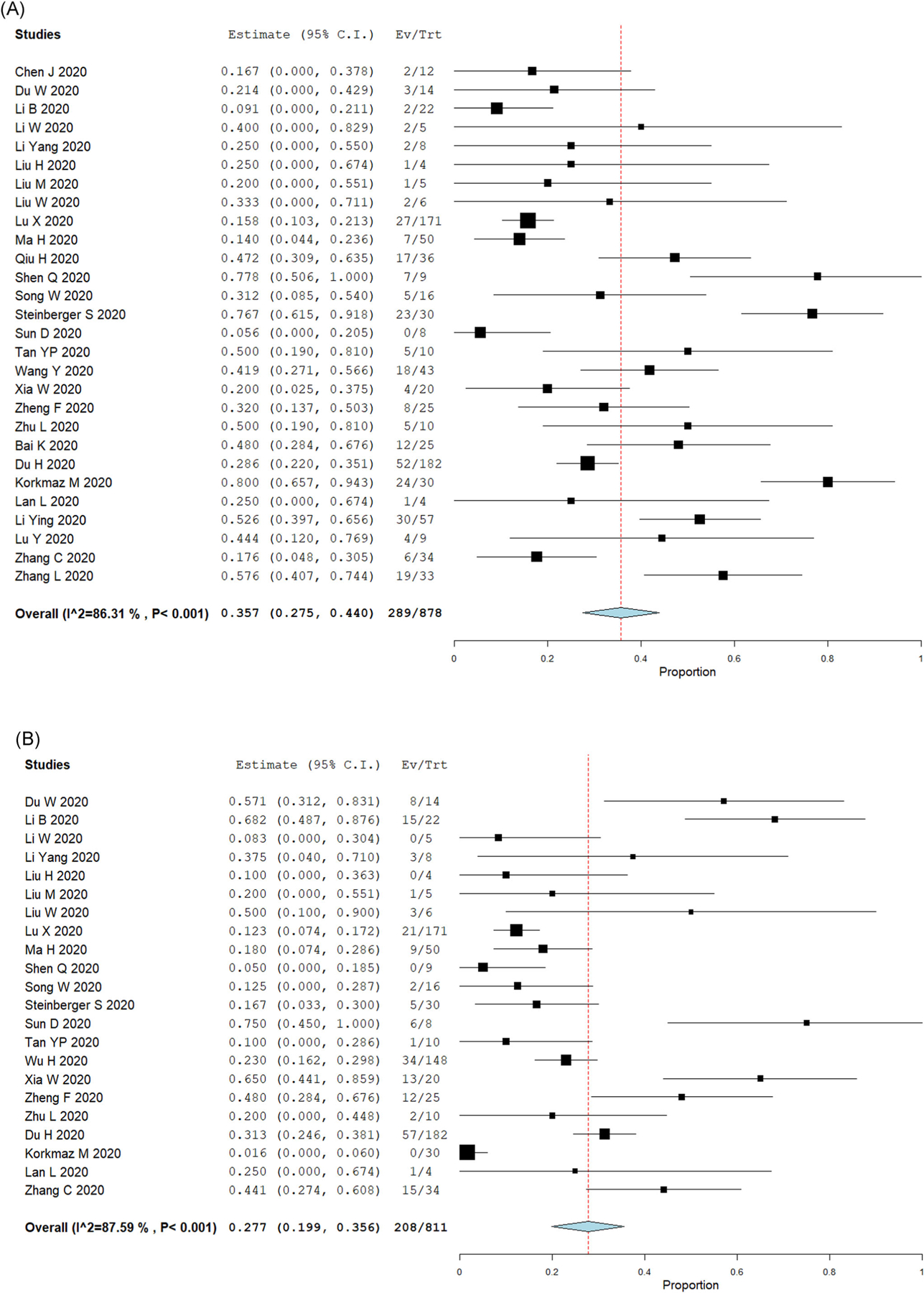
Lung imaging results in pediatric COVID-19 cases. Forest plots for single-arm meta-analysis of the pediatric COVID-19 studies reporting (A) normal lung imaging; or (B) bilateral compromise. Data presented as the 95% confidence interval (CI) of the proportion of subjects with normal findings or bilateral compromise in each study. COVID-19, coronavirus disease 2019 [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
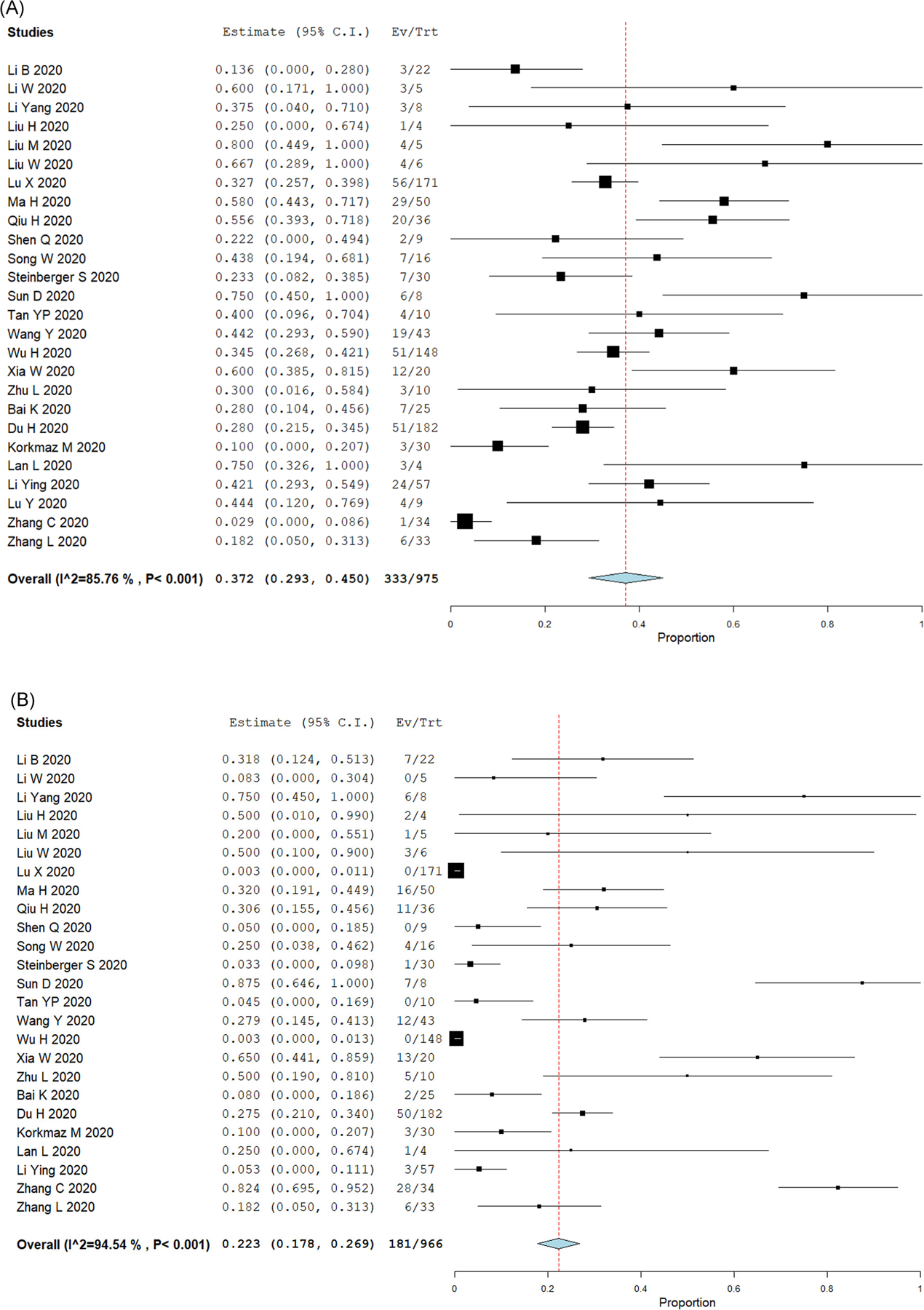
Top lung imaging abnormalities in pediatric COVID-19 cases. Forest plots for single-arm meta-analysis of the pediatric COVID-19 studies reporting (A) ground-glass opacifications (GGO); or (B) consolidations or pneumonic infiltrates. Data presented as the 95% confidence interval (CI) of the proportion of subjects with these lung abnormalities in each study. COVID-19, coronavirus disease 2019 [Color figure can be viewed at wileyonlinelibrary.com]
All the other lung imaging findings reported were very uncommon and included halo sign 0.9% (95% CI: 0.1%–1.8%), interstitial abnormalities and interlobular septal thickening, 0.7% (95% CI: 0.2%–1.2%), bronchovascular bundle thickening 0.6% (95% CI: 0.1%–1.1%), crazy paving pattern 0.5% (95% CI: 0.1%–0.9%), lymphadenopathy 0.5% (95% CI: 0.1%–0.9%), and pleural effusion or adjacent pleura thickening 0.5% (95% CI: 0.1%–0.9%). Atypical CT imaging findings previously reported in adults with COVID-19, such as bronchiectasis, air bronchogram, and pericardial effusion, were not identified in the pediatric COVID-19 cases included (Table 1). The typical findings of pediatric viral respiratory infections (e.g., hyperinflation48–50) were not reported in children with COVID-19. An example of the typical radiological appearance of the most common abnormalities identified in pediatric COVID-19 cases is shown in Figure 4.
FIGURE 4.
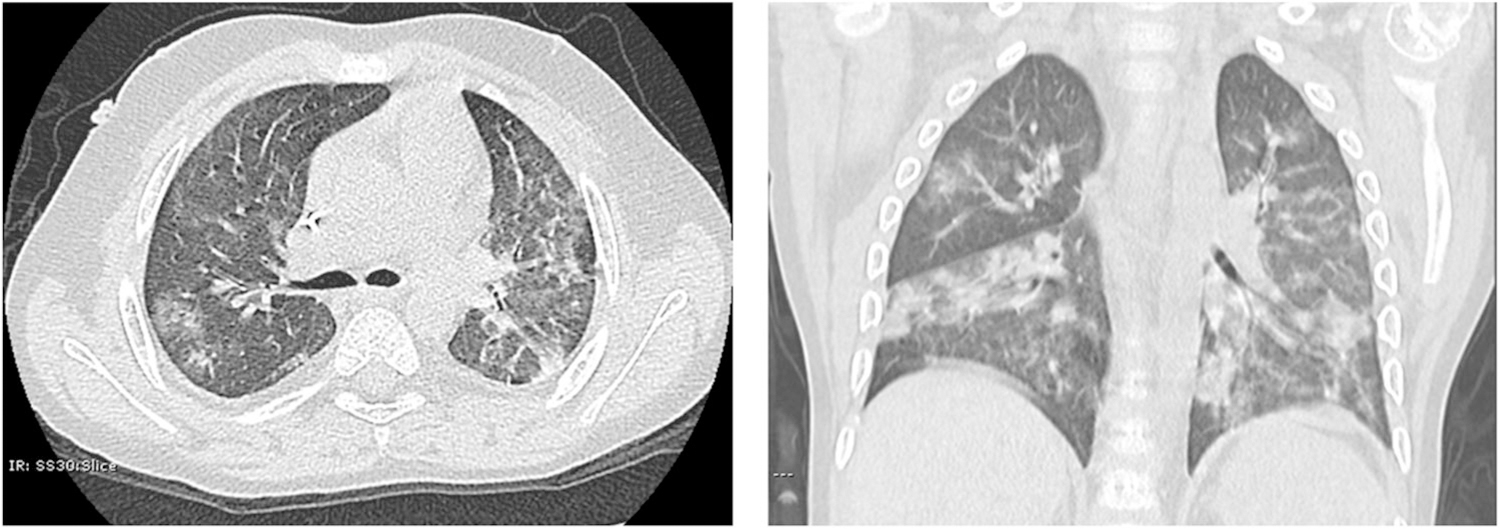
Typical radiological appearance of the most common abnormalities identified in pediatric COVID-19 cases. Chest CT of a 5-year-old male with PCR-confirmed SARS-CoV-2 infection. Images show multifocal scattered GGO and patchy consolidations with a peripheral distribution. There is no hyperinflation, increased perihilar markings, pleural thickening, pleural effusion, or bronchiectasis present. COVID-19, coronavirus disease 2019; CT, computerized tomography; GGO, ground-glass opacity; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
3.3 |. Comparison of lung imaging features of pediatric and adult patients with COVID-19
Lung imaging features of pediatric with COVID-19 were compared with findings presented in two recently published systematic reviews and meta-analyses of the COVID-19 lung imaging of adult patients.13,14 This comparison showed that pediatric patients were about three times more likely to have a normal CT scan compared to adults with COVID-19 (35.7% of children vs. 8.4%–10.24% of adults, Table 3). Bilateral compromise during COVID-19 was much more common in adults than in children (73.8%–78.8% of adults vs. 27.7% of children, Table 3). Although GGO was the most frequent lung disease signature in both children and adults, it was more commonly reported in adults than in children with COVID-19 (68.1%–83.3% of adults vs. 37.2% of children, Table 3). Several common lung imaging findings reported in adults were extremely rare or not found in the pediatric studies (e.g., crazy-paving pattern, air bronchogram, and pleural thickening). Overall, all types of lung abnormalities were less frequent in children with COVID-19 relative to adult patients (Table 3).
TABLE 3.
Comparison of lung imaging features of pediatric and adult patients with COVID-19
| Children with COVID-19 (n = 1026) |
Adults with COVID-1914 (n = 4121) |
Adults with COVID-1913 (n = 2738) |
|
|---|---|---|---|
| Number of studies | 29 | 34 | 13 |
| Normal lung imaging (%) | 35.7 | 8.4 | 10.24 |
| Bilateral compromise (%) | 27.7 | 73.8 | 78.2 |
| GGO (%) | 37.2 | 68.1 | 83.3 |
| Consolidation, infiltrates (%) | 22.3 | 32 | 44 |
| Crazy-paving pattern (%) | 0.5 | 35.6 | 14.8 |
| Pleura thickening (%) | 0.5 | 27.1 | 52.5 |
| Lymphadenopathy (%) | 0.5 | 5.4 | 3.38 |
| Air bronchogram (%) | NR | 44.7 | 46.5 |
| Bronchiectasis (%) | NR | NR | 5.42 |
Abbreviations: COVID-19, coronavirus disease 2019; GGO, ground-glass opacity; NR, not reported.
4 |. DISCUSSION
Our study is the first comprehensive summary of the published studies thus far describing COVID-19 lung imaging data in the pediatric population. This systematic review and meta-analysis comprised data from 1026 children, from newborns to 18 years of age, with PCR-confirmed SARS-CoV-2 infection and lung CT imaging at the time of diagnosis. The main results of this comprehensive analysis are as follows: (1) over a third of pediatric patients with COVID-19 had normal chest CT scans and only 27.7% had bilateral lesions; (2) the most typical pediatric chest CT findings of COVID-19 were GGO (37.2%) and the presence of consolidations or pneumonic infiltrates (22.3%); (3) the lung imaging findings in children with COVID-19 were overall less frequent and less severe than in adult patients; and (4) typical lung imaging features of viral respiratory infections in the pediatric population (hyperinflation 49–51) were not reported in children with COVID-19.
COVID-19 is a heterogeneous condition in children with a wide phenotype and variable presentations in chest imaging. No more than 37.2% of the cases shared any of the disease manifestations, such as the presence of GGO, which was the most common finding linked to COVID-19 in children. This is a major difference from what has been consistently observed in adults with COVID-19 who often share the same lung findings, including GGO, in more than 80% of the cases.13–16 Moreover, although viral respiratory infections are usually more severe in children,9,10 there is strong epidemiological evidence showing that children infected with SARS-CoV-2 not only have less severe clinical manifestations, but are also frequently asymptomatic.4–6,8 In agreement with this clinical evidence, we found that 35.7% of children with PCR-confirmed SARS-CoV-2 infections did not have any lung imaging abnormality in chest CT, which is similar to the results reported in a recent literature review conducted by Duan et al.51 Notably, we also found that most of the studies reporting pediatric COVID-19 cases did not include subjects with negative PCR, so a true sensitivity and specificity analysis of CT scanning could not be systematically assessed in our pediatric systematic review and meta-analysis. In the adult population, a large study of 1014 patients (mean age 51 years) found that lung CT scanshad a sensitivity of 97% but a low specificity value of 25% for detecting COVID-19.52 However, other COVID-19 lung CT studies have reported much lower sensitivity values. Based on a literature review mostly focused on the adult population, Merkus and Klein53 recently estimated the sensitivity of a chest CT to detect abnormalities in proven COVID-19 cases as 44%–97% (median 69%). This value may be even lower in children as they often have normal lung CT scans during PCR-confirmed SARS-CoV-2 infections, as reported in our study (Figure 2).
An important conclusion of our study is that the abnormalities reported on the chest CT scans of children infected with SARS-CoV-2 are distinct from the typical lung images seen during other viral respiratory infections in the pediatric population. GGO is an uncommon radiological feature of viral infections in children, except for cytomegalovirus, usually an opportunistic pathogen in this population.54 Common viral respiratory pathogens in children, such as respiratory syncytial virus (RSV),10 are known to cause airway mucosal edema, mucous plugging, bronchoconstriction, and bronchial luminal obstruction.10 These pathological features manifest in lung imaging as increased perihilar markings with distal air-trapping and hyperinflation.48–50 In contrast, we found that children with COVID-19 present with alveolar compromise leading to a GGO pattern characterized by increased lung attenuation in CT without increased perihilar markings or air trapping (Figure 4). Given that the findings described in children with COVID-19 are different from the lung imaging features of acute viral respiratory infections in the pediatric population, we believe that the detection of nontypical lung imaging patterns (e.g., GGO) and complementary clinical features such as hypoxemia due to impaired alveolar gas exchange,50 could potentially be used for early identification and prompt intervention in pediatric cases of COVID-19.
We also compared the results of our pediatric study with two comprehensive meta-analyses recently published in the adult literature.13,14 The most typical findings in the pediatric population were GGO and the presence of consolidation or pneumonic infiltrates. Overall, these findings are similar to the lung abnormalities reported in adult patients, but they were less frequent and less severe in children. Other findings present in the adult COVID-19 populations, such as halo sign, interstitial abnormalities, bronchovascular bundle thickening, crazy paving pattern, lymphadenopathy, and pleural compromise, were reported in less than 1% of the children. Less typical findings reported in adults, such as bronchiectasis, air bronchogram, and pericardial effusion were not identified in children. There are several potential explanations for these age-related differences. First, some of the lung features of adult COVID-19 may represent more severe and/or advanced pathology. Second, differences in the pathobiology of COVID-19 between adults and children may result in a distinct pattern of disease. For instance, SARS-CoV-2 infection in children was much less likely to affect the pleural space. Third, elderly adults with COVID-19 may have underlying chronic lung changes not present in previously healthy children with SARS-CoV-2 infections (e.g., bronchiectasis). Future studies are still needed to compare pediatric and adult SARS-CoV-2 infections by integrating lung imaging features with individual clinical and molecular factors to better define the mechanisms mediating the reported age-related differences in COVID-19 and the potential clinical implications for pediatric and adult patients.
While there have been many reports of COVID-19 lung imaging and literature reviews in adults, our meta-analysis is the first to our knowledge to focus only on pediatric COVID-19 lung imaging. Furthermore, our study included case series from different hospitals in different countries, allowing for generalization of the results. However, the majority of these studies were based on data collected in China, particularly nearby Wuhan, Hubei Province, the initial epicenter of the global COVID-19 pandemic.1 It is also noteworthy that we were able to pool a relatively large number of pediatric cases with chest CT imaging available during PCR-confirmed SARS-CoV-2 infection. Although lung CT findings are known to play an important role in the detection and determination of the severity of COVID-19,13–16 in the pediatric population, CT scans are typically performed much less frequently than in adults, predominantly to decrease the risk of sedation and radiation exposure.55 Lung CT scans may also not be widely available in low- and middle-income settings. These concerns limit the value of CT of the chest in the diagnosis and management of children with SARS-CoV-2 infection. Nonetheless, we believe lung CT scans may be useful to quantify disease extension and to consider other respiratory pathologies in severe COVID-19 cases.
Given the potential concerns and limitations of lung CT scans in children, the use of CXRs may be better suited for screening and serial analysis (within few hours) of lung compromise in confirmed or suspected pediatric COVID-19 cases. We found that CXR abnormalities are detectable in some pediatric patients with COVID-19 and, to some extent, resemble those described in CT scans. The most common CXRs findings reported included opacities, pneumonic infiltrates, and lung haziness or “white lung,”20,24,42 which may be radiographic manifestations of GGO caused by SARS-CoV-2 infection. However, these CXR findings are often subtle and nonspecific in children. Thus novel software technology and artificial intelligence tools may be useful in detecting and quantifying lung signatures in pediatric cases of SARS-CoV-2 infection.56 Computer-assisted analyses and scoring of pediatric CXRs have been developed for other respiratory viruses;48,49 thus this approach could potentially be used to aid in the diagnosis of pediatric COVID-19. This would be an important development since COVID-19 identification in the pediatric population can be challenging due to the presence of mild symptomatology and atypical lung features (e.g., GGO). The results of our systematic review and meta-analyses indicate that there is an emerging need to improve pulmonary imaging technology for children to enable identification and severity quantification of pediatric SARS-CoV-2 infections in chest CT scans and CXRs.
Our systematic review also has several limitations. First, we could not correlate our findings to clinical manifestations and symptom severity. Although some papers did report their imaging findings related to the patient’s symptoms, this was not done ubiquitously and thus was not included in the analysis. On the same spectrum, many studies did not distinguish between clinically silent disease or mild, moderate, and severe symptoms in these patients. Second, radiologists from different parts of the world have varying vernacular and different terminology for certain CT findings, which could be a cause of diverging or overlapping CT-based reporting. For example, the term “shadow” was often used to describe lung opacities, which could be related to GGO and/or pneumonic infiltrates. Third, our analyses only included chest CT findings since there have been very few studies on CXRs, despite being often the mainstay for pediatric imaging. The role of CXR in the diagnosis and management of pediatric COVID-19 continues to be defined. Lastly, our systematic review and meta-analysis did not include lung imaging findings of the Multisystem Inflammatory Syndrome in Children (MIS-C), which have only recently been published.7 The lung features of this condition were not found in the articles included at the moment of this publication. MIS-C syndrome is a potentially serious illness, which is still being fully elucidated and reported in developing literature.
5 |. CONCLUSION
The literature on COVID-19 imaging is rapidly expanding, albeit at a much lower rate in pediatrics than adults. Based on heterogeneous data, pediatric chest CT findings are to some extent, similar to adults, although less frequent and less severe. The top abnormalities identified in pediatric COVID-19 cases (n = 1026) were GGO, and the presence of consolidations or pneumonic infiltrates. However, over a third of children with COVID-19 demonstrate normal chest CTs. Future directions include developing robust severity scoring systems for COVID-19 lung imaging in children, including CT scans and CXRs, as well as further analysis of the pulmonary manifestations of MIS-C.
ACKNOWLEDGMENTS
The study was funded by NIH Grant Numbers HL145669 and HL141237.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: HL141237 and, HL145669
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Chen R, Liang W, Medical Treatment Expert Group for COVID-19, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382: 2302–2315. 10.1056/NEJMoa2006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team. Coronavirus disease 2019 in Children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422–426. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020;April 8:e201346. 10.1001/jamapediatrics.2020.1346 [Epub ahead of print]. PubMed PMID: 32267485; PubMed Central PMCID: PMC7142799. [DOI] [PMC free article] [PubMed]
- 5.Morand A, Fabre A, Minodier P, et al. COVID-19 virus and children: What do we know? Arch Pediatr. 2020;27(3):117–118. 10.1016/j.arcped.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Zhang L, Chinese Pediatric Novel Coronavirus Study Team, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382: 1663–1665. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents [published online ahead of print, 2020 Jun 29]. N Engl J Med. 2020; NEJMoa2021680. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed]
- 8.Liu W, Zhang Q, Chen J, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14): 1370–1371. 10.1056/NEJMc2003717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissner HC. Viral bronchiolitis in children. N Engl J Med. 2016; 374(1):62–72. 10.1056/NEJMra1413456 [DOI] [PubMed] [Google Scholar]
- 11.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest. 2020;158:106–116. 10.1016/j.chest.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society [published online ahead of print, 2020 Apr 7]. Radiology. 2020:201365. 10.1148/radiol.2020201365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–709. 10.1016/j.jacr.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Zhong Z, Li H, et al. CT imaging features of 4121 patients with COVID-19: a meta-analysis. J Med Virol. 2020;92(7):891–902. 10.1002/jmv.25910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan S, Li M, Ye Z, et al. Manifestations and clinical characteristics of 1115 patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Acad Radiol. 2020;27(7):910–921. 10.1016/j.acra.2020.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 2020;92:1449–1459. 10.1002/jmv.25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute website. Study Quality Assessment Tools. http://www.nhlbi.nih.gov/health-topics/study-qualityassessment-tools. Accessed February 16, 2020.
- 19.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. 10.1186/1471-2288-9-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020;16(3):251–259. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Zhang ZZ, Chen YK, et al. The clinical and immunological features of pediatric COVID-19 patients in China [published online ahead of print, Apr 14]. Genes Dis. 2020. 10.1016/j.gendis.2020.03.008 [DOI] [PMC free article] [PubMed]
- 22.Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng F, Liao C, Fan Q, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei China. Curr Med Sci. 2020;40(2): 275–280. 10.1007/s11596-020-2172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Wen H, Rong D, Zhou Z, Liu H. Clinical characteristics and radiological features of children infected with the 2019 novel coronavirus. Clin Radiol. 2020;75(7):520–525. 10.1016/j.crad.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Shen J, Li L, Yu C. Radiographic and clinical features of children with coronavirus disease (COVID-19) pneumonia. Indian Pediatr. 2020;57(5):423–426. 10.1007/s13312-020-1816-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020; 50(6):796–799. 10.1007/s00247-020-04656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Cao J, Zhang X, Liu G, Wu X, Wu B. Chest CT imaging characteristics of COVID-19 pneumonia in preschool children: a retrospective study. BMC Pediatr. 2020;20(1):227. 10.1186/s12887-020-02140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Liu F, Li J, Zhang, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7–e13. 10.1016/j.jinf.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Hu J, Tian J, et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med. 2020;18(1):123. 10.1186/s12916-020-01596-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberger S, Lin B, Bernheim A, et al. CT features of coronavirus disease (COVID-19) in 30 pediatric patients [published online ahead of print, 2020 May 22]. AJR Am J Roentgenol. 2020:1–9. 10.2214/AJR.20.23145 [DOI] [PubMed]
- 31.Wang Y, Zhu F, Wang C, et al. Children hospitalized with severe COVID-19 in Wuhan. Pediatr Infect Dis J. 2020;39(7):e91–e94. 10.1097/INF.0000000000002739 [DOI] [PubMed] [Google Scholar]
- 32.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169–1174. 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du W, Yu J, Wang H, et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection. 2020;48(3):445–452. 10.1007/s15010-020-01427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Song Z, Xiao K. High-resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr. 2020;44(3):311–313. 10.1097/RCT.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55(6):1424–1429. 10.1002/ppul.24762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19). J Clin Virol. 2020;127:104377. 10.1016/j.jcv.2020.104377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan YP, Tan BY, Pan J, Wu J, Zeng SZ, Wei HY. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. 10.1016/j.jcv.2020.104353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3(6): e2010895. 10.1001/jamanetworkopen.2020.10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020; 55(6):1430–1432. 10.1002/ppul.24767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du H, Dong X, Zhang J, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status [published online ahead of print, 2020 Jun 10]. Allergy. 2020. 10.1111/all.14452 [DOI] [PMC free article] [PubMed]
- 42.Korkmaz MF, Türe E, Dorum BA, Kılıç ZB. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci. 2020;35(25):e236. 10.3346/jkms.2020.35.e236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Wang H, Wang F, et al. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years [published online ahead of print, 2020 Jun 11]. Int J Infect Dis. 2020;98:80–83. 10.1016/j.ijid.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Gu J, Chen Q, et al. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: a multi-center case series. PLOS Med. 2020;17(6):e1003130. 10.1371/journal.pmed.1003130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Huang S. Clinical features of 33 cases in children infected with SARS-CoV-2 in Anhui Province, China—a multi-center retrospective cohort study. Front Public Health. 2020;8:255. 10.3389/fpubh.2020.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai K, Liu W, Liu C, et al. Clinical analysis of 25 COVID-19 infections in children. Pediatr Infect Dis J. 2020;39(7):e100–e103. 10.1097/INF.0000000000002740 [DOI] [PubMed] [Google Scholar]
- 47.Lan L, Xu D, Xia C, Wang S, Yu M, Xu H. Early CT findings of coronavirus disease 2019 (COVID-19) in asymptomatic children: a single-center experience. Korean J Radiol. 2020;21(7):919–924. 10.3348/kjr.2020.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansoor A, Perez G, Nino G, Linguraru MG. Automatic tissue characterization of air trapping in chest radiographs using deep neural networks. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 01 August 2016, 2016:97–100. 10.1109/EMBC.2016.7590649. PMID: 28324924; PMCID: PMC5459489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada K, Golbaz M, Mansoor A, et al. Severity quantification of pediatric viral respiratory illnesses in chest X-ray images. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:165–168. 10.1109/EMBC.2015.7318326. PMID: 26736226; PMCID: PMC4704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arroyo M, Salka K, Perez GF, et al. Phenotypical sub-setting of the first episode of severe viral respiratory infection based on clinical assessment and underlying airway disease: a pilot study. Front Pediatr. 2020;8:121. 10.3389/fped.2020.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan YN, Zhu YQ, Tang LL, Qin JCT. features of novel coronavirus pneumonia (COVID-19) in children. Eur Radiol. 2020;30(8): 4427–4433. 10.1007/s00330-020-06860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merkus PJFM, Klein WM. The value of chest CT as a COVID-19 screening tool in children. Eur Respir J. 2020;55(6):2001241. 10.1183/13993003.01241-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Restrepo-Gualteros SM, Gutierrez MJ, Villamil-Osorio M, Arroyo MA, Nino G. Challenges and clinical implications of the diagnosis of cytomegalovirus lung infection in children. Curr Infect Dis Rep. 2019; 21(7):24. 10.1007/s11908-019-0681-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harmon SA, Sanford TH, Xu S, et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat Commun. 2020;11(1):4080. 10.1038/s41467-020-17971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


