Abstract
Three-dimensional (3D) bioprinting has become a promising strategy for bone manufacturing, with excellent control over geometry and microarchitectures of the scaffolds. The bioprinting ink for bone and cartilage engineering has thus become the key to developing 3D constructs for bone and cartilage defect repair. Maintaining the balance of cellular viability, drugs or cytokines’ function, and mechanical integrity is critical for constructing 3D bone and/or cartilage scaffolds. Photo-crosslinkable hydrogel is one of the most promising materials in tissue engineering; it can respond to light and induce structural or morphological transition. The biocompatibility, easy fabrication, as well as controllable mechanical and degradation properties of photo-crosslinkable hydrogel can meet various requirements of the bone and cartilage scaffolds, which enable it to serve as an effective bio-ink for 3D bioprinting. Here, in this review, we first introduce commonly used photo-crosslinkable hydrogel materials and additives (such as nanomaterials, functional cells, and drugs/cytokine), and then discuss the applications of the 3D bioprinted photo-crosslinkable hydrogel scaffolds for bone and cartilage engineering. Finally, we conclude the review with future perspectives about the development of 3D bioprinting photo-crosslinkable hydrogels in bone and cartilage engineering.
Keywords: Bone and cartilage engineering, Hydrogel, Photo-crosslinking, Three-dimensional printing
1. Introduction
The incidence of skeletal disorders involving both bone and cartilage caused by trauma, injuries, and dysfunction has significantly increased in recent decades, creating a demand for more effective treatment[1]. Despite decades of study, the treatments for large bone and cartilage defects remain a significant clinical problem[2]. Although traditional methods such as autografts, allografts and xenografts have been developed for repairing these defects, they all suffer corresponding restrictions, including limited supply, insufficient function, and immune response[3]. Hence, synthetic biomaterials are developed as alternatives; however, they often fail to be properly integrated into the recipient sites due to the lack of native tissue-mimicking structure and the inability to establish a three-dimensional (3D) niche for different cell types (osteoblasts, osteoclasts, and endothelial cells [EC])[4]. Bone and cartilage are highly complex anisotropic tissues with distinctive structures, various compositions, and excellent mechanical properties. The repair of bone and cartilage involves cell migration, extracellular matrix (ECM) remodeling, and tissue regeneration, of which both functional details and structures should be carefully considered when developing bone and cartilage constructs[5].
3D bioprinting, which not only provides adjustable 3D organizational structures but also encapsulates cells and growth factors, brings forth a new strategy to design biomimetic scaffolds for bone and cartilage repair[6]. 3D bioprinting can be considered an additive manufacturing technique where biomaterials, cells and growth factors, often referred to as “bio-ink,” are printed to create tissue-like structures that imitate natural tissues[7]. It has been applied in bone and cartilage tissue engineering as it can precisely fabricate the 3D scaffolds by controlling the pore size, porosity, and interconnectivity[8,9]. To date, there are four leading 3D printing technologies: Inkjet-based bioprinting, extrusion-based bioprinting, laser-assisted bioprinting, and stereolithography (SLA) bioprinting. In extrusion-based bioprinting, bio-inks are extruded as filaments and undergo fast crosslinking to maintain the desired shape and structure. Laser-assisted bioprinters use a laser pulse to produce a cell suspended bio-ink and deposit it into the substrate in an orderly manner. In inkjet-based bioprinting, a certain volume of bio-inks was injected onto a substrate to form a precise pattern with either thermal or piezoelectric energy. In SLA bioprinting, a digital projector is used to selectively crosslink bio-ink plane-by-plane into desired shapes. The four primary bioprinting techniques each have specific strengths, weaknesses, and limitations. Although no single bioprinting technology can achieve the complete replication of complexities of various tissues, extrusion and SLA bioprinting are commonly used for preparing bone and cartilage scaffolds due to their good biocompatibility and easy combination of multiple crosslinking mechanisms[4,10].
One of the key elements for 3D bioprinting is the bio-ink. Unlike conventional 3D printing process in which inks can be printed in melt form at high temperature (ceramics and alloys) or as a polymer solution dissolved in organic solvents, elevated temperature or organic solvents are unfortunately not cytocompatible with depositing living cells and growth factors in a 3D bioprinting process. Hydrogels, which can provide nutritious environments suitable for cell survival, proliferation, and differentiation, have unique advantages in 3D printing living cells and/or growth factors. In general, click chemistry, enzymatic reactions, Schiff’s base reaction, and photo-polymerization can be used to crosslink hydrogels. In this review, we would like to focus on photo-crosslinking due to its rapid in situ gelling, good cytocompatibility and low toxicity[11,12]. Besides, the crosslinking density and physicochemical properties of photo-crosslinkable hydrogel could be precisely controlled through adjusting the intensity of light and exposure time to promote cell proliferation and differentiation[12,13]. For example, by controlling crosslinking density of the photo-crosslinkable hydrogel, Li et al. regulated the morphology of chondrocytes in 3D gelatin methacrylate (GelMA) environment. Cells exhibited round shape in hard hydrogel with stiffness at ~30 kPa; elongated shape in soft hydrogel (~4 kPa) and chondrogenic phenotype in hydrogels with medium stiffness (~17 kPa)[14]. Cell proliferation could also be influenced by cross-linking density. In one study, Bryant et al. found that the increase in crosslinking density of poly(ethylene glycol) diacrylate (PEGDA) hydrogel resulted in a decrease of chondrocytes proliferation and protein expression; in another study, Marklein et al. discovered that human mesenchymal stem cells (hMSCs) exhibited increased cell proliferation on a stiff hyaluronic acid methacrylate (HAMA) hydrogel compared to a softer one in 2D condition[15,16]. In terms of cell differentiation, Bian et al. investigated the effect of crosslinking density of photo-crosslinkable hydrogels on encapsulated mesenchymal stem cells (MSCs)[17]. They found that the degree of photo-crosslinking can regulate the differentiation of MSCs: Enhanced chondrogenesis in a soft environment and osteogenesis in a stiff environment, which provides important clues for the design of photo-crosslinking hydrogels for cartilage and bone repair. Due to these unique features, photo-crosslinkable hydrogels have shown a wide range of applications in biomedical fields including organ printing, tissue engineering, disease modeling, and high-throughput drug screening[18].
In this review, we summarize the classification, crosslinking mechanism and application of photo-crosslinkable hydrogels for 3D bone and cartilage bioprinting (Figure 1). The cell types and additives encapsulated in hydrogels to promote bone and cartilage reconstruction are additionally discussed. Finally, the future prospects of bone and cartilage 3D bioprinting are outlined.
Figure 1.
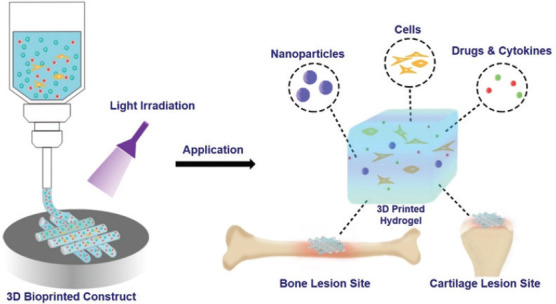
Schematic illustration of photo-crosslinkable hydrogels for bioprinting bone and cartilage tissues.
2. Photo-crosslinkable hydrogel bio-ink systems
In this section, we focus on photo-crosslinkable hydrogels, and introduce additives (such as nanomaterials, functional cells, and drugs or cytokines) which can improve the physical and biological properties of hydrogels.
2.1. Photo-crosslinkable hydrogels
For photo-crosslinkable materials, when they are exposed to a suitable light, the liquid state solution can be solidified. In general, photo-polymerization includes free-radical initiated chain polymerization and bio-orthogonal click reaction. In free radical photo-polymerization, the functionalization of hydrogel prepolymers with active groups (such as methacrylates, vinyl esters, and acrylates) is an essential step. Under light irradiation, the absorbed photons of photoinitiator promote its cleavage, thereby encouraging the generation of free-radical molecules. Then, these molecules will react with the vinyl bonds, leading to the formation of chemical crosslinks between prepolymers[19]. In photo-click reaction, the thiol-ene reaction is widely used in hydrogel fabrication, where the addition of thiols to carbon-carbon double bonds contributes to polymerization[20]. Derived from natural or synthetic polymers, several hydrogel materials undergoing photo-polymerization have been explored. These materials contain various functional groups, which enable different functionalization strategies for photo-crosslinking.
Hyaluronic acid (HA) is a linear glycosaminoglycan[21]. As one of the main components of ECM and important glycosaminoglycan in connective tissues, HA plays a key role in rheological and structural function[22]. Furthermore, due to the interactions between certain cell receptors, HA can regulate cell proliferation, migration and differentiation[23]. Due to many abundant functional groups in its chemical structure such as N-acetyl group, carboxylic acid, and primary/secondary hydroxyl groups, photo-polymerizable groups such as methacrylates and norbornenes can be modified on HA[24,25]. The most popular chemical modification for HA is using methacrylic anhydride (MA), which can be introduced into carboxy or hydroxy groups of HA in mild conditions (referred to as HAMA). The properties of HAMA can be regulated by tuning the polysaccharide derivatization degree and the concentration of pre-polymer or illumination time[26]. For example, the elastic modulus of HAMA hydrogel can be enhanced when increasing the polymer concentration. Furthermore, varying the polymer concentration can also regulate the degradation rate of HAMA hydrogel, where the high degradability is essential for cell remodeling in 3D tissue applications[27].
Gelatin is an FDA-approved food processing material comprising a mixture of peptide sequences (arginine-glycine-aspartic acid) that enhance cell attachment[28]. As one of the popular substrates of hydrogels, gelatin has strong biocompatibility and low antigenicity[29]. Meanwhile, it can be easily obtained by denaturing collagen through acid or base treatment[21]. However, gelatin is a thermo-responsive and water-soluble material which can undergo a reversible sol-gel transition below body temperature[30]. To prevent the degradation of gelatin, forming chemical crosslinks by photo-polymerization is an effective method, which is usually achieved through modifying primary amines with ethylenic/thiol groups or methacrylate groups. The norbornene/thiol-functionalized gelatin can form crosslinked hydrogels through photo-click reaction, and by controlling the concentration of thiol/ene substitution, crosslinking degree of hydrogels can be tuned[31]. Meanwhile, functionalization with MA is also an ideal way to counteract its fast degradation and poor mechanical property[32]. Similar to HAMA hydrogel, by controlling the amount of MA and degrees of methylation, the hydrogels (referred to as GelMA) will possess different mechanical properties. Besides, increasing the degrees of methacrylation can enhance the matrix density, thereby increasing the compressive modulus and reducing the degradation rate of GelMA[6,33]. Furthermore, the elastic modulus and adhesive strength of GelMA can be controlled by regulating the polymer concentration[12,34,35].
Chitosan (CS) is derived from chitin, which can be obtained from the exoskeleton of insects and crustaceans as well as the cell walls of fungi[36]. As a natural material, CS has good biodegradability, and it can stop bleeding and promote tissue regeneration, thus having wide usage in wound healing and scar prevention[37]. Due to active amino and hydroxyl groups in CS, there are many opportunities to introduce photoactive groups into CS through the chemical reactions between -NH2/-OH and substituting groups, such as azido-functionalized CS, vinylated CS, and MA-CS[38]. However, this mechanical strength of azido or vinylated-functionalized hydrogel is relatively low, and the insufficient biocompatibility limits further applications. At present, the most common method for preparing photo-crosslinkable CS is by grafting MA groups to provide good mechanical properties as well as suitable biocompatibility[39].
Polyethylene glycol (PEG) has high hydrophilicity with abundant functional groups for chemical modification and adjustable properties, which is widely used in various biomedical applications. PEG hydrogel formed by photo-click reaction is one of the common methods to achieve photo-polymerization. The biophysical or biochemical properties in the thiol-ene PEG hydrogel can be tuned with the degree of substitution[40]. Meanwhile, PEGDA, PEG dimethacrylate (PEGDMA), and ethylene linkage multi-arm PEG (n-PEG) are the most commonly used macromolecules in photo-polymerization and of which, PEGDA is very popular in terms of bioprinting. Under ultraviolet (UV) or visible light exposure, the double-bond acrylate groups in PEGDA can initiate rapid photo-polymerization to form a 3D polymer network and in the presence of a photoinitiator[41], and the mechanical strength can be controlled by changing the molecular weight or the concentration of the hydrogel[42].
2.2. Control of photo-crosslinkable structures with various parameters
The integrity of photo-crosslinkable scaffolds is determined by both the strength of the individual polymer chain as well as the density of crosslinking networks within the hydrogel matrix. Naturally, high molecular weight hydrogels possess higher matrix stiffness due to the larger proportion of less bendable polymer backbones compared with the more flexible connective ends between polymer chains. The increase in polymer concentration naturally enhances matrix strength as the density of inter-linkage between polymer chains increase. Yet increase in hydrogel concentration in aqueous solutions often exhibits high viscosity, which impedes the extrusion process due to the shear stress. Alternatively, the density of crosslinks can be chemically modified through the degree of substitution, which is the ratio of substituent group to unmodified group in a photopolymer. A high degree of substitution greatly increases the chance of successful crosslinks throughout the hydrogel matrix without actual addition of polymer chains, and contributes toward increased complexity of the polymer network as well as improved stiffness and lowered porosity of printed structures[43].
By modulating the light intensity and exposure time, photo-polymerization controls the formation process of the hydrogel and its properties, including the crosslinking density and matrix stiffness[12]. This can be attributed to energy-dependent regulation of photoinitiator activation and crosslinker reactivity[44]. While the presence of photoinitiators can greatly lower the energy barrier for initiating the often-simple click-reactions of crosslinking groups such as acrylates, under appropriate photo-crosslinking conditions, sufficient photonic energy is required to complete the photo-crosslinking process, whereas by intentionally limiting light exposure intensity and duration, partially crosslinked matrixes are formed with reduced stiffness and strength to mimic softer tissues. This creates a two-way array to regulate the mechanical features of photo-crosslinkable hydrogels entirely from tuning the light source alone. To achieve complete crosslinking, light intensity and exposure time are interdependent as the amount to the total energy supplied to the reaction between polymer chains and crosslinkers. Herein, a high intensity enables low exposure time, and vice versa. This creates an interesting dynamic between these two parameters when applied in photo-bioprinting as vigorous limitations exist in terms of biocompatibility in additive manufacturing[45]. In addition, cells are highly sensitive to external changes in the microenvironment including shear, heat, and radiation. As radioactive damage and heat generated by light sources such as lasers are unavoidable when crosslinking cell-embedded hydrogels, a balance of intensity and exposure is required to preserve cell viability of printed structures. For example, alginate/GelMA bio-inks can be crosslinked at as low as 4 mW cm−2 with UV light source to ensure 80% survival of cells in printed scaffolds, spanning over 20 – 60 kPa modulus under various UV exposure[46]. Yet the same approach faces difficulties in SLA, where light intensity decays as it goes through the medium in which it is absorbed in accordance with the Beer–Lambert equation. This creates dilemma where light intensity cannot be maintained in deeper areas of the hydrogel matrix to surmount the energy barrier required to achieve photo-crosslinking. In addition, a decrease in intensity impedes resolution as it increases diffraction of light, which greatly limits the fidelity of printed structures in reservoir-based printing. Hence, to elucidate the effects on light intensity, exposure time, and cell density, the previous studies have created GelMA-printed model phase diagram between 7 – 16 mW/cm2 and 15 – 45 s, where areas for underexposure and overexposure were plotted[47]. With the advancement in neural network technology, machine learning has also been used to predict cell viability in SLA bioprinting, with exceptional accuracies in predictions at as low as 10% of total data supplied[48]. The learning algorithm concluded that exposure time had the greatest effect on cell viability, followed by layer thickness, GelMA concentration, and light intensity. Thus, the adjustment of light source alone in terms of intensity and exposure time has high controllability as well as predictability in tuning mechanical properties and maintaining cell viability.
2.3. Additives in photo-crosslinkable hydrogel systems
To fabricate functional hydrogel platforms, many additives such as nanomaterials, functional cells, drugs, and/or cytokine are introduced into the hydrogel systems, bestowing on those hydrogels improved physical and biological properties.
(1) Nanomaterials
The majority of human bone tissues range from cancellous to cortical structures. It is difficult to design an ideal scaffold by a single pure hydrogel material due to the diversity in geometric mechanics and mechanical strength of bone tissues. Herein, nanomaterials can be integrated into hydrogels with multiple gradients and good mechanical properties, which may help solve this challenge. Nanomaterials can control the micro- and nano-scale structures of hydrogel as well as regulate hydrogels’ mechanical properties without hindering the exchange of nutrients with the surrounding environment[49]. Meanwhile, the nanomaterial itself can also work as a drug delivery system.
For example, Zuo et al. combined GelMA with hydroxyapatite (Hap) for osteon biofabrication[50]. As the main inorganic composite material of bone matrix, Hap can guide and induce bone formation. Furthermore, the introduction of Hap into GelMA network could enhance the mechanical rigidity. The results demonstrated that compared to the pure hydrogel, this composite hydrogel showed a lower swelling behavior, higher mechanical modulus, and better biocompatibility, which had a prospective application for bone reconstruction. In another example, Zhang et al. prepared a self-assembled metallic-ion nanocomposite hydrogel[49]. This hydrogel consisted of bisphosphonate-grafted HA (HABP) and magnesium chloride (MgCl2). The coordination between BP and magnesium ions (Mg2+) contributed to the formation of acrylated-BP-Mg-nanoparticles (Ac-BP-Mg NPs), which would stabilize the hydrogel network as multi-valent crosslinker, increasing the mechanical properties and contributing to the injectability as well as self-healing characteristics. The acrylate groups could be crosslinked under UV irradiation, and allowed for better control over stiffness. This nanocomposite hydrogel allowed for encapsulation of stem cells, which could be used for bone engineering. In a study of Shi et al., the authors fabricated a rapidly photo-crosslinkable hydrogel with Kartogenin (KGN)-loaded nanoparticles to prepare cartilage[51]. The small molecule KGN (which could induce bone marrow-derived MSCs [BMSCs]) into chondrocytes) was encapsulated into poly (lactic-co-glycolic acid) (PLGA) nanoparticles. Then, the KGN-PLGA were incorporated into photo-crosslinkable acrylated HA (m-HA), and this system could form a hydrogel scaffold in situ under UV light treatment. In vivo experiments demonstrated that the regenerated tissue was close to the natural hyaline cartilage.
(2) Cells
For bone engineering, BMSCs are the most widely used cells since it possesses the ability to differentiate into functional bone cells[52]. Meanwhile, articular cartilage-resident chondroprogenitor cells (ACPCs) represent an opportunity for cartilage regeneration[53]. In addition, some bone cells such as osteoblasts and chondrocytes have also been introduced into hydrogels[54], while other cells associated with bone growth such as human umbilical vein EC (HUVECs) provide abundant opportunities for bone tissue repair[55]. Vascularization is also essential for bone regeneration process especially for large bone defects. ECs are the main cells with angiogenic ability. Three types of ECs, HUVECs, human dermal microvascular EC (HDMVECs), and endothelial progenitor cells (EPC), have been reported to create vessel-like structures in in vitro culture[56]. Incorporating ECs in the hydrogel scaffolds could induce the regeneration of new blood vessels by creating capillary networks. These prevascularized constructs could provide nutrients to surrounding cells and reduce the time to anastomose with host vasculature, which would promote angiogenesis and osteogenesis for bone regeneration[57]. To fabricate a suitable microenvironment, photo-polymerized hydrogels can be used for cell transplantation, where the hydrogel materials provide a cell-favorable environment which allows for diffusion of nutrients, oxygen, and metabolic products.
For example, in a study of Zhai et al., the authors developed a biodegradable two-channel 3D bioprinting ink consisting of both PEGDA and Laponite nanoclay in channel A, and rat osteoblasts (ROBs)-laden HA in channel B[58]. The bio-ink A, composed of a PEG-clay nanocomposite crosslinked hydrogel, was used to prepare 3D-bioprinting and effectively deliver oxygen and nutrients to cells. Meanwhile, it could promote osteogenesis due to the released silicon ions (Si4+) and Mg2+, while the bio-ink B, ROBs-ladened HA, was adopted to improve distribution uniformity, deposition efficiency and cell viability. The two inks were alternately extruded through a two-channel 3D-bioprinting machine to construct osteoblast-laden nanocomposite hydrogel for bone regeneration. The printed scaffolds showed excellent osteogenic potential in in vivo experiments. In another study, Annika et al. co-encapsulated EC (HDMVECs) and osteogenic cells (human adipose-derived stem cells) in GelMA hydrogel for engineering vascularized bone. Results showed that this co-culture system could promote the formation of vascular networks and enhance bone regeneration[59].
(3) Drugs and cytokines
Some drugs or cytokines play a key role in promoting bone cell growth, modulating cell proliferation, and differentiation as well as regulating the formation of ECM. Statins[55], osteoprotegerin[60], αvβ3 integrin antagonists[61], cathepsin K inhibitors[62], parathyroid hormone[63], transforming growth factor-β, and bone morphogenetic protein (BMP)[64] have all been considered for stimulation of bone growth. Apart from drugs and cytokines for bone formation, angiogenic growth factors, vascular endothelial growth factors (VEGF), fibroblast growth factor, hepatocyte growth factor, and platelet-derived growth factor have been used for the modulation of vessel formation[65,66]. For delivering drugs or therapeutic agents to bone sites, various approaches have been investigated, such as nanoparticles, nanofibers, and films. Among them, hydrogel attracts much attention owing to its compatibility and hydrophilicity, and it can regulate release performance by controlling swelling or degradation[67]. Photo-polymerized hydrogels have also been used for localized drug delivery depots due to the in situ formation of hydrogels and the direct adhesion to the targeted tissue. As a bulk reservoir, hydrogels allow for encapsulation of cells, drugs or nanoparticles, and provide physical support at bone lesion site.
In a report by Kim et al., they fabricated PEGDA/chondroitin sulfate-based hydrogels and studied them as biomineralizing 3D scaffolds[68]. Chondroitin sulfate has negative charge on its sulfate group, so it binds with charged ions such as calcium and phosphate to form an osteogenically favorable microenvironment, which will induce the biomineralization and osteogenic differentiation of stem cells. The results proved that the ion binding and distribution in hydrogel were related to the concentration of chondroitin sulfate, and PEGDA/chondroitin sulfate-based hydrogels induced osteogenic differentiation of stem cells in vitro. After transplanting this hydrogel into a critically sized cranial defect model, 10% chondroitin sulfate hydrogel induced effective bone regeneration with the highest bone density.
In another study, Xin et al. prepared recombinant human BMP-2 (rhBMP-2)-laden GelMA hydrogel to accelerate bone repair[69]. rhBMP-2 can localize BMSCs to the site of bone injury, promoting proliferation, and osteogenic differentiation. Mesoporous bioglass nanoparticles (MBGNs) were used to load rhBMP-2 by grafting, and then it would photo-crosslink with GelMA hydrogel (GelMA/MBGNs-rhBMP-2), where rhBMP-2 could be controllably released at an early stage of bone regeneration, and calcium/silicon ions in MBGNs would be released to keep promoting osteogenesis in a long term. Incorporation of MBGNs-rhBMP-2 in GelMA hydrogel could better control the release rate as well as local concentration of rhBMP-2, while the MBGNs could be fixed at the lesion site without clearance by body fluids. This hydrogel showed strong ability in osteogenesis and bone tissue regeneration in vivo.
In summary, photo-crosslinkable hydrogels have incorporated nanomaterial components, cells, drugs, and/or cytokines to produce viable bone tissue scaffolds. With the improved physical and biological properties, it is of great significance to apply them in 3D printing technology to broaden its application.
4. Applications of 3D bioprinted photo-crosslinkable hydrogels for bone and cartilage regeneration
Over the last decade, there has been immense progress in 3D bioprinting skeletal systems using photo-crosslinkable hydrogels. In this part, we review the applications of 3D bioprinted photo-crosslinkable hydrogels for bone and cartilage tissue engineering.
4.1. Bioprinted hydrogels in bone tissue engineering
Bone has a highly specialized structure with a mineral matrix, multiple cells, and vascular networks. Reconstruction of large-scale bone defects remains challenging due to the lack of biomimetic architectural, bioactive factors, and functional vasculature. With the advance of 3D bioprinting, more complicated bionic 3D constructs could be bioprinted, with different cell types and growth factors with hydrogel for better bone regeneration to imitate the hierarchical structure and function of natural bone. Cui et al. developed a mimetic bone structure, with a hard mineral matrix, a soft organic matrix and vascularized networks (Figure 2A)[70]. The structure was constructed through a dual 3D bioprinter including a Fused Deposition Modeling (FDM) and SLA 3D bioprinter, by alternately depositing cell-loaded GelMA hydrogel (hMSCs and HUVECs), and biodegradable polylactide (PLA) (Figure 2Ai). This capillary structure allows cells to evolve and expand uniformly in the 3D space during culture period (Figure 2Aii). Moreover, bioactive growth factors such as BMP-2 and VEGF peptides were added into the scaffold designs to further facilitate osteogenesis and angiogenesis. Results indicated that the 3D printed biomimetic bone constructs could integrate with surrounding native bone tissue, showing an excellent bone regeneration and significant angiogenesis ability. This study also provides a feasible strategy for the construction of a hierarchical structure with multiple functions, thereby meeting the current challenges for large bone repair.
Figure 2.
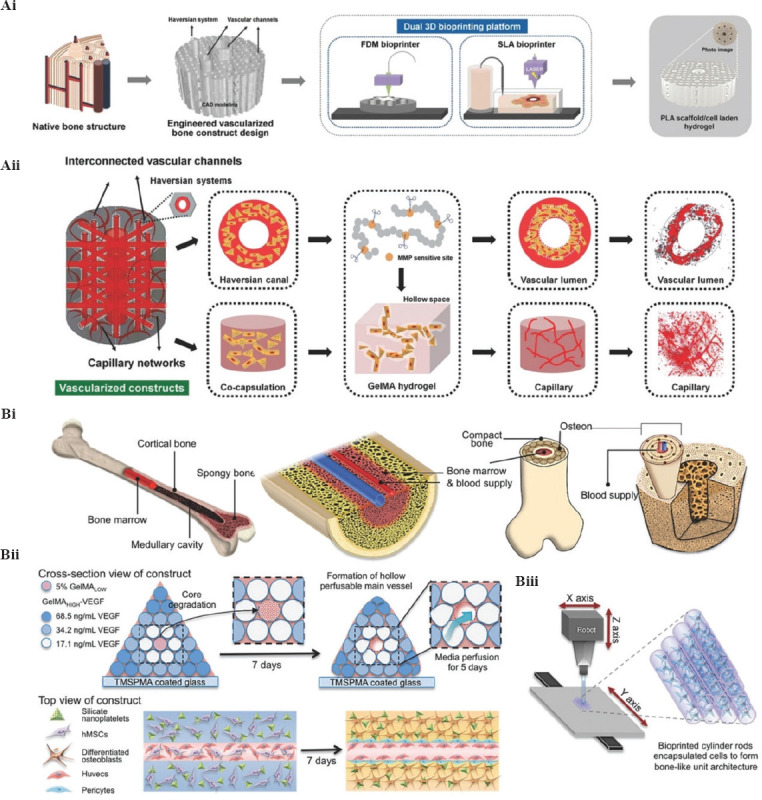
Construction of 3D mimetic bone tissue by 3D bioprinting. (Ai) Schematic diagram of the structure of a native bone; design and fabrication of the engineered vascularized bone structure. (Aii) Schematic diagram of the microstructure of vascularized construct based on matrix metalloproteinase (MMP)-sensitive GelMA hydrogel, the formation of vascular lumen, and capillary network in different regions[70]. (Reproduced from H. Cui, W. Zhu, M. Nowicki, et al., Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs Via Dual 3D Bioprinting: Integrating Regional Bioactive Factors Into Architectural Design, Wiley. © 2016 WILEY-VCH Verlag GmbH Co. KgaA Weinheim). (Bi) Schematic diagram of a complex bone structure. (Bii) Schematic diagram of the fabrication process of complex bone construct through 3D bioprinting strategy. (Biii) Illustration of bioprinted cell-laden hydrogel cylinders through an SLA bioprinter[6]. (Reproduced from B. Byambaa, N. Annabi, K. Yue, et al., Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue Wiley. © 2017 WILEY-VCH Verlag GmbH Co. KGaA, Weinheim).
To address the challenges of large-scale bone tissue with a 3D vascularization network, Khademhosseini et al. fabricated a biomimetic bone tissue construct comprised of a perfusable vascular lumen through an extrusion-based bioprinting strategy (Figure 2B)[6]. First, they used bioprinting technology to imitate the overall structure of native bone tissue (Figure 2 Bi). Then, the GelMA hydrogel cylinders were individually printed (Figure 2Bii). After piling up these cylinders, pyramidal constructs were formed (Figure 2Biii). They also encapsulate angiogenic cells, and osteogenic cells and silicate nanoplatelets into the GelMA hydrogel simultaneously to promote bone regeneration ability. Through optimization of bioprinting conditions, they could print a well-defined, multicellular bone tissue construct in a large scale. Results indicated that the biomimetic bone construct has high structural stability and promotes cell proliferation during in vitro culture. Immunostaining and reverse transcription quantitative polymerase chain reaction (RT-qPCR) proved the formation of new bone tissue in the HUVEC/hMSC group after in vitro culture. Demonstrably, this study provides a superior strategy for the construction of large biomimetic bone-like structures with vasculature meeting the clinical demands of large-scale bone defects.
4.2. Bioprinted hydrogels in cartilage tissue engineering
Natural cartilage is a smooth and elastic tissue with poor self-repair ability. Usually, super-physiological shock load and osteoarthritis result in cartilage defects. The regeneration of cartilage is limited by its low number of cells[71]. The difficulties of fabricating artificial cartilaginous tissue can be solved by bioprinting because this printing technique allows for encapsulation of implanting cells. Meanwhile, the hydrogel has a striking similarity to the ECM of natural cartilage[72]. Thus, 3D bioprinted hydrogels become a potential alternative therapy for cartilage repair. Duchi et al. developed a hand-held extrusion-based bioprinter for 3D bioprinting cartilage (Figure 3A). They printed a core-shell structure with a cell-free but photo-crosslinkable hydrogel shell and a cell-loaded core hydrogel[73]. The printed structure was cross-linked by UV light for 10 s for photo-polymerization and the viability of cell remained above 90% after 7 days of printing. This study served as a proof-of-concept for the in situ cartilage bioprinting and cartilage regeneration. In another study, Burdick et al. proposed an in situ crosslinking method for 3D bioprinting photo-crosslinkable hydrogel (Figure 3Bi)[74]. They further used a norbornene-modified HA (NorHA) as a representative bio-ink for cartilage regeneration. By adjusting the printing parameters (i.e. light intensity, exposure time, and printing speed), they obtained an optimal curing condition. The whole bioprinting process was cytocompatible. Post-printed MSCs distributed homogenously in the printed construct with high cell viability. After 56 days of culture in vitro, the bioprinted cartilage constructs showed an increase in moduli, biochemical expression, and cartilage regeneration (Figure 3Bii).
Figure 3.
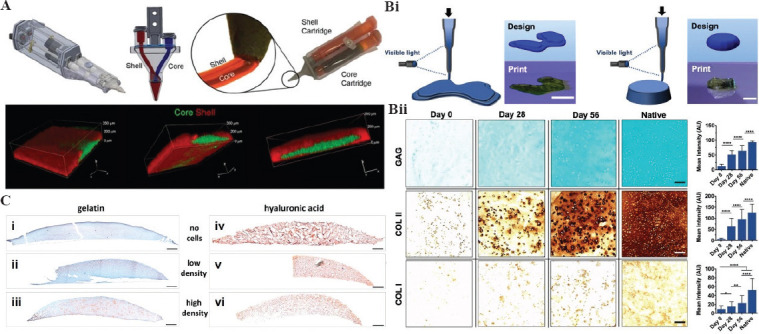
Construction of 3D mimetic cartilage tissue by 3D bioprinting. (A) Schematic representation of a core/shell 3D printing by co-axial extrusion printer and confocal images of 3D printed core/shell structure[73] (from ref.[73] licensed under Creative Commons Attribution 4.0 license with permission). (Bi) Representative images of 3D constructs printed through an in situ crosslinking method. (Bii) Histological staining of printed cartilage constructs; left image shows the representative staining images and right image shows quantification analysis in different culture time. Scale bar = 100 μm[74] (from ref.[74] licensed under Creative Commons Attribution 4.0 license). (C) Detection of proteoglycans in printed constructs after 14 days of incubation (red: proteoglycans; green to blue: nuclei and other ECM/bio-ink). Porcine chondrocytes were embedded in gelatin (i, ii, iii) and hyaluronic acid (iv, v, vi) bio-inks. Scale bar = 500 μm[75] (from ref.[75] licensed under Creative Commons Attribution-Non Commercial 4.0).
In a 3D bioprinting process, the interaction between cell patterning and hydrogel matrix has a great effect on the performance of printed cells. For instance, Lutz Klok et al. fabricated different 3D cartilage tissue constructs with different cell concentrations (Figure 3C)[75]. The bio-inks were prepared by homogeneously mixing cell suspensions with GelMA or HAMA hydrogel separately and then printed in a layer by layer process through SLA-based bioprinting. Histological staining indicated that cells distributed homogeneously in both GelMA (Figure 3Ci-iii) and HAMA constructs (Figure 3Civ-vi). A noticeable difference in cell number can be observed between low and high cell density constructs. Results indicated that both GelMA scaffolds and HAMA scaffolds supported the recovery of chondrocyte phenotype and formation of cartilage ECM with uniform cellular distribution. Meanwhile, the increase in cartilage-specific genes expression, type II collagen, and aggrecan, in cell loaded hydrogels proved successful cartilage formation. Compared with HAMA, GelMA showed more chondrocyte phenotypes as confirmed by the higher expression of cartilage genes. Another element that affects cartilage formation is the cell density; a high cell density (2.5×107 cells/mL) showed better cartilage regeneration ability compared with a low cell density (5×106 cells/mL).
4.3. Bioprinted hydrogels in osteochondral tissue engineering
Osteochondral defect involving both the cartilage and subchondral layer is difficult to repair due to their difference in physiological structures and bioactive properties. The cartilage tissues do not have vasculature and nervous systems but the subchondral bone tissues are rich in blood and nerves. In addition, the structure and function of cartilage and subchondral bone are also different[76]. Thus, how to promote cartilage and subchondral bone regeneration simultaneously has become quite the challenge for osteochondral repair. The rise of 3D bioprinting, allowing for the fabrication of complicated 3D scaffolds with precise control of intricate geometry composition and functions, is a promising treatment for osteochondral repair. Meanwhile, photo-crosslinking hydrogels are promising bio-inks for osteochondral regeneration as we discussed in the previous chapters[77].
In an earlier study, Cui et al. bioprinted a photo-crosslinkable 3D osteochondral tissue using PEGDMA and human chondrocytes. Simultaneous photo-polymerization maintained the precise positions of deposited cells and reduced photo toxicity[78]. This bioprinted cell-laden hydrogel constructs integrated excellently with the native tissue and promote osteochondral repair. In another study by the same group, Cui et al. further printed mechanically strong and tissue differentiated bone and cartilage constructs using photo-crosslinkable PEG-GelMA hydrogel with hMSCs[55]. The printed constructs provided strong mechanical support and showed an excellent osteogenic and chondrogenic regeneration capacity, suggesting their desirable potential in osteochondral repair. Recently, Gao et al. developed a biohybrid gradient construct for osteochondral regeneration by 3D bioprinting (Figure 4A)[79]. By incorporating cleavable poly (nacryloyl 2-glycine) (PACG) into GelMA, the mechanical properties of hydrogels had been significantly improved with a tensile strength of up to 1.1 MPa and a compressive strength up to 12.4 MPa (Figure 4B). Results demonstrated that the photo-crosslinkable gradient hydrogel scaffold could not only facilitate the regeneration of cartilage (Figure 3Ci-ii) but also promote the regeneration of subchondral bone (Figure 4Ciii-iv).
Figure 4.
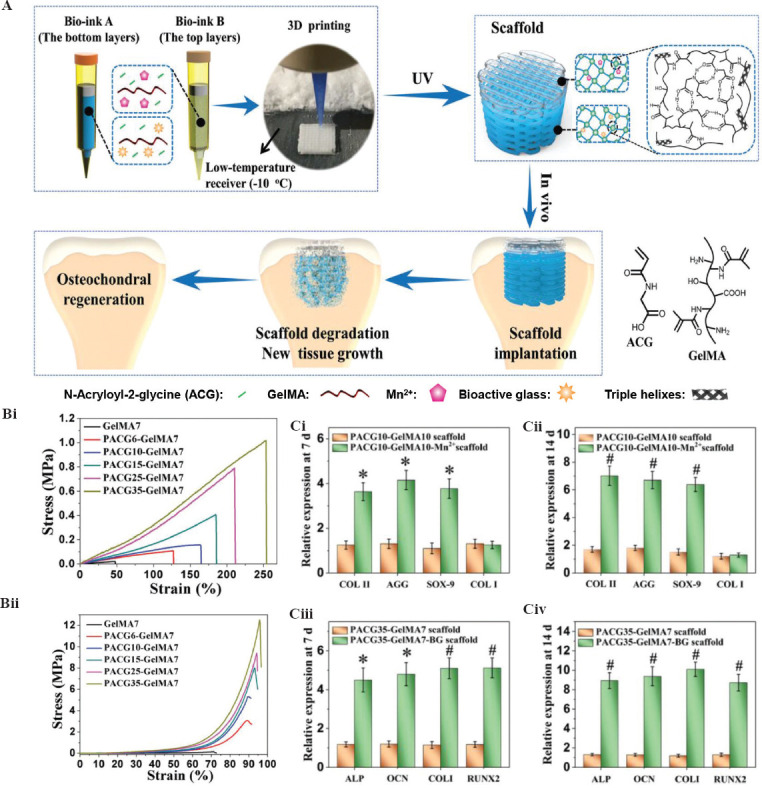
Construction of 3D mimetic osteochondral tissue by 3D bioprinting. (A) Schematic diagram of the biohybrid gradient scaffolds for osteochondral regeneration. (B) Mechanical properties of poly (nacryloyl 2-glycine) -GelMA hydrogels: (i) tensile strength and (ii) compressive strength. (C) Genetic analysis for osteochondral differentiation: (i and ii) Expression of cartilage-related genes (COL II, aggrecan, SOX-9, and COL I) and (iii-iv) expression of osteogenesis-related genes (ALP, OCN, COL I, and RUNX2) after 7 and 14 days of culture, respectively[79] (from ref.[79] licensed under Creative Commons Attribution 4.0 license).
4.4. Bioprinted hydrogels in bone disease model
Apart from the above discussed applications, 3D bioprinted hydrogels can also be used to create bone disease models and drug screening research. At present, most bone disease models are still 2D, which fails to reconstruct the complex of the in vivo environment[80]. Conversely, 3D bioprinting has the promising potential to create 3D mimic bone models as discussed in the previous sections, allowing for cell-cell or cell-matrix interactions and integration of a vascular system[81]. Hence, these bioprinted models could be better used for bone disease study and drug screening purposes[82].
For example, 3D-printed tissue models mimicking the native bone tissues can be applied in drug screening to test the efficacy and toxicity of new drugs, and promoting the translation of new therapeutic molecules in clinic[83]. Recently, Amir K et al. designed and bioprinted a musculoskeletal junction model mimicking the musculoskeletal interface by an SLA-based bioprinting platform (Figure 5Ai)[84]. Photo-crosslinkable GelMA was selected as a bio-ink due to its high biocompatibility for cell spreading and functionality. The musculoskeletal interface is loaded with three different cell types, MSCs, fibroblasts, and osteoblasts (Figure 5Aii). As shown in Figure 5Aiii-iv, the printed 3D pattern has a well-defined construct showing a close similarity with the designed one (Figure 5Aii). The proposed musculoskeletal junctions provide a multi-material microstructure on demand for multi-applications in skeletal related tissue engineering and regenerative medicine, thus facilitating new drug discovery and clinical use.
Figure 5.
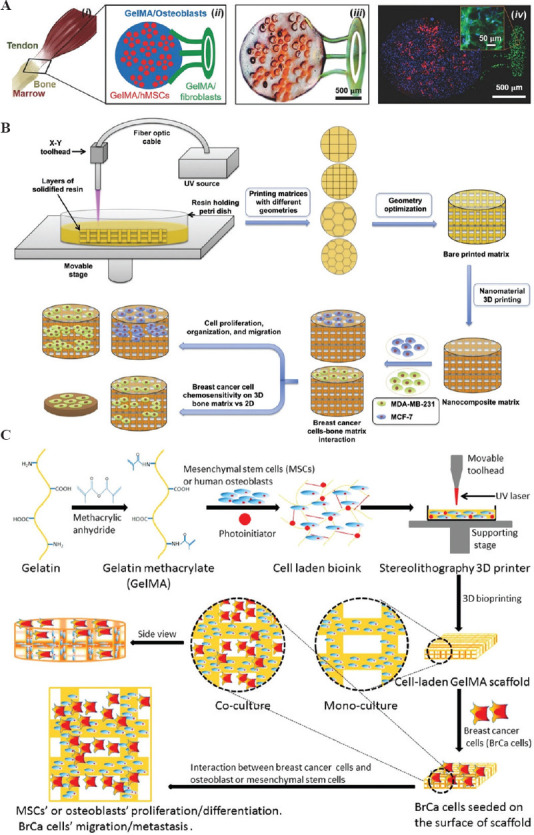
Construction of 3D bioprinted hydrogels in bone disease model. (A) Fabrication of a musculoskeletal interface model: (i) Schematic diagram of a native insertion site; (ii) illustration of the printing model; (iii) a representative image showing the bioprinted structure; (iv) fluorescent image of the bioprinted structure; blue: osteoblasts, red: MSCs, and green: Fibroblasts[84]. (Reproduced from A. K. Miri, D. Nieto, L. Iglesias, et al., Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting from Wiley. © 2018 WILEY-VCH Verlag GmbH Co. KgaA Weinheim). (B) Illustration of 3D bioprinted PEGDA bone matrix model for breast cancer cell invasion research[85]. (Reprinted from Nanomed-Nanotechnol,12(1), W. Zhu, B. Holmes, R. I. Glazer, et al., 3D Printed Nanocomposite Matrix for The Study of Breast Cancer Bone Metastasis, 69 – 79, Copyright (2016), with permission from Elsevier). (C) Schematic diagram of 3D bioprinted GelMA bone matrix model for breast cancer metastasis study[86]. (Reprinted with permission from X. Zhou, W. Zhu, M. Nowicki, et al., Acs Appl Mater Inter, 2016, 8(44): 30017 – 30026, Copyright (2016) American Chemical Society).
In addition, 3D-printed tissue models could also be applied in creating cancer models for cancer metastasis research. One of the primary transfer sites for breast carcinoma is bone; however, the fundamental mechanisms are still unknown because of the lack of 3D biomimetic models for cancer research. Therefore, Zhu et al. created a series of 3D bionic bone models for breast cancer bone metastasis study through 3D bioprinting[85]. In their early study, the authors printed the 3D bone matrix using a nano-bioink consisting of Hap and PEGDA (Mn 700) hydrogel with 0.5% photo-initiator (Figure 5B). Results indicated that the cocultivation of tumor cells and BMSCs increased the formation of spheroid clusters. Zhu et al. further investigated the interplay between the cancer cells and bone stromal cells (fetal osteoblasts and MSCs) on a GelMA-based bionic bone matrix fabricated by 3D bioprinting (Figure 5C)[86]. These 3D bionic bone models also exhibited better drug fastness of breast cancer cells compared with 2D culture, suggesting its potential for assessing drug sensitivity. Results demonstrated that the 3D bionic bone models, with breast cancer cells and bone stromal cells, offer a suitable 3D model for the research of the interaction between cells and a bionic bone microenvironment, and thus, might serve as a desirable model for the studying the progression of metastatic breast cancer and drug screening in bone.
5. Conclusion and future perspectives
Since 3D printing can control the structure of tissue scaffolds with high resolution, accuracy and reproducibility, it has become a key fabrication process for bone and cartilage engineering[87]. Although there are numerous advantages of 3D printing in biomedical field, the involvement of cells and sensitive biomolecules leads to rigorous requirements for ink selection. Hydrogels are gold standard materials for bioprinting because of its elastic and hydrated properties as well as the ECM-mimetic crosslinked network structure, which enable cell survival and retention of their functions. Among various crosslinking methods, photo-polymerization has been widely used due to low toxicity, high crosslinking efficiency and in situ gelling capability. Through modification by computer-aided design/manufacturing or medical imaging systems, photo-crosslinkable hydrogels can be personalized at different bone length scale by 3D printing. With the application of UV light, the photo-crosslinking hydrogels undergo rapid formation immediately after printing[88]. By changing the UV intensity and exposure time, the performance of hydrogels including crosslinking density and matrix stiffness can be exceptionally controlled. In addition, the concentration, degree of substitution or molecular weight of chemical modified precursors, as well as introduction of nanomaterials can further regulate the mechanical properties and swelling behavior of photo-crosslinkable hydrogels to meet different therapeutic needs. Moreover, cells, drugs and biochemical entities (e.g., growth factors) can also be added into photo-crosslinkable bio-inks, thereby endowing the bone scaffold with excellent biological functions and promoting bone repairing as well as regeneration. Up to now, this strategy has been explored in various bone tissues (such as hard bone, osteochondral, and cartilage tissue), and it can reconstruct bone disease models to investigate disease mechanisms and drug screening.
Looking forward, the development of 3D bioprinting bone tissues needs further investigation of the physical properties of bio-ink and combination with other technologies to fabricate functional and optimized bone scaffolds. For example, bioelectrical effect in natural bone has been proven to play a significant role in bone development and fracture healing. The endogenous electric field of living bone contributes to the regulation of cell metabolism, such as proliferation, differentiation, and migration[89]. Consequently, conductive photo-crosslinkable hydrogel can be developed for the construction of a biomimetic electro-microenvironment for bone tissue engineering. In general, conductive hydrogels can be constructed by adding nano-conductive fillers such as metal/carbon/graphene nanoparticles into a hydrogel matrix or by crosslinking with conductive polymers including polyaniline, polypyrrole, and polythiophene[90]. In addition, self-healing enables hydrogels to withstand repeated damage. Self-healing mechanism such as host-guest interactions, Schiff’s base reaction, ionic bonding, and hydrogen bonding can be incorporated into photo-crosslinking hydrogel for bone and cartilage tissue engineering to prevent the potential risks of repeated rupture and to restore its original properties after being damaged. In one study, Wei et al. introduced the host-guest non-covalent interaction into photo-crosslinked HA hydrogel to develop a double network dynamic hydrogel with excellent self-healing ability, enhanced toughness, and greater collagen deposition to support chondrogenesis and cartilage regeneration[91]. Moreover, to improve the toughness of photo-crosslinking hydrogel, composite hydrogels have been fabricated with enhanced mechanical performance by addition of organic or inorganic additives (e.g., clay, Hap, grapheme, and carbon nanotubes) for bone and cartilage engineering. Another barrier for the application of hydrogel to bone and cartilage repair is the adhesion and integration with surrounding tissues. The surrounding of most tissues, such as cartilage, is slippery and hard to bond with hydrogels; therefore, the integration with surrounding tissue is crucial for hydrogel retention and new tissue development. To address this challenge, adhesive hydrogel, for example, by modifying HAMA hydrogel with 3,4-dihydroxyphenylalanine groups[92], can be developed to improve the mechanical integrity between the hydrogels and the tissues. Meanwhile, the adhesive hydrogel provides sites for cell adhesion, proliferation and thus promotes tissue regeneration in vivo. Moreover, piezoelectricity can modulate cellular functions which assist the repair and regeneration of bone tissue[93]. Hence, introducing piezoelectric biomaterials (such as zinc oxide[93], lithium sodium potassium niobate[94], and barium titanate[95]) into 3D bioprinting hydrogels can blaze a new pathway for bone engineering.
When 3D printing is combined with microfluidics, vascularized bone scaffolds can be fabricated for organ-on-a-chip devices. The microfluidics technique can manipulate and regulate fluid at a small scale, and it can combine the geometry of channels (controlled by 3D printing) and cell confinement with fluid flow to reconstruct a physiological environment[96], which will reconstruct a structure similar to that of a natural bone and investigate the biological responses of cells in specific microenvironment. Moreover, as a new emerging concept, 4D bioprinting has attracted significant attention for constructing a smart and multi-functional bone scaffold. With “time” as a fourth dimension, this bone scaffold will respond and adjust its structure and function to the disease development process as well as mimic the dynamics of the native tissues[97]. To achieve 4D bioprinting, both bio-inks and related technologies should be further explored. Bio-inks should be sensitive to physical, chemical, or biological changes in microenvironment, necessitating the design of smart or stimuli-responsive materials. Meanwhile, advanced scanning system, updated model processing software, and improved printing technologies (such as lithography, femtosecond et al.) can simplify the operation process and optimize printed bone tissues. By investigating bio-inks combined with other advanced technologies and transitioning to 4D printing, scientists could construct scaffolds more similar to natural tissues for bone engineering.
In conclusion, 3D bioprinting is a rapidly advancing research field for bone engineering. With the advance of new technologies and biomaterials, we believe that this technique may be the key to giving patients with bone or cartilage defects a chance to improve their quality of life.
Acknowledgments
This work was supported by the grant from the Guangdong Basic and Applied Basic Research Foundation (2020B1515130002).
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
X.Z. supervised the entire writing process of the review. Q.M., J.R., and H.P.B. wrote the manuscript. Y.L. edited the manuscript. All the authors approved the review for publication.
References
- 1.Midha S, Dalela M, Sybil D, et al. Advances in Three-dimensional Bioprinting of Bone:Progress and Challenges. J Tissue Eng Regen Med. 2019;13:925–45. doi: 10.1002/term.2847. https://doi.org/10.1002/term.2847. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Luo D, Wang T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small. 2016;12:4611–32. doi: 10.1002/smll.201600626. https://doi.org/10.1002/smll.201600626. [DOI] [PubMed] [Google Scholar]
- 3.Bai X, Gao M, Syed S, et al. Bioactive Hydrogels For Bone Regeneration. Bioact Mater. 2018;3:401–17. doi: 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandrycky C, Wang Z, Kim K, et al. 3D Bioprinting for Engineering Complex Tissues. Biotechnol Adv. 2016;34:422–34. doi: 10.1016/j.biotechadv.2015.12.011. https://doi.org/10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen MR, Burr DB. Basic and Applied Bone Biology. Amsterdam, Netherlands: Elsevier; 2014. Bone Modeling and Remodeling; pp. 75–90. https://doi.org/10.1016/b978-0-12-416015-6.00004-6. [Google Scholar]
- 6.Byambaa B, Annabi N, Yue K, et al. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv Healthc Mater. 2017;6:1700015. doi: 10.1002/adhm.201700015. https://doi.org/10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groll J, Boland T, Blunk T, et al. Biofabrication:Reappraising the Definition of an Evolving Field. Biofabrication. 2016;8:013001. doi: 10.1088/1758-5090/8/1/013001. https://doi.org/10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 8.Ashammakhi N, Hasan A, Kaarela O, et al. Advancing Frontiers in Bone Bioprinting. Adv Healthc Mater. 2019;8:1801048. doi: 10.1002/adhm.201801048. https://doi.org/10.1002/adhm.201801048. [DOI] [PubMed] [Google Scholar]
- 9.Keriquel V, Oliveira H, Rémy M, et al. In Situ Printing of Mesenchymal Stromal Cells, by Laser-assisted Bioprinting, for In Vivo Bone Regeneration Applications. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-01914-x. https://doi.org/10.1038/s41598-017-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Zhang Q, Xu T, et al. Photocrosslinkable Nanocomposite Ink for Printing Strong, Biodegradable and Bioactive Bone Graft. Biomaterials. 2020;263:120378. doi: 10.1016/j.biomaterials.2020.120378. https://doi.org/10.1016/j.biomaterials.2020.120378. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen QV, Park JH, Lee DS. Injectable Polymeric Hydrogels for the Delivery of Therapeutic Agents:A Review. Eur Polym J. 2015;72:602–619. https://doi.org/10.1016/j.eurpolymj.2015.03.016. [Google Scholar]
- 12.Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. https://doi.org/10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayavenkataraman S, Yan WC, Lu WF, et al. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv Drug Deliv Rev. 2018;132:296–332. doi: 10.1016/j.addr.2018.07.004. https://doi.org/10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Chen S, Li J, et al. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers (Basel) 2016;8:269. doi: 10.3390/polym8080269. https://doi.org/10.3390/polym8080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant SJ, Chowdhury TT, Lee DA, et al. Crosslinking Density Influences Chondrocyte Metabolism in Dynamically Loaded Photocrosslinked Poly (Ethylene Glycol) Hydrogels. Ann Biomed Eng. 2004;32:407–17. doi: 10.1023/b:abme.0000017535.00602.ca. https://doi.org/10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 16.Marklein RA, Burdick JA. Spatially Controlled Hydrogel Mechanics to Modulate Stem Cell Interactions. Soft Matter. 2010;6:136–43. https://doi.org/10.1039/b916933d. [Google Scholar]
- 17.Bian L, Hou C, Tous E, et al. The Influence of Hyaluronic Acid Hydrogel Crosslinking Density and Macromolecular Diffusivity on Human MSC Chondrogenesis and Hypertrophy. Biomaterials. 2013;34:413–21. doi: 10.1016/j.biomaterials.2012.09.052. https://doi.org/10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen KT, West JL. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials. 2002;23:4307–14. doi: 10.1016/s0142-9612(02)00175-8. https://doi.org/10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 19.Annabi N, Tamayol A, Uquillas JA, et al. 25th Anniversary Article:Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv Mater. 2014;26:85–124. doi: 10.1002/adma.201303233. https://doi.org/10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Chen J, Deng C, et al. Click Hydrogels, Microgels and Nanogels:Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials. 2014;35:4969–85. doi: 10.1016/j.biomaterials.2014.03.001. https://doi.org/10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Van Vlierberghe S, Dubruel P, Schacht EJ. Biopolymer-based Hydrogels as Scaffolds for Tissue Engineering Applications:A Review. Biomacromolecules. 2011;12:1387–408. doi: 10.1021/bm200083n. https://doi.org/10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 22.Collins MN, Birkinshaw CJ. Hyaluronic Acid Based Scaffolds for Tissue Engineering a review. Rom J Morphol Embryol. 2013;92:1262–79. doi: 10.1016/j.carbpol.2012.10.028. https://doi.org/10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Sherman L, Sleeman J, Herrlich P, et al. Hyaluronate Receptors:Key Players in Growth, Differentiation, Migration and Tumor Progression. Curr Opin Cell Biol. 1994;6:726–33. doi: 10.1016/0955-0674(94)90100-7. https://doi.org/10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 24.Prestwich GD. Hyaluronic Acid-based Clinical Biomaterials Derived for Cell and Molecule Delivery in Regenerative Medicine. J Control Release. 2011;155:193–9. doi: 10.1016/j.jconrel.2011.04.007. https://doi.org/10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gramlich WM, Kim IL, Burdick JA. Synthesis and Orthogonal Photopatterning of Hyaluronic Acid Hydrogels with Thiol-Norbornene Chemistry. Biomaterials. 2013;34:9803–11. doi: 10.1016/j.biomaterials.2013.08.089. https://doi.org/10.1016/j.biomaterials.2013.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oudshoorn MH, Rissmann R, Bouwstra JA, et al. Synthesis of Methacrylated Hyaluronic Acid with Tailored Degree of Substitution. Polymer. 2007;48:1915–20. https://doi.org/10.1016/j.polymer.2007.01.068. [Google Scholar]
- 27.Poldervaart MT, Goversen B, De Ruijter M, et al. 3D Bioprinting of Methacrylated Hyaluronic Acid (MeHA) Hydrogel with Intrinsic Osteogenicity. PLoS One. 2017;12:e0177628. doi: 10.1371/journal.pone.0177628. https://doi.org/10.1371/journal.pone.0177628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Chan-Park MB. A Biomimetic Hydrogel Based on Methacrylated Dextran-graft-lysine and Gelatin for 3D Smooth Muscle Cell Culture. Biomaterials. 2010;31:1158–70. doi: 10.1016/j.biomaterials.2009.10.040. https://doi.org/10.1016/j.biomaterials.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Lai JY, Li YT. Functional Assessment of Cross-linked Porous Gelatin Hydrogels for Bioengineered Cell Sheet Carriers. Biomacromolecules. 2010;11:1387–97. doi: 10.1021/bm100213f. https://doi.org/10.1021/bm100213f. [DOI] [PubMed] [Google Scholar]
- 30.Schuurman W, Levett PA, Pot MW, et al. Gelatin-methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-engineered Cartilage Constructs. Macromol Biosci. 2013;13:551–61. doi: 10.1002/mabi.201200471. https://doi.org/10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 31.Mũnoz Z, Shih H, Lin CC. Gelatin Hydrogels Formed by Orthogonal Thiol-Norbornene Photochemistry for Cell Encapsulation. Biomater Sci. 2014;2:1063–72. doi: 10.1039/c4bm00070f. https://doi.org/10.1039/c4bm00070f. [DOI] [PubMed] [Google Scholar]
- 32.Chen YC, Lin RZ, Qi H, et al. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv Funct Mater. 2012;22:2027–39. doi: 10.1002/adfm.201101662. https://doi.org/10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Lang Q, Zhang H, et al. Electrospun Photocrosslinkable Hydrogel Fibrous Scaffolds for Rapid In Vivo Vascularized Skin Flap Regeneration. Adv Funct Mater. 2017;27:1604617. https://doi.org/10.1002/adfm.201604617. [Google Scholar]
- 34.Zhao X, Lang Q, Yildirimer L, et al. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. PLoS One. 2016;5:108–18. doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Sun X, Yildirimer L, et al. Cell Infiltrative Hydrogel Fibrous Scaffolds for Accelerated Wound Healing. Acta Biomater. 2017;49:66–77. doi: 10.1016/j.actbio.2016.11.017. https://doi.org/10.1016/j.actbio.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellá MC, Lima-Tenório MK, Tenório-Neto ET, et al. Chitosan-based Hydrogels:From Preparation to Biomedical Applications. Carbohydr Polym. 2018;196:233–45. doi: 10.1016/j.carbpol.2018.05.033. https://doi.org/10.1016/j.carbpol.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 37.He M, Han B, Jiang Z, et al. Synthesis of a Chitosan-based Photo-Sensitive Hydrogel and its Biocompatibility and Biodegradability. Carbohydr Polym. 2017;166:228–35. doi: 10.1016/j.carbpol.2017.02.072. https://doi.org/10.1016/j.carbpol.2017.02.072. [DOI] [PubMed] [Google Scholar]
- 38.Pei M, Mao J, Xu W, et al. Photocrosslinkable Chitosan Hydrogels and their Biomedical Applications. J Polym Sci. 2019;57:1862–71. [Google Scholar]
- 39.Elizalde-Peña E, Flores-Ramirez N, Luna-Barcenas G, et al. Synthesis and Characterization of Chitosan-g-glycidyl Methacrylate with Methyl Methacrylate. Eur Polym J. 2007;43:3963–9. https://doi.org/10.1016/j.eurpolymj.2007.06.004. [Google Scholar]
- 40.Lin CC, Raza A, Shih HJ. PEG Hydrogels Formed by Thiol-ene Photo-Click Chemistry and their Effect on the Formation and Recovery of Insulin-Secreting Cell Spheroids. Biomaterials. 2011;32:9685–95. doi: 10.1016/j.biomaterials.2011.08.083. https://doi.org/10.1016/j.biomaterials.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hockaday L, Kang K, Colangelo N, et al. Rapid 3D Printing of Anatomically Accurate and Mechanically Heterogeneous Aortic Valve Hydrogel Scaffolds. Biofabricatio. 2012;4:035005. doi: 10.1088/1758-5082/4/3/035005. https://doi.org/10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim WS, Chen K, Chong TW, et al. A Bilayer Swellable Drug-eluting Ureteric Stent:Localized Drug Delivery to Treat Urothelial Diseases. Biomaterials. 2018;165:25–38. doi: 10.1016/j.biomaterials.2018.02.035. https://doi.org/10.1016/j.biomaterials.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 43.Shin H, Olsen BD, Khademhosseini A. The Mechanical Properties and Cytotoxicity of Cell-laden Double-network Hydrogels Based on Photocrosslinkable Gelatin and Gellan Gum Biomacromolecules. Biomaterials. 2012;33:3143–52. doi: 10.1016/j.biomaterials.2011.12.050. https://doi.org/10.1016/j.biomaterials.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur M, Srivastava A. Photopolymerization:A review. J Macromol Sci Part C Polym Rev. 2002;42:481–512. [Google Scholar]
- 45.Yan Q, Dong H, Su J, et al. A Review of 3D Printing Technology for Medical Applications. Engineering. 2018;4:729–42. [Google Scholar]
- 46.Colosi C, Shin SR, Manoharan V, et al. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-viscosity Bioink. Adv Mater. 2016;28:677–84. doi: 10.1002/adma.201503310. https://doi.org/10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadnap S, Krishnamoorthy S, Zhang Z, et al. Biofabrication of 3D Cell-encapsulated Tubular Constructs Using Dynamic Optical Projection Stereolithography. J Mater Sci Mater Med. 2019;30:1–10. doi: 10.1007/s10856-019-6239-5. https://doi.org/10.1007/s10856-019-6239-5. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Liu Q, Casillas J, et al. Prediction of Cell Viability in Dynamic Optical Projection Stereolithography-based Bioprinting Using Machine Learning. J Intell Manuf, 2020. 2020:1–11. https://doi.org/10.1007/s10845-020-01708-5. [Google Scholar]
- 49.Zhang K, Feng Q, Xu J, et al. Self-assembled Injectable Nanocomposite Hydrogels Stabilized by Bisphosphonate-magnesium (Mg2+) Coordination Regulates the Differentiation of Encapsulated Stem Cells Via Dual Crosslinking. Adv Funct Mater. 2017;27:1701642. https://doi.org/10.1002/adfm.201701642. [Google Scholar]
- 50.Zuo Y, Liu X, Wei D, et al. Photo-cross-linkable Methacrylated Gelatin and Hydroxyapatite Hybrid Hydrogel for Modularly Engineering Biomimetic Osteon. 2015;7:10386–94. doi: 10.1021/acsami.5b01433. https://doi.org/10.1021/acsami.5b01433. [DOI] [PubMed] [Google Scholar]
- 51.Shi D, Xu X, Ye Y, et al. Photo-cross-linked Scaffold with Kartogenin-encapsulated Nanoparticles for Cartilage Regeneration. ACS Nano. 2016;10:1292–9. doi: 10.1021/acsnano.5b06663. https://doi.org/10.1021/acsnano.5b06663.s001. [DOI] [PubMed] [Google Scholar]
- 52.de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and are Safe for Single-stage Cartilage Repair in Humans Upon Mixture with Recycled Autologous Chondrons. Stem Cells. 2017;35:256–64. doi: 10.1002/stem.2475. https://doi.org/10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 53.Levato R, Webb WR, Otto IA, et al. The Bio in the Ink:Cartilage Regeneration with Bioprintable Hydrogels and Articular Cartilage-derived Progenitor Cells. Acta Biomater. 2017;61:41–53. doi: 10.1016/j.actbio.2017.08.005. https://doi.org/10.1016/j.actbio.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan S, Wang T, Feng L, et al. Injectable In Situ Self-cross-linking Hydrogels Based on Poly (L-glutamic Acid) and Alginate for Cartilage Tissue Engineering. Biomacromolecules. 2014;15:4495–508. doi: 10.1021/bm501313t. https://doi.org/10.1021/bm501313t. [DOI] [PubMed] [Google Scholar]
- 55.Gao G, Schilling AF, Hubbell K, et al. Improved Properties of Bone and Cartilage Tissue from 3D Inkjet-bioprinted Human Mesenchymal Stem Cells by Simultaneous Deposition and Photocrosslinking in PEG-GelMA. 2015;37:2349–55. doi: 10.1007/s10529-015-1921-2. https://doi.org/10.1007/s10529-015-1921-2. [DOI] [PubMed] [Google Scholar]
- 56.Rao RR, Stegemann JP. Cell-based Approaches to the Engineering of Vascularized Bone Tissue. Cytotherapy. 2013;15:1309–22. doi: 10.1016/j.jcyt.2013.06.005. https://doi.org/10.1016/j.jcyt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters EB. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng Part B Rev. 2018;24:1–24. doi: 10.1089/ten.teb.2017.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai X, Ruan C, Ma Y, et al. 3D-bioprinted Osteoblast-laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis Both In Vitro and In Vivo. Adv Sci (Weinh) 2018;5:1700550. doi: 10.1002/advs.201700550. https://doi.org/10.1002/advs.201700550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenz A, Tjoeng I, Schneider I, et al. Improved Vasculogenesis and Bone Matrix Formation through Coculture of Endothelial Cells and Stem Cells in Tissue-specific Methacryloyl Gelatin-Based Hydrogels. Biotechnol Bioeng. 2018;115:2643–53. doi: 10.1002/bit.26792. https://doi.org/10.1002/bit.26792. [DOI] [PubMed] [Google Scholar]
- 60.Doschak MR, Kucharski CM, Wright JE, et al. Improved Bone Delivery of Osteoprotegerin by Bisphosphonate Conjugation in a Rat Model of Osteoarthritis. Mol Pharm. 2009;6:634–40. doi: 10.1021/mp8002368. https://doi.org/10.1021/mp8002368. [DOI] [PubMed] [Google Scholar]
- 61.Park D, Park CW, Choi Y, et al. A Novel Small-molecule PPI Inhibitor Targeting Integrin avb3-osteopontin Interface Blocks Bone Resorption In Vitro and Prevents Bone Loss in Mice. Biomaterials. 2016;98:131–42. doi: 10.1016/j.biomaterials.2016.05.007. https://doi.org/10.1016/j.biomaterials.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Teno N, Masuya K, Ehara T, et al. Effect of Cathepsin K Inhibitors on Bone Resorption. J Med Chem. 2008;51:5459–62. doi: 10.1021/jm800626a. [DOI] [PubMed] [Google Scholar]
- 63.Arrighi I, Mark S, Alvisi M, et al. Bone Healing Induced by Local Delivery of an Engineered Parathyroid Hormone Prodrug. Biomaterials. 2009;30:1763–71. doi: 10.1016/j.biomaterials.2008.12.023. https://doi.org/10.1016/j.biomaterials.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 64.Rodan GA, Martin TJ. Therapeutic Approaches to Bone Diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Gand Suggs L J J A d d r. Matrices and scaffolds for drug delivery in vascular tissue engineering. Adv Drug Deliv Rev. 2007;59:360–73. doi: 10.1016/j.addr.2007.03.018. https://doi.org/10.1016/j.addr.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Nomi M, Atala A, De Coppi P, et al. Principals of Neovascularization for Tissue Engineering. Mol Aspects Med. 2002;23:463–83. doi: 10.1016/s0098-2997(02)00008-0. https://doi.org/10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 67.An Y, Hubbell JA. Intraarterial Protein Delivery Via Intimally-adherent Bilayer Hydrogels. J Control Release. 2000;64:205–15. doi: 10.1016/s0168-3659(99)00143-1. https://doi.org/10.1016/s0168-3659(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 68.Kim HD, Lee EA, An YH, et al. Chondroitin Sulfate-based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2017;9:21639–50. doi: 10.1021/acsami.7b04114. https://doi.org/10.1021/acsami.7b04114. [DOI] [PubMed] [Google Scholar]
- 69.Xin T, Mao J, Liu L, et al. Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic ion Composite Hydrogel as Artificial Periosteum. ACS Appl Mater Interfaces. 2020;12:6840–51. doi: 10.1021/acsami.9b18496. https://doi.org/10.1021/acsami.9b18496. [DOI] [PubMed] [Google Scholar]
- 70.Cui H, Zhu W, Nowicki M, et al. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs Via Dual 3D Bioprinting:Integrating Regional Bioactive Factors into Architectural Design. Adv Healthc Mater. 2016;5:2174–81. doi: 10.1002/adhm.201600505. https://doi.org/10.1002/adhm.201600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boere KW, Visser J, Seyednejad H, et al. Covalent Attachment of a Three-dimensionally Printed Thermoplast to a Gelatin Hydrogel for Mechanically Enhanced Cartilage Constructs. Acta Biomater. 2014;10:2602–11. doi: 10.1016/j.actbio.2014.02.041. https://doi.org/10.1016/j.actbio.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 72.Dhawan A, Kennedy PM, Rizk EB, et al. Three-dimensional Bioprinting for Bone and Cartilage Restoration in Orthopaedic Surgery. J Am Acad Orthop Surg. 2019;27:e215–26. doi: 10.5435/JAAOS-D-17-00632. https://doi.org/10.5435/jaaos-d-17-00632. [DOI] [PubMed] [Google Scholar]
- 73.Duchi S, Onofrillo C, O'Connell CD, et al. Handheld Co-axial Bioprinting:Application to In Situ Surgical Cartilage Repair. Scientific Reports. 2017;7:1–12. doi: 10.1038/s41598-017-05699-x. https://doi.org/10.1038/s41598-017-05699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galarraga JH, Kwon MY, Burdick JA. 3D Bioprinting Via an In Situ Crosslinking Technique towards Engineering Cartilage Tissue. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-56117-3. https://doi.org/10.1038/s41598-019-56117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lam T, Dehne T, Krüger JP, et al. Photopolymerizable Gelatin and Hyaluronic Acid for Stereolithographic 3D Bioprinting of Tissue-engineered Cartilage. J Biomed Mater Res. 2019;107:2649–57. doi: 10.1002/jbm.b.34354. https://doi.org/10.1002/jbm.b.34354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdollahiyan P, Oroojalian F, Mokhtarzadeh A, et al. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. 2020;15:2000095. doi: 10.1002/biot.202000095. https://doi.org/10.1002/biot.202000095. [DOI] [PubMed] [Google Scholar]
- 77.Askari M, Naniz MA, Kouhi M, et al. Recent Progress in Extrusion 3D Bioprinting of Hydrogel Biomaterials for Tissue Regeneration:A Comprehensive Review with Focus on Advanced Fabrication Techniques. Biomater Sci. 2021;9:535–73. doi: 10.1039/d0bm00973c. https://doi.org/10.1039/d0bm00973c. [DOI] [PubMed] [Google Scholar]
- 78.Cui X, Breitenkamp K, Finn M, et al. Direct Human Cartilage Repair Using Three-dimensional Bioprinting Technology. Tissue Eng Part A. 2012;18:1304–12. doi: 10.1089/ten.tea.2011.0543. https://doi.org/10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao F, Xu Z, Liang Q, et al. Osteochondral Regeneration with 3D-Printed Biodegradable High-strength Supramolecular Polymer Reinforced-gelatin Hydrogel Scaffolds. Adv Sci. 2019;6:1900867. doi: 10.1002/advs.201900867. https://doi.org/10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Genova T, Roato I, Carossa M, et al. Advances on Bone Substitutes Through 3D Bioprinting. Int J Mol Sci. 2020;21:7012. doi: 10.3390/ijms21197012. https://doi.org/10.3390/ijms21197012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiao H., Tang TJ. Engineering 3D Approaches to Model the Dynamic Microenvironments of Cancer Bone Metastasis. Bone Res. 2018;6:1–12. doi: 10.1038/s41413-018-0008-9. https://doi.org/10.1038/s41413-018-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozbolat IT, Peng W, Ozbolat VJ. Application Areas of 3D Bioprinting. Drug Discov Today. 2016;21:1257–71. doi: 10.1016/j.drudis.2016.04.006. https://doi.org/10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Ozbolat IT, Hospodiuk MJ. Current Advances and Future Perspectives in Extrusion-based Bioprinting. Biomaterials. 2016;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 84.Miri AK, Nieto D, Iglesias L, et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv Mater. 2018;30:e1800242. doi: 10.1002/adma.201800242. https://doi.org/10.1002/adma.201⇾01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu W, Holmes B, Glazer RI, et al. 3D Printed Nanocomposite Matrix for the Study of Breast Cancer Bone Metastasis. Nanomedicine. 2016;12:69–79. doi: 10.1016/j.nano.2015.09.010. https://doi.org/10.1016/j.nano.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Zhou X, Zhu W, Nowicki M, et al. 3D Bioprinting a Cell-laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl Mater Interfaces. 2016;8:30017–26. doi: 10.1021/acsami.6b10673. https://doi.org/10.1021/acsami.6b10673. [DOI] [PubMed] [Google Scholar]
- 87.Stichler S, Jungst T, Schamel M, et al. Thiol-ene Clickable Poly(glycidol) Hydrogels for Biofabrication. Ann Biomed Eng. 2017;45:273–85. doi: 10.1007/s10439-016-1633-3. https://doi.org/10.1007/s10439-016-1633-3. [DOI] [PubMed] [Google Scholar]
- 88.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 89.Baxter FR, Bowen CR, Turner IG, et al. Electrically Active Bioceramics:A Review of Interfacial Responses. Ann Biomed Eng. 2010;38:2079–92. doi: 10.1007/s10439-010-9977-6. https://doi.org/10.1007/s10439-010-9977-6. [DOI] [PubMed] [Google Scholar]
- 90.Jiang L, Wang Y, Liu Z, et al. Three-dimensional Printing and Injectable Conductive Hydrogels for Tissue Engineering Application. Tissue Eng Part B Rev. 2019;25:398–411. doi: 10.1089/ten.TEB.2019.0100. https://doi.org/10.1089/ten.teb.2019.0100. [DOI] [PubMed] [Google Scholar]
- 91.Wei K, Zhu M, Sun Y, et al. Robust Biopolymeric Supramolecular “Host Guest Macromer”Hydrogels Reinforced by In Situ Formed Multivalent Nanoclusters for Cartilage Regeneration. Macromolecules. 2016;49:866–75. https://doi.org/10.1021/acs.macromol.5b02527.s001. [Google Scholar]
- 92.Salzlechner C, Haghighi T, Huebscher I, et al. Adhesive Hydrogels for Maxillofacial Tissue Regeneration Using Minimally Invasive Procedures. Adv Healthc Mater. 2020;9:1901134. doi: 10.1002/adhm.201901134. https://doi.org/10.1002/adhm.201901134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khare D, Basu B, Dubey AK. Electrical Stimulation and Piezoelectric Biomaterials for Bone Tissue Engineering Applications. Biomaterials. 2002;258:120280. doi: 10.1016/j.biomaterials.2020.120280. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q, Chen X, Zhu J, et al. Porous Li-Na-K Niobate Bone-substitute Ceramics:Microstructure and Piezoelectric Properties. Mater Lettt. 2008;62:3506–8. https://doi.org/10.1016/j.matlet.2008.03.024. [Google Scholar]
- 95.Zhang X, Zhang C, Lin Y, et al. Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment. ACS Nano. 2016;10:7279–86. doi: 10.1021/acsnano.6b02247. https://doi.org/10.1021/acsnano.6b02247.s001. [DOI] [PubMed] [Google Scholar]
- 96.Mestres G, Perez RA, D'Elía NL, et al. Advantages of Microfluidic Systems for Studying Cell-biomaterial Interactions Focus on Bone Regeneration Applications. Biomed Phys Eng Express. 2019;5:032001. https://doi.org/10.1088/2057-1976/ab1033. [Google Scholar]
- 97.Kirillova A, Maxson R, Stoychev G, et al. 4D Biofabrication Using Shape-morphing Hydrogels. Adv Mater. 2017;29:1703443. doi: 10.1002/adma.201703443. https://doi.org/10.1002/adma.201703443. [DOI] [PubMed] [Google Scholar]


