Abstract
Heart diseases have become the main killer threatening human health, and various methods have been developed to study heart disease. Among them, heart-on-a-chip has emerged in recent years as a method for constructing disease (or normal) models in vitro and is considered as a promising tool to study heart diseases. Compared with other methods, the advantages of heart-on-a-chip include the high portability, high throughput, and the capability to mimic microenvironments in vivo. It has shown a great potential in disease mechanism study and drug screening. In this paper, we review the recent advances in heart-on-a-chip, including the fabrication methods (e.g., 3D bioprinting) and biomedical applications. By analyzing the structure of the existing heart-on-a-chip, we proposed that a highly integrated heart-on-a-chip includes four elements: Microfluidic chips, cells/microtissues, microactuators to construct the microenvironment, and microsensors for results readout. Finally, the current challenges and future directions of heart-on-a-chip are discussed.
Keywords: Heart-on-a-chip, Microfluidic chip, Disease model, In vitro culture, Drug screening
1. Introduction
Heart is one of the most important organs in human body. It provides power for blood circulation which supplies oxygen and nutrients to organs and removes metabolic waste. In recent years, with the change in dietary structure and the increase of life pressure, heart diseases have become the leading cause of death worldwide[1,2]. To study the pathogenesis of heart diseases and pursue the effective treatments, it is of need to establish the disease (or normal) models. Overall, animal model and cell culture are the two frequently used methods.
These two methods have their own advantages and limitations. For animal model, it is different with human being in physiological conditions and organ functionalities[3]. Thus, the results of animal experiments may not precisely predict the response and/or functionalities of humans. In addition, animal experiments have other limitations, such as time-consuming investigations, high cost, and low efficiency. Moreover, there are ethical issues to consider when conducting research. In recent years, Society for the Prevention of Cruelty to Animals has been established around the world, which strongly opposes animal experiments. Therefore, research communities are under increasing public pressure as to whether animals can be used in experiments.
As to the Petri dish-based cell culture, it is now widely used due to the simple operation and good controllability. Despite its merits, there are still some drawbacks to this method. For instance, the morphologies are different for cells in human body and those in Petri dish. The cells in vivo usually assemble into three-dimensional (3D) structures, while cells in a petri dish lack the 3D scaffolds and thus form a two-dimensional (2D) flat structure. In addition, Petri dish is unable to provide the microenvironments that are required for cell growth and differentiation, and cannot well simulate the cell-cell and cell-extracellular matrix (ECM) interactions. Cells in vivo are usually subjected to some mechanical and electrical stimuli. These stimuli are of importance for the maturation and functionalities of cells. However, it is difficult to impose the stimuli in Petri dish.
Since these two methods are not satisfying, a novel method is required. With the advances of bio-fabrication, microfluidics, and biosensing techniques, organ-on-a-chip has emerged as a new technology. Organ-on-a-chip is a device for in vitro cell culture which utilizes microfluidic technology to create a highly controllable microenvironment for cells. Various organs-on-chips, for example, lung-on-a-chip, kidney-on-a-chip, intestine-on-a-chip, and even tumor-on-a-chip, have been developed for different biomedical applications[4]. Among them, heart-on-a-chip, as a method to construct heart models in vitro, has attracted considerable attention. Various fabrication methods have been developed and different applications, for example, drug screening, physiology study and disease modeling, have been proposed[5].
There are some well-written review papers on heart-on-a-chip[6-9]. However, most of them focused on the biomedical applications. In this paper, we review the recent advances of heart-on-a-chip from the viewpoint of biofabrication. By analyzing the structures of heart-on-a-chip, we found that a highly integrated heart-on-a-chip includes four elements: Microfluidic chips, cells/microtissues, microactuators for constructing the microenvironment, and microsensors for results detection. The fabrication methods for these elements are introduced in details, and the applications of heart-on-a-chip in the field of biomedical engineering are discussed. In the end, the challenges and possible solutions, as well as the future directions of heart-on-a-chip are summarized.
2. The structures of heart-on-a-chip
By reviewing the existing literature, we propose that a highly integrated heart-on-a-chip includes four elements: microfluidic chip, cells/microtissues, microactuators for physical/chemical stimuli, and microsensors for monitoring cells status (Figure 1). In practical experiments, a heart-on-a-chip may not include all these four elements, but the microfluidic chip and cells/microtissues are necessary. In recent years, with the advances in manufacturing technology (e.g., 3D bioprinting), microactuators and microsensors have been integrated within heart-on-a-chip. The purpose of microactuators is to promote cardiac cells maturation and functionalization, while microsensors are to detect cells status. In the following, we introduce these four elements sequentially.
Figure 1.
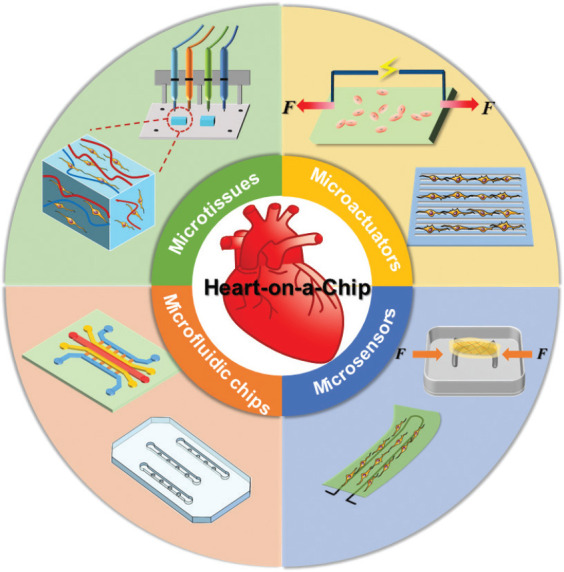
Structures of the heart-on-a-chip. A highly integrated heart-on-a-chip may include four components: microfluidic chips, microtissues, microactuators and microsensor.
2.1. Microfluidic chips
Microfluidic chip is the major part of heart-on-a-chip, and it provides habitat for cells. To date, various materials and fabrication methods have been proposed for microfluidic chips.
(1) Materials of microfluidic chips
The traditional materials for microfluidic chips include glass, silicon, polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and papers[10]. Silicon and glass are compatible to the standard micro- or nano-fabrication methods (photolithography, etching, etc.). The advantages of silicon and glass include high precision, reproducibility, and resistance to organic solvents. As to the disadvantages, silicon has poor light transmittance, which renders direct observation of the cells difficult. As a polymer organic silicon compound, PDMS is the most popular material for microfluidic chips. Its main advantages are non-toxic, chemically inert, and optically transparent properties as well as simple to use and low cost. The major disadvantage of PDMS is that it has a high absorption rate for small molecule chemicals. If the heart-on-a-chip is used for drug screening, the absorption problem should be addressed. PMMA is a transparent thermoplastic, which has the advantage of high biocompatibility, low cost, superior mechanical strength, and transparency. Papers are also used for microfluidic chips. The merits include good biocompatibility, ease of fabrication, environmental friendliness, and self-powered feature[11].
With the advances in 3D bioprinting, some printable materials have been developed for microfluidic chips. Some representative materials include thermoplastic polyurethane (TPU)[12], polycaprolactone (PCL)[13,14], and silicone[15]. TPU is a versatile multiphase block copolymer. When heated, TPU becomes soft and easy to print. If the temperature decreases, it becomes flexible with elasticity[12]. The advantages include high transparency, resistance to chemical solvents, high strength, low melting point, and high abrasion resistance. PCL is a thermoplastic material which has been approved by the Food and Drug Administration (FDA)[14]. It has a low melting temperature (~60°C), thus it is suitable for 3D bioprinting[13]. Besides, the silicon material can be modified for fast crosslinking. The modified material is 3D printable and can be used for heart-on-a-chip.
(2) Fabrication of microfluidic chips
Various methods have been used to fabricate the microfluidic chips, including photolithography, soft lithography, laser etching, hot embossing, and machining process. As a well-developed technique, photolithography enables the micro/nano structures with a high resolution[16]. The extreme ultraviolet (EUV) lithography enables the fabrication of microstructures down to 10 nm[17]. However, the facility of photolithography is expensive. As to the soft lithography, the PDMS is poured on the pre-fabricated mold. After being cured and peeling off from the mold, the PDMS slab is then bonded to the glass substrate. Laser etching enables the fabrication of microstructures in polymers. Materials are sublimated due to high temperature from the laser, and the depth of microstructures can be controlled by the laser intensity. In hot embossing, the hot mold is pressed into thermoplastic materials, for example, PMMA. Under the high temperature and pressure, microstructures can be obtained in PMMA. This technique is rather straightforward and suitable for mass production. However, it may cause the deformation of microfluidic chips due to high temperature. Machining process is another fabrication method for microfluidic chips. The materials are precisely milled in a programmable manner with a machine tool. In this method, the resolution can reach up to tens of micrometers.
With the improvement of resolution, some researchers used 3D bioprinting to fabricate microfluidic chips[18]. In 3D bioprinting, the microfluidic chip is fabricated in a layer-by-layer manner. The major advantage of 3D bioprinting is that it is an integrated fabrication method, and enables 3D complex structures. Using 3D bioprinting, the microfluidic chip can be fabricated in one-step. The assembling errors in chip assembly can be avoided[19]. To date, various microfluidic chips have been fabricated with high definition and high quality.
2.2. Microtissues
The second component of heart-on-a-chip is the microtissues. According to the dimension, microtissue can be divided into 2D microtissue and 3D microtissue. Based on the cells in microtissue, it can be divided into single type of cells and co-culture of multiple types of cells (see Table 1 for the details). Both dimension and cell types have significant influence on cell shape, alignment, expression of structural protein, and sarcomere length and beating.
Table 1.
Some representative microtissues in heart-on-a-chip
| Features | Fabrication methods | Cells | Materials | Functionalities |
|---|---|---|---|---|
| Patterned microtissues | Soft lithography[22,27] Microcontact printing[22-25] Electrospinning[44] 3D bioprinting[28] |
HUVECs[22] Rat CMs[24,25,2828] Fibroblasts[24] |
PAAm[22] Collagen[24] GelMA[28] MeTro[25] |
To study the influence of stiffness on cell morphology[22] To promote the attachment, spreading, alignment, and intercellular communication[24,25,28] |
| Vascularized microtissues | 3D bioprinting[30,34,100] Photocuring[32] Lithography[34] |
HUVECs[30,34,98] iPSC-CMs[30] Rat CMs[32] |
Alginate[30] GelMA[30,98] PEGDA[98], hydroxybutyl chitosan (HBC)[34] |
To align cardiomyocytes that can contract synchronously[30,32] |
| Microtissues with multiple cells | 3D bioprinting[31,33] | NIH/3T3[33], C2C12[33] iPSC-CMs[29,31] Endothelial cells[29] |
GelMA[31,33] Alginate[31] Gelatin, Fibrinogen[29] |
Biomimetic heterogeneous tissues[31,33] Multiple-organs-on-a-chip[29] |
(1) Cells in heart-on-a-chip
Cell type and source are the key parameters that may affect the performance of heart-on-a-chip. The adult heart is mainly composed of cardiomycytes (CMs), human cardiac fibroblasts (hCFs), endothelial cells, and others, which can perform different functions to make the heart work. Although CMs only account for ~33% of the total number of cells, they account for ~75% of the heart volume[20]. CMs are responsible for heart beating and considered as the most important cells. Thus, CMs are usually adopted in heart-on-a-chip. Usually, human cells are favorable since the purpose of heart-on-a-chip is to study heart disease of human beings. Unfortunately, it is difficult to obtain human cells directly. To date, the frequently used cells in heart-on-a-chip are animal’s primary cells and/or commercial cell lines. The animal cells are different from human cells in term of species. One possible solution to the dilemma is the use of induced pluripotent stem cells (iPSCs) which offer an almost unlimited source of cells[21]. As a novel technique, deriving CMs from iPSCs are still unsatisfying as the cells are usually phenotypically immature. Improving the maturity of CMs is the challenging part of developing heart-on-a-chip.
(2) Fabrication of microtissues
As aforementioned, microtissues can be divided into 2D and 3D types. In 2D microtissues, cells are cultured in a planner layer. Patterning cells are an important task for 2D microtissues fabrication[22]. Micro-contact printing is the most frequently used technique for cell patterning[23,24]. In this method, the pattern of liquid biomaterials is realized on the surface of a substrate through conformal contact with the soft mold (Figure 2A)[25]. The area with liquid biomaterial (e.g. fibronectin and hyaluronic acid) is adhesive to living cells. Thus, cells proliferate on these patterning areas only. Gabriele et al. dipped fibronectin with PDMS mold and then transferred to the substrate[26]. Due to the good biocompatibility of fibronectin, cells tend to adhere and grow on the area with fibronectin. In this manner, they obtained 2D microtissues with well-controlled shape.
Figure 2.
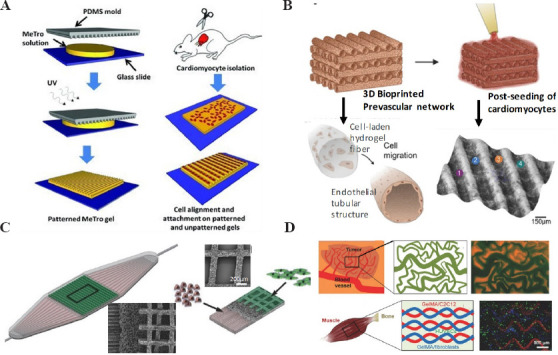
Microtissues in heart-on-a-chip. (A) 2D patterned microtissues by micro-contact printing (Republished with permission from Annabi N, Tsang K, Mithieux SM, et al., Advanced Functional Materials, © 2013 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim). (B) 3D vascularized microtissue by 3D bioprinting (Republished with permission from Colosi C, Shin SR, Manoharan V, et al., Advanced Materials, © 2015 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim.) (C) 3D hydrogel scaffold for the co-culture of CMs and vascular endothelial cells (Republished with permission from Morgan KY, Sklaviadis D, Tochka ZL, et al., Advanced Functional Materials, © 2016 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim.) (D) Microtissues with multiple cells by 3D bioprinting (Republished with permission from Miri AK, Daniel N, Luis I, et al., Adv Mater, ©2018 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim)
The tissues in vivo are usually in 3D morphology. It has been found that the 2D and 3D microtissues are different in protein expression and drug response. To fabricate the 3D microtissues, various methods have been proposed, including photolithography, electrospinning, and 3D bioprinting. Among them, 3D bioprinting is considered a promising technique to fabricate 3D microtissues. The cell-laden bioinks are printed through the nozzle of a 3D printer. Bioinks are of great importance for 3D bioprinting of microtissues. So far, the most frequently used bioinks are hydrogels. Hydrogels have similar structure with ECM and porous properties as well as good diffusion of biomolecules. Thus, they are promising candidates for the fabrication of microtissues and simulation of the growth environment for cardiac cells in vivo[27]. Their stiffness is adjustable and the biocompatibility is good after modification.
Some researchers used 3D bioprinting to fabricate microtissues in heart-on-a-chip. Chen et al. prepared CMs-laden GelMA and fabricated the cardiac microtissues using 3D bioprinting[28]. The phonotype of cells is similar with the cells in vivo. Hydrogel based 3D bioprinting has shown a great potential in fabrication of microtissues. Due to the good biocompatibility, hydrogel-based microtissues provide ideal microenvironments for cell growth and proliferation. Recently, some researchers have attempted to print the microtissues together with microfluidic chips in one step. For example, Lewis et al. used multi-materials (six functional bioinks) and fabricated the heart-on-a-chip by 3D bioprinting technology[12]. Using this chip, the alignment of cells can be well controlled by fabricating some microgrooves in the chip.
(3) Multiple cells in heart-on-a-chip
Native tissues are generally composed of multiple cells, which are necessary to implement the complex functions. To better mimic the heart function, some researchers used multiple cells to fabricate the microtissues[29,30]. To fabricate the vascularized cardiac microtissues, Colosi et al. developed a 3D bioprinting platform with a coaxial printhead. The human umbilical vein endothelial cells (HUVECs)-laden bioink (including alginate and GelMA) was squeezed out of the printhead, and formed the 3D scaffold. Then, cardiomyocytes were seeded in the scaffold. After a few days of culture, the vascularized cardiac microtissues were obtained (Figure 2B)[31]. Morgan et al. fabricated polymer scaffold with multiscale porous structure and then co-cultured cardiomyocytes and HUVECs on the structure. They successfully obtained the vascularized cardiac microtissue (Figure 2C)[32]. Miri et al. developed a printing platform to fabricate the multi material heterogeneous hydrogel microstructures[33]. This platform can quickly switch between different nozzles that have loaded different hydrogels and cells, and thus, enable multi-material printing (Figure 2D). The system provides a platform for high-resolution printing of microtissues.
Besides 3D bioprinting, some other methods have been employed to fabricate 3D cardiac microtissues. Fenech et al. used photolithography to fabricate the network of blood vessels with microvessel diameter ranging from 5 mm to 120 mm[34]. The microvessel caters to the needs of the large-scale cardiac tissues by providing nutrition and discharging waste. Bartholoma et al. have fabricated a scaffold-free functional cardiac spheroids which can perform spontaneous beating and synchronous contraction[35]. Since the fabrication process is simple, the cardiac spheroids have been widely used in drug screening.
2.3. Microactuators
The third component of heart-on-a-chip is the microactuators, which are used to impose external stimuli to cells/microtissues. In the native tissues, cells are subjected to various chemical, mechanical, and electrical cues, which significantly affect the cell behaviors. In heart-on-a-chip, the main function of microactuator is to stimulate the cells/microtissues to promote their maturation. In this section, we discuss the fabrication of microactuators for electrical and mechanical stimulation.
(1) Electrical stimulation microactuators
It is known that CMs are responsive to electrophysiological stimulation. Electrical stimulation can increase the percentage of cells that beat spontaneously and assist in cell synchronization and calcium processing. In a heart-on-a-chip, electrical stimulation is usually performed through electrodes that are in contact with cells[36]. A variety of materials have been applied to fabricate electrical actuators in heart-on-a-chip, including copper, graphite, titanium, silver, platinum, and alloys[37]. As a commonly used electrode, graphite has good machining performance, and is wear-resistant and low-cost. Margari et al. fabricated an engineered heart-on-a-chip which includes graphite electrodes and gold electrodes[38]. The hPSC-CMs were cultured on the electrodes and the CMs became mature after 14 days. Platinum and titanium electrodes are also used in heart-on-a-chip. Zhang et al. fabricated a 3D cylindrical platinum electrode, which can electrically stimulate the cells for a few weeks[39]. The advantage of using platinum and titanium is that they have high corrosion resistance.
Some metal electrodes may cause toxic reaction in the culture, and the electrodes may degrade due to the electrochemical reaction with the culture medium. An alternative solution is the indium tin oxide (ITO) film, which has good conductivity, optical transparency, stability, and non-toxicity. Kang et al. used ITO to electrically stimulate neonatal rat ventricular myocytes. It was found that electrical stimulation results in a well-organized sarcomere structure and upregulates the heart-specific gene expression[40]. Nieto et al. fabricated a chip and stimulated the H9C2 cells by depositing an aluminum film on soda lime glass with physical vapor deposition (PVD) method[41]. Recently, 3D bioprinting has been used to fabricate electrodes in heart-on-a-chip. Lin et al. 3D printed the microprobes with the conductive polymer poly (3,4-ethylenediox-ythiophene): polystyrene sulfonate (PEDOT: PSS)[42]. Adly et al. fabricated microelectrode arrays (MEAs) with a high resolution by ink-jet printing. In this method, the conductive material was deposited the substrate which was composed of PDMS, agarose, and gelatin[43].
In natural myocardial tissues, CMs show synchronous contraction through intercellular electrical communication, resulting in a strong contraction force. It has been found that it can promote electrical communication and maturation of myocardial tissues by adding conductive materials, for example, carbon nanotubes (CNTs), to cell culture scaffolds. The directional conduction of CNTs can regulate the alignment of CMs and improve their beating and contractility. Some researchers have fabricated CNTs or silk scaffolds by electrospinning and found that the materials can improve the maturity of CMs[44].
(2) Mechanical stimulation microactuators
In addition to the electrical stimulation, mechanical stimulation also plays an important role in heart. It has been found that the external mechanical forces can affect the alignment, phenotype, calcium concentration, and contractile properties of CMs[45]. In this section, we mainly discuss two types of mechanical stimuli, mechanical stress, and mechanical properties of substrate.
A variety of devices have been developed to impose mechanical stress to CMs with the purpose to mimic the physiological functions of heart. We would introduce two types of mechanical stress microactuators, the electromagnetic microactuators, and pneumatic microactuators. Electromagnetic microactuators are widely used due to its stability and high sensitivity. Li et al. fabricated a device to impose mechanical stress to cells. In their experiments, the magnetic particles were mixed to the cell-laden hydrogels. The electromagnetic microactuators were placed at the ends of hydrogel structures. In this manner, the non-contact electromagnetic force can be applied to the cells[46]. The force is well controlled in magnitude, frequency, and duration by the electromagnetic microactuators. This method was successful in promoting the maturation of CMs. As to the pneumatic actuators, Rasponi et al. have developed a heart-on-a-chip that can mimic the mechanical microenvironment of native CMs[47]. The chip includes a pneumatic system that can impose uniform uniaxial cyclic strain to the 3D microtissues.
The substrate stiffness is another mechanical stimulus in heart-on-a-chip. In the native myocardial tissues, the mechanical property, for example, stiffness is spatially non-uniform which has been observed in the tissue slices[48]. The stiffness would affect the cells in growth, differentiation, gene expression, and protein secretion. It has been found that the CMs would have sarcomere in 48 h when cultured on the soft substrate. However, the sarcomere was not observed when cells were cultured on the medium stiffness substrate. Studies have been conducted to evaluate the effects of 2D substrate stiffness on CMs contraction rate, stress, and intracellular calcium concentration. Experimental results indicate that the contraction force of CMs in vitro would change with matrix stiffness in a time-dependent manner. Bajaj et al. cultured embryonic chicken CMs on PAAm gels with different stiffness. They have found that the stiffness of gels affects the beating frequency in 24 h[49].
Moreover, the surface morphology of substrate also affects the behavior of cells. In heart-on-a-chip, the microstructure on substrate can regulate the alignment of CMs, which is a critical factor influencing the contraction force. Lewis et al. fabricated a heart-on-a-chip by 3D bioprinting. In this chip, they fabricated microgroove structures on the surface of substrate. The CMs were cultured on the substrate surface and cells tend to orient along the grooves. By regulating the alignment of CMs, the contraction force would be enhanced since cells tend to contract along the same long axis[12].
Some researchers have simultaneously imposed the electrical and mechanical stimuli to cells. Murry et al. found that if both electrical and mechanical stimuli are imposed, the hESC-CMs can mature at a faster pace[50]. Later, they used hiPSC-CMs and collagen to construct the engineered cardiac microtissues and studied the effect of mechanical conditions and electrical stimulation on the maturation of cardiac tissue[51]. Rasponi et al. developed a biological reactor which can provide a uniform electric field and periodic uniaxial strain to the 3D cardiac microtissues[52].
2.4. Microsensors
Microsensors are the fourth elements in heart-on-a-chip. The function is to monitor the status of cells/microtissues in heart-on-a-chip. At the early stage, some biochemical reagents were used to stain cells to characterize their status and functionalities. The disadvantage is that this method is destructive to cells, and it is difficult to implement the real-time monitoring. In recent years, some researchers have begun to integrate microsensors in heart-on-a-chip to monitor the physiological status of cardiac cells noninvasively[53].
(1) Measurement of contraction force
One property of CMs is that it can generate contraction force. The contraction force is an important indicator of CMs status. The contraction of CMs is visible in the microscope, but it is difficult to quantify the contraction force by direct observation. The atomic force microscopy (AFM) and traction force microscopy (TFM) are capable to quantify the contraction force. However, it may affect behaviors of the cells[54]. Moreover, the instruments are expensive and complex to operate. Some researchers integrated microsensors in heart-on-a-chip to measure the contraction force of CMs (see Table 2 for the details).
Table 2.
Microsensors for contraction force measurement in heart-on-a-chip
| Mechanism | Structures | Materials | Cells | Applications | Measured values |
|---|---|---|---|---|---|
| Visible deformation | Biowires[62,63,9393] Cantilever[59,6464] Micropillars[57] Helical structure[56] |
POMaC[62] PDMS[59] Hydrogel[57,64] |
iPSC-CMs[62,63] Rat CMs[59,64] HCFs[63] |
Cell maturation[57,62] Drug screening [57,59,62,63] Disease model[63] |
Contraction force 2.89-5.09 µN[64] ~40 µN[62] Contractile stress ~15.4 kPa[59] ~1.7 kPa[57] |
| Electrical sensors | Piezoelectric[69] Crack sensor[74] Piezoresistive[12,67,68] |
AlN[69] Pt-PDMS[74] CNTs-PDMS[68] CB:TPU[12] |
Rat CMs[12,67,72,74] iPSC-CMs[68,69] | Drug screening[9,12,67-69] | Contraction force ~107 nN[74] Contractile stress ~2.34 kPa[68] 7-15 kPa[12] |
| Structural color | Cantilever[56] Biological-Crawling robot[66] |
GelMA[66] | Rat CMs[66] | Drug screening[56,6666] Biological soft robots[66] |
crawling speed 20 µm/s[66] |
One commonly used method is to fabricate an elastic component in heart-on-a-chip. The CMs cultured on the elastic component would cause the visible deformation of the component[55-58]. The elastic component could be in different shape, for example, film, cantilever, or micropillars. The contraction force of CMs would bend the elastic component, and the deformation is observed and recorded by a microscope. Then, the contraction force can be calculated by image processing. Parker et al. fabricated the PDMS films in a heart-on-a-chip, and they cultured cells on these films which are referred to as muscular thin films (MTFs)[59]. The deformation caused by the contraction force can be read out by the optical signal. Using this platform, they studied the influence of temperature and electrical stimulation on the contraction force (Figure 3Ai). Some researchers used micropillars to measure the contraction force. Cells cultured on the micropillars tend to adhere to the tips[60]. When the cell contracts, it would deflect the surrounding micropillars (Figure 3Aii)[61]. The micropillars can be fabricated by soft lithography or other methods. Another similar structure that can measure contraction force is the biowires[62,63]. In this method, the microtissues tend to enlace the two wires and the contraction force drags them to each other (Figure 3Aiii). Bashir et al. fabricated microcantilevers using 3D bioprinting with cross-linkable hydrogels. The cantilevers have similar mechanical properties with native myocardial tissues and can be used to measure the contraction force. Using this device, they studied the influence of substrate stiffness on the contraction force[64]. Chen et al. fabricated similar microsensors by 3D bioprinting. They obtained microcantilevers with CMs-laden GelMA hydrogel and measured the contraction force[28].
Figure 3.
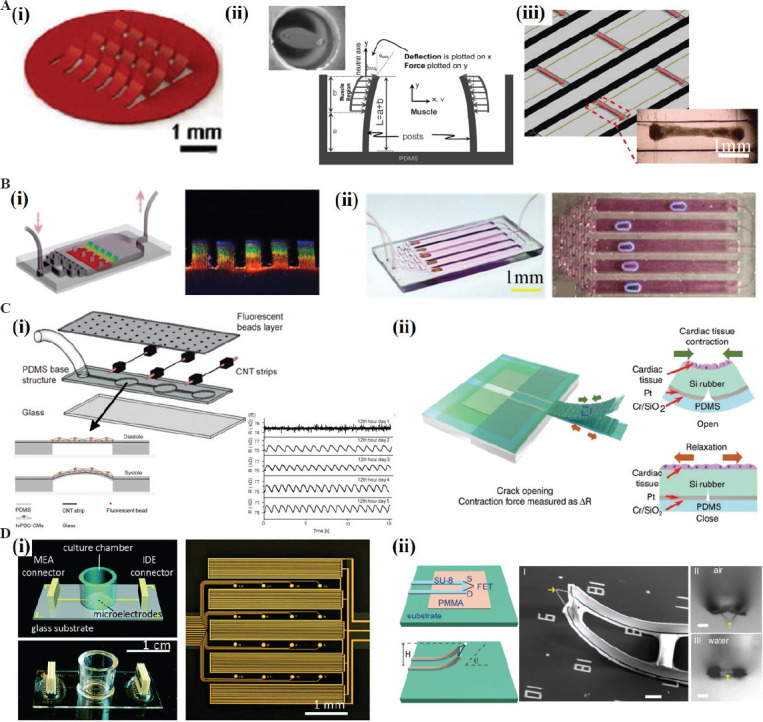
Microsensers in heart-on-a-chip. (A) Mechanical stress microsensors based on the visible deformation. The CMs can be cultured on different structures and result in the deformation. (i) MTFs (Reproduced from ref.[59] with permission from The Royal Society of Chemistry); (ii) micropillars (Republished with permission from Vandenburgh H, Shansky J, Benesch-Lee F, et al., Muscle Nerve, © 2008 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim). (iii) biowires (Republished with permission from Zhao Y, Wang EY, Davenport LH, et al., Adv Healthcare Mater, © 2019 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim). (B) Mechanical stress microsensors based on structure color. The microsensors can be designed to different structures. (i) MTFs (Republished with permission from Fu F, Shang L, Chen Z, et al., Science Robotics, 2018, 3(16): aar8580); (ii) microrobots. (Republished with permission from Sun L, Chen Z, Bian F, et al., 2020., Advanced Functional Materials, 2019, Weinheim) (C) Mechanical stress microsensors based on the electrical sensors. (i) Piezoresistive microsensors embedded inside the membrane (Adapted with permission from Wang L, Dou W, Malhi M, et al., 2018, Microdevice Platform for Continuous Measurement of Contractility, Beating Rate, and Beating Rhythm of Human-iPSCs-Cardiomyocytes inside a Controlled Incubator Environment. ACS Applied Materials and Interfaces, 10(25): 21173-83. Copyright (2018) American Chemical Society); (ii) crack-based microsensors (from ref.[74] licensed under Creative Commons Attribution 4.0 license) (D) Electrophysiology microsensors. (i) 2D MAE array for action potential and impedance readout (Reproduced from ref.[76] with permission from The Royal Society of Chemistry); (ii) 3D nanoelectrodes, scale bar = 5 mm (Tian B, Cohen-Karni T, Qing Q, et al., Science, 2010,329(5993): 830-4).
Another method to measure the contraction force is to use the structure coloration. Zhao et al. fabricated a heart-on-a-chip with thin films which was made of the material inverse opal structure GelMA hydrogel[65]. The material would change its color when subjected to external force. In this manner, the thin films performed as microsensors and the contraction of myocardial cells would cause a visible color change (Figure 3Bi). Such microsensors can be used to characterize the beating frequency of CMs. Zhao et al. also fabricated a micro-robot which was powered by the CMs. The materials of micro-robot include CNT and the inverse opal structure GelMA hydrogel (structure color material)[66]. The micro-robot can mimic the crawling behavior of a caterpillar (Figure 3Bii). The crawling speed and structural color are the indicators of myocardial tissues status.
The above-mentioned methods are referred to as direct methods. The contraction force-induced deformation is usually observed under a microscope, making it inconvenient to some extent since the heart-on-a-chip is usually laid in the incubator for culture. An alternative is to embed piezoelectric or piezoresistive materials into the microsensors. When the contraction force causes the deformation in the elastic component, the electrical voltage or electrical resistance would be changed. In other words, the contraction force is transferred to the electrical signal by the microsensors[67].
Sun et al. developed a deformable PDMS membrane with CNT (a piezoresistive material) embedded inside. They cultured hiPSC-CMs on the membrane. After a period of incubation, the cell begun to contract and the contraction force causes the change of electrical resistance of CNT (Figure 3Ci). This platform can continuously measure the contraction force and beating rate of CMs in a long term (14 days)[68]. Another choice is to use the piezoelectric material to measure the contraction force[69-71]. When the contraction force triggered the deformation, an electrical voltage (and thus a measurable current) is generated in the piezoelectric material. No external voltage is required for using this method.
The limitation of the piezoelectric and piezoresistive microsensors is that they have low sensitivity[69,72]. Crack-based microsensors may overcome this limitation[73]. Under the same contraction force, the sensitivity of crack-based microsensor is 900 times higher than that of piezoresistive microsensor. Lee et al. fabricated an integrated high-sensitivity crack microsensor within a PDMS cantilever. The contraction force of CMs leads to the deformation of cantilever and further causes the distance change in the crack[74]. When used in heart-on-a-chip, the crack-based microsensors have high sensitivity and accuracy (Figure 3Cii).
For the aforementioned microsensors in heart-on-a-chip, the fabrication is still challenging. 3D bioprinting is a powerful technique and can be used to fabricate the microsensors. Lewis et al. developed a multi-materials 3D bioprinting platform to fabricate the heart-on-a-chip[12]. They prepared six functional bioinks, including polyurethane (TPU), carbon black (CB), PDMS, and dextran, to print the chips. In this heart-on-a-chip, they integrated a flexible microsensor which performed well in monitoring the contraction of CMs. To fabricate the microsensors by 3D bioprinting, choosing the conductive material is a key task. Recently, a conductive polymer, poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT: PSS), has attracted considerable attention[42]. This material has shown excellent printability, high conductivity, high resolution (~30 mm), and good biocompatibility. This material is a promising candidate to fabricate the microsensors in heart-on-a-chip.
(2) Measurement of electrophysiological signals
Electrophysiological signal is another important indicator of CMs. Some researchers fabricated microsensors in heart-on-a-chip to detect the electrical activity of CMs. The planar MEA made of metal electrodes (gold or platinum) is a conventional tool to monitor the electrophysiology of CMs. Besides metal, some other conductive materials are also available for MEA. Offenhäusser et al. proposed a graphene-based MEA microsensors and monitored the extracellular action potential of CMs[75]. The MEA can detect local action potentials with high spatial resolution and high sensitivity, but failed to measure the contractility of CMs. Some researchers combined the MEA and interdigital electrode (IDE) together to measure the action potential, contraction force, beating rate, and other parameters (Figure 3Di)[76]. The MEA is an electrically stable, high-throughput, and non-invasive microsensor for recording the electrophysiology of CMs.
Sometimes, the microtissues in heart-on-a-chip are in 3D and thus 3D electrodes are required to measure the electrophysiological signals in the microtissues. To date, various 3D nanoelectrode structures have been developed, including nanotubes, nanopillars, and mushroom-shaped electrodes[77]. Lieber et al. fabricated a nanoscale field-effect transistor device which shows a high sensitivity in water and acid/alkali solution. They modified the transistor surface with phospholipid bilayers and implemented the real-time monitoring of intracellular potentials of single cell (Figure 3Dii)[77]. Abbott et al. fabricated nanoscale intracellular electrodes and realized a high-fidelity electrophysiological image for neonatal rat cardiomyocytes[78]. Some researchers used 3D plasma nanoelectrodes to record the electrical activities inside and outside the cells for a long term. Lieber et al. introduced a 3D nanoelectrode array that mimics the tissue scaffold. The device can simultaneously map the action potential in three dimensions in real time. This heart-on-a-chip with 3D nanoelectrode array can realize the monitoring of electrophysiological activity in the process of culture and development[79].
3. Biomedical applications of heart-on-a-chip
Heart-on-a-chip has found various applications, including physiology study, disease modeling, and drug screening. Compared with the traditional techniques, heart-on-a-chip can better mimic the cellular microenvironment and promotes the maturation of microtissues. In addition, heart-on-a-chip enables the real-time monitoring of the status of cells/microtissues. In this section, we discuss the applications of heart-on-a-chip in physiology study, disease modeling, and drug screening.
3.1. Physiology study
With the assistance of heart-on-a-chip, we can enhance our understanding in the physiological characteristics of heart. One feature of CMs is that they can rhythmically beat and are responsive to external stimuli such as force and electricity. Yasuda et al. used agarose material and fabricated a heart-on-a-chip with microchamber array[80]. They cultured single CM in each microchamber, and observed the beating cycle of CM. By changing the distance between two adjacent microchambers, they confirmed the clustering effect of CMs. Another feature of CMs is contraction, and the contraction force is an important indicator of cell physiological state. Varghese et al. fabricated a heart-on-a-chip, and studied the influence of electrical stimulation on the contraction force of CMs[81].
Some researchers fabricated heart-on-a-chip with the biowire structures. Two flexible wires fabricated from a poly(octamethylene maleate (anhydride) citrate) (POMaC) polymer are secured with adhesive glue. The chip was used to study the response of immature CMs to electrical stimulation. It was found that electrical stimulation could increase the microstructure of myofibrils, increase the electrical conduction, and change the properties of electrophysiology and calcium ion transients. The human circulatory system is composed of the heart and a complex network of blood vessels. Blood pressure is an important issue of blood circulation, and it can be studied by heart-on-a-chip. Sethu et al. designed a chip which can accurately simulate the hemodynamic stress, and they found the stress can promote the maturation of CMs[82]. Wu et al. developed a heart-on-a-chip to simulate the circulation system[83]. The chip includes four pump units representing the four heart chambers, and the pressure is controllable to study its influence on the cells (Figure 4A).
Figure 4.
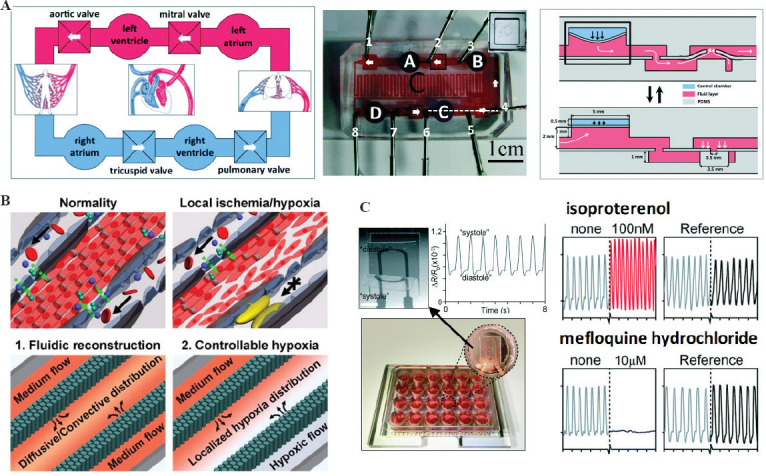
Biomedical applications of heart-on-a-chip. (A) Physiology study. The chip was composed of four microchambers to mimic the heart and the microfluidic channels to mimic the network of blood vessels. Its function is to study the working mechanism of circulatory system (Reproduced from ref. [83] with permission from The Royal Society of Chemistry). (B) Disease model. The heart-on-a-chip was designed to model the hypoxia-induced myocardial injury (Adapted with permission from (Ren L, Liu W, Wang Y, et al., 2013, Investigation of Hypoxia-Induced Myocardial Injury Dynamics in a Tissue Interface Mimicking Microfluidic Device. Analytical Chemistry, 85(1): 235-244). Copyright (2013) American Chemical Society.) (C) Drug screening. The high-throughput chip with iPSC-CMs microtissues was used to test the effect of isoproterenol and mefloquine hydrochloride (Reproduced from ref.[72] with permission from The Royal Society of Chemistry).
3.2 Disease modeling
Disease modeling is an important step in analyzing disease mechanisms and developing drugs for treatments[84]. Coronary heart disease refers to the stenosis or blockage of the vascular lumen caused by coronary atherosclerosis. Coronary heart disease may lead to the myocardial infarction in the later stage. For a better understanding of the coronary heart disease and for exploring effective treatments, Wang et al. designed a heart-on-a-chip in which the oxygen was well controlled to study the myocardial damage caused by hypoxia (Figure 4B)[85]. Liu et al. fabricated the heart-on-a-chip to model the non-uniform oxygen distribution. The model can mimic the blockage of coronary arteries and study the electrophysiological response of myocardial hypoxia[86]. For CMs, the activity of Ca2+ channels would affect the contraction. Elvassore et al. designed a heart-on-a-chip and found that the hypoxia would induce the reversible change of Ca2+ concentration in CMs[87].
Cardiac fibrosis could form a large number of fibrosis scar tissue, and lead to heart failure. Heart-on-a-chip can be used to simulate the cardiac fibrosis model by controlling the number of fibroblasts and the concentration of collagen in the engineered microtissues. Experimental results showed that increasing the fibroblast density can reduce the contraction force[12]. Some researchers used 3D hydrogel microtissues to model the cardiac fibroblasts[88]. The advantage is that the microenvironment is close to the native tissues and the mechanical properties are controllable. These models in heart-on-a-chip can be used to study the pathology of cardiac fibrosis, and therefore, lay a basis for exploring the effective treatments.
Another cause of heart failure is the arrhythmia, which refers to the disturbed heartbeat rhythm caused by the abnormal cardiac electrical activity[89]. Heart-on-a-chip has been used to model the arrhythmia and related cardiovascular diseases. Healy et al. constructed a 3D in vitro arrhythmia model by heart-on-a-chip. They used the iPSCs-derived CMs and filamentous matrix to fabricate the 3D microtissues[90]. They studied the electrophysiological signals and contraction force related to arrhythmia. Using the chip, the response to a group of drugs was also investigated. In addition, there have been some studies using heart-on-a-chip to model other cardiovascular conditions, such as hypertension or hypotension and hypertrophy[91].
3.3. Drug screening
Drug screening is one of the most important applications of heart-on-a-chip. For some drugs, the side effect may cause heart damage or even heart failure. Thus, it is necessary to study the drug-induced cardiotoxicity. Heart-on-a-chip as an effective and accurate in vitro model can be used to evaluate cardiotoxicity. Parker et al. have found that the isopropylnoradrenaline has a positive influence on the contraction force of CMs using heart-on-a-chip[59]. Ren et al. fabricated a heart-on-a-chip for high-throughput drug screening[92]. They chose clinically approved doxorubicin and cyclophosphamide as model drugs to examine dose-dependent cardiotoxicity, and ivabradine and carbachol as candidates for ameliorating cardiotoxicity.
As discussed in the previous section, the 3D microtissues are different from 2D microtissues in morphology and functionality. Some researchers suggested to use 3D microtissues for drug response in heart-on-a-chip. Compared with the 2D microtissues, the heart-on-a-chip with 3D microtissues is complex in structure and thus, the throughput is lower. However, the advantage is that the functionalities are close to native tissues and the maturation is improved. Nakayama et al. fabricated a heart-on-a-chip with 3D microtissues using 3D bioprinter. The cell spheroids were printed on the micropillar array, and the contraction force of the 3D microtissues was evaluated by measuring the micropillar deformation. The platform can be used for drug screening by adding drugs into the device and observing the response afterward. Chen et al. fabricated the 3D microtissues using 3D bioprinting, and monitored the concentration of calcium. They found that the isopropylnoradrenaline can affect the calcium concentration[28]. Zhao et al. designed a heart-on-a-chip with biowire structures, which can be used for drug screening and gene expression testing. This chip can record the contraction force and calcium concentration in real-time[93]. Paker et al. fabricated a heart-on-a-chip with 24 MTFs and each MTF can detect the contraction force and beating frequency of cardiomyocytes. Using this heart-on-a-chip, they tested the influence of 12 drugs (e.g. Isradipine, Nicardipine, Clofilium, and Flecainide) and demonstrated the application of this chip in drug screening (Figure 4C)[72]. Wan et al. fabricated a heart-on-a-chip with 3D microtissues and used it for cardiotoxicity studies of drugs (antibiotics, antidiabetic drugs, and anticancer drugs). Compared with the 2D model, the 3D microtissues performed better in drug screening. The results by 3D microtissues are consistent with clinical observations[5].
4. Summary and outlook
In this paper, we review the recent advances of heart-on-a-chip, including the history, structures, fabrication methods, and the biomedical applications. Collectively, we propose that a highly integrated heart-on-a-chip includes four elements: Microfluidic chip, cells/microtissues, microactuators, and microsensors. The microfluidic chip and microtissues are necessary for a heart-on-a-chip. The microactuators can be used to impose electrical and/or mechanical stimuli to cells. The microsensors are designed to monitor the performance of cells in heart-on-a-chip. Various methods have been proposed to fabricate heart-on-a-chip. We shed light on the 3D bioprinting which is a promising technique and can enable the one-step fabrication of heart-on-a-chip. 3D bioprinting has greatly improved the complexity, functionality, and efficiency of heart-on-a-chip. Heart-on-a-chip has found broad applications in biomedical engineering, including physiology study, disease modeling, and drug screening. At present, the development and applications of heart-on-a-chip are still in its early stage, and facing some challenges.
An important challenge for heart-on-a-chip is to better mimic the microenvironment of native cells[94]. The challenges include addressing the issues pertaining to cell alignment, multiple cells co-culture and external stimuli which are key factors to improve cell maturation and functionalities. They can be implemented by fabricating micro-patterned substrate, complex 3D scaffold, and vascularized tissues. It is still challenging to fabricate these structures. Perhaps, 3D bioprinting is a possible solution which has shown a great potential in biofabrication. To date, 3D bioprinting has enabled cell alignment, co-culture of multiple cells, and vascularized myocardial tissue. Nevertheless, precise regulation of cell microenvironment (especially the spatial-temporal anisotropic microenvironment) still needs further exploration.
There are considerable research on the microsensors in heart-on-a-chip[53]. However, the accuracy and sensitivity are still unsatisfying. PDMS as an elastic material is widely used in microsensors to measure conTFM. The modulus of PDMS is still high and thus the deformation caused by CMs is relatively small. For the microsensors based on optical method, it is complex to measure the contraction force. The strain microsensors can read out the contraction force directly, but the stability, linearity, and accuracy of the microsensors are not satisfying[7]. With the advances of wearable and flexible electronics, more microsensors have been invented. These microsensors could be used in heart-on-a-chip, and monitor the status of cells.
As a promising technique, 3D bioprinting has shown a great potential in fabricating highly integrated heart-on-a-chip in one step[95]. Using 3D bioprinting, it becomes possible to fabricate the microfluidic chip, microtissues, microactuators, and microsensors on the same platform. On the basis of 3D bioprinting, some researchers developed 4D bioprinting method[96]. In 4D bioprinting, smart materials were used and the printed objects can change their shapes or functionalities with time. This technique can be used to fabricate microactuators and impose mechanical stress to cells. For 3D/4D bioprinting, there are still challenges, such as on how to improve the resolution and develop novel materials with good printability and mechanical properties.
Another challenge for heart-on-a-chip is the commercialization. To ensure the physiological state of the cardiac cells, the storage and transportation should be considered which are necessary for the off-the-shelf use. One possible solution is to use the vitrifying method which is commonly used in reproductive medicine[97]. The chip together with the microtissues is frozen under a low temperature, and the physiological activity of the cells is paused. When it is ready to be used, the heart-on-a-chip is rewarmed to activate physiological activity of the cells. Such vitrifying process necessitates strict demands for the design and materials of heart-on-a-chip.
To sum up, the development of heart-on-a-chip is still in its early stage, and there are barriers to overcome in its commercialization and clinical applications. However, it is believed that heart-on-a-chip is a promising technique and has a great potential in various biomedical applications.
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (51911530694, 11902245), the Key Research and Development Program of Shaanxi (2021GY-294), the Zhejiang Provincial Natural Science Foundation of China (LQ21E050009), and Beilin District Applied Technology Research and Development Projects in 2020 (GX2027).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Yusuf SW, Cipolla C, Durand JB, et al. Cancer and Cardiovascular Disease. Cardiol Res Pract, 2011. 2011:943748. doi: 10.4061/2011/943748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update:A Report from the American Heart Association. Circulation. 2020;141:E33. doi: 10.1161/CIR.0000000000000746. https://doi.org/10.1161/cir.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 3.Denayer T, StöHr T, Roy MT. Animal Models in Translational Medicine:Validation and Prediction. New Horiz Transl Med. 2014;2:5–11. [Google Scholar]
- 4.Chi CW, Lao YH, Ahmed AH, et al. High-Throughput Tumor-on-a-Chip Platform to Study Tumor-Stroma Interactions and Drug Pharmacokinetics. Adv Healthc Mater. 2020;9:2000880. doi: 10.1002/adhm.202000880. https://doi.org/10.1002/adhm.202000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu HF, Leong MF, Lim TC, et al. Engineering a Functional Three-dimensional Human Cardiac Tissue Model for Drug Toxicity Screening. Biofabrication. 2017;9:025011. doi: 10.1088/1758-5090/aa6c3a. https://doi.org/10.1088/1758-5090/aa6c3a. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Aleman J, Arneri A, et al. From Cardiac Tissue Engineering to Heart-on-a-chip:Beating Challenges. Biomed Mater. 2015;10:034006. doi: 10.1088/1748-6041/10/3/034006. https://doi.org/10.1088/1748-6041/10/3/034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei X, Zhuang L, Li H, et al. Advances in Multidimensional Cardiac Biosensing Technologies:From Electrophysiology to Mechanical Motion and Contractile Force. Small. 2020;16:2005828. doi: 10.1002/smll.202005828. https://doi.org/10.1002/smll.202005828. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Yang Y, Qi Y, et al. Engineered Circulatory Scaffolds for Building Cardiac Tissue. J Thorac Dis. 2018;10:S2312–28. doi: 10.21037/jtd.2017.12.92. https://doi.org/10.21037/jtd.2017.12.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Rafatian N, Wang E, et al. Towards Chamber Specific Heart-on-a-chip for Drug Testing Applications. Adv Drug Deliv Rev. 2020;165:60–76. doi: 10.1016/j.addr.2019.12.002. https://doi.org/10.1016/j.addr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerzoni L, Tsukamoto Y, Gehlen DB, et al. A Layer-by-Layer Single-Cell Coating Technique To Produce Injectable Beating Mini Heart Tissues via Microfluidics. Biomacromolecules. 2019;20:3746–54. doi: 10.1021/acs.biomac.9b00786. https://doi.org/10.1021/acs.biomac.9b00786. [DOI] [PubMed] [Google Scholar]
- 11.Derda R, Laromaine A, Mannoto A, et al. Paper-supported 3D Cell Culture for Tissue-based Bioassays. Proc Natl Acad Sci U S A. 2009;106:18457–62. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind JU, Busbee TA, Valentine AD, et al. Instrumented Cardiac Microphysiological Devices via Multimaterial Three-dimensional Printing. Nat Mater. 2017;16:303. doi: 10.1038/nmat4782. https://doi.org/10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BS, Jang J, Chae S, et al. Three-dimensional Bioprinting of Cell-laden Constructs with Polycaprolactone Protective Layers for Using Various Thermoplastic Polymers. Biofabrication. 2016;8:035013. doi: 10.1088/1758-5090/8/3/035013. https://doi.org/10.1088/1758-5090/8/3/035013. [DOI] [PubMed] [Google Scholar]
- 14.Yi HG, Choi YJ, Kang KS, et al. A 3D-printed Local Drug Delivery Patch for Pancreatic Cancer Growth Suppression. J Control Release. 2016;238:231–41. doi: 10.1016/j.jconrel.2016.06.015. https://doi.org/10.1016/j.jconrel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Kolesky DB, TRuby RL, Gladman AS, et al. 3D Bioprinting of Vascularized, Heterogeneous Cell-laden Tissue Constructs. Adv Mater. 2014;26:3124–30. doi: 10.1002/adma.201305506. https://doi.org/10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 16.Yashiro M, Oouchi T, Tsushima H, et al. Excimer Laser Gas Usage Reduction Technology for Semiconductor Manufacturing. SPIE Adv Lithogr. 2017;10147:1014710. https://doi.org/10.1117/12.2257972. [Google Scholar]
- 17.Randall JN, Owen JH, Lake J, et al. Next Generation of Extreme-Resolution Electron Beam Lithography. J Vac Sci Technol. 2019;37:061605. https://doi.org/10.1116/1.5119392. [Google Scholar]
- 18.He Y, Wu Y, Fu JZ, et al. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology:A Review. Electroanalysis. 2016;28:1658–78. https://doi.org/10.1002/elan.201600043. [Google Scholar]
- 19.He Y, Qiu J, Fu J, et al. Printing 3D Microfluidic Chips with a 3D SUGAR Printer. Microfluid Nanofluidics. 2015;19:447–56. https://doi.org/10.1007/s10404-015-1571-7. [Google Scholar]
- 20.Caulfield JB, Borg TK. The Collagen Network of the Heart. Lab Invest. 1979;40:364–72. [PubMed] [Google Scholar]
- 21.Ramme AP, Koenig L, Hasenberg T, et al. Towards an Autologous iPSC-Derived Patient-on-a-Chip 2018 [Google Scholar]
- 22.Grevesse T, Verseavel M, Circelli G, et al. A Simple Route to Functionalize Polyacrylamide Hydrogels for the Independent Tuning of Mechanotransduction Cues. Lab Chip. 2013;13:777–80. doi: 10.1039/c2lc41168g. https://doi.org/10.1039/c2lc41168g. [DOI] [PubMed] [Google Scholar]
- 23.Cimetta E, Pizzato S, Bollini S, et al. Production of Arrays of Cardiac and Skeletal Muscle Myofibers by Micropatterning Techniques on a Soft Substrate. Biomed Microdevices. 2009;11:389–400. doi: 10.1007/s10544-008-9245-9. https://doi.org/10.1007/s10544-008-9245-9. [DOI] [PubMed] [Google Scholar]
- 24.Camelliti P, Gallagher JO, Kohl P, et al. Micropatterned Cell Cultures on Elastic Membranes as an In Vitro Model of Myocardium. Nat Protoc. 2006;1:1379–91. doi: 10.1038/nprot.2006.203. https://doi.org/10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- 25.Annabi N, Tsang K, Mithieux SM, et al. Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv Funct Mater. 2013;23:4949–59. doi: 10.1002/adfm.201300570. https://doi.org/10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Versaevel M, Grevesse T, Gabriele S. Spatial Coordination Between Cell and Nuclear Shape within Micropatterned Endothelial Cells. Nat Commun. 2012;3:671. doi: 10.1038/ncomms1668. https://doi.org/10.1038/ncomms1668. [DOI] [PubMed] [Google Scholar]
- 27.Mccain ML, Agarwal A, Nesmith HW, et al. Micromolded Gelatin Hydrogels for Extended Culture of Engineered Cardiac Tissues. Biomaterials. 2014;35:5462–71. doi: 10.1016/j.biomaterials.2014.03.052. https://doi.org/10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Miller K, Ma X, et al. Direct 3D Bioprinting of Cardiac Micro-Tissues Mimicking Native Myocardium. Biomaterials. 2020;256:120204. doi: 10.1016/j.biomaterials.2020.120204. https://doi.org/10.1016/j.biomaterials.2020.120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skardal A, Murphy SV, Devarasetty M, et al. Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform. Sci Rep. 2017;7:8837. doi: 10.1038/s41598-017-08879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YS, Arneri A, Bersini S, et al. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. https://doi.org/10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colosi C, Shin SR, Manoharan V, et al. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28:677–84. doi: 10.1002/adma.201503310. https://doi.org/10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan KY, Sklaviadis D, Tochka ZL, et al. Multi-Material Tissue Engineering Scaffold with Hierarchical Pore Architecture. Adv Funct Mater. 2016;26:5873–83. doi: 10.1002/adfm.201601146. https://doi.org/10.1002/adfm.201601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miri AK, Daniel N, Luis I, et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv Mater. 2018;30:1800242. doi: 10.1002/adma.201800242. https://doi.org/10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenech M, Girod V, Claveria V, et al. Microfluidic Blood Vasculature Replicas using Backside Lithography. Lab Chip. 2019;19:2096106. doi: 10.1039/c9lc00254e. https://doi.org/10.1039/c9lc00254e. [DOI] [PubMed] [Google Scholar]
- 35.Bartholomaand P, Gorjup E, Monz D, et al. Three-Dimensional In Vitro Reaggregates of Embryonic Cardiomyocytes:A Potential Model System for Monitoring Effects of Bioactive Agents. J Biomol Screen. 2005;10:814–22. doi: 10.1177/1087057105280070. https://doi.org/10.1177/10∁7105280070. [DOI] [PubMed] [Google Scholar]
- 36.Ronaldson-Bouchard K, Ma SP, Yeager K, et al. Advanced Maturation of Human Cardiac Tissue Grown from Pluripotent Stem Cells. Nature. 2018;556:239–43. doi: 10.1038/s41586-018-0016-3. https://doi.org/10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandon N, Marsano A, Maidhof R, et al. Optimization of Electrical Stimulation Parameters for Cardiac Tissue Engineering. J Tissue Eng Regen Med. 2011;5:E115–25. doi: 10.1002/term.377. https://doi.org/10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valls-Margarit M, Iglesias-Garcia O, di Guglielmo C, et al. Engineered Macroscale Cardiac Constructs Elicit Human Myocardial Tissue-like Functionality. Stem Cell Reports. 2019;13:207–20. doi: 10.1016/j.stemcr.2019.05.024. https://doi.org/10.1016/j.stemcr.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang N, Stauffer F, Simona BR, et al. Multifunctional 3D Electrode Platform for Real-Time In Situ Monitoring and Stimulation of Cardiac Tissues. Biosens Bioelectron. 2018;112:149–55. doi: 10.1016/j.bios.2018.04.037. https://doi.org/10.1016/j.bios.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 40.Moon SH, Cho YW, Shim HE, et al. Electrically Stimulable Indium Tin Oxide Plate for Long-Term In Vitro Cardiomyocyte Culture. Biomater Res. 2020;24:10. doi: 10.1186/s40824-020-00189-0. https://doi.org/10.21203/rs.3.rs-22137/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyunbaatar NE, Shanmugasundaram A, Jeong YJ, et al. Micro-Patterned SU-8 Cantilever Integrated with Metal Electrode for Enhanced Electromechanical Stimulation of Cardiac Cells. Colloids Surf B Biointerfaces. 2020;186:110682. doi: 10.1016/j.colsurfb.2019.110682. https://doi.org/10.1016/j.colsurfb.2019.110682. [DOI] [PubMed] [Google Scholar]
- 42.Yuk H, Lu B, Lin S, et al. 3D Printing of Conducting Polymers. Nat Commun. 2020;11:1604. doi: 10.1038/s41467-020-15316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adly N, Weidlich S, Seyock S, et al. Printed Microelectrode Arrays on Soft Materials:From PDMS to Hydrogels. NPJ Flex Electron. 2018;2:15. https://doi.org/10.1038/s41528-018-0027-z. [Google Scholar]
- 44.Zhao G, Zhang X, Li B, et al. Solvent-Free Fabrication of Carbon Nanotube/Silk Fibroin Electrospun Matrices for Enhancing Cardiomyocyte Functionalities. ACS Biomater Sci Eng. 2020;6:1630–40. doi: 10.1021/acsbiomaterials.9b01682. https://doi.org/10.1021/acsbiomaterials.9b01682. [DOI] [PubMed] [Google Scholar]
- 45.Stoppel WL, Kaplan DL, Black LD. Electrical and Mechanical Stimulation of Cardiac Cells and Tissue Constructs. Adv Drug Deliv Rev. 2016;96:135–55. doi: 10.1016/j.addr.2015.07.009. https://doi.org/10.1016/j.addr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mihic A, Li J, Miyagi Y, et al. The Effect of Cyclic Stretch on Maturation and 3D Tissue Formation of Human Embryonic Stem Cell-Derived Cardiomyocytes. Biomaterials. 2014;35:2798–808. doi: 10.1016/j.biomaterials.2013.12.052. https://doi.org/10.1016/j.biomaterials.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 47.Marsano A, Conficconi C, Lemme M, et al. Beating Heart on a Chip:A Novel Microfluidic Platform to Generate Functional 3D Cardiac Microtissues. Lab Chip. 2016;16:599–610. doi: 10.1039/c5lc01356a. https://doi.org/10.1039/c5lc01356a. [DOI] [PubMed] [Google Scholar]
- 48.Chinali M, Simone GD, Roman MJ, et al. Left Atrial Systolic Force and Cardiovascular Outcome. The Strong Heart Study. Am J Hypertens. 2005;18:1570–6. doi: 10.1016/j.amjhyper.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 49.Bajaj P, Tang X, Saif TA, et al. Stiffness of the Substrate Influences the Phenotype of Embryonic Chicken Cardiac Myocytes. J Biomed Mater Res Part A. 2010;95A:1261–9. doi: 10.1002/jbm.a.32951. https://doi.org/10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- 50.Hong SP, Song S, Cho SW, et al. Generation of PDGFRa+Cardioblasts from Pluripotent Stem Cells. Sci Rep. 2017;7:41840. doi: 10.1038/srep41840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruan JL, Tulloch NL, Razumova MV, et al. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation. 2016;134:1557–67. doi: 10.1161/CIRCULATIONAHA.114.014998. https://doi.org/10.1161/circulationaha.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Visone R, Talò G, Occhetta P, et al. A Microscale Biomimetic Platform for Generation and Electro-Mechanical Stimulation of 3D Cardiac Microtissues. APL Bioeng. 2018;2:046102. doi: 10.1063/1.5037968. https://doi.org/10.1063/1.5037968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho KW, Lee WH, Kim BS, et al. Sensors in Heart-on-a-Chip:A Review on Recent Progress. Talanta. 2020;219:121269. doi: 10.1016/j.talanta.2020.121269. https://doi.org/10.1016/j.talanta.2020.121269. [DOI] [PubMed] [Google Scholar]
- 54.Plotnikov SV, Sabass B, Schwarz U, et al. High-Resolution Traction Force Microscopy. Methods Cell Biol. 2014;123:367–94. doi: 10.1016/B978-0-12-420138-5.00020-3. https://doi.org/10.1016/b978-0-12-420138-5.00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polacheck W, William J, Chen CS. Measuring Cell-Generated Forces:A Guide to the Available Tools. Nat Methods. 2016;13:415–23. doi: 10.1038/nmeth.3834. https://doi.org/10.1038/nmeth.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Y, Fu F, Shang L, et al. Bioinspired Helical Microfibers from Microfluidics. Adv Mater. 2017;29:1605765. doi: 10.1002/adma.201605765. https://doi.org/10.1002/adma.201605765. [DOI] [PubMed] [Google Scholar]
- 57.Ma X, Dewan S, Liu J, et al. 3D Printed Micro-Scale Force Gauge Arrays to Improve Human Cardiac Tissue Maturation and Enable High Throughput Drug Testing. Acta Biomat. 2018;95:319–27. doi: 10.1016/j.actbio.2018.12.026. https://doi.org/10.1016/j.actbio.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan JL, Tien J, Pirone DM, et al. Cells Lying on a Bed of Microneedles:An Approach to Isolate Mechanical Force. Proc Natl Acad Sci U S A. 2003;100:1484–9. doi: 10.1073/pnas.0235407100. https://doi.org/10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal A, Goss JA, Cho A, et al. Microfluidic Heart on a Chip for Higher Throughput Pharmacological Studies. Lab Chip. 2013;13:3599–608. doi: 10.1039/c3lc50350j. https://doi.org/10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oyunbaatar NE, Shanmugasundaram A, Lee DW. Contractile Behaviors of Cardiac Muscle Cells on Mushroom-Shaped Micropillar Arrays. Colloids Surf B Biointerfaces. 2019;174:103–9. doi: 10.1016/j.colsurfb.2018.10.058. https://doi.org/10.1016/j.colsurfb.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 61.Vandenburgh H, Shansky J, Benesch-Lee F, et al. Drug-Screening Platform Based on the Contractility of Tissue-Engineered Muscle. Muscle Nerve. 2008;37:438–47. doi: 10.1002/mus.20931. https://doi.org/10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Wang EY, Davenport LH, et al. A Multimaterial Microphysiological Platform Enabled by Rapid Casting of Elastic Microwires. Adv Healthc Mater. 2019;8:1801187. doi: 10.1002/adhm.201801187. https://doi.org/10.1002/adhm.201801187. [DOI] [PubMed] [Google Scholar]
- 63.Mastikhina O, Moon BU, Williams K, et al. Human Cardiac Fibrosis-on-a-Chip Model Recapitulates Disease Hallmarks and Can Serve as a Platform for Drug Testing. Biomaterials. 2020;233:119741. doi: 10.1016/j.biomaterials.2019.119741. https://doi.org/10.1016/j.biomaterials.2019.119741. [DOI] [PubMed] [Google Scholar]
- 64.Chan V, Jeong JH, Bajaj P, et al. Multi-Material Bio-Fabrication of Hydrogel Cantilevers and Actuators with Stereolithography. Lab Chip. 2011;12:88–98. doi: 10.1039/c1lc20688e. https://doi.org/10.1039/c1lc20688e. [DOI] [PubMed] [Google Scholar]
- 65.Fu F, Shang L, Chen Z, et al. Bioinspired Living Structural Color Hydrogels. Sci Robot. 2018;3:aar8580. doi: 10.1126/scirobotics.aar8580. [DOI] [PubMed] [Google Scholar]
- 66.Sun L, Chen Z, Bian F, et al. Bioinspired Soft Robotic Caterpillar with Cardiomyocyte Drivers. Adv Funct Mater. 2020;30:1907820. https://doi.org/10.1002/adfm.201907820. [Google Scholar]
- 67.Kim DS, Jeong YJ, Lee BK, et al. Piezoresistive Sensor-Integrated PDMS Cantilever:A New Class of Device for Measuring the Drug-Induced Changes in the Mechanical Activity of Cardiomyocytes. Sens Actuators B Chem. 2017;240:566–72. https://doi.org/10.1016/j.snb.2016.08.167. [Google Scholar]
- 68.Wang L, Dou W, Malhi M, et al. Microdevice Platform for Continuous Measurement of Contractility, Beating Rate, and Beating Rhythm of Human-Induced Pluripotent Stem Cell-Cardiomyocytes inside a Controlled Incubator Environment. Acs Appl Mater Interfaces. 2018;10:21173–83. doi: 10.1021/acsami.8b05407. https://doi.org/10.1021/acsami.8b05407.s001. [DOI] [PubMed] [Google Scholar]
- 69.Coln EA, Colon A, Long CJ, et al. Piezoelectric bioMEMS Cantilever for Measurement of Muscle Contraction and for Actuation of Mechanosensitive Cells. MRS Commun. 2019;9:1186–92. doi: 10.1557/mrc.2019.129. https://doi.org/10.1557/mrc.2019.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang A, Liu Z, Hu M, et al. Piezoelectric Nanofibrous Scaffolds as In Vivo Energy Harvesters for Modifying Fibroblast Alignment and Proliferation in Wound Healing. Nano Energy. 2018;43:63–71. https://doi.org/10.1016/j.nanoen.2017.11.023. [Google Scholar]
- 71.Sakamiya M, Fang Y, Mo X, et al. A Heart-on-a-Chip Platform for Online Monitoring of Contractile Behavior Via Digital Image Processing and Piezoelectric Sensing Technique. Med Eng Phys. 2020;75:36–44. doi: 10.1016/j.medengphy.2019.10.001. https://doi.org/10.1016/j.medengphy.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Lind JU, Yadid M, Perkins I, et al. Cardiac Microphysiological Devices with Flexible Thin-Film Sensors for Higher-Throughput Drug Screening. Lab Chip. 2017;17:3692–703. doi: 10.1039/c7lc00740j. https://doi.org/10.1039/c7lc00740j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang D, Pikhitsa PV, Choi YW, et al. Ultrasensitive Mechanical Crack-Based Sensor Inspired by the Spider Sensory System. Nature. 2014;516:222–6. doi: 10.1038/nature14002. https://doi.org/10.1038/nature14002. [DOI] [PubMed] [Google Scholar]
- 74.Kim DS, Choi YW, Shanmugasundaram A, et al. Highly Durable Crack Sensor Integrated with Silicone Rubber Cantilever for Measuring Cardiac Contractility. Nat Commun. 2020;11:535. doi: 10.1038/s41467-019-14019-y. https://doi.org/10.1038/s41467-019-14019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kireev D, Seyock S, Lewen J, et al. Graphene Multielectrode Arrays as a Versatile Tool for Extracellular Measurements. Adv Healthc Mater. 2017;6:1601433. doi: 10.1002/adhm.201601433. https://doi.org/10.1002/adhm.201601433. [DOI] [PubMed] [Google Scholar]
- 76.Qian F, Huang C, Lin YD, et al. Simultaneous Electrical Recording of Cardiac Electrophysiology and Contraction on Chip. Lab Chip. 2017;17:1732–9. doi: 10.1039/c7lc00210f. https://doi.org/10.1039/c7lc00210f. [DOI] [PubMed] [Google Scholar]
- 77.Tian B, Cohen-Karni T, Qing Q, et al. Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science. 2010;329:830–4. doi: 10.1126/science.1192033. https://doi.org/10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbott J, Ye T, Qin L, et al. CMOS Nanoelectrode Array for All-Electrical Intracellular Electrophysiological Imaging. Nat Nanotechnol. 2017;12:460–6. doi: 10.1038/nnano.2017.3. https://doi.org/10.1038/nnano.2017.3. [DOI] [PubMed] [Google Scholar]
- 79.Dai X, Zhou W, Gao T, et al. Three-Dimensional Mapping and Regulation of Action Potential Propagation in Nanoelectronics-Innervated Tissues. Nat Nanotechnol. 2016;11:776–82. doi: 10.1038/nnano.2016.96. https://doi.org/10.1038/nnano.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaneko T, Kojima K, Yasuda K. An On-Chip Cardiomyocyte Cell Network Assay for Stable Drug Screening Regarding Community Effect of Cell Network Size. Analyst. 2007;132:892–8. doi: 10.1039/b704961g. https://doi.org/10.1039/b704961g. [DOI] [PubMed] [Google Scholar]
- 81.Aung A, Bhullar IS, Theprungsirikul J, et al. 3D Cardiac Mu Tissues Within a Microfluidic Device with Real-Time Contractile Stress Readout. Lab Chip. 2016;16:153–62. doi: 10.1039/c5lc00820d. https://doi.org/10.1039/c5lc00820d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mai-Dung N, Tinney JP, Yuan F, et al. Cardiac Cell Culture Model As a Left Ventricle Mimic for Cardiac Tissue Generation. Anal Chem. 2013;85:8773–9. doi: 10.1021/ac401910d. https://doi.org/10.1021/ac401910d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Chan HN, Michael SA, et al. A Microfluidic Circulatory System Integrated with Capillary-Assisted Pressure Sensors. Lab Chip. 2017;17:653–62. doi: 10.1039/c6lc01427e. https://doi.org/10.1039/c6lc01427e. [DOI] [PubMed] [Google Scholar]
- 84.Savoji H, Mohammadi MH, Rafatian N, et al. Cardiovascular Disease Models:A Game Changing Paradigm in Drug Discovery and Screening. Biomaterials. 2019;198:3–26. doi: 10.1016/j.biomaterials.2018.09.036. https://doi.org/10.1016/j.biomaterials.2018.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ren L, Liu W, Wang Y, et al. Investigation of Hypoxia-Induced Myocardial Injury Dynamics in a Tissue Interface Mimicking Microfluidic Device. Anal Chem. 2013;85:235–44. doi: 10.1021/ac3025812. https://doi.org/10.1021/ac3025812. [DOI] [PubMed] [Google Scholar]
- 86.Liu H, Bolonduro OA, Hu N, et al. Heart-on-a-Chip Model with Integrated Extra-and Intracellular Bioelectronics for Monitoring Cardiac Electrophysiology under Acute Hypoxia. Nano Lett. 2020;20:2585–93. doi: 10.1021/acs.nanolett.0c00076. https://doi.org/10.1021/acs.nanolett.0c00076. [DOI] [PubMed] [Google Scholar]
- 87.Martewicz S, Michielin F, Serena E, et al. Reversible Alteration of Calcium Dynamics in Cardiomyocytes during Acute Hypoxia Transient in a Microfluidic Platform. Integr Biol. 2012;4:153–64. doi: 10.1039/c1ib00087j. https://doi.org/10.1039/c1ib00087j. [DOI] [PubMed] [Google Scholar]
- 88.Sadeghi AH, Shin SR, Deddens JC, et al. Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling. Adv Healthc Mater. 2017;6:1601434. doi: 10.1002/adhm.201601434. https://doi.org/10.1002/adhm.201601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tse G. Mechanisms of Cardiac Arrhythmias. J Arrhythm. 2016;32:75–81. doi: 10.1016/j.joa.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma Z, Koo S, Finnegan MA, et al. Three-Dimensional Filamentous Human Diseased Cardiac Tissue Model. Biomaterials. 2014;35:1367–77. doi: 10.1016/j.biomaterials.2013.10.052. https://doi.org/10.1016/j.biomaterials.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brandão KO, Tabel VA, Atsma DE, et al. Human Pluripotent Stem Cell Models of Cardiac Disease:From Mechanisms to Therapies. Dis Model Mech. 2017;10:1039–59. doi: 10.1242/dmm.030320. https://doi.org/10.1242/dmm.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren L, Zhou X, Nasiri R, et al. Combined Effects of Electric Stimulation and Microgrooves in Cardiac Tissue-on-a-Chip for Drug Screening. Small Methods. 2020;4:2000438. doi: 10.1002/smtd.202000438. https://doi.org/10.1002/smtd.202000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y, Rafatian N, Feric NT, et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell. 2019;176:913–27.e18. doi: 10.1016/j.cell.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang G, Li F, Zhao X, et al. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem Rev. 2017;117:12764–850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu F, Choudhury D. Microfluidic Bioprinting for Organ-on-a-Chip Models. Drug Discov Today. 2019;24:1248–57. doi: 10.1016/j.drudis.2019.03.025. https://doi.org/10.1016/j.drudis.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 96.Gao B, Yang Q, Zhao X, et al. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016;34:746–56. doi: 10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 97.Shi M, Ling K, Yong KW, et al. High-Throughput Non-Contact Vitrification of Cell-Laden Droplets Based on Cell Printing. Sci Rep. 2015;5:17928. doi: 10.1038/srep17928. https://doi.org/10.1038/srep17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs. Lab Chip. 2014;14:2202–11. doi: 10.1039/c4lc00030g. https://doi.org/10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]


