Abstract
Aims:
To retrospectively analyse the Bernese radiologically isolated syndrome (RIS) cohort with the goal of developing a prediction score for conversion to multiple sclerosis (MS).
Methods:
A total of 31 patients with RIS were identified by screening medical records of neurological patients seen at the University Hospital of Bern between 2004 and 2017 for the diagnoses ‘radiologically isolated syndrome’ and ‘RIS’ adhering to 2009 Okuda recommendations. We analysed clinical, paraclinical and magnetic resonance imaging data during a maximum follow-up period of 3 years and identified significant predictors of conversion to MS.
Results:
Data were available for 31 patients meeting 2009 Okuda RIS criteria. During the 3 years of follow up, 5/31 RIS patients converted to relapsing-remitting (RR) MS. In our univariate analysis, gadolinium (Gd) enhancement, brainstem and cerebellar hemisphere lesions, immune cell count and albumin concentration in cerebrospinal fluid (CSF), and anti-nuclear antibody (ANA) positivity in serum were identified as significant predictors of conversion to MS. Integrating these factors into our ‘RIS–MS prediction score’ enabled us to calculate a cut-off for prediction of conversion to MS within 3 years with high specificity [1.0, 95% confidence interval (CI) 0.84–1.00) and acceptable sensitivity (0.6, 95% CI 0.17–0.93)].
Conclusion:
Our RIS–MS prediction score, if validated in an independent cohort, integrating radiological (Gd enhancement, brainstem and cerebellar hemisphere lesions) and paraclinical factors (ANA in serum, cell count and albumin in CSF) could be a useful prognostic tool for early recognition of RIS patients with a high risk of clinical progression to MS.
Keywords: conversion, multiple sclerosis, prediction score, radiologically isolated syndrome
Introduction
The term ‘radiologically isolated syndrome’ (RIS) was first coined in 2009 by Okuda et al. and is applied to the incidental finding of lesions suggestive of multiple sclerosis (MS) during brain magnetic resonance imaging (MRI). 1 These lesions demonstrate dissemination in space and occur in subjects with a normal neurological examination and no history of typical MS symptoms. Since then, the risk of RIS evolving into MS has become probably the most studied RIS topic. Several independent observational cohort studies have shown that, within 5 years after detection of RIS, up to 30% of patients will experience a symptomatic demyelinating event and nearly two-thirds will progress radiologically with new lesions visible on magnetic resonance imaging (MRI).2–4 Various predictors of increased risk of MS or clinically isolated syndrome (CIS) conversion have been described, such as male sex, 4 infratentorial and spinal lesions,2–4 a higher absolute number of lesions, pathological visual evoked potentials, 3 a younger age at RIS diagnosis,3,4 CSF-specific oligoclonal bands (OCB), 5 a pathological cerebrospinal fluid (CSF) immunoglobulin G (IgG) index and recently also CSF neurofilament light chain concentration, measured by enzyme-linked immunosorbent assay.3,5 Recently, the consortium studying the epidemiology of RIS worldwide (RISC) presented their 10-year follow-up results, identifying age, infratentorial and spinal cord lesions, positive oligoclonal bands in CSF at baseline and the presence of gadolinium (Gd)-enhancing lesions during follow up as significant predictors for the development of a first clinical event. 6 However, despite the clinical need, there is so far no predictive score to estimate the risk of conversion of RIS to MS with a high enough specificity to avoid false negative results. The potential importance of early recognition of the subgroup of RIS patients at a high risk of developing MS is emphasized by existing data on CIS/MS. This data from multiple placebo-controlled trials shows that the early introduction of disease modifying therapies (DMTs) prevents future disability.7–12 As RIS can also convert to MS, an analogous situation can be assumed and a reduced conversion rate in RIS patients treated with DMT is likely. Two large multi-centre, double-blinded, randomised clinical trials (TERIS) in Europe, 13 and (ARISE) in the United States (US), 14 are ongoing to find out whether treatment of RIS reduces the risk of conversion to MS. The present study aimed to develop a clinical score to predict conversion of RIS to MS in a large monocentric cohort of patients with RIS.
Methods
A total of 31 patients with RIS were identified retrospectively by screening medical records of all patients seen at the neurological department of the University Hospital of Bern (Switzerland), a tertiary care centre, for the diagnoses ‘radiologically isolated syndrome’ and ‘RIS’ between 2004 and 2017. Diagnosis of RIS adhered to Okuda’s criteria (2009). 1 The following data, recorded during routine clinical examinations, were extracted for our study: five sociodemographic variables (female sex, age, abnormal neurological status at RIS-diagnosis, family history of MS, and of other autoimmune disease), four serological markers [anti-nuclear antibody (ANA) in serum, perinuclear anti-neutrophil cytoplasm antibodies (p-ANCA), cytoplasmic anti-neutrophil cytoplasm antibodies (c-ANCA) and cardiolipin IgG], five CSF parameters (CSF albumin, CSF protein, CSF immune cells, CSF-specific oligoclonal bands and IgG in CSF) and seven MRI parameters (Gd-enhancing lesions, cerebellar hemisphere lesions, periventricular lesions, brainstem lesions, juxtacortical lesions, deep white matter lesions and white matter lesion load). In an exploratory approach, we pre-selected the above-mentioned 23 sociodemographic, serological, CSF- and MRI-characteristics to be investigated as possible predictors of conversion to MS from all patient information given in the digital hospital information system. These variables were chosen because either they were investigated in previous studies or, in the opinion of the authors, could be biologically plausible factors of autoimmunity.
For coding of variables, we referred to the codebook (Supplemental material 1). CSF and serum parameters were analysed in the main laboratory of the University Hospital Bern, Switzerland. MRIs were acquired at the University Hospital Bern using 3 and 1.5 Tesla MRI scanners (Siemens Healthcare, Erlangen, Germany) following our in-house standard operating procedures (see Supplemental material 2). The involvement of the following regions of the central nervous system in the MRI was assessed qualitatively: corpus callosum, brainstem and cerebellar hemisphere, juxtacortical and periventricular. Gd-enhancing lesions were identified on post-contrast T1-weighted scans. MS diagnosis followed the 2010 revised McDonald criteria. 12 Patients were followed up until conversion to MS or, in non-converters, for a maximum period of 3 years. The time to conversion to MS was assessed by calculating the interval between the date of the MRI from which RIS was diagnosed and the date of conversion.
Statistical analysis
Ordinal or continuous variables are presented as median and 25th–75th percentiles, whereas dichotomous variables are presented as frequencies. An exception to this rule was made for sensitivity, specificity and area under the curve (AUC), which are presented as mean and standard deviation (SD). Sensitivity and specificity were calculated using the ‘VassarStats research calculator’ (Poughkeepsie, NY, USA). All other analyses were performed with SPSS 25 (IBM, Armonk, NY, USA).
The significant predictors of conversion to MS in RIS patients were identified statistically using Kaplan–Meier log rank statistic for comparison of two groups. Therefore, classification of variables was necessary prior to analysis. Here, continuous variables with biological plausible cut off values for pathological values were classified using the upper limit of the respective reference range, e.g., CSF protein concentration >0.4 g/l. Continuous variables without such a given cutoff, like age, were analysed using receiver operating characteristic curves to identify the value which differentiates best between converters and non-converters. Finally, all significant predictors of conversion to MS were integrated into a score called ‘the RIS–MS prediction score’.
For this purpose, the significant predictors were added up by their chi-squared value. This sum was divided by the number of available tests in order to adjust for missing variables in some patients. Again, an ROC analysis was performed to identify the optimal cut-off to differentiate between RIS patients who convert to MS and those who do not. Specificity and sensitivity were then calculated together with 95% confidence intervals (CIs). The aim of this mathematical procedure was to be able to predict a high likelihood of RIS conversion to MS to develop a tool that would justify early initiation of immunotherapy. Therefore, specificity was rated more important than sensitivity to keep the rate of false positives low.
Ethical approval
This study was approved by the responsible cantonal ethics committee (registration no. KEK-BE 2017-01369). Because of the retrospective nature of the analysis with pseudonymised patient data, separate informed consent was waived by the committee. This corresponds to the local legislation. For patients seen after the introduction of the general consent (February 2015), the presence of the patients’ consent was checked before inclusion in the analysis.
Results
Patient population and conversion to MS
Retrospective analysis of medical records identified 31 patients fulfilling the 2009 Okuda criteria who were diagnosed in University Hospital Bern, Switzerland between 2004 and 2017 (Table 1). Within the follow-up period of up to 3 years, 5 of these RIS patients (16.1%) converted to MS. The times of conversion were distributed as follows: first year: 4/31; second year: 0/27; third year 1/27.
Table 1.
Baseline characteristics.
| Baseline characteristics | Median | IQR | n | Missing |
|---|---|---|---|---|
| Age at RIS diagnosis (years) | 41.0 | 24.0 | 31/31 | 0 |
| CSF cell count per µl | 1.0 | 3.0 | 27/31 | 0 |
| CSF protein (g/l) | 0.33 | 0.16 | 27/31 | 0 |
| CSF albumin (mg/l) | 210 | 101.5 | 26/31 | 0 |
| CSF IgG (mg/l) | 26.0 | 22.0 | 26/31 | 0 |
| Time to MS conversion (years) | 0.67 | 2.41 | 5/31 | 0 |
| Follow up in non-converters a (years) | 0.58 | 1.42 | 22/26 | 4 |
| % | N | |||
| Female | 67.7 | 21/31 | 0 | |
| Abnormal neurological status at RIS b | 9.7 | 3/31 | 0 | |
| Frequency of CSF analysis | 87.10 | 27/31 | 0 | |
| CSF-specific OCB (+) | 36.0 | 9/25 | 6 | |
| ANA positivity (serum) | 32.3 | 10/26 | 5 | |
| c-ANCA positivity (serum) | 0.0 | 0/31 | 0 | |
| p-ANCA positivity (serum) | 0.0 | 0/27 | 4 | |
| Cardiolipin IgG positivity (serum) | 0.0 | 0/16 | 15 | |
| Number of cMRIs performed | 100 | 31/31 | 0 | |
| MRI lesion location | ||||
| Lesions in corpus callosum | 67.7 | 21/31 | 0 | |
| Infratentorial | 45.2 | 14/31 | 0 | |
| Gd enhancement | 29.0 | 9/31 | 0 | |
MS conversion was defined using clinical and MRI parameters and applied the 2010 McDonald criteria (see Methods).
Unrelated to RIS and not suggestive of MS, see Supplemental material 3.
ANA, anti-nuclear antibodies; c-/p-ANCA, cytoplasmic/perinuclear anti-neutrophil cytoplasm antibodies; cMRI, magnetic resonance imaging of the cerebrum; CSF, cerebrospinal fluid; Gd, gadolinium; IQR, interquartile range; n, number of observations; OCB, oligoclonal bands.
Role of sociodemographic characteristics and disability in RIS patients converting to MS
To identify sociodemographic factors associated with conversion to MS in our RIS cohort, Kaplan–Meier curves were plotted and log rank tests performed for sex (female 21/31), age (<40 years 16/31), abnormal neurological findings at RIS diagnosis (3/31), family history of MS (4/31) and family history of any other autoimmune disease including MS (7/31). Within this set of variables, only female sex demonstrated a trend towards earlier and more frequent conversion to MS (Table 2). Three of our patients formally had an abnormal neurological exam at diagnosis of RIS. However, none of the abnormal findings were related to this diagnosis and they were not suggestive of MS (for details, see Supplemental material 3). Fittingly, abnormal neurological findings at RIS diagnosis did not reach statistical significance in further analysis (Table 2).
Table 2.
Log-rank test statistic.
| Variable | χ2 | p value | N |
|---|---|---|---|
| Sociodemographic variables | |||
| Female sex | 3.04 | 0.08 | 31 |
| Age (⩽40 versus >40 years) | 1.91 | 0.17 | 31 |
| Abnormal neurological status at RIS (no versus yes) | 1.26 | 0.26 | 31 |
| Family history of: | |||
| MS | 0.05 | 0.82 | 31 |
| Any other AID | 1.26 | 0.26 | 31 |
| Serological immune parameters | |||
| ANA positivity (serum) | 4.66 | 0.03 | 26 |
| CSF parameters | |||
| Elevated albumin (>300 mg/l) | 5.15 | 0.02 | 26 |
| Elevated protein (>0.4 g/l) | 0.33 | 0.56 | 27 |
| Elevated immune cells (>5 M/l) | 8.42 | 0.004 | 27 |
| CSF-specific OCB (positive) | 3.32 | 0.07 | 25 |
| Elevated IgG (⩾40 mg/l) | 0.47 | 0.49 | 26 |
| MRI parameters | |||
| Callosal lesion (yes) | 0.005 | 0.943 | 31 |
| Gadolinium enhancement (yes) | 8.158 | 0.004 | 31 |
| Lesion load WM (⩽10 versus >10) | 1.07 | 0.301 | 31 |
| Cerebellar hemisphere lesions | 14.727 | <0.001 | 31 |
| Periventricular lesions | 0.09 | 0.761 | 31 |
| Brainstem lesions | 9.847 | 0.002 | 31 |
| Juxtacortical lesions | 1.787 | 0.181 | 31 |
| Deep WM lesions | 0.426 | 0.514 | 31 |
| RIS–MS prediction score | 28.82 | <0.001 | 31 |
AID, autoimmune disease; ANA, anti-nuclear antibody; CSF, cerebrospinal fluid; Ig, immunoglobulin; MRI, magnetic resonance imaging; MS, multiple sclerosis; OCB, oligoclonal bands; RIS, radiologically isolated syndrome; WM, white matter.
Values in bold indicate variables predicting conversion to ms.
Role of serological immune parameters in RIS patients converting to MS
Analyses were performed for p-ANCA in 27 patients, c-ANCA in 31, cardiolipin IgG in 16 and ANA in 26 of the 31 RIS patients. Two of the ANA-positive subjects presented autoimmune comorbidities [psoriasis (n = 1), relapsing uveitis (n = 1)]. Except ANA positivity (10/26), no other immunological parameter was detectable in any of the RIS patients (Table 1). Moreover, ANA positivity also predicted RIS conversion to MS (Table 2).
Role of CSF parameters in RIS patients converting to MS
CSF data was available for 27 of the 31 RIS patients. Analysis of albumin concentration (26/27), protein concentration (27/27), IgG concentration (26/27), OCBs (25/27) and number of immune cells in the CSF (27/27) showed that only CSF immune cell count and CSF albumin concentration demonstrated significant potential for predicting conversion to MS (Table 1).
Role of MRI parameters in RIS patients converting to MS
MRI of the cerebrum with T1 post-contrast sequences was obtained for all (31/31) patients. The presence of Gd enhancement, lesions in the corpus callosum, cerebellar hemisphere, brainstem, periventricular lesions, deep white matter and juxtacortical lesions, as well as the absolute number of lesions dichotomised according to whether there were ⩽10 or >10 was assessed. Of these parameters, Gd enhancement, cerebellar hemisphere lesions and brainstem lesions were associated with a risk of conversion to MS in survival analysis (Table 1).
RIS–MS prediction score
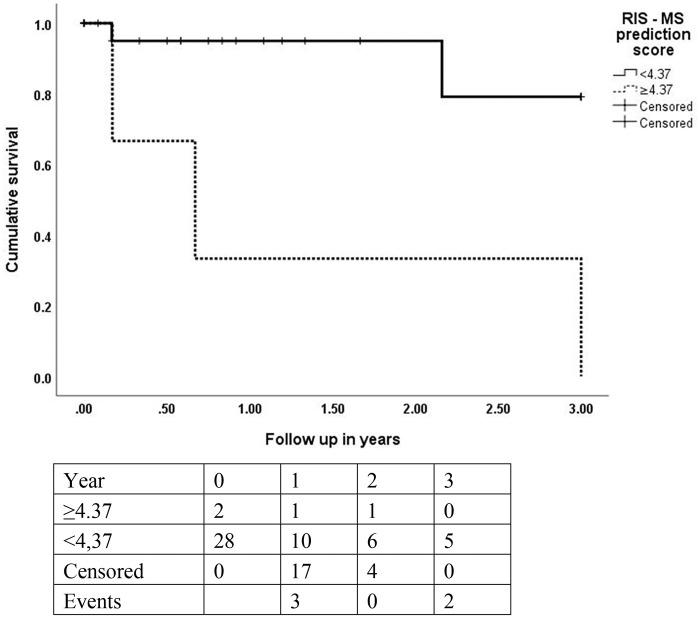
All six significant predictors of conversion to MS were included in the RIS–MS prediction score (Table 2). Running a ROC analysis demonstrated that a cut-off of >4.37 points had the highest specificity (1.0, 95% CI 0.84–1.00) and an acceptable sensitivity (0.6, 95% CI 0.17–0.93) for prediction of RIS conversion to MS (Figure 1). Furthermore, the survival analysis yielded a significant difference of RIS patients converting to MS over a maximum follow up of 3 years between those above and those below the cut-off value of 4.37 (Figure 2).
Figure 1.
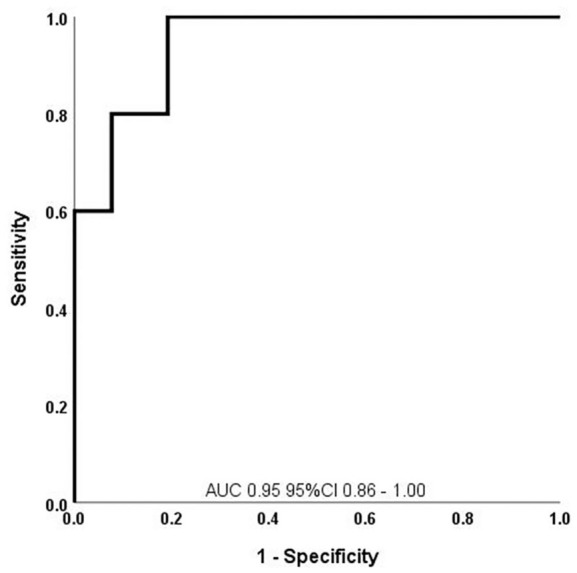
ROC analysis to predict conversion to MS in RIS patients using the RIS–MS prediction score.
AUC, area under the curve; CI, confidence interval; MS, multiple sclerosis; RIS, radiologically isolated syndrome; ROC, receiver operating characteristic.
Figure 2.
Kaplan–Meier curve after stratification by RIS–MS prediction score (cut-off ⩾4.37 points): conversion to MS within 3 years; statistic, log rank test: p value = 0.004.
MS, multiple sclerosis; RIS, radiologically isolated syndrome.
Discussion
Identifying accurate predictors of the risk of RIS patients experiencing a clinical event and progressing to MS is a considerable challenge. The ability to define these risk predictors could be particularly useful because treatment with disease-modifying agents may be more beneficial for RIS patients before conversion to MS. To answer the question, if RIS patients should be treated with DMT two large multi-centre, double-blind, randomised clinical trials (TERIS) in Europe and (ARISE) in the US are ongoing.13,14 However, a potential therapy of RIS patients is only feasible if these predictors can be defined accurately given that RIS patients are an especially vulnerable population as they are free of MS symptoms. Thus, false positive predictions should be avoided to prevent unnecessary medical testing, treatment and psychological stress.
Against this background, we tried to develop and statistically design a prediction score focussing on high specificity. Univariate analysis identified six significant predictors of conversion of RIS to MS that were included in the RIS–MS prediction score (variables: Gd enhancement, brainstem and cerebellar hemisphere lesions, CSF immune cell count, CSF albumin concentration and ANA positivity of serum). With a cut-off of ⩾4.37 points, this score can predict the conversion of RIS to MS with a specificity of 1.0 (95% CI 0.84–1.00) and a sensitivity of 0.6 (95% CI 0.17–0.93).
Looking at the six predictors in more detail, infratentorial involvement seen on baseline imaging, and known to be important in CIS, 15 was predictive of RIS conversion to MS in our cohort too, as we found brainstem and cerebellar hemisphere lesions to be significant risk predictors. Infratentorial lesions have long been considered typical of MS and this topography was incorporated into the Fazekas and the Barkhof criteria and, more recently, the MAGNIMS consensus criteria. Lebrun et al. 2 and Okuda et al. 3 recognized infratentorial lesions as a possible prognostic factor for clinical progression in RIS patients. Furthermore, a recent publication of the 10-year follow up of the consortium studying the epidemiology of RIS worldwide (RISC) identified infratentorial lesions as a predictor for the development of a first clinical event. 6 Thus, our findings fit well with the literature.
Gd enhancement in brain lesions reflects disturbances of the blood–brain barrier (BBB) with high sensitivity. Disruption of the BBB is an early event in the development of inflammatory lesions in MS and a robust predictor of the occurrence of relapses. 16 Lebrun et al. 2 demonstrated that RIS patients with Gd-enhancing lesions in baseline imaging had a substantially increased risk of developing new lesions, which we also found in our study. In another study the presence of gadolinium-enhancing lesions during follow up was also associated with the risk of first clinical event. 6 Furthermore, in accordance with the 2017 McDonald criteria, the finding of enhancing lesions in combination with non-enhancing lesions meets the criterion of dissemination in time, even in the first MRI scan, independently of clinical symptoms. 17
The prognostic role of CSF parameters in predicting conversion of RIS to MS was studied in 451 RIS patients. The authors identified a pathological CSF IgG index or the presence of CSF-specific OCB as risk factors. 4 Our finding that elevated CSF immune cell count and elevated CSF albumin concentration may predict conversion of RIS to MS emphasizes the importance of CSF analysis. Our findings are in line with those of Lotan et al., who studied a cohort of MS patients with CSF pleocytosis. 18 These patients had a higher annualised relapse rate and a steeper slope of the Expanded Disability Status Scale score throughout the follow-up period. We suggest that this finding in MS patients can also be applied to RIS patients, as our data showed a higher likelihood of conversion to MS in RIS patients with CSF pleocytosis. The elevated CSF albumin levels reflect disturbances of the BBB and is in line with our above-mentioned finding that Gd enhancement in MRI may also play a predictive role. In our study, CSF OCB showed a trend at a 90% level of significance towards early conversion. Interestingly, another study also found no statistically significant predictive value of CSF OCB. 2
In our study, elevated CSF albumin predicted RIS conversion to MS, as did a pathological albumin quotient. However, the pathological albumin quotient showed a weaker correlation to RIS conversion compared with elevated CSF albumin (p = 0.031 versus p = 0.02) and a lower specificity (0.86, 95% CI 0.63–0.96 versus 0.95, 95% CI 0.74–0.9975). As both variables have a strong correlation (Pearson correlation coefficient 0.99 p < 0.001), it is not possible to include both of them in our score. Therefore, we chose to include elevated CSF albumin in our score and to omit a pathological albumin quotient as another potential variable, as in this study we rated specificity more important than sensitivity to keep the rate of false positives low.
Finally, the presence of ANA in our RIS cohort is noteworthy. Collard et al. observed that ANA-positivity was more frequent in MS patients than in healthy controls and suggested an association with MS disease activity. 19 These investigators hypothesized that ANA is a product of systemic immune dysregulation, which is known to occur not only in systemic autoimmune diseases but also in MS. Further, they postulated that the presence of ANA might be a marker of disease activity or of response to therapy, which is also supported by the work of Spadaro et al. 20 ANA also occurred commonly in patients with RIS. 21 However, ANA are generally thought to be a nonspecific and unimportant marker of autoimmunity in people with MS. In our analysis, we found ANA positivity as a risk factor for conversion of RIS to MS. In 26 patients with ANA measurements, 10 patients had positive ANA values. Of those 10 patients, 4 patients eventually converted from RIS to MS, whereas 6 did not. Therefore, ANA positivity was 2.8 times more frequent in converters than in non-converters in our cohort. Due to the small number of patients, the ANA titre did not reach a statistical significance as a risk factor for conversion of RIS to MS in our cohort. If we go back and look at the literature, Collard et al. found ANA in a frequency of 22.5% in their MS cohort and in 16 patients tested twice, ANA occurrence also correlated with MS disease activity. 19 They denominated the ANA in terms of disease specificity as ‘false positive’ as they are not associated directly with MS. However, ANA might reflect the ‘non-sense’ antibody production 19. We hypothesize that presence of ANA might be indicative for the state of activation of the dysregulated immune system in people with MS. However, as mentioned before, more than the association between positive and negative ANA and RIS conversion cannot be shown by our data due to overall small number of cases. In our opinion, in addition to obvious markers of disease activity, matching of multicentric study cohorts to increase sample size and inclusion of broader non-CNS specific markers of autoimmunity could be highly valuable in studies about RIS. We therefore hope that our article, with all the limitations of a retrospective study and small sample size, stimulates researchers to join forces to investigate immunological markers, like ANA, on their predictive value for conversion to MS in people with RIS. The ongoing prospective clinical trials, to which our centre also contributes in terms of the TERIS study, would give an ideal opportunity to conduct broader immunological studies in people with RIS. Thus, further research in this field is needed.
Our study has several significant limitations. First, it was a retrospective, non-standardized study resulting in different intervals and examinations/paraclinical tests for clinical RIS surveillance in routine care.
Another significant limitation is the absence of spinal MRI in the analysis, which was shown to be an important predictor of conversion to MS in other studies, including the largest RIS series by Okuda et al. with 451 patients. 4 Due to the retrospective, non-standardized nature of our study, this data was missing in the majority of our patients (29/31), as a spinal MRI was not performed routinely in our centre at that point in time.
Thirdly, four patients who did not convert to MS during the study period were lost to follow up. Furthermore, our maximal follow-up period was only 3 years, so we cannot rule out the possibility that patients with RIS converted to MS later on. The short follow-up period is because a significant number (12 of 31) of the included subjects was diagnosed with RIS in 2017 or at the end of 2016; therefore, at the time this manuscript was written (2020) follow up was only available for up to 3 years for these patients.
As we studied a monocentric cohort with a small number of subjects, our statistical results need verification in a larger, multicentric cohort especially as, in total, only 5 of 31 patients converted from RIS to MS. Thus, caution in the interpretation of our data is required, which is reflected in the fact, that key risk factors previously identified in larger RIS studies did not reach statistical significance or where not included in our study due to lack of data (such as spinal cord lesions). Moreover, the results are based on a univariate analysis which is statistically weaker, compared with multivariate analysis. However, as for example spinal lesions (representing one of the major risk factors) were not included into the statistical procedure, our data could potentially be compared with univariate analyses of other RIS cohort studies. Therefore, standardized studies with a larger number of patients are needed to verify our findings and help improve estimates of risk as standardized diagnosis and treatment of RIS patients is crucial.
Lastly, two of the variables identified as significant risk factors for conversion to MS (ANA positivity and high CSF albumin) lack specificity for CNS demyelination from a biological point of view. Concerning elevated CSF albumin, a possible explanation for our results is that this value reflects disturbances of the BBB, which is in line with our finding that Gd enhancement in MRI may also play a predictive role. Nonetheless, to the best of our knowledge, no other study has investigated these variables as possible predictors of conversion to MS.
In conclusion, in our monocentric cohort, our RIS–MS prediction score, which consists of readily available MRI variables (Gd enhancement, brainstem and cerebellar hemisphere lesions), CSF variables (CSF immune cell count and CSF albumin concentration) and serum variables (ANA) allowed prediction of conversion of RIS to MS with high specificity and acceptable sensitivity. The present study has an exploratory character and several limitations inherent to the retrospective nature, small sample size and investigated number of variables with the possibility that our observations might be coincidental. Thus, our work should not be regarded as a stand-alone diagnostic tool for prediction of MS-risk in RIS, but rather serve as an input for further research in the field of RIS to predict conversion to MS by using multiple domain prediction scores. Therefore, the score presented here should first be verified by independent cohorts, and in the future a validated prediction score could help to identify the patients who need a more careful monitoring for conversion to MS and therefore, help in deciding which patients might benefit from initiation of early treatment, as no approved therapeutic options are currently available.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211030664 for Predicting conversion to multiple sclerosis in patients with radiologically isolated syndrome: a retrospective study by Panagiotis Chaloulos-Iakovidis, Franca Wagner, Lea Weber, Lara Diem, Andrew Chan, Anke Salmen, Christoph Friedli and Robert Hoepner in Therapeutic Advances in Neurological Disorders
Footnotes
Author contributions: RH and PC-I were involved in the conception and design of the study. RH and CF organized the database. PC-I performed the data extraction. RH performed the statistical analysis. PC-I, RH, CF and FW analysed and interpreted the patient data. PC-I, CF and RH were major contributors in writing the manuscript. All authors contributed to manuscript revision, read and approved the final manuscript.
Conflict of interest statement: PC-I, FW and LW report no disclosures or conflicts of interest in relation to this manuscript.
LD has received travel grants from Bayer, Biogen, Roche and Merck, as well as speaker honoraria from Merck and Biogen, not related to this work. She reports no conflicts of interest related to this manuscript.
AC has served on advisory boards for, and received funding for travel or speaker honoraria from, Actelion-Janssen, Almirall, Bayer, Biogen, Celgene, Sanofi-Genzyme, Merck, Novartis, Roche and Teva, all for hospital research funds; and research support from Biogen, Genzyme and UCB. AC is associate editor of the European Journal of Neurology and serves on the editorial board for Clinical and Translational Neuroscience and as topic editor for the Journal of International Medical Research. He reports no conflicts of interest related to this manuscript.
AS has received speaker honoraria and/or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche and Sanofi Genzyme, and research support of the Swiss MS society not related to this work. She reports no conflicts of interest related to this manuscript.
RH has received research and travel grants from Roche, Novartis and Biogen Idec and speaker honoraria from Biogen, Novartis, Merck, Celgene and Almirall, not related to this work. He reports no conflicts of interest related to this manuscript.
CF has received travel grants from Biogen and Sanofi Genzyme, as well as speaker honoraria from Biogen and Merck, not related to this work. He reports no conflicts of interest related to this manuscript.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chaloulos-Iakovidis Panagiotis  https://orcid.org/0000-0002-4620-9155
https://orcid.org/0000-0002-4620-9155
Diem Lara  https://orcid.org/0000-0001-6171-5761
https://orcid.org/0000-0001-6171-5761
Salmen Anke  https://orcid.org/0000-0002-4751-299X
https://orcid.org/0000-0002-4751-299X
Hoepner Robert  https://orcid.org/0000-0002-0115-7021
https://orcid.org/0000-0002-0115-7021
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Panagiotis Chaloulos-Iakovidis, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Freiburgstrasse, Bern, CH-3010, Switzerland.
Franca Wagner, Department of Neuroradiology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
Lea Weber, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
Lara Diem, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
Andrew Chan, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
Anke Salmen, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
Christoph Friedli, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
Robert Hoepner, Department of Neurology, Inselspital, Bern University Hospital, and University of Bern, Bern, Switzerland.
References
- 1. Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 2009; 72: 800–805. [DOI] [PubMed] [Google Scholar]
- 2. Lebrun C, Bensa C, Debouverie M, et al. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Arch Neurol 2009; 66: 841–846. [DOI] [PubMed] [Google Scholar]
- 3. Okuda DT, Mowry EM, Cree BA, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011; 76: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One 2014; 9: e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matute-Blanch C, Villar LM, Álvarez-Cermeño JC, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018; 141: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 6. Lebrun-Frenay C, Kantarci O, Siva A, et al. Radiologically Isolated Syndrome: 10-Year Risk Estimate of a Clinical Event. Ann Neurol 2020; 88: 407–417. [DOI] [PubMed] [Google Scholar]
- 7. Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000; 343: 898–904. [DOI] [PubMed] [Google Scholar]
- 8. Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006; 67: 1242–1249. [DOI] [PubMed] [Google Scholar]
- 9. Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009; 374: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 10. Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 977–986. [DOI] [PubMed] [Google Scholar]
- 11. Leist TP, Comi G, Cree BA, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol 2014; 13: 257–267. [DOI] [PubMed] [Google Scholar]
- 12. Edan G, Kappos L, Montalbán X, et al. Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT. J Neurol Neurosurg Psychiatry 2014; 85: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ClinicalTrials.gov. Multi-center, randomized, double-blinded assessment of teriflunomide in extending the time to a first clinical event in radiologically isolated syndrome (RIS) The TERIS study, https://clinicaltrials.gov/show/NCT03122652. NLM identifier: NCT03122652 (accessed 1 November 2020).
- 14. ClinicalTrials.gov. A multi-center, randomized, double-blinded assessment of dimethyl fumarate in extending the time to a first attack in radiologically isolated syndrome (RIS) (ARISE Trial), https://clinicaltrials.gov/show/NCT02739542. NLM identifier: NCT02739542 (accessed 1 November 2020).
- 15. Tintore M, Rovira A, Arrambide G, et al. Brainstem lesions in clinically isolated syndromes. Neurology 2010; 75: 1933–1938. [DOI] [PubMed] [Google Scholar]
- 16. Kappos L, Moeri D, Radue EW, et al. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Gadolinium MRI Meta-Analysis Group. Lancet 1999; 353: 964–969. [DOI] [PubMed] [Google Scholar]
- 17. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 18. Lotan I, Benninger F, Mendel R, et al. Does CSF pleocytosis have a predictive value for disease course in MS? Neurol Neuroimmunol Neuroinflamm 2019; 6: e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collard RC, Koehler RP, Mattson DH. Frequency and significance of antinuclear antibodies in multiple sclerosis. Neurology 1997; 49: 857–861. [DOI] [PubMed] [Google Scholar]
- 20. Spadaro M, Amendolea MA, Mazzucconi MG, et al. Autoimmunity in multiple sclerosis: study of a wide spectrum of autoantibodies. Mult Scler 1999; 5: 121–125. [DOI] [PubMed] [Google Scholar]
- 21. Gabelić T, Radmilović M, Posavec V, et al. Differences in oligoclonal bands and visual evoked potentials in patients with radiologically and clinically isolated syndrome. Acta Neurol Belg 2013; 113: 13–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211030664 for Predicting conversion to multiple sclerosis in patients with radiologically isolated syndrome: a retrospective study by Panagiotis Chaloulos-Iakovidis, Franca Wagner, Lea Weber, Lara Diem, Andrew Chan, Anke Salmen, Christoph Friedli and Robert Hoepner in Therapeutic Advances in Neurological Disorders