Abstract
Organoids developed from pluripotent stem cells or adult stem cells are three-dimensional cell cultures possessing certain key characteristics of their organ counterparts, and they can mimic certain biological developmental processes of organs in vitro. Therefore, they have promising applications in drug screening, disease modeling, and regenerative repair of tissues and organs. However, the construction of organoids currently faces numerous challenges, such as breakthroughs in scale size, vascularization, better reproducibility, and precise architecture in time and space. Recently, the application of bioprinting has accelerated the process of organoid construction. In this review, we present current bioprinting techniques and the application of bioinks and summarize examples of successful organoid bioprinting. In the future, a multidisciplinary combination of developmental biology, disease pathology, cell biology, and materials science will aid in overcoming the obstacles pertaining to the bioprinting of organoids. The combination of bioprinting and organoids with a focus on structure and function can facilitate further development of real organs.
Keywords: Bioprinting, Organoid, Tissue engineering, Organ development
1. Introduction
An organoid is a three-dimensional (3D) model, similar to the source tissue or organ in vivo, of an in vitro cell culture system.[1]. In addition, an organoid is a collection of organ-specific cells that are developed from pluripotent stem cells (PSCs) or adult stem cells (AdSCs). They can self-form in a similar manner to the body through cell sorting and spatially restricted lineage differentiation[2-4] (Figure 1). At present, animal models are typically used for experiments, such as drug screening, disease modeling, and tissue regeneration and repair; however, such models do not accurately reflect the physiological characteristics of the human body[5]. The new in vitro model organoid bridges the gap between animal models and humans by replicating the cellular composition and behavior of a normal organism meticulously to recreate the physiological structure of human organs. The construction of organoids offers the advantages of individualization, short modeling times, high-throughput genetic or drug screening, and the possibility of gene editing[6]. The organoid compensates for the shortcomings of simple cellular models and complex animal models, and provides an important experimental basis for studying key functions of living organisms. Organoids have become a current research hotspot with great theoretical significance and broad development prospects in basic biology research, drug testing, and molecular medicine[6]. However, the construction of organoids poses certain limitations, such as the inability to fully simulate the in vivo microenvironment, inadequate vascularization, the slightly different size of the self-organization of organoids from that of normal organs and lack of precise spatial ordering, and unestablished co-culture system with other types of cells. Recent bioprinting techniques have been used to overcome some of these shortcomings.
Figure 1.
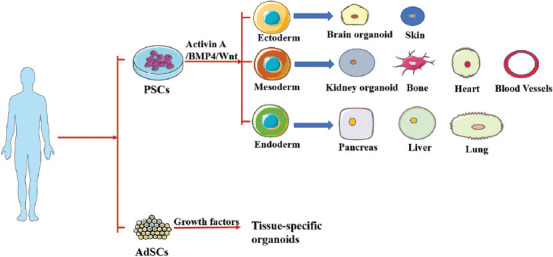
Organoids can be identified based on PSCs or AdSCs that are created autotrophically. PSC-derived organoids need to pass through the endoderm, mesoderm, or ectoderm, and then be induced and matured by applying certain growth signals and then differentiate into the phenotype required for a particular organ. A few PSC-derived organoids may be determined from numerous distinctive germ layers of cells. AdSC-derived organoids require segregation of tissue-specific stem cell populations, which are at that point implanted in extracellular matrix (ECM) and engendered in combination with particular tissue development components.
Bioprinting is an additive manufacturing technology that can design and selectively distribute cells, bioactive materials, and cytokines to construct 3D living organs and tissues[7]. Bioprinting defines two types of fabricated structural properties or smart surface properties of cell-free constructs characterized by layering, which guides cellular activity and cell-filled biological constructs[7]. Before the rise of biomanufacturing technologies, the fusion of developmental biology and cellular self-organization has emerged as a new paradigm for advancing tissue engineering. The formation of self-organizing multicellular modules is a key step in this technology; however, the ability to assemble intermediate modules into larger tissue units in a controlled manner is a major challenge[8]. Thus, the synergistic development of bioprinting and cellular self-organization technologies, working in tandem, can significantly facilitate the functionalization of organ tissues. Bioprinting can apply a specific spatial architecture design similar to actual organs for PSCs or AdSCs so that the specific structure of organoids can be quickly printed with high-precision and high-throughput. Accordingly, they can self-organize better and faster to form organoids. In addition, stem cell suspensions can self-organize into millimeter-scale structures, which contain only moderate complexity, and can be further printed into more complex tissues and organs, with the use of the resulting geometry to guide organoid formation.
Here, we review existing bioprinting methods and bioinks, highlight the recent success stories in the field of organ bioprinting, and summarize strategies and possible directions for future organoid bioprinting. Organoid construction and bioprinting have become research hotspots in the scientific community, and they can facilitate the development of truly artificial organs in the future, signifying a substantial step forward in the field of regenerative medicine.
2. Technologies of organoid bioprinting
3D printing is also known as a layer-by-layer stacking, additive manufacturing method, and the printing technology associated with cell printing is known as 3D bioprinting. According to different molding principles and printing materials, existing bioprinting methods can be classified into extrusion bioprinting (pneumatic, piston, and spiral), inkjet bioprinting (temperature control and piezoelectric), laser-assisted bioprinting, and photo-curing bioprinting. Biological 3D printing techniques use biomaterials, cells, and/or cytokines as bio-inks to build human tissues and organs. However, to date, no biological printing technology can produce synthetic tissues of all sizes and complexities. The aforementioned four primary biological printing techniques pose certain advantages, disadvantages, and limitations (Table 1).
Table 1.
Performance comparison between inkjet-based bioprinting, laser-based assisted bioprinting, extrusion-based bioprinting, and photo-curing bioprinting.
| Inkjet-based bioprinting | Laser-assisted bioprinting | Extrusion-based bioprinting | Photo-curing bioprinting | |
|---|---|---|---|---|
| Cost | Low | High | Medium | Low |
| Speed | Fast | Medium | Slow | Fast |
| Resolution | 75 µm | 10-100 µm | 200-500 µm | 50 µm |
| Cell density | 106-107 cells/ml | >108 cells/ml | 108-109cells/ml | >107 cells/ml |
| Cell Viability | High | High (>95%) | Low | High |
| Nozzle | Exist | No | Exist | No |
| Features | • Piezoelectric or thermodynamic driven nozzles • Can be equipped with multiple nozzles • Requires the printed biomaterial to be in liquid form |
• No clogging of the cell/biomaterial nozzle • Does not cause mechanical damage to cells, thus increasing cell survival rates (typically above 95%) • Multiple biomaterials can be used to print high-viscosity bioinks |
• Printable high-viscosity biomaterials • Wide range of printable biocompatible materials • Most commonly used |
• Selective cross-linking of bioink using light and layer-by-layer solidification to form a 3D structure • High efficiency • Simpler device • Easy to control |
| Limitations | • Low drive pressure • Cannot print high-viscosity materials or high concentration of cells • May cause mechanical or thermal damage to cells during printing • Print head is also prone to wear and clogging |
• Complex control of laser printing systems • Few hydrogel materials suitable for laser bioprinting • Low printing efficiency • Side effects of laser irradiation on cells are not fully understood |
• High mechanical pressure and shear stress • Relatively low cell viability |
• Ultraviolet light and its initiator can damage cells |
2.1. Inkjet-based bioprinting
The inkjet-based bioprinting method was the first bioprinting method used to print cells[9]. It is a contactless printing process based on traditional inkjet printing technology, which uses piezoelectric or thermal driving nozzles to form a series of liquid droplets according to a predetermined 3D structure of biological ink (a mixture of hydrogels and cells). Inkjet-based bioprinting has the advantages of high cell activity, fast printing speed, higher resolution, and low cost[10,11]. In addition, inkjet-based bioprinting can use multiple nozzles simultaneously, enabling the simultaneous printing of different bioactive materials, cells, or cytokines. Using inkjet-based bioprinting technology, scientists have made significant progress in drawing the patterns of molecules, cells, and organs. Researchers have reported the use of inkjet-based bioprinting and fibroblasts to design curved, vascular-like suspension structures without scaffolds[12]. A method of applying high-throughput inkjet printing to control cell attachment and proliferation through precise, automated deposition of collagen was also reported[13]. However, due to the low driving pressure, inkjet biological printing cannot print high-viscosity materials or high-concentrations of cells. Therefore, it is often difficult to fabricate complex biomimetic tissues or organoids with high cell density using the inkjet bioprinting method. Low-viscosity biomaterials can reduce the mechanical properties of bioprinted structures but fail to provide a normal or similar physiological environment for cells to the extent that subsequent in vitro and in vivo cultures are less effective. In addition, the nozzles are prone to wear and clogging during inkjet bioprinting, and cells may also suffer thermal or mechanical damage, limiting the widespread use of inkjet-based bioprinting technologies.
2.2. Laser-assisted bioprinting
Laser-assisted bioprinting utilizes laser direct-write and laser-induced transfer technologies[14]. A focused laser pulse is used to generate high-pressure bubbles on the ribbon absorption layer, and the suspended bioink is pushed onto the receiving substrate and then cross-linked. Compared with other printing techniques, non-nozzle printing methods, such as laser-assisted bioprinting, can avoid direct contact between the inkjet and bioink, thereby preventing the cell/biomaterial from clogging the nozzle and mechanical damage to the cell[15]. Thus, laser-assisted bioprinting allows the printing of highly viscous biomaterials as well as printing with a high cell density. The constructed organoids exhibit high cellular activity, high cell density, and improved functionality. The use of laser-based bioprinting to prepare 3D patterns for spinal cord repair with axon-like extensions and high cellular activity has been reported[16]. Moreover, laser-assisted bioprinting has been used to deposit human umbilical vein endothelial cells (HUVECs) onto the surface of biopaper using a simple crossover technique; these cells were differentiated and stretched into a network of vascularized tissues[17]. However, laser-assisted bioprinting pose several shortcomings. First, laser-assisted bioprinting devices are difficult to commercialize because the cost is relatively high, the control of the laser printing system is complex, and there are limited hydrogel materials suitable for laser-assisted bioprinting. Second, the printing efficiency is low, and each layer of ink is coated repeatedly. In addition, uniformity cannot be guaranteed, and the process is time-consuming and laborious. This makes it difficult to apply to complex structure printing. Moreover, the side effects of laser exposure on cells are not fully understood, which limits the use of this technology.
2.3. Extrusion-based bioprinting
Extrusion bioprinting is currently the most widely used bioprinting strategy that uses air pressure or mechanical stress to control the extrusion of bioink through a nozzle. It can print high-viscosity biomaterials and high-density cell suspensions[18]. Its greatest advantage is that it features a wide range of printable biocompatible materials, covering biomaterials with viscosities extending from 30 to 6×107 mPa/s, particularly hydrogels with shear diminishing, and fast cross-linking properties. In contrast to the aforementioned two techniques, the biomaterial or cell suspension is squeezed by continuous squeezing pressure to form uninterrupted deposits of fibrillated filaments, rather than just individual droplets, resulting in greater mechanical pressure, and shear stress on biomaterials and cells. Therefore, using this technique can reduce the survival rate of printed cells, which is more obvious when printing bioinks with high cell density. Extrusion bioprinting is currently a common method used to construct organoids, and new bioprinting methods have emerged based on traditional extrusion bioprinting methods. Researchers have built an extrusion bioprinter equipped with two nozzles and a motorized X-Z robot. Using hepatocyte- and fibroblast-loaded GelMA hydrogels, the feasibility of the technique was demonstrated for bioprinting organoids or cellular aggregates that maintained a certain level of cellular activity over time[19]. In addition, a prominent study has recently proposed a printing method referred to as bioprinting-assisted tissue emergence (BATE) that combines an extrusion printing system and a microscope system to build a printing system with its own microscope image for real-time observation and precise control of tissue development temporally and spatially[20].
2.4. Photo-curing bioprinting
Photo-curing bioprinting is a biological 3D printing method based on surface projection, which is now often subdivided into stereolithography (SLA) and digital light processing (DLP). Both methods use light-induced photopolymer molding. SLA applies this molding to light-cure using laser light from point to line and line to surface, while DLP uses a projector to irradiate the photopolymer and light-cure it layer-by-layer[21]. The photo-curing printing device uses a digital light projector to solidify the entire surface of the bioink with high efficiency. Regardless of the complexity of the single-layer structure, the printing time is the same, and the printing accuracy is high[22]. The printer requires only a vertically moving platform. Compared with other methods, the device is relatively simple and easy to control, and the printing mode without nozzles does not cause problems, such as nozzle blockage and shear force affecting cell activity[23]. Photo-curing bioprinting is a promising printing method for cell assemblies and organoid construction because of its ability to guide cell self-organization and relatively controlled differentiation. Creff et al. used SLA technology and a photosensitive polymer hydrogel (polyethylene glycol diacrylate/acrylic acid polymer) that supports the growth of intestinal cell lines to construct intestinal epithelial structures, demonstrating that these structures support small intestinal epithelial cell proliferation and differentiation for 3 weeks[24]. However, the disadvantage is that ultraviolet light and its initiator can damage the cells. Photo-curing bioprinting is a progressively imperative part of cell printing strategies, and it is anticipated to supplant extrusion bioprinting as the foremost standard biological 3D printing in the future.
3. Bioinks for bioprinting organoids
Bioinks are crucial for bioprinting. The ideal bioink should meet certain printability requirements, demonstrate suitable mechanical properties; and possess sufficient levels of biodegradability, biocompatibility, and cytocompatibility. For bioprinting organoids, the bioink is selected based on the printability of the ink and its effect on cell behavior. Printability implies that during bioprinting, bioinks are required to exhibit proper flowability and the capability to quickly mold into a shape after printing and cross-linking (photo cross-linking, chemical cross-linking, and physical cross-linking). Increasing the hydrogel concentration can accelerate the curing time and improve the hydrogel strength, which is conducive for better forming. However, it reduces the gel water content and narrows the micro-pore size inside the gel, which is not conducive for cell survival and deposition of the extracellular matrix (ECM). In addition, different types of bioinks produce cells with different microenvironments, affecting cell proliferation, differentiation, migration, and self-organization. Therefore, bioinks have a suitable printing window for printing complex geometric organ shapes.
Both natural and synthetic polymeric materials can be used as raw materials for bioprinting. The commonly used bioinks are agarose-based, alginate-based, collagen-based, hyaluronic acid-based, fibrin-based, cellulose-based, silk protein-based, and ECM bioinks. Each bioink possess its own advantages and disadvantages (Table 2). Agarose, a marine polysaccharide extracted from seaweed, exhibits suitable mechanical properties. However, its ability to support cell growth is limited, which is not conducive to organoid construction. Therefore, its use often requires mixing with other biomaterials to improve its biocompatibility. The earliest reported strategy for bioprinting blood vessels by Norotte et al. was the use of 300–500 mm diameter blood vessels and supporting cell spheres. These spheres were then allowed to deposit on each other on molds printed with agarose to form a single blood vessel[25]. Alginate is a negatively charged polysaccharide that can be transiently cross-linked with divalent cations to form hydrogels. However, it lacks cell adhesion sites[26], and different polymers, such as PCL and gelatin, are often mixed with alginate to form different structures for 3D printed tissues. Zhang et al. used alginate and nano-hydroxyapatite to promote osteochondral repair[27]. Collagen is the primary component of the ECM, which exhibits appropriate biocompatibility. It can be cross-linked by changing the temperature or pH. Using a mixture of collagen and alginate bioink produces a stronger effect than using alginate ink alone. The application of bioink with two or even three biomaterials will improve the stability of polymer systems, tissues, and organoid constructs and will be more beneficial for cell proliferation, differentiation, and self-organization. Hyaluronic acid (HA) is a natural ECM. HA gels slowly, have low mechanical properties after gel formation, and are usually double cross-linked or chemically modified to improve its mechanical properties. Skardal et al. developed a versatile HA and gelatin-based hydrogel system to print primary liver spheroids[28]. Carboxymethylcellulose (CMC) is a semi-flexible polysaccharide derived from cellulose. CMC can be converted into environmentally sensitive hydrogels by changing its concentrations and molecular weights, as appropriate. Markstedt et al. combined nanofibrillated cellulose–alginate complexes and chondrocytes to prepare ear-shaped and curved-moon scaffolds[29]. Fibrin is a pro-coagulant protein. It is enzymatically thrombinized to prepare hydrogels with adequate biocompatibility and biodegradability. Gruene et al.[30] used laser-assisted bioprinting to produce stable vascular networks using natural hydrogels composed of fibrin precursors and HA as cell carriers and environmental materials. Gelatin is a protein obtained by the partial hydrolysis of collagen and is homologous with collagen.
Table 2.
Performance comparison of different bioinks for organoids
| Bioink types | Cell types | Printing methods | Gelation method | Properties | References |
|---|---|---|---|---|---|
| Collagen-based | Human stem cell–derived cardiomyocytes | Extrusion | pH | Better biocompatibility Slow gelation rate Low mechanical properties |
[33] |
| ECM-based | Human induced pluripotent stem cells (hiPSCs) | Extrusion | Light | Better biocompatibility Better functionality |
[36] |
| Alginate-based | HepaRG | Extrusion | Temperature/ion | Easy to prepare Fast gelation Better cytocompatibility |
[52] |
| Hyaluronic acid-based | Primary cell liver | Extrusion | Chemical crosslinking | Better biocompatibility Slower gelation lower Mechanical properties |
[28] |
| Agarose-based | Human umbilical vein smooth muscle cells (HUVSMCs) Human skin fibroblasts (HSFs) |
Extrusion | Chemical crosslinking | Good gel forming ability Good mechanical properties and biological tolerance Limited ability to support cell growth |
[25] |
| Fibrin-based | Human adipose-derived stem cells (ASCs) Endothelial colony-forming cells (ECFCs) |
Laser-assisted | Thrombin | Better biocompatibility, biodegradability Poor mechanical properties |
[30] |
| Cellulose-based | Human nasoseptal chondrocytes cells (hNCs) | Inkjet | Temperature | Environmentally sensitive Easy to gel |
[29] |
| Gelatin-based | HepG2 cells | Extrusion | Light | Better biodegradability and remodeling | [31] |
Its strength depends on the concentration of the solution. Gelatin exhibits sufficient degradability and remodeling. ECM bioink, formed by crushing the removed cellular tissue, dissolving it in buffer, and adding other easy-to-form gels, is the most suitable bioink for cell survival. Matrigel™, an ECM secreted from murine Engelbreth–Holm–Swarm tumors, is the most commonly used ECM for bioprinting. Salvador et al. used hydrogels composed of alginate, gelatin, and matrix gel-controlled fractions for bioprinting tumor models to maintain and prolong patient-derived tumor spheres in culture without disrupting tumor sphere formation[31].
4. Bioprinting organoids applications
Organoids and bioprinting are two of the most popular areas of tissue engineering. Although the use of bio-3D printers to print organoids is nascent, the combination of bioprinting and organoids has demonstrated successful examples, indicating their promising future. Here, we present the current state of research on bioprinting of organoids.
4.1. Heart
The heart is one of the most important organs in the human body, providing power to support the flow of blood, supplying various nutrients and oxygen to other organs and tissues, and eliminating the waste products of metabolism so that the body can function properly. A mature heart contains 9 billion cells, including fibroblasts, cardiomyocytes, smooth muscle cells, connective tissue cells, and immune cells[32,33]. Furthermore, unlike other parts of the body, the heart tissue cannot heal itself from damage. The current challenge faced in bioprinting cardiac organs is that the biomaterials used in bioprinting cardiac organs are primarily the soft materials that possess low mechanical strength and weak support, making it difficult to print and shape layer by layer. Another challenge is the fact that current cardiac organ constructs are either lacking in cells[34,35] or have no evidence of electromechanical function and lack functionality[36].
Certain studies have been conducted for heart organoid construction. Ishino Fumitoshi’s team from Tokyo Medical and Dental University developed a 3D heart organoid, similar to the developing heart, using mouse embryonic stem cells in the presence of FGF4 and LN/ET complexes[37]. Zweigerdt et al. of the Hannover Medical School, Germany, encapsulated free-suspended human PSCs in a matrix gel (Matrigel) in suspension culture in response to the classical Wnt signaling pathway, thereby effectively differentiating them into highly purified cardiomyocytes and establishing a 3D heart-like structure of a certain size with different cell layer patterns and a foreground endoderm structure[38]. However, the organoid thus constructed successfully replicates some aspects of heart tissue, including stromal cells, endothelial cell network, and epicardial layer, and even resembles to early heart developmental morphology, but the macroscopic structure of the organoid moderately differs from that of the real organ. The incorporation of bioprinting has shown satisfactory results. In April 2019, scientists in Israel successfully 3D printed an “artificial heart,” which is the first successfully designed and printed heart comprising cells, blood vessels, ventricles, and atria (Figure 2A, B and C). Although the cells in this heart appear to contract, they cannot beat and pump blood like a normal heart[36]. Recently, Professor Adam Feinberg’s team at Carnegie Mellon University used collagen as a freely embeddable suspension hydrogel (FRESH) to bioprint heart organoids, combining MRI images of coronary arteries and 3D images of the heart to achieve fine structures at different structural scales from capillaries to the entire heart organ, as well as high-resolution printing of heart organoids with systolic function.[34] (Figure 2D, E, and F). Previously constructed organoids are almost always millimeter-sized. However, researchers at the University of Minnesota in the U.S. have recently 3D printed the first ever centimeter-sized heart organoids. They optimized a specialized bioink, made from ECM proteins and human stem cells, to print into ventricular structures. The corresponding stem cells were first expanded to high cell density on the ventricular structures. Then, the cells were differentiated into cardiomyocytes, with critical cell density and the ability to make the cells beat like a heart[39]. This is a major advance in organoid studies of the heart to bioprint stem cells in a tissue synergistic manner and to be able to direct their differentiation into cardiomyocytes in similar situations with in vivo stem cells adjacent to each other. While their printed cardiac muscle models demonstrated encouraging results in small kinetic models, this is insufficient in large animal models with thicker myocardial walls and more demanding vascularization; therefore, further exploration is required.
Figure 2.
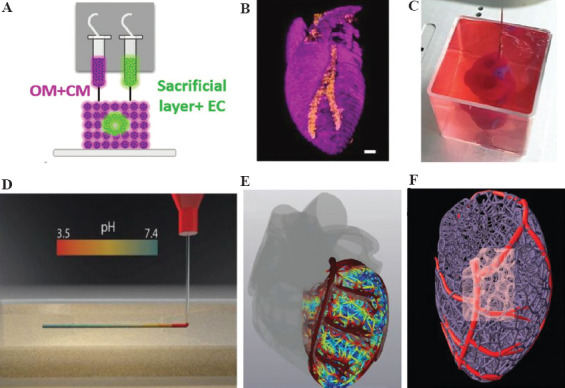
(A) Side view of the bioprinting concept and the unique cellular bioink. (B) 3D confocal image of a bioprinted heart (CM in pink, EC in orange), scale bar =1 mm. (C) Bioprinted heart in a support bath. (D) A schematic diagram of fast cross-linking by squeezing the collagen solution in a support bath with a pH of 7.4. (E and F) Magnetic resonance imaging (MRI) post-processed images of the heart model, showing it has a coronary vascular network. (Adapted with permission from Lee et al, Science, 2019, 482–487 (2019)[34]) and (from ref.[36] licensed under Creative Commons Attribution 4.0 license).
4.2. Kidney
Kidney organoids primarily comprise metanephros (MM) cells, which have been successfully used for nephron-related disease modeling and drug screening. Significant barriers in using the current systemic approach persist, such as in experimental modeling and kidney transplantation. scRNA-seq and transcriptomic studies have identified renal organoids as a very premature renal system. Cultured kidney organoids do not produce all kidney cells, specifically a wide variety of mesenchymal cells, and do not allow the formation of advanced renal structures with a vascular system[40]. Kidney organoids cannot grow above the millimeter level because they become necrotic internally as they develop and have difficulty developing a higher form of the dermal medulla. In addition, the main limitation of kidney organoids is the lack of a functional vascular system.
To construct kidney organoids, based on the finding that Metanephric Mesenchyme (MM) Ureteric Bud (UB) have distinctive roots, Taguchi et al. established a method to extract MM from mouse ESCs and human iPSCs cultured into 3D spheres and promoted the development of mesoderm with Wnt agonists, retinoic acid, etc., thereby producing pedunculated, Bowman’s capsule cells, and tubular epithelial cells[41]. Takasato et al. used human embryonic stem cells in 3D spheroids to develop kidney cells[42]. They first performed induced culture in a 2D plane and then subjected the stem cells to aggregated culture at a 3D level to produce human iPSC-derived kidney organs containing renal progenitor cell-derived podocytes, Bowman’s capsule, and tubules, as well as UB-like cells, stromal cells, and endothelial cells. However, kidney organoids constructed using these methods often suffer from poor reproducibility and high inter-group variability. This is true even in the case of a single iPSC using a single protocol. Kidney unit patterns and cell ratios may also fluctuate between experiments. In the field of bioprinting, the construction of kidney organoids likely yields satisfactory results. Jennifer et al. used bioprinting to construct a functional 3D kidney structure containing living human epithelial cells that form the surface of the renal tubules[43] (Figure 3A, B and C). Organovo recently developed a proximal tubule-like tissue that was bioprinted as a layered structure in a well membrane by mixing fibroblasts and HUVECs with a proprietary heat-responsive hydrogel. After 3 days of culture, renal PTECs were inoculated onto the bioimprinted layer. On maturation, the kidney cells exhibited a microvascular network with tight junctions and cell polarization (Figure 3D, E, and F). In nephrotoxicity tests of mature tissues, the metabolism of renal cells and cellular activity produced greater adverse effects with increasing concentrations of cisplatin[44]. Bioprinting facilitates the precise control of cell deposition in a 3D space in terms of the speed and scale, which could lead to a significant reduction in variability between batches of constructed kidney organoids and even a breakthrough in scale from millimeters to centimeters. Recently, Melissa H. Little’s team at the University of Melbourne, Australia, reported the application of extrusion-based bioprinting technology to rapidly prepare a large number of kidney organoids. Extrusion bioprinting was used to prepare human pluripotent stem cells (hPSCs) derived from renal progenitor cells in 6-well and 96-well plates and they developed into initial cellular microclusters of kidney organoids, which were then cultured for 20 days to obtain kidney organoids with morphology, cell type, and gene expression levels comparable to those previously reported for kidney organoids in artificial culture. This study provides high-quality control of cell number, tissue diameter, and cell viability through bioprinting[45]. Extrusion-based automated bioprinting has shown the ability to produce kidney organoids with improved throughput, controlled quality, and scale-up, signaling the potential of this technique in the fabrication of kidney organoids at the scale of actual kidney organs in future.
Figure 3.
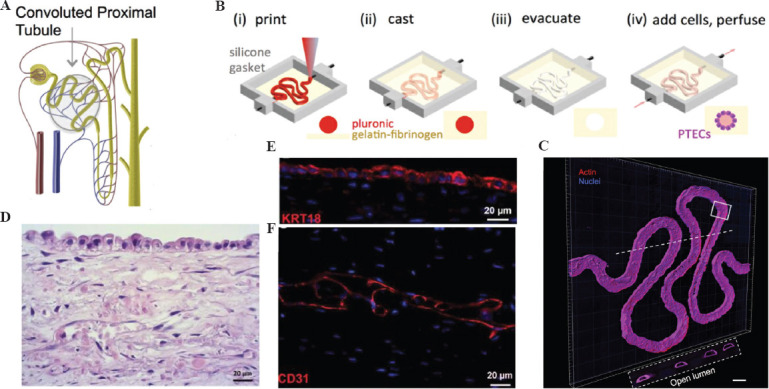
(A) Schematic diagram of the proximal renal tubule. (B) Corresponding schematic diagrams of the different steps for making 3D convoluted perfusable proximal tubules. (C) 3D rendering of a confocal image of the proximal tubule, with actin colored red and the nucleus colored blue, scale bar = 200 μm (D) Hematoxylin and eosin (HE) staining showed complete cellular organization as well as organization of the mesenchymal and epithelial layers. (E) RPTECs form a monolayer and express cytokeratin 18 (red). (F) The interstitial layer exhibits an extensive network of endothelial cell linings (red, CD31), scale bar = 20 mm. (from ref.[43] licensed under Creative Commons Attribution 4.0 license) and (from[44] Creative Commons Attribution license).
4.3. Liver
The liver is the largest gland in the body and contains hepatocytes (HCs), hepatic stellate cells (HSCs), hepatic sinusoidal cells (LSECs), Kupffer cells (KCs), and biliary epithelial cells (BECs), which are densely and orderly arranged in the hexagonal hepatic lobules[46]. Although the liver has an innate ability to regenerate, the hepatocytes survive only 2-3 days once they are removed from the body and rapidly lose their characteristic self-replicating proliferative function. With the rapid development of the field of cellular biology, the 3D culture system significantly promotes the maturation of hepatocytes in vitro. This implies that the spatial location, pressure signals, and matrix adhesion information provided to the cells in a 3D culture environment play an important role in the survival and function of hepatocytes[47,48]. The use of bioprinted liver tissue can better simulate the in vivo static microenvironment and dynamic microenvironment of the liver, which overcomes the limitations of 2D cultures and can better simulate the complexity of the in vivo microenvironment.
Over the past decade, researchers have demonstrated that hepatocytes exhibit a high activity and functional capacity when grown and differentiated in assembled spheres[49]. Skardal et al. used a multifunctional HA and gelatin-based hydrogel system to print specific primary liver spheroids to create in vitro liver constructs with high cell viability and measurable functional albumin and urea output[28]. Conventionally, the most commonly used liver organoids in 3D culture are spheroids. However, spheroids are limited in size due to diffusion barriers in their dense structure, limiting the supply of nutrients, and oxygen. Recently, researchers have attempted to prepare liver organoids using SLA. Grix et al. combined HepaRG and human stellate cells using stereolithography to produce bioprinted liver organoids which exhibited the basic properties of liver[50]. The highly vascularized complex liver tissue is divided into lobes, lobules, hepatocytes, and hepatic sinusoids, covering both microscopic and macroscopic scales. To fabricate multiscale heterogeneous tissues, Song et al. used a pre-defined extrusion bioprinting technique (Figure 4A) to create arrays of liver lobules that could simultaneously create heterogeneous, multicellular, and multi-material structures[51] (Figure 4B). Liver vascularization is an important aspect, which should be considered when bioprinting liver models. Recently, Mao et al. used a mixture of hepatocyte suspension and 4% sodium alginate solution as bioink (cell density: 1×106/ml), then printed and coated in a culture dish, collected the printed structures, and prepared liver tissue layer-by-layer to build liver organoids. Three-dimensionally printed liver organoids formed clusters and exhibited the ability to accumulate hepatic glycogen and transport indole green and acetylated LDL. Remarkably, the mouse liver spontaneously developed a vascular network system 14 days after transplantation[52]. The study of 3D bioprinting liver organoids holds great promise and significance. If 3D printed liver organoids can be mass-produced and survive in large quantities in vitro, expensive biologics, such as albumin and clotting factors, can be left to them to produce.
Figure 4.
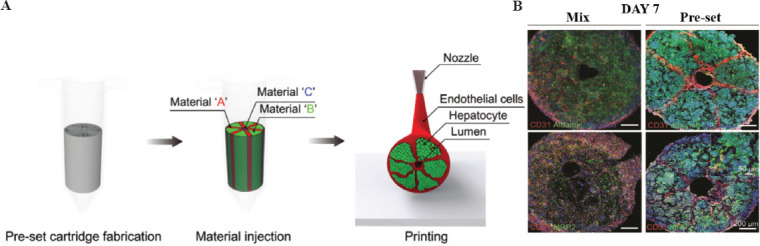
(A) Schematic diagram of the pre-set extrusion 3D bioprinting technique for liver lobule printing. (B) MIX and preset structures were compared to assess liver function, and immunostaining for CD31 (red), albumin (green), MRP2 (green), and DAPI cell nuclei (blue) was performed; scale bar = 200 μm. (Adapted with permission from Kang et al, Small, Copyright 2020 Wiley-VCH Verlag[51]).
4.4. Intestinal tract
Intestinal organoids are derived from intestinal tissue stem cells or PSCs and possess a 3D structure. Organoids of the intestine are widely used in scientific research and drug development because of their stable culture system and relatively well-defined developmental processes. At present, intestinal organs, particularly small intestinal organs, are widely studied models, and the establishment of intestinal organs provides a basis for the establishment of other organs. By adding growth factors for the growth and development of different organs based on the intestinal organ culture system, other organs derived from the digestive tract epithelium, such as the liver[47] and pancreas[53], have been established, in addition to other epithelial organs from non-digestive tract sources, such as the prostate[54] and breast[55]. Intestinal organs can simulate the relationship between cells in vivo and can be used to study the characteristics of stem cells; they are also widely used in studying ulcerative colitis (UC)[56] and other intestinal diseases.
Deng et al. constructed a new intestinal organ culture system that can simulate the regeneration process of proliferative crypts after intestinal epithelial injury and revealed the key role and mechanism of two epigenetic regulators (VPA and EPZ6438) in regulating regeneration after intestinal injury (Figure 5A and B)[57]. Meanwhile, a novel engineered plant-based nanocellulose hydrogel was recently reported as a culture medium for small intestine organoids, which has the advantages of clear composition and low cost compared with the currently used organoid culture medium, Matrigel[58] (Figure 5C). However, many questions remain unanswered. For example, there are multiple phenotypes (symmetrical, budding, mixed, etc.) that can occur during the culture of human intestinal organoids, and varying phenotypes make experiments less reproducible[59]. The introduction of bioprinting has improved the function and structure of intestinal organoids and reproducibility of experiments. The intestinal surface possesses a microvilli structure that provides a large surface area for efficient digestion and absorption. Using collagen and cell-loaded bioink from the submucosa of the small intestine, microscale villi structures with better permeability coefficients and glucose uptake were prepared through a vertically moving bioprinting method[60]. To overcome the limitations of current 3D culture systems, researchers have attempted to use bioprinting technology to prepare 3D intestinal tissues composed of human primary small intestinal epithelial cells and myofibroblasts. These tissues possess physiological barrier function and damage response to toxicity and inflammation[61]. In the past decade, researchers have cultured intestinal organoids that often assemble hepatocytes into micrometer to millimeter spheres. In a recent study, however, researchers prepared centimeter-sized intestinal organoids using bioprinting technology. Jonathan et al. developed a unique 3D bioprinting technique referred to as BATE, which is a combination of a microscope and an extrusion printing system[20]. Using microscopy for continuous monitoring of the process, the researchers combined organoid technology to deposit intestinal stem cells approximately a few centimeters long into the gel to obtain centimeter-scale gastrointestinal tissues with self-organizing features (e.g. lumen, branching blood vessels, and crypt and villi structures of the tubular intestinal epithelium). This study provides new tools for drug discovery, disease diagnosis, and regenerative medicine research.
Figure 5.
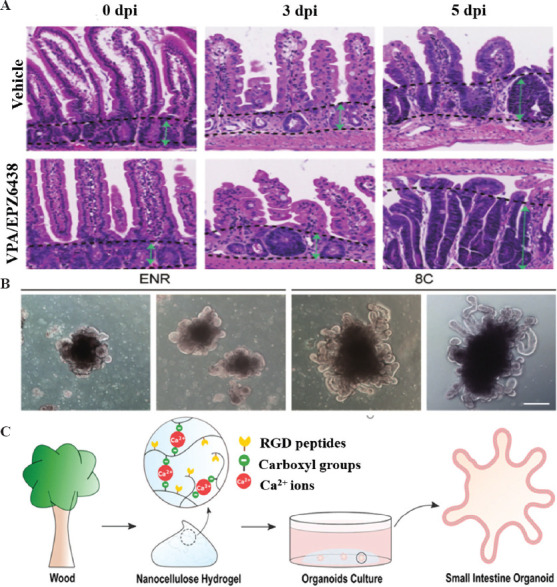
(A) HE staining of the small intestinal crypt was performed on days 3 and 5 (dpi) after irradiation. The green arrow between the two dashed lines indicates the length of the crypt. (B) Typical morphology of intestinal organoids cultured under the indicated conditions. (C) Schematic diagram of small intestine organoid culture in plant-based nanocellulose hydrogel. (from ref.[57] licensed under Creative Commons Attribution 4.0 license) and (from ref.[58] licensed under Creative Commons Attribution 4.0 license).
4.5. Tumor model
Stem cells used in tumor organoid model cultures can be derived from tissue stem cells and PSCs[62], as well as from tumor stem cells. The tumor organoid model provides a new approach for personalized cancer treatment. It not only simulates tumor characteristics [63] and tumor cell heterogeneity[62] but also better reflects human changes compared with traditional animal tumor models. Bioprinting has been applied to alter the tumor microenvironments by precisely controlling the combination of tumor-associated cells and ECM components and organizing them into well-defined spatial distributions. As the field of precision medicine and the development of organoid culture techniques continue to advance, tumor organoid models are being studied at an increasingly rapid pace.[64]
Recently, various tumor organoid models have been successfully established. Clevers et al. described a strategy for producing 3D prostate organoid cultures from healthy mice and human prostate cells (single lumen and basal cells sorted in bulk or FACS), metastatic prostate cancer lesions, and circulating tumor cells[65]. This strategy allows for the growth of intraluminal and basal prostate epithelial cell lines, as well as that of advanced prostate cancer. Fujii et al. established a model library containing 55 colorectal tumor organoids (including different tumor subtypes) that can help in the understanding of the genesis and pathogenesis of colorectal tumors, and provide insights for promoting patient-centered treatment[66]. However, the currently established tumor models do not accurately simulate the natural ECM components and interactions between tissue, cell, and matrix molecules. The introduction of bioprinting has slightly enhanced the structure of blood vessels and lymphatic vessels in tumor models, as well as the lesion characteristics. Zhao et al. used bioprinting to construct the first in vitro 3D tumor model of HeLa cells (a type of cervical cancer cell)[67]. The tumor model better reflected the growth and development of the tumor in vivo and approximated the lesion characteristics of the cancer cells in vivo (Figure 6A, B, C and D). To accurately simulate the complex microenvironment of a tumor, Zhang et al. designed a coaxial bioprinting technique to construct a tumor organ model with perfusable hollow blood and lymphatic vessels closed at one end and integrated a gelatin hydrogel gel containing breast cancer cells to form a pair of tumor organs containing both blood vessels and lymphatic vessels (Figure 6E and F)[68]. To better simulate the tumor microenvironment, tumor models must be constructed by focusing on the establishment of gradients of physical and chemical properties. Researchers printed tumor cells, vascular endothelial cells, and porcine-derived brain tissue ECM into concentric cancer-stromal rings to form a regionalized structure of vascular matrix surrounding tumor tissue and an oxygen gradient within the tumor tissue[69]. The use of bioprinting technology to construct 3D tumor microenvironments has shown some advantages in reconstructing cellular functions, signaling pathways, and drug screening.
Figure 6.
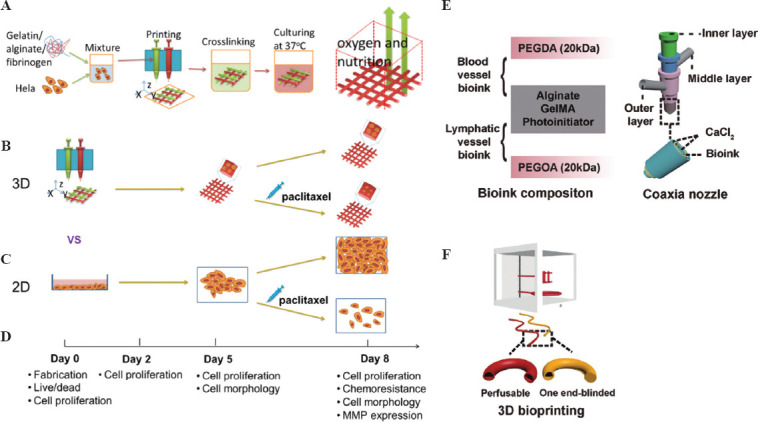
(A) Schematic diagram of the method of 3D bioprinting tumor models with HeLa cells. (B) The plan of the 3D HeLa/hydrogel builds. (C) Both 3D HeLa/hydrogel constructs and 2D planar samples were incubated for 5 and 3 days with/without paclitaxel. The final results were compared. (D) Composition of bioink for bioprinting of blood vessels and lymphatic vessels. (E) Multi-layer coaxial nozzle design for bioink printing as well as cross-linking. (F) Two different hollow tubes were bioprinted using a perfusable hollow tube that mimics a blood vessel and an end-blind hollow tube that mimics a lymphatic vessel. (Adapted with permission from Yu Zhao et al, Biofabrication, 2014, 6 035001[67]) and (Adapted with permission from Cao X, Ashfaq R, Cheng F, et al., Adv Funct Mater, ©2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim[68]).
4.6. Brain organoids
Brain organoids are microscopic organs of embryonic stem cells (ESCs) or PSCs that are artificially cultured and have a functional structure similar to that of brain tissue. Brain tissue is composed of tightly packed glial and neuronal cells, and the ability of cells to self-organize depends mostly on the influence of component gradients and intercellular interactions[70,71]. Brain organoids can be used to study neurophysiology and neurodevelopment and can mimic various neurological diseases.
The development of human PSCs into brain organoids encompasses the formation of embryoid bodies, neural induction, neuroepithelial expansion, and maturation of the organoid. In a new study, Trevino et al. constructed human forebrain organoids for the first time using PSCs in a 3D culture and increased their lifespan for up to 300 days[72] (Figure 7A). However, the challenge with current 3D organoid and spheroid models grown in culture dishes is the insufficient control over cellular localization and diversity. Accordingly, Jodat et al. recently designed a photocrosslinkable bioink and a thermotherapeutic support bath using embedded 3D bioprinting to distribute heterogeneous neural populations with neurospheres and glial cell specificity while supporting the formation of self-organizing spheroids in 3D network structures[73]. Bioprinted brain organoids can be used for drug target screening in neurological diseases. Moreover, researchers described how bioprinting could provide a high-throughput and reproducible preparation of neural tissue, as an alternative to expensive animal experiments, to screen potential drug targets for treating Alzheimer’s disease[74]. In the past, the act of culturing brain-like organs often produced only one cell type of interest when transcription factors were overexpressed, rather than the multiple cell type structures found in natural tissues. Therefore, Mark et al. used a multi-material bioprinting technique in which they differentiated on-demand orthologous regions composed of neural stem cells, endothelium, and neurons from a mixed class of embryos overexpressing transcription factors and wild-type human induced stem cells (hiPSCs)[75]. When conducting drug screening, a protocol that is simple to operate and highly reproducible is required. Recently, researchers printed PCL scaffolds to culture brain-like organs and designed them into structures with favorable diffusion conditions for engineered flat brain organoids (efBOs) (Figure 7B). The efBOs were fabricated in a highly simplified manner. In addition, this was the first study to report the preparation of an in vitro model of neural tissue with an intrinsic gyrus[7].
Figure 7.
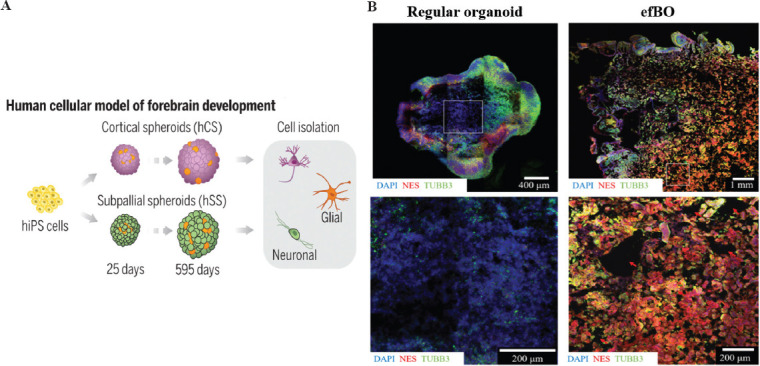
(A) Schematic representation of the flow of human induced PSCs developing into forebrain cells. (B) Comparison of tissue core between regular brain organoids and efBOs. Immunohistochemical staining of NES and TUBB3 was performed to visualize cells. DAPI was used as counterstain. (Adapted with permission from Trevino AE, et al., Science, 367: eaay1645, Copyright 2020, The American Association for the Advancement of Science[72]) and (Adapted with permission from Theresa S P Rothenbücher et al., Biofabrication, 2021,13 011001, IOP Publishing Ltd[76]).
4.7. Other organoids
The cornea possesses a complex structure. It is divided into five layers from anterior to posterior: the epithelial cell layer, preelastic layer, stromal layer, posterior elastic layer, and endothelial cell layer. Therefore, culturing corneal organoids poses certain challenges. Considering the complex spatial structure, bioprinting may have some advantages. Isaacson et al. designed corneas with the structure of natural human corneal stroma by obtaining stem cells from the corneas of healthy donors and mixing them with gel (Figure 8A)[77]. The pancreas is a relatively small organ, but its functional and structural complexity have always made it difficult to make mechanistic breakthroughs. Kim et al. attempted to develop pancreatic tissue constructs enriched with 3D islets for use as a source to enhance key functions of pancreatic tissue[78]. The formation of lung organoids begins with the differentiation of hPSC into a stereotyped endoderm, followed by differentiation into a foregut endoderm, and finally into lung organoids. Recently, Han et al. constructed the first lung organoids for the study of COVID-19 and screened therapeutic agents[79]. In a previous report, Grigoryan et al. used stereolithography as a 3D bioprinting technique to create a small 3D printed lung model with a multivessel network and “breathing” function (Figure 8B)[80]. Conventional breast organoid 3D culture involves mixing dispersed mammary epithelial cells in an ECM matrix before gelling and subsequent self-organization into organoid structures[81,82]. However, there is a large variation between batches of such fabricated mammary organoids. To address this issue, John et al. reported the bioprinting of mammary organoids in collagen with minimal variation from batch to batch (Figure 8C and D)[83]. More recently, more bionic assemblies have been proposed based on the concept of organoids. Professor Kunyoo Shin’s team at Pohang University of Science and Technology in South Korea used bio-3D printing technology to reconstruct bladder assemblies and develop patient-specific bladder tumor assemblies that accurately mimicked the pathological features of tumors in vivo[84].
Figure 8.
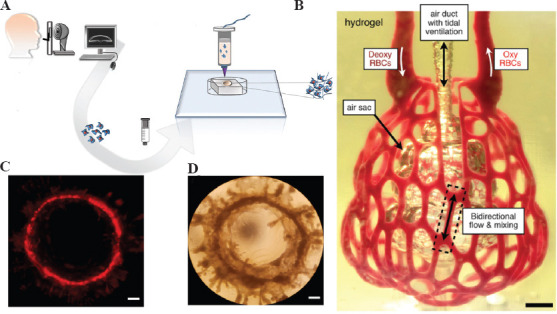
(A) Schematic diagram of the bioprinted cornea process. (B) Photographs of printed hydrogels containing distal lung subunits during red blood cell perfusion when the balloon is ventilated with oxygen, scale bar = 1 mm. (C) Images of red fluorescent protein-positive (RFP+) MCF12A cells forming a large mammary circular organoid at 14 days after printing. (D) Example of a large mammary round-like organ with a diameter of approximately 4 mm at 24 days after printing, scale bar = 500 μm. (from ref.[77] licensed under Creative Commons Attribution 4.0 license), (Adapted with permission from Grigoryan B, et al., 2019, Science, 364:458–64, Copyright 2019, The American Association for the Advancement of Science[80]) and (from ref.[83] licensed under Creative Commons Attribution 4.0 license).
5. Future outlook for bioprinting organoids
Bioprinting is a developing technology that has the potential for tissue and organ development because of its capacity to accurately control the spatial dissemination of cells and encompassing microenvironment. Organoids are 3D cellular self-organization cultures exhibiting some key characteristics of the corresponding organs and are uniquely similar to actual human organs[85-88]. Since the first successful cell bioprinting experiment in 2003[89], organoid construction and bioprinting have brought hopes, although significant efforts are required before bioprinting of organs actually is put into practical use.
There are numerous problems with the current construction of organoids. Organoids are millimeter-scale 3D culture systems formed by intercellular self-organization. However, certain structural features of organs range from a few hundred microns to a few centimeters. In addition, a large gap remains in the scale of actual organs. Vascularization is crucial for organoid construction. Oxygen and nutrients may successfully sustain organoid development in the early stages of 3D organoid culture. However, in the later stages, a single vasculature may fail to keep up with the organoid’s requirements by supplying sufficient nutrients and oxygen[90]. Therefore, a complex vascular system is required. Traditional organoid construction also suffers from variation between culture batches and is less reproducible. The yield of the corresponding organoids is then limited. Bioprinting addresses these problems to some extent. As a breakthrough in addressing the traditional 3D culture-scale limitations, bioprinting has been reported for producing centimeter-level intestinal organoids[20,34]. Bioprinting can also build complex vascular systems for organoids[91-94], and increase the yield of organoids by a factor of nine, while reducing the size variability of organoids, that is, only 1–4%[45,95].
There are a series of difficulties in bioprinting organoids. For example, although bioprinting technology can effectively control the deposition process of cells in 3D space, it is impossible to construct macroscopic tissues through cellular self-organization. To address the current issues pertaining to the scale size of organoids, future studies will focus on how to print small functional units of organoids and then how to deposit and assemble these functional units into larger structures. Therefore, we should find a balance between the architecture of space, vascular network, and self-organization of organoid cells in the bioprinting process. In the precise arrangement of cells, sufficient resolution is also required. However, a considerably high resolution indicates an increase in the density of cells, and an extremely high shear force will aggravate cell damage. In the future, with breakthroughs in bioprinting technology, biomaterials, and a better understanding of the molecular mechanisms of organ development, more bioprinting strategies for organoid development will emerge. We believe that the future of bioprinting research will revolve around the studies on early developmental stages of organs and tissues. Bioprinting organoid technology will potentially play an extraordinary role in developmental biology, disease pathology, cell biology, regenerative mechanisms, precision medicine, and drug screening.
6. Conclusion
Both bioprinting and organoids are intriguing research topics in the field of regenerative medicine. Bioprinting emphasizes on the reconstrution of tissue structures, while organoids fouces on the rebiulding of biological functions. When these two technologies are combined, bioprinted organs with both physiological function and structure may come into reality in the near future. Although organoid bioprinting is still in its infancy phase, this technique has brought us one step closer to truly 3D printing personalized organs.
Acknowledgments
This research was supported by the Key R and D Program of the Ministry of Science and Technology of China (No. 2018YFB1105600/2020YFC2008700/2018YFB11 07000), National Natural Science Foundation of China (No. 92048205/81902195/82072228), the Project of Shanghai Science and Technology Commission (No. 19XD1434200), and Shanghai Municipal Commission of Economy and Information (No. 202001007).
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Fatehullah A, Tan SH, Barker N. Organoids as an in Vitro Model of Human Development and Disease. Nat Cell Biol. 2016;18:246–54. doi: 10.1038/ncb3312. https://doi.org/10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 2.Matano M, Date S, Shimokawa M, et al. Modeling Colorectal Cancer Using CRISPR-Cas9-Mediated Engineering of Human Intestinal Organoids. Nat Med. 2015;21:256–62. doi: 10.1038/nm.3802. https://doi.org/10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 3.Ballard DH, Boyer CJ, Alexander JS. Organoids-Preclinical Models of Human Disease. N Engl J Med. 2019;380:1981–2. doi: 10.1056/NEJMc1903253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huch M, Koo BK. Modeling Mouse and Human Development using Organoid Cultures. Development. 2015;142:3113–25. doi: 10.1242/dev.118570. https://doi.org/10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 5.Lui JH, Hansen DV, Kriegstein AR. Development and Evolution of the Human Neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. https://doi.org/10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Koo BK, Knoblich JA. Human Organoids:Model Systems for Human Biology and Medicine. Nat Rev Mol Cell Biol. 2020;21:571–84. doi: 10.1038/s41580-020-0259-3. https://doi.org/10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groll J, Boland T, Blunk T, et al. Biofabrication:Reappraising the Definition of an Evolving Field. Biofabrication. 2016;8:5. doi: 10.1088/1758-5090/8/1/013001. https://doi.org/10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich MA, Liu WJ, Jimenez A, et al. 3D Bioprinting:From Benches to Translational Applications. Small. 2019;15:47. doi: 10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuan RS, Boland G, Tuli R. Adult Mesenchymal Stem Cells and Cell-Based Tissue Engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu T, Gregory CA, Molnar P, et al. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials. 2006;27:3580–8. doi: 10.1016/j.biomaterials.2006.01.048. https://doi.org/10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Liu B, Pei B, et al. Inkjet Bioprinting of Biomaterials. Chem Rev. 2020;120:10793–833. doi: 10.1021/acs.chemrev.0c00008. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Chai W, Yong H, et al. Scaffold-Free Inkjet Printing of Three-Dimensional Zigzag Cellular Tubes. Biotechnol Bioeng. 2015;109:3152–60. doi: 10.1002/bit.24591. https://doi.org/10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- 13.Lee YB, Polio S, Lee W, et al. Bio-Printing of Collagen and VEGF-Releasing Fibrin Gel Scaffolds for Neural Stem Cell Culture. Exp Neurol. 2010;223:645–52. doi: 10.1016/j.expneurol.2010.02.014. https://doi.org/10.1016/j.expneurol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Mandrycky C, Wang Z, Kim K, et al. 3D Bioprinting for Engineering Complex Tissues. Biotechnol Adv. 2016;34:422–34. doi: 10.1016/j.biotechadv.2015.12.011. https://doi.org/10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayavenkataraman S, Wei-Cheng Y, Lu WF, et al. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv Drug Deliv Rev. 2018;132:296–332. doi: 10.1016/j.addr.2018.07.004. https://doi.org/10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Othon CM, Wu X, Anders JJ, et al. Single-Cell Printing to form Three-Dimensional Lines of Olfactory Ensheathing Cells. Biomed Mater. 2008;3:034101. doi: 10.1088/1748-6041/3/3/034101. https://doi.org/10.1088/1748-6041/3/3/034101. [DOI] [PubMed] [Google Scholar]
- 17.Pirlo RK, Wu P, Liu J, et al. PLGA/Hydrogel Biopapers as a Stackable Substrate for Printing HUVEC Networks Via BioLP. Biotechnol Bioeng. 2011;109:262–73. doi: 10.1002/bit.23295. https://doi.org/10.1002/bit.23295. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Chen M, Fan X, et al. Recent Advances in Bioprinting Techniques:Approaches, Applications and Future Prospects. J Transl Med. 2016;14:271. doi: 10.1186/s12967-016-1028-0. https://doi.org/10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertassoni LE, Cardoso JC, Manoharan V, et al. Direct-Write Bioprinting of Cell-Laden Methacrylated Gelatin Hydrogels. Biofabrication. 2014;6:024105. doi: 10.1088/1758-5082/6/2/024105. https://doi.org/10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brassard JA, Nikolaev M, Hübscher T, et al. Recapitulating Macro-Scale Tissue Self-Organization Through Organoid Bioprinting. Nat Mater. 2020;20:22–9. doi: 10.1038/s41563-020-00803-5. https://doi.org/10.1038/s41563-020-00803-5. [DOI] [PubMed] [Google Scholar]
- 21.Daly AC, Prendergast ME, Hughes AJ, et al. Bioprinting for the Biologist. Cell. 2021;184:18–32. doi: 10.1016/j.cell.2020.12.002. https://doi.org/10.1016/j.cell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui H, Nowicki M, Fisher JP, et al. 3D Bioprinting for Organ Regeneration. Adv Healthc Mater. 2016;6:1601118. doi: 10.1002/adhm.201601118. https://doi.org/10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan H, Zhang T, Xu H, et al. Photo-Curing 3D Printing Technique and Its Challenges. Bioact Mater. 2020;5:110–5. doi: 10.1016/j.bioactmat.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creff J, Courson R, Mangeat T, et al. Fabrication of 3D Scaffolds Reproducing Intestinal Epithelium Topography by High-Resolution 3D Stereolithography. 45th International Conference on Micro and Nano Engineering (MNE 2019) 2019 doi: 10.1016/j.biomaterials.2019.119404. https://doi.org/10.1016/j.biomaterials.2019.119404. [DOI] [PubMed] [Google Scholar]
- 25.Norotte C, Marga FS, Niklason LE, et al. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. https://doi.org/10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair LS, Laurencin CT. Biodegradable Polymers as Biomaterials. Prog Polym Sci. 2007;32:762–98. [Google Scholar]
- 27.Zhang H, Huang H, Hao G, et al. 3D Printing Hydrogel Scaffolds with Nanohydroxyapatite Gradient to Effectively Repair Osteochondral Defects in Rats. Adv Funct Mater. 2021;31:2006697. https://doi.org/10.1002/adfm.202006697. [Google Scholar]
- 28.Skardal A, Devarasetty M, Kang HW, et al. Bioprinting Cellularized Constructs Using a Tissue-specific Hydrogel Bioink. J Vis Exp. 20162016:e53606. doi: 10.3791/53606. https://doi.org/10.3791/53606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markstedt K, Mantas A, Tournier I, et al. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015;16:1489–96. doi: 10.1021/acs.biomac.5b00188. https://doi.org/10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 30.Gruene M, Pflaum M, Hess C, et al. Laser Printing of Three-Dimensional Multicellular Arrays for Studies of Cell-Cell and Cell-Environment Interactions. Tissue Eng Part C Methods. 17:973–82. doi: 10.1089/ten.tec.2011.0185. https://doi.org/10.1089/ten.tec.2011.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flores-Torres S, Peza-Chavez O, Kuasne H, et al. Alginate-gelatin-Matrigel hydrogels enable the development and multigenerational passaging of patient-derived 3D bioprinted cancer spheroid models. Biofabrication. 2021;13:025001. doi: 10.1088/1758-5090/abdb87. https://doi.org/10.1088/1758-5090/abdb87. [DOI] [PubMed] [Google Scholar]
- 32.Tirziu D, Giordano FJ, Simons M. Cell Communications in the Heart. Circulation. 2010;122:928–37. doi: 10.1161/CIRCULATIONAHA.108.847731. https://doi.org/10.1161/circulationaha.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergmann O, Zdunek S, Felker A, et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–75. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Lee A, Hudson AR, Shiwarski DJ, et al. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science. 2019;365:482–7. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 35.Hinton TJ, Jallerat Q, Palchesko RN, et al. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. https://doi.org/10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noor N, Shapira A, Edri R, et al. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv Sci. 2019;6:1900344. doi: 10.1002/advs.201900344. https://doi.org/10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Sutani A, Kaneko R, et al. In Vitro Generation of Functional Murine Heart Organoids via FGF4 and Extracellular Matrix. Nat Commun. 2020;11:4283. doi: 10.1038/s41467-020-18031-5. https://doi.org/10.1038/s41467-020-18031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drakhlis L, Biswanath S, Farr CM, et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol. 39:737–746 (2021). doi: 10.1038/s41587-021-00815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupfer ME, Lin WH, Ravikumar V, et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ Res. 2020;127:207–24. doi: 10.1161/CIRCRESAHA.119.316155. https://doi.org/10.1161/circresaha.119.316155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishinakamura R. Human Kidney Organoids:Progress and Remaining Challenges. Nat Rev Nephrol. 2019;15:613–24. doi: 10.1038/s41581-019-0176-x. https://doi.org/10.1038/s41581-019-0176-x. [DOI] [PubMed] [Google Scholar]
- 41.Taguchi A, Kaku Y, Ohmori T, et al. Redefining the In Vivo Origin of Metanephric Nephron Progenitors Enables Generation of Complex Kidney Structures from Pluripotent Stem Cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. https://doi.org/10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Takasato M, Er PX, Chiu HS, et al. Kidney Organoids from Human iPS Cells Contain Multiple Lineages and Model Human Nephrogenesis. Nature. 2016;536:238. doi: 10.1038/nature17982. https://doi.org/10.1038/nature17982. [DOI] [PubMed] [Google Scholar]
- 43.Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Scic Rep. 2016;6:34845. doi: 10.1038/srep34845. https://doi.org/10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King SM, Higgins JW, Nino CR, et al. 3D Proximal Tubule Tissues Recapitulate Key Aspects of Renal Physiology to Enable Nephrotoxicity Testing. Front Physiol. 2017;8:18. doi: 10.3389/fphys.2017.00123. https://doi.org/10.3389/fphys.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawlor KT, Vanslambrouck JM, Higgins JW, et al. Cellular Extrusion Bioprinting Improves Kidney Organoid Reproducibility and Conformation. Nat Mater. 20:260–71. doi: 10.1038/s41563-020-00853-9. https://doi.org/10.3390/mi10100676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng J, Wei WB, Chen ZZ, et al. Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications:A Review. Micromachines. 2019;10:26. doi: 10.3390/mi10100676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huch M, Dorrell C, Boj SF, et al. In Vitro Expansion of Single Lgr5(+) Liver Stem Cells Induced by Wnt-Driven Regeneration. Nature. 2013;494:247–50. doi: 10.1038/nature11826. https://doi.org/10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takebe T, Sekine K, Enomura M, et al. Vascularized and Functional Human Liver from an iPSC-Derived Organ Bud Transplant. Nature. 2013;499:481–4. doi: 10.1038/nature12271. https://doi.org/10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 49.Tostões RM, Leite SB, Serra M, et al. Human Liver Cell Spheroids in Extended Perfusion Bioreactor Culture for Repeated-Dose Drug Testing. Hepatology. 2012;55:1227–36. doi: 10.1002/hep.24760. https://doi.org/10.1002/hep.24760. [DOI] [PubMed] [Google Scholar]
- 50.Tobias G, Alicia R, Alexander T, et al. Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-On-A-Chip Applications. Genes. 2018;9:176. doi: 10.3390/genes9040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang D, Hong G, An S, et al. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small. 2020;16:1905505. doi: 10.1002/smll.201905505. https://doi.org/10.1002/smll.201905505. [DOI] [PubMed] [Google Scholar]
- 52.Kaur S, Tripathi DM, Ghosh S. Three-Dimensional Bioprinted Hepatorganoids in Liver Failure. Gut. 2020;70:998–9. doi: 10.1136/gutjnl-2020-322317. https://doi.org/10.1136/gutjnl-2020-322317. [DOI] [PubMed] [Google Scholar]
- 53.Boj SF, Hwang CI, Baker LA, et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell. 2015;160:324–38. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chua CW, Shibata M, Lei M, et al. Single Luminal Epithelial Progenitors Can Generate Prostate Organoids in Culture. Nat Cell Biol. 2014;16:951. doi: 10.1038/ncb3047. https://doi.org/10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linnemann JR, Miura H, Meixner LK, et al. Quantification of Regenerative Potential in Primary Human Mammary Epithelial Cells. Development. 2015;142:3239–51. doi: 10.1242/dev.123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarvestani SK, Signs S, Hu B, et al. Induced Organoids Derived from Patients with Ulcerative Colitis Recapitulate Colitic Reactivity. Nat Commun. 2021;12:262. doi: 10.1038/s41467-020-20351-5. https://doi.org/10.1038/s41467-020-20351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu ML, Xiong L, Lyu YL, et al. Establishment of Intestinal Organoid Cultures Modeling Injury-Associated Epithelial Regeneration. Cell Res. 31:259–71. doi: 10.1038/s41422-020-00453-x. https://doi.org/10.1038/s41422-020-00453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curvello R, Kerr G, Micati DJ, et al. Engineered Plant-Based Nanocellulose Hydrogel for Small Intestinal Organoid Growth. Adv Sci. 2021;8:2002135. doi: 10.1002/advs.202002135. https://doi.org/10.1002/advs.202002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lukonin I, Serra D, Meylan LC, et al. Phenotypic Landscape of Intestinal Organoid Regeneration. Nature. 2020;586:275–80. doi: 10.1038/s41586-020-2776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim WJ, Kim GH. An Intestinal Model with a Finger-Like Villus Structure Fabricated Using a Bioprinting Process and Collagen/SIS-Based Cell-Laden Bioink. Theranostics. 2020;10:2495–508. doi: 10.7150/thno.41225. https://doi.org/10.7150/thno.41225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madden LR, Nguyen TV, Garcia-Mojica S, et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. Iscience. 2018;2:156–67. doi: 10.1016/j.isci.2018.03.015. https://doi.org/10.1016/j.isci.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang L, Holtzinger A, Jagan I, et al. Ductal Pancreatic Cancer Modeling and Drug Screening Using Human Pluripotent Stem Cell-and Patient-Derived Tumor Organoids. Nat Med. 2015;21:1364–71. doi: 10.1038/nm.3973. https://doi.org/10.1158/1538-7445.panca16-b45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dominijanni A, Mazzocchi A, Shelkey E, et al. Bioengineered Tumor Organoids. Curr Opin Biomed Eng. 2020;13:168–73. doi: 10.1016/j.cobme.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L, Li Y, Wu Y, et al. The Construction of In Vitro Tumor Models Based on 3D Bioprinting. Biodes Manuf. 2020;3:227–36. [Google Scholar]
- 65.Drost J, Karthaus W R, Gao D, et al. Organoid Culture Systems for Prostate Epithelial and Cancer Tissue. Nat Protoc. 11:347–58. doi: 10.1038/nprot.2016.006. https://doi.org/10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujii M, Shimokawa M, Date S, et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell. 2016;18:827–38. doi: 10.1016/j.stem.2016.04.003. https://doi.org/10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Yao R, Ouyang L, et al. Three-Dimensional Printing of Hela Cells for Cervical Tumor Model In Vitro. Biofabrication. 2014;6:035001. doi: 10.1088/1758-5082/6/3/035001. https://doi.org/10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 68.Cao X, Ashfaq R, Cheng F, et al. A Tumor-On-A-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv Funct Mater. 2019;29:1807173. doi: 10.1002/adfm.201807173. https://doi.org/10.1002/adfm.201807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi HG, Jeong YH, Kim Y, et al. A Bioprinted Human-Glioblastoma-On-A-Chip for the Identification of Patient-Specific Responses to Chemoradiotherapy. Nat Biomed Eng. 3:509–19. doi: 10.1038/s41551-019-0363-x. https://doi.org/10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- 70.Bonneh-Barkay D, Wiley CA. Brain Extracellular Matrix in Neurodegeneration. Brain Pathol. 2009;19:573–85. doi: 10.1111/j.1750-3639.2008.00195.x. https://doi.org/10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stiles J, Jernigan TL. The Basics of Brain Development. Neuropsychol Rev. 2010;20:327–48. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trevino AE, Sinnott-Armstrong N, Andersen J, et al. Chromatin Accessibility Dynamics in a Model of Human Forebrain Development. Science. 2020;367:eaay1645. doi: 10.1126/science.aay1645. https://doi.org/10.1126/science.aay1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li YC, Jodat YA, Samanipour R, et al. Toward a Neurospheroid Niche Model:Optimizing Embedded 3D Bioprinting for Fabrication of Neurospheroid Brain-Like Co-Culture Constructs. Biofabrication. 2021;13:015014. doi: 10.1088/1758-5090/abc1be. https://doi.org/10.1088/1758-5090/abc1be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willerth SM. Bioprinting Neural Tissues using Stem Cells as a Tool for Screening Drug Targets for Alzheimer's Disease. J 3D Print Med. 2018;2:1–4. [Google Scholar]
- 75.Skylar-Scott M, Huang J, Lu A, et al. An Orthogonal Differentiation Platform for Genomically Programming Stem Cells, Organoids, and Bioprinted Tissues. bioRxiv. 2020 https://doi.org/10.1101/2020.07.11.198671. [Google Scholar]
- 76.Rothenbücher T, Gürbüz H, Emnéus J, et al. Next Generation Human Brain Models:Engineered Flat Brain Organoids Featuring Gyrification. Biofabrication. 2021;13:011001. doi: 10.1088/1758-5090/abc95e. https://doi.org/10.1088/1758-5090/abc95e. [DOI] [PubMed] [Google Scholar]
- 77.Abigail I, Stephen S, Connon CJ. 3D Bioprinting of a Corneal Stroma Equivalent. Exp Eye Res. 2018;173:188–93. doi: 10.1016/j.exer.2018.05.010. https://doi.org/10.1016/j.exer.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J, Shim IK, Hwang DG, et al. 3D Cell Printing of Islet-Laden Pancreatic Tissue-Derived Extracellular Matrix Bioink Constructs for Enhancing Pancreatic Functions. J Mater Chem B. 2019;7:4592. doi: 10.1039/c8tb02787k. https://doi.org/10.1039/c9tb90097g. [DOI] [PubMed] [Google Scholar]
- 79.Han Y, Yang L, Duan X, et al. Identification of Candidate COVID-19 Therapeutics using hPSC-Derived Lung Organoids. bioRxiv 2020 [Google Scholar]
- 80.Grigoryan B, Paulsen SJ, Corbett DC, et al. Biomedicine Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science. 2019;364:458–64. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vichas A, Zallen JA. Translating Cell Polarity into Tissue Elongation. Semin Cell Dev Biol. 2011;22:858–64. doi: 10.1016/j.semcdb.2011.09.013. https://doi.org/10.1016/j.semcdb.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis GE, Cleaver OB. Outside in:Inversion of Cell Polarity Controls Epithelial Lumen Formation. Dev Cell. 2014;31:140–2. doi: 10.1016/j.devcel.2014.10.011. https://doi.org/10.1016/j.devcel.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reid JA, Mollica PM, Bruno RD, et al. Consistent and Reproducible Cultures of Large-Scale 3D Mammary Epithelial Structures using an Accessible Bioprinting Platform. Breast Cancer Res. 2018;20:122. doi: 10.1186/s13058-018-1045-4. https://doi.org/10.1186/s13058-018-1045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim E, Choi S, Kang B, et al. Creation of Bladder Assembloids Mimicking Tissue Regeneration and Cancer. Nature. 2020;588:664–9. doi: 10.1038/s41586-020-3034-x. https://doi.org/10.1038/s41586-020-3034-x. [DOI] [PubMed] [Google Scholar]
- 85.Fujii M, Matano M, Toshimitsu K, et al. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell. 2018;23:787–93. doi: 10.1016/j.stem.2018.11.016. https://doi.org/10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Hu H, Gehart H, Artegiani B, et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175:1591–606.e19. doi: 10.1016/j.cell.2018.11.013. https://doi.org/10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 87.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures In Vitro Without a Mesenchymal Niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. https://doi.org/10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 88.Sato T, Stange DE, Ferrante M, et al. Long-Term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium. Gastroenterology. 2011;141:1762–72. doi: 10.1053/j.gastro.2011.07.050. https://doi.org/10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 89.Wilson WC, Boland T. Cell and Organ Printing 1:Protein and Cell Printers. Anat Rec A Discov Mol Cell Evol Biol. 2003;272A:491–6. doi: 10.1002/ar.a.10057. https://doi.org/10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 90.Rawal P, Tripathi DM, Ramakrishna S, et al. Prospects for 3D Bioprinting of Organoids. Biodes Manuf. 4:627–40. https://doi.org/10.1007/s42242-020-00124-1. [Google Scholar]
- 91.Yap KK, Gerrand YW, Dingle AM, et al. Liver Sinusoidal Endothelial Cells Promote the Differentiation and Survival of Mouse Vascularised Hepatobiliary Organoids. Biomaterials. 2020;251:120091. doi: 10.1016/j.biomaterials.2020.120091. https://doi.org/10.1016/j.biomaterials.2020.120091. [DOI] [PubMed] [Google Scholar]
- 92.Silvestri V L, Henriet E, Linville R M, et al. A Tissue-Engineered 3D Microvessel Model Reveals the Dynamics of Mosaic Vessel Formation in Breast Cancer. Cancer Res. 2020;80:4288–301. doi: 10.1158/0008-5472.CAN-19-1564. https://doi.org/10.1158/0008-5472.can-19-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Markou M, Kouroupis D, Badounas F, et al. Tissue Engineering Using Vascular Organoids From Human Pluripotent Stem Cell Derived Mural Cell Phenotypes. Front Bioeng Biotechnol. 2020;8:278. doi: 10.3389/fbioe.2020.00278. https://doi.org/10.3389/fbioe.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song KH, Highley CB, Rouff A, et al. Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv Funct Mater. 2018;28:1801331. https://doi.org/10.1002/adfm.201801331. [Google Scholar]
- 95.Humphreys BD. Bioprinting Better Kidney Organoids. Nat Mater. 2021;20:128–30. doi: 10.1038/s41563-020-00881-5. [DOI] [PubMed] [Google Scholar]


