Granulocyte-macrophage colony-stimulating factor (GM-CSF), which belongs to the colony-stimulating factor (CSF) superfamily and was originally identified as a hemopoietic growth factor, is mainly produced by lymphocytes and innate lymphoid cells. Nonhematopoietic cell populations such as fibroblasts, endothelial cells, and epithelial cells may also secrete GM-CSF in response to an activating stimulus. Currently, GM-CSF is considered to play a smaller role in homeostatic myelopoiesis in a steady state because GM-CSF-gene-deficient mice exhibit steady-state hematopoiesis and a virtually normal lifespan of granulocytes and monocytes [1]. During the course of inflammation, systemic GM-CSF dramatically increases monocyte and neutrophil production from the bone marrow and plays an important role in the expansion and differentiation of myeloid cells [2]. Moreover, GM-CSF is deemed essential for resistance to local infection and normal pulmonary physiology. GM-CSF deficiency results in pulmonary alveolar proteinosis characterized by the failure to clear surfactant from the lungs due to a shortage of macrophages induced by GM-CSF.
GM-CSF is rarely detectable in the peripheral blood of healthy individuals and is expressed basally in nonsterile tissues such as the lung, gut, and skin. During inflammation, GM-CSF is principally produced by T helper (Th) cells and acts as a communication conduit between myeloid cells and tissue-invading lymphocytes. In an experimental autoimmune encephalomyelitis mouse model, the interleukin (IL)-7-STAT5 axis promotes the generation of GM-CSF/IL-3-producing Th cells, which are designated Th-GMs, exhibit a distinct transcriptional profile, and represent a novel subset of Th cells [3]. In another experimental autoimmune encephalomyelitis mouse model, it was reported that GM-CSF is critical for the proinflammatory functions of Th17 cells; GM-CSF blockade, but not IL-17 depletion, may efficiently inhibit tissue inflammation [4]. Therefore, GM-CSF is considered an important proinflammatory cytokine and not only a supplementary medication for patients with neutropenia secondary to chemotherapy-induced myelosuppression.
Sepsis is a life-threatening medical condition caused by the entry of various microorganisms into the human bloodstream that triggers an uncontrolled inflammatory reaction [5]. The systemic inflammatory response syndrome/compensatory anti-inflammatory response state model or persistent inflammation-immunosuppression catabolism syndrome model are used to depict the immune status of sepsis patients. An increased number of peripheral GM-CSF-producing CD4+ T cells were shown to serve as a marker of severity in patients with sepsis [6]. However, exogenous GM-CSF administration improved the survival of patients with sepsis by enhancing phagocytosis by neutrophils and monocytes and increasing reactive oxygen species generation. Therefore, a better stratification of the immune status with a tailored approach is essential for the treatment of patients with sepsis. The expression of monocytic human leukocyte antigen DR (mHLA-DR) in fewer than 30% of cells, reduction in the lipopolysaccharide (LPS)-induced TNF-α level to <200 pg/mL in whole blood, and an absolute lymphocyte count <1000 cells/mm3 are significant factors for immunoparalysis [7]. GM-CSF stimulates the production of neutrophils and monocytes by the bone marrow and maintains the activation and survival of monocytes. GM-CSF restored cytokine secretion in monocytes from sepsis patients by reducing apoptosis and increased CD71 and HLA-DR expression on monocytes upon synergistic coordination with LPS in vitro [8]. Many clinical studies have found that GM-CSF treatment has a potential benefit for patients with sepsis. In a prospective, randomized, open-label clinical study, GM-CSF treatment facilitated the recovery of the TNF-α response and prevented nosocomial infection in children with nonneutropenic, nontransplant, and severe multiple organ dysfunction syndrome [9]. Strong evidence has shown that mHLA-DR and plasma IL-6 levels are closely related to the outcome of recombinant human GM-CSF administration, and sepsis patients treated with GM-CSF have a shorter length of stay in the hospital or intensive care units [10]. In another randomized phase II trial, although GM-CSF-treated patients with sepsis showed improvement in PaO2/FiO2 and increased peripheral blood neutrophil counts, GM-CSF therapy did not reverse acute respiratory distress syndrome and had no effect on 30-day survival [11]. As GM-CSF application might lead to different results in severe sepsis at different stages, we suggest that biomarker-guided, individualized precision therapies based on the immune status may work in a well-defined patient population (Fig. 1).
Fig. 1. Immunological response in sepsis across time.
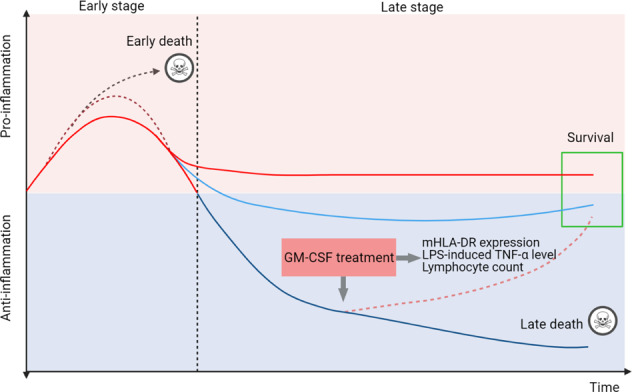
Sepsis initiates a complex immune response with the concomitant occurrence of excessive systemic inflammation at an early stage and relative immunosuppression at the late stage. Some patients die in the early stage of the uncontrolled proinflammatory response, whereas some patients die during the late stage of irreversible immune suppression. Different markers are used to distinguish the immunological thresholds, including mHLA-DR expression in <30% of cells, LPS-induced TNF-α level <200 pg/mL in whole blood, and absolute lymphocyte counts <1000 cells/mm3. GM-CSF treatment is preferred for use in patients with severe immunosuppression. mHLA-DR monocytic human leukocyte antigen DR, LPS lipopolysaccharide.
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 and has become a global pandemic. As a proinflammatory cytokine and myelopoietic growth factor, GM-CSF plays an essential role in mediating alveolar macrophage homeostasis and lung inflammation in COVID-19. Under homeostatic conditions, a low GM-CSF level is critical for the development and long-term maintenance of alveolar macrophages. For this reason, an inhaled GM-CSF formulation is being assessed for patients with COVID-19-related acute hypoxic respiratory failure (NCT04326920). In the later stages of COVID-19, increased GM-CSF levels may serve as a link between T cell-mediated acute pulmonary inflammation and a self-amplifying cytokine loop, which leads to the activation of monocytes and macrophages and contributes to the induction of proinflammatory cytokine production [12]. The increased GM-CSF levels in bronchoalveolar fluid may indirectly contribute to acute respiratory distress syndrome through the suppression of neutrophil apoptosis, which plays a major role in microvascular damage, thereby contributing to lung damage. The presence of emergency myelopoiesis, disease severity, and higher 28-day mortality was associated with an increased level of GM-CSF in patients with COVID-19 [13]. Clinical trials have demonstrated the efficacy of GM-CSF blockade in the treatment of patients with COVID-19. GM-CSF neutralization with lenzilumab prevents immune hyperstimulation and is associated with faster clinical improvements and slower progression to severe disease or death in some high-risk COVID-19 patients with severe pneumonia [14]. For non-mechanically ventilated patients with severe COVID-19 pneumonia, mavrilimumab, as an antagonist of GM-CSFα, is associated with improved clinical outcomes [15]. Other studies targeting GM-CSF in COVID-19 are ongoing, and the safety and effectiveness of anti-GM-CSF treatment need to be evaluated.
In summary, treatment targeting GM-CSF, either with GM-CSF supplementation or inhibition, was implemented in patients with sepsis and COVID-19. Efficient discrimination between excessive systemic inflammation and immunosuppressive processes was used to determine the timing of GM-CSF implementation, and multiple clinical trials are still needed.
Acknowledgements
We acknowledge funding support from the National Natural Innovation Fund (Project 81721002).
Author contributions
XM and RX wrote the manuscript; XM, RX, HL, and KL constructed the figures. F-SW revised the manuscript and figures.
Competing interests
The authors declare no competing interests.
Contributor Information
Fu-Sheng Wang, Email: fswang302@163.com.
Ruonan Xu, Email: xuruonan2004@aliyun.com.
References
- 1.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–6. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 2.Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 2016;45:963–73. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Sheng W, Yang F, Zhou Y, Yang H, Low PY, Kemeny DM, et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24:1387–402. doi: 10.1038/cr.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012;72:1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Wang S, Jiang T, Fan R, Zhang Z, Mu J, et al. High levels of circulating GM-CSF(+)CD4(+) T cells are predictive of poor outcomes in sepsis patients: a prospective cohort study. Cell Mol. Immunol. 2019;16:602–10. doi: 10.1038/s41423-018-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carcillo JA. Critical Illness Stress-induced Immune Suppression. In: Intensive Care Medicine. Yearbook of Intensive Care and Emergency Medicine, 217–28 (2007).
- 8.Williams MA, Withington S, Newland AC, Kelsey SM. Monocyte anergy in septic shock is associated with a predilection to apoptosis and is reversed by granulocyte-macrophage colony-stimulating factor ex vivo. J. Infect. Dis. 1998;178:1421–33. doi: 10.1086/314447. [DOI] [PubMed] [Google Scholar]
- 9.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–32. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 2009;180:640–8. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 11.Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am. J. Respir. Crit. Care Med. 2002;166:138–43. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 12.Mehta P, Porter JC, Manson JJ, Isaacs JD, Openshaw P, McInnes IB, et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir. Med. 2020;8:822–30. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hue S, Beldi-Ferchiou A, Bendib I, Surenaud M, Fourati S, Frapard T, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;202:1509–19. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temesgen Z, Assi M, Shweta F, Vergidis P, Rizza SA, Bauer PR, et al. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin. Proc. 2020;95:2382–94. doi: 10.1016/j.mayocp.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


