Abstract
Blood vessels are essential in transporting nutrients, oxygen, metabolic wastes, and maintaining the homeostasis of the whole human body. Mass of engineered microvessels is required to deliver nutrients to the cells included in the constructed large three-dimensional (3D) functional tissues by diffusion. It is a formidable challenge to regenerate microvessels and build a microvascular network, mimicking the cellular viabilities and activities in the engineered organs with traditional or existing manufacturing techniques. Modular tissue engineering adopting the “bottom-up” approach builds one-dimensional (1D) or two-dimensional (2D) modular tissues in micro scale first and then uses these modules as building blocks to generate large tissues and organs with complex but indispensable microstructural features. Building the microvascular network utilizing this approach could be appropriate and adequate. In this review, we introduced existing methods using the “bottom-up” concept developed to fabricate microvessels including bio-assembling powered by different micromanipulation techniques and bioprinting utilizing varied solidification mechanisms. We compared and discussed the features of the artificial microvessels engineered by these two strategies from multiple aspects. Regarding the future development of engineering the microvessels from the bottom up, potential directions were also concluded.
Keywords: Microvessels, Bio-assembling, Bioprinting, Bottom-up, Tissue engineering
1. Introduction
Tissues such as bone and skin have the ability to repair slight injuries by themselves[1-3]. However, many serious tissue injuries and multiple organ failures can hardly be effectively cured by conventional interventional, pharmacological, and surgical therapies, except organ transplantation[4-6]. Unfortunately, organ transplantation in the clinic is greatly limited by the shortage of donors[7,8]. Tissue engineering was introduced by Langer 30 years before targeting to build artificial functional tissue substitutes through combining specific cell lines, molecules, and organic materials[4]. In the past two decades, significant advances have been achieved in tissue engineering. Many different kinds of tissues have been fabricated, but most of these engineered tissues feature thin slices and simple architectures[9,10]. The main reason is the challenges in including the microvascular networks in the large functional regenerated tissues[11].
Blood vessels are essential in transporting nutrients, oxygen, metabolic wastes, and maintaining the homeostasis of the whole human body. Mass of engineered microvessels is required to deliver nutrients to the cells included in the constructed large three-dimensional (3D) functional tissues by diffusion which is limited within an area of smaller than 200 mm[12-15]. Traditional tissue engineering strategies adopt the “top-down” approach, in which cells are seeded on a biodegradable scaffold. The seeded cells then populate on the scaffold and generate the appropriate extracellular matrix[16-20]. However, this approach can only fabricate vessels larger than 6 mm, which are mainly used to replace the damaged vessels of patients with cardiovascular diseases[21]. It is a formidable challenge to regenerate microvessels and build a microvascular network, mimicking the cellular viabilities and activities in the engineered organs, such as the liver, the heart, and the kidney, with traditional, or existing manufacturing techniques[9-11,21].
Modular tissue engineering adopting the “bottom-up” approach builds one-dimensional (1D) or two-dimensional (2D) modular tissues in micro scale first and then uses these modules as building blocks to generate large tissues and organs[22-32]. It allows recreating complex but indispensable microstructural features of the engineered tissues. Building the microvascular network using this approach could be appropriate and adequate. However, fabricating the basic modular tissues and building the microvessels with these modules in micro scale face tough challenges in precision, efficiency, and configuration complexity. Existing methods using the “bottom-up” concept developed to fabricate microvessels can be divided into bio-assembling powered by developing micromanipulation techniques and bioprinting utilizing varied solidification mechanisms. Some researchers tend to treat bioprinting as a particular assembly approach in modular tissue engineering. The main difference of bioprinting from the common bio-assembling is its ability to create modular tissues and build 3D structures simultaneously[33].
In this review, we describe the bio-assembling and bioprinting strategies for engineering the microvessels. First, we introduce the 1D or 2D modular tissues with different geometries for assembling the microvessels and the bioinks used in bioprinting. Then, assembly methods applying different micromanipulation techniques and bioprinting devices adopting different mechanisms are reviewed. Finally, we compare and discuss the features of the artificial microvessels constructed by these two strategies from the aspects of the fabrication efficiency, the sizes of the engineered microvessels, and the ability to construct the complex 3D microvascular networks.
2. Engineering microvessels from the bottom up
At present, there are two construction strategies in tissue engineering, which are “top-down” and “bottom-up” approaches. In most “top-down” approaches, a scaffold is fabricated first, and cells are then seeded on the scaffold. In the following culture process, cells populate on the scaffold and generate the appropriate extracellular matrix with the external chemical and mechanical stimulations. While developing the “top-down” approaches, researchers focus on improving the fabrication of scaffold or testing various combinations of the cell sources and stimulation ways during culture[16-21]. Nowadays, the main challenge in applying “top-down” approaches to the construction of the independent vascular networks or tissues including the microvascular architectures is that “top-down” approach is by nature a 2D construction strategy. The cells are seeded on the surface of the scaffold which is a 2D space. Along with increasing the complexity of the scaffold architectures, it becomes difficult for seeding the cells on the scaffold and giving the necessary support or stimulations to the seeded cells. Moreover, “top-down” approach features extremely low flexibilities in constructing tissues with varied sizes and architectures.
Different from the “top-down” approaches, “bottom-up” approaches start from constructing fabricating the micro modular tissues with cells and biocompatible materials[22-32]. As shown in Figure 1, 1D or 2D modules such as spheroids, rings especially for engineering microvessels, fibers, plates with arbitrary shapes, and cell sheets can be fabricated through cell aggregation and microfabrication techniques with mass production. Then, these micromodules as the basic blocks can be used to build the large tissues with desired micro architectures including the microvascular networks. The existing “bottom-up” approaches for engineering microvessels can be divided into bio-assembling and bioprinting. Some researchers also classify the bioprinting into a particular bio-assembling way. In engineering microvessel by bioassembling, the key is the geometry design of the micro modules, which governs the selection of the micromanipulation methods in the assembling procedure. The geometry of the micromodules and the micromanipulation utilized in the assembling determines the fabrication efficiency, complexity, and size of the constructed microvessels. In engineering microvessel by bioprinting, the bioink compositions and solidification mechanisms are the two major factors as they influence the mechanical property, curing time, curing degree, printing speed, and printing resolution. In the following sections, we will introduce existing bio-assembling approaches utilizing various micro modular tissues and bioprinting approaches based on different printing mechanisms.
Figure 1.
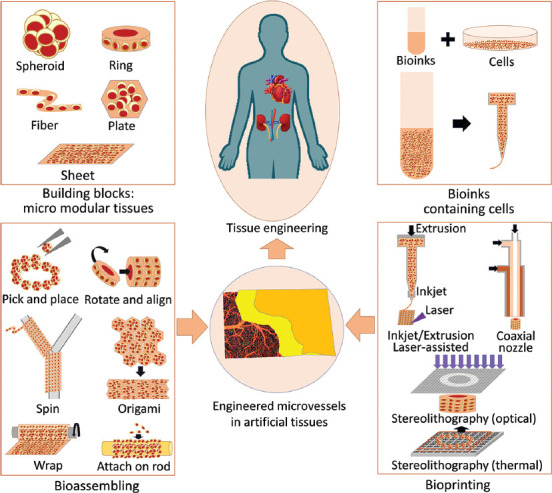
Artificial microvessels by modular tissue engineering: bio-assembling and bioprinting microvessels from the bottom up.
3. Modules and bioinks
According to the morphology, we divide modules for assembling microvessels into five categories: fiber, sheet, plates, rings, and spheroid. The bioinks can be divided into the pure polymer and composite polymer. In this section, we focus on introducing the existing fabrication method of the micro modular tissues for assembling microvessels, and bioinks that can be utilized in bioprinting the microvessels.
3.1. Cell spheroids
Cell spheroids usually refer to cell clusters composed of multiple cells that do not adhere to any culture substrate. In the cell spheroids, cells are typically surrounded by the extracellular matrix, which is a substance that affects cell growth and proliferation[34-36]. In most biological research, cells adhere to cell culture-compatible polystyrene and proliferate in a 2D monolayer. However, this in vitro environment is thought significantly different from the natural cellular environment. Nowadays, with novel microfabrication techniques, cells can be cultured as spherical aggregates or be embedded in spherical structures made by biocompatible materials. With these cell culturing methods, cells stay in the extracellular matrix interacting with each other just like in their natural environment. The biological research conducted with these cell culturing methods is considered more convincing. Moreover, these cell spheroids are also considered as ideal basic building blocks in “bottom-up” tissue engineering since they offer a stable natural cellular microenvironment in the assembling process. The fabrication of cell spheroid is closely related to cell 3D culture, as shown in Figure 2A. The traditional cell spheroid fabrication methods are hanging drop and cell suspension. These methods achieve aggregation by avoiding cell contact with the substrate, but the size of cell spheroids cannot be well controlled. Microwell hanging drop technique was developed to address this issue because it has pores that can be used to filter the size. It ensured that the collected cell spheroids are small enough with a similar outer diameter. Besides, using photolithography or customized channels to make and collect cell spheroids can also yield products with an unfirm size because cells can gather into clusters in a chamber with a fixed size. However, the fabrication efficiency of the latter two methods is low, and the cost is high.
Figure 2.
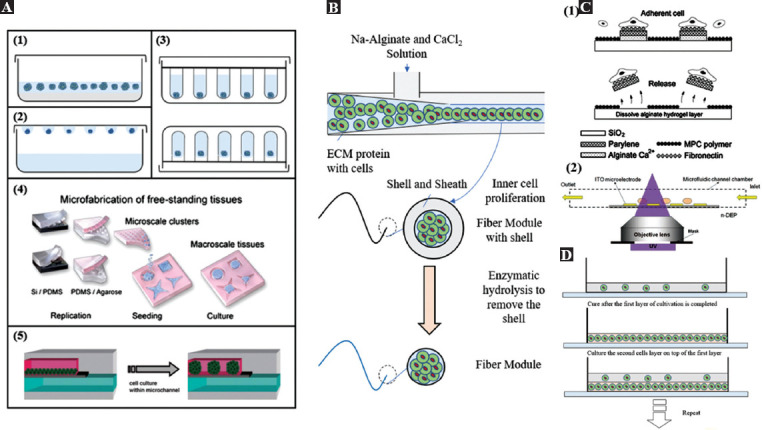
The fabrication process of several kinds of modules. (A) Six fabrication methods of cell spheroids modules[34], including (A1) suspension cell culture, (A2) hanging drop, (A3) microwell, (A4) microwell array from micropatterned agarose wells (Republished with permission from Rivron NC, Vrij EJ, Rouwkema J, et al., Proc Natl Acad Sci, 2012, 109:6886–91.[35]), and finally, (A5) microchannel forming (Reproduced from ref. 36 with permission from The Royal Society of Chemistry). (B) Fabrication process of fiber module by microchannels[30]. (C) Fabrication process of microplates and rings by (C1) photolithography (Republished with permission from Teshima T, Onoe H, Kuribayashiashiashias K, et al., Small, © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim[38]). and (C2) dielectrophoresis (from ref.[39] licensed under Creative Commons Creative Commons Attribution License). (D) Layer-by-layer fabrication process of cell sheets[44].
3.2. Cell-embedded fibers
Fiber is popular in “bottom-up” tissue engineering as the basic module. The advantage of using the fibers as the basic modules to construct large 2D and 3D structures (mainly clothes) can be traced back thousands of years ago. Furthermore, inspired by the ancient fiber production methods and being assisted by the recent microfabrication techniques, researchers have developed many methods to fabricate cell-embedded fiber modules. Nearly all these methods follow the same way, which is to make biocompatible materials (together with some other materials) pass through narrow channels or pipes, and finally fabricate the fibers close to the outlet by solidification. The microfluidic manufacturing method is recognized as an efficient solution, as shown in Figure 2B derived from the paper by Onoe et al.[30], which depicts an advanced fiber manufacturing process. The extracellular matrix protein with cells in the pre-gel state is covered by stiff Ca-alginate hydrogel formed by the chemical reaction between the Na-alginate solution and the CaCl2 solution. Since the alginate in the outer layer forms a shell to avoid the diffusion of the inner extracellular matrix protein gel, the cells inside can proliferate and form the cell-embedded fibers under suitable conditions. Finally, the outer alginate shell is removed with enzymes, and the fibers as the building blocks in “bottom-up” tissue engineering can be obtained. In addition to the method of microfluidic spinning introduced in the above, electrospinning, wet spinning, biospinning, and melt spinning are all feasible methods for fabricating microfibers[37]. Using these fabrication methods, encapsulation of cells, fabrication efficiency, damages to cells during the fabrication, size control, and mechanical property of the fabricated fibers should be well considered.
3.3. Cell-laden 2D modules
Compared with the 1D cell spheroids and fibers embedded cells, 2D modules with designed geometry could accelerate the following assembling process. For example, assembling the ring-shaped 2D modules to microvascular structures could be more efficient than assembling the spheroids. Nowadays, photolithography and soft lithography are commonly utilized in the fabrication of 2D modules with arbitrary shapes. Figure 2C shows the fabrication of cell-laden microplates using photolithography[38,39]. The expected microplate is composed of the cell-adhesion layer, the core layer, and the sacrificial layer. During the manufacturing process, first, the core layer with a metal plate is covered to ensure that the required part can be left. Second, MPC polymer, the hard-to-attach layer, is covered first. The metal part is washed off then. Thus, the adhesion layer can stay on the surface of the core layer to allow the cells to attach. Finally, to make the microplate fall off from the substrate, the sacrificial layer is washed away, and the standardized cell-attached microplates can be obtained. Different from the cells that adhere to the surfaces of the microplates, cells can also be maintained inside 2D structures. As shown in Figure 2C, mixing cells with photo-cross-linkable resin (PEGDA, polyethylene glycol diacrylate), and ring-shaped (arbitrary shape is available) cellular modules can be fabricated by exposing the resin inside a thin microchannel to the patterned Ultraviolet light[40]. Here, dielectrophoresis is employed to array the cells in the resin to ensure the uniform distribution of cells in the fabricated 2D modules. Moreover, in the fabrication of the 2D modules utilizing soft lithography, the template patterns are first fabricated. Then, by molding the templates, poly(dimethylsiloxane) (PDMS) microwell arrays are obtained. The PDMS microwell array also serves as the template for shaping the curable solutions. After solidification of the solution, the 2D modules with arbitrary shapes can be fabricated[41,42].
3.4. Cell sheets
Researchers have a wide variety of choices available in the fabrication of the cell sheets which can also act as the modular tissues in the “bottom-up” tissue engineering. The typical methods include the layer-by-layer covering method and the textile method. The layer-by-layer covering method is one of the simplest approaches to fabricate the sheet modules. In the research of Yokoyama et al., they used elastin to wrap smooth muscle cells arranged in a multilayer spiral[43]. In the fabrication process, Yokoyama et al. used the layer-by-layer method, as shown in Figure 2D, which cultivates a layer of smooth muscle cells in the elastin. When the cells are proliferated to the required number, they are gelled, and then the second layer of cells is cultivated on it, and so on. Hinds et al. used a similar method to fabricate a cell sheet and then used it to build a vascular structure[44]. Although the fabrication was conducted on a cylinder mold, all the contents are uploaded layer-by-layer through a syringe. Electrospun uses the electric field to gel the woven elastin fiber. After weaving the fiber gels, the final output is the sheet module.
3.5. Bioinks: pure polymers
In “bottom-up” tissue engineering by bioprinting, the bioinks are important. The concept of bioinks comes with the development of bioprinting technology. Many studies on bioinks in recent years have discovered or developed various materials suitable for bioprinting. In general, bioinks need to have the following characteristics: bioinks should protect cells from extrusion, inappropriate environments, and other damages in the fabricating processes[45]; they should have suitable mechanical properties, such as high mechanical integrity, viscoelasticity, and stability; they should consider the biocompatibility, such as non-toxicity, and non-immunogenicity, and also cell adhesion promotion properties; and they should also own other necessary characteristics including printability, insolubility in the culture medium, commercial feasibility, and manufacturing efficiency.
Researchers such as Saito et al., Jia et al., and Mobaraki et al. have made a summary of the materials suitable to be used as bioinks[20,46,47]. Here, we only introduce the inductive nature of various materials. Almost all bioinks used currently are composed of polymer components, most of which are used in the form of hydrogels. Pure polymers can be used in bioprinting, but some composite polymers are also used to improve the mechanical properties of materials or expand the other physical properties. Some popular pure polymers are as follows. Collagen is a triple helix biocompatible protein of natural origin, which has the smallest immune response[48]. Collagen can also promote cell culture and enhance cell adhesion and attachment to the culture medium[49]. Collagen stays in a liquid state at a low temperature and will gel when the temperature rises, but the solidification speed is slow; it takes 30 min at 37°C to gel totally, which is a barrier to 3D bioprinting[46]. Fibrin is a kind of bioink with considerable mechanical properties (especially elasticity) and short gelation time. It is also biocompatible and able to promote cell growth and is thus a choice to fabricate microvessels. However, the price of fibrin is also high. Silk is one of the most common biological protein materials, derived from biological activities such as spider silk or silkworm cocoons. Silk has ideal mechanical properties while being smooth, non-toxic, and not easy to be contaminated by bacteria. However, it usually needs to be mixed with other substances to optimize some other important printing properties, such as transparency and fluidity. Alginate is one of the most popular natural hydrogels. It is a natural polysaccharide with special characteristics such as high biocompatibility, low price, different crosslinking options, and high compatibility with various printing methods[46]. Low cell adhesion and average mechanical properties are its two main shortcomings. Chitosan, similar to cellulose, is also a natural polymer polysaccharide, which is generally used to make tissues or organs with high mechanical strength (resistance to tension and compression). Its price is also very low, and it is often used in the medical field as well. Gelatin[50] is mainly derived from the hydrolysis of collagen. Therefore, it can be directly extracted from animal connective tissue and other materials. It has low immunogenicity, no cytotoxicity, and low cost, but its mechanical strength is average. Hyaluronic acid is well known to many people as a material in the fields of medicine, hygiene, and beauty. However, it also can make flexible hydrogels. When subjected to force, it is prone to deformation.
3.6. Bioinks: composite polymers
Compared with pure polymers, composite polymers are utilized to improve the mechanical properties, modify other physical properties of the printed construes, and retain the excellent biocompatibility of pure polymers. According to the operation mode, composite materials can be divided into five types: multimaterial, stimuli-responsive, biomolecular, self-assembling, and materials based on nanotechnology. Many pure bioinks, as described before, have insufficient deformability during use, or their nature is not conducive to 3D printing operations. Therefore, they can be doped with some other substances. For example, calcium ions can add to alginate to improve the mechanical strength and crosslinking performance[51]; chitosan hydrogel and hydroxyapatite can mix as artificial cartilage material[52]. Biomolecular material is a vital branch of the bioinks for our research, which is widely used in the fabrication of engineered vessels. This material includes cytoplasmic matrix, decellularized extracellular matrix, or DNA and other living tissues elements as a bioink, which can simulate the life state and mechanical properties of cells to the greatest extent[47]. Essentially, these materials are also a mixture of polysaccharides, lipids, and proteins. In addition to simple doping methods, controllable combinations of multiple materials also play an important role in enhancing the mechanical properties of materials and expanding other physical properties (such as magnetism), where nanotechnology is required, including nanofabrication and nanoparticle reinforced polymer composites. Self-assembling materials are a kind of hydrogel materials with better mechanical properties by catalyzing the automatic combination of proteins or peptides in a specific way or shape[53]. Besides, current popular bioprinting targets are not only limited to satisfy sufficient mechanical properties and biocompatibility but also to provide obvious features so that external field forces can manipulate these structures. Researchers gave bioinks additional characteristics such as magnetism. Using the magnetic force can expand the methods of printing and further operations, achieve higher precision, and manufacture more stable artificial blood vessels. Nanotechnology is urgently needed among the above two materials. It is still very important to explore and develop new excellent bioinks in the future.
4. Bio-assembling powered by micromanipulations
Micromanipulation has been widely used to construct artificial tissues[54]. Adopting the “bottom-up” approach, bio-assembling powered by micromanipulation techniques provides a robust and highly scalable method to build 3D engineered tissue through assembling micro modular tissues[20,28,55]. This section lists the typical bio-assembling methods depending on the morphology of the fabricated micromodules.
4.1. Pick-and-place of spheroids
The concept of “cell as a material” proposed by Kasza et al. implies that micro-spherical modular tissues can be considered as the basic building blocks of blood vessels, which is a more intuitive and feasible way to realize the construction of artificial microvascular networks or any other engineered tissues with complex architectures[56]. Using this method, the diameter of the microvessels can be controlled under 1 mm. In the process of assembling the micro spheroids into microvessels, the most commonly adopted method is using a high-precision and high-speed motorized micromanipulator that can pick and place the spherical modules to the planned locations. This primitive manipulation has high flexibility in the construction of microvessels with varied sizes and branches. However, assembling a huge number of spherical modules can hardly allow efficient construction. Figure 3A shows an a schematic diagram of building microvascular structures by assembling spherical micro modular tissues[33]. In the relevant research, picking progress was avoided to improve the fabrication efficiency. Arai et al. designed a high-speed piezo-driven two-finger microhand system, which can realize extremely high-speed automated assembly of the spherical structures with the assistance of computer vision technique[57,58]. Although the pick-and-place operations in micro scale become faster and faster, the fabrication efficiency is still the main challenge in building microvessels through assembling spherical modules.
Figure 3.
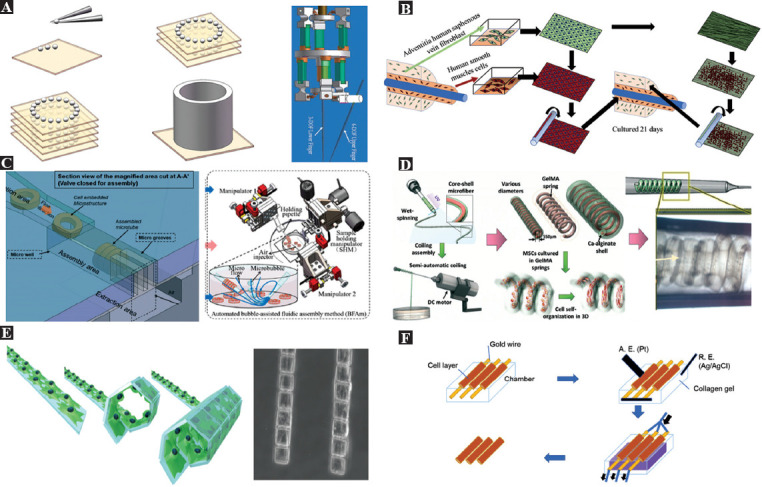
Bio-assembling powered by micromanipulations. (A) Building microvessels by pick-and-place of the cell spheroids and the piezo-driven two-finger microhand for high speed pick-and-place assembly (Republished with permission from Ramadan AA, Takubo T, Mae Y, et al., IEEE Trans Ind Electron, 56:1121–35.[58]). (B) Construction of the microvessels by wrapping a cell sheet[60]. (C) A 4-layer PDMS microfluidic device for microfluidic self-assembly of ring-shaped modules (left, reproduced from ref 63 with permission from The Royal Society of Chemistry), and automated assembly of microvascular structures using a multimicromanipulator system (Republished with permission, from Liu X, Shi Q, Wang H, et al. IEEE/ASME Trans Mechatron, 2018, 23:667–78[65]). (D) Semi-automatic fabrication of microvascular structures by spinning fibers containing cells (Reproduced from ref. 66 with permission from the Royal Society of Chemistry). (E) Construction of microvessels using cell origami based on the self-folding driven by cell traction force (from ref.[39] licensed under Creative Commons Creative Commons Attribution License). (F) The cells are electrically assembled to a microvascular structure on the capillary surface by applying an electrical potential and extracting the rods from the collagen gel[22,76].
4.2. Wrapping sheets
Due to the tubular structure of the blood vessels, directly assembling the planar 2D cell sheet into microvascular structures features high fabrication efficiency. Heureux et al. first achieved cell-sheet-based tissue-engineered vascular structures in 1998 without the use of any synthetic or exogenous biomaterials[59]. Based on this approach, many more efficient methods have been explored to fabricate engineered vessels with smaller diameters. Bourget et al. developed a decellularized matrix scaffold generated from dermal fibroblasts or saphenous vein fibroblasts to implant smooth muscle cells and produce tissue-engineered vascular media to shorten the time required for their generation[60]. This approach reduced the total production time from 6 weeks to 4 weeks (Figure 3B). Moreover, assisted by micromanipulation tools, it allows the fabrication of sub-millimeter vascular structures directly with the smooth muscle cells, regardless of their ability to synthesize extracellular matrix.
4.3. Rotation and alignment of rings
Cell-embedded 2D ring-shaped units are important building blocks for engineering the microvessels from the bottom up. Du et al. first realized the efficient assembly of the ring-shaped micromodules utilizing the surface tension force to drive rotation and alignment of the modules during the interaction between each other[61]. The interaction was initialed by manually swiping a needle to generate physical forces and fluidic shear. Wang et al. developed a dual-micromanipulator system to achieve the automated rotation and alignment of the ring-shaped modules[62]. The modules were rotated and picked up using the micropipette fixed on one micromanipulator to press the modules and then pushed up along the micropipette utilizing the micropipette fixed on another micromanipulator. Depending on the specific shape, the assembly process can also be carried out in the microchannels using microfluidic forces. On-chip assembly driven by fluidic force is a promising efficient method for fabricating artificial microvessels with ring-shaped modules[22,63,64]. Figure 3C shows the assembly of ring-shaped micromodules conducted in the 4-layer PDMS microfluidic device. The microfluidic device provides a closed environment for biological applications while allowing integration with other functional components for multiple tasks, including the rotation area, aligning area, and collection area. However, the whole assembly process is not as flexible as the robotic assembly using based on the micromanipulator. It can only assemble the ring-shaped modules with fixed sizes. Liu et al. integrated robotic assembly and fluidic assembly in the bubble-based fluidic assembly method[65]. The method allows the assembly of ring-shaped modules in an open environment using microbubble-excited microflow and automates the assembly process by a multi-micromanipulator system with the assistance of computer vision techniques. This non-contact assembly method does not involve any picking and releasing operations, thus minimizing the time spent in precise micromanipulation. The operations in the open environment and the robotic system also allow the flexible assembly of the microvessels with varied sizes. The outer diameter of the assemble microvessels ranged 150-500 mm. The reported longest microvessels adopting this method were longer than 3 mm. This research suggests that the combination of fluidic assembly and robotic assembly can be a potential solution for micro-assembly where high throughput and flexibility are required.
4.4. Spinning fibers
In the fabrication of artificial microvessels, spinning the cell-laden microfibers into tubular structures is also an important assembly approach. As shown in Figure 3D, Sun et al. successfully constructed a spring-like microstructure for promoting the formation of the microvascular structures in a 3D environment[66]. By including magnetic materials inside the microfibers, Sun et al. set a magnetic tweezer system to operate and assemble these magnetic microfibers by direct mechanical contact. The magnetic microfibers can be guided to move around a rod to form microvascular structures directly[67-69]. This method required high-precision manipulation, and the efficiency was extremely low. In another research work, magnetic alginate microfibers were used to fabricate the ring-shaped microstructures by spinning first. Then, ring-shaped modules were picked by the magnetic tweezers in a non-contact way and subsequently stacked along the micropillars[70,71]. This method has higher operational efficiency than the direct fiber spinning by the magnetic tweezer and no damage caused by mechanical contact. The diameter of the microvessels assembled by microfibers can be controlled to smaller than 1 mm.
4.5. Self-folding origami of microplates
The traditional art of origami has always been popular because it allows the production of various 3D sculptures by folding only 2D pages. In recent years, engineers inspired by have applied the art of origami in a variety of ways, including the spatial deployment of solar panels for manufacturing, flexible medical scaffolds, microrobots, and DNA objects[29,72,73]. Kuribayashi-Shigetomi et al. introduced a technique called cellular origami using living cells as the driving force for self-folding to create a variety of different 3D cellular microstructures, including microvascular structures[39]. They mentioned that cells could naturally generate cellular traction as a contractile force, which is generated by actin-globin interactions and actin polymerization, pulling toward the center of the cell body. Thus, various 3D microstructures can be generated by changing the geometric design of the 2D template. Figure 3E shows a schematic diagram of the microvascular structure generation based on such a principle. It is a highly biocompatible, simple, and efficient technique for encapsulating cells into microstructures with just one step. It is particularly suitable for the efficient production of simple microvessels. The outer diameter of the microvessels fabricated by this method can be as small as 50 mm.
4.6. Attaching on rod
Based on the research on the electrochemical detachment and patterning of the self-assembled cellular monolayers[74,75], Seto et al. proposed a microvessel fabrication method combining the self-assembly of the cells based on chemical bond and electrochemical detachment (Figure 3F)[22,76]. Human umbilical vein endothelial cells are attached to the gold surface by an oligopeptide. In this research, the oligopeptide CCRRGDWLC chemically adhered to the gold surface of the rod driven by the gold–thiolate bond. Then, the cells were automatically assembled on the rod and formed a tubular cellular monolayer. Finally, to separate the microvascular structure from the rod, the cells are detached by applying a negative potential to the rod for reductively splitting the gold–thiolate bond. This technique can detach more than 90% of the attached cells within minutes of applying a negative potential.
5. Devices for direct bioprinting
5.1. Inkjet, extrusion, and laser-assisted bioprinting
The inkjet, extrusion, and laser-assisted bioprinting all adopt a point-by-point printing approach, featuring excellent flexibility in constructing large high-resolution tissues with complex microarchitectures. Figure 4A shows the general concept of using these bioprinting methods for fabricating microvascular structures[77]. The inkjet bioprinting is developed by modifying the conventional 2D inkjet printer[78-81]. The common ink is replaced by the bioink, and a Z motorized stage is employed to expand the 2D printing to 3D. Due to the well-developed 2D inkjet printing devices, inkjet bioprinting costs the least among the existing bioprinting methods. It is capable of printing microvessels with outer diameter smaller than 300 mm[80,81]. According to the working mechanism of the inkjet, the inkjet bioprinting can be divided into two types categorized by the use of either the piezoelectric actuator or the thermal actuator. With multiple inkjets and varied inks containing different cell sources, inkjet bioprinting is capable of composite bioprinting. However, its disadvantages are low cell density and low mechanical properties of the printed structures.
Figure 4.
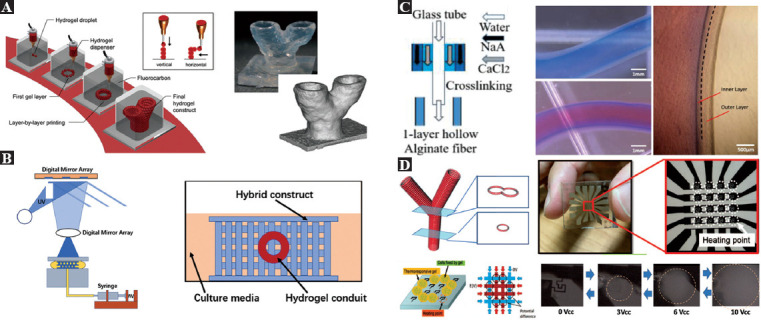
Devices developed for bioprinting microvessels. (A) Microvascular structures could be built by bioprinting based on inkjet, extraction, and direct laser writing (from ref.[77] licensed under Creative Commons Attribution License. Copyright © Mary Ann Liebert). (B) Direct bioprinting of microvascular structures based on the coaxial nozzle, and microscopic view of L929 mouse fibroblasts encapsulated by tubular alginate (Republished with permission from reference[88]). (C) Experimental setup and fabrication of engineered tissues containing microvascular structures using optical stereolithography[93]. (D) Microscopic photograph of a microheater array used to perform thermal stereolithography (Republished with permission, from Kojima M, Horade M, Takata S, et al., IEEE International Conference on Cyborg and Bionic Systems, IEEE, 2018.[97]).
Targeting to printing the materials with better mechanical properties, researchers developed extrusion bioprinting to deposit viscous materials[82]. The extrusion is commonly actuated by air pump screw plunger. Similar to inkjet bioprinting, extrusion bioprinting is also capable of composite bioprinting with multiple bioinks. It also allows high cell density. However, the relatively low cell viability limited its application, since the large pressure is harmful to the living cells[83-85].
Laser-assisted bioprinting is based on laser direct writing and laser-induced transfer techniques[86,87]. The laser-assisted bioprinting devices’ core is a three-layer laser-responsive plate. The plate contains a top energy-absorbing layer, a middle donor layer, and a bottom bioink layer. When the focused laser is at a small local area of the energy-absorbing layer, a small part of the donor layer under the laser exposure will be vaporized to form a high-pressure microbubble. The bubble will impel the bioinks, and the formed small droplet of bioink will fall onto the substrate. In laser-assisted bioprinting, cells are protected from the damages of the high pressure, thereby achieving high cell viability. However, the system is complex and expensive. Its unable to print multiple materials.
5.2. Coaxial nozzle
Considering the special structure of microvessels, it is possible to prepare hydrogel 3D structures with built-in microchannels by controlling the crosslinking time sequence and using the fusion of adjacent hollow filaments. As shown in Figure 4B, a novel 3D bioprinting method was proposed for fabricating cell-loaded built-in hollow hydrogel structures in the study by Arai et al. and Gao et al.[88,89]. The inner diameter of the fabricated microvessels could be smaller than 200 mm and longer than 10 cm. Coaxial nozzles are used to fabricate hollow alginate fibers that are able to move in the XY direction, with a Z-shaped platform, and raw materials such as calcium chloride solution to precisely control the XY direction to determine the coaxial nozzle position for planar feature printing. In contrast to other bioprinting methods used to fabricate built-in microchannel structures, this method allows for simultaneous printing of scaffolds and microchannels[88-90].
5.3. Optical stereolithography
Bioprinting based on stereolithography technique belongs to layer-by-layer printing featuring high efficiency. Stereolithography is a technique applying the selective solidification of curable bioinks[91,92]. As shown in Figure 4C, the digital mirror device (DMD) is most commonly utilized in optical stereolithography to pattern the 2D parallel light. The printing resolution can be as high as 1 mm. Compared with the extrusion-based bioprinting using high pressure, optical stereolithography can achieve relatively higher cell viability. In the process of engineering the microvessels using this method, researchers commonly printed the microvascular structures together with other microarchitectures simultaneously. However, stereolithography originally does not allow the printing of multiple materials. Shanjani and Yan developed special devices to print more than one kind of material[93,94]. The printed artificial tissue including microvascular structure is shown in Figure 4C. However, patterned light utilized in bioprinting based on optical stereolithography requires the bioink to be transparent, and the system is extremely complex and expensive.
5.4. Thermal stereolithography
Thermally responsive gels have the potential to build large artificial tissues with complex architectures in the presence of precise local heating[95-97]. As shown in Figure 4D, to realize layer-by-layer curing and printing of microvessels, a microheater array is fabricated to shape the thermally responsive gel in 2D plane. Specifically, a microheater with a 2D arrangement structure is used as a DMD that allows area-selective heating at any position[79]. Since the temperature distribution on the glass substrate can be digitally processed, when combined with a thermally responsive polymer, its temperature variation can be controlled, and high-speed graphing can be achieved. This system allows reversible pasting and liquefaction at arbitrary locations. The relatively simpler system and low cost make it an extremely promising solution for building microvascular structures.
6. Discussion and prospects
Emerging microfabrication techniques have significantly advanced the bio-assembling for engineering microvessels[28,29,33,39,54-76]. Since the smaller and more precise fabricated micro modular tissues are now available, we can build larger artificial tissues without sacrificing the necessary microarchitectural feature[30,34-44]. The newly developed micromanipulation methods are necessary for the building of microvessels by modules with varied geometries. Fully automated assembly utilizing the robotic micromanipulation, self-assembly, and on-chip assembly by microfluidic devices significantly improve the efficiency of fabricating the microvessels by bio-assembling approaches[61-71].
Engineering microvessels by bioprinting highly relies on the overall development of bioprinting[77-100]. With the advanced bioinks featuring short curing time and better mechanical property, and efficient respective bioprinting devices, we can now achieve fabrication of the microvessels with high throughput[98,99]. Conventional inkjet-based bioprinting holds advantages for printing microvessels together with the other tissues simultaneously with composite bioprinting techniques while the 3D stereolithography can only deal with one bioink containing the same cell source[77-81,91-94,100]. However, 3D stereolithography has incomparable efficiency. Moreover, bioprinting devices specifically designed for printing microvessels are expected to achieve more attractive efficiency[88-90]. The extrusion-based bioprinting has relatively low printing speed and poor cell viability[82-85]. Except the extrusion-based bioprinting, engineering microvessels using bioprinting can achieve satisfied cell viability[98,99]. When the high mechanical property is required, the extrusion-based bioprinting strategies are recommended. A disadvantage of using the inkjet-based bioprinting to engineer microvessels is the weak mechanical property of the fabricated artificial microvessels. The optical stereolithography can guarantee the resolution of printing the microvessels to as low as 1 mm, but the system setup is much more complex than the bioprinting based on inkjet and extrusion. Laser-assisted bioprinting also needs a complex system setup.
Although bio-assembling and bioprinting techniques for engineering microvessels are all based on the “bottom-up” concept[21-32,98,99,101], they still have significant differences in the following aspects: resolution, fabrication efficiency, mechanical property, and complexity of fabricated microvessels. Limited by the speed of integrating the most recent advanced microfabrication techniques into the bioprinting devices, the bio-assembled microvessels still hold a higher resolution than the bioprinted structure, but the gap will be bridged by the effort in developing the bioprinting devices over time[30,34-44,77-97,100]. At present, bioprinting has much higher fabrication efficiency in engineering the microvessels from the bottom up; however, it is not absolute. Recent advances in micromanipulation keep improving the efficiency of the bio-assembling[61-71]. The self-assembly and on-chip assembly achieved satisfied fabrication efficiency but inevitably sacrificed the complexity of the engineered microvessels. Fully automated robotic microassembly provides a solution to the problem caused by the conflict of the fabrication efficiency and the complexity of the engineered microvessels. Although the fabricated micromodules have excellent mechanical properties, the overall mechanical property highly relies on the secondary crosslinking quantity. Moreover, smaller gaps between the assembled micromodules achieved by the precise assembly contribute to better mechanical property[61-67]. The mechanical property of the printed microvessels depends on the utilized bioinks and the respective solidification mechanisms[51-53,98,99]. Sometimes, researchers are in a dilemma of either choosing higher efficiency to reduce the curing time or opting for better mechanical property in the products at the expense of longer curing time. Both bio-assembling and bioprinting hold the potential in constructing complex 3D microvascular networks. Bio-assembling can construct complex microvessels by fabricating the complex 2D modules at the beginning and through further dexterous micromanipulations, while bioprinting realizes this through composite bioprinting techniques.
Recent review articles related with the fabrication of artificial blood vessels mainly focus on artificial vascular grafts required in coronary angioplasty and coronary artery bypass grafting for treating coronary artery disease[20,21,22,98,99,101]. These artificial blood vessels with diameter larger than 2 mm are used for blood circulation instead of delivering nutrients and oxygen at single cell level. Fabricating mass of microvessels required in transporting nutrients, oxygen, and metabolic wastes in the constructed large 3D functional tissues requires the use of more advanced microfabrication techniques. Building the microvascular network using “bottom-up” approach could be appropriate and adequate. Existing methods using the “bottom-up” concept developed to fabricate microvessels can be divided into bio-assembling powered by different micromanipulation techniques and bioprinting utilizing different solidification mechanisms. Existing reviews mainly revolve around the use of bioprinting methods in the fabrication of microvessels, while many research works on bio-assembling for engineering microvessels have not been summarized in any reviews. Our review has summarized all the important bio-assembling methods and bioprinting methods for engineering microvessels and compared them in different aspects.
With regard to the future development of microvessel engineering through bioprinting and bio-assembling methods from the bottom up, we conclude with the future directions as follows: (i) fabricating microvessels across the micro to macro with higher efficiency; (ii) fabricating multilayer structure with stem cells and other cell sources; (iii) using noncytotoxic biodegradable materials with the strong mechanical property; (iv) building microvascular networks and other tissues simultaneously; (v) achieving 3D structure design and optimization of the microvascular network; and (vi) attempting in creating clinically relevant engineered microvessels for implantation and the treatment of disease on a small scale.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grants (61903039, 61873037, 61803250), China Postdoctoral Science Foundation (BX20190035, 2020M680015), the Grant-in-Aid for Scientific Research (JP19H02093, JP19H02097) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Shanghai Science and Technology Committee Rising-Star Program (19QA1403700).
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
X.L. conceived the ideas and drafted the manuscript. Q.H. and T.A. advised the organization of the main contents. M.K. and T.Y. collected the detailed research results.
References
- 1.Hillsley MV, Frangos JA. Bone Tissue Engineering:The Role of Interstitial Fluid Flow. Biotechnol Bioeng. 1994;43:573–81. doi: 10.1002/bit.260430706. http://doi.org/10.1002/bit.260430706. [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound Healing--Aiming for Perfect Skin Regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. http://doi.org/10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Bello YM, Falabella AF, Eaglstein WH. Tissue-Engineered Skin. Current Status in Wound Healing. Am J Clin Dermatol. 2001;2:305–13. doi: 10.2165/00128071-200102050-00005. http://doi.org/10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. http://doi.org/10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 5.Stock UA, Vacanti JP. Tissue Engineering:Current State and Prospects. Annu Rev Med. 2001;52:443–51. doi: 10.1146/annurev.med.52.1.443. http://doi.org/10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 6.Vacanti CA. The History of Tissue Engineering. J Cell Mol Med. 2006;10:569–76. doi: 10.1111/j.1582-4934.2006.tb00421.x. http://doi.org/10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aikawa E, Nahrendorf M, Sosnovik D, et al. Multimodality Molecular Imaging Identifies Proteolytic and Osteogenic Activities in Early Aortic Valve Disease. Circulation. 2007;115:377–86. doi: 10.1161/CIRCULATIONAHA.106.654913. http://doi.org/10.1161/circulationaha.106.654913. [DOI] [PubMed] [Google Scholar]
- 8.Walther G, Gekas J, Bertrand OF. Amniotic Stem Cells for Cellular Cardiomyoplasty:Promises and Premises. Catheter Cardiovasc Interv. 2009;73:917–24. doi: 10.1002/ccd.22016. http://doi.org/10.1002/ccd.22016. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Yan, Y, Zhang R. Gelatin-Based Hydrogels for Controlled Cell Assembly. New York: Springer; 2010. [Google Scholar]
- 10.Wang X. Intelligent Freeform Manufacturing of Complex Organs. Artif Organs. 2012;36:951–61. doi: 10.1111/j.1525-1594.2012.01499.x. http://doi.org/10.1111/j.1525-1594.2012.01499.x. [DOI] [PubMed] [Google Scholar]
- 11.Orive G, Hernández RM, Rodríguez Gascón A, et al. History, Challenges and Perspectives of Cell Microencapsulation. Trends Biotechnol. 2004;22:87–92. doi: 10.1016/j.tibtech.2003.11.004. http://doi.org/10.1016/j.tibtech.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Khademhosseini A, Langer R, Borenstein J, et al. Microscale Technologies for Tissue Engineering and Biology. Proc Natl Acad Sci U S A. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. http://doi.org/10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung BG, Kang L, Khademhosseini A. Micro-and Nanoscale Technologies for Tissue Engineering and Drug Discovery Applications. Expert Opin Drug Discov. 2007;2:1653–68. doi: 10.1517/17460441.2.12.1653. http://doi.org/10.1517/17460441.2.12.1653. [DOI] [PubMed] [Google Scholar]
- 14.Miller JS, Stevens KR, Yang MT, et al. Rapid Casting of Patterned Vascular Networks for Perfusable Engineered Three-dimensional Tissues. Nat Mater. 2012;11:768–74. doi: 10.1038/nmat3357. http://doi.org/10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue T, Zhao D, Phan DT, et al. A Modular Microfluidic System Based on a Multilayered Configuration to Generate Large-Scale Perfusable Microvascular Networks. Microsyst Nanoeng. 2021;7:4. doi: 10.1038/s41378-020-00229-8. http://doi.org/10.1038/s41378-020-00229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niklason LE, Gao J, Abbott WM, et al. Functional Arteries Grown In Vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. http://doi.org/10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 17.Gooch KJ, Blunk T, Courter DL, et al. Bone Morphogenetic Proteins-2, -12, and -13 Modulate In Vitro Development of Engineered Cartilage. Tissue Eng. 2002;8:591–601. doi: 10.1089/107632702760240517. http://doi.org/10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 18.Tranquillo RT. The Tissue-engineered Small-diameter Artery. Ann N Y Acad Sci. 2002;961:251–4. doi: 10.1111/j.1749-6632.2002.tb03094.x. http://doi.org/10.1111/j.1749-6632.2002.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 19.Boublik J, Park H, Radisic M, et al. Mechanical Properties and Remodeling of Hybrid Cardiac Constructs Made From Heart Cells, Fibrin, and Biodegradable, Elastomeric Knitted Fabric. Tissue Eng. 2005;11:1122–32. doi: 10.1089/ten.2005.11.1122. http://doi.org/10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- 20.Saito J, Kaneko M, Ishikawa Y, et al. Challenges and Possibilities of Cell-Based Tissue-Engineered Vascular Grafts. Cyborg Bionic Syst, 2021. 2021:1532103. doi: 10.34133/2021/1532103. http://doi.org/10.34133/2021/1532103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng HY, Lee KA, Kuo CN, et al. Bioprinting of Artificial Blood Vessels. Int J Bioprint. 2018;4:140. doi: 10.18063/IJB.v4i2.140. http://doi.org/10.18063/IJB.v4i2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai T, Arai F, Yamato M. Hyper Bio Assembler for 3D Cellular Systems. Japan: Springer; 2015. https://doi.org/10.1007/978-4-431-55297-0. [Google Scholar]
- 23.Yang J, Yamato M, Sekine H, et al. Tissue Engineering Using Laminar Cellular Assemblies. Adv Mater. 2009;21:3404–9. doi: 10.1002/adma.200801990. http://doi.org/10.1002/adma.200801990. [DOI] [PubMed] [Google Scholar]
- 24.Kinstlinger IS, Saxton SH, Calderon GA, et al. Generation of Model Tissues with Dendritic Vascular Networks via Sacrificial Laser-sintered Carbohydrate Templates. Nat Biomed Eng, 2020. 2020:1–17. doi: 10.1038/s41551-020-0566-1. https://doi.org/10.1038/s41551-020-0566-1. [DOI] [PubMed] [Google Scholar]
- 25.Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs. Lab A Chip. 2014;14:2202–11. doi: 10.1039/c4lc00030g. https://doi.org/10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsunaga YT, Morimoto Y, Takeuchi S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Adv Mater. 2011;23:H90–4. doi: 10.1002/adma.201004375. http://doi.org/10.1002/adma.201004375. [DOI] [PubMed] [Google Scholar]
- 27.Tan A, Fujisawa K, Yukawa Y, et al. Bottom-up Fabrication of Artery-Mimicking Tubular Co-cultures in Collagen-based Microchannel Scaffolds. Biomater Sci. 2016;4:1503–14. doi: 10.1039/c6bm00340k. http://doi.org/10.1039/c6bm00340k. [DOI] [PubMed] [Google Scholar]
- 28.Nichol JW, Khademhosseini A. Modular Tissue Engineering:Engineering Biological Tissues from the Bottom Up. Soft Matter. 2009;5:1312–9. doi: 10.1039/b814285h. http://doi.org/10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurkan UA, Tasoglu S, Kavaz D, et al. Emerging Technologies for Assembly of Microscale Hydrogels. Adv Healthc Mater. 2012;1:149–58. doi: 10.1002/adhm.201200011. https://doi.org/10.1002/adhm.201200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onoe H, Okitsu T, Itou A, et al. Metre-long Cell-laden Microfibres Exhibit Tissue Morphologies and Functions. Nat Mater. 2013;12:584–90. doi: 10.1038/nmat3606. https://doi.org/10.1038/nmat3606. [DOI] [PubMed] [Google Scholar]
- 31.Connon CJ. Approaches to Corneal Tissue Engineering:Top-down or Bottom-up? Proc Eng. 2015;110:15–20. https://doi.org/10.1016/j.proeng.2015.07.004. [Google Scholar]
- 32.Bova L, Billi F, Cimetta E. Mini-review:Advances in 3D Bioprinting of Vascularized Constructs. Biol Direct. 2020;15:22. doi: 10.1186/s13062-020-00273-4. http://doi.org/10.1186/s13062-020-00273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mironov V, Visconti RP, Kasyanov V, et al. Organ Printing:Tissue Spheroids as Building Blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. http://doi.org/10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fennema E, Rivron N, Rouwkema J, et al. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013;31:108–15. doi: 10.1016/j.tibtech.2012.12.003. https://doi.org/10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Rivron NC, Vrij EJ, Rouwkema J, et al. Tissue Deformation Spatially Modulates VEGF Signaling and Angiogenesis. Proc Natl Acad Sci. 2012;109:6886–91. doi: 10.1073/pnas.1201626109. https://doi.org/10.1073/pnas.1201626109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torisawa YS, Chueh BH, Huh D, et al. Efficient Formation of Uniform-sized Embryoid Bodies Using a Compartmentalized Microchannel Device. Lab Chip. 2007;7:770–6. doi: 10.1039/b618439a. https://doi.org/10.1039/B618439A. [DOI] [PubMed] [Google Scholar]
- 37.Tamayol A, Akbari M, Annabi N, et al. Fiber-based Tissue Engineering:Progress, Challenges, and Opportunities. Biotechnol Adv. 2013;31:669–87. doi: 10.1016/j.biotechadv.2012.11.007. https://doi.org/10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teshima T, Onoe H, Kuribayashiashiashias K, et al. Parylene Mobile Microplates Integrated with an Enzymatic Release for Handling of Single Adherent Cells. Small. 2014;10:912–21. doi: 10.1002/smll.201301993. https://doi.org/10.1002/smll.201301993. [DOI] [PubMed] [Google Scholar]
- 39.Kuribayashi-Shigetomi K, Onoe H, Takeuchi S. Cell Origami:Self-Folding of Three-Dimensional Cell-Laden Microstructures Driven by Cell Traction Force. PLoS One. 2012;7:e51085. doi: 10.1371/journal.pone.0051085. https://doi.org/10.1371/journal.pone.0051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue, T, Nakajima M, Tajima H, et al. Fabrication of Microstructures Embedding Controllable Particles inside Dielectrophoretic Microfluidic Devices. Int J Adv Robot Syst. 2013;10:132–40. https://doi.org/10.5772/55598. [Google Scholar]
- 41.Gach PC, Wang Y, Phillips C, et al. Isolation and Manipulation of Living Adherent Cells by Micromolded Magnetic Rafts. Biomicrofluidics. 2011;5:32002–12. doi: 10.1063/1.3608133. https://doi.org/10.1063/1.3608133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Takeuchi M, Nakajima M, et al. Batch Fabrication of Microscale Gear-Like Tissue by Alginate-Poly-L-lysine (PLL) Microcapsules System. IEEE Robot Autom Lett. 2016;1:206–12. https://doi.org/10.1109/lra.2016.2514500. [Google Scholar]
- 43.Yokoyama U, Tonooka Y, Koretake R, et al. Arterial Graft with Elastic Layer Structure Grown From Cells. Sci Rep. 2017;7:140–55. doi: 10.1038/s41598-017-00237-1. https://doi.org/10.1038/s41598-017-00237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinds MT, Rowe RC, Ren Z, et al. Development of a Reinforced Porcine Elastin Composite Vascular Scaffold. J Biomed Mater Res Part A. 2006;77A:458–69. doi: 10.1002/jbm.a.30571. https://doi.org/10.1002/jbm.a.30571. [DOI] [PubMed] [Google Scholar]
- 45.Rutz AL, Lewis PL, Shah RN. Toward Next-generation Bioinks:Tuning Material Properties Pre-and Post-printing to Optimize Cell Viability. MRS Bull. 2017;42:563–70. https://doi.org/10.1557/mrs.2017.162. [Google Scholar]
- 46.Mobaraki M, Ghaffari M, Yazdanpanah A, et al. Bioinks and Bioprinting:A Focused Review. Bioprinting. 2020;18:e00080–95. https://doi.org/10.1016/j.bprint.2020.e00080. [Google Scholar]
- 47.Jia J, Richards DJ, Pollard S, et al. Engineering Alginate as Bioink for Bioprinting. Acta Biomater. 2014;10:4323–31. doi: 10.1016/j.actbio.2014.06.034. https://doi.org/10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guvendiren M, Molde J, Soares RM, et al. Designing Biomaterials for 3D Printing. ACS Biomater Sci Eng. 2016;2:1679–93. doi: 10.1021/acsbiomaterials.6b00121. https://doi.org/10.1021/acsbiomaterials.6b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hospodiuk M, Dey M, Sosnoski D, et al. The Bioink:A Comprehensive Review on Bioprintable Materials. Biotechnol Adv. 2017;35:217–39. doi: 10.1016/j.biotechadv.2016.12.006. https://doi.org/10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Guillen MC, Turnay J, Fernandez-Dıaz MD, et al. Structural and Physical Properties of Gelatin Extracted from Different Marine Species:A Comparative Study. Food Hydrocoll. 2002;16:25–34. https://doi.org/10.1016/S0268-005X(01)00035-2. [Google Scholar]
- 51.Rowley JA, Madlambayan G, Mooney DJ. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. https://doi.org/10.1016/S0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 52.Demirtas TT, Irmak G, Gümüs M, et al. A Bioprintable form of Chitosan Hydrogel for Bone Tissue Engineering. Biofabrication. 2017;9:35003. doi: 10.1088/1758-5090/aa7b1d. https://doi.org/10.1088/1758-5090/aa7b1d. [DOI] [PubMed] [Google Scholar]
- 53.Loo Y, Hauser CA. Bioprinting Synthetic Self-assembling Peptide Hydrogels for Biomedical Applications. Biomed Mater. 2015;11:14103. doi: 10.1088/1748-6041/11/1/014103. https://doi.org/10.1088/1748-6041/11/1/014103. [DOI] [PubMed] [Google Scholar]
- 54.Du Y, Lo E, Ali S, et al. Directed Assembly of Cell-laden Microgels for Fabrication of 3D Tissue Constructs. Proc Natl Acad Sci. 2008;105:9522–7. doi: 10.1073/pnas.0801866105. https://doi.org/10.1073/pnas.0801⇕05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elbert DL. Bottom-up Tissue Engineering. Curr Opin Biotechnol. 2011;22:674–80. doi: 10.1016/j.copbio.2011.04.001. https://doi.org/10.1016/j.copbio.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasza KE, Rowat AC, Liu J, et al. The Cell as a Material. Curr Opin Cell Biol. 2007;19:101–7. doi: 10.1016/j.ceb.2006.12.002. https://doi.org/10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Avci E, Ohara K, Nguyen CN, et al. High-Speed Automated Manipulation of Microobjects Using a Two-Fingered Microhand. IEEE Trans Ind Electron. 2015;62:1070–9. https://doi.org/10.1109/TIE.2014.2347004. [Google Scholar]
- 58.Ramadan AA, Takubo T, Mae Y, et al. Developmental Process of a Chopstick-Like Hybrid-Structure Two-Fingered Micromanipulator Hand for 3-D Manipulation of Microscopic Objects. IEEE Trans Ind Electron. 2009;56:1121–35. https://doi.org/10.1109/TIE.2008.200∱. [Google Scholar]
- 59.L'Heureux N, Quet SP, Labbe R, et al. A Completely Biological Tissue-Engineered Human Blood Vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. https://doi.org/10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 60.Bourget JM, Gauvin R, Larouche D, et al. Human Fibroblast-derived ECM as a Scaffold for Vascular Tissue Engineering. Biomaterials. 2012;33:9205–13. doi: 10.1016/j.biomaterials.2012.09.015. https://doi.org/10.1016/j.biomaterials.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Du Y, Ghodousi M, Qi H, et al. Sequential Assembly of Cell-laden Hydrogel Constructs to Engineer Vascular-like Microchannels. Biotechnol Bioeng. 2011;108:1693–703. doi: 10.1002/bit.23102. https://doi.org/10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Huang Q, Shi Q, et al. Automated Assembly of Vascular-like Microtube with Repetitive Single-step Contact Manipulation. IEEE Trans Biomed Eng. 2015;62:2620–8. doi: 10.1109/TBME.2015.2437952. https://doi.org/10.1109/TBME.2015.2437952. [DOI] [PubMed] [Google Scholar]
- 63.Yue T, Nakajima M, Takeuchi M, et al. On-chip Self-assembly of Cell Embedded Microstructures to Vascular-like Microtubes. Lab Chip. 2014;14:1151. doi: 10.1039/c3lc51134k. https://doi.org/10.1039/c3lc51134k. [DOI] [PubMed] [Google Scholar]
- 64.Yue T, Liu N, Liu Y, et al. On-Chip Construction of Multilayered Hydrogel Microtubes for Engineered Vascular-Like Microstructures. Micromachines. 2019;10:840. doi: 10.3390/mi10120840. https://doi.org/10.3390/mi10120840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Shi Q, Wang H, et al. Automated Fluidic Assembly of Microvessel-Like Structures Using a Multimicromanipulator System. IEEE/ASME Trans Mechatron. 2018;23:667–78. https://doi.org/10.1109/TMECH.2018.2796182. [Google Scholar]
- 66.Sun T, Shi Q, Liang Q, et al. Fabrication of Vascular Smooth Muscle-like Tissues Based on Self-organization of Circumferentially Aligned Cells in Microengineered Hydrogels. Lab Chip. 2020;20:3120–31. doi: 10.1039/d0lc00544d. https://doi.org/10.1039/D0LC00544D. [DOI] [PubMed] [Google Scholar]
- 67.Sun T, Wang H, Shi Q, et al. Micromanipulation for Coiling Microfluidic Spun Alginate Microfibers by Magnetically Guided System. IEEE Robot Autom Lett. 2016;1:808–13. https://doi.org/10.1109/LRA.2016.2524991. [Google Scholar]
- 68.Sun T, Shi Q, Huang Q, et al. Magnetic Alginate Microfibers as Scaffolding Elements for the Fabrication of Microvascular-like Structures. Acta Biomater. 2017;66:272–81. doi: 10.1016/j.actbio.2017.11.038. https://doi.org/10.1016/j.actbio.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 69.Sun T, Huang Q, Shi Q, et al. Magnetic Assembly of Microfluidic Spun Alginate Microfibers for Fabricating Three-dimensional Cell-laden Hydrogel Constructs. Microfluid Nanofluid. 2015;19:1169–80. https://doi.org/10.1007/s10404-015-1633-x. [Google Scholar]
- 70.Sun T, Shi Q, Yao Y, et al. Engineered Tissue Micro-rings Fabricated from Aggregated Fibroblasts and Microfibres for a Bottom-up Tissue Engineering Approach. Biofabrication. 2019;11:035029. doi: 10.1088/1758-5090/ab1ee5. https://doi.org/10.1088/1758-5090/ab1ee5. [DOI] [PubMed] [Google Scholar]
- 71.Sun T, Yao Y, Shi Q, et al. Template-based Fabrication of Spatially Organized 3D Bioactive Constructs Using Magnetic Low-concentration Gelation Methacrylate (GelMA) Microfibers. Soft Matter. 2020;16:3902–13. doi: 10.1039/c9sm01945f. https://doi.org/10.1039/C9SM01945F. [DOI] [PubMed] [Google Scholar]
- 72.Onoe H, Takeuchi S. Microfabricated Mobile Microplates for Handling Single Adherent Cells. J Micromech Microeng. 2008;18:095003. https://doi.org/10.1088/0960-1317/18/9/095003. [Google Scholar]
- 73.Yoshida S, Sato K, Takeuchi S. Three-Dimensional Microassembly of Cell-Laden Microplates by In Situ Gluing with Photocurable Hydrogels. Int J Automation Technol. 2014;8(1):95–101. https://doi.org/10.20965/ijat.2014.p0095. [Google Scholar]
- 74.Jiang X, Ferrigno R, Mrksich M, et al. Electrochemical Desorption of Self-Assembled Monolayers Noninvasively Releases Patterned Cells from Geometrical Confinements. J Am Chem Soc. 2016;125:2366–7. doi: 10.1021/ja029485c. https://doi.org/10.1021/ja029485c. [DOI] [PubMed] [Google Scholar]
- 75.Inaba R, Khademhosseini A, Suzuki H, et al. Electrochemical Desorption of Self-assembled Monolayers for Engineering Cellular Tissues. Biomaterials. 2009;30:3573–9. doi: 10.1016/j.biomaterials.2009.03.045. https://doi.org/10.1016/j.biomaterials.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 76.Seto Y, Inaba R, Okuyama T, et al. Engineering of Capillary-like Structures in Tissue Constructs by Electrochemical Detachment of Cells. Biomaterials. 2010;31:2209–15. doi: 10.1016/j.biomaterials.2009.11.104. https://doi.org/10.1016/j.biomaterials.2009.11.104. [DOI] [PubMed] [Google Scholar]
- 77.Blaeser A, Campos DF, Weber M, et al. Biofabrication Under Fluorocarbon:A Novel Freeform Fabrication Technique to Generate High Aspect Ratio Tissue-Engineered Constructs. Biores Open Access. 2013;2:374–84. doi: 10.1089/biores.2013.0031. https://doi.org/10.1089/biores.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saunders RE, Derby B. Inkjet Printing Biomaterials for Tissue Engineering:Bioprinting. Int Mater Rev. 2014;59:430–48. https://doi.org/10.1179/1743280414Y.0000000040. [Google Scholar]
- 79.Ramiah P, du Toit LC, Choonara YE, et al. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front Mater. 2020;7:76. https://doi.org/10.3389/fmats.2020.00076. [Google Scholar]
- 80.Liu X, Wang X, Zhang L, et al. A Novel Method for Generating 3D Constructs with Branched Vascular Networks Using Multi-materials Bioprinting and Direct Surgical Anastomosis. bioRxiv. 2021;3:436268. https://doi.org/10.1101/2021.03.21.436268. [Google Scholar]
- 81.Kolesky DB, Truby RL, Gladman AS, et al. Bioprinting: 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv Mater. 2014;26:3124–30. doi: 10.1002/adma.201305506. https://doi.org/10.1002/adma.201470124. [DOI] [PubMed] [Google Scholar]
- 82.Iwan Z, Dietmar WH, et al. Fused Deposition Modeling of Novel Scaffold Architectures for Tissue Engineering Applications. Biomaterials. 2002;23:1169–85. doi: 10.1016/s0142-9612(01)00232-0. https://doi.org/10.1016/S0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim TO, Monika H. Current Advances and Future Perspectives in Extrusion-based Bioprinting. Biomaterials. 2016;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 84.Fielding GA, Bandyopadhyay A, Bose S. Effects of Silica and Zinc Oxide Doping on Mechanical and Biological Properties of 3D Printed Tricalcium Phosphate Tissue Engineering Scaffolds. Dent Mater. 2012;28:113–22. doi: 10.1016/j.dental.2011.09.010. https://doi.org/10.1016/j.dental.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Huang X, Shen Y, et al. Direct Writing Alginate Bioink Inside Pre-polymers of Hydrogels to Create Patterned Vascular Networks. J Mater Sci. 2019;54:883–7892. https://doi.org/10.1007/s10853-019-03447-2. [Google Scholar]
- 86.Bohandy J, Kim BF, Adrian FJ. Metal Deposition from a Supported Metal Film Using an Excimer Laser. J Appl Phys. 1986;60:1538–9. https://doi.org/10.1063/1.337287. [Google Scholar]
- 87.Duocastella M, Colina M, Fernández-Pradas JM, et al. Study of the Laser-induced Forward Transfer of Liquids for Laser Bioprinting. Appl Surf Sci. 2007;253:7855–9. https://doi.org/10.1016/j.apsusc.2007.02.097. [Google Scholar]
- 88.Kihara T, Kojima M, Horade M, et al. A Channel Device Generating Multilayer Tubular Structure In Situ Delivering Nutrients. The Proceedings of JSME annual Conference on Robotics and Mechatronics (Robomec) 2016:p2A2–19b5. https://doi.org/10.1299/jsmermd.2016.2A2-19b5. [Google Scholar]
- 89.Gao Q, He Y, Fu J, et al. Coaxial Nozzle-assisted 3D Bioprinting with Built-in Microchannels for Nutrients Delivery. Biomaterials. 2015;61:203–15. doi: 10.1016/j.biomaterials.2015.05.031. https://doi.org/10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 90.Li S, Wang K, Jiang X, et al. Rapid Fabrication of Ready-to-Use Gelatin Scaffolds with Prevascular Networks using Alginate Hollow Fibers as Sacrificial Templates. ACS Biomater Sci Eng. 2020;6:2297–311. doi: 10.1021/acsbiomaterials.9b01834. https://doi.org/10.1021/acsbiomaterials.9b01834. [DOI] [PubMed] [Google Scholar]
- 91.Gauvin R, Chen YC, Lee JW, et al. Microfabrication of Complex Porous Tissue Engineering Scaffolds using 3D Projection Stereolithography. Biomaterials. 2012;33:3824–34. doi: 10.1016/j.biomaterials.2012.01.048. https://doi.org/10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raman R, Bhaduri B, Mir M, et al. High-Resolution Projection Microstereolithography for Patterning of Neovasculature. Adv Healthc Mater. 2016;5:610–9. doi: 10.1002/adhm.201500721. https://doi.org/10.1002/adhm.201500721. [DOI] [PubMed] [Google Scholar]
- 93.Shanjani Y, Pan CC, Elomaa L, et al. A Novel Bioprinting Method and System for Forming Hybrid Tissue Engineering Constructs. Biofabrication. 2015;7:045008. doi: 10.1088/1758-5090/7/4/045008. https://doi.org/10.1088/1758-5090/7/4/045008. [DOI] [PubMed] [Google Scholar]
- 94.Yan J, Huang Y, Chrisey DB. Laser-assisted Printing of Alginate Long Tubes and Annular Constructs. Biofabrication. 2012;5:015002. doi: 10.1088/1758-5082/5/1/015002. https://doi.org/10.1088/1758-5082/5/1/015002. [DOI] [PubMed] [Google Scholar]
- 95.Tsuda Y, Yamato M, Kikuchi A, et al. Thermoresponsive Microtextured Culture Surfaces Facilitate Fabrication of Capillary Networks. Adv Mater. 2007;19:3633–6. https://doi.org/10.1002/adma.200700988. [Google Scholar]
- 96.Horade M, Kojima M, Kamiyama K, et al. Development of Micro-heater Array Device with Regional Selective Heating for Biochemical Applications. International Conference 2014 [Google Scholar]
- 97.Kojima M, Horade M, Takata S, et al. Development of Micro Heater Array System for Cell Manipulation. IEEE International Conference on Cyborg and Bionic Systems, IEEE 2018 [Google Scholar]
- 98.Heinrich MA, Liu W, Jimenez A, et al. 3D Bioprinting:From Benches to Translational Applications. Small. 2019;15:1805510. doi: 10.1002/smll.201805510. http://dx.doi.org/10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mandrycky C, Wang Z, Kim K, et al. 3D Bioprinting for Engineering Complex Tissues. Biotechnol Adv. 2016;34:422–34. doi: 10.1016/j.biotechadv.2015.12.011. https://doi.org/10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular Networks and Functional Intravascular Topologies Within Biocompatible Hydrogels. Science. 2019;364:458–64. doi: 10.1126/science.aav9750. http://dx.doi.org/10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu LB, Wang XH. Creation of a Vascular System for Organ Manufacturing. Int J Bioprint. 2015;1:77–86. http://dx.doi.org/10.18063/IJB.2015.01.009. [Google Scholar]


