Version Changes
Revised. Amendments from Version 1
In Abstract and Conclusion we reduced the conclusion of the transcription factor analysis, eliminating the “co-regulation”, since it was too speculative. We added vtRNA2-2P pseudogen to the vtRNA3-1P analysis, to include the two vtRNA pseudogenes currently annotated (introduction, results, materials and methods, figure legends were modified accordingly). We included a study of Y-RNY genes ATAC-seq as an external family of RNApol III transcripts (results, materials and methods, figure legends were modified accordingly). The addition of vtRNA2-2P and Y-RNY genes required an new version of SF3, and an updated ST1. “MicroRNA precursor” was substituted to “generate small RNA” since the evidence of vtRNA processing and function in the microRNA pathway is not demonstrated for all of them. “Expression” of vtRNA was substituted to "promoter DNA methylation, chromatin status or inferred expression” to make clear that the chromatin status just a surrogate of vtRNA expression but not their expression itself. “Chromatin polymorphism” was substituted for “epigenetic variation” to describe the chromatin variability of vtRNA2-1. “High Chromatin accessibility” was substituted for “Low DNA methylation” on the results section title to be accurate. A comment about antecedents of RNApol III-RNApol II interaction was added to transcription factor result section to further justify the pertinency of the analysis. The involvement of RNApol III in innate immune response was also commented. TGFB1 was substituted by TFGBI. Zenodo link was updated to include the new supplementary figure. http://doi.org/10.5281/zenodo.4784353
Abstract
Background: The vault RNAs (vtRNAs) are a class of 84-141-nt eukaryotic non-coding RNAs transcribed by RNA polymerase III, associated to the ribonucleoprotein complex known as vault particle. Of the four human vtRNA genes, vtRNA1-1, vtRNA1-2 and vtRNA1-3, clustered at locus 1, are integral components of the vault particle, while vtRNA2-1 is a more divergent homologue located in a second locus. Gene expression studies of vtRNAs in large cohorts have been hindered by their unsuccessful sequencing using conventional transcriptomic approaches.
Methods: VtRNA expression in The Cancer Genome Atlas (TCGA) Pan-Cancer cohort was estimated using the genome-wide DNA methylation and chromatin accessibility data (ATAC-seq) of their genes as surrogate variables. The association between vtRNA expression and patient clinical outcome, immune subtypes and transcriptionally co-regulated gene programs was analyzed in the dataset.
Results: VtRNAs promoters are enriched in transcription factors related to viral infection. VtRNA2-1 is likely the most independently regulated homologue. VtRNA1-1 has the most accessible chromatin, followed by vtRNA1-2, vtRNA2-1 and vtRNA1-3. VtRNA1-1 and vtRNA1-3 chromatin status does not significantly change in cancer tissues. Meanwhile, vtRNA2-1 and vtRNA1-2 expression is widely deregulated in neoplastic tissues and its alteration is compatible with a broad oncogenic role for vtRNA1-2, and both tumor suppressor and oncogenic functions for vtRNA2-1. Yet, vtRNA1-1, vtRNA1-2 and vtRNA2-1 promoter DNA methylation predicts a shorter patient overall survival cancer-wide. In addition, gene ontology analyses of vtRNAs co-regulated genes identify a chromosome regulatory domain, epithelial differentiation, immune and thyroid cancer gene sets for specific vtRNAs. Furthermore, vtRNA expression patterns are associated with cancer immune subtypes and vtRNA1-2 expression is positively associated with cell proliferation and wound healing.
Conclusions: Our study presents the landscape of vtRNA chromatin status cancer-wide, identifying co-regulated gene networks and ontological pathways associated with the different vtRNA genes that may account for their diverse roles in cancer.
Keywords: vault RNA, vtRNA1-1, vtRNA1-2, vtRNA1-3, vtRNA2-1, nc886, mir-886, cancer, TCGA, DNA methylation, chromatin accessibility
Abbreviations
vtRNA vault RNA
TCGA The Cancer Genome Atlas
ATAC-seq Assay for Transposase Accessible Chromatin with high-throughput sequencing
NoMe-seq Nucleosome Occupancy and Methylome sequencing
OG Oncogene
TSG Tumor Suppressor Gene
TCGA The Cancer Genome Atlas
TSS Transcription Start Site
Ave Average
SD Standard Deviation
ACC Adrenocortical carcinoma
BLCA Bladder Urothelial Carcinoma
BRCA Breast invasive carcinoma
CESC Cervical squamous cell carcinoma and endocervical adenocarcinoma
CHOL Cholangiocarcinoma
COAD Colon adenocarcinoma
DLBC Lymphoid Neoplasm Diffuse Large B-cell Lymphoma
ESCA Esophageal carcinoma
GBM Glioblastoma multiforme
HNSC Head and Neck squamous cell carcinoma
KICH Kidney Chromophobe
KIRC Kidney renal clear cell carcinoma
KIRP Kidney renal papillary cell carcinoma
LGG Brain Lower Grade Glioma
LIHC Liver hepatocellular carcinoma
LUAD Lung adenocarcinoma
LUSC Lung squamous cell carcinoma
MESO Mesothelioma
OV Ovarian serous cystadenocarcinoma
PAAD Pancreatic adenocarcinoma
PCPG Pheochromocytoma and Paraganglioma
PRAD Prostate adenocarcinoma
READ Rectum adenocarcinoma
SARC Sarcoma
SKCM Skin Cutaneous Melanoma
STAD Stomach adenocarcinoma
TGCT Testicular Germ Cell Tumors
TGCT Testicular Germ Cell Tumors
THCA Thyroid carcinoma
THYM Thymoma
UCEC Uterine Corpus Endometrial Carcinoma
UCS Uterine Carcinosarcoma
UVM Uveal Melanoma
Introduction
The vault RNAs (vtRNAs) are a class of eukaryotic middle size non-coding RNAs (84-141 nt) transcribed by the RNA polymerase III, which associates to the vault particle (<5 % of the total mass of the vault particle) ( Boivin et al., 2019; Kedersha & Rome, 1986; Kedersha et al., 1991). Although this particle is the largest cellular ribonucleoprotein complex, its function remains scarcely understood. Most mammals have a single vtRNA gene (i.e., mouse and rat), but the human genome has three copies, annotated as vtRNA1-1 (98bp), vtRNA1-2 and vtRNA1-3 (88bp) genes, which are located in the chromosome region 5q31.3 (locus vtRNA1) and code for the RNAs that are integral components of the vault particle. Lately, a transcript initially annotated as the human microRNA precursor hsa-mir-886 ( Landgraf et al., 2007), was re-classified as another human vtRNA homologue and consequently renamed as vtRNA2-1 ( Stadler et al., 2009). VtRNA2-1 is also located in chromosome 5 (5q31.1, locus vtRNA2) at 0.5Mb distance from the vtRNA1 cluster, where is placed between SMAD5 and TGFBI genes in an antisense direction. Existing evidence indicates that despite being a duplication of the vtRNAs of locus 1, the vtRNA2-1 is neither associated with the vault particle nor co-regulated with the vtRNA1 locus ( Lee et al., 2011; Stadler et al., 2009). Nevertheless, the realization that only 5–20% of all the cellular vtRNA transcripts are associated to the vault particle suggests additional roles for the majority of vtRNA transcripts, independent of the vault particle ( Kickhoefer et al., 1998; Nandy et al., 2009; van Zon et al., 2001). The vtRNA3-1P and vtRNA2-2P are two additional vault RNA genes situated in chromosome X and 2 respectively, but were classified as a pseudogenes due to the absence of expression in cell lines and the presence of silencing mutations at the promoter ( Büscher et al., 2020; van Zon et al., 2001).
Most of the current knowledge of vtRNA function focuses on vtRNA1-1 and vtRNA2-1 roles in viral infection and cancer. Functional studies found that vtRNA1-1 and vtRNA1-2 (but not vtRNA1-3) can interact directly with drugs as mitoxantrone, doxorubicin and etoposide ( Gopinath et al., 2005; Gopinath et al., 2010; Mashima et al., 2008). In addition, p62 protein interacts with vtRNA1-1 and inhibits the p62 dependent autophagy in vitro and in vivo ( Horos et al., 2019). The vault RNAs have also been linked to native immune response, because of their strong upregulation during the infection of Influenza A virus (IAV) and Epstein-Barr virus (EBV) ( Amort et al., 2015; Li et al., 2015; Nandy et al., 2009). These studies proposed a viral activation of host vtRNAs as a mechanism to maintain the inhibition of PKR signaling pathway, representing a viral strategy to circumvent host innate immunity.
Initial reports about vtRNA2-1 (locus vtRNA2) revealed that it is abundant in normal tissues while lowly expressed in cancer cell lines from different tissue origin (breast, melanoma, cervix, lung, oral and prostate), which is consistent with a tumor suppressive role in cancer ( Lee et al., 2011; Treppendahl et al., 2012). Moreover, vtRNA2-1 was proposed as a new type of ncRNA functioning as a tumor suppressor gene (TSG) that inhibits PKR, and was consequently renamed as “nc886” ( Golec et al., 2019; Jeon et al., 2012a; Jeon et al., 2012b; Lee et al., 2011). Then, its anti-proliferative and TSG function was described in prostate ( Aakula et al., 2016; Fort et al., 2018; Ma et al., 2020), skin ( Lee et al., 2019a), gastric ( Lee et al., 2014b) esophageal ( Im et al., 2020; Lee et al., 2014a) and cholangiocarcinoma cells ( Kunkeaw et al., 2012). Conversely, a pro-proliferative and anti-apoptotic oncogenic role was proposed for vtRNA2-1 in renal ( Lei et al., 2017), ovarian ( Ahn et al., 2018), thyroid ( Lee et al., 2016), cervical ( Li et al., 2017) and endometrial ( Hu et al., 2017) tissues. A recent finding proposing a vtRNA2-1/PKR loss mediated doxorubicin cytotoxicity introduced a novel view about its contribution to chemotherapy response ( Kunkeaw et al., 2019). In addition, a potential TSG role has been put forward for vtRNA1-3 in Myelodysplastic syndrome (MDS) ( Helbo et al., 2015).
Apart from their role as full length RNAs, vtRNAs are precursors of small RNAs. Indeed, small RNAs with microRNA like function were demonstrated to derive from vtRNA1-1 (svRNAs) and to repress the expression CYP3A4, a key enzyme of drug metabolism ( Meng et al., 2016; Persson et al., 2009). VtRNA2-1 has also been shown to generate small RNAs that could act like microRNAs in different tissues, serving both as TSG in prostate ( Aakula et al., 2016; Fendler et al., 2011; Fort et al., 2020), bladder ( Nordentoft et al., 2012), breast ( Tahiri et al., 2014), colon ( Yu et al., 2011), lung ( Bi et al., 2014; Cao et al., 2013; Gao et al., 2011; Shen et al., 2018) and thyroid cancers ( Dettmer et al., 2014; Xiong et al., 2011) and as oncogene (OG) in renal ( Yu et al., 2014), colorectal ( Schou et al., 2014) and esophagus cancer ( Okumura et al., 2016) through the repression of specific mRNA transcripts in human cancer.
Different lines of evidence revealed that the vtRNA transcription can be controlled by promoter DNA methylation. The epigenetic control of vtRNA2-1 is complex and owns clinical relevance in several solid tumors including breast, lung, colon, bladder, prostate, esophagus, hepatic and stomach cancer ( Cao et al., 2013; Fort et al., 2018; Lee et al., 2014a; Lee et al., 2014b; Romanelli et al., 2014; Treppendahl et al., 2012; Yu et al., 2020). Intriguing aspects of the epigenetic regulation of vtRNA2-1 locus comprise its dependence on the parental origin of the allele ( Joo et al., 2018; Paliwal et al., 2013), and its sensitivity to the periconceptional environment (( Silver et al., 2015) and subsequent independent studies ( Richmond et al., 2018; van Dijk et al., 2018)). In addition, vtRNA1-3 promoter methylation was associated with significantly poor outcome in lower risk myelodysplastic syndrome patients ( Helbo et al., 2015).
Currently, transcriptomic sequencing is the benchmark technique to study global RNA expression ( Stark et al., 2019). Nonetheless, some classes of RNAs are elusive to the standard transcriptomics due to their stable RNA structure and the presence of modified bases or ends, which impair the cDNA synthesis and/or adapter ligation during the sequencing library preparation ( Sendler et al., 2011; Zheng et al., 2015). The vtRNAs belong to this group due to their conserved stable stem/hairpin loop secondary structure. The latter, together with the lack of sequencing of 40–200bp-long RNAs in the conventional transcriptomic studies, which are mostly intended for small and long RNAs, probably delayed their study in comparison with other regulatory RNAs. In fact, the 50–300 nucleotides long RNAs are considered the black hole of RNA biology ( Boivin et al., 2018; Steitz, 2015). Nonetheless, since chromatin accessibility plays a critical role in the regulation of gene expression, at some extent the transcription of an RNA can be inferred from the chromatin status of its promoter ( Corces et al., 2018; Duren et al., 2017; Liu et al., 2018; Park et al., 2017b). Since mounting evidence has shown that vtRNAs expression is tightly controlled by chromatin accessibility, dependent on nucleosome positioning and promoter DNA methylation ( Ahn et al., 2018; Fort et al., 2018; Helbo et al., 2015; Helbo et al., 2017; Lee et al., 2014a; Lee et al., 2014b; Park et al., 2017a; Park et al., 2017b; Sallustio et al., 2016; Treppendahl et al., 2012), the chromatin structure is a suitable surrogate marker of vtRNA transcription and could be used as a proxy of their expression.
The expression, regulation, and role of the four human vtRNAs in normal and disease conditions are still poorly understood, and available knowledge indicates that they hold diverse tissue specific activities. The availability of genomic data of large sets of human tissues provided by The Cancer Genome Atlas (TCGA), allows the study of the human vtRNAs across 16 tissue types and normal/cancer conditions. Here, we analyze vtRNA genes chromatin of the TCGA patient cohort withdrawn from two approaches: the assay for transposase-accessible chromatin followed by NGS sequencing (ATAC-seq) to analyze chromatin accessibility ( Buenrostro et al., 2013; Buenrostro et al., 2015; Corces et al., 2018; Thurman et al., 2012) and the Illumina Infinium Human Methylation 450K BeadChip to analyze the CpG methylation of DNA ( Moran et al., 2016). This led us to determine the patterns of transcriptional regulation of the four human vtRNAs throughout cancer tissues. We also evaluate the association between vtRNA promoter DNA methylation and the patient clinical outcome in all the available cancer types. Finally, looking for functional discoveries, we analyze vtRNA relations with immune subtypes and transcriptionally co-regulated gene programs. Our study reveals specific patterns of inferred expression for each vtRNA that support previous evidence and poses potential new roles and molecular programs in which they may participate across and intra cancer types, increasing the comprehension of the role of the human vtRNAs in health and disease.
Methods
ATAC-seq data
The ATAC-seq data obtained by The Cancer Genome Atlas (TCGA) consortium were retrieved from UCSC Xena Browser ( Goldman et al., 2020) on 03/10/2018. It comprises the genomic matrix TCGA_ATAC_peak_Log2Counts_dedup_promoter with normalized count values for 404 samples (385 primary solid tumor samples across 23 tissues). As is described in UCSC Xena Browser to calculate the average ATAC-seq values a prior count of 5 was added to the raw counts, then put into a "counts per million", then log2 transformed, then quantile normalized; the result is the average value in the file (log2(count+5)-qn values) across all technical replicates and all biospecimens belonging to the same TCGA sample group. The gene promoter for ATAC-seq is defined as a region within -1000 to +100bp from the TSS site. Assignment of promoter peak to gene mapping information derives from the peak summit within the promoter region. Peak location information of the ATAC-seq values for 500 bp gene promoter regions was retrieved from the file TCGA_ATAC_Log2Counts_Matrix.180608.txt.gz. All the analyses were performed for tissues with at least five primary tumor samples (21 tissues), which resulted in the exclusion of CESC (2 primary tumor samples) and SKCM (4 primary tumor samples). Correlations between ATAC-seq and DNA promoter methylation were performed for the 329 primary tumors that are studied by both strategies ( Extended Data: Tables S1 and S2).
DNA methylation data
The DNA methylation data obtained from The Cancer Genome Atlas (TCGA) consortium was retrieved from UCSC Xena Browser ( Goldman et al., 2020) on date 03/10/2018. It comprises the normalized beta-value of DNA methylation obtained using Illumina Infinium Human Methylation 450 BeadChip arrays of the total (9149 samples) normal adjacent tissues (746 samples across 23 tissues) and primary solid tumors (8403 samples across 32 tissues) of the Pan-Cancer TCGA cohort. The current study is limited to solid tumors since only AML DNA methylation is available at the TCGA. All the analyses were performed for tissues with at least five samples. Normal adjacent tissues comprise 16 tissues, excluding CESC (two samples), GBM (two samples), PCPG (three samples), SARC (four samples), SKCM (two samples), STAD (two samples) and THYM (two samples). Primary tumors comprise 32 tissues. The normalized promoter average beta-values (500 bp bin) comprise the following CpG sites for vtRNA1-1: cg12532653, cg05913451, cg14633504, cg16615348, cg25602765, cg13323902, cg18296956; for vtRNA1-2: cg21161173, cg13303313, cg00500100, cg05174942, cg25984996, cg11807153, cg15697852; for vtRNA1-3: cg23910413, cg19065177, cg02053188, cg01063759, cg07379832, cg07741016; and for vtRNA2-1: cg16615357, cg08745965, cg00124993, cg26896946, cg25340688, cg26328633, cg06536614, cg18678645, cg04481923 ( Extended Data: Tables S2–S5).
Analysis of transcription factors associated to vtRNA genes
The transcription factors (TFs) occupancy analysis of vtRNAs was performed at a ± 3000 bp region centered at each vtRNA gene. The CHIP-seq data was retrieved from the UCSC genome browser (GRCh37/hg19) ( Kent et al., 2002) and the K562 chronic myelogenous leukemia cell line was chosen since it has the richest TFs data of ENCODE dataset (ENCODE Transcription Factor Binding tracks - ENCODE 3 TFBS) ( Euskirchen et al., 2007). The KEGG pathways enrichment analysis of the core 23 TFs common to all vtRNAs was done using STRING software ( Szklarczyk et al., 2017) ( Extended Data: Table S6).
Overall survival analysis
The Kaplan Meier curve analysis ( Bland & Altman, 1998) was performed with software GraphPad Prism 6 using Log-ranked Mantel-Cox test. We analyzed the ATAC-seq normalized values and DNA methylation average normalized beta-values for vtRNA promoters and survival data until 4000 days (99% of data). The log-rank statistics was calculated for quartiles of vtRNAs promoter chromatin accessibility comparison groups (25 th and 75 th) ( Extended Data: Tables S7 and S8).
Pathway enrichment and cluster chromosome localization analyses
The genome wide correlation analysis of promoter chromatin accessibility (ATAC-seq normalized values) for each vtRNA in comparison with all the annotated genes was performed using the genomic matrix TCGA_ATAC_peak_Log2Counts_dedup_promoter retrieved from UCSC Xena Browser (385 primary tumor samples across 23 tissues) ( Goldman et al., 2020) on date 03/10/2018. The protein coding genes showing a Spearman correlation r ≥ 0.4 were selected for pathway and cluster location analysis. The pathway enrichment analysis was done using STRING software for the Gene Ontology Biological Process and KEGG pathways categories ( Szklarczyk et al., 2017). The cluster localization analysis was performed with two approaches, the Cluster Locator algorithm ( Pazos Obregón et al., 2018) and the Enrichr software ( Kuleshov et al., 2016) ( Extended Data: Tables S9 and S10).
Immune subtypes data
The data of Immune Subtypes defined by Thorsson et al. ( Thorsson et al., 2018) is available at the Pan-Cancer TCGA and was retrieved from UCSC Xena Browser ( Goldman et al., 2020) on date 03/10/2018 and from the supplementary material of Thorsson et al. article ( Thorsson et al., 2018). ATAC-seq data of vtRNA promoters for the six Immune Subtype comprised a total of 364 samples: wound healing (105 samples), IFN-g dominant (93 samples), inflammatory (110 samples), lymphocyte depleted (43 samples), immunologically quiet (nine samples), TGF-b dominant (four samples). DNA methylation average normalized beta-values of vtRNA promoters for the six Immune Subtype comprised 7693 samples including wound healing (1963 samples), IFN-g dominant (2137 samples), inflammatory (2126 samples), lymphocyte depleted (933 samples), immunologically quiet (383 samples), TGF-b dominant (151 samples) ( Extended Data: Tables S11–S14).
Statistical analysis
Unless specified all the variables are expressed as average value ± standard deviation (SD). Statistical analyses were performed with one-way ANOVA for multiple comparison tests, including Sidak’s Honest Significant Difference test as a post-hoc test, as referred in the legend of the Figures. Spearman equations were applied to determine the correlations. All the analyses were done in R software (version 3.6) using libraries ggcorrplot, corrplot, xlsx, heatmap.2 (clustering distance measured by Euclidean and Ward clustering algorithms) and software GraphPad Prism 6. The statistical significance of the observed differences was described using the p-value (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p-value < 0.0001). Differences with a p-value of < 0.05 were considered significant.
Results
VtRNA genes have different chromatin accessibility at the promoter region
The Cancer Genome Atlas consortium incorporated ATAC-seq data for tumor samples of the GDC Pan-Cancer dataset (385 primary tumors samples across 23 cancer types ( Corces et al., 2018)). The ATAC-seq strategy is a genome-wide sequencing profiling approximation to chromatin accessibility using the hyperactive Tn5 transposase that simultaneously cut and ligate adapters preferentially to the accessible DNA regions. The counts of sequencing reads in a particular genomic region provides a direct measurement of chromatin accessibility ( Buenrostro et al., 2015; Tsompana & Buck, 2014). For those gene promoters regulated by DNA methylation, ATAC-seq is a more direct approximation to chromatin accessibility than promoter CpG DNA methylation measurements because the latter is well correlated but not an exact measurement of chromatin accessibility. Indeed, the effect of DNA methylation on chromatin structure depends on the gene and the distribution of the methylation sites along it. It is also important to mention that gene expression interpretations based solely on chromatin accessibility are blind to post-transcriptional regulatory events such as processing, localization, and stability of the RNA transcript. Nevertheless, since the chromatin status of the different vtRNA promoters has been positively correlated with the abundance of their transcripts ( Ahn et al., 2018; Fort et al., 2018; Helbo et al., 2015; Lee et al., 2014a; Lee et al., 2014b; Park et al., 2017a; Park et al., 2017b; Sallustio et al., 2016; Treppendahl et al., 2012), ATAC-seq is likely a good surrogate of vtRNA expression. DNA CpG methylation data is available for 746 normal adjacent (across 23 tissues) and 8403 primary tumor (across 32 tissues) tissue samples of the Pan-Cancer TCGA cohort ( Extended Data: Tables S1–S5 and Figure S1). The full data is listed in Tables S1–S5 and the tissue composition of the three datasets of the cohort, comprising tumor ATAC-seq, normal and tumor DNA methylation, is shown in Figure S1. The DNA methylation data has nine more tissue types and a higher number of samples than the others, while the abundance of some tissues is skewed to twice enrichment in 1-4 specific tissue types.
Although several reports demonstrated that the DNA methylation of vtRNA promoters is well correlated to vtRNA transcript expression in various tissues ( Ahn et al., 2018; Fort et al., 2018; Helbo et al., 2015; Helbo et al., 2017; Lee et al., 2014a; Lee et al., 2014b; Park et al., 2017b; Sallustio et al., 2016; Treppendahl et al., 2012), the association between the DNA methylation and chromatin accessibility at their promoters was not previously investigated.
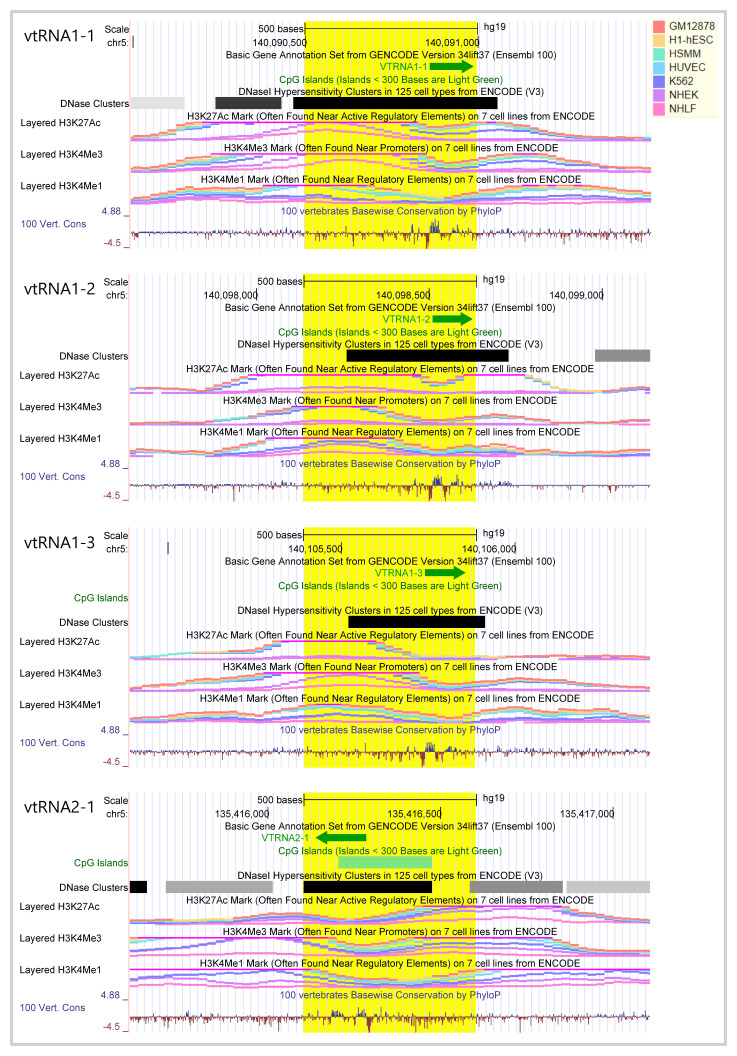
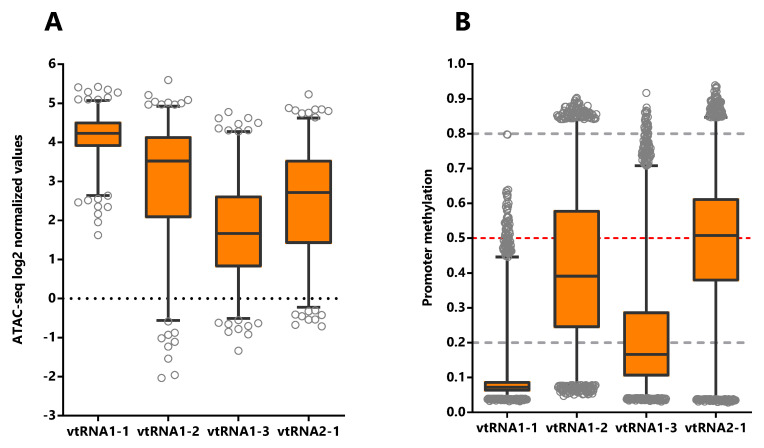
To assess the expression of vtRNAs in cancer tissues, we analyzed a 500 bp region, containing the full vtRNA genes, the proximal promoter region and the RNA polymerase lII A and B box elements located downstream of the transcription start site (TSS) ( Figure 1). Indeed, Vilalta et al., 1994 demonstrated that the deletion of the 300bp 5´-flanking region of the rat vtRNA transcript largely reduced its transcription greater than 30-fold ( Vilalta et al., 1994). As depicted in Figure 1, this region bears epigenetic marks of transcriptionally active chromatin ( Li et al., 2007), including histone H3K27ac, H3K4me1, H3K4me3 at the promoters and DNaseI hypersensitivity clusters around the TSS in the cell types compiled by UCSC browser ( Goldman et al., 2013). Interestingly, vtRNA2-1 is the only vtRNA immersed in a CpG island and vtRNA1-1 displays the strongest epigenetic marks of transcriptional activity ( Figure 1). The analysis of the ATAC-seq values of this 500 bp region of all the TCGA primary tumors reveals that the four human vtRNAs have different levels of chromatin accessibility ( Figure 2A). Moreover, the average DNA methylation of the promoters ( Figure 2B) in primary tumor samples supports this finding (32 tissues, Extended Data: Figure S1 and Tables S2 and S4). VtRNA1-1 promoter has the highest chromatin accessibility and the lowest DNA methylation, showing the smallest dispersion of values among the samples (Ave. 4.1, 0.6 SD) relative to the other three vtRNAs. Meanwhile, vtRNA1-2 (Ave. 2.9, 1.6 SD), vtRNA1-3 (Ave. 1.7, 1.2 SD) and vtRNA2-1 (Ave. 2.5, 1.4 SD) have broader ranges of ATAC-seq and DNA methylation values ( Figure 2, Table 1, Extended Data: Tables S1 and S2 and Figure S1). A comparison among the vtRNAs shows that the average chromatin accessibility of their promoters is inversely proportional to their average DNA methylation ( Table 1 and Figure 2) (relative ATAC-seq values are vtRNA1-1 (4.1) > vtRNA1-2 (2.9) > vtRNA2-1 (2.5) and accordingly, relative DNA methylation are vtRNA1-1 (0.09) < vtRNA1-2 (0.4) < vtRNA2-1 (0.5)). Yet vtRNA1-3 average low chromatin accessibility is not accompanied by a comparatively denser promoter methylation (values 1.7 and 0.22 respectively). Nevertheless, the intra-sample correlation between the ATAC-seq and DNA methylation for the vtRNAs is between -0.24 and -0.78 (see rs-values in Table 1 and Extended Data: Figure S2), suggesting that regardless of the relatively smaller average effect of DNA methylation on vtRNA1-3 promoter accessibility, all the vtRNAs are regulated by DNA methylation to some extent ( Table 1). In addition, the CpG DNA methylation negatively correlates with ATAC-seq values in the 500bp of the promoters of the four vtRNAs ( Table 1 and Extended Data: Figure S2). The strength of the correlation for each vtRNA is higher for the two vtRNAs that have a broader spectrum of DNA methylation, i.e. vtRNA1-2 and vtRNA2-1 (rs = -0.74 and rs = -0.78 respectively) ( Table 1 and Extended Data: Figure S2B and S2D). Meanwhile, vtRNA1-1 and vtRNA1-3 promoters, which have narrower range of DNA methylation (vtRNA1-1 has also a small range of chromatin accessibility) present a smaller association between both values (rs = -0.24 and rs = -0.54 respectively) ( Table 1 and Extended Data: Figure S2A and S2C). Technical differences in ATAC-seq and DNA methylation array approaches, as well as naturally occurring non-linear correlation between average DNA methylation and chromatin structure may account for these differences ( Corces et al., 2018; Liu et al., 2018). The low ATAC-seq value of the pseudogenes vtRNA3-1P (Ave. -1.2, 0.6 SD) and vtRNA2-2P (Ave. 0.1, 0.6 SD), represents a proof of concept of the analyses (Extended Data: Figure S3 and Tables S1-S5). The same approach was performed for the Y-RNAs genes RNY1 (Ave. 3.7, 0.4 SD), RNY3 (Ave. 2.1, 0.6 SD, RNY4 (Ave. 1.9, 0.6 SD) and RNY5 (Ave. 3.1, 0.4 SD) ( Canella et al., 2010), which code for ubiquitously expressed RNA polymerase III transcripts ( Extended Data: Figure S3 and Tables S1).
Figure 1. Genomic view of human vtRNA genes epigenetic features.
Genomic view of 1.5 kb region of human vtRNA genes in UCSC Genome browser (GRCh37/hg19) centered at the 500bp bin highlighted in yellow, which was used for ATAC-seq and CpG methylation analyses. VtRNAs of locus 1 cluster (vtRNA1-1, vtRNA1-2, vtRNA1-3) and of locus 2 (vtRNA2-1) are in the sense and antisense orientation respectively. Several Gene annotation and ENCODE Project tracks for seven cell lines (GM12878, H1-hESC, HSMM, HUVEC, K562, NHEK, NHL) are displayed: DNA accessibility (DNaseI hypersensitivity clusters (color intensity is proportional to the maximum signal strength)), DNA methylation (for CpG islands length greater than 200 bp), histone modification (H3K27Ac, H3K4me1, H3K4me3 marks), conservation of the region in 100 Vertebrates (log-odds Phylop scores). The vertical viewing range of the tracks displays the default settings of the browser for each variable.
Figure 2. Chromatin accessibility and DNA methylation of the vtRNA promoters.
Analysis of vtRNA 500bp promoter region. A. Average chromatin accessibility ATAC-seq values for the 385 primary tumors available at the Pan-Cancer TCGA dataset (23 tissues types). B. Average DNA methylation beta-values for the 8403 primary tumors (32 tissues types). Dashed horizontal lines denote unmethylated (bottom gray, average beta-value ≤ 0.2), 50% methylated (middle red, average beta-value = 0.5) and highly methylated (top gray, average beta-value ≥ 0.8) promoters. The box plots show the median and the lower and upper quartile, and the whiskers the 2.5 and 97.5 percentile of the distribution.
Table 1. Correlation between vtRNA promoters ATAC-seq and DNA methylation values in the Pan-Cancer TCGA samples.
| Average ± SD | Spearman
correlation (ATAC-seq vs DNA methylation) |
|||
|---|---|---|---|---|
| vtRNA | Promoter
ATAC-seq |
Promoter DNA
methylation |
Rs | p-value |
| vtRNA1-1 | 4.1 ± 0.6 | 0.09 ± 0.07 | -0.24 | <0.0001 |
| vtRNA1-2 | 2.9 ± 1.6 | 0.42 ± 0.20 | -0.74 | <0.0001 |
| vtRNA1-3 | 1.7 ± 1.2 | 0.22 ± 0.15 | -0.54 | <0.0001 |
| vtRNA2-1 | 2.5 ± 1.4 | 0.47 ± 0.21 | -0.78 | <0.0001 |
The Spearman correlation was calculated for the 329 primary tumors studied by ATAC-seq and DNA methylation.
Chromatin accessibility, DNA methylation and transcription factor binding at the four vtRNA promoters are correlated
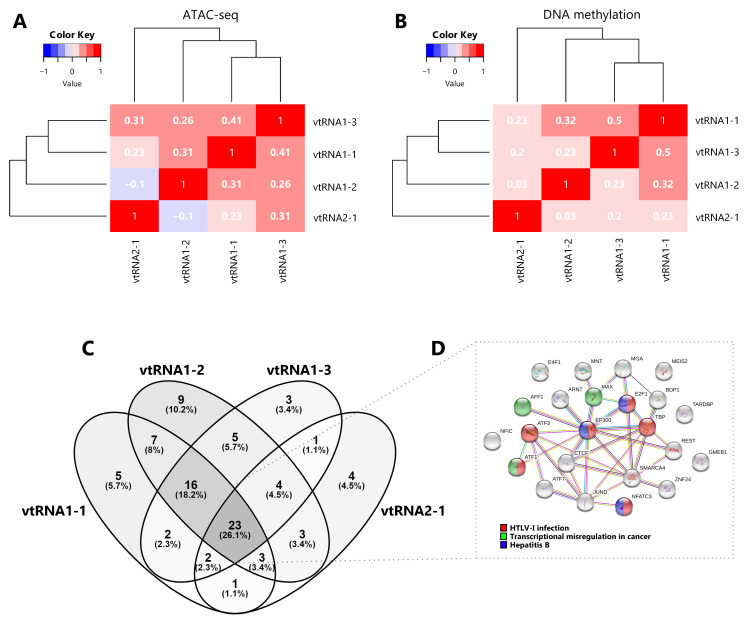
Aiming to investigate a possible transcriptional co-regulation of vtRNA genes, we determined the pairwise correlations in chromatin accessibility and DNA methylation among the four vtRNA promoters. We found that all pairs of vtRNAs, except vtRNA1-2 and vtRNA2-1, show positive correlations in both datasets ( Figure 3A and 3B) (rs-values 0.23-0.41 for ATAC-seq and rs-values 0.20-0.50 for DNA methylation). The unsupervised clustering of these epigenetic marks indicates that the three vtRNAs clustered at vtRNA1 locus (vtRNA1-1, vtRNA1-2 and vtRNA1-3), which are the vault particle associated vtRNAs, are more similar than vtRNA2-1 ( Figure 3A and 3B).
Figure 3. Comparative chromatin accessibility and transcription factor occupancy at the vtRNA loci.
A–B Matrix of pairwise Spearman correlations (two-way hierarchical clustering distance measured by Euclidean and Ward clustering algorithms) of vtRNA 500 bp promoter region for ATAC-seq data (385 primary tumors samples across 23 tissues) ( A) and DNA methylation average beta-values data (8403 primary tumors samples across 32 tissues) ( B). C. Venn diagram of transcription factors identified as ChIP-seq Peaks by ENCODE 3 project in the cell line K562 (Venny 2.1; https://bioinfogp.cnb.csic.es/tools/venny/index.html). The region for TFs assignment was defined as ±3000 bp from the vtRNA transcript sequence boundaries. D. Interaction cluster of the core 23 TFs common to all vtRNAs performed with STRING ( Szklarczyk et al., 2017). The colored labels of the top 3 enriched KEGG pathway terms (FDR < 0.05) are indicated.
Given that the chromatin accessibility of a DNA region is partially controlled by transcription and chromatin remodeler’s factors ( Corces et al., 2018; Klemm et al., 2019), we analyzed transcription factors (TFs) occupancy at a ± 3000 bp region centered at each vtRNA gene. Since there is no such study of the TCGA samples, we investigated the CHIP-seq data of the K562 chronic myelogenous leukemia cell line, which has the richest TF data at ENCODE dataset ( Euskirchen et al., 2007; Goldman et al., 2013). We observed that the four vtRNAs share a common core of 23 TFs (26%). The three members of the vtRNA1 cluster share 16 additional TFs (18%), reaching 39 common TFs (44%), while only 2-4 TFs with vtRNA2-1 ( Figure 3C and 3D and Extended Data: Table S6). We finally asked whether this common core of TFs is linked to a specific biological process. The STRING enrichment analysis of the core of 23 TFs common to all vtRNAs identified HTLV-I infection (ATF1, ATF3, E2F1, EP300, NFATC3, TBP), Hepatitis B (E2F1, EP300, NFATC3) and Transcriptional misregulation in cancer (AFF1, ATF1, MAX) as the top three enriched KEGG pathway terms (FDR < 0.05) ( Figure 3D and Extended Data: Table S6).
Taken together these findings indicate that the transcriptional activity of the vtRNAs is gene specific, being vtRNA1-1 the most accessible and possibly the most expressed. In addition, the data suggest that the regulatory status of the vtRNA promoters could be coordinately controlled, and the three vtRNA1s are more co-regulated among themselves. Yet, the core of TFs shared by the four vtRNAs is associated with viral infection and cancer related terms in the myeloid cell line K562.
The vtRNA promoters present gene and tissue specific patterns of chromatin accessibility in primary tumors
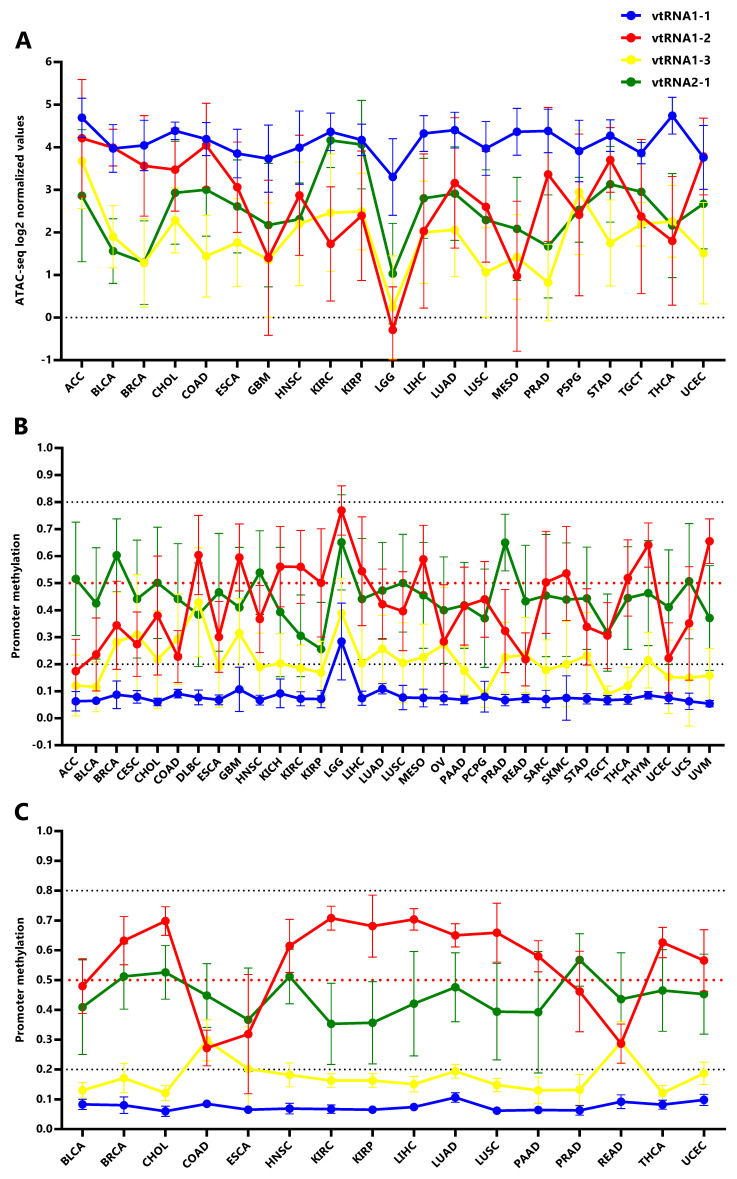
In order to investigate the expression of vtRNAs in different tissues we analyzed the Pan-Cancer TCGA ATAC-seq data discriminating the tissue of origin. As expected, vtRNA1-1 has the highest promoter chromatin accessibility among tissues and the smallest variation ( Figure 4A, Extended Data: Tables S1-S5 and Figure S1). Remarkably, low-grade glioma tumor samples (LGG) show a global reduction in chromatin accessibility at the four vtRNA gene promoters ( Figure 4A). An opposite pattern is seen in adrenocortical carcinoma tissue (ACC), where the vtRNAs have the highest concerted chromatin accessibility ( Figure 4A). Individually, vtRNAs promoter accessibility is maximum and minimum in THCA (4.7) and LGG (3.3) for vtRNA1-1, ACC (4.2) and LGG (0.29) for vtRNA1-2, ACC (3.7) and LGG (0.24) for vtRNA1-3 and KIRC (4.2) and LGG (1.0) for vtRNA2-1 ( Figure 4A). VtRNA3-1P reaches a maximum of promoter chromatin accessibility in Testicular Germ Cell Tumors (TGCT) (0.65) with an extremely low average ATAC-seq value and its minimum in PRAD (-1.5) ( Extended Data: Figure S3C). VtRNA2-2P reaches a maximum and minimum promoter chromatin accessibility in Lung Adenocarcinoma (LUAD) (1.0) and ACC (-0.34) respectively ( Extended Data: Figure S3C). Remarkably, although vtRNA1-1 and vtRNA1-3 have the highest and lowest promoter accessibility in the majority of tissues respectively, vtRNA1-2 and vtRNA2-1 are the most variable (vtRNA1-1 SD = 0.34, vtRNA1-2 SD = 1.4, vtRNA1-3 SD = 0.75 and vtRNA2-1 SD = 0.78) ( Figure 4A). In addition, in 12 tissues the chromatin accessibility of vtRNA1-2 is higher than vtRNA2-1 (57%) whereas in nine tissues the chromatin accessibility of vtRNA1-2 is lower than vtRNA2-1 (43%) ( Figure 4A).
Figure 4. Chromatin accessibility and DNA methylation of the vtRNA promoters in tumor and normal tissues.
A. ATAC-seq values for vtRNA promoters in 21 primary tumor tissues. B. Average beta-values of promoter DNA methylation for vtRNAs in 32 primary tumors tissues. C. Average beta-values of promoter DNA methylation for vtRNAs in 16 normal adjacent tissues. The charts show the average value and standard deviation of each vtRNAs for the tissues with at least five samples available at Pan-Cancer TCGA dataset.
Using the vtRNAs promoter DNA methylation data, we extended the analysis to 32 primary tumor tissues, incorporating nine more tissues than ATAC-seq samples (DLBC, KICH, OV, PAAD, READ, SARC, THYM, UCS, UVM) ( Figure 4B, Extended Data: Figure S1) and 16 normal adjacent tissues ( Figure 4C, Extended Data: Figure S1). Again, the DNA methylation profile mirrors the chromatin accessibility in the individual tissue types ( Figure 4). Remarkably, the association between the average vtRNA promoter chromatin accessibility and DNA methylation of the vtRNA1 cluster in each tissue type is higher than that observed for the average of all tissues (vtRNA1-1 rs = -0.28, vtRNA1-2 rs = -0.90 vtRNA1-3 rs = -0.74 and vtRNA2-1 rs = -0.58) ( Table 1 and Extended Data: Figure S4). The opposite finding for vtRNA2-1 may be explained by the impact of the previously recognized epigenetic variation in the locus ( Silver et al., 2015), which may prevail over the tissue specific variation for this gene.
In primary tumor tissues, promoter DNA methylation of vtRNA2-1 is higher than vtRNA1-2 in 17 tissues (53%) and the opposite is observed in the remaining 15 tissues (47%) ( Figure 4B). Meanwhile in normal tissues, promoter DNA methylation of vtRNA2-1 is higher than vtRNA1-2 in 4 tissues (25%) and lower in 12 tissues (75%) ( Figure 4C), indicating that although vtRNA1-2 is more methylated in normal samples, vtRNA2-1 gains methylation in neoplastic tissue, becoming more methylated than vtRNA1-2 (in 7 tissue types and vtRNA1-2 loose methylation: BLCA, BRCA, CHOL, HNCSC, LUAD, LUSC, UCEC). Indeed, the Fisher's exact test identifies a mirrored profile of chromatin accessibility between normal (4 tissues with high vtRNA1-2 and 12 tissues with high vtRNA2-1 from 16 total tissues) and tumor tissues (17 tissues with high vtRNA1-2 and 15 tissues with high vtRNA2-1 from 32 total tissues) for vtRNA1-2 and vtRNA2-1 (p-value = 0.07). Despite this deregulation in transformed tissues, we wondered if the tissue specific differences in vtRNA promoters were explained by their pre-existing status in the normal tissues. The correlation among average vtRNA promoter DNA methylation in normal and tumor tissues suggest that the variation in chromatin accessibility among tissue types is already established in the normal tissue counterparts (vtRNA1-1 rs = 0.53, vtRNA1-2 rs = 0.84, vtRNA1-3 rs = 0.47 and vtRNA2-1 rs = 0.75, Extended Data: Figure S5).
VtRNA promoter´s DNA methylation is associated with tumor stage and tissue of origin
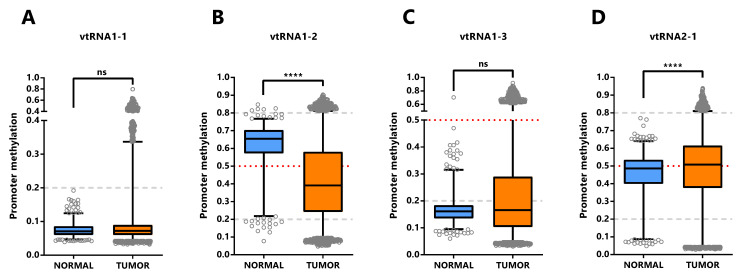
The assessment of differential vtRNA expression from normal to tumor condition at the TCGA cohort can only be performed using DNA methylation dataset since no ATAC-seq analyses were performed in the normal tissues. The average beta-value of CpG sites in the 500bp vtRNAs promoter of normal and tumor tissue evidenced gene specific patterns of deregulation in neoplastic tissues ( Figure 5). As depicted in Figure 4, vtRNA1-1 and vtRNA1-3 promoter DNA methylation and chromatin accessibility is low and high tissue-wide respectively, in both normal and tumor tissues (yet, there are highly methylated tumor outliers as LGG, that lack normal counterpart) ( Figure 4 and Figures 5A and 5C). Conversely, vtRNA1-2 and vtRNA2-1 revealed significant differences in average promoter DNA methylation among the tissue categories ( Figures 5B and 5D). In agreement with previous results, the methylation of vtRNA1-2 promoter decreases from normal to tumor samples, while vtRNA2-1´s increases ( Figures 5B and 5D and Figure 4).
Figure 5. Promoter DNA methylation of vtRNAs in Normal and Tumor tissues of Pan-Cancer TCGA dataset.
Average beta-values of promoter DNA methylation of vtRNA1-1 ( A), vtRNA1-2 ( B), vtRNA1-3 ( C) and vtRNA2-1 ( D), assessed in 746 normal and 8403 tumor tissues respectively. The box plots show the median line and lower and upper quartile and the whiskers the 2.5 and 97.5 percentile. Horizontal grey striped and red dotted lines denote unmethylated (average beta-value ≤ 0.2), 50% methylated (average beta-value = 0.5) and highly methylated (average beta-value ≥ 0.8) promoters. One-way ANOVA multiple test analysis with Sidak as posthoc test was performed. **** p-value < 0.0001.
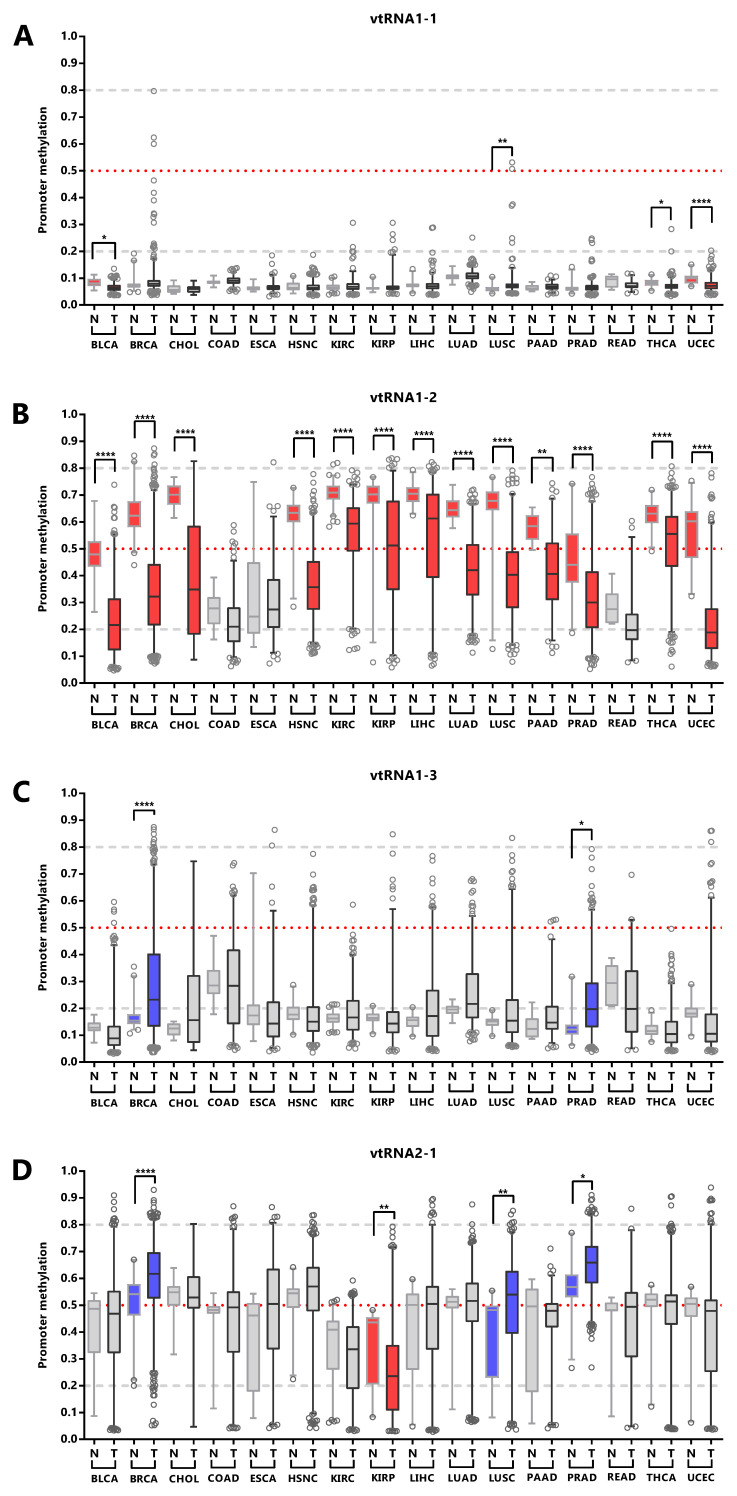
We next asked whether the promoter status of the vtRNAs is deregulated during the normal and tumor transformation of different tissue types. This comparison was restricted to the 16 tissues with normal samples (with data of at least five samples, Figure 6 and Extended Data: Figure S1, Table S5). The results revealed that vtRNA1-1 is globally unmethylated in normal and tumor from different tissue origins, but presents small but statistically significant changes in 4 tissue types (OG profile in BLCA, THCA UCEC and TSG in LUSC), whose biological impact remains to be determined ( Figure 6A). Similarly, vtRNA1-3 is globally unmethylated, showing larger variability and significant upregulation in BRCA and PRAD, compatible with a TSG function (TSG trends for LIHC p-value = 0.08 and LUSC p-value = 0.09, Figure 6C). In agreement with the literature, vtRNA2-1 presents a TSG pattern of deregulation in PRAD, LUSC and BRCA ( Cao et al., 2013; Fort et al., 2018; Romanelli et al., 2014), and an OG pattern in KIRP that has not been previously described ( Figure 6D). Finally, a statistically significant dysregulation of vtRNA1-2 promoter DNA methylation across almost all cancer types (13 out of 16 tissues analyzed) poses it as a candidate OG in cancer ( Figure 6B).
Figure 6. VtRNA promoters DNA methylation in Normal vs. Tumor samples.
Average beta-values of promoter DNA methylation for vtRNA1-1 ( A) vtRNA1-2 ( B), vtRNA1-3 ( C) and vtRNA2-1 ( D). Acronyms indicate the tissue condition (normal (N) and tumor (T)). Blue and red colors indicate an increase or reduction in promoter methylation in tumor vs their normal tissues counterparts. The box plots show the median and the lower and upper quartile, and the whiskers the 2.5 and 97.5 percentile of the distribution. Horizontal, lines denote the methylation level of the promoters: grey striped bottom and top for unmethylated (average beta-value ≤ 0.2) or highly methylated (average beta-value ≥ 0.8) respectively, and red dotted for 50% methylated (average beta-value = 0.5)) promoters. One-way ANOVA multiple test analysis with Sidak as posthoc test was performed. * p-value < 0.05; ** p-value < 0.01; **** p-value < 0.0001.
Overall, the data shows different DNA methylation changes in the vtRNAs promoters upon malignant transformation in several tissues. In addition, the deregulation of each vtRNA occurs mostly in a gene specific direction, where vtRNA1-2 has an OG like epigenetic de-repression while vtRNA2-1 (and to a lesser extent vtRNA1-1, vtRNA1-3) has a TSG like repressive methylation in tumor tissues.
Low DNA methylation at the promoter of vtRNA1-1, vtRNA1-2 and vtRNA2-1 is associated with low patient overall survival
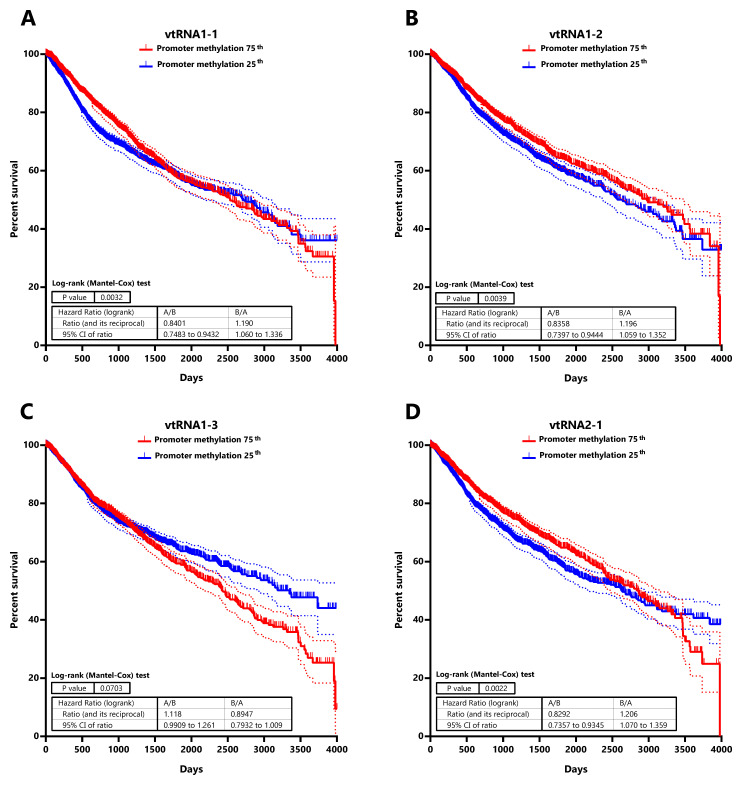
Seeking for a possible clinical significance of the chromatin accessibility change of the vtRNA promoters, we studied its association with the overall survival of patients ( Figure 7 and Extended Data: Tables S7 and S8). The analysis of patients stratified by vtRNA promoter accessibility quartiles did not show differences in overall survival (data available at Extended Data: Table S8). However, a significantly lower patient survival probability when patient tumors have lower promoter DNA methylation is observed for vtRNA1-1 (p-value = 0.003), vtRNA1-2 (p-value = 0.004) and vtRNA2-1 (p-value = 0.002) (patient stratified in quartiles of the promoter DNA methylation cohort values, Figure 7). Therefore, a lower promoter DNA methylation of vtRNA1-1, vtRNA1-2 and vtRNA2-1, a surrogate of their high expression, might be associated with poor patient overall survival cancer-wide.
Figure 7. Association between patient Overall survival and vtRNA promoters DNA methylation status in Pan-Cancer TCGA dataset.
Kaplan-Meier curves of overall patient survival probability over the time (4000 days) discriminating the primary tumors into two cohorts with relatively higher (75 th) and lower (25 th) DNA methylation at each vtRNA promoter. Promoter DNA methylation data of the 8060 primary tumors was used to stratify the patients in two quartiles, based on the expression of vtRNA1-1 ( A) vtRNA1-2 ( B), vtRNA1-3 ( C) and vtRNA2-1 ( D). Patient survival probability of the two groups was analyzed using Log-rank (Mantel-Cox) test. The dotted lines represent the 95% confidence interval for each curve.
Genome-wide chromatin accessibility correlations with vtRNA promoters reveal their link to specific cancer related functions
Seeking to get insight into the function of the vtRNAs from their transcription regulatory marks, we searched for the genes co-regulated at chromatin level. We calculated the correlation of the ATAC-seq promoter values of each vtRNA and all the individual genes in the 385 primary tumor cohort and selected those showing a Spearman correlation rs ≥ 0.4. Using STRING software analysis, we then investigated their connection with Biological Process categories and KEGG pathways ( Szklarczyk et al., 2017). A few enriched terms for the genes correlated with vtRNA1-2, vtRNA1-3 and vtRNA2-1 is identified (FDR < 1×10 -3) ( Extended Data: Tables S9 and S10). The 196 protein coding genes correlated with vtRNA1-2 are enriched in the Biological Process “Epithelial cell differentiation” (FDR < 1×10 -4) ( Extended Data: Table S10). Meanwhile, the 358 protein coding genes correlated with vtRNA2-1 are enriched in the Biological Processes “Immune system process”, “Inflammatory response” and “Immune response” (FDR < 1×10 -5) and the KEGG pathways term “Cytokine-cytokine receptor interaction” (FDR < 1×10 -4) ( Extended Data: Table S10). The only six protein coding genes correlated with vtRNA1-3 are enriched in the “Thyroid cancer” KEGG pathway term (FDR < 1×10 -4). Remarkably, although STRING analysis of the vtRNA1-1 associated 37 protein coding genes did not find enriched process or pathways, 36 of the 37 correlated genes are located at chromosome 5q region. Using the Cluster Locator algorithm ( Pazos Obregón et al., 2018), we found a statistically significant non-random clustering behavior for the chromosomal location of the genes co-regulated with vtRNA1-1 (p-value < 1×10 -10). Indeed, when the analysis was extended to 173 protein coding genes with Spearman correlation rs ≥ 0.3, 139 genes of 173 were situated at chromosome 5q region (p-value < 1×10 -10). These genes are positioned in particular regions at chr5q31, chr5q35, chr5q23, chr5q14, chr5q32, chr5q34 (FDR < 1×10 -4) ( Kuleshov et al., 2016) ( Extended Data: Table S10). We did not find a similar phenomenon for the other three vtRNAs. In summary, vtRNA1-3, vtRNA1-2 and vtRNA2-1 transcription may be co-regulated with genes belonging to specific biological processes located elsewhere, while vtRNA1-1 transcription may be controlled by a locally specialized chromatin domain at chromosome 5q region lacking apparent functional relatedness.
Immune Subtypes associated with the vtRNAs profile
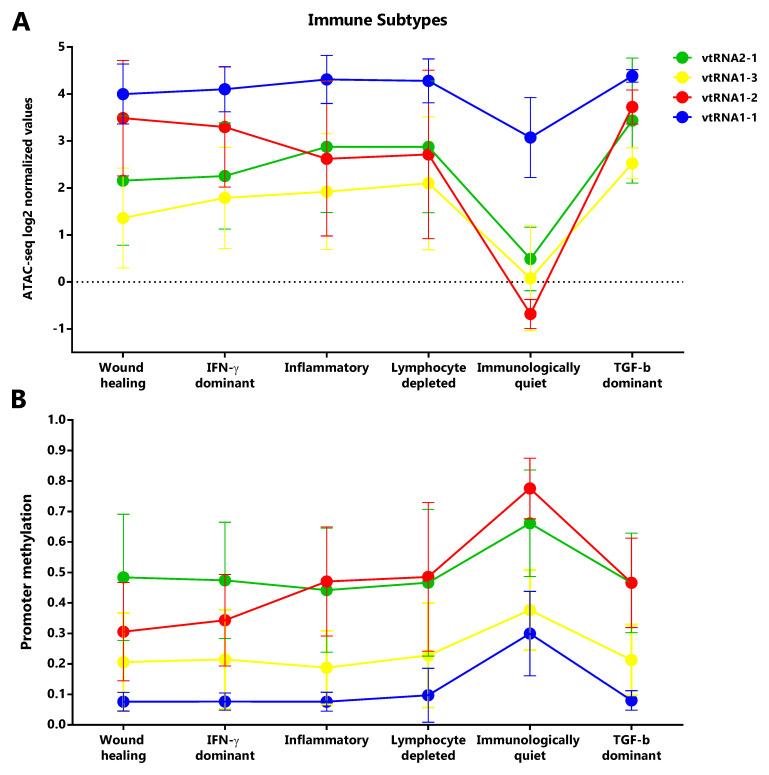
Taking into account that vtRNAs have been previously related to the immune response ( Golec et al., 2019; Li et al., 2015; Nandy et al., 2009), and that we found an association of vtRNA2-1 with immune related terms, we sought to investigate a putative relation between the vtRNAs expression and the six Immune Subtypes defined by Thorsson et al. ( Thorsson et al., 2018), compiled at Pan-Cancer TCGA ( Extended Data: Tables S11-S14). These six Immune Subtypes are named because of the foremost immune characteristic, comprising wound healing, IFN-g dominant, lymphocyte depleted, inflammatory, immunologically quiet and TGF-b dominant types ( Thorsson et al., 2018). As was expected, mirrored patterns were observed for promoter ATAC-seq and DNA methylation data of the six groups ( Figure 8). The immunologically quiet subtype shows a concerted shutdown of all vtRNA promoters ( Figure 8), which is largely composed by LGG samples showing low vtRNA ATAC-seq and high promoter methylation average values in LGG tumors ( Figures 4A and 4B). The immunological quiet subtype exhibits the lowest leukocyte fraction (very low Th17 and low Th2) and the highest macrophage:lymphocyte ratio (high M2 macrophages) ( Thorsson et al., 2018). The remaining subtypes present a similar vtRNA profile except for the inversion of vtRNA1-2/vtRNA2-1 accessibility ratio, which is higher than 1 for the wound healing and IFN-g dominant subtypes while is lower than 1 for the lymphocyte depleted and inflammatory subtypes ( Figure 8A). As expected, a mirrored pattern was observed for vtRNA1-2 and vtRNA2-1 at DNA methylation data ( Figure 8B). Since the wound healing and IFN-g dominant subtype have high proliferation rate opposite to the lymphocyte depleted and inflammatory subtypes ( Thorsson et al., 2018), we evaluated the correlation between vtRNA1-2 and vtRNA2-1 chromatin status and proliferation rate or wound healing status defined by Thorsson et al. ( Extended Data: Tables S12 and S14) ( Thorsson et al., 2018). A negative correlation between vtRNA1-2 promoter DNA methylation and the proliferation rate (rs = -0.44) and wound-healing features of the tumors is revealed (rs = -0.48) ( Extended Data: Tables S12 and S14). These findings suggest that vtRNA transcriptional regulation may be associated with tumor immunity.
Figure 8. Chromatin accessibility and DNA methylation of vtRNA promoters in the Six Immune Subtypes analyzed in the PANCAN TCGA dataset.
A. ATAC-seq values expressed as log2 normalized values for vtRNA promoters grouped in six Immune Subtype: wound healing (105 samples), IFN-g dominant (93 samples), inflammatory (110 samples), lymphocyte depleted (43 samples), immunologically quiet (9 samples), TGF-b dominant (4 samples). B. DNA methylation average beta-values for vtRNA promoters grouped in six Immune Subtype: wound healing (1963 samples), IFN-g dominant (2137 samples), inflammatory (2126 samples), lymphocyte depleted (933 samples), immunologically quiet (383 samples), TGF-b dominant (151 samples) ( Thorsson et al., 2018). The charts show the average and standard deviation for each vtRNA promoter in each Immune Subtype group.
Discussion
Although important insight into the vtRNA function has been recently gained, the landscape of vtRNA expression in human tissues and cancer was unknown, perhaps for the absence of vtRNA data in transcriptomic studies. Here we used ATAC-seq and methylation arrays data from the Pan-Cancer TCGA consortium ( Weinstein et al., 2013), as surrogate variables to uncover the expression of vtRNAs across multiple cancer types. A limitation of our study is its blindness to post-transcriptional controls of vtRNA expression, such as RNA processing, transport, localization and/or stability. Indeed, there is evidence of vtRNA transcript regulation via RNA methylation by NSUN2, or via its association to other proteins like DUSP11, DIS3L2, SSB, SRSF2 and DICER ( Burke et al., 2016; Hussain et al., 2013; Kickhoefer et al., 2002; Łabno et al., 2016; Persson et al., 2009; Sajini et al., 2019). Nonetheless, the chromatin status of the vtRNA promoters has been correlated with the expression of their transcripts in several reports ( Ahn et al., 2018; Fort et al., 2018; Helbo et al., 2015; Helbo et al., 2017; Lee et al., 2014a; Lee et al., 2014b; Sallustio et al., 2016; Treppendahl et al., 2012). These evidences unequivocally stand for the use of chromatin structure as a suitable proxy for vtRNA expression.
An integrated genomic view of the regulatory features of the vtRNA genes compiled in UCSC genome browser indicates that the four vtRNAs have chromatin signs of active transcription in different cell lines and defines a proximal promoter regulatory region of approximately 500 bp ( Nikitina et al., 2011; Vilalta et al., 1994). High resolution chromatin accessibility mapping studies by NOMe-Seq of the vtRNA promoters in cell lines validate this assumption ( Helbo et al., 2017). Particularly, vtRNA2-1 is singular for being immersed in a CpG island, for lacking the TATA box, DSE or PSE elements characteristic of the others vtRNA promoters, and for bearing a cAMP-response (CRE) element ( Canella et al., 2010; Helbo et al., 2017; Nandy et al., 2009). Since ATAC-seq approach is currently the best high throughput method to determine the chromatin accessibility of a DNA region directly ( Chen et al., 2016; Sun et al., 2019; Tsompana & Buck, 2014), we interrogated the ATAC-seq data of TCGA samples as a surrogate marker of vtRNA expression ( Corces et al., 2018). Although DNA methylation is a less direct and less accurate measurement of chromatin accessibility compared to ATAC-seq, 25 times more TCGA samples have been analyzed by DNA Methylation Arrays, including matched normal tissues samples, thus this dataset is of great worth for the current study. Our analyses revealed that vtRNA1-1 has accessible chromatin and small variation in all the evaluated tissues, which is consistent with its identity as the ancestor vtRNA gene and its significance as the major RNA component of the ubiquitous vault particle ( Stadler et al., 2009; van Zon et al., 2001). In addition, vtRNA1-2, vtRNA1-3 and vtRNA2-1 have lower chromatin accessibility at their promoters and a larger variation among the samples. As expected, the average DNA methylation assessed by microarrays is negatively correlated with the chromatin accessibility assessed by ATAC-seq. Therefore, the landscape of vtRNA chromatin status was corroborated by both approaches. Yet, the low chromatin accessibility of vtRNA1-3 promoter is not reflected by a concomitant high DNA methylation, suggesting that additional regulatory features of this gene, acting in trans or cis, play a major influence in its chromatin structure. As was reported by Helbo et al. using NOMe-seq assay (Nucleosome Positioning), the chromatin at vtRNA1-3 may be poised for transcription, and still require TFs to achieve physiological levels of transcription ( Helbo et al., 2017).
The relative abundance of the four vtRNAs determined in the current study has been previously observed in various cell lines, including H157, H1299, CRL2741, WI-38 and NTera-2 ( Lee et al., 2011), HSC CD34+, HL60 ( Helbo et al., 2015), T24, colorectal RKO, human diploid fibroblasts IMR90 ( Helbo et al., 2017) and MCF7 ( Chen et al., 2018). Yet, others found different comparative levels of the vtRNAs in MCF7, SW1573, GLC4, 8226, KB, SW620, MCF-7, HeLa, HEK-293 and L88/5 ( van Zon et al., 2001), CBL, BL2 and BL41 ( Nandy et al., 2009), EBV-negative Burkitt's lymphoma cell lines and A549 ( Li et al., 2015) and in LNCaP, PC3, DU145, RWPE-1, HEK, HeLa and MCF-7 ( Stadler et al., 2009) cell lines, suggesting that the expression of vtRNAs may differ in laboratory cell lines compared to tissues. Alternatively, the methods used to quantify the vtRNAs are not comparable among the studies.
Seeking to investigate regulatory connections between the vtRNAs as a valuable tool to study their function, we determine the pairwise correlations between the promoters of the vtRNAs. The positive correlations found are compatible with a coordinated transcriptional control of the vtRNAs, that is greater for the three members of the vtRNA1 cluster (specially vtRNA1-1 and vtRNA1-3). The exception is the pair vtRNA1-2 and vtRNA2-1, whose chromatin accessibility and promoter methylation are not linked in the cohort and can be indeed negatively associated.
As an alternative approach to investigate the vtRNA transcriptional co-regulation, we analyzed transcription and chromatin remodeling factors associated with their proximal promoter region. It is worth to mention that RNA polymerase II TFs clustered near TSS of RNA polymerase III transcripts could modulate occupancy and transcription rate of RNA polymerase III through RNA pol II activity ( Campbell & White, 2014; Gerber et al., 2020; Raha et al., 2010; Zhang et al., 2019). Specifically, RNA polymerase II could assist chromatin opening, thus allowing accessibility of RNA polymerase III or its associated factors in the human genome ( Raha et al., 2010). Interestingly, the 23 TFs common to all vtRNAs are related to viral infections and cancer. In agreement with their higher chromatin correlation, the vtRNA1 clustered genes have a larger core of common TFs (39) in comparison with vtRNA2-1 (23 TFs). The divergence of the transcriptional control of vtRNA2-1 was previously recognized given its unique regulatory elements and the complex developmental methylation it undergoes ( Canella et al., 2010; Carpenter et al., 2018; Helbo et al., 2017; Nandy et al., 2009). The upregulation of the four vtRNAs upon viral infection has been reported for several viruses, so it is possible that this core of TFs participate in the coordinated expression of vtRNA during viral infection and related responses ( Amort et al., 2015; Li et al., 2015; Nandy et al., 2009). These TFs regulate cell cycle and translational arrest as well as the inhibition of host innate immune response ( Attar & Kurdistani, 2017; Ertosun et al., 2016; Horsley & Pavlath, 2002; Jean et al., 2000; Persengiev & Green, 2003; Rohini et al., 2018). Since these processes are also hallmarks of cancer, the dysregulation of the normal function of vtRNAs in viral response may provide a selective advantage for cancer cell to overcome the many sources of stress faced during neoplastic evolution. If that holds true, the core vtRNA TFs may be co-opted in the malignant context to tune vtRNA expression favoring tumor progression. In agreement with that hypothesis, ATF1 and ATF3 were associated with CREB response and increased cell viability ( Persengiev & Green, 2003). Likewise, Golec et al. showed that altered levels of vtRNA2-1 modulate PKR activation and consequently altered the levels of CREB phosphorylation during T cell activation, which is a prerequisite for IFN-g activity ( Golec et al., 2019). Furthermore, E2F1, a cell cycle regulator, was described as a direct transcriptional regulator of vtRNA2-1 in cervical cancer cells ( Li et al., 2017). Moreover, it was shown that MYC binding to vtRNA2-1 promoter raises its expression and could be the explanation for the increased levels of vtRNA2-1 in some tumors ( Park et al., 2017a). Similarly, TGFB1 provokes the demethylation of vtRNA2-1 promoter and consequently increases its expression in ovarian cancer ( Ahn et al., 2018).
Alternatively, since RNA polymerase III transcripts are involved in virus replication and immune response ( Graczyk et al., 2015), it is possible that the TF bound to vtRNA vicinity are part of a global and not a vtRNA specific viral response.
Due to its importance for cancer biology, vtRNA2-1 transcriptional regulation was recently more investigated ( Yeganeh & Hernandez, 2020). Various studies reported tissue specific roles of vtRNA2-1 in normal ( Golec et al., 2019; Lee et al., 2019; Miñones-Moyano et al., 2013; Sallustio et al., 2016; Suojalehto et al., 2014) and cancer tissues ( Ahn et al., 2018; Fort et al., 2018; Hu et al., 2017; Im et al., 2020; Jeon et al., 2012a; Kunkeaw et al., 2012; Lee et al., 2011; Lee, 2015; Lee et al., 2016; Lee et al., 2014a; Lee et al., 2014b; Lei et al., 2017; Li et al., 2017; Ma et al., 2020; Treppendahl et al., 2012) and epigenetic alterations of the locus have been described in different malignancies ( Ahn et al., 2018; Cao et al., 2013; Fort et al., 2018; Helbo et al., 2015; Helbo et al., 2017; Joo et al., 2018; Lee et al., 2014a; Lee et al., 2014b; Park et al., 2017a; Romanelli et al., 2014; Treppendahl et al., 2012). Meanwhile, except for Kirsten Grønbæk group´s contributions on the chromatin characterization of vtRNA1-3 and vtRNA1-2 promoters ( Helbo et al., 2015; Helbo et al., 2017), little is known about the transcriptional regulation and expression of the other vtRNAs. Likewise, to our knowledge, the global landscape of vtRNA expression across different cancer types has not been investigated. Our study shows that, except for vtRNA1-1, which has a high promoter chromatin accessibility and low DNA methylation in all the tissues, the other vtRNA promoters exhibit different levels of chromatin accessibility and DNA methylation among the tissue types. The relative status of the chromatin in different tissues is accurately mirrored by both approaches in the tissues examined by ATAC-seq and Illumina DNA methylation arrays. Interestingly, the same analysis performed in the normal tissue samples reveals that the tissue specific variation in DNA methylation of vtRNA promoters in the neoplastic tissues is already established in their normal tissue counterparts, indicating that the regulation of vtRNA expression is tuned during normal development and cell differentiation.
While there is no apparent concerted modulation of the chromatin accessibility of the vtRNA promoters in most tissues, ACC and LGG tumors seem to be the exception. Concordantly, a global hypomethylation of malignant ACC tumors ( Legendre et al., 2016; Rechache et al., 2012), and an aberrantly methylation processes associated to altered DNMTs activity in LGG tumors were reported ( Nomura et al., 2019). However, more research is necessary to understand the molecular basis of these observations.
The differential expression of the vtRNAs from normal to primary tumor tissues revealed different patterns depending on tissue origin. The average DNA methylation of the vtRNA1-1 and vtRNA1-3 promoters is not significantly deregulated, whereas vtRNA1-2 and vtRNA2-1 are decreased and increased respectively in tumor tissues. The latter finding favors a candidate OG and TSG function of vtRNA1-2 and vtRNA2-1 in cancer, respectively. Indeed, the epigenetic repression of vtRNA2-1 was previously reported by Romanelli et al. in BLCA, BRCA, COAD and LUSC analyzing the same data from TCGA ( Romanelli et al., 2014). Furthermore, functional studies in LUSC, PRAD, ESCA and AML provided experimental support to that hypothesis ( Cao et al., 2013; Fort et al., 2018; Lee et al., 2014a; Treppendahl et al., 2012). Yet, vtRNA2-1 has been also proposed as an OG in ovarian, thyroid, endometrial, cervical and renal cancer ( Ahn et al., 2018; Hu et al., 2017; Lee et al., 2016; Lei et al., 2017; Li et al., 2017; Yeganeh & Hernandez, 2020). In agreement with the functional data found by Lei et al. ( Lei et al., 2017), we found an epigenetic de-repression of vtRNA2-1 promoter DNA methylation in renal carcinoma (KIRP). Likewise, a closer look at THCA samples, shows an average decrease in the DNA methylation of vtRNA2-1 promoter that supports the OG function described in this tissue ( Lee et al., 2016). Unfortunately, normal ovarian, cervical and endometrial tissues are not available at the TCGA. Finally, vtRNA1-2 has an oncogenic pattern of inferred expression between normal and tumor tissues for 13 of 16 tissues analyzed, raising a possible oncogenic function for this RNA.
Our study found also an association of DNA methylation at the promoter with a shorter overall survival for vtRNA1-1, vtRNA1-2 and vtRNA2-1. This is surprising for vtRNA2-1, since it has both TSG and OG roles and TSG compatible expression profiles in three cancer types, inferred by DNA methylation of its promoter in tumor vs normal tissues and the average promoter DNA methylation is increased in tumor compared to normal tissue. A higher impact of vtRNA2-1 expression in patient survival in the oncogenic context may justify this discrepancy. On the contrary, the association between vtRNA1-2 low promoter DNA methylation and low patient survival is in agreement with its OG expression in cancer. The lack of survival association with the ATAC-seq values may be due to the small number of patients studied by this approach.
VtRNA expression in individual cancer types, inferred from the epigenetic status of their promoters, has been associated with patient survival in several studies of vtRNA2-1, one of vtRNA1-3 and none for vtRNA1-1 or vtRNA1-2. Low methylation or high expression of vtRNA2-1 promoter were associated with good prognosis or overall survival in lung ( Cao et al., 2013), esophageal ( Lee et al., 2014a), prostate ( Fort et al., 2018), AML ( Treppendahl et al., 2012), gastric ( Lee et al., 2014b) and liver ( Yu et al., 2020). Conversely, a worse prognosis or overall survival association of vtRNA2-1 was reported in thyroid ( Lee et al., 2016) and ovarian ( Ahn et al., 2018). From these reports, only prostate ( Fort et al., 2018) and gastric ( Lee et al., 2014b) studies used the TCGA data. Likewise, the methylation status of the vtRNA1-3 promoter associates with overall survival in the lower risk Myelodysplastic Syndrome patients ( Helbo et al., 2015). Additionally, vtRNA1-1 and vtRNA1-2 correlate to chemotherapeutic resistance by direct interaction with drugs (doxorubicin, etoposide and mitoxantrone) and the modulation of vtRNA1-1 confirmed this finding in osteosarcoma cell lines ( Gopinath et al., 2005; Gopinath et al., 2010; Mashima et al., 2008; van Zon et al., 2001). Furthermore, increased levels of vtRNA1-1 were associated with increased proliferation and chemoresistance due to GAGE6 induction in MCF-7 cells ( Chen et al., 2018). Besides, Norbert Polacek group showed that vtRNA1-1 expression confers apoptosis resistance in several human cell lines (BL2, BL41, HS578T, HEK293, A549 and HeLa) and revealed it capacity to repressed intrinsic and extrinsic apoptosis pathway ( Amort et al., 2015; Bracher et al., 2020). The later findings are in agreement with the lower patient survival associated with high levels of vtRNA1-1 expression cancer-wide observed in our analysis.
Seeking to get insights into the cancer related function of the vtRNAs we performed a genome wide search for genes co-regulated at the level of promoter chromatin accessibility. Remarkably, we identified immune and cytokine related terms for the genes co-regulated with vtRNA2-1. This association agrees with its proposed roles in innate immune modulation via PKR repression or OAS1 regulation and in cytokine production ( Calderon & Conn, 2018; Golec et al., 2019; Lee et al., 2020; Li et al., 2015). Furthermore, vtRNA2-1 has been associated with autoimmune disorders ( Renauer et al., 2015; Weeding & Sawalha, 2018) and tumor engraftment in prostate cancer ( Ma et al., 2020). This enrichment in viral infection pathways, together with the viral infection involvement of the core TFs common to all vtRNAs, reinforces the hypothesis of the re-utilization of a regulatory RNA induced upon viral response in favor of cancer development at upstream and downstream regulatory steps. Additionally, we found a non-random clustering localization at chromosome arm 5q for genes co-regulated with vtRNA1-1. The chromosome arm 5q and regions chr5q31 and chr5q32-q33 were found frequently deleted in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) ( Fuchs, 2012; Treppendahl et al., 2012). Furthermore, the modulation of vtRNA2-1 and vtRNA1-3 was previously associated with human bone marrow CD34+ cells, AML and MDS ( Helbo et al., 2015; Treppendahl et al., 2012). Yet, our analysis points to vtRNA2-1 and vtRNA1-2, and to a lesser extent to vtRNA1-1 and vtRNA1-3, as the most relevant drivers across cancer types.
Support for the involvement of the vtRNAs in the immune responses in the context of cancer came from the association of their chromatin status with the immune subtype categories defined by Thorsson et al. ( Thorsson et al., 2018). Our findings suggest a possible role of the vtRNAs in lymphocyte response in tumor samples, since the immunologically quiet subtype shows the lowest leukocyte fraction and vtRNAs promoter chromatin accessibility ( Thorsson et al., 2018). Meanwhile, the vtRNA2-1 and vtRNA1-2 expression distinguishes two groups among the remaining four subtypes. The participation of vtRNA2-1 in the induction of the IFN-g and IL-2 expression in activated T cells through PKR modulation ( Golec et al., 2019) goes in agreement with this hypothesis. Although the same study demonstrated that vtRNA1-1 expression was unchanged during T cell activation, it is remarkable that the 5q31-q33 region, comprising the vtRNA1 locus and chromatin co-regulated genes, encodes several cytokines that regulate the differentiation of Th1 and Th2 lymphocytes and has been linked with susceptibility to infections ( Jeronimo et al., 2007; Lacy et al., 2000; Naka et al., 2009; Rodrigues et al., 1999). Furthermore, vtRNA2-1 levels were recently associated with macrophages M1/M2 fates in prostate PC3 cell line mice xenografts via TGF-b ( Ma et al., 2020), two pathways that define the six immune subtypes ( Thorsson et al., 2018). Finally, we found that the proliferation rate and wound healing of the tumors are associated with the levels of vtRNA1-2 chromatin accessibility, which reinforces an OG role of vtRNA1-2 in cancer. Nonetheless, the participation of the vtRNAs in immune cells response inside the neoplastic niche needs to be further investigated.
Conclusion
Taken together our analysis reveals the pattern of chromatin accessibility and DNA methylation at the four vtRNA promoters, analyzed by tissue of origin in the TCGA cohort. The agreement between both dataset and previous related literature endorses the use of these variables as surrogates of the vtRNA transcripts expression. VtRNA genes are enriched in viral related TFs. The comparison of TFs and chromatin status of the vtRNAs suggest that vtRNA2-1 is likely the most independently regulated vtRNA locus. The pattern of chromatin accessibility and DNA promoter methylation in normal vs tumor tissue and the association with cancer cell pathways and patient survival suggest that vtRNA2-1 and vtRNA1-2 are possibly the more relevant contributors to cancer. The results favor tissue specific TSG and OG roles for vtRNA2-1, a still not investigated oncogenic role of vtRNA1-2 and a limited cancer driver effect of vtRNA1-1/3 in specific tissue types. Lastly, we uncovered new evidence linking the vtRNAs with the immune response, cell proliferation and overall survival in cancer, which guarantees further investigation.
Data availability
Underlying data
All raw data used in this study can be downloaded from Xena Browser, a tool to explore functional genomic data sets ( Goldman et al., 2020) ( https://xenabrowser.net/), and UCSC genome browser ( Kent et al., 2002) ( https://genome.ucsc.edu/).
Extended data
Zenodo: Pan-Cancer chromatin analysis of the human vtRNA genes - Supplementary Figures, http://doi.org/10.5281/zenodo.4784353 ( Fort & Duhagon, 2021a)
This project contains the following extended data:
Figure S1.Data type and tissue sample distribution. Sample distribution per tissue type in the ATAC-seq, DNA methylation, ATAC-seq & DNA methylation and Normal adjacent tissue sample groups. A. Number of samples. B. Percentage of samples. The color reference, the number of samples of this tissue and the accounted percentage are presented to each tissue. Names references: ACC (Adrenocortical carcinoma), BLCA (Bladder Urothelial Carcinoma), BRCA (Breast invasive carcinoma), CESC (Cervical squamous cell carcinoma and endocervical adenocarcinoma), CHOL (Cholangiocarcinoma), COAD (Colon adenocarcinoma), DLBC (Lymphoid Neoplasm Diffuse Large B-cell Lymphoma), ESCA (Esophageal carcinoma), GBM (Glioblastoma multiforme), HNSC (Head and Neck squamous cell carcinoma), KICH (Kidney Chromophobe), KIRC (Kidney renal clear cell carcinoma), KIRP (Kidney renal papillary cell carcinoma), LGG (Brain Lower Grade Glioma), LIHC (Liver hepatocellular carcinoma), LUAD (Lung adenocarcinoma), LUSC (Lung squamous cell carcinoma), MESO (Mesothelioma), OV (Ovarian serous cystadenocarcinoma), PAAD (Pancreatic adenocarcinoma), PCPG (Pheochromocytoma and Paraganglioma), PRAD (Prostate adenocarcinoma), READ (Rectum adenocarcinoma), SARC (Sarcoma), SKCM (Skin Cutaneous Melanoma), STAD (Stomach adenocarcinoma), TGCT (Testicular Germ Cell Tumors), THCA (Thyroid carcinoma), THYM (Thymoma), UCEC (Uterine Corpus Endometrial Carcinoma), UCS (Uterine Carcinosarcoma) and UVM (Uveal Melanoma).
Figure S2.Correlation between ATAC-seq values and promoter methylation values of vtRNAs in Pan-Cancer TCGA dataset.A–D. The correlation between ATAC-seq and DNA methylation average normalized beta values was calculated for vtRNAs (vtRNAs1-1 ( A), vtRNA1-2 ( B), vtRNA1-3 ( C) and vtRNA2-1 ( D)), in 329 primary tumors samples. The box plots show the median line and lower and upper quartile and the whiskers the 2.5 and 97.5 percentile. Horizontal grey striped and red dotted lines are shown to denote unmethylated (average beta-value ≤ 0.2), 50% methylated (average beta-value = 0.5) and highly methylated (average beta-value ≥ 0.8) levels. Spearman correlation value and the best-fit line (red line) with 95% confidence bands (black dot lines) are shown.
Figure S3.Genomic context and chromatin accessibility of human vtRNA3-1P, vtRNA2-2P and RNYs (RNY1, RNY3, RNY4 and RNY5).A. Genomic view of the 1.5 kb region of human vtRNA3-1P, vtRNA2-2P and RNYs (RNY1, RNY3, RNY4 and RNY5) genes in UCSC Genome browser (GRCh37/hg19) centered at the 500bp of the ATAC-seq. Highlighted in yellow is the 500bp ATAC-seq region used in the posterior analyses. The following Gene annotation and ENCODE Project tracks for seven cell lines (GM12878, H1-hESC, HSMM, HUVEC, K562, NHEK, NHL) are displayed: DNA accessibility (DNaseI hypersensitivity clusters (color intensity is proportional to the maximum signal strength)), DNA methylation (CpG islands length greater than 200 bp), histone modification (H3K27Ac, H3K4me1, H3K4me3 marks), conservation of the region in 100 Vertebrates (log-odds Phylop scores). The vertical viewing range of the tracks displays the default settings of the browser for each variable. B. The distribution of ATAC-seq values for of human vtRNA3-1P, vtRNA2-2P and RNYs (RNY1, RNY3, RNY4 and RNY5) in Pan-Cancer TCGA dataset (385 tumors samples across 23 cancer types) expressed as log2 normalized values. The box plots show the median and the lower and upper quartile, and the whiskers the 2.5 and 97.5 percentile of the distribution. C. ATAC-seq values for of human vtRNA3-1P, vtRNA2-2P and RNYs (RNY1, RNY3, RNY4 and RNY5) in 21 different tumors with at least 5 tumor samples available in Pan-Cancer TCGA dataset (385 tumors samples) expressed as log2 normalized values. The chart shows the average and standard error for each vtRNAs in each tumor type.
Figure S4.VtRNA promoter’s chromatin accessibility (ATAC-seq and DNA methylation) average values for tumor tissuesfrom Pan-Cancer TCGA dataset. The average vtRNA promoters ATAC-seq and DNA methylation values for each primary tumor tissue type (21 tissues). VtRNA1-1 ( A), vtRNA1-2 ( B), vtRNA1-3 ( C) and vtRNA2-1 ( D). The Spearman correlation between averages ATAC-seq and DNA methylation values was calculated for each vtRNA. The chart shows the average and standard deviation for each tissue with at least five samples available at Pan-Cancer TCGA.
Figure S5. VtRNA promoter’s DNA methylation for normal and tumor tissues from Pan-Cancer TCGA dataset. The average promoter DNA methylation beta-values of normal adjacent (Normal) and primary tumor (Tumor) tissues (16 tissue types) for vtRNA1-1 ( A), vtRNA1-2 ( B), vtRNA1-3 ( C) and vtRNA2-1 ( D) is shown. The Spearman (r) correlation between normal and tumor values was calculated. The chart shows the average and standard deviation for each tissue with at least five samples available at Pan-Cancer TCGA.
Zenodo: Pan-Cancer chromatin analysis of the human vtRNA genes - Supplementary Tables, http://doi.org/10.5281/zenodo.4784473 ( Fort & Duhagon, 2021b)
This project contains the following extended data:
Tables S1–S5.ATAC-seq and DNA methylation data for vtRNA promoters in primary tumors and normal adjacent tissues.CSV spreadsheets: Table_S1_ATAC-seq_data_500bp: All ATAC-seq data of vtRNAs promoter (500bp) data for primary tumor samples; Table_S2_DNA_methylation_500bp: All DNA methylation data and ATAC-seq data of vtRNAs promoter (500bp) data for total primary tumor and normal adjacent samples; Table_S3_DNA_methylation_NORMAL: All DNA methylation data of vtRNAs promoter (500bp) data for normal adjacent samples; Table_S4_DNA_methylation_TUMOR: All DNA methylation data of vtRNAs promoter (500bp) data for primary tumor samples; Table_S5_Normal_&_Tumor_matched: All DNA methylation data of vtRNAs promoter (500bp) data for primary tumor and normal adjacent samples.
Table S6.VtRNAs Transcription Factor Binding and KEEG enriched terms.CSV spreadsheets: Table_S6_Binding_Factors: Transcription factors identified in the cell line K562 as ChIP-seq Peaks by ENCODE 3 project and KEEG_terms: enriched KEGG pathway terms (FDR < 0.05).
Tables S7–S8.DNA methylation, ATAC-seq data and associated survival data for primary tumors.CSV spreadsheets: Table_S7_DNA-methylation_Survival_data: All DNA methylation data of vtRNAs promoter (500bp) and survival data for primary tumor samples; Table_S8_ATAC-seq_Survival_data: ATAC-seq data of vtRNAs promoter (500bp) and survival data for primary tumor samples.
Tables S9–S10.Correlation of ATAC-seq values between vtRNA and all genome promoters in primary tumor samples.CSV spreadsheets: Table_S9_ATAC-seq_gene_promoter_spearman_correlation: Spearman correlation values of all promoter genes and vtRNAs in primary tumors samples; Table_S10_vtRNAs_pathway_enrichment_and_cluster_chromosome_localization_analysis: vtRNAs vtRNA1-1, vtRNA1-2, vtRNA1-3 and vtRNA2-1 pathway enrichment and cluster chromosome localization data.
Tables S11–S14.ATAC-seq and DNA methylation data for vtRNA promoters in primary tumors and the associated Immune Subtypes data.CSV spreadsheets: Table_S11_Immune_Subtypes_DNA_methylation_data: All DNA methylation data of vtRNAs promoter (500bp) and Immune Subtypes data for primary tumor samples; Table_S12_Spearman_corr_vtRNAs_Immune_Subtypes_DNA_methylation_data: Spearman correlation values of DNA methylation data of vtRNAs promoter (500bp) and Immune Subtypes data for primary tumor samples; Table_S13_Immune_Subtypes_ATAC-seq_data: All ATAC-seq data of vtRNAs promoter (500bp) and Immune Subtypes data for primary tumor samples; Table_S14_Spearman_corr_vtRNAs_Immune_Subtypes_ATAC-seq_data: Spearman correlation values of ATAC-seq data of vtRNAs promoter (500bp) and Immune Subtypes data for primary tumor samples.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Acknowledgments
A previous version of this article is available on BioRxiv: https://doi.org/10.1101/2020.10.05.324723
Funding Statement
This work was supported by Comision Academica de Posgrado, UDELAR (Student Fellowship RF 2020); Comisión Sectorial de Investigación Científica (CSIC, UDELAR) (Research Grant MAD I+D 2016 #487); Agencia Nacional de Investigación e Innovación (ANII) (Student Fellowship RF 2016-2019); and Programa para el Desarrollo de las Ciencias Basicas (PEDECIBA, MEC).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved
References
- Aakula A, Kohonen P, Leivonen SK, et al. : Systematic Identification of MicroRNAs That Impact on Proliferation of Prostate Cancer Cells and Display Changed Expression in Tumor Tissue. Eur Urol. 2016;69(6):1120–1128. 10.1016/j.eururo.2015.09.019 [DOI] [PubMed] [Google Scholar]
- Ahn JH, Lee HS, Lee JS, et al. : Nc886 is induced by TGF-β and suppresses the microRNA pathway in ovarian cancer. Nat Commun. 2018;9(1):1166. 10.1038/s41467-018-03556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amort M, Nachbauer B, Tuzlak S, et al. : Expression of the vault RNA protects cells from undergoing apoptosis. Nat Commun. 2015;6:7030. 10.1038/ncomms8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attar N, Kurdistani SK: Exploitation of EP300 and CREBBP lysine acetyltransferases by cancer. Cold Spring Harb Perspect Med. 2017;7(3):a026534. 10.1101/cshperspect.a026534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi N, Cao J, Song Y, et al. : A MicroRNA signature predicts survival in early stage small-cell lung cancer treated with surgery and adjuvant chemotherapy. PLoS One. 2014;9(3):e91388. 10.1371/journal.pone.0091388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG: Survival probabilities (the Kaplan-Meier method). BMJ. 1998;317(7172):1572. 10.1136/bmj.317.7172.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin V, Deschamps-Francoeur G, Couture S, et al. : Simultaneous sequencing of coding and noncoding RNA reveals a human transcriptome dominated by a small number of highly expressed noncoding genes. RNA. 2018;24(7):950–965. 10.1261/rna.064493.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin V, Faucher-Giguère L, Scott M, et al. : The cellular landscape of mid-size noncoding RNA. Wiley Interdiscip Rev RNA. 2019;10(4):e1530. 10.1002/wrna.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher L, Ferro I, Pulido-Quetglas C, et al. : Human vtRNA1-1 levels modulate signaling pathways and regulate apoptosis in human cancer cells. Biomolecules. 2020;10(4):614. 10.3390/biom10040614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, et al. : Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–1218. 10.1038/nmeth.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Chang HY, et al. : ATAC-seq: A method for assaying chromatin accessibility genome-wide. Curr Protoc Mol Biol. 2015;109:21.29.1–21.29.9. 10.1002/0471142727.mb2129s109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Kincaid RP, Nottingham RM, et al. : DUSP11 activity on triphosphorylated transcripts promotes Argonaute association with noncanonical viral microRNAs and regulates steady-state levels of cellular noncoding RNAs. Genes Dev. 2016;30(18):2076–2092. 10.1101/gad.282616.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher M, Horos R, Hentze MW: 'High vault-age': non-coding RNA control of autophagy. Open Biol. 2020;10(2):190307. 10.1098/rsob.190307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon BM, Conn GL: A human cellular noncoding RNA activates the antiviral protein 2’-5’-oligoadenylate synthetase 1. J Biol Chem. 2018;293(41):16115–16124. 10.1074/jbc.RA118.004747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KJ, White RJ: MYC regulation of cell growth through control of transcription by RNA polymerases I and III. Cold Spring Harb Perspect Med. 2014;4(5):a018408. 10.1101/cshperspect.a018408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canella D, Praz V, Reina JH, et al. : Defining the RNA polymerase III transcriptome: Genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res. 2010;20(6):710–721. 10.1101/gr.101337.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Song Y, Bi N, et al. : DNA methylation-mediated repression of miR-886-3p predicts poor outcome of human small cell lung cancer. Cancer Res. 2013;73(11):3326–3335. 10.1158/0008-5472.CAN-12-3055 [DOI] [PubMed] [Google Scholar]
- Carpenter BL, Zhou W, Madaj Z, et al. : Mother-child transmission of epigenetic information by tunable polymorphic imprinting. Proc Natl Acad Sci U S A. 2018;115(51):E11970–E11977. 10.1073/pnas.1815005115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, OuYang H, An X, et al. : Vault RNAs partially induces drug resistance of human tumor cells MCF-7 by binding to the RNA/DNA-binding protein PSF and inducing oncogene GAGE6. PLoS One. 2018;13(1):e0191325. 10.1371/journal.pone.0191325 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen X, Shen Y, Draper W, et al. : ATAC-see reveals the accessible genome by transposase-mediated imaging and sequencing. Nat Methods. 2016;13(12):1013–1020. 10.1038/nmeth.4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Granja JM, Shams S, et al. : The chromatin accessibility landscape of primary human cancers. Science. 2018;362(6413):eaav1898. 10.1126/science.aav1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer MS, Perren A, Moch H, et al. : MicroRNA profile of poorly differentiated thyroid carcinomas: New diagnostic and prognostic insights. J Mol Endocrinol. 2014;52(2):181–189. 10.1530/JME-13-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duren Z, Chen X, Jiang R, et al. : Modeling gene regulation from paired expression and chromatin accessibility data. Proc Natl Acad Sci U S A. 2017;114(25):E4914–E4923. 10.1073/pnas.1704553114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertosun MG, Hapil FZ, Nidai OO: E2F1 transcription factor and its impact on growth factor and cytokine signaling. Cytokine Growth Factor Rev. 2016;31:17–25. 10.1016/j.cytogfr.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Euskirchen GM, Rozowsky JS, Wei CL, et al. : Mapping of transcription factor binding regions in mammalian cells by ChIP: Comparison of array- and sequencing-based technologies. Genome Res. 2007;17(6):898–909. 10.1101/gr.5583007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler A, Jung M, Stephan C, et al. : miRNAs can predict prostate cancer biochemical relapse and are involved in tumor progression. Int J Oncol. 2011;39(5):1183–1192. 10.3892/ijo.2011.1128 [DOI] [PubMed] [Google Scholar]
- Fort RS, Duhagon MA: Pan-Cancer chromatin analysis of the human vtRNA genes - Supplementary Figures.2021a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort RS, Duhagon MA: Pan-Cancer chromatin analysis of the human vtRNA genes - Supplementary Tables.2021b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort RS, Mathó C, Geraldo MV, et al. : Nc886 is epigenetically repressed in prostate cancer and acts as a tumor suppressor through the inhibition of cell growth. BMC Cancer. 2018;18(1):127. 10.1186/s12885-018-4049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort RS, Garat B, Sotelo-Silveira JR, et al. : vtRNA2-1/nc886 Produces a Small RNA That Contributes to Its Tumor Suppression Action through the microRNA Pathway in Prostate Cancer. Noncoding RNA. 2020;6(1):7. 10.3390/ncrna6010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs O: Important genes in the pathogenesis of 5q- syndrome and their connection with ribosomal stress and the innate immune system pathway. Leuk Res Treatment. 2012;2012:179402. 10.1155/2012/179402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Shen H, Liu L, et al. : MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–566. 10.1007/s00432-010-0918-4 [DOI] [PubMed] [Google Scholar]
- Gerber A, Ito K, Chu CS, et al. : Gene-Specific Control of tRNA Expression by RNA Polymerase II. Mol Cell. 2020;78(4):765–778.e7. 10.1016/j.molcel.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MJ, Craft B, Hastie M, et al. : Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–678. 10.1038/s41587-020-0546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Craft B, Swatloski T, et al. : The UCSC Cancer Genomics Browser: update 2013. Nucleic Acids Res. 2013;41(Database issue):D949–D954. 10.1093/nar/gks1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golec E, Lind L, Qayyum M, et al. : The Noncoding RNA nc886 Regulates PKR Signaling and Cytokine Production in Human Cells. J Immunol. 2019;202(1):131–141. 10.4049/jimmunol.1701234 [DOI] [PubMed] [Google Scholar]
- Gopinath SCB, Matsugami A, Katahira M, et al. : Human vault-associated non-coding RNAs bind to mitoxantrone, a chemotherapeutic compound. Nucleic Acids Res. 2005;33(15):4874–4881. 10.1093/nar/gki809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SCB, Wadhwa R, Kumar PKR: Expression of noncoding vault RNA in human malignant cells and its importance in mitoxantrone resistance. Mol Cancer Res. 2010;8(11):1536–1546. 10.1158/1541-7786.MCR-10-0242 [DOI] [PubMed] [Google Scholar]
- Graczyk D, White RJ, Ryan KM: Involvement of RNA Polymerase III in Immune Responses. Mol Cell Biol. 2015;35(10):1848–59. 10.1128/MCB.00990-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbo AS, Lay FD, Jones PA, et al. : Nucleosome Positioning and NDR Structure at RNA Polymerase III Promoters. Sci Rep. 2017;7:41947. 10.1038/srep41947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbo AS, Treppendahl M, Aslan D, et al. : Hypermethylation of the VTRNA1-3 promoter is associated with poor outcome in lower risk myelodysplastic syndrome patients. Genes (Basel). 2015;6(4):977–990. 10.3390/genes6040977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horos R, Büscher M, Kleinendorst R, et al. : The Small Non-coding Vault RNA1-1 Acts as a Riboregulator of Autophagy. Cell. 2019;176(5):1054–1067.e12. 10.1016/j.cell.2019.01.030 [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK: NFAT: Ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156(5):771–774. 10.1083/jcb.200111073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang H, Tang L, et al. : Silencing nc886, a Non-Coding RNA, Induces Apoptosis of Human Endometrial Cancer Cells-1A In Vitro. Med Sci Monit. 2017;23:1317–1324. 10.12659/msm.900320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Sajini AA, Blanco S, et al. : NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4(2):255–261. 10.1016/j.celrep.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im WR, Lee HS, Lee YS, et al. : A Regulatory Noncoding RNA, nc886, Suppresses Esophageal Cancer by Inhibiting the AKT Pathway and Cell Cycle Progression. Cells. 2020;9(4):801. 10.3390/cells9040801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean D, Tellez C, Huang S, et al. : Inhibition of tumor growth and metastasis of human melanoma by intracellular anti-ATF-1 single chain Fv fragment. Oncogene. 2000;19(22):2721–2730. 10.1038/sj.onc.1203569 [DOI] [PubMed] [Google Scholar]
- Jeon SH, Johnson BH, Lee YS: A tumor surveillance model: A non-coding RNA senses neoplastic cells and its protein partner signals cell death. Int J Mol Sci. 2012a;13(10):13134–13139. 10.3390/ijms131013134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SH, Lee K, Lee KS, et al. : Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR. FEBS Lett. 2012b;586(19):3477–3484. 10.1016/j.febslet.2012.07.076 [DOI] [PubMed] [Google Scholar]
- Jeronimo SMB, Holst AKB, Jamieson SE, et al. : Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection. Genes Immun. 2007;8(7):539–551. 10.1038/sj.gene.6364422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JE, Dowty JG, Milne RL, et al. : Heritable DNA methylation marks associated with susceptibility to breast cancer. Nat Commun. 2018;9(1):867. 10.1038/s41467-018-03058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Rome LH: Isolation and characterization of a novel ribonucleoprotein particle: Large structures contain a single species of small RNA. J Cell Biol. 1986;103(3):699–709. 10.1083/jcb.103.3.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Heuser JE, Chugani DC, et al. : Vaults. III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J Cell Biol. 1991;112(2):225–235. 10.1083/jcb.112.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, et al. : The Human Genome Browser at UCSC. Genome Res. 2002;12(6):996–1006. 10.1101/gr.229102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickhoefer VA, Rajavel KS, Scheffer GL, et al. : Vaults are up-regulated in multidrug-resistant cancer cell lines. J Biol Chem. 1998;273(15):8971–8974. 10.1074/jbc.273.15.8971 [DOI] [PubMed] [Google Scholar]
- Kickhoefer VA, Poderycki MJ, Chan EKL, et al. : The La RNA-binding protein interacts with the vault RNA and is a vault-associated protein. J Biol Chem. 2002;277(43):41282–41286. 10.1074/jbc.M206980200 [DOI] [PubMed] [Google Scholar]
- Klemm SL, Shipony Z, Greenleaf WJ: Chromatin accessibility and the regulatory epigenome. Nat Rev Genet. 2019;20(4):207–220. 10.1038/s41576-018-0089-8 [DOI] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, et al. : Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkeaw N, Jeon SH, Lee K, et al. : Cell death/proliferation roles for nc886, a non-coding RNA, in the protein kinase R pathway in cholangiocarcinoma. Oncogene. 2012;32(32):3722–3731. 10.1038/onc.2012.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkeaw N, Lee YS, Im WR, et al. : Mechanism mediated by a noncoding RNA, nc886, in the cytotoxicity of a DNA-reactive compound. Proc Natl Acad Sci U S A. 2019;116(17):8289–8294. 10.1073/pnas.1814510116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łabno A, Warkocki Z, Kulinski T, et al. : Perlman syndrome nuclease DIS3L2 controls cytoplasmic non-coding RNAs and provides surveillance pathway for maturing snRNAs. Nucleic Acids Res. 2016;44(21):10437–10453. 10.1093/nar/gkw649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy DA, Wang ZE, Symula DJ, et al. : Faithful expression of the human 5q31 cytokine cluster in transgenic mice. J Immunol. 2000;164(9):4569–4574. 10.4049/jimmunol.164.9.4569 [DOI] [PubMed] [Google Scholar]
- Landgraf P, Rusu M, Sheridan R, et al. : A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. 10.1016/j.cell.2007.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS: A Novel Type of Non-coding RNA, nc886, Implicated in Tumor Sensing and Suppression. Genomics Inform. 2015;13(2):26–30. 10.5808/GI.2015.13.2.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Hong SH, Shin S, et al. : Nc886, a non-coding RNA and suppressor of PKR, exerts an oncogenic function in thyroid cancer. Oncotarget. 2016;7(46):75000–75012. 10.18632/oncotarget.11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee K, Jang HJ, et al. : Epigenetic silencing of the non-coding RNA nc886 provokes oncogenes during human esophageal tumorigenesis. Oncotarget. 2014a;5(11):3472–3481. 10.18632/oncotarget.1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Park JL, Lee K, et al. : nc886, a non-coding RNA of anti-proliferative role, is suppressed by CpG DNA methylation in human gastric cancer. Oncotarget. 2014b;5(11):3944–3955. 10.18632/oncotarget.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kunkeaw N, Jeon SH, et al. : Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA. 2011;17(6):1076–1089. 10.1261/rna.2701111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Shin S, Cho E, et al. : nc886, a non-coding RNA, inhibits UVB-induced MMP-9 and COX-2 expression via the PKR pathway in human keratinocytes. Biochem Biophys Res Commun. 2019;512(4):647–652. 10.1016/j.bbrc.2019.01.068 [DOI] [PubMed] [Google Scholar]
- Lee YS, Kunkeaw N, Lee YS: Protein kinase R and its cellular regulators in cancer: An active player or a surveillant? Wiley Interdiscip Rev RNA. 2020;11(2):e1558. 10.1002/wrna.1558 [DOI] [PubMed] [Google Scholar]
- Legendre CR, Demeure MJ, Whitsett TG, et al. : Pathway Implications of Aberrant Global Methylation in Adrenocortical Cancer. PLoS One. 2016;11(3):e0150629. 10.1371/journal.pone.0150629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Xiao JH, Zhang SH, et al. : Non-coding RNA 886 promotes renal cell carcinoma growth and metastasis through the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Mol Med Rep. 2017;16(4):4273–4278. 10.3892/mmr.2017.7093 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL: The Role of Chromatin during Transcription. Cell. 2007;128(4):707–719. 10.1016/j.cell.2007.01.015 [DOI] [PubMed] [Google Scholar]
- Li F, Chen Y, Zhang Z, et al. : Robust expression of vault RNAs induced by influenza A virus plays a critical role in suppression of PKR-mediated innate immunity. Nucleic Acids Res. 2015;43(21):10321–37. 10.1093/nar/gkv1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Wang M, Zhang R, et al. : E2F1-directed activation of nc886 mediates drug resistance in cervical cancer cells via regulation of major vault protein. Int J Clin Exp Pathol. 2017;10(9):9233–9242. [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang W, Hu S, et al. : Inherited DNA methylation primes the establishment of accessible chromatin during genome activation. Genome Res. 2018;28(7):998–1007. 10.1101/gr.228833.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wang M, Zhou Y, et al. : Noncoding RNA 886 alleviates tumor cellular immunological rejection in host C57BL/C mice. Cancer Med. 2020;9(14):5258–5271. 10.1002/cam4.3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T, Kudo M, Takada Y, et al. : Interactions between antitumor drugs and vault RNA. Nucleic Acids Symp Ser (Oxf). 2008; (52):217–218. 10.1093/nass/nrn110 [DOI] [PubMed] [Google Scholar]
- Meng C, Wei Z, Zhang Y, et al. : Regulation of cytochrome P450 3A4 by small vault RNAb derived from the non-coding vault RNA1 of multidrug resistance-linked vault particle. Mol Med Rep. 2016;14(1):387–93. 10.3892/mmr.2016.5228 [DOI] [PubMed] [Google Scholar]
- Miñones-Moyano E, Friedländer MR, Pallares J, et al. : Upregulation of a small vault RNA (svtRNA2-1a) is an early event in Parkinson disease and induces neuronal dysfunction. RNA Biol. 2013;10(7):1093–1106. 10.4161/rna.24813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran S, Arribas C, Esteller M: Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics. 2016;8(3):389–399. 10.2217/epi.15.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka I, Nishida N, Patarapotikul J, et al. : Identification of a haplotype block in the 5q31 cytokine gene cluster associated with the susceptibility to severe malaria. Malar J. 2009;8:232. 10.1186/1475-2875-8-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy C, Mrázek J, Stoiber H, et al. : Epstein-Barr Virus-Induced Expression of a Novel Human Vault RNA. J Mol Biol. 2009;388(4):776–784. 10.1016/j.jmb.2009.03.031 [DOI] [PubMed] [Google Scholar]
- Nikitina TV, Tischenko LI, Schulz WA: Recent insights into regulation of transcription by RNA polymerase III and the cellular functions of its transcripts. Biol Chem. 2011;392(5):395–404. 10.1515/BC.2011.049 [DOI] [PubMed] [Google Scholar]
- Nomura M, Saito K, Aihara K, et al. : DNA demethylation is associated with malignant progression of lower-grade gliomas. Sci Rep. 2019;9(1):1903. 10.1038/s41598-019-38510-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordentoft I, Birkenkamp-Demtroder K, Agerbæk M, et al. : miRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med Genomics. 2012;5:40. 10.1186/1755-8794-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T, Kojima H, Miwa T, et al. : The expression of microRNA 574-3p as a predictor of postoperative outcome in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2016;14(1):228. 10.1186/s12957-016-0985-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal A, Temkin AM, Kerkel K, et al. : Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation. PLoS Genet. 2013;9(8): e1003622. 10.1371/journal.pgen.1003622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JL, Lee YS, Kunkeaw N, et al. : Epigenetic regulation of noncoding RNA transcription by mammalian RNA polymerase III. Epigenomics. 2017a;9(2):171–187. 10.2217/epi-2016-0108 [DOI] [PubMed] [Google Scholar]
- Park JL, Lee YS, Song MJ, et al. : Epigenetic regulation of RNA polymerase III transcription in early breast tumorigenesis. Oncogene. 2017b;36(49):6793–6804. 10.1038/onc.2017.285 [DOI] [PubMed] [Google Scholar]
- Pazos Obregón F, Soto P, Lavín JL, et al. : Cluster Locator, online analysis and visualization of gene clustering. Bioinformatics. 2018;34(19):3377–3379. 10.1093/bioinformatics/bty336 [DOI] [PubMed] [Google Scholar]
- Persengiev SP, Green MR: The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis. 2003;8(3):225–228. 10.1023/a:1023633704132 [DOI] [PubMed] [Google Scholar]
- Persson H, Kvist A, Vallon-Christersson J, et al. : The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat Cell Biol. 2009;11(10):1268–1271. 10.1038/ncb1972 [DOI] [PubMed] [Google Scholar]
- Raha D, Wang Z, Moqtaderi Z, et al. : Close association of RNA polymerase II and many transcription factors with Pol III genes. Proc Natl Acad Sci U S A. 2010;107(8):3639–44. 10.1073/pnas.0911315106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechache NS, Wang Y, Stevenson HS, et al. : DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J Clin Endocrinol Metab. 2012;97(6): E1004–13. 10.1210/jc.2011-3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauer P, Coit P, Jeffries MA, et al. : DNA methylation patterns in naïve CD 4+ T cells identify epigenetic susceptibility loci for malar rash and discoid rash in systemic lupus erythematosus. Lupus Sci Med. 2015;2(1): e000101. 10.1136/lupus-2015-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RC, Sharp GC, Herbert G, et al. : The long-term impact of folic acid in pregnancy on offspring DNA methylation: follow-up of the Aberdeen Folic Acid Supplementation Trial (AFAST). Int J Epidemiol. 2018;47(3):928–937. 10.1093/ije/dyy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V, Jr, Piper K, Couissinier-Paris P, et al. : Genetic control of schistosome infections by the SM1 locus of the 5q31-q33 region is linked to differentiation of type 2 helper T lymphocytes. Infect Immun. 1999;67(9):4689–4692. 10.1128/IAI.67.9.4689-4692.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohini M, Haritha Menon A, Selvamurugan N: Role of activating transcription factor 3 and its interacting proteins under physiological and pathological conditions. Int J Biol Macromol. 2018;120(Pt A):310–317. 10.1016/j.ijbiomac.2018.08.107 [DOI] [PubMed] [Google Scholar]
- Romanelli V, Nakabayashi K, Vizoso M, et al. : Variable maternal methylation overlapping the nc886/vtRNA2-1 locus is locked between hypermethylated repeats and is frequently altered in cancer. Epigenetics. 2014;9(5):783–790. 10.4161/epi.28323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajini AA, Choudhury NR, Wagner RE, et al. : Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun. 2019;10(1):2550. 10.1038/s41467-019-10020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallustio F, Serino G, Cox SN, et al. : Aberrantly methylated DNA regions lead to low activation of CD 4+ T-cells in IgA nephropathy. Clin Sci (Lond). 2016;130(9):733–746. 10.1042/CS20150711 [DOI] [PubMed] [Google Scholar]
- Schou JV, Rossi S, Jensen BV, et al. : miR-345 in Metastatic Colorectal Cancer: A Non-Invasive Biomarker for Clinical Outcome in Non-KRAS Mutant Patients Treated with 3rd Line Cetuximab and Irinotecan. PLoS One. 2014;9(6): e99886. 10.1371/journal.pone.0099886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendler E, Johnson GD, Krawetz SA: Local and global factors affecting RNA sequencing analysis. Anal Biochem. 2011;419(2):317–322. 10.1016/j.ab.2011.08.013 [DOI] [PubMed] [Google Scholar]
- Shen J, Zhou W, Bi N, et al. : MicroRNA-886-3P functions as a tumor suppressor in small cell lung cancer. Cancer Biol Ther. 2018;19(12):1185–1192. 10.1080/15384047.2018.1491505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MJ, Kessler NJ, Hennig BJ, et al. : Independent genomewide screens identify the tumor suppressor VTRNA2-1 as a human epiallele responsive to periconceptional environment. Genome Biol. 2015;16(1):118. 10.1186/s13059-015-0660-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler PF, Chen JJL, Hackermüller J, et al. : Evolution of vault RNAs. Mol Biol Evol. 2009;26(9):1975–1991. 10.1093/molbev/msp112 [DOI] [PubMed] [Google Scholar]
- Stark R, Grzelak M, Hadfield J: RNA sequencing: the teenage years. Nat Rev Genet. 2019;20(11):631–656. 10.1038/s41576-019-0150-2 [DOI] [PubMed] [Google Scholar]
- Steitz J: RNA-RNA base-pairing: Theme and variations. RNA. 2015;21(4):476–477. 10.1261/rna.050427.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Miao N, Sun T: Detect accessible chromatin using ATAC-sequencing, from principle to applications. Hereditas. 2019;156:29. 10.1186/s41065-019-0105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suojalehto H, Lindström I, Majuri ML, et al. : Altered microRNA expression of nasal mucosa in long-term asthma and allergic rhinitis. Int Arch Allergy Immunol. 2014;163(3):168–178. 10.1159/000358486 [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Morris JH, Cook H, et al. : The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiri A, Leivonen SK, Lüders T, et al. : Deregulation of cancer-related miRNAs is a common event in both benign and malignant human breast tumors. Carcinogenesis. 2014;35(1):76–85. 10.1093/carcin/bgt333 [DOI] [PubMed] [Google Scholar]
- Thorsson V, Gibbs DL, Brown SD, et al. : The Immune Landscape of Cancer. Immunity. 2018;48(4):812–830.e14. 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, et al. : The accessible chromatin landscape of the human genome. Nature. 2012;489(7414):75–82. 10.1038/nature11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treppendahl MB, Qiu X, Søgaard A, et al. : Allelic methylation levels of the noncoding VTRNA2-1 located on chromosome 5q31.1 predict outcome in AML. Blood. 2012;119(1):206–216. 10.1182/blood-2011-06-362541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsompana M, Buck MJ: Chromatin accessibility: A window into the genome. Epigenetics Chromatin. 2014;7(1):33. 10.1186/1756-8935-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk SJ, Peters TJ, Buckley M, et al. : DNA methylation in blood from neonatal screening cards and the association with BMI and insulin sensitivity in early childhood. Int J Obes (Lond). 2018;42(1):28–35. 10.1038/ijo.2017.228 [DOI] [PubMed] [Google Scholar]
- van Zon A, Mossink MH, Schoester M, et al. : Multiple human vault RNAs. Expression and association with the vault complex. J Biol Chem. 2001;276(40):37715–37721. 10.1074/jbc.M106055200 [DOI] [PubMed] [Google Scholar]
- Vilalta A, Kickhoefer VA, Rome LH, et al. : The rat vault RNA gene contains a unique RNA polymerase III promoter composed of both external and internal elements that function synergistically. J Biol Chem. 1994;269(47):29752–29759. [PubMed] [Google Scholar]
- Weeding E, Sawalha AH: Deoxyribonucleic acid methylation in systemic lupus erythematosus: Implications for future clinical practice. Front Immunol. 2018;9:875. 10.3389/fimmu.2018.00875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Collisson EA, Mills GB, et al. : The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120. 10.1038/ng.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang L, Holloway AK, et al. : MiR-886-3p regulates cell proliferation and migration, and is dysregulated in familial non-medullary thyroid cancer. PLoS One. 2011;6(10):e24717. 10.1371/journal.pone.0024717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh M, Hernandez N: RNA polymerase III transcription as a disease factor. Genes Dev. 2020;34(13–14):865–882. 10.1101/gad.333989.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Chen D, Su Z, et al. : miR‑886‑3p upregulation in clear cell renal cell carcinoma regulates cell migration, proliferation and apoptosis by targeting PITX1. Int J Mol Med. 2014;34(5):1409–1416. 10.3892/ijmm.2014.1923 [DOI] [PubMed] [Google Scholar]
- Yu MC, Lee CW, Lin CH, et al. : Differential hypermethylation of the VTRNA2-1 promoter in hepatocellular carcinoma as a prognostic factor: Tumor marker prognostic study. Int J Surg. 2020;79:282–289. 10.1016/j.ijsu.2020.05.016 [DOI] [PubMed] [Google Scholar]
- Yu XF, Zou J, Bao ZJ, et al. : miR-93 suppresses proliferation and colony formation of human colon cancer stem cells. World J Gastroenterol. 2011;17(42):4711–4717. 10.3748/wjg.v17.i42.4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XO, Gingeras TR, Weng Z: Genome-wide analysis of polymerase III-transcribed Alu elements suggests cell-type-specific enhancer function. Genome Res. 2019;29(9):1402–1414. 10.1101/gr.249789.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Qin Y, Clark WC, et al. : Efficient and quantitative high-throughput tRNA sequencing. Nat Methods. 2015;12(9):835–837. 10.1038/nmeth.3478 [DOI] [PMC free article] [PubMed] [Google Scholar]