Abstract
Introduction
Measuring linkage after community-based testing, particularly HIV self-testing (HIVST), is challenging. Here, we use data from studies of community-based HIVST distribution, conducted within the STAR Initiative, to assess initiation of antiretroviral therapy (ART) and factors driving differences in linkage rates.
Methods
Five STAR studies evaluated HIVST implementation in Malawi, Zambia and Zimbabwe. New ART initiations during the months of intervention at clinics in HIVST and comparison areas were presented graphically, and study effects combined using meta-analysis. Meta-regression was used to estimate associations between the impact of community-based HIVST distribution and indicators of implementation context, intensity and reach. Effect size estimates used (1) prespecified trial definitions of ART timing and comparator facilities and (2) exploratory definitions accounting for unexpected diffusion of HIVST into comparison areas and periods with less distribution of HIVST than was expected.
Results
Compared with arms with standard testing only, ART initiations were higher in clinics in HIVST distribution areas in 4/5 studies. The prespecified meta-analysis found positive but variable effects of HIVST on facility ART initiations (RR: 1.14, 95% CI 0.93 to 1.40; p=0.21). The exploratory meta-analysis found a stronger impact of HIVST distribution on ART initiations (RR: 1.29, 95% CI 1.08 to 1.55, p=0.02).
ART initiations were higher in studies with greater self-reported population-level intensity of HIVST use (RR: 1.12; 95% CI 1.04 to 1.21; p=0.02.), but did not differ by national-level indicators of ART use among people living with HIV, number of HIVST kits distributed per 1000 population, or self-reported knowledge of how to link to care after a reactive HIVST.
Conclusion
Community-based HIVST distribution has variable effect on ART initiations compared with standard testing service alone. Optimising both support for and approach to measurement of effective and timely linkage or relinkage to HIV care and prevention following HIVST is needed to maximise impact and guide implementation strategies.
Keywords: HIV
Key questions.
What is already known?
Measuring facility-based linkage after community-based HIV self-test (HIVST) distribution is challenging because of the inherent confidentiality of the HIVST process, and the difficulty of predicting when and where HIVST users will link to care.
The STAR Initiative included five large-scale investigations of community HIVST distribution, and all included facility-based measurement of antiretroviral therapy (ART) initiation. These studies provide a unique opportunity to assess ART initiation after HIVST.
What are the new findings?
In some STAR Initiative studies assessing the change in facility ART initiations following community HIVST distribution, intervention effects seemed to diffuse into areas without HIVST distribution. In others, HIVST distribution was not consistent over time, or intervention impacts were more brief than expected.
Combining five STAR studies, we found that the impact of HIVST on facility ART initiations was highly variable. After accounting for unexpected diffusion of the intervention into comparison areas and timing of distribution or linkage, there was a clearer positive impact of HIVST on ART initiations.
ART initiations were higher in studies where more people self-reported using an HIVST during the intervention period. Number of HIVST distributed per population and knowledge of how to link following HIVST did not predict greater ART initiations following HIVST distribution.
What do the new findings imply?
HIVST programmes should consider integrating some measure of population uptake of testing, for example, contacting test users to assess whether testing has been used successfully, to help assess the effectiveness of HIVST in promoting ART initiations.
All STAR Initiative HIVST distribution included support for linkage, but actual linkage varied across studies, suggesting that there are barriers and enablers of linkage across multiple levels of influence. This complexity should be considered in programme planning.
Background
Recent large-scale trials of universal testing-and-treatment (UTT) for HIV prevention have confirmed that widespread HIV testing followed by successful linkage to antiretroviral therapy (ART) for persons with an HIV diagnosis can reduce HIV transmission across the general population.1–5 Biomedical prevention options like pre-exposure prophylaxis (PrEP) and voluntary medical male circumcision (VMMC) are also highly effective for preventing HIV transmission and require clients to be linked to additional services following an HIV test.6 Universal HIV test and treat and biomedical prevention scale-up both require that HIV tests be widely available across a variety of community-based settings, not just within healthcare facilities.3 Linkage to care in this context is a multi-step process requiring that programmes help users to link from testing in the community into health facilities to initiate treatment or prevention options.7
HIV self-testing (HIVST) is an important strategy for expanding the reach of HIV testing outside of healthcare facilities, and community distribution of HIVST has been shown to be particularly effective in supporting young people and men who wish to test for HIV.8 9 Measuring facility-based linkage after community-based testing, particularly HIVST, is challenging, however, and HIVST is less amenable to standard results-based cohort analysis due to the intrinsic confidentiality of results.10 While self-reports suggest that linkage to follow-on care is similar following HIVST as for standard HIV testing services (HTS), these responses may be prone to social desirability bias.10 Data on ART initiations collected from healthcare facilities are less susceptible to reporting bias. However, HIVST use is not always routinely captured in facility data, making it difficult to count users who accessed facility services as a result of HIVST. Finally, studies of linkage among general populations tend to have small numbers of new diagnoses, making robust statistical inference difficult as countries approach 90-90-90 targets set by the Joint United Nations Programme on HIV/AIDS (UNAIDS).
In this study, we have combined HIVST distribution and ART initiation data collected across studies sponsored by the STAR Initiative (hivstar.lshtm.ac.uk). The STAR Initiative supported HIVST research and implementation across in southern Africa, including large cluster randomised trials evaluating the effectiveness of community HIVST distribution on increasing HIV testing coverage and linkage to additional services in rural communities in Malawi, Zambia and Zimbabwe. In each study, we collected monthly data on ART initiations from clinics within communities where HIVST kits were distributed and similar data from comparison clinics where HIVST was not available. Using the date and location of HIVST distribution, we were able to measure the increase in ART initiations at clinics during HIVST distribution.
This analysis capitalises on the harmonised methods and outcomes within STAR, using descriptive analysis, meta-analysis and meta-regression to assess patterns of linkage over time and to measure whether community-based HIVST distribution to users in the general population (16 years and older) in three countries in Southern Africa was associated with increased ART demand at facilities compared with provision of facility-based HTS. We also examined the effect of the local testing and treatment context, aspects of intervention delivery, and whether population members knew where to link to care, to identify characteristics of HIVST interventions with successful linkage outcomes.
Methods
Studies included
Only studies funded through the UNITAID/PSI STAR Initiative (hivstar.lshtm.ac.uk) were eligible for inclusion, reflecting their common methodologies outlined earlier. A systematic review was not conducted, but meta-analysis and meta-regression tools were used to pool data across eligible STAR studies. Other elements of the meta-analysis have been reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.11
Within STAR, we used the following inclusion criteria: (1) an intervention including the distribution of HIVST outside of health facilities to the general adult population by lay health workers or community members; (2) a prespecified comparison area (either through randomisation or not) receiving local standard HTS; (3) study outcomes including monthly ART initiation measured using facility data.
Five studies from three countries (Malawi, Zambia and Zimbabwe) were included in this analysis (table 1), of which three were cluster randomised trials that included a standard of care arm and two were cluster randomised trials comparing two different HIVST distribution strategies that also included non-randomised comparisons of ART uptake in clinics from catchment areas where HIVST distribution and from clinics where HIVST was not being delivered in the catchment area. A study-specific assessment of bias was completed12 (online supplemental table 1).
Table 1.
Summary of STAR Initiative-funded studies of community-based HIVST distribution
| Study author | Location | Eligible population | Study design | Primary outcome and evaluation | Intervention description | Intervention duration and dates | ART initiation measurement duration and dates | Main and exploratory analyses |
| Indravudh et al17 | Blantyre, Machinga, Mwanza, and Neno districts, Malawi | Adult (≥16 years) residents of 22 HCF catchment areas | Cluster RCT with baseline and endline household surveys | HIV testing within past 12 months evaluated with baseline and endline household survey | Door-to-door distribution of HIVST by resident distributors over at least 12 months | At least 12 months per location, September 2016–January 2018 | ART initiations listing study villages as place of residence were extracted from health facility registers. Data were modelled as initiations per 1000 population. No data collected for the period prior to the intervention | Main analysis compared ART initiations in HIVST and non-HIVST clinics during full 12-month intervention period. Exploratory analysis showed most pronounced effects at months 6–12, corresponding to peak distribution period |
| Indravudh et al8 | Mangochi district, Malawi | Adult (≥15 years) residents of 30 group village head clusters | Cluster RCT evaluated with endline household survey | Self-reported lifetime HIV testing among adolescents, evaluated with endline survey | Engage established community health groups to lead the HIVST campaigns in their areas, with suggested emphasis on door-to-door | 7-day campaigns in each location, October 2018–January 2019 | Staff at health facilities in intervention and comparison areas interviewed new ART initiators during and for 6 months following the intervention. ART initiations were modelled as cumulative incidence per 1000 over the 6 months period | Main analysis compared ART initiations in HIVST and non-HIVST clinics for 6 months following ST distribution campaign. Exploratory analysis showed most pronounced effects at months 1–3 with minimal effect in months 4–6 |
| Neuman et al18 | Lusaka, Choma, Kapiri Mposhi, and Ndola districts, Zambia | Adult (≥16 years) residents of 16 healthcare facility catchment areas | Pair-matched cluster RCT evaluated with baseline and endline household survey | HIV testing within past 12 months evaluated with baseline and endline household survey | HIVST distribution by community distributors door-to-door, in high-density public areas, in health facilities, and by trained VMMC promoters in 3 of 6 clusters | At least 12 months per location, September 2016–May 2018 | Research staff collected data on ART initiations from records kept by health facilities in intervention and comparison districts. Data were collected for at least 5 months before the intervention and at least 11 months during the intervention | Main analysis compared ART initiations in HIVST and non-HIVST clinics during full 12-month intervention period. No exploratory analysis |
| Sibanda et al13 | Buhera, Bulilima, Chivi, Gutu, Gweru, Mazowe, and Masvingo districts, Zimbabwe | Adult (≥16 years) residents of 38 wards | Quasi-experimental design* | Proportion of individuals who report attending health facility following HIV self-test kit distribution, evaluated with endline household survey | HIVST distribution by community distributors with and without a financial incentive for each client linked to additional services | 4–6 weeks of distribution per cluster, September 2016–July 2017 | Catchment areas of all public sector facilities providing ART in each district were mapped in relation towards of both trial arms, categorised into clinics with and without catchment-area HIVST distribution, and paired with one ward receiving HIVST distribution for the purposes of defining before-during-after HIVST time points. ART initiations per month were extracted from clinic registers for the period 6 months before HIVST distribution, during distribution and 3 months following completion of distribution | Main analysis compared ART initiations in HIVST and non-HIVST clinics before, during and after distribution campaigns. No exploratory analysis |
| Sibanda et al16 | Mutoko, Muzarabani, Shamva, Shurugwi Umguza, and Zvimba districts, Zimbabwe |
Adult (≥16 years) residents of 40 village head man units | Quasi-experimental design† | Self-reported linkage to confirmatory testing, VMMC or PrEP among self-testers, and proportion of individuals reporting a new HIV diagnosis, both measured using endline household survey | HIVST distribution by community distributors or in campaigns led by community members | Four weeks of distribution per cluster, March–October 2019 | Data on monthly numbers of ART initiations from facilities within and outside HIVST distribution areas were collected from registers at all facilities in the six districts for the periods 6 months before HIVST distribution, during distribution and 3 months postdistribution | Main analysis compared ART initiations in HIVST and non-HIVST clinics before, during and after distribution campaigns. Exploratory analysis compares all facilities before, during and after distribution due to substantial diffusion of intervention into non-HIVST clinics |
*A cluster-randomised trial assessed the effectiveness of a linkage incentive paid to HIVST distributors versus no incentive and found no impact. While both trial arms received HIVST distribution, a non-randomised comparison area (no community distribution of HIVST kits) was used to assess the association between HIVST distribution and ART initiation.
†A cluster-randomised trial assessed the effectiveness of a community-led HIVST intervention compared with distribution by lay health workers and found no impact on ART initiations. While both trial arms received HIVST distribution, a non-randomised comparison area (no community distribution of HIVST kits) was used to assess the association between HIVST distribution and ART initiation.
ART, antiretroviral therapy; HIVST, HIV self-testing; PLHIV, people living with HIV; PrEP, pre-exposure prophylaxis; RCT, randomised controlled trial; VMMC, voluntary medical male circumcision.
bmjgh-2021-004986supp001.pdf (113.2KB, pdf)
HIVST distribution models evaluated in STAR included both community-based distribution, in which community-based distribution agents (CBDAs) were paid to distribute HIVST kits, and community-led (CL) designs with greater engagement from community members in designing, implementing and overseeing HIVST distribution and less direct supervision from programme implementers. Duration of distribution ranged from 7 days to 12 months. In all studies, distributors were trained to facilitate correct use of kits and to support users linking to health facilities for HIV prevention or care after HIVST.8 13 14 All studies were reported using Consolidated Standards of Reporting Trials guidelines for reporting of cluster randomised trials.15
Outcome measure
Outcomes for all trials included clinical-based ART initiations per month over the intervention period, measured using medical register data collected from health facilities in intervention and comparison areas. In four of five trials, data on ART initiations before HIVST distribution were also collected (period ranging from 6 to 12 months), while one study collected postintervention data only. (Data collection is detailed in table 1.) Data on whether HIVST were used before the ART initiation were not collected in medical registers in study areas and so are not available for this analysis.
Published estimates of the increase in ART initiation rate during HIVST distribution were included in this study. In these published estimates, ART initiations were standardised using the number of days on which data were collected for studies based in Zimbabwe,13 16 or the population of the clinic catchment area for studies based in Malawi or Zambia.8 17 18
Descriptive analysis and meta-analysis
For each trial, monthly ART initiation data were graphed and trends in both intervention and comparison areas were identified. The main meta-analysis combined the results of ART initiation analyses from all five trials as prespecified in analysis plans finalised before data collection was complete. A sensitivity meta-analysis included the results of exploratory analyses that were presented together with the main study findings. These exploratory analyses were conducted after data collection was complete and accounted for unexpected complications in measuring HIVST impact. In these studies, the prespecified analysis of ART initiations included months in which there was less HIVST distribution than expected,17 less sustained impact after distribution than expected,8 or insufficient distance between HIVST and non-HIVST areas leading to likely diffusion of the intervention into comparison areas.16 (A complete description of prespecified and exploratory analyses by study is presented in table 1.)
MN extracted study-specific rate ratios for outcomes reported in the prespecified trial analyses and exploratory analyses using monthly level data. Because we assumed that the effect of HIVST distribution varied based on distribution model and local context, we used random effects meta-analysis methods. The meta-analysis was conducted using Stata V.16.1,19 with additional visualisations completed in R.20
Meta-regression
We used meta-regression to identify factors associated of ART uptake following community HIVST distribution as measured using the prespecified analysis only. Meta-regression used the Hartnung and Knapp method for adjusting standard errors and test statistics in meta-analyses with small numbers of studies.21
Measures used
To understand why HIVST distribution was more effective at increasing ART initiations in some settings than others, we explored key measures of the context of the intervention, intervention implementation, and the mechanisms of impact that may be associated with intervention success22 (online supplemental figure 1 and table 2).
Table 2.
Data on intervention context, implementation and mechanisms used in meta-regression analysis
| Study author | Country and main year of implementation | National estimates of % PLHIV on ART | Total population in intervention area | HIVST distributed (total and per 1000 residents of intervention areas) | Reported HIVST use in previous 12 months at endline survey (%) | Know where to access care if test HIV-positive |
| Indravudh et al17 | Malawi, 2017 | 72.6 | Total population: 93 640 adults; 44 390 in intervention area | Total: 220 314 (including intervention and other distribution areas). No population denominator available for HIVST distribution in this study | 37.7* | 74.7% |
| Indravudh et al8 | Malawi, 2019 | 78.5 | Total population: 84 392 residents; 44 519 in intervention area | Total: 24 316 Per 1000 intervention population: 546 |
74.7† | Data not available |
| Neuman et al18 | Zambia, 2017 | 72.2 | Total population: 308 822; 148 541 in intervention area | Total: 65 585 Per 1000 intervention population: 442 |
23.2* | 78.7% |
| Sibanda et al13 | Zimbabwe, 2017 | 67.9 | Total population: 195 076 | Total: 80 378 Per 1000 intervention population: 412 |
50.2†* | 57.4% |
| Sibanda et al16 | Zimbabwe, 2019 | 76.5 | Total population of HIVST wards: 242 096 | Total: 59 631 Per 1000 intervention population: 246 |
24.5†* | 76.7% |
*HIVST uptake ascertained after 12–16 months of continuous distribution.
†HIVST uptake ascertained 3 months after the start of campaign-style distribution.
‡HIVST update ascertained during campaign-style distribution.
ART, antiretroviral therapy; HIVST, HIV self-testing; PLHIV, people living with HIV.
As ART initiations will become more infrequent as populations move closer to achieving universal HIV testing and treatment, we used Spectrum estimates of the proportion of PLHIV that are on ART23 24 (Personal communication with A Jahn, 30 September 2020), measured as the proportion of PLHIV who know their status and are on ART. These are the first two indicators of the ‘90-90-90’ target set by UNAIDS shaping national HIV testing and treatment strategies.25 We used general population estimates for country and year in which all or most intervention occurred.
Effectiveness of the HIVST intervention at encouraging ART initiations may also be related to the intensity of HIVST distribution, and whether the HIVST were used by intended populations as expected. Intervention intensity, defined as the number of HIVST kits distributed per 100 population, was estimated using the count of kits distributed by implementing partners and study author estimates of adult population size. These data were available for four of five studies. Intervention reach was measured as the proportion of endline household survey participants in intervention areas who self-reported using HIVST kits to test for HIV.
Finally, knowledge of how to link to care may be important in predicting whether HIVST users go on to access ART. We included a measure of knowledge of how to link to care following a reactive HIV test result to assess whether respondents’ perceived understanding of linkage affected intervention outcomes. This was measured using endline survey data as the proportion of survey participants in HIVST-distribution communities who responded positively to a question asking whether they knew where to access appropriate follow-up services following a positive HIV test.
Patient and public involvement
Communities collaborated in the design of CL interventions in Zimbabwe and Malawi.8 16 In other studies, communities were sensitised to interventions and participated in social harms reporting. The public was not involved in the analysis of this study.
Results
Descriptive analysis and meta-analysis
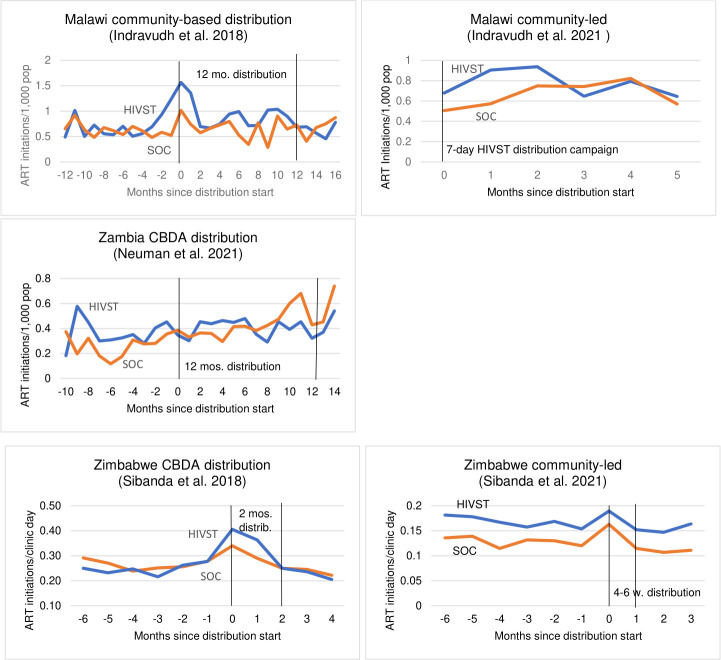
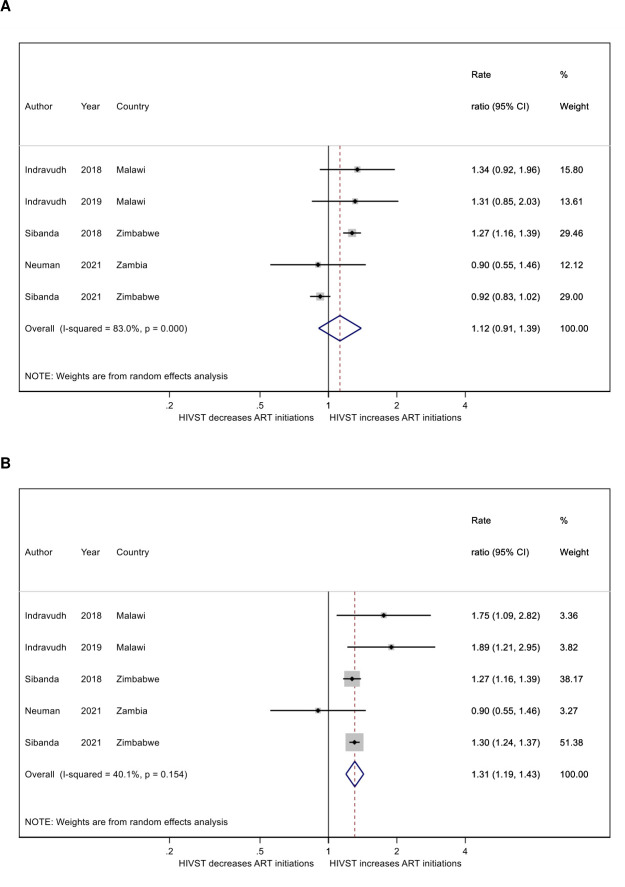
In studies in Malawi and Zimbabwe, ART initiations were greater in clinics associated with HIVST distribution areas during the distribution period, but there was no visual evidence of any difference between HIVST areas over time in the Zambia-based study (figure 1). This is consistent with the results of prespecified trial analyses for these studies, which identified no difference between HIVST and non-HIVST areas in Zambia (RR: 0.90; 95% CI 0.55 to 1.46; p=0.67), but greater ART initiations in HIVST areas in all other studies (Malawi CBDA study: RR: 1.34, 95% CI 0.92 to 1.96, p=0.12; Malawi CL study; RR: 1.31; 95% CI 0.84 to 2.03, p=0.34; Zimbabwe CBDA study: RR: 1.27, 95% CI 1.17 to 1.39, p<0.001; Zimbabwe CL study: RR: 0.92, 95% CI 0.83 to 1.02, p=0.098) (figure 2).
Figure 1.
Monthly ART initiations in HIVST distribution and non-HIVST distribution areas. ART, antiretroviral therapy; CBDA, community-based distribution agents; HIVST, HIV self-testing; mos, months; SOC, standard of care (no HIVST); w, weeks.
Figure 2.
Meta-analyses of the association between community HIVST distribution and clinic ART initiation using primary findings (A) and exploratory analysis findings (B). ART, antiretroviral therapy; HIVST, HIV self-testing.
However, investigating the trajectory of ART initiations and HIVST distribution over time shows that these results were not consistent across the entire distribution period. In several studies, ART initiations increased within the first 2 months of distribution and then fall afterward. This is particularly pronounced in the Malawi CL study,8 but also somewhat apparent in the Zimbabwe-based studies.13 16 The Malawi community-based distribution study8 shows a sharp increase in initiations at the beginning of the distribution period, then sustained increase during the second half of the distribution period. The data visualisations also show that ART initiations appear to be higher in both the HIVST and non-HIVST areas in both studies based in Zimbabwe. The increase in ART initiations in non-HIVST areas is particularly noticeable during the Zimbabwe CL distribution, suggesting possible diffusion of HIVST into the non-HIVST areas with onward effect on ART initiations. Post-hoc analyses accounting for these issues have been published,8 13 17 and generally find a more consistent positive association between HIVST distribution and local ART initiations.
In the meta-analysis combining findings of prespecified analyses, HIVST distribution in the community was associated with a 14% increase in ART initiations while distribution was ongoing (RR: 1.12, 95% CI 0.91 to 1.39; p=0.29; figure 2), but with a highly variable strength of association as reflected in high I2 (I2=83%). In a sensitivity meta-analysis incorporating exploratory analyses accounting for unexpectedly low HIVST distribution during parts of the intervention period17; lack of sustained impact of intervention8 and diffusion of intervention into comparison areas,16 the effect of distribution increases (RR: 1.31, 95% CI 1.19 to 1.43, p<0.001 I2=40%; figure 2).
Meta-regression
There was no evidence of a difference in the effectiveness of HIVST distribution by national-level indicators of ART use among PLHIV (10% increase in proportion PLHIV on ART: RR: 0.81, 95% CI 0.41 to 1.61, p=0.40; table 3), Intensity of HIVST distribution (100 kits per 10 000 residents) in the population was not associated with effectiveness (RR: 1.14, 95% CI 0.82 to 1.57, p=0.23), nor was knowledge of how to link to care following a positive HIV test (10% increase, RR: 0.87; 95% CI 0.61 to 1.24; p=0.24).
Table 3.
Meta-regression of indicators of intervention context, delivery and potential mechanism of impact on intervention outcomes
| Variable description | RR | 95% CI LB 95% CI UB |
P value | N (studies) |
| Context | ||||
| Proportion PLHIV on ART (10% change) | 0.81 | 0.41 to 1.61 | 0.40 | 5 |
| Intervention delivery | ||||
| 100 HIVST distributed per 10K population | 1.14 | 0.82 to 1.57 | 0.23 | 4 |
| Proportion taking up HIVST using survey data (10% change) | 1.12 | 1.04 to 1.21 | 0.02 | 5 |
| Mechanism of impact | ||||
| Proportion knowing where to link with an HIV-positive result (10% change) | 0.87 | 0.61 to 1.24 | 0.24 | 4 |
However, there is a small positive association between HIVST distribution and linkage and the percentage of survey participants self-reporting using an HIVST during the intervention period (10% increase, RR: 1.12; 95% CI 1.04 to 1.21; p=0.02).
Discussion
Combining prespecified analyses of ART initiations, we found weak evidence of an impact of community-based HIVST distribution on linkage to ART in facilities, but substantial heterogeneity in effect across studies made it difficult to reach a firm conclusion. A combination of exploratory results incorporating results reflecting a shorter linkage period and diffusion issues resulted in a clearer increase in ART initiations following community HIV distribution, still with substantial heterogeneity. There is strong evidence that HIVST distribution efforts that reach eligible populations, as measured using population endline survey data, are associated with greater facility linkage. However, other measures of context, intervention implementation and mechanisms of impact are not associated with ART initiation.
Systematic reviews of linkage to care following HIVST have found limited but encouraging evidence of successful linkage among HIVST users in the general population.26–28 A review across community-based HTS modes found that linkage was most likely to occur if supported by a counsellor.29 Literature on the determinants of linkage to care following HIV testing note that multiple levels of influence affect linkage, including patient and household-level factors as well as broader clinical-level qualities. At the patient and client level, perceived stigma and failure to disclose status are barriers to linkage.30 Clinical-level factors, including travel time to clinic, waiting time at clinic, and immediate availability of treatment, have also been highlighted as factors encouraging linkage following testing29; no information on these clinical-level factors were available for these studies. The heterogeneity reflected in the findings presented above may be caused by a combination of these individual and community-level factors mentioned in the literature, reflecting real differences in the linkage process in different places and populations. This highlights the complexity of the linkage process and the importance of considering linkage over multiple levels, from individual to clinic and community, when developing and evaluating testing programmes.
In all studies included in this analysis, ART initiation data were captured10 31 monthly during the period of ART initiation: while modelled results presented an overall change in ART initiations during the intervention, descriptive figures were used to identify when increases in ART initiations were largest. The descriptive results presented above suggest that there is a distinct uptick in ART initiations within weeks or the first month following the beginning of HIVST distribution, rather than a steady increase over time. This is somewhat different from the findings of UTT intervention studies, which have defined timely linkage to care as linkage within 6 months;32 one UTT trial found that around 50% of those identified by UTT actually link within 6 months.33 This difference in time to linkage between UTT interventions and HIVST distribution may be related to the self-directed nature of HIVST uptake. UTT interventions are intensive testing programmes designed to find even the hardest-to-reach populations, including those who might find accessing follow-up care difficult. In contrast, individuals who take an HIVST from a community distribution programme may be more ready to test and receive treatment quickly. Because the designs used in the STAR studies did not follow a cohort of HIVST users through the linkage process, it is difficult to disentangle these issues here; however, understanding the time to linkage is necessary for estimating the public health impact of testing interventions on community viral load and HIV incidence.
The findings of this analysis have several implications for future planning of HIVST programmes. First, when assessing intervention implementation, programmes should consider integrating some measure of population uptake of testing by contacting test users to assess whether testing has been used successfully within the targeted population. In contrast, measuring the number of HIVST distributed is likely to be less useful for assessing the success of a programme at linking. The Zambia trial findings, in which there was no impact of HIVST distribution on ART initiation, is an example: in this trial, only 23.2% of household survey respondents reported using an HIVST despite substantial numbers of HIVST distributed, explaining the lack of intervention impact.18 34
Second, programmatic assessments of the capability of respondents to link following testing should include measures of barriers and enablers of linkage across multiple levels of influence. While all HIVST interventions in this meta-analysis included additional support for linkage from community-based HIVST distributors or community members, there was substantial variation across included studies in the proportion of the population knowing how to link following HIVST. This indicates that, even when support exists, potential users may not be aware of support or may face other barriers to successful linkage. This is consistent with a previous trial showing significant impact on post-test confirmation and ART registrations when HIVST was accompanied by a linkage intervention of home-based assessment and initiation of ART if eligible.35 Finally, recording HIVST use in health facility registers would help understand how HIVST users access both HIV care and prevention services, and would provide additional useful data to further improve HIV services. This should be facilitated where possible.31
This study reviewed data collected from five studies conducted in three countries as part of the STAR Initiative. The similarity of protocols and distribution models across STAR Initiative studies, is a strength of this study, both because these studies particularly suited to pooling, and because outcomes and meta-regression predictors were measured using similar techniques. These studies cover over 615 000 rural residents of Malawi, Zambia and Zimbabwe; the size of the combined evaluations is an additional strength. The outcome measure used across all studies was collected from health facility records, so is less prone to social desirability bias than self-reported linkage and is an additional study strength.
However, there are several important limitations to this study. This study was not based on a systematic review, so did not include the universe of HIVST interventions. However, it did include all studies of community-based HIVST distribution compared with standard HTS among adults during this period in Malawi, Zambia and Zimbabwe.28 Other recent reviews of linkage following HTS and HIVST that include studies covering a wider geography have recently been completed.27 28 Because all STAR studies linked community HIVST distribution with linkage in nearby health facilities, they have excluded individuals who used HIVST and linked at a clinic outside their community, as well as users who linked after the end of the study. Thus, we assume that our linkage estimates underestimate the true number of HIVST users who eventually linked to ART. This study does not measure linkage to prevention or other HIV services apart from ART initiation, so only accounts for a limited number of actions following HIVST. While STAR studies shared similar data collection tools and methodologies, the duration of HIVST distribution differed across studies, as did the number of months of data collected after HIVST distribution began. This makes it difficult to use these data to estimate conclusive findings around the amount of time required for individuals to link to ART after using HIVST. Additional research on how best to support HIVST users to link quickly after testing and to understand linkage trajectories following HIVST remains necessary.
Additional limitations include the measures of PLHIV who are on ART: while national-level estimates were used to calculate this indicator, these likely masks substantial variation in testing and treatment uptake by district. Finally, these results reflect findings of a small number of studies (N=5) based in rural settings in a limited number of countries in Southern Africa. These results may not be generalisable across all settings in the region, particularly urban areas or areas where the characteristics of the HIV epidemic differ.
This study identified a positive but highly variable impact of community HIVST distribution on facility ART initiations. These results highlight the complexity of measuring linkage following community-based HIV testing, and suggest that additional linkage strategies may be needed to maximise the population benefits of HIV testing.
Acknowledgments
The authors declare that they have no conflicts of interests. This work is supported by Unitaid (grant number: PO#8477-0-600). ELC is supported by the Wellcome Trust (grant number: WT091769). ELC, KLF and MN conceptualised the study and identified the research question. MN extracted data, conducted statistical analyses and wrote first draft of manuscript. HA, FMC, BH, PI, CJ, ELS and KH contributed to revisions of the manuscript, and all authors have reviewed the final manuscript.
Footnotes
Handling editor: Seye Abimbola
Twitter: @ccasejohn, @Euphemia4
Contributors: ELC, KF and MN conceptualised the study and identified the research question. MN extracted data, conducted statistical analyses and wrote first draft of manuscript. HA, FMC, BH, PI, CJ, ES and KH contributed to revisions of the manuscript, and all authors have reviewed the final manuscript.
Funding: This work is supported by Unitaid (grant number: PO#8477–0–600). ELC is supported by the Wellcome Trust (grant number: WT091769).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon request. All data used in meta-analyses comes from peer-reviewed published manuscripts or manuscripts in preparation. Survey data used to estimate proportion taking up an HIVST or knowing how to link to care are available from datacompass.lshtm.ac.uk. Data on ART use among PLHIV are based on public Spectrum estimates or personal communication. Programmatic data on number of HIVST distributed in each study and other extracted data are available upon request from the corresponding author.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Hayes RJ, Donnell D, Floyd S, et al. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART). N Engl J Med Overseas Ed 2019;381:207–18. 10.1056/NEJMoa1814556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwuji C, Ome-Gliemann J, Balestre E. The impact of universal test and treat on HIV incidence in a rural South African population: ANRS 12249 TASP trial, 2012-2016. J Int AIDS Soc 2016;19. [Google Scholar]
- 3.Havlir D, Lockman S, Ayles H, et al. What do the universal test and treat trials tell us about the path to HIV epidemic control? J Int AIDS Soc 2020;23:e25455–e55. 10.1002/jia2.25455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havlir DV, Balzer LB, Charlebois ED, et al. Hiv testing and treatment with the use of a community health approach in rural Africa. N Engl J Med 2019;381:219–29. 10.1056/NEJMoa1809866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019;381:230–42. 10.1056/NEJMoa1812281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnaratne S, Hensen B, Cordes J, et al. Interventions to strengthen the HIV prevention cascade: a systematic review of reviews. Lancet HIV 2016;3:e307–17. 10.1016/S2352-3018(16)30038-8 [DOI] [PubMed] [Google Scholar]
- 7.Herce ME, Chi BH, Liao RC, et al. Re-thinking linkage to care in the era of universal test and treat: insights from implementation and behavioral science for achieving the second 90. AIDS Behav 2019;23:120–8. 10.1007/s10461-019-02541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indravudh PP, Fielding K, Kumwenda MK, et al. Effect of community-led delivery of HIV self-testing on HIV testing and antiretroviral therapy initiation in Malawi: a cluster-randomised trial. PLoS Med. In Press 2021;18:e1003608. 10.1371/journal.pmed.1003608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choko AT, Corbett EL, Stallard N, et al. Hiv self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: an adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med 2019;16:e1002719. 10.1371/journal.pmed.1002719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman M, Taegtmeyer M, Hatzold K, et al. Challenges in measurement of linkage following HIV self-testing: examples from the StAR project. J Int AIDS Soc 2019;22 Suppl 1:e25238. 10.1002/jia2.25238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibanda E, Neuman M, Tumushime M. Linkage to care after HIV self-testing in Zimbabwe: a cluster-randomized trial. BMJ Global Health. In Press 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuman M, Indravudh P, Chilongosi R, et al. The effectiveness and cost-effectiveness of community-based lay distribution of HIV self-tests in increasing uptake of HIV testing among adults in rural Malawi and rural and peri-urban Zambia: protocol for StAR (self-testing for Africa) cluster randomized evaluations. BMC Public Health 2018;18:1234. 10.1186/s12889-018-6120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 16.Sibanda E, Neuman M, Mangenah C. Comparison of community-led distribution of HIV self-tests kits with distribution by paid distributors: a cluster randomised trial in rural Zimbabwean communities. BMJ Global Health 2021;6:e005000. 10.1136/bmjgh-2021-005000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indravudh P, Fielding K, Neuman M. Increasing knowledge of HIV status and demand for art using community-based HIV self-testing: a cluster randomised trial in rural Malawi. AIDS 2018. Amsterdam 2018. [Google Scholar]
- 18.Neuman M, Hensen B, Mwinga A. Does community-based distribution of HIV self-tests increase uptake of HIV testing? results of pair-matched cluster randomised trial in Zambia. BMJ Global Health 2021;6:e004543. 10.1136/bmjgh-2020-004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corp S. Stata statistical software V. 16.1. TX, USA: College Station, 2020. [Google Scholar]
- 20.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS . National HIV estimates file Geneva: UNAIDS, 2020. Available: https://www.unaids.org/en/dataanalysis/datatools/spectrum-epp
- 24.Stover J, Andreev K, Slaymaker E, et al. Updates to the spectrum model to estimate key HIV indicators for adults and children. AIDS 2014;28 Suppl 4:S427–34. 10.1097/QAD.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNAIDS . Accelerating towards 90–90–90 2018.
- 26.Johnson CC, Kennedy C, Fonner V, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc 2017;20:21594. 10.7448/IAS.20.1.21594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Web Annex B. GRADE table: should HIV self-testing be offered as an additional HIV testing approach?In: Consolidated guidelines on HIV testing services. Geneva: World Health Organization, 2019. [Google Scholar]
- 28.Eshun-Wilson I, Jamil MS, T Charles W, et al. A systematic review and network meta-analyses to assess the effectiveness of HIV self-testing distribution strategies. Clin Infect Dis 2021. doi: 10.1093/cid/ciab029. [Epub ahead of print: 20 Jan 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma M, Ying R, Tarr G, et al. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 2015;528:S77–85. 10.1038/nature16044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanga ES, Mukumbang FC, Mushi AK, et al. Understanding factors influencing linkage to HIV care in a rural setting, Mbeya, Tanzania: qualitative findings of a mixed methods study. BMC Public Health 2019;19:383. 10.1186/s12889-019-6691-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choko AT, Jamil MS, MacPherson P, et al. Measuring linkage to HIV treatment services following HIV self-testing in low-income settings. J Int AIDS Soc 2020;23:e25548. 10.1002/jia2.25548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabapathy K, Mubekapi-Musadaidzwa C, Mulubwa C, et al. Predictors of timely linkage-to-ART within universal test and treat in the HPTN 071 (PopART) trial in Zambia and South Africa: findings from a nested case-control study. J Int AIDS Soc 2017;20:e25037. 10.1002/jia2.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Uptake of home-based HIV testing, linkage to care, and community attitudes about art in rural KwaZulu-Natal, South Africa: descriptive results from the first phase of the ANRS 12249 TASP cluster-randomised trial. PLoS Med 2016;13:e1002107. 10.1371/journal.pmed.1002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Does community distribution of HIV self-test kits increase uptake of HIV testing at population level? results of a cluster-randomised trial in Zambia. AIDS research and human retroviruses Mary Ann liebert, Inc 140 huguenot Street, 3rd fl, new rochelle, NY 10801 USA. 2018.
- 35.MacPherson P, Lalloo DG, Webb EL, et al. Effect of optional home initiation of HIV care following HIV Self-testing on antiretroviral therapy initiation among adults in Malawi. JAMA 2014;312:372–9. 10.1001/jama.2014.6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-004986supp001.pdf (113.2KB, pdf)
Data Availability Statement
Data are available upon request. All data used in meta-analyses comes from peer-reviewed published manuscripts or manuscripts in preparation. Survey data used to estimate proportion taking up an HIVST or knowing how to link to care are available from datacompass.lshtm.ac.uk. Data on ART use among PLHIV are based on public Spectrum estimates or personal communication. Programmatic data on number of HIVST distributed in each study and other extracted data are available upon request from the corresponding author.