Abstract
Background:
Prematurity presents a diagnostic challenge in interpreting primary immunodeficiency (PID) testing.
Methods:
We retrospectively reviewed the charts of all infants in our level IV referral NICU in Massachusetts with immunologic testing performed from 2006–2018.
Results:
The overall rate of PID testing was enriched in our population, with 1% of admitted patients having extended immunologic testing. The addition of TREC newborn screening in Massachusetts in 2009 increased the proportion of infants tested for PID in our NICU by 3-fold (1.21% post-NBS vs 0.46% pre-NBS). A majority of the term and late preterm (T/LP, ≥ 34 weeks) infants (31 of 41, 76%) and very premature (VP, 29–33 weeks) infants (12 of 17, 71%) who had immune testing had a genetic diagnosis associated with secondary immunodeficiency or a PID. Most infants who were born extremely premature (EP, < 29 weeks) (25 of 29, 86%) had no identifiable cause of immunodeficiency besides prematurity, despite a mean postmenstrual age of 40.1 weeks at the time of testing.
Conclusion:
Persistent immune derangements were present within a subgroup of the extremely premature population through term postmenstrual age. EP infants with significant infectious history and abnormal immune testing at term-corrected age should be considered for genetic testing.
Introduction
Primary immunodeficiency disorders (PID, also referred to as inborn errors of immunity/IEI) are a heterogeneous group of genetic diseases that cause dysfunction of the immune system (1). They typically present with an increased frequency or severity of infections, and those that cause the most severe immune dysfunction generally present in infancy. Diagnosing PID, and particularly severe combined immunodeficiency (SCID), as early as possible improves clinical outcomes (2–4). The premature population presents a challenge for diagnosing PID given a state of relative immunocompromise when compared with term infants and adults (5–11). In addition, premature infants typically have comorbidities and tend to be critically ill, which can further alter immunologic function and composition. These infants also frequently have in-dwelling central lines and endotracheal tubes, both of which predispose to infections. Lastly, given these confounding factors, identifying clinical features that warrant immune investigation in this population is challenging.
Recent studies have shown that premature infants appear to have lasting delays in T cell maturation when compared with term infants (12), suggesting that prematurity may cause persistent impairments in immunity. It is not known whether the incidence of PID in premature infants is equivalent to that of term born infants. Immunodeficiency on the part of the baby could lead to an abnormal uterine immune interface that actually increases the risk of premature birth in the setting of PID, this is unknown. There are many considerations that need further clarity.
While newborn screening for severe combined immunodeficiency (SCID) is undoubtedly beneficial for term infants, it is difficult to interpret in premature infants, and frequently leads to false positive or equivocal results in this population (13). In this study, we examined the results of immunologic testing in premature infants with abnormal newborn screens as well as those that were tested based on clinical concern in an effort to determine the best way to identify preterm infants likely to have clinically significant immunocompromise. We retrospectively reviewed all of the medical charts of any patient admitted to our level IV NICU who had undergone any type of immunologic testing (beyond a CBC) during their admission from 2006 through 2018. It should be noted that newborn screening (NBS) for severe combined immunodeficiency (SCID) using T cell receptor excision circles (TRECs) was implemented in 2009 in Massachusetts. While TREC screening is designed to be sensitive for SCID, it is not specific and also can detect other forms of T cell lymphopenia, however, it does not detect all forms of immunodeficiency.
Methods
This research protocol was approved by the IRB at Boston Children’s Hospital, protocol IRB-P00029621. The research was carried out under the ethical principles of the Belmont Report and in accordance with the Declaration of Helsinki. The study was granted an informed consent waiver by our IRB; consent was not required.
Patient selection:
We queried the medical record to identify all patients who had any of the following tests performed during the time of admission to the NICU: Immunoglobulins (IgG, IgA, IgM, or IgE), T/B/NK cell panels, T cell memory panels, B cell memory panels, T cell mitogen testing, T cell antigen testing, or neutrophil oxidative burst assays. We assumed anyone who then underwent any more specific immune testing (genetic testing, etc) would be captured by querying these categories.
Data collection:
For each patient, we collected demographic and clinical information including gestational age, age at immune testing, results of immune testing, diagnoses and underlying conditions, reasons for immune testing, TREC results, recommendations from the immunology consult team if a consult was obtained, and disposition of the patient at time of discharge.
NICU statistics:
NICU census and admission data from 2006 and 2007 was not available. Admission data from 2008–2018 was obtained from our historical database. Since admissions were relatively consistent over time (average 651.9, std dev 51.6) and they size of the unit was stable, we estimated the 2007 admission number to be 652, in order to calculate the percentage of infants referred for testing in that year.
Results
A total of 119 neonates met the criteria for inclusion in the study (Fig. 1A, Table 1). Of those, thirty-two infants had only testing for IgG without any other studies, typically done for consideration of immunoglobulin replacement in the setting of chylous effusions or in patients with congenital heart disease. Because there is a weak evidence basis for this practice (14), and because no additional immune studies were done to give further insight, we did not consider these patients further in our assessment of testing for PID. Also, any infant with a true clinical concern for an immunologic problem would have had additional testing done, such as flow cytometry. Outside of infants with an IgG only, there were eighty-seven infants who had immunologic testing due to concern for PID. During the years of the study, admissions to the NICU and average census remained relatively stable (Fig. 1B). Forty-three patients (49%) of those tested for concern for PID were tested based on a clinical history of concern for infection, the remainder (n=44, 51%) were tested primarily due to low T cell receptor excision circles (TRECs) on NBS (Fig. 1C). The proportion of PID testing done for TREC screening rather than clinical indications increased over time. Nine of the patients tested for PID based on clinical scenario were born prior to the initiation of NBS for SCID in Massachusetts (2009). TREC screening did increase the rate of additional immunologic testing in the NICU by roughly 2-fold. The pre-TREC screening proportion of immune testing was 0.53% (9 patients out of an estimated 1965 admissions), the post-TREC screening rate was 1.02% (78 patients out of confirmed 7171 admissions).
Figure 1. Study Population.
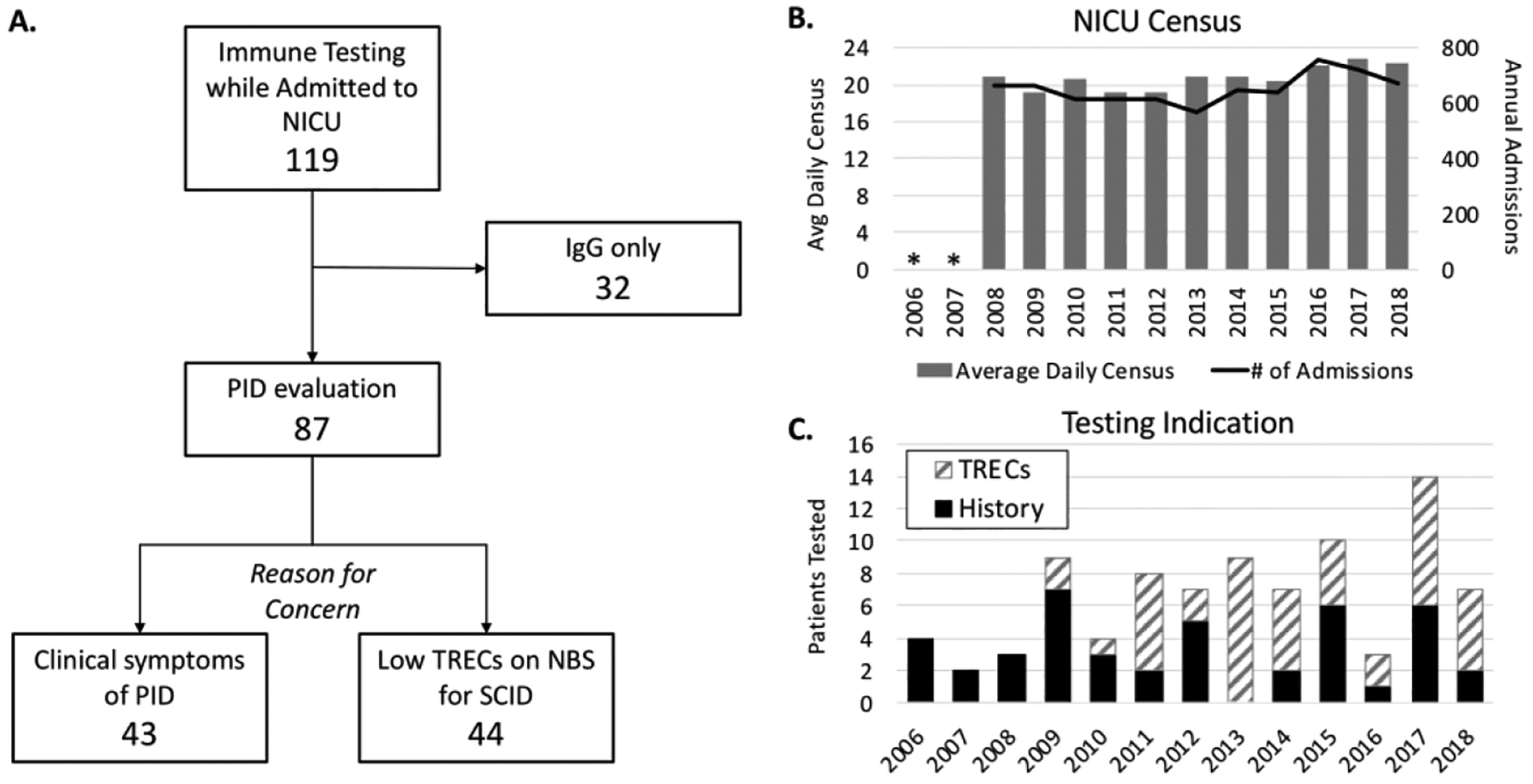
A. Study inclusion and evaluation numbers. B. Total NICU census over study period (admission data for 2006 and 2007 not available). C. Number of patients with immune testing per year, broken down by indication for testing. PID - primary immunodeficiency, TRECs – T cell receptor excision circles, SCID – severe combined immunodeficiency.
Table 1.
Overview of PID testing by gestational age group.
| Gestational age group | Mean GA at birth (wk) | Mean PMA at testing (wk) | Testing indication | Total | |
|---|---|---|---|---|---|
| Clinical History | Low TRECs | ||||
| Extremely premature (<29 wk) | 25.8 | 40.1 | 15 | 14 | 29 |
| Very Premature (29–<34wk) | 30.8 | 38.8 | 11 | 6 | 17 |
| Late Premature or Term (34wk or more) | 37.4 | 42.5 | 17 | 24 | 41 |
Prematurity is a known condition of relative immunodeficiency and is associated with false positive newborn screening for SCID (13, 15). We therefore sub-analyzed the patients evaluated for PID by gestational age at birth. Of the cohort of patients evaluated for possible PID, twenty-nine were extremely premature (EP, < 29 weeks GA at birth). Of these, fifteen were referred for immune testing due to clinical scenarios and the remaining fourteen were due to low TRECs on newborn screening (Table 1, Table S1). Of the fifteen EP patients that were tested due to low TRECs, many had a significant infectious history, however most of these patients had reassuring flow cytometry not suggestive of PID (Table S2). Patient P-5 had idiopathic T cell lymphopenia that persisted through 3 years of age. Another patient, P-18 had hepatic and cerebral abscesses, and similarly had low recent thymic emigrants, low CD4 T cells, and a poor response to anti-CD3 mitogen stimulation. This anti-CD3 response was judged to be due to lab artifact, and PID risk was considered low. Repeat testing was recommended at 4 months of age, however the patient ultimately died from liver failure in setting of hepatic abscess and multiple comorbidities.
There were fifteen EP Patients that had immunologic evaluation done due to clinical concerns. Compared to the patients tested due to low TRECs, these patients were born at a slightly later gestational age, 26 2/7 weeks compared to 25 1/7 weeks for the infants screened based on TRECs. They were also older at the time of testing (43 2/7 weeks PMA vs. 37 1/7 weeks for patients with low TRECs) Of these infants, patient P-4 had a known diagnosis of DiGeorge syndrome. Patients P-11 and P-23 only had CBCs and immunoglobulin levels checked in the setting of known losses of proteinaceous fluids: post-operative chylothoraces and peritoneal dialysis for patient P-11 and congenital nephrotic syndrome for P-23. Patient P-14 had MRSA sepsis and a MRSA infection of the anterior chamber of the right eye in the setting of severe B cell and NK cell deficiency and other cytopenias. This patient had TRECS of <252 on the first four newborn screens, but then these levels subsequently normalized. This patient was ultimately diagnosed with bone marrow failure and did well after stem cell transplantation. Patient P-26 had an initial normal TREC followed by recurrent low TRECs and abnormal flow with T cell lymphopenia and low switched B cells as well as absent proliferation to CD3. While PID was initially considered, it was recommended to repeat testing once the patient was less lymphopenic. Repeat testing with a higher absolute lymphocyte count (ALC) showed the same defects, however the patient died only a few days later from fulminant sepsis. Exome sequencing was sent after the patient died and sequencing showed heterozygous deleterious mutations in a gene related to immune synapse formation. Confirmatory testing for causality is still underway. Thus, we had a likely explanation for immunocompromise in four of fifteen (27%) of EP infants tested due to clinical concern and none of the patients tested for low TRECS alone.
Seventeen infants referred for PID testing were very premature (VP, 29–33 weeks). Six of the VP infants had low TREC screens, and all of those six patients were already known to have conditions which are associated with secondary immunodeficiency at the time of extended testing, including hydrops, CHARGE association, Noonan-like syndrome, and Niemann Pick Type C.
Forty-one late preterm or term infants (LP/T, ≥ 34 weeks) underwent PID testing. Seventeen of those were referred solely for clinical indications and the remaining twenty-four were solely or initially referred for abnormal newborn screening. A majority of the LP/T infants who had immune testing had other co-diagnoses that have been known to cause low TRECs, such trisomy 21 (T21), other trisomies, chylous loses/hydrops, history of ECMO, or history of DiGeorge syndrome in patient or parent. One patient was found to have combined immunodeficiency due to homozygous in trans mutations in RAC2 and another patient was diagnosed with combined immunodeficiency later in like due to homozygous biallelic BCL11B mutations. Another term patient whose mother had a clinical diagnosis of incontinentia pigmenti presented with and died from disseminated MSSA infection. The patient also had low TRECs with T cell lymphopenia and severely diminished naïve T cells. This patient was ultimately found to have a hemizygous IKBKG mutation causing NEMO deficiency.
The majority of the VP (71%) and LP/T (76%) infants had a diagnosis that is known to cause secondary lymphopenia or a diagnosis that causes primary immune dysfunction or immunodeficiency (Table 1). In contrast, only 4 of the 29 (14%) EP infants had any other identifiable cause of immunodeficiency aside from prematurity, and all of these patients had immunologic testing done due to clinical concern. While prematurity is known to be a cause of secondary immunodeficiency and T cell lymphopenia, this is characterized as happening while the infants are still premature. However, the mean postmenstrual age for testing in this cohort was at term-corrected gestational age, or 40 weeks. Despite this, most of the infants who had T cell profiles done, had CD4+ T cell lymphopenia even after 37 weeks when they would be considered term equivalent (Figure 2). Only two of the EP patients who had T cell profiles done had underlying diagnoses that might affect immune profiles, patients P-4 with DiGeorge syndrome and P-14 (bone marrow failure), the rest had no identifiable risks other than prematurity.
Figure 2. Premature infants without other diagnoses had T cell lymphopenia after term corrected age.
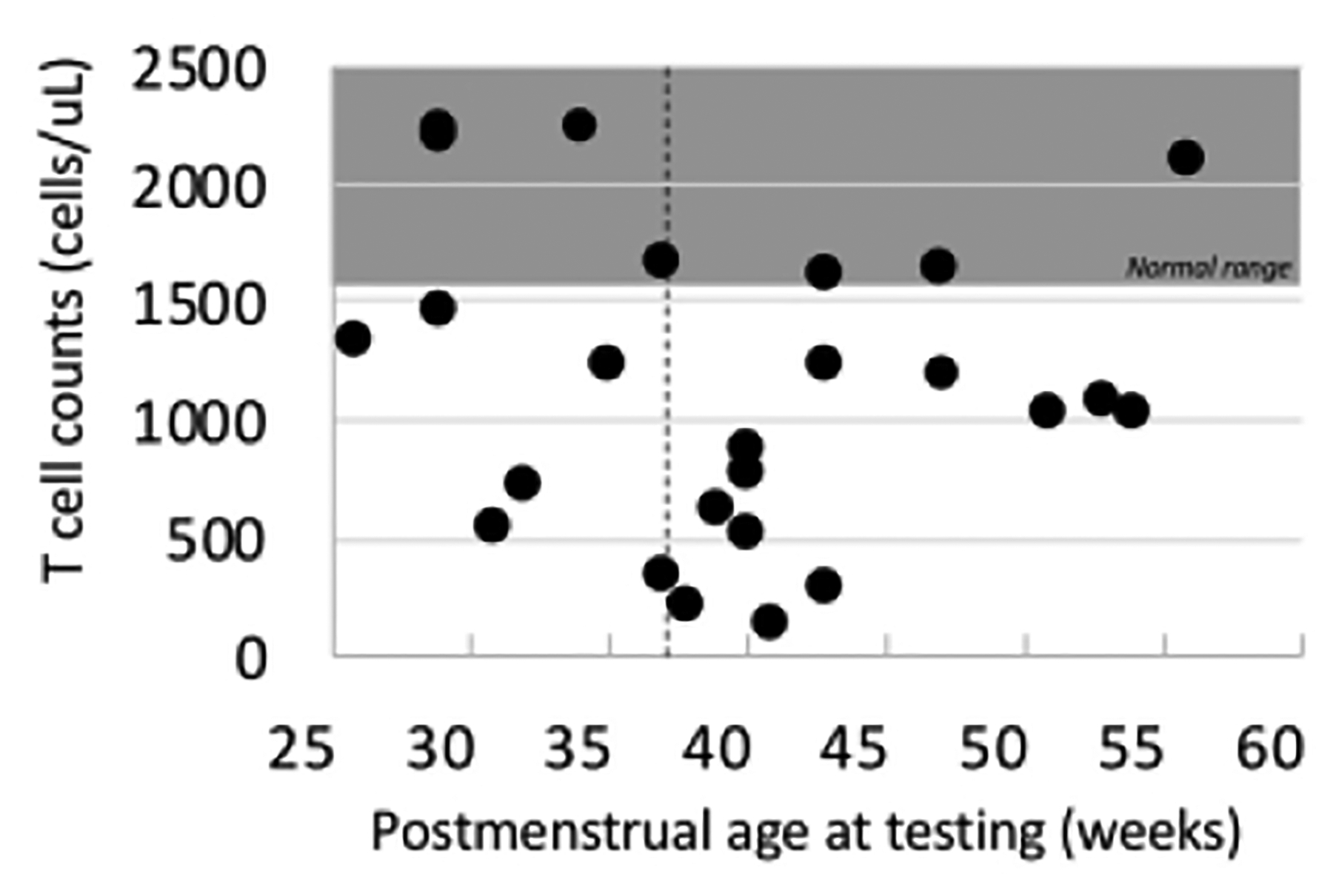
CD4+ T cell counts for premature infants who had immune testing. The shaded area represents the normal range for age and the dashed vertical line is term age.
Corticosteroids are often administered to premature infants in the NICU for treatment of lung disease and ionotropic effects, but they can also cause immunosuppression. We found that 54% (15 of 28) of EP infants received corticosteroids within 48 hours or less of immunologic testing (data was not available for 1 patient). We compared CD3+CD4+ T cell counts between EP patients who did and did not receive recent corticosteroid treatment and found no difference between the groups (P=0.93, Mann-Whitney U test, Figure 3, Table S2). None of the EP patients were on immunomodulators other than corticosteroids at the time of testing.
Figure 3. Steroid exposed infants did not have lower T cell counts than non-exposed infants.
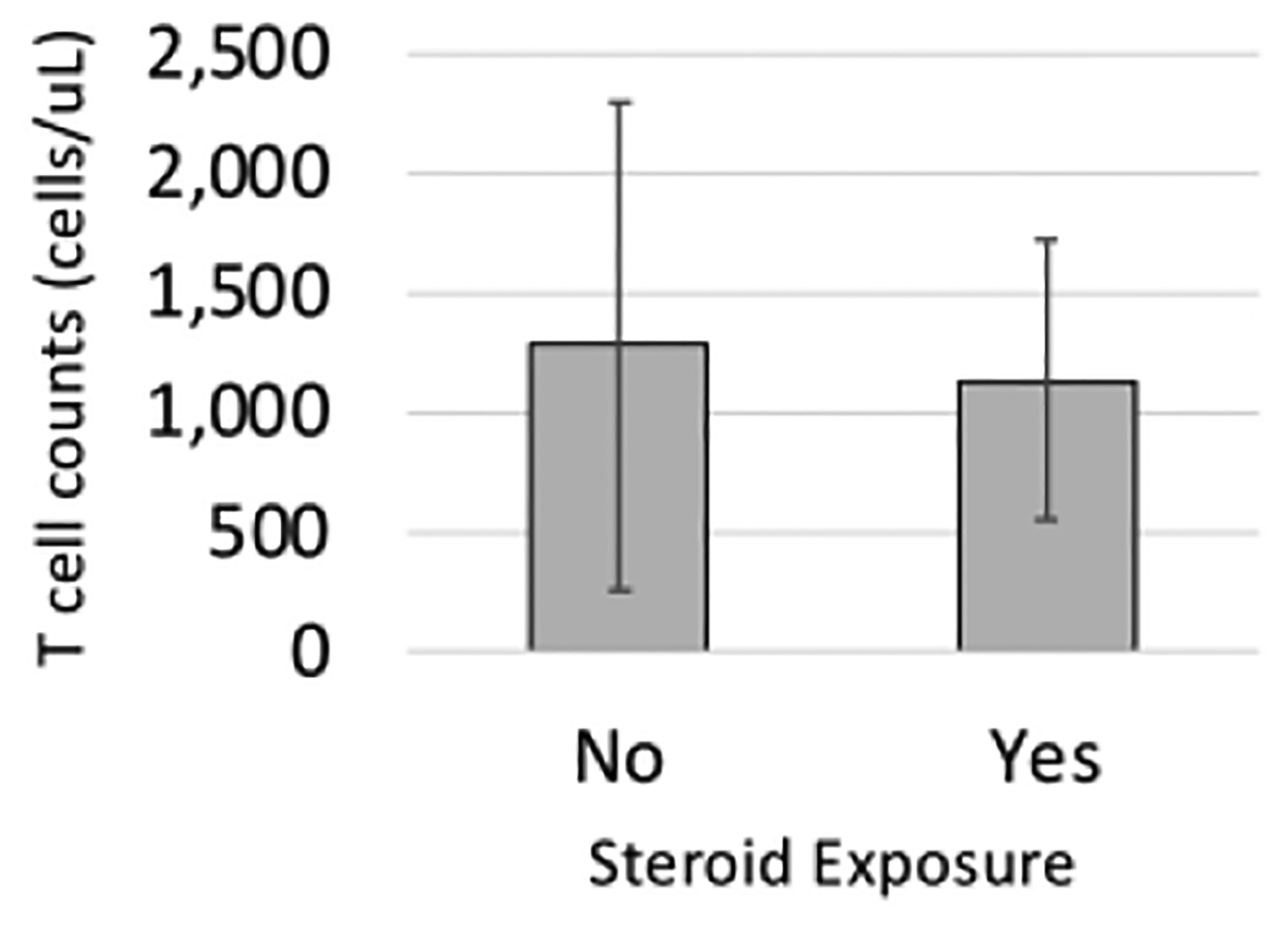
CD4+ T cell counts (cells/mm3) for the premature infant groups were subdivided into those who were not exposed to steroid therapy within 48 hours of immune testing (No) and those who were (Yes). Data is the mean for the group with the standard deviation depicted by the error bars.
Discussion
In our cohort of eighty-seven babies receiving immune testing over a twelve-year period, none were definitively diagnosed with SCID. This is not surprising, given the low incidence of SCID in the population and previous studies showing that a majority of patients with SCID were healthy term infants who were not in the NICU (16). We did identify several patients with known PID, however, including a patient with Rac2 deficiency, which has previously been reported to be detected by neonatal TREC screening (16, 17). Another patient was identified to have NEMO deficiency, one patient had complete DiGeorge syndrome, and a fourth patient was eventually determined to have BCL11B mutations causing immunodeficiency.
In the first seven years of TREC-based newborn screening in California, which used an initial TREC cut off of 40 copies/μL, they screened 3.25 million infants, and of those, thirty-three premature infants were found to have T cell lymphopenia (TCL, <1500 CD3+ T cells/m3) (16). In Massachusetts the cut off for low TRECs is higher, at 252 copies/μL. In our single NICU over a period of 10 years, we evaluated twenty very or extremely premature infants with flow cytometry after low TRECs were detected on the NBS. Assuming similar detection rates of TCL to California (33 out of 3.25 million or 1:98,000), we should have identified approximately seven preterm infants with TCL of an estimated 725,000 babies born in Massachusetts during this time period. In our center alone, we identified five premature babies who were referred for additional testing due to low TRECs and found to have TCL who had no other known contributory conditions. Because our NICU sees a very low proportion of the ELBW and VLBW infants born in the state of Massachusetts, it seems likely that we are capturing more premature infants with TCL in Massachusetts. Because these infants’ TCL tends to resolve over time, it is unclear whether the use of a higher TREC cut off in premature infants is beneficial. Nonetheless, at our center only twenty-nine EP infants had extended immune testing over the ten years of the study, meaning an average of 2–3 EP infants annually.
Surprisingly, the average time of testing for this population at 40 weeks’ postmenstrual age, or term-corrected gestational age. We suspect this is because of the known contribution of prematurity to low TREC levels, and the typical recommendation to simply repeat testing at 2-week intervals when low TRECs are detected in extremely premature infants. Moreover, in some infants who had flow cytometry earlier, the conclusion of the immunology team was that the patient was too small, ill, or lymphopenic to reliably interpret the tests, and the clinical recommendation was to repeat testing when the patient was more stable or had a higher lymphocyte count.
Several EP infants with clinical symptoms of PID in the form of recurrent or unusual infections were ultimately found to have abnormal immune function testing. The March of Dimes and the CDC quote the rate of very premature birth at 1.5–2%, however premature infants have been historically underrepresented in identification of SCID. That means that some premature infants with SCID and other severe PIDs are likely going undetected and potentially dying before the severity of their immunodeficiency is identified. In the multi-state cohort report of TREC screening, no cases of SCID were noted to have been identified in very or extremely premature infants (13). It is unknown whether SCID might precipitate premature delivery, which would further compound this number. One other study closely scrutinized premature infants with abnormal TRECs, and they also found a high incidence of inconclusive lymphocyte subsets testing and a high rate of death in premature infants (18). They and concluded the deaths were due to prematurity without additional substantiation. Ultimately, data to establish appropriate thresholds for TREC levels in this population would lead clearer designations on how to interpret neonatal PID screening in premature infants. Recent studies have begun to tackle establishing normal thresholds for T cell numbers in premature infants (12, 19), however, whether the standard TREC cut off is appropriate in premature infants remains yet to be determined.
This study is limited by the small number of patients reviewed, and by applicability of the findings in a tertiary, all outborn NICU population. We are further limited in translatability to other states in terms of the impact of TREC screening because of Massachusetts’ distinctly higher TREC screening cut off of 252 copies/μL. Finally, the retrospective nature of the study limits additional data collection.
Based on this study, we propose that EP and VP infants with both persistent low TREC levels as they approach term postmenstrual age and significant clinical history of infection undergo additional testing for PID expediently, rather than delaying definitive testing. Based on our experience, tracheitis is common in this population, and should not be a concerning factor. We recommend that clinical concern be triggered for recurrent bloodstream infections (especially when an indwelling central line is not present), unusual organisms, or unusual types of infection such as abscesses. The distinction here is that abnormal immunodeficiency screening in a premature infant with a clinical history of significant infection should proceed to genetic testing for potential primary immunodeficiency, rather than having the abnormality ascribed to prematurity alone and not investigated further.
Supplementary Material
Table 2.
Diagnoses by gestational group.
| Extremely Premature | Very premature | Late premature/Term | ||||
|---|---|---|---|---|---|---|
| Patients | 29 | Diagnoses | 17 | Diagnoses | 41 | Diagnoses |
| Confirmed genetic disorder | 0 | 5 | T21 (2), CHARGE (2), Neimann Pick type C | 9 | T21 (4), CHARGE, T18, T13, Goldenhar syndrome, 48XXY + 22qdup | |
| Likely genetic disorder | 0 | 2 | Multiple anomalies, Noonan-like syndrome | 2 | Multiple anomalies (MCA) with septo-optic dysplasia, MCA with renal anomalies | |
| Hydrops | 0 | 3 | 4 | Congenital heart block (2), congenital hydrops NOS (2) | ||
| Immunodeficiency | 2 | DiGeorge, congenital bone marrow failure | 2 | DiGeorge, T cell lymphopenia not specified | 9 | Jacobsen’s syndrome (3), Bcl11b mutation, DiGeorge (2), NEMO deficiency, benign neutropenia, Rac2 mutation |
| Lymphatic losses | 2 | d-TGA/chylothorax and peritoneal dialysis, Congenital nephropathy | 0 | 7 | Nephrotic syndrome, chylothorax after heart surgery (2, ToF/PS, TA), s/p ECMO (2), chylous effusions NOS (2) | |
| Congenital viral infection | 2 | Disseminated HSV, congenital CMV | ||||
| Inflammatory disorder | 5 | HLH (4), Degos disease | ||||
| Unknown | 25 | 5 | 10 | |||
| Patients with diagnosis (%) | 4 (14 %) | 12 (71%) | 31 (76%) | |||
IMPACT.
The role of immunologic testing in the premature population is unclear, we therefore reviewed the records of all infants in our NICU who had immunologic testing to rule out immunodeficiency done in the years from 2006–2018.
The addition of newborn screening for SCID in 2009 doubled the number of infants who had immune investigations.
The extremely premature cohort included many infants with persistent immune derangements through term-corrected gestational age, suggesting a persistent effect of prematurity on immune development and potentially function.
We propose that former premature infants with clinical evidence of immunodeficiency and sustained immune abnormalities by term corrected age undergo genetic testing for immunodeficiency.
Acknowledgements
The authors would like to thank Dr. Henry Feldman for his critical review of the manuscript. We would also like to thank the patients and families who contributed to this study. Dr. O’Connell is supported by NIH NIDDK K08 award PA-19-117.
Statement of financial support:
Dr. O’Connell’s work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. Dr. Frazer’s work was supported by the National institute of Child Health and Human Development of the National Institutes of Health under award number T32HD09061. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Disclosure statement: The authors declare no conflicts of interest.
Patient consent statement: The study was granted an informed consent waiver by our IRB; consent was not required.
References:
- 1.Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Sullivan KE 2018. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol 38:96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, Notarangelo LD, Neven B, Cavazzana M, Puck JM 2015. Severe combined immunodeficiencies and related disorders. Nat Rev Dis Primers 1:15061. [DOI] [PubMed] [Google Scholar]
- 3.Buelow BJ, Verbsky JW, Routes JM 2016. Newborn screening for SCID: lessons learned. Expert Rev Hematol 9:579–584. [DOI] [PubMed] [Google Scholar]
- 4.Heimall J, Cowan MJ 2017. Long term outcomes of severe combined immunodeficiency: therapy implications. Expert Rev Clin Immunol 13:1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling DJ, Levy O 2014. Ontogeny of early life immunity. Trends Immunol 35:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drossou V, Kanakoudi F, Tzimouli V, Sarafidis K, Taparkou A, Bougiouklis D, Petropoulou T, Kremenopoulos G 1997. Impact of prematurity, stress and sepsis on the neutrophil respiratory burst activity of neonates. Biol Neonate 72:201–209. [DOI] [PubMed] [Google Scholar]
- 7.Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M 2011. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huenecke S, Fryns E, Wittekindt B, Buxmann H, Konigs C, Quaiser A, Fischer D, Bremm M, Klingebiel T, Koehl U, Schloesser R, Bochennek K 2016. Percentiles of Lymphocyte Subsets in Preterm Infants According to Gestational Age Compared to Children and Adolescents. Scand J Immunol 84:291–298. [DOI] [PubMed] [Google Scholar]
- 9.Kollmann TR, Levy O, Montgomery RR, Goriely S 2012. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy O 2002. Impaired innate immunity at birth: deficiency of bactericidal/permeability-increasing protein (BPI) in the neutrophils of newborns. Pediatr Res 51:667–669. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Tanaka S, Bonno M, Ido M, Kawai M, Yamamoto H, Komada Y 2014. Cord blood CD4(+)CD25(+) regulatory T cells fail to inhibit cord blood NK cell functions due to insufficient production and expression of TGF-beta1. Cell Immunol 290:89–95. [DOI] [PubMed] [Google Scholar]
- 12.Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, Brodin P 2018. Stereotypic Immune System Development in Newborn Children. Cell 174:1277–1292 e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, Baker M, Ballow M, Bartoshesky LE, Bonilla FA, Brokopp C, Brooks E, Caggana M, Celestin J, Church JA, Comeau AM, Connelly JA, Cowan MJ, Cunningham-Rundles C, Dasu T, Dave N, De La Morena MT, Duffner U, Fong CT, Forbes L, Freedenberg D, Gelfand EW, Hale JE, Hanson IC, Hay BN, Hu D, Infante A, Johnson D, Kapoor N, Kay DM, Kohn DB, Lee R, Lehman H, Lin Z, Lorey F, Abdel-Mageed A, Manning A, McGhee S, Moore TB, Naides SJ, Notarangelo LD, Orange JS, Pai SY, Porteus M, Rodriguez R, Romberg N, Routes J, Ruehle M, Rubenstein A, Saavedra-Matiz CA, Scott G, Scott PM, Secord E, Seroogy C, Shearer WT, Siegel S, Silvers SK, Stiehm ER, Sugerman RW, Sullivan JL, Tanksley S, Tierce MLt, Verbsky J, Vogel B, Walker R, Walkovich K, Walter JE, Wasserman RL, Watson MS, Weinberg GA, Weiner LB, Wood H, Yates AB, Puck JM, Bonagura VR 2014. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 312:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orange JS, Geha RS, Bonilla FA 2003. Acute chylothorax in children: selective retention of memory T cells and natural killer cells. J Pediatr 143:243–249. [DOI] [PubMed] [Google Scholar]
- 15.Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, Lewis DB, McGhee SA, Moore TB, Stiehm ER, Porteus M, Aznar CP, Currier R, Lorey F, Puck JM 2013. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol 132:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AA, Agarwal-Hashmi R, Aznar CP, Butte MJ, Cowan MJ, Dorsey MJ, Dvorak CC, Kapoor N, Kohn DB, Markert ML, Moore TB, Naides SJ, Sciortino S, Feuchtbaum L, Koupaei RA, Puck JM 2019. Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010–2017. Pediatrics 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accetta D, Syverson G, Bonacci B, Reddy S, Bengtson C, Surfus J, Harbeck R, Huttenlocher A, Grossman W, Routes J, Verbsky J 2011. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol 127:535–538 e531–532. [DOI] [PubMed] [Google Scholar]
- 18.Accetta DJ, Brokopp CD, Baker MW, Verbsky J, Routes JM 2011. Cause of death in neonates with inconclusive or abnormal T-cell receptor excision circle assays on newborn screening. J Clin Immunol 31:962–967. [DOI] [PubMed] [Google Scholar]
- 19.Amatuni GS, Sciortino S, Currier RJ, Naides SJ, Church JA, Puck JM 2019. Reference intervals for lymphocyte subsets in preterm and term neonates without immune defects. J Allergy Clin Immunol 144:1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


