Abstract
Objective
To determine whether communicating personalised statin therapy-effects obtained by prognostic algorithm leads to lower decisional conflict associated with statin use in patients with stable cardiovascular disease (CVD) compared with standard (non-personalised) therapy-effects.
Design
Hypothesis-blinded, three-armed randomised controlled trial
Setting and participants
303 statin users with stable CVD enrolled in a cohort
Intervention
Participants were randomised in a 1:1:1 ratio to standard practice (control-group) or one of two intervention arms. Intervention arms received standard practice plus (1) a personalised health profile, (2) educational videos and (3) a structured telephone consultation. Intervention arms received personalised estimates of prognostic changes associated with both discontinuation of current statin and intensification to the most potent statin type and dose (ie, atorvastatin 80 mg). Intervention arms differed in how these changes were expressed: either change in individual 10-year absolute CVD risk (iAR-group) or CVD-free life-expectancy (iLE-group) calculated with the SMART-REACH model (http://U-Prevent.com).
Outcome
Primary outcome was patient decisional conflict score (DCS) after 1 month. The score varies from 0 (no conflict) to 100 (high conflict). Secondary outcomes were collected at 1 or 6 months: DCS, quality of life, illness perception, patient activation, patient perception of statin efficacy and shared decision-making, self-reported statin adherence, understanding of statin-therapy, post-randomisation low-density lipoprotein cholesterol level and physician opinion of the intervention. Outcomes are reported as median (25th– 75th percentile).
Results
Decisional conflict differed between the intervention arms: median control 27 (20–43), iAR-group 22 (11–30; p-value vs control 0.001) and iLE-group 25 (10–31; p-value vs control 0.021). No differences in secondary outcomes were observed.
Conclusion
In patients with clinically manifest CVD, providing personalised estimations of treatment-effects resulted in a small but significant decrease in decisional conflict after 1 month. The results support the use of personalised predictions for supporting decision-making.
Trial registration
NTR6227/NL6080.
Keywords: vascular medicine, public health, cardiology, preventive medicine, lipid disorders
Strengths and limitations of the study.
Patients were provided with estimations of their personalised causal therapy-effects, unlike many previous studies which used hypothetical therapy-effects.
Performance bias was limited by hypothesis blinding.
Because the control group had a low decisional conflict at baseline, the effect seen in the study is possibly underestimated compared with the general population.
The personalised effects were not used directly during a clinical consultation, but provided prior to any potential consultation with a physician.
Some questionnaires were created for this study and not externally validated.
Introduction
Several online tools have recently become available that calculate the personalised therapy effects for various cardiovascular disease (CVD) prevention strategies. These calculators can express the therapy-benefit in terms absolute 10-year CVD risk reduction, or more recently, gain in healthy life-expectancy.1
The use of decision tools is associated with increased knowledge and less decisional conflict. Providing therapy-related information increases patient participation in medical decision-making.1–5 However, most decision aids do not provide the actual personalised benefits and harms an indivudual can expect,6 but use hypothetical or population-based effects of CVD-prevention. One obstacle in providing these causal effects is that patients often desire a far greater therapy-benefit than can be expected from preventive therapy.7–9 One survey showed that patients desire an increase in life-expectancy of around 42 months from life-long statin-use, whereas the actual benefit is often less than half this amount.7 8 Being presented with an actual predicted therapy-benefit far smaller than the benefit desired might discourage patients from using medication. Moreover, the effect seen may also differ according to which metric is used to communicate the therapy-benefit.10–13
We conducted a hypothesis blinded, three-armed, randomised controlled trial (RCT) to determine whether communication strategies involving personalised therapy-effects of statins obtained by algorithm, expressed as change in CVD-free life-expectancy or absolute 10-year CVD-risk reduction, lead to improved decisional certainty regarding the use of statins compared with standard communication strategies and with one another.
Methods
Population
The SMART-Inform study was nested within the previously described Secondary Manifestations of ARTerial disease (SMART) study, an ongoing, single-centre, prospective cohort of patients referred to the University Medical Center Utrecht in the Netherlands for CVD screening.14 All patients invited to participate in a SMART-examination were telephonically informed of the SMART-Inform substudy and sent further information by mail. Additional inclusion criteria for SMART-Inform were current statin use, being between 45 and 80 years old, and having CVD (ie, coronary artery disease, cerebrovascular disease and peripheral artery disease and abdominal aortic aneurysm). Additional exclusion criteria for SMART-Inform were terminal malignancy and not returning the baseline questionnaires.
Design, blinding, and randomisation
The SMART-Inform study was a three-armed, hypothesis-blinded, RCT. Hypothesis blinding entailed informing patients and their general practitioners (GPs) that all patients would receive at least standard SMART-protocol practice and that the study goal was to investigate if information about cholesterol-lowering medications would impact motivation for use. Patients were unaware what aspect of the received content was additional to standard practice, and what the primary and secondary outcomes were. Researchers and outcome assessors were not blinded. A computer-generated random allocation sequence was used to assign each patient after inclusion by order of inclusion. The investigator generating the random sequence was not involved in other aspects of the study. All other investigators had no access to the sequence.
Patient and public involvement
The study design and goal was discussed at an open conference of patient-organisations held in Amstelveen, the Netherlands in April 2016 to gain and incorporate input from patients at an early stage.
Description of standard practice
All participants received cardiovascular care as usual from their own referring GP or medical specialist and written information consisting of general lifestyle advice based on the treatment targets recommended by the European Society of Cardiology (SMART-study standard practice online supplemental file 1A).15
bmjopen-2020-041673supp001.pdf (853.6KB, pdf)
Description of intervention arms
There were three intervention arms: control group, individualised 10-year absolute risk (iAR-group) and individualised CVD-free life-expectancy (iLE-group). The control group received only standard practice.
Both intervention arms received standard practice plus: (1) a leaflet entitled personalised health profile (online supplemental file 1B and 1C provides examples for fictional patients); (2) a USB device containing educational videos and (3) a structured telephone consultation enforcing uptake of the information (online supplemental file 2). The ‘personal health profile’ outlined the individual effect of the following treatment options: (1) continue with the type and dose of statin-therapy (‘current prognosis’); (2) discontinue statin therapy (‘stop statins’) and (3) intensify to maximum statin-therapy, defined as once-daily atorvastatin 80 mg (‘increase statins’). The only difference between the intervention arms was the measure used to communicate the prognostic change associated with the therapy-effects: either in terms of change in iAR-or iLE. The USB-device contained intervention-group specific educational videos on how to read and interpret the ‘personal health profile’ and on the effect of statin-medications on CVD. The structured telephone consultation for the intervention arms ensured that the information was well received and understood by the patients. In the SMART-study, patients are encouraged to discuss the results with their own doctor and decide whether or not to change their statin prescription. Participants are free to decide whether to follow this advice or not. In the SMART-Inform study, the received information was not designed to replace a doctor’s advice and there was no extra face-to-face contact; however, all patients were strongly encouraged to visit their GP within 2 weeks to discuss the received information. Follow-up questionnaires were sent by mail 1 and 6 months postintervention, with telephone reminders ensuing after 2 weeks if the questionnaires were not returned.
Predicted therapy-effects
The estimations in the ‘personal health profile’ were obtained with the SMART-REACH score, an internationally validated model predicting the personalised effects of secondary CVD-prevention for patients aged 45–80 years. The model was developed using data from the SMART (Secondary Manifestations of Arterial Disease) and REACH (Reduction of Atherothrombosis for Continued Health) cohorts (http://U-Prevent.com).2 The model combines hazard ratios (HRs) derived from meta-analyses with a prediction algorithm incorporating individual patient characteristics to derive the personalised therapy effects. A 1 mmol/L reduction in low-density lipoprotein cholesterol (LDL-c) was modelled to correspond to the CVD-specific HR of 0.80 and the expected LDL-c-reduction for each statin was derived from a previous meta-analysis.16 17 Subgroup analyses in literature provide no evidence for differences of treatment effects on a relative effect scale for statins. Therefore, the treatment effect estimates based on the SMART-REACH score differ only on an absolute effect scale. Online supplemental figure 1 shows the distribution of the predicted therapy-effects for the trial patients.
Primary outcome
The study’s primary outcome was the intergroup difference in experienced decisional conflict at 1 month regarding the decision to continue, discontinue or intensify statin therapy. Decisional conflict was measured using the Decisional Conflict Scale (DCS), a validated and translated measure of patient perception of uncertainty in choosing between options.18 19 The DCS consists of 16 statements pertaining to the decision to use statins as prescribed (eg, ‘I am clear about which benefits matter most to me’). The scale measures the amount of internal conflict a patient feels regarding a medical decision. Summary scores range from 0 (no decisional conflict) to 100 (extremely high decisional conflict). Scores>37.5 are associated with feeling unsure about implementation of the decision, possibly leading to discontinuation of or fretting about the chosen option (ie, using statins as prescribed by the physician). Scores <25 are associated with following through with a decision.
Patient reported secondary outcomes
Secondary outcomes reported only at 6 months were the DCS and quality of life measured using the eight subscales of the RAND Medical Outcomes Study Short Form Survey (SF-36).20 Other patient-reported secondary outcomes were reported at both 1 and 6 months. The Brief Illness Perception Questionnaire (Brief-IPQ) was used to measure the degree to which CVD was considered threatening by patients.21 A Visual Analogue Scale (VAS) was used to measure how effective patients perceived statin therapy (online supplemental file 3). The 13 question Patient Activation Measure (PAM-13) was used to assess patient knowledge, skills and confidence for self-management of health.22 Due to limitations on maximum population size of academic use licenses, PAM-13 was only used for the last 213 study participants. Patient’s perception of shared decision-making was measured with the Shared Decision Making Questionnaire (SDMQ-9).23 Self-reported statin adherence was determined with the 2003 Brief Medication Questionnaire (BMQ).24 Patient understanding of statin-therapy was measured with a questionnaire developed for the trial (online supplemental file 4). The possible numeric ranges and interpretation of the secondary outcomes are shown in online supplemental file 5.
Physician reported secondary outcomes
Patients’ GPs’ received a copy of the personalised health profile. On enrolment of the first patient from their practice, GPs were provided a short telephonic explanation of the study and asked to fill in a questionnaire (online supplemental file 6). Questionnaire results and the last known postintervention LDL-value at 6 months were secondary outcomes. Interviewed GPs were blinded to study outcomes and treatment arm differences. GPs were not approached forsubsequent patients as receiving material from multiple patients unblinded them to treatment arm differences.
Sample size
The aim of this study was a pairwise comparison between study arms. To limit the overall probability of type 1 errors to 0.05, an analysis of variance (ANOVA) was used to detect the presence of any differences between the three groups. If the ANOVA detected a difference, subsequent t-tests were performed. Therefore, the sample-size was calculated to detect a difference in two groups using the t-test. Sample-size calculations were conducted using G*Power V.3.1. Sample size was based on an effect size (Cohen’s d=mean difference/SD) of 0.43 and a standard deviation of 0.80 to detect a mean difference of 0.34 on the 5-point scale (ranging from 0 to 4).19 25 A power of 80% and a two-tailed alpha of 0.05 was used. A minimum of 86 patients per arm was needed.
Statistical analyses
An intention-to-treat analysis was performed. Differences among the three arms were detected with ANOVA, or a Kruskal-Wallis one-way ANOVA to deal with heteroscedasticity. Assumptions of normal (residual) distribution and homoscedasticity were visually inspected. If ANOVA p<0.05, pairwise comparisons between arms were determined using a t-test or with the Wilcoxon-rank sum test for the difference in ranked means if ANOVA assumptions were not met after transformation attempts. Analyses were performed using R-Statistical Software 1.0.14.
Subgroups
Prespecified subgroup analyses were performed using an analysis of covariance (ANCOVA) test to investigate whether the effect of the intervention on DCS at 1 month differed according to the following: gender; age (<65 versus >65); years since first CVD event (<1 versus >1 years); educational level (low, medium or high);26 low versus high patient activation (low a PAM-13 level of 1–2 and high a PAM-13 level of 3–4 based on a conversion of the 100-point PAM-13 score to a 4-point scale);22 27 health literacy categories based on the Dutch version of the Newest Vital Sign (NVS)28 and disutility defined as the minimum gain in life-expectancy desired to offset the inconvenience of taking a lifelong, hypothetical, idealised daily tablet.8 The study was not powered to detect any subgroup differences. A Bonferroni correction corresponding to the 21 secondary outcome analyses was applied; the new p-value for statistical significance was 0.002.
Sensitivity analyses
Sensitivity analyses were performed to account for possible differences in baseline characteristics for missing outcomes between trial arms by conducting an ANCOVA with gender, age, smoking status, diabetes status, LDL-cholesterol (mmol/L), creatinine (umol/L), disutility score, NVS health literacy and number of medications used daily.
Results
Participant flow
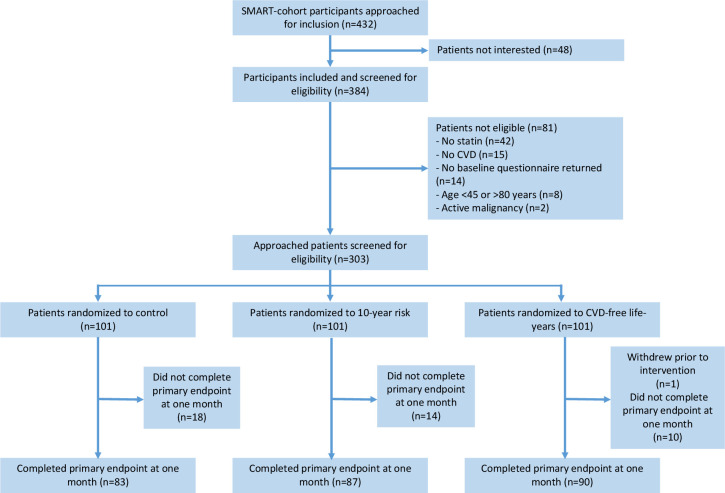
Between March 2017 and August 2018, 303 participants were enrolled. Baseline characteristics are shown in table 1 and the flow of participants throughout the trial in figure 1. The primary outcome was collected in 260 participants (86%) (control=83, iAR group=87, iLE group=90). Online supplemental table 1 displays characteristics for those with and without the primary endpoint. At 1 month, 12% (n=10) of control, 8% (n=7) of iAR and 11% (n=9) of iLE patients reported increasing their statin dose after the intervention. Respective numbers for decreased statin dose were 2% (n=2) in the control arm, 1% (n=1) in the iAR arm and 3% (n=3) in the iLE arm.
Table 1.
Baseline characteristics
| Control-group n=101 |
iAR-group n=101 |
iLE-group n=101 |
|
| Age | 66 (59–70) | 66 (58–71) | 64 (59–71) |
| Gender (male) | 86% | 82% | 85% |
| More than one CVD location | 11% | 10% | 10% |
| Current smoker | 17% | 16% | 9% |
| Years clinically manifest CVD | 0 (0–10) | 0 (0–10) | 3 (0–10) |
| Diabetes mellitus | 14% | 27% | 23% |
| LDL-cholesterol (mmol/L) | 2.0 (1.7–2.4) | 2.0 (1.6–2.4) | 2.0 (1.6–2.5) |
| LDL-cholesterol>1.8 mmol/L | 65% | 67% | 60% |
| Already on maximum statin therapy | 1.3% | 1.0% | 1.0% |
| Creatinin (umol/L) | 84 (78–93) | 83 (75–96) | 85 (75–94) |
| Systolic blood pressure (mm Hg) | 131 (121–142) | 131 (121–143) | 129 (122-142) |
| Number of medications per day | 5 (4–6) | 6 (4–9) | 6 (4–8) |
| Disutility | 61 (9–97) | 61 (5–97) | 61 (9–97) |
| Adequate health literacy | 83% | 83% | 81% |
Data are reported as mean±SD, median (IQR) or (%). CVD locations defined as coronary artery disease, peripheral artery disease or abdominal aortic aneurysm in addition to cerebrovascular disease. Health literacy was based on the Newest Vital Sign score in the baseline questionnaire.28 Disutility is months required to offset inconvenience of daily pill-taking of an idealised medication.8 Number of medications excludes over the counter medications, (nasal) sprays and topical medications. Maximum therapy was atorvastatin 80 mg.
CVD, cardiovascular disease; iAR-group, 10-year risk; iLE-group, CVD-free life-expectancy; LDL, low-density lipoprotein.
Figure 1.
Participant flow during the trial. CVD, cardiovascular disease; SMART, Secondary Manifestations of ARTerial.
DCS at one month
There was a significant difference between the groups (ANOVA χ2, p=0.002) with a median (25th–75th percentile) DCS of 27 (20–43) for control arm, 22 (11–30) for the iAR arm and 25 (10–31) for iLE arm. Subsequent Wilcoxon-rank sum tests showed the difference between the control and iAR arm (W=2707, p=0.001) and the control and iLE arm (W=4219, p=0.021) to be significant. The difference between iAR and iLE arms was not significant (W=3317, p=0.208, figure 2). All groups showed a DCS of around 25, the value associated with following through with a decision.
Figure 2.
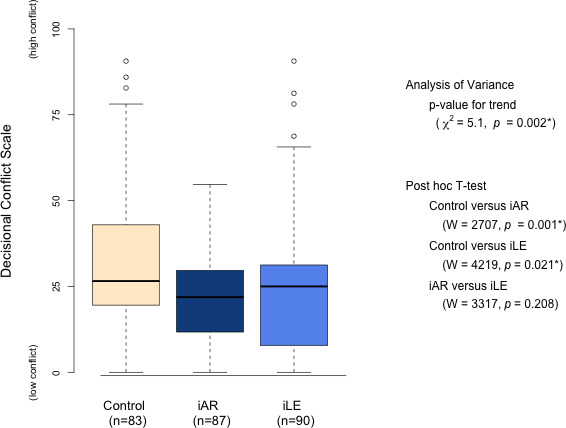
DCS at 1 month and Kruskal-Wallis analysis of variance and pos-hoc Wilcoxon-rank sum t-test. Boxes denote the median (25th–75th percentiles). DCS, Decisional Conflict Scale; iAR-group, 10-year risk; iLE-group, CVD-free life-expectancy.
Patient reported secondary outcomes
After 6 months, there was no longer a significant difference between the groups in DCS score (ANOVA χ2, p=0.10) with a median (25th–75th percentile) DCS of 25 (16–38) for control arm, 22 (9–29) for the iAR arm and 25 (7–31) for iLE arm. All other secondary outcomes also showed no intergroup differences (table 2).
Table 2.
Patient-reported secondary outcomes
| Median (25th–75th percentile) | P value | |||
| Control-group | iAR-group | iLE-group | ||
| DCS (6) | 25 (16–38) | 22 (9–29) | 25 (7–31) | p=0.10* |
| Brief-IPQ (1) | 36 (26–42) | 34 (28–44) | 37 (30–42) | p=0.68 |
| Brief-IPQ (6) | 34 (26–43) | 35 (30–41) | 37 (29–44) | p=0.19 |
| PAM (1) | 60 (51–70) | 58 (53–68) | 63 (56–75) | p=0.20 |
| PAM (6) | 64 (54–77) | 63 (56–77) | 63 (56–78) | p=0.48 |
| Perceived Statin Efficacy (1) | 8 (7–9) | 8 (7–9) | 8 (7–9) | p=0.92* |
| Perceived Statin Efficacy (6) | 8 (7–9) | 8 (7–9) | 8 (7–9) | p=0.98* |
| Understanding of therapy-effects (1) | 88 (75–88) | 88 (75–100) | 88 (75–100) | p=0.07* |
| Understanding of therapy-effects (6) | 88 (75–100) | 88 (75–100) | 88 (63–100) | p=0.60* |
| BMQ Adherence Risk Scale (1) | 1 (0–1) | 1 (0–1) | 1 (0–1) | p=0.60* |
| BMQ Adherence Risk Scale (6) | 1 (0–1) | 1 (0–1) | 1 (0–1) | p=0.41* |
| SDMQ-9 (1); reported visiting GP (n) | 44 (9–69); (46) | 42 (18–62); (58) | 58 (22–76); (55) | p=0.40* |
| SDMQ-9 (6); reported visiting GP (n) | 44 (24–73); (60) | 48 (32–63); (49) | 62 (22–84); (47) | p=0.28* |
| RAND-36 Quality of life (6) | ||||
| Physical functioning | 80 (70–85) | 75 (60–85) | 80 (65–85) | p=0.11* |
| Role limitations due to physical health | 80 (70–85) | 75 (60–85) | 80 (65–85) | p=0.57* |
| Role limitations due to emotional problems | 100 (100–100) | 100 (100–100) | 100 (100–100) | p=0.80* |
| Energy/fatigue | 75 (65–80) | 70 (60–80) | 73 (55–80) | p=0.20* |
| Emotional well-being | 84 (73–92) | 84 (72–92) | 80 (72–88) | p=0.11* |
| Social functioning | 88 (75–88) | 88 (63–88) | 88 (75–88) | p=0.49* |
| Pain | 90 (78–100) | 90 (68–100) | 100 (78–100) | p=0.93* |
| General health | 70 (55–75) | 60 (49–75) | 65 (50–74) | p=0.10* |
Data are for 1 or 6 months. Bonferroni p-value for significance was 0.002.
*A non-parametric test was applied.
BMQ, Brief Medication Questionnaire; Brief-IPQ, Brief Illness Perception Questionnaire; DCS, Decisional Conflict Scale; PAM, Patient Activation Measure; SDMQ-9, Shared Decision Making Questionnaire.
Physician reported secondary outcomes
Physician-reported secondary outcomes are shown in online supplemental table 2. Between randomisation and 6 months, 119 patients had their LDL-c values determined (control n=51, iAR n=48, iLE=39), with no difference in median serum LDL-c levels found (median 1.9 mmol/L in all groups) between study-arms. In total, 267 physicians were approached after the inclusion of their first patient of which 141 (53%) participated in the questionnaire. Physicians viewed statin-medication as equally worthwhile for patients in all study-arms. There was no difference of opinion between how iAR and iLE formats could positively influence doctor–patient communication, consultation efficiency, and therapy-adherence.
Subgroup analysis
No evidence of subgroup effects was found for sex (p-value for interaction=0.32), age (p=0.90), years since first CVD-event (p=0.24), months gain in CVD-free life-expectancy desired prior to taking an idealised medication daily (ie, disutility, p=0.54) and educational level (p=0.09). An interaction was found for health literacy (p=0.02). The median (25th–75th percentile) DCS scores for all subgroups are shown in online supplemental table 3, and a t-test for differences in each health literacy group is shown in online supplemental figure 2. Across health literacy categories, decisional conflict was lower in the intervention arms than in the control arm, with the largest differences found in people with a low health literacy.
Sensitivity analyses
Online supplemental table 4 shows the sensitivity analyses which corrected for baseline characteristics. After correction, none of the outcomes were significant.
Discussion
Providing personalised estimates of the prognostic changes associated with statin use in terms of 10-year CVD risk and CVD-free life-years (compared with a control group) resulted in lower decisional conflict after 1 month. After 6 months, no differences were found. Likewise, no differences were found in secondary outcomes, which included the degree to which people perceived their CVD to be threatening, how effective patients viewed their statin-medications, and LDL-c levels after 6 months. Although the actual benefit from CVD-prevention is smaller than people initially report acceptable, communicating this benefit resulted in lower decisional conflict without many people discontinuing treatment. However, the effect was small.
Many tools designed for decision-support report DCS differences of 8–10 points immediately after an intervention in favour of the decision-aid.6 We measured the outcomes after 1 month to provide time for patients to visit their physician. The already low decisional conflict in the control arm possibly explains the relatively small absolute differences found in this study (2–5 points). The loss of statistical significance at 6 months is in line with previous studies investigating the long-term effects of decision-support tools for statin-medications which show that positive results of such interventions fade over time.29
The use of patient communication-aids is known to make people feel better informed and help them form accurate opinions of benefit–harm ratios.6 30 A number of studies have examined the effect of providing estimations of hypothetical or generalised therapy-benefit to patients with clinically manifest CVD.11 31–33 One study examined the effect of providing primary care patients without any prior statin-exposure with the approximated personalised effect of statin-medications.12 Receiving the predictions in the form of absolute risk reduction estimates resulted in a greater likelihood to redeem statin-medications compared with prolongation of life. However, no differences were found in patient satisfaction and decision confidence. A possible explanation for this discrepancy between literature and our study could be that patients already using medication may respond differently to personalised estimations than patients initiating a new medication. As opposed to first-time statin-users, all patients in our study already used statins, and would know if they have experienced sside-effects. Willingness to use a new therapy may be more sensitive to the perceived side-effects than the perceived benefits.34 Similarly, worry about side-effects is a stronger determinant of intentional non-adherence than belief in the effectiveness of statin-medications.35
Similar to our study, previous literature shows that patients often overestimate the relative effects of medication and desire a greater absolute therapy-benefit than clinically feasible.7 8 Although the majority of patients in our study desired more benefit than clinically feasible (median disutility score 61 months), statin discontinuation was minimal and there was no evidence of subgroup effects based on baseline disutility. Although physicians may also overestimate the effects of preventive therapy,7 there were no intergroup differences in how physicians perceived the necessity of statin-medications.
Strengths of this study include providing patients with estimations of their actual causal therapy-effects, in contrast to pre-existing decision aids which present participants with either hypothetical or population-based effects. As we assessed current statin users, we were able to provide information on multiple treatment options. Systematically approaching cohort patients who were already due to receive physical examinations minimised the risk of preferentially selecting patients likely to respond to personalised predictions. Moreover, it was possible to select a structured and well-defined control group. The structured telephonic consultations ensured that patients had each interventional format explained in a similar fashion. Performance bias was limited by hypothesis blinding. A number of study limitations must also be highlighted. First, the control group of this clinically stable cohort population has low decisional-conflict and high belief in the effectivity of statin-medications. The effects described here may thus be different in patients who have pre-existing negative associations with statins due to adverse effects, or who are considering a more intensive strategy with additional medication such as blood pressure reduction or antithrombotic treatment. Second, the personalised effects were not used directly during a clinical consultation, but provided prior to any potential consultation with a physician. The effects may therefore be different in patients who are involved in a clinical consultation in which statin initiation is discussed. Third, the loss to follow-up was 14% for the primary outcome. This is however lower than other communication-trials involving follow-up questionnaires12 and baseline characteristics of missing and non-missing individuals were relatively similar. Correction for baseline health literacy, a characteristic which may have differed between missing and non-missing individuals did not level-off the effects. Fourth, self-reported measures may be subject to recall and reporting biases, in particular for questions relating to adherence. Fifth, a number of questionnaires were created specific for this study, and were thus not externally validated.
A number of risk-prediction tools capable of estimating treatment-effects for lipid-lowering, blood pressure-lowering and antithrombotic medications are now readily available in clinical practice for patients with and without CVD.1 Statins are usually prescribed to patients with CVD during hospital admission for the first CVD event. Outpatient decision-making regarding statins in this population usually pertain to continuing or altering the current statin dose. In the present study, we aimed to examine a setting closely resembling outpatient practice. However, only a small effect was found. Therefore, future studies could focus on populations with higher baseline decisional conflict such as patients experiencing adverse effects or considering intensifying preventive treatment with additional medication such as blood-pressure lowering or antithrombotic treatment.
In conclusion, providing statin users with clinically manifest CVD with personalised estimations of treatment-effects, both in terms of 10-year absolute risk and CVD-free life-expectancy, resulted in small but significant decrease in decisional conflict associated with statin use after 1 month. This effect disappeared after 6 months of follow-up. The results support the use of personalised predictions of therapy benefit in clinical practice. Future studies may focus on decisions associated with higher decisional conflict such as the addition of more intensive preventive treatment options on top of standard treatment.
Supplementary Material
Acknowledgments
Participants of the April 2016 Amstelveen PGO Support conference; SMART research nurses; R van Petersen (data-manager); BGF Dinther (cohort manager) and the SMART Study Group: A Algra MD, PhD; DE Grobbee, MD, PhD; GJ de Borst, MD, PhD, Department of Vascular Surgery; LJ Kappelle, MD, PhD, Department of Neurology; T Leiner, MD, PhD, Department of Radiology; HM Nathoe, MD, PhD, Department of Cardiology.
Footnotes
Contributors: NEMJ, FLJV, YvdG, OD, CB and JAND contributed to the conception, design of the work and interpretation of the data; YMS and GR contributed to the interpretation. NEMJ drafted the work and performed the analyses; FLJV, YvdG, OD, CB, JAND, YMS and GR critically revised the work. All authors gave final approval and agreed to be accountable for the work.
Funding: Partially funded by a Netherlands Heart Foundation grant (2016T026). Funder was not involved in the design or assessment of the study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Some individual deidentified participant data (including data dictionaries) may be shared upon reasonable request pending approval by the department and institution on a case-by-case basis. Approval will be based the scientific question to be answered with the data, ability of the authors of this manuscript to co-operate on the project and compliance with contracts acquired for the questionnaires in this study and hospital and SMART-cohort policy.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Medical Ethics Review Committee of the UMCU approved the study (16-665/D). All participants provided written informed consent.
References
- 1.Jaspers NEM, Ridker PM, Dorresteijn JAN, et al. The prediction of therapy-benefit for individual cardiovascular disease prevention: rationale, implications, and implementation. Curr Opin Lipidol 2018;29:436–44. 10.1097/MOL.0000000000000554 [DOI] [PubMed] [Google Scholar]
- 2.Kaasenbrood L, Bhatt DL, Dorresteijn JAN, et al. Estimated life expectancy without recurrent cardiovascular events in patients with vascular disease: the SMART-REACH model. J Am Heart Assoc 2018;7:e009217. 10.1161/JAHA.118.009217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Huffman MD, Karmali KN, et al. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare patients: the million hearts longitudinal ASCVD risk assessment tool: a special report from the American heart association and American College of cardiology. J Am Coll Cardiol 2017;69:1617–36. 10.1016/j.jacc.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.JBS3 Board . Joint British societies' consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100 Suppl 2:ii1–67. 10.1136/heartjnl-2014-305693 [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann TC, Del Mar C. Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2015;175:274–86. 10.1001/jamainternmed.2014.6016 [DOI] [PubMed] [Google Scholar]
- 6.Stacey D, Légaré F, Lewis K, et al. Decision AIDS for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaspers NEM, Visseren FLJ, Numans ME, et al. Variation in minimum desired cardiovascular disease-free longevity benefit from statin and antihypertensive medications: a cross-sectional study of patient and primary care physician perspectives. BMJ Open 2018;8:e021309. 10.1136/bmjopen-2017-021309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana M, Asaria P, Moraldo M, et al. Patient-accessible tool for shared decision making in cardiovascular primary prevention: balancing longevity benefits against medication disutility. Circulation 2014;129:2539–46. 10.1161/CIRCULATIONAHA.113.007595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trewby PN, Reddy AV, Trewby CS, et al. Are preventive drugs preventive enough? A study of patients' expectation of benefit from preventive drugs. Clin Med 2002;2:527–33. 10.7861/clinmedicine.2-6-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med 2014;161:270–80. 10.7326/M14-0295 [DOI] [PubMed] [Google Scholar]
- 11.Jegan NRA, Kürwitz SA, Kramer LK, et al. The effect of a new lifetime-cardiovascular-risk display on patients' motivation to participate in shared decision-making. BMC Fam Pract 2018;19:84. 10.1186/s12875-018-0766-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmsen CG, Kristiansen IS, Larsen PV, et al. Communicating risk using absolute risk reduction or prolongation of life formats: cluster-randomised trial in general practice. Br J Gen Pract 2014;64:e199–207. 10.3399/bjgp14X677824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misselbrook D, Armstrong D. Patients' responses to risk information about the benefits of treating hypertension. Br J Gen Pract 2001;51:276–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Simons PC, Algra A, van de Laak MF, et al. Second manifestations of arterial disease (smart) study: rationale and design. Eur J Epidemiol 1999;15:773–81. 10.1023/A:1007621514757 [DOI] [PubMed] [Google Scholar]
- 15.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–81. 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423. 10.1136/bmj.326.7404.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor A. User Manual - Decisional conflict scale 1993, 2010. Available: http://decisionaid.ohri.ca/evaldcs.html
- 19.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. 10.1177/0272989X9501500105 [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Sherbourne CD. The mos 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 21.Broadbent E, Petrie KJ, Main J, et al. The brief illness perception questionnaire. J Psychosom Res 2006;60:631–7. 10.1016/j.jpsychores.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 22.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918–30. 10.1111/j.1475-6773.2005.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriston L, Scholl I, Hölzel L, et al. The 9-item shared decision making questionnaire (SDM-Q-9). development and psychometric properties in a primary care sample. Patient Educ Couns 2010;80:94–9. 10.1016/j.pec.2009.09.034 [DOI] [PubMed] [Google Scholar]
- 24.Svarstad BL, Chewning BA, Sleath BL, et al. The brief medication questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns 1999;37:113–24. 10.1016/S0738-3991(98)00107-4 [DOI] [PubMed] [Google Scholar]
- 25.Schwalm J-D, Stacey D, Pericak D, et al. Radial artery versus femoral artery access options in coronary angiogram procedures: randomized controlled trial of a patient-decision aid. Circ Cardiovasc Qual Outcomes 2012;5:260–6. 10.1161/CIRCOUTCOMES.111.962837 [DOI] [PubMed] [Google Scholar]
- 26.Statistiek CBvd . Opleidingsniveau. Available: https://www.cbs.nl/nl-nl/artikelen/nieuws/2008/16/bijna-evenveel-hoogopgeleide-als-laagopgeleide-nederlanders/opleidingsniveau2019
- 27.Hibbard JH, Greene J, Shi Y, et al. Taking the long view: how well do patient activation scores predict outcomes four years later? Med Care Res Rev 2015;72:324–37. 10.1177/1077558715573871 [DOI] [PubMed] [Google Scholar]
- 28.Fransen MP, Leenaars KEF, Rowlands G, et al. International application of health literacy measures: adaptation and validation of the newest vital sign in the Netherlands. Patient Educ Couns 2014;97:403–9. 10.1016/j.pec.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 29.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med 2007;167:1076–82. 10.1001/archinte.167.10.1076 [DOI] [PubMed] [Google Scholar]
- 30.Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2018;7:CD006732. 10.1002/14651858.CD006732.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halvorsen PA, Selmer R, Kristiansen IS. Different ways to describe the benefits of risk-reducing treatments: a randomized trial. Ann Intern Med 2007;146:848–56. 10.7326/0003-4819-146-12-200706190-00006 [DOI] [PubMed] [Google Scholar]
- 32.Adarkwah CC, Jegan N, Heinzel-Gutenbrunner M, et al. Time-to-event versus ten-year-absolute-risk in cardiovascular risk prevention - does it make a difference? Results from the Optimizing-Risk-Communication (OptRisk) randomized-controlled trial. BMC Med Inform Decis Mak 2016;16:152. 10.1186/s12911-016-0393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krones T, Keller H, Sönnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med 2008;6:218–27. 10.1370/afm.854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried TR, Tinetti ME, Towle V, et al. Effects of benefits and harms on older persons' willingness to take medication for primary cardiovascular prevention. Arch Intern Med 2011;171:923–8. 10.1001/archinternmed.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wouters H, Van Dijk L, Geers HCJ, et al. Understanding statin Non-Adherence: knowing which perceptions and experiences matter to different patients. PLoS One 2016;11:e0146272. 10.1371/journal.pone.0146272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041673supp001.pdf (853.6KB, pdf)
Data Availability Statement
Some individual deidentified participant data (including data dictionaries) may be shared upon reasonable request pending approval by the department and institution on a case-by-case basis. Approval will be based the scientific question to be answered with the data, ability of the authors of this manuscript to co-operate on the project and compliance with contracts acquired for the questionnaires in this study and hospital and SMART-cohort policy.