Abstract
Background
Current worldwide pandemic coronavirus disease 2019 (COVID-19) with high numbers of mortality rates and huge economic problems require an urgent demand for safe and effective vaccine development. Inactivated SARS-CoV2 vaccine with alum. Hydroxide can play an important role in reducing the impacts of the COVID-19 pandemic. In this study, vaccine efficacy was evaluated through the detection of the neutralizing antibodies that protect mice from challenge with SARS-CoV 2 3 weeks after the second dose. We conclude that the vaccine described here has safety and desirable properties, and our data support further development and plans for clinical trials.
Methods
Characterized SARS-COV-2 strain, severe acute respiratory syndrome coronavirus 2 isolates (SARS-CoV-2/human/EGY/Egy-SERVAC/2020) with accession numbers; MT981440; MT981439; MT981441; MT974071; MT974069; and MW250352 at GenBank were isolated from Egyptian patients SARS-CoV-2-positive. Development of inactivated vaccine was carried out in a BSL-3 facilities and the immunogenicity was determined in mice at two doses (55 and 100 μg per dose).
Results
The distinct cytopathic effect induced by SARS-COV-2 propagation on Vero cell monolayers and the viral particles were identified as Coronaviridae by transmission electron microscopy and RT-PCR on infected cells cultures. Immunogenicity of the developed vaccine indicated the high antigen-binding and neutralizing antibody titers, regardless of the dose concentration, with excellent safety profiles and no deaths or clinical symptoms in mice groups. The efficacy of the inactivated vaccine formulation was tested by the wild virus challenge of the vaccinated mice and viral replication detection in lung tissues.
Conclusions
Vaccinated mice recorded complete protection from challenge infection via inhibition of SARS-COV-2 replication in the lung tissues of mice following virus challenge, regardless of the level of serum neutralizing antibodies. This finding will support future trials for the evaluation of an applicable SARS-CoV-2 vaccine candidate.
Keywords: SARS-CoV-2, isolation, inactivation, emerging vaccine
STATEMENT OF SIGNIFICANCE
Evaluation of the immunogenicity induced by inactivated whole SARS-CoV-2 candidate vaccine is achieved through immunization of mice groups with different doses of inactivated antigen with alum. Hydroxide as adjuvant. Regardless of dose concentration, high levels of neutralizing antibody titers were detected in vaccinated mice that provide reliable protection from wild virus challenge.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an emerging respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that had infected >16 million individuals and caused >656 000 deaths worldwide [1]. Safe and effective vaccines against COVID-19 were established [2]. SARS-CoV-2—a member of the Betacoronavirus genus—is closely related to severe acute respiratory syndrome coronavirus (SARS-CoV) and several bat coronaviruses [3–5]. Compared with SARS-CoV and the Middle East respiratory coronavirus (MERS-CoV), SARS-CoV-2 appears to undergo more rapid transmission [6, 7] leading to the urgent demand for a vaccine. There are currently >160 COVID-19 candidate vaccines in development worldwide, and 25 are in different phases of clinical trials using different platforms [8]. Several vaccines, such as a recombinant adenovirus type-5 (Ad5)–vectored vaccine, a chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19), and the mRNA vaccines, have been published or made available on preprint servers [9, 10]. Inactivated vaccines have been widely used for the prevention of emerging respiratory diseases for decades [11], and the relatively high speed of the development of this kind of vaccine makes it a promising strategy for COVID-19 vaccine development. Moreover, results from preclinical studies of the inactivated COVID-19 vaccines have shown that the vaccines could protect against SARS-CoV-2 with varying efficacy [1].
In Egypt, the efforts piloted to fight against COVID-19 through precautionary measures and trails to produce effective vaccines have been reported. Thus, successful experimental steps for isolation and characterization of SARS-CoV-2 from patients are crucial for vaccine development. In our study, we evaluated the safety, efficacy, and immunogenicity of a β-propiolactone (BPL)-inactivated the whole virus SARS-CoV 2 vaccine in mice. We examined the response to two doses of two antigen concentrations (55 and100 μg) and protection rate, which was evaluated by challenge virus infection to vaccinated mice. The results were assessed in mice as preclinical evaluation of the vaccine candidate for further animal’s model and clinical trial approach.
MATERIALS AND METHODS
Sample collection
Nasopharyngeal and oropharyngeal swabs were collected in 5 ml viral transport media from six COVID-19 patients with age > 45 years (2 males and 4 females), who were positively diagnosed using rRT-PCR. Swabs were transferred to Egypt Center for Research and Regenerative Medicine (ECRRM) BSL-3 laboratory and stored at 4°C for immediate virus isolation. With the approval of the ethics institutional review board (IRB) of the Ministry of Defense, written informed consent was obtained from the participants.
Biosafety containment
Using the precautions that adhered to or exceeded the requirements by WHO [12], all experiments with suspected samples, infected cells, and infected fluids were performed in ECRRM Biosafety Level 3 laboratory and were conducted under appropriate conditions.
Virus isolation, propagation, and identification
Virus isolation was applied on the Vero cell line of kidney epithelial cells derived from the African green monkey (ATCC No. CCL-81, Manassas, Virginia USA) supplied from Veterinary Serum Vaccine Research Institute (VSVRI). Confluent monolayer of Vero cells was grown in Dulbecco’s modified minimum essential medium (DMEM, Gibco UK) supplemented with penicillin (100 units/ml), streptomycin (100 mg/ml), 0.2% sodium bicarbonate, and 10% fetal bovine serum (FBS). The prepared cell culture was infected with 1 ml of suspected SARS-COV2 samples for ~45–60 min, then a maintenance medium (MEM supplemented with 2% fetal bovine calf serum) was added and followed by incubation at 37 ± 1°C. The cells were examined twice daily for cytopathic effects (CPE) formed by the inoculated virus. Three blind passages [13] followed by seven successive serial passages were obtained and tissue culture suspensions were collected for virus detection and quantification by real-time PCR. Virus replication and isolation were confirmed through CPEs, gene detection, and electron microscopy.
Real-time PCR detection
Total RNA was extracted using a Viral RNA Extraction kit (Qiagen, CA) following the manufacturer’s instructions. Extracted RNA concentration and purity were tested with a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). One step real-time PCR was achieved using TaqPath™ COVID-19 CE-IVD RT-PCR Combo Kit (Thermo Fischer Scientific, USA) following manufacturer’s instructions. The reaction was incubated in real-time PCR ABI 7500 (Thermo Fisher Scientific, USA) at 50°C for 15 min for reverse transcriptase step, 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s. Three primers/probes were used targeting ORF1ab, Nucleocapsid (N), and Spike (S) regions. Primers/probes specific for bacteriophage MS2 were used as a positive control. The cycle threshold value below 33 was stately to be positive. The result is valid when two of the three targeted genes and the MS2 showed positive results.
Whole Genome sequencing
Extracted RNAs were quantified using Qubit RNA High Sensitivity Kit (Invitrogen, USA). Libraries were prepared using Ion AmpliSeq SARS-CoV-2 Kit (Thermo Scientific, USA) following the manufacturer’s Protocol. Clonal amplification of the libraries was done using the Ion-PI-Hi-Q Sequencing 200 Kit (Thermo Scientific, USA) PCR emulsions. Purified libraries were qualified and quantified by Agilent Bioanalyzer and Qubit 4 Fluorometer (Thermo Scientific, USA). Libraries were sequenced on the Ion proton NGS platform (Thermo Scientific, USA). Virus sequence assembly was performed using The Ion Torrent package (v.5.12) followed by genome mapping using tmap program (v.512) against complete SRAS-CoV-2 genome sequences retrieved from the GISAID website. All of the strains were isolated from Vero cells, which have been certified by WHO for vaccine production. Vero cell monolayers were infected via the swabs of patients to prevent possible mutations during viral culture and isolation.
Transmission electron microscopy
Infected Vero cells were scraped from the flask, pelleted, and cell pellet was rinsed with 0.1 M phosphate buffer (Sigma Aldrich, Germany). Cell suspensions collected from inoculated Vero Cell monolayers were first fixed with 2% formaldehyde in phosphate-buffered saline for 1 h before ultracentrifugation (1 h, 25 000 rpm), loading sample on carbon coating grid stained with 2% phosphor-tungstic acid for 30 s then examined. Bar:100 nm.
Virus titration
The 50% tissue culture infectious dose (TCID50) per ml was determined in Vero cell monolayers on 24 and 96-well plates. Serial dilutions of virus samples were incubated at 37°C for 4 days and subsequently examined for CPE in infected cells. TCID50 assay was performed according to [14]. The infectious titer was calculated using an in-house method adapted by Spearman and Kärber and expressed in TCID50 units [15].
Virus inactivation and vaccine production
For vaccine preparation, the virus was propagated in Vero cells with a dilution of 1:100 (v:v) of the SARS-CoV-2 virus in a serum-free medium. The cells were incubated at 37°C for 72 h. On the third day of infection, when the CPE was visible, the virus was harvested by collecting cell supernatants. The infectious titer of the virus was determined using a 50% cell culture infectious dose as described above. Vaccine purification was then performed with low-speed centrifugation (1000 rpm) to clarify the cell harvest followed by filtration. SARS-CoV-2 was inactivated with β—propiolactone at 2–8°C for 24–32 h [16, 17]. The final bulk was prepared as a liquid formulation containing 55 or 100 μg total protein with aluminum hydroxide (Alhydrogel® CRODA health care Corp.) as adjuvant (0.45 mg/0·5 ml).
Validation of the inactivation
Effective inactivation of the virus was validated by inoculations of Vero monolayers in 75 cm2 flasks with 10 ml of inactivated virus and then cultured at 36 ± 1°C for 4 days. No CPE was observed for three passages, besides, quantitative PCR (Q-PCR) performed at several time points during passage confirmed the absence of amplification of virus genomes [18].
Safety test
The vaccine formulations at two different concentrations and the adjuvant have been evaluated for safety in groups of Swiss albino mice (n = 10/group; 5  and 5
and 5  ). Safety has been documented in repeat-dose toxicity studies in mice (6–8 weeks old) which were vaccinated intraperitoneally (i.p.) with three doses (N + 1) at 55 and 100 μg/dose of inactivated vaccine candidate without adjuvant on Days 0, 7, and 14 [19]. In contrast, the mice group was treated (i.p.) with a single dose of alum. Hydroxide at the dose of 5 mg of Al (OH)3/mouse, which equivalent to ~25–8 human doses than recommended (0.2–0.8 mg). Another negative mice group was injected with phosphate-buffered saline (PBS) as a control group. All animals were observed for mortality during the experimental period. Animals were euthanized on Days 21 and 28 and necropsied and organs were evaluated for macroscopic and microscopic findings. Evaluations for histopathology marked as to the vaccine/dosage to assess the extent of pathologic damage and the eosinophilic component of the inflammatory infiltrates.
). Safety has been documented in repeat-dose toxicity studies in mice (6–8 weeks old) which were vaccinated intraperitoneally (i.p.) with three doses (N + 1) at 55 and 100 μg/dose of inactivated vaccine candidate without adjuvant on Days 0, 7, and 14 [19]. In contrast, the mice group was treated (i.p.) with a single dose of alum. Hydroxide at the dose of 5 mg of Al (OH)3/mouse, which equivalent to ~25–8 human doses than recommended (0.2–0.8 mg). Another negative mice group was injected with phosphate-buffered saline (PBS) as a control group. All animals were observed for mortality during the experimental period. Animals were euthanized on Days 21 and 28 and necropsied and organs were evaluated for macroscopic and microscopic findings. Evaluations for histopathology marked as to the vaccine/dosage to assess the extent of pathologic damage and the eosinophilic component of the inflammatory infiltrates.
Immunization of mice
Mice groups (6–8 weeks old) were obtained from VSVRI (n = 10/group 5  and 5
and 5  ) were immunized intramuscularly (i.m.) with candidate vaccine at two different antigen concentrations, 55 and 100 μg total protein, formulated with aluminum hydroxide (alum). A booster immunization was carried out 14 days post first immunization with the same formulation and dosage as primary inoculation. Control groups received the same volume of buffered saline. Sera were then drawn from all groups every week to quantitative evaluation of SARS-COV2 neutralizing antibodies in vaccinated mice (20).
) were immunized intramuscularly (i.m.) with candidate vaccine at two different antigen concentrations, 55 and 100 μg total protein, formulated with aluminum hydroxide (alum). A booster immunization was carried out 14 days post first immunization with the same formulation and dosage as primary inoculation. Control groups received the same volume of buffered saline. Sera were then drawn from all groups every week to quantitative evaluation of SARS-COV2 neutralizing antibodies in vaccinated mice (20).
SARS-CoV-2 challenge test
Three weeks post the booster dose, all mice of the groups that received vaccine doses of 55 and 100 μg and the control groups were challenged. Before the challenge, a blood sample was drawn for the determination of neutralizing antibody titers. For the challenge, mice were anesthetized with isofluran and inoculated intranasally with 60 μl of SARS-CoV-2 virus (106 TCID50) according to animal care and use guidelines in an approved animal BSL-3 laboratory [20]. The isolated SARS-CoV2 that has been propagated five times on serum protein-free Vero SF cells were used for the homologous challenge. On Days 3 and 7 post-challenge, mice were euthanized, before lung and trachea were removed and frozen at −80°C. Tissue samples were thawed and homogenized in 1 ml of Vero cell culture medium supplemented with antibiotics for titration in the TCID50 assay [21].
Histopathological analysis
The lung tissues of challenged mice were immediately fixed in 10% buffered formalin and embedded in paraffin wax. Histopathological changes caused by isolated SARS-CoV-2 virus infection were examined by H&E staining and viewed under the light microscope as described previously [22].
Determination of neutralizing antibody titers
Serum samples were heat‐inactivated for 30 min at 56°C and serially diluted with cell culture medium in twofold dilution. The serum dilutions were mixed at a ratio of 1:1 with a SARS‐CoV‐2 virus stock suspension adjusted to 100 TCID50/ml, incubated for 1 h at 37°C in a humidified atmosphere with 5% CO2, and transferred (eight replicates per dilution) to a 96-well tissue culture plate seeded with Vero cells. The plates were incubated for 5 days at 37°C in a CO2-incubator before the cultures were inspected under a light microscope for the presence of a CPE caused by SARS-CoV2, i.e. cell rounding and detachment. Neutralizing antibody titers were expressed as the reciprocal of the last dilution of serum that completely inhibited virus-inducedCPE.
Statistical analysis
Neutralizing antibody titers were compared in vaccinated groups. Differences were considered statistically significant when P < 0.05 and the P values were calculated through the unpaired Student’s t-test.
RESULTS
Isolation and propagation SARS-COV2 virus for vaccine candidate development
A primary virus seed was prepared from a human isolate by 10 passages on Vero cell cultures. Distinct CPE in cell monolayers infected by SARS-CoV-2 was observed following an incubation period of 2–3 days post virus inoculation (Fig. 1) indicated that the virus grows well on Vero cells that were approved by the WHO for the production of human vaccines, so it was reflected for rapid vaccine development. This primary seed was further amplified to generate a seed virus bank, a working virus bank, and a production virus bank (for vaccine manufacturing). The virus was then used to infect serum protein-free Vero cell cultures resulted in the generation of high viral titers (107.5 TCID50/ml).
Figure 1.
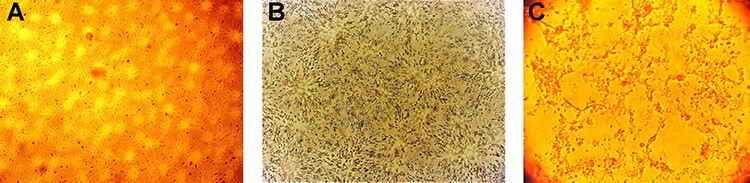
Normal Vero cells monolayer (A). Vero cell monolayer infected with SARS-CoV-2/48 h post-inoculation showed rounding of Vero cells (the first sign of CPE of virus infection) (B). Marked detachment of cells 72 h post-SARS-COV2 cell cultures infection (C). (X 100).
Determination of SARS-COV2 virus titer
Titration of the virus isolate revealed a gradual increase in the virus titer through the successive passages (Fig. 2). The virus titer was 5 log10 TCID50 at the fourth passage and reached 6.5 log10 TCID50 by the eighth passage. At the final passage, the virus titer reached 7.5 log10 TCID50.
Figure 2.
Titer of isolated SARS-COV2 in Vero cell culture.
Whole Genome sequencing
complete viral genome sequences of the isolate’s SARS-CoV-2/human/EGY/Egy-SERVAC/2020 with accession numbers; MT981440; MT981439; MT981441; MT974071; MT974069; and MW250352 at GenBank revealed that the virus was most closely related (99.5% nucleotide similarity) to USA/VA-CDC-6377/2020 strain (MT325612.1) and USA/FL-BPHL-0259/2020 strain (MT704077.1).
Virus inactivation
The harvested SARS-COV2 infected cells supernatant was inactivated by β—propiolactone treatment at 2–8°C for 24. Inactivation was confirmed by three passages into Vero cell cultures’. No CPE was observed in the inactivated virus-infected cell monolayers.
An electron micrograph of the purified inactivated virus showed virion particles belonging to the Coronaviridae Family were observed. Negatively stained electron microscopy image visualized oval viral particles with spikes having diameters of ~100 nm (Fig. 3) confirmed that virus particles being demonstrated to present well-defined spikes on the virus membrane.
Figure 3.
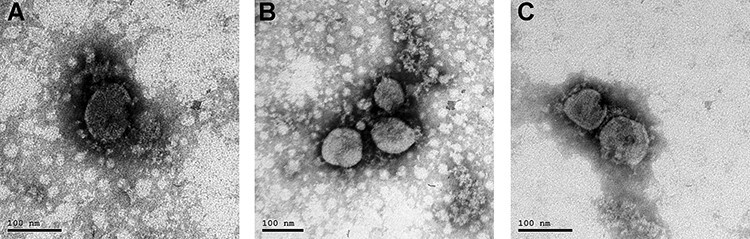
Virion particle belonging to the Coronaviridae Family observed by electron microscopy (A). Electron micrograph (187 000-fold magnification) of purified inactivated SARS-CoV-2 candidate vaccine after staining with uranyl acetate. Spikes formed by S protein project from the viral surface (B and C).
Safety test
No mortality or morbidity with normal clinical signs was observed in mice inoculated intra-peritoneal with repeated doses of the vaccine and alum adjuvant. Microscopic examination recorded normal organ tissues of all vaccinated animals (Fig. 4). The experiment revealed that the inactivation steps were independently capable of inactivating this titer with a large margin of safety.
Figure 4.
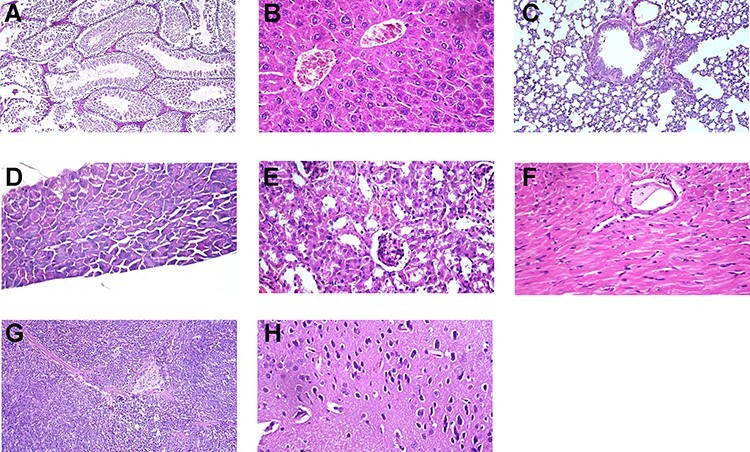
Normal organs tissue of mice vaccinated with three doses (N + 1) of candidate inactivated SARS-COV2 vaccine. Testes showing normal seminefrous tubules with normal spermatogonia cells. H&E X 200 (A). Liver showing normal polyhedral hepatocytes with normal cytoplasm and nucleus, H&E X400 (B). Lung from showing normal bronchi and normal alveoli. H&E X200 (C). Pancreas showing normal islets of Langerhans. H&E X400 (D). Kidneys showing normal renal glomeruli and renal tubules. H&E X 400 (E). Heart showing normal cardiac muscle with normal striation and nucleation. H&E X400 (F). Spleen showing normal lymphoid follicles, white bulbs, and red bulbs. H&E X200 (G). Brain showing normal cerebrum with normal neurons and nerve cells H&E X400 (H).
Immunogenicity in mice
The immunogenicity of the candidate vaccine with two different doses of antigen content (55 and 100 μg) was investigated in mice. Neutralizing antibodies titer determined for each group of animals is presented in (Fig. 5) No dosage effect was noted as the mean neutralizing antibody titers of the low and high doses of inactivated vaccine groups were non-significant different (P ≥ 0.05) in vaccinated mice groups pre-challenge test. These data demonstrate that the candidate vaccine is highly immunogenic in mice. A single immunization with 55 or 100 μg induced SARS-COV2 specific antibody titers up to 1:160 and 1:213 2 weeks post first vaccination. Following the booster immunization, titers were substantially increased up to 1:2560 with the dose 100 μg at third week post-second dose. Mice vaccinated with high dose induced significant-high antibodies titer (P = 0.0001) 3 days post-challenge virus infection in compared with low dose immunized mice group (Table 1). High neutralizing antibody titers (1:1067 and 1:1707) were recorded one-week post-challenge test in 55 and 100 μg/dose, respectively without significant differences. All of the vaccinated animals had significantly high serum neutralizing antibody titers than the non-vaccinated challenged mice group.
Figure 5.
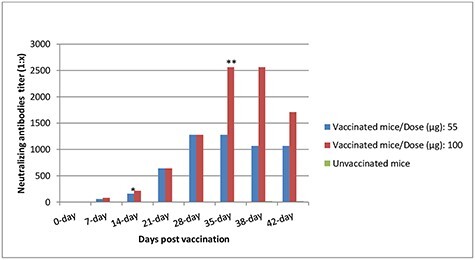
Mean neutralizing-antibody responses to the SARS-COV2 inactivated vaccine in mice. *Mice were re-immunized with a booster dose. **Mice were intranasally challenged with 106 TCID50 of SARS-CoV2.
Table 1.
Mean neutralizing-antibody responses to the inactivated SARS-COV 2 candidate vaccine in mice groups
| Mice group | Neutralization titer ± SD/day post-vaccination | |||||||
|---|---|---|---|---|---|---|---|---|
| (Dose) | 0 d | 7 d | 14 d* | 21 d | 28 d | 35 d** | 38 d | 42 d |
| 55 μg | <1:10 | 1:60 ± 28.2 | 1:160 ± 0.00 | 1:640 ± 0.00 | 1:1280 ± 0.00 | 1:1280 ± 0.00 | 1:1067a ± 33.4 | 1:1067 ± 36.4 |
| 100 μg | <1:10 | 1:80 ± 0.00 | 1:213 ± 92.3 | 1:640 ± 0.00 | 1:1280 ± 0.00 | 1:2560 ± 0.00 | 1:2560b ± 0.00 | 1:1707 ± 73.0 |
| control | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | 1:10 ± 0.00 | 1:20 ± 0.00 |
The results with different letters “a,b” have statistically significant differences (p < 0.05)
*Mice were re-immunized with a boosterdose.
**Mice were intranasally challenged with 106 TCID50 of SARS-CoV2.
Efficacy in mice
Mice were monitored daily post-challenge for morbidity, loss weight, and mortality (Table 2). No clinical signs were observed in vaccinated groups compared with the unvaccinated challenged group. The unvaccinated group showed rough hair with arched back 3 days post-challenge. The challenge virus replicated to titers 102.5 TCID50/ml and 105.4 TCID50 in Vero cell culture infected with homogenized lung tissues from mice at 3 and 7 days post-infection, respectively. Replication of the challenge virus was not detected in the lung tissues of all mice that received the candidate vaccine by Real-time PCR and absence of CPE in cell culture monolayer (Table 3) revealed that candidate vaccine could be highly efficacious in mice at the two doses (55 and 100 μg).
Table 2.
Clinical signs observation post-vaccination (A) and challenge test (B)
| A. Post-vaccination | |||||
|---|---|---|---|---|---|
| Weight Gm ± SD |
Body temp. 0°C |
Heart rate | Sleeping hours | Daily activities | |
| First week | 17.3 ± 0.41 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Second week* | 17.6 ± 0.41 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Third week | 18.2 ± 0.62 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Fourth week | 19.3 ± 0.9 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Fifth week** | 20.6 ± 0.94 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Sixth week | 21.75 ± 0.34 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| B. Post challenge test (Control unvaccinated) | |||||
| Weight Gm ± SD |
Body temp (average) |
Heart rate | Sleeping hours (average) |
Daily activities | |
| Day 0 | 18.2 ± 0.62 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Day 1 | 17.6 ± 0.47 | 36.9 ± 0.5 | 400–600/m | 12–14 | Normal |
| Day 2 | 17.3 ± 0.47 | 36.9 ± 0.5 | 400–600/m | 14–16 | Moderate |
| Day 3 | 17.3 ± 0.41 | 36.9 ± 0.5 | 500–600/m | 16–18 | Low |
| Day 4 | 16.8 ± 0.54 | 36.9 ± 0.5 | 500–600/m | 16–18 | Low |
| Day 5 | 16.6 ± 0.41 | 36.9 ± 0.5 | 500–600/m | 16–18 | Low |
| Day 6 | 16.6 ± 0.41 | 36.9 ± 0.5 | 600–700/m | 16–18 | Low |
| Day 7 | 16 ± 0.70 | 36.9 ± 0.5 | 600–700/m | 16–18 | Low |
*Boosterdose.
**Challengetest.
Table 3.
Virus replication in lung tissue of vaccinated and control mice groups post-SARS-COV 2 challenge infection
| lungs | Vero cell cultures | ||||
|---|---|---|---|---|---|
| Mice groups | Days post-challenge | No. infected/ no. tested |
Viral RNA |
No. infected/ no. tested |
Mean virus titer* |
| Unvaccinated | 3 | 4/4 | +VE | 4/4 | 2.5 |
| 7 | 3/3 | +VE | 3/3 | 5.4 | |
| Vaccinated (55 μg/dose) |
3 7 |
0/4 0/3 |
−VE −VE |
0/4 0/3 |
< 1** |
| Vaccinated (100 μg/dose) |
3 7 |
0/4 0/3 |
−VE −VE |
0/4 0/3 |
< 1** |
*Virus titers are expressed as log10 TCID50/ml of homogenized lung tissue.
**Virus not detected by the absence of CPE in infected Vero cell monolayer cultures.
Histological evidence of protective efficacy post challenge
Histopathological findings did not vary within either low dose (55 μg) or high dose (100 μg) vaccinated groups among tissues collected post-challenge (Fig. 6). In addition to the reduction in viral titers detected in the lungs, histopathological findings in the lungs of immunized mice indicate that the candidate inactivated vaccine produced protection from SARS-CoV-2 after 3 and 7 days post-challenge. It is meaning that vaccinated groups that had detectable levels of serum neutralizing antibodies to SARS-CoV-2 at the time of challenge were protected from severe lung lesions. The unvaccinated control animals that lacked detectable levels of SARS-CoV-2 neutralizing antibodies, had severe lung lesions, mice lung at 3 and 7 days post-challenge test showing diffuse thickening in the interstitial tissue, congested peri-alveolar blood capillaries, and lymphocytic cells infiltrations.
Figure 6.
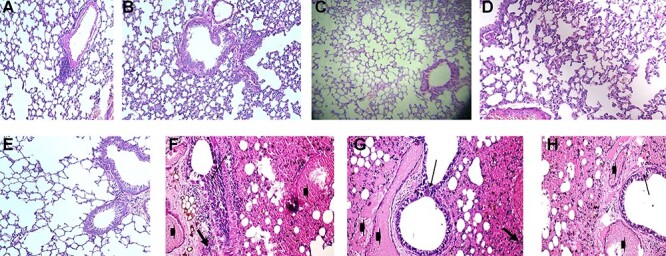
Normal lung tissue. H&E X 100 (A). Vaccinated mice with 55 μg after 3 post-SARS-COV-2 challenge showing normal lung tissues. H&E X 100 (B). Vaccinated mice with 100 μg after 3 days post-SARS-COV-2 challenge showing normal lung tissues H&E X 100 (C). Vaccinated mice with 55 μg after 7 days post-SARS-COV-2 challenge showing normal lung tissues H&E X 200 (D). Vaccinated mice with 100 μg after 7 days post-SARS-COV-2 challenge showing normal lung tissues. H&E X 200 (E). Unvaccinated mice lung after 3 days post-challenge test showing hyperplasia of the lining epithelium (thin arrow), interstitial blood vessel congestion (arrowhead), and diffuse thickening in the interstitial tissue and lymphocytic cells infiltrations (thick arrows). H&E X 200 (F). Unvaccinated mice lung after 7 days post-challenge test showing hyperplasia of the bronchial wall and the lining epithelium (thin arrow), interstitial blood vessel congestion (arrowhead), and diffuse thickening in the interstitial tissue lymphocytic cells infiltrations (thick arrows). H&E X 400 (G). Unvaccinated mice lung after 7 days post-challenge test showing hyperplasia of the bronchial wall and the lining epithelium (thin arrow), interstitial blood vessel congestion (arrowhead), and diffuse thickening in the interstitial tissue and lymphocytic cells infiltrations (thick arrows). H&E X 400 (H).
DISCUSSION
Novel COVID-19 has shown a rapid spread since December 2019 causing a huge outbreak in China [23]. Isolating and studying the causative virus is crucial for developing diagnostic tools, therapeutics, and effective vaccines. The development of vaccines with high immunogenicity and safety is paramount for controlling the pandemic COVID-19 and prevent further infection spread. Many different strategies have previously been reported for the development of experimental and candidate human SARS vaccines and these include inactivated whole virus vaccine using large-scale serum protein-free Vero cell cultures [24]. The whole-genome sequence of isolated SARS-CoV-2 strain was closely related to most available sequences, representing to some extent circulating SARS-CoV-2 populations. As well as the development of the SARS-CoV-2 vaccine was based on the adaption of this isolated virus, to establish optimal conditions for growth, inactivation, and purification of the inactivated virus. Inactivated vaccine derived from isolated SARS-CoV-2 strain which is closely related to Wuhan/WIV04/2019 was reported [25]. The SARS-CoV-2 had been reported to grow effectively on Vero cells [26, 27], considering that this was probably the optimal cell matrix for rapid vaccine development. Propagation of the isolated SARS-COV-2 virus on Vero cell monolayers, yield a titer of 7.5 log10 post 10 passages with distinct CPE within 72 h post-infection. This was in agreement with Gao et al. [25] who discussed that Vero cell cultures showed SARS-COV 2 replication efficiently and reached a peak titer of 6–7 log10 TCID50/ml by 3 or 4 days pi. Previous findings recorded the SARS-CoV CPEs with monolayers of Vero cells 3 days post the blind passages [8]. In contrast, no specific CPEs were observed in the Vero E6 cells until 6 days after inoculation as reported by Zhu et al. [19]. Most processes for inactivated whole virus vaccines have utilized β-Propiolactone as an inactivating agent. Our study report development of an inactivated SARS-CoV-2 vaccine (EgyCoVax) showed intact, oval-shaped particles with diameters of 90–150 nm, which were embellished with crown-like spikes, representing a pre-fusion state of the virus (Fig. 3). These results are consistent with the previous report for transmission electron microscopy analysis of stained samples demonstrated that the inactivated virion presented well-defined spike structures on the virus particle with no apparent structural alterations resulting from the inactivation procedures [24]. Also, candidate vaccine toxicity and safety evaluation showed no adverse or clinical signs in vaccinated mice. Inactivated whole virus vaccine would be most efficient in inducing neutralizing antibodies, which are possibly critical in preventing SARS-CoV infection [24]. Since mice are a model of SARS-CoV infection but not disease so, Balb/C or Swiss Albino mice were used in the evaluation of the developed SARS-COV2 vaccine as previously reported [28]. It was reported that the Whole Killed vaccine convened more protection against pulmonary SARS-CoV replication in mice lung tissue vaccinated with 50 μg inactivated virus in 0.2 ml [21]. Other previous studies have shown that mice and hamsters that immunized IM twice with 3 weeks interval using doses of 0.5, 1.0, 1.5, and 2.0 μg SARS S-protein (5, 10, 15, or 20 μg of total protein) in the absence of adjuvant had higher serum neutralizing antibody titers than mice that had recovered from infection with 105 TCID50 of SARS-CoV [29]. Our results show that the candidate vaccine formulations induced significantly elevated antigen neutralizing antibody responses in the vaccinated mice with low and high doses, protecting them against SARS-CoV-2 infection. These were in agreement with previous results of development inactivated SARS-COVI 2 vaccines, BBIBP-CorV and PiCoVacc from China, and BBV152 whole virion inactivated vaccine [25, 28, 30, 31]. Alum. hydroxide gel which the most frequently used as vaccine adjuvant with an extensive safety record desired to have a COVID-19 vaccine that can generate both humoral and cell-mediated immune responses. The response generated from alum is primarily Th2-biased with the induction of strong humoral responses via neutralizing antibodies [32]. Although previous studies in mice have shown that low levels of neutralizing antibodies are sufficient to prevent detectable viral replication following challenge [29, 33]. In our study, the viral RNA was detected by Real-time PCR in mice lung tissues harvested from the unvaccinated mice post-infection as well as the mean SARS-CoV-2 titer of homogenized lung tissues in Vero cell culture from the control group were 102.5 TCID50/ml and 105.4 TCID50 at 3 and 7 days post-infection, respectively. Our data also demonstrate complete protection against the SARS-CoV-2 challenge by inhibition of virus replication in lung tissue post-challenge test. These results concluded that this vaccine will not cause antibody-dependent enhancement (ADE) as all the data obtained in this trial support the safety and immunogenicity [the ability to incite an immune response] of this inactivated vaccine. As well as replication of the challenge virus was not detected in the lung tissues of mice that received the vaccine. As ADE of virus infection is a phenomenon in which virus-specific antibodies enhance the entry of virus, and this occurs in case of nonsufficient antibodies that bind to the surface proteins but do not inactivate the virus [34].
These data were confirmed by the absence of histopathological findings in the lungs of vaccinated mice groups. The same finding was recorded in mice experimentally infected with SARS-COV and SARS-COV2 virus as virus challenge was successfully established in animal models [25]. It was reported that a combination of high neutralizing antibody titers elicited against inactivated antigen alone and the presence of intact spike protein on the surface of the virus confirms that the antigen is in the right formula and can itself may act as a Th1 inducer with its surface glycoproteins, intracellular viral proteins [30]. Therefore, inactivated SARS-CoV-2 candidate vaccine (EgyCoVax) described here provided a potential solution to fight against the COVID-19 pandemic and has desirable properties that support further development and studies for clinical trials.
DATA AVAILABILITY STATEMENT
Data are contained within the article can be available online.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
COMPLIANCE WITH ETHICS REQUIREMENTS
All Institutional and National Guidelines for the care and use of animals were followed.
REFERENCES
- 1.Xia, S, Duan, K, Zhang, Y et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020 Sep 8 2020; 324: 951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO coronavirus disease (COVID-19) dashboard. 2021. (30 July 2020, last accessed), [PubMed]
- 3.Tan, WJ, Zhao, X, Ma, XJ et al. A novel coronavirus genome identified in a cluster of pneumonia cases - Wuhan, China 20192020. China CDC Weekly 2020; 2: 61–2. [PMC free article] [PubMed] [Google Scholar]
- 4.Lu, R, Zhao, X, Li, J et al. Genomic characterization and epidemiology of 2019novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai, CC, Shih, TP, Ko, WC et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020; 55: 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, JF, Yuan, S, Kok, KH et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, N, Zhou, M, Dong, X et al. Epidemiological and clinical characteristics of 99cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Draft landscape of COVID-19 candidate vaccines. 2021. (30 July 2020, last accessed),
- 9.Zhu, FC, Li, YH, Guan, XH et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomized, first-in-human trial. Lancet 2020; 395: 1845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folegatti, PM, Ewer, KJ, Aley, PK et al. Oxford COVID vaccine trial group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet 2020; 396: 467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern, PL. Key steps in vaccine development. Ann Allergy Asthma Immunol 2020; 125: 17–27. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Fifteenth Laboratory biosafety guidance related to coronavirus disease (COVID-19), WHO/WPE/GIH/2020.3. laboratory‐biosafety‐guidance‐related‐to‐coronavirus‐disease‐(covid‐19), 2020
- 13.Ge, XY, Li, JL, Shi, ZHL. Isolation and characterization of bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013; 535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ksiazek, T, Erdman, D, Goldsmith, C et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1953–66. [DOI] [PubMed] [Google Scholar]
- 15.Ramakrishnan, M. Determination of 50% endpoint titer using a simple formula. World J Virol 2016; 5: 85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnella, M, Subbaraob, K, Feinstonea, S et al. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods 2004; 12: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darnell, M, Plant, E, Watanabe, H et al. Severe acute respiratory syndrome coronavirus infection in vaccinated ferrets. J Infect Dis 2007; 196: 1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson, E, Prince, T, Anderson, ER et al. Methods of inactivation of SARS-COV-2 for downstream biological assays. Infect Dis 2020; 222: 1462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Muttaqien, S, Mardiyati, E, Rahmani, S et al. Intraperitoneal acute toxicity of aluminum hydroxide nanoparticles as an adjuvant vaccine candidate in mice. J Pharmacol Toxicol 2020; 15: 22–35. [Google Scholar]
- 20.T. Tseng, E. Sbrana, N. Iwata-Yoshikawa N, et al. , Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus, Plos One, 7 (2012) pp. e35421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.See, R, Zakhartchouk, A, Petric, M et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol 2006; 87: 641–50. [DOI] [PubMed] [Google Scholar]
- 22.Zheng, B, Ng, M, Chan, K et al. A single dose of oral DNA immunization delivered by attenuated Salmonella typhimurium down-regulates transgene expression in HBsAg transgenic mice. Eur J Immunol 2002; 32: 3294–304. [DOI] [PubMed] [Google Scholar]
- 23.WHO guidelines on non-clinical evaluation of vaccines, 2005. No . 719 927, 2005. /biologicals/publications/trs/areas/vaccines/nonclinical_evaluation/ANNEX%201Nonclinical.721 P31–63.pdf), refer to section 4.2.2 (Developmental toxicity studies) pg 49-50.
- 24.Spruth, M, Kistner, O, Savidis-Dacho, H et al. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralizing and protective antibody responses. Vaccine 2006; 24: 652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao, Q, Bao, L, Mao, H et al. Rapid development of an inactivated vaccine for SARS-CoV-2. bioRxiv 2020; 369: 77–81. 10.1101/2020.04.17.046375 April 19, 2020, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calderao, A, Arcangeletti, MC, Conto, FD et al. SARS-COV-2 infection diagnosed only by cell culture isolation before the local outbreak in an Italian seven-week-old suckling baby. Inter J Inf Dise 2020; 96: 386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harcourt, J, Tamin, A, Lu1, X et al. Isolation and characterization of SARS-CoV-2 from the first US COVID-19 patient. bioRxiv 2020. March 03, 2020. 10.1101/2020.03.02.972935 preprint: not peer reviewed. [DOI] [Google Scholar]
- 28.Gao, Q, Bao, L, Mao, H et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020; 369: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisht, H, Roberts, A, Vogel, L et al. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology 2005; 334: 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganneru, B, Jogdand, H, Dharam, VK et al. Evaluation of safety and immunogenicity of an adjuvanted, TH-1 skewed, whole Virion inactivated SARS-CoV-2 vaccine - BBV152. bioRxiv 2020September 09, 2020. 10.1101/2020.09.09.285445 preprint: not peer reviewed. [DOI] [Google Scholar]
- 31.Wang, H, Zhang, Y, Huang, B et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 2020; 82: 713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He, P, Zou, Y, Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother 2015; 11: 477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapadia, S, Rose, J, Lamirande, E et al. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 2005; 340: 174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaume, M, Yip, MS, Cheung, CY et al. Anti‑severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH‑ and cysteine protease‑independent Fc R pathway. J Virol 2011; 85: 10582–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article can be available online.


