Abstract
Background
Xinjiang wild apple is an important tree of the Tianshan Mountains, and in recent years, it has undergone destruction by many biotic and abiotic stress and human activities. It is necessary to use new technologies to research its genomic function and molecular improvement. The clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) system has been successfully applied to genetic improvement in many crops, but its editing capability varies depending on the different combinations of the synthetic guide RNA (sgRNA) and Cas9 protein expression devices.
Results
In this study, we used 2 systems of vectors with paired sgRNAs targeting to MsPDS. As expected, we successfully induced the albino phenotype of calli and buds in both systems.
Conclusions
We conclude that CRISPR/Cas9 is a powerful system for editing the wild apple genome and expands the range of plants available for gene editing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13007-021-00769-8.
Keywords: CRISPR/Cas9, Gene editing, Malus sieverii, PDS gene
Background
Wild apple (Malus sieversii (Ledeb.) Roem.), is native to the mountains of Central Asia and is widely distributed in the wild fruit forests of the Tianshan Mountains. Wild apple forests are estimated to account for 92% of the fruit forests of the Chinese Tianshan Mountains, whereas wild apple forests make up approximately 78% of the wild apple forests in the Central Asian Tianshan Mountains [1]. Evolutionarily, the wild apple population has evolved cold-tolerant and disease-resistant varieties with diverse fruit colourations, shapes, and flavours [1]. Many genetic studies have shown that the wild apple M. sieversii is the distant ancestor species of cultivated domesticated apple species [2–4]. Unfortunately, in the last two decades, wild apple trees in Tianshan forests have been heavily damaged by biotic stresses and human activities that have resulted in severe forest destruction [5–7]. This tree has been listed as an endangered second-class protected plant in China [8]. Therefore, this valuable germplasm urgently needs effective protection, and it is necessary to create new stress-resistant germplasms. Due to the self-incompatibility of wild apples and their longer time to maturity [9], conventional breeding methods cannot easily meet the urgent needs.
Genome editing tools include zinc-finger nucleases (zinc-fingernuclease), transcription activator-like effector nuclease (TALEN), and the clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) system (CRISPR/Cas) [10]. Compared with the other techniques, CRISPR/Cas9 has become a powerful technology for gene editing because of its easy construction and high specific efficiency. The CRISPR/Cas system can be divided into six types (types I–VI) [11]. Type II Cas9 protein has been widely adopted as a simple and highly efficient targeted genome editing tool for multiplex genome engineering using CRISPR/Cas systems [12]. In this system, the mature dual CRISPR RNA consists of a crRNA and small trans-activating CRISPR RNA (tracrRNA), which forms a functional complex with the endonuclease Cas9. Scientists combined the two small RNAs into a single guide RNA (sgRNA), which was more convenient to use. The Cas9 ribonucleoprotein complex then binds to DNA at a typical protospacer adjacent motif (PAM) sequence and a protospacer matching the crRNA through Watson–Crick pairing [13, 14]. Cleavage occurs at the targeted site concerning the PAM on both strands, mediated by the Cas9 endonuclease domains RuvC and HNH, introducing precise double-strand breaks (DSBs) [13]. DSBs can be repaired in two ways, nonhomologous end joining (NEHJ) and homology-directed repair (HDR), depending on the cell type, target site, and DNA repair machinery [15]. NHEJ is responsible for the vast majority of repair and may occur in almost all types of cells as well as in different phases of the cell cycle (G1, S, and G2 phases), introducing various indels and substitutions at DSB sites that cause gene knockout or knockdown [16, 17]. HDR events occur mainly during the S and G2 periods if a homologous DNA donor is available to serve as a repair template [18, 19]. HDR-based repair has the greatest potential for precise genome editing but currently suffers from very low efficiencies. For eukaryotic genome editing, nuclear localization signals (NLSs) are added to the Cas9 protein to encourage nuclear localization [12, 20]. The Cas9 gene is driven by a constituent promoter with high transcriptional activity, such as the Cauliflower mosaic virus (CaMV) 35S promoter or the Arabidopsis ubiquitin 10 (AtUbi) promoter, to drive Cas9 expression in dicots, Maize or rice ubiquitin (ZmUbi; OsUbi) promoters in monocots [15]. Some organ-specific promoters are also used to drive Cas9 expression, such as the female gametophyte specificity promoter [21], male gametophyte-specific promoter [22], meristem promoter [23], and yao promoter [24]. sgRNAs are typically expressed under small nuclear RNA promoters, such as the U6 and U3 promoters constitutively transcribed by RNA polymerase III (Pol III) [25–29]. To edit multiple genes simultaneously, multiple sgRNA expression cassettes harbouring a small RNA promoter (U6 or U3), sgRNA spacer targeting sequence, sgRNA scaffold sequence, and 3’ terminator element were combined in series by the Golden Gate cloning method [29–31]. In planta expression of multiplexed gRNAs can also be based on ribozyme processing, Csy4 processing, or tRNA processing [15]. A polycistronic tRNA–sgRNA system generates multiple sgRNAs by using tRNA precursor sequences (pre-RNA) to link the sgRNA sequences under transcription by a U3 or U6 promoter [32]. CRISPR/Cas9 technology has been applied to wheat, rice, citrus, lettuce, and other fruits, and some new germplasms have been created, such as anti-powdery mildew wheat, ulcer-resistant citrus, and herbicide-resistant rice [33–38]. Phytoene desaturase (PDS) gene is a key gene in chlorophyll synthesis, and its mutant plants with albino phenotype and has served as a model gene for CRISPR/Cas9-mediated targeted gene editing [34, 37, 39]. In this study, we used two different systems to construct 8 different vectors with pairing sgRNAs targeting the MsPDS gene. Some calli and buds with mutant were successfully obtained. This is the first successful application of the CRISPR/Cas9 gene editing system in this species, which will provide strong technical support for its gene function research, utilization, and conservation.
Results
Cloning of MsPDS and selection of target sites
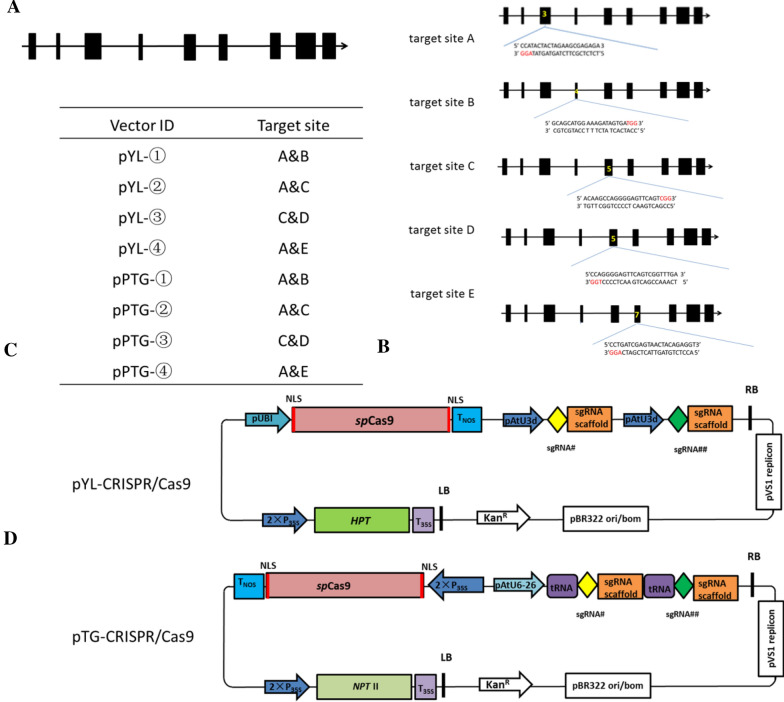
Two pairs of primers were used to clone the approximately full-length MsPDS gene sequence 5232 bp (supplementary sequence). The intron and exon sequences of this gene have been determined by the sequence alignment of multiple species. Five target sites (Fig. 1B) in exons 3, 4, 5, and 7 were selected and termed as target sites A, B, C, D, and E, respectively. According to the target site sequence, a 20 bp sgRNA was designed.
Fig. 1.
The structure of the MsPDS gene, target site selection, and schematic diagram of the Cas9/sgRNA vector. A The structure of the MsPDS gene. The black rectangles represent the different exons. B The target sites of different exons. The yellow number is the position of the exon. The red text indicates the PAM. C The vector ID of paired-sgRNA/Cas9 binary vectors for both the CRISPR/Cas9 and pTG-CRISPR/Cas9 systems. D Schematic depicting the paired sgRNA expression cassettes of both the CRISPR/Cas9 and pTG-CRISPR/Cas9 systems. Diamonds of different colours represent sgRNA, rectangles represent the sgRNA scaffold, and purple rounded rectangles represent tRNA
Constructs for the targeted genome editing of wild apple
To edit the target gene, we selected two different systems, pYL-CRISPR/Cas9 and pTG-CRISPR/Cas9, and constructed 8 different plasmids harbouring 2 sgRNAs according to their methods (Fig. 1C) [33, 40, 41]. For the pYL system, Cas9 endonuclease was under the maize ubiquitin promoter (PUBI), and a sgRNA expression cassette was under the AtU3d promoter. This system used the hygromycin resistance cassette in the vector for the selection of wild apple transformants. In contrast, for the pTG-CRISPR/Cas9 system, the same Cas9 endonuclease was located under the cauliflower mosaic virus 35S promoter (P35S) promoter, and 2 sgRNA expression cassettes were located under the AtU6-26 promoter and blanked by tRNAGly, which may function as a transcriptional enhancer for pol III promoters. This system used the NPT II resistance cassette in the vector for the selection of wild apple transformants. All 8 constructs with different paired sgRNAs (Fig. 1C) were transformed into wild apple leaf discs separately using the Agrobacterium method.
Efficiency of calli editing
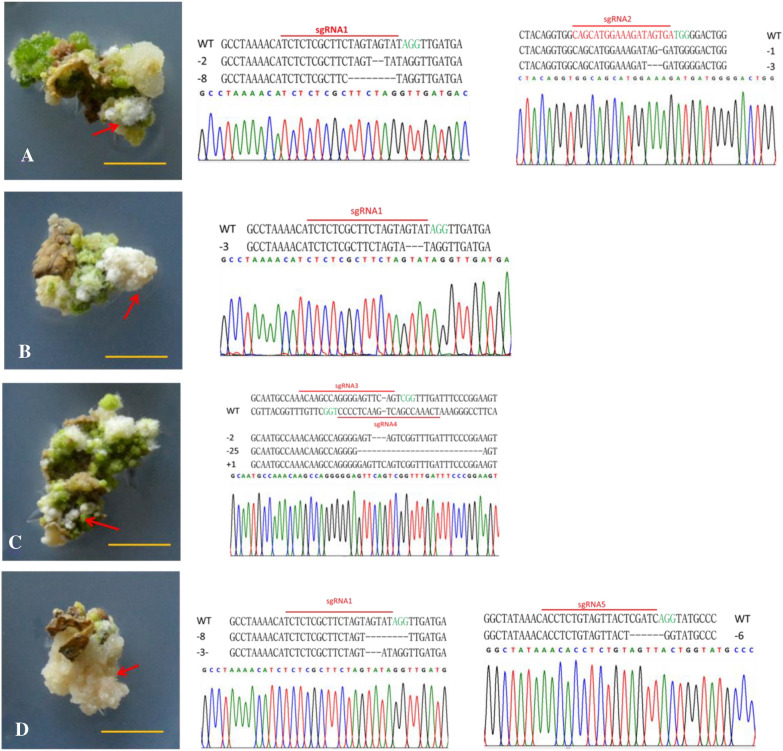
After 4 months of screening, some 1 cm2 resistant calli were obtained (Fig. 2). Typically, most of the wild-type calli were green after exposure to light. As expected, there were some white calli in the screening medium (Fig. 2). The calli were selected randomly to identify the mutation. The specific primers SP-L1/SP-R-L or SP-DL/SP-R-w of the T-DNA region and the Agrobacterium-specific primers VCF/VCR were used in PCR amplification, which revealed that the T-DNA region was successfully and efficiently inserted into the wild apple genome by both the pYL-CRISPR/Cas9 and pTG-CRISPR/Cas9 systems. Most of the resistant calli selected were transgenic. A total of 87.5% of the calli from hygromycin plates had the Cas9 gene inserted successfully, and 96.2% of the calli from kanamycin plates had the Cas9 gene inserted successfully (Additional file 1: Fig. S1A). Further, we detected whether the mutation was produced in these T-DNA insertion samples. PCR was performed using site-specific primers, and the PCR products were denatured and reannealed for the subsequent T7 endonuclease I assay (T7E1) (Additional file 1: Fig. S1B–D). The mutation efficiency of different loci was different (20–80%) (Table 1). The pYL-CRISPR/Cas9 system performed better than the pTG-CRISPR/Cas9 system in wild apple genome editing. The average target efficiency of pYL-CRISPR/Cas9 was 70.2%, while that of the pTG-CRISPR/Cas9 system was 40.3%. The efficiency of the two targets simultaneous editing was 59.5% for pYL-CRISPR/Cas9 and 26.6% for pTG-CRISPR/Cas9. The efficiency of different targets was different: target C had the highest average mutation rate, at 70%, and the mutation rate of E targets was 31.5%. To precisely identify the mutations in the resistant callus lines, the PCR products were purified using a cycle pure kit gel and cloned into a pMD20T vector followed by Sanger sequencing for validation. Mutation types were diverse and included not only 1–28 bp deletions but also 1–2 bp inserts (Fig. 2, Additional file 5).
Fig. 2.
Mutant calli and Some Sanger sequencing of the target site. A–D left: Calli transformed by pYL-①, pYL-②, C: pTG-③, and pTG-④. Right: mainly mutation types and one sequencing chromatogram among them. Red arrow: some white calli in which MsPDS was edited
Table 1.
Summary of site-specific mutagenesis frequencies in M. sieverii callus
| Vector ID | Target site | Number of callus lines analysed | Number of mutated callus lines | Mutation frequency (%) |
|---|---|---|---|---|
| pYL-① | A | 18 | 13 | 72.2 |
| B | 18 | 10 | 55.6 | |
| A&B | 18 | 10 | 55.6 | |
| pYL-② | A | 21 | 14 | 66.7 |
| C | 21 | 19 | 90.0 | |
| A&C | 21 | 14 | 66.7 | |
| pYL-③ | C&D | 23 | 17 | 73.9 |
| pYL-④ | A | 19 | 18 | 94.0 |
| E | 19 | 8 | 42.0 | |
| A&E | 19 | 8 | 42.1 | |
| pTG-① | A | 10 | 4 | 40.0 |
| B | 10 | 0 | 0 | |
| A&B | 10 | 0 | 0 | |
| pTG-② | A | 20 | 9 | 45.0 |
| C | 20 | 10 | 50.0 | |
| A&C | 20 | 9 | 45.0 | |
| pTG-③ | C&D | 5 | 2 | 40 |
| pTG-④ | A | 14 | 12 | 85.7 |
| E | 14 | 3 | 21.4 | |
| A&E | 14 | 3 | 21.4 |
Mutation in wild apple buds
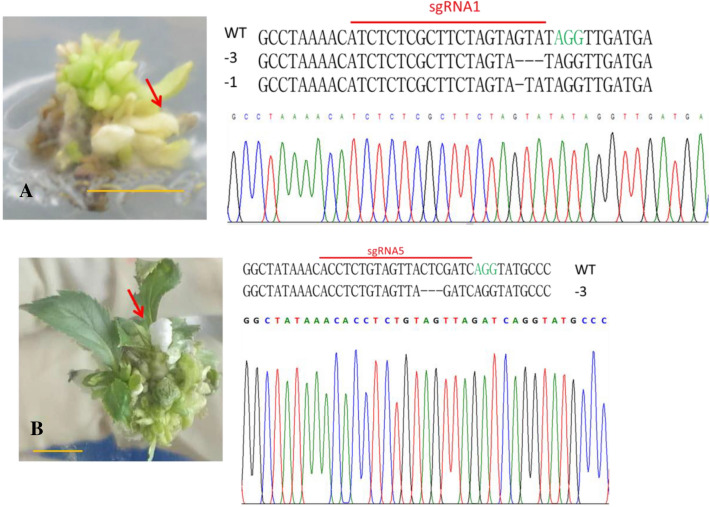
For the pYL system, we obtain 1 bud which PDS gene was edited through transformation method 1. Half of it is green, and the other is pale. Sanger sequencing shows there is 2 mutant type in the albino parts of bud (Fig. 3A), while one mutant is same as calli. For the pTG system, through method 2, we only obtained 1bud with one leaf albino (Fig. 3B). Because screening pressure was not applied in the cultured early stage, the bud is chimeric. Through Sanger sequencing, there is 3 base deletion in target 5 (Fig. 3B).
Fig. 3.
Mutant buds and Sanger sequencing of the target site. A, B left: Buds transformed by pYL-② and pTG-④, respectively. Right: mutation types and one sequencing chromatogram among them. Red arrow: albino parts in which MsPDS was edited
Off-target probability
To date, it is difficult to evaluate the off-target results of gene editing because the whole genome of M. sieversii has not been published. We designed 5 different sgRNAs using an online website and evaluated their potential off-target rate (< 0.6, good) (Additional file 3) by comparison to the apple genome (GDDH13, Version 1.1). Eighteen of the 54 potential off-target sites are in the CDS region (Additional file 3). For a preliminary evaluation of the off-target, we cloned ~ 300 bp upstream and downstream of each of these genes from the M. sieversii genome (Additional file 3, Additional file 4). Comparing with apple, these sequences are also highly conserved in wild apple. Sanger sequencing revealed no mutation in these DNA samples(Additional file 4), which were randomly selected from samples with the PDS gene edited by CRISPR/Cas9. Preliminarily, it was proven that these two systems have high fidelity in M. sieversii. In the future, it will be necessary to sequence the genome of M. sieversii and comprehensively evaluate the off-target rate caused by gene editing.
Discussion
The CRISPR/Cas9 system is a powerful genetic editing tool. To date, rice, corn, wheat, tomato, apple, grape, kiwifruit, and other food or cash crops have been edited [35, 42]. pYL-CRISPR/Cas9 is a multi-target system developed by Pro. Liu [36], and the pTG-CRISPR/Cas9 system is another paired targeting system designed by Dr. Huang that was used for kiwifruit efficiency editing [33]. Both systems were used for the genome editing of wild apple, and our results show that gene editing technology can be applied in the wild apple. Our data revealed consistently high editing efficacy in both systems, which is different from the previous results in kiwifruit. Perhaps the PUBI is as efficient as the P35S in wild apple. In our study, it was easy to obtain edited callus, while only a few buds with edited were obtained. The regeneration of plant becomes a limiting factor to apply this technology. Woody plant is hard to separate CRISPR/Cas9 by breeding in contrast to crops owing to it need long time to maturity. Some scientists used RNPs to edit genomic genes in apple protoplast [35]. On the other hand, some scientists have tried to use the loxp or FLP/FRT method to delete the Cas9 and sgRNA genes [43, 44]. But, there are some residual T-DNA sequences in a plant. T-DNA free is another limiting factor to apply this technology. Researching key genes concerning plant regeneration and T-DNA integration into the chromosome is the way to solve these problems.
In spite of this, it is easy to obtain edited calli, which is also the material for gene function study. Chen used apple calli to analyze the functions of MdMYBPA1and MdSYP121 in anthocyanin synthesis and the activation of disease resistance against Botryosphaeria dothidea [45, 46]. Using our experimental method, gene-edited calli can be obtained within 4 months. The establishment of this method contributes to the study of the genetic function of Malus plants.
Conclusions
In summary, we have used the CRISPR/Cas system to edit the genome of Xinjiang wild apple and for the first time edited two targets at the same time. The success of this experiment expanded the range of plant species that can be edited by the CRISPR/Cas9 system and will provide technical support for the creation of new germplasms and research on functional genes in M. sieversii.
Methods
Cloning of the PDS gene of M. sieversii
Genomic DNA was extracted from wild-type plants using the cetyltrimethyl ammonium bromide (CTAB) method [47]. Two pairs of primers (Ms-PDS-F1/Ms-PDS-R1 and Ms-PDS-F2/Ms-PDS-R2) were used to clone this gene. To analyse the intron–exon structure, the sequence was compared with reported apple PDS ESTs (accession nos. GO517828.1, GO499218.1, GO523095.1, GO514137.1, GO547261.1) and PDS ESTs from Arabidopsis (L16237), tomato (X59948), pepper (X68058), and soybean (M64704) by the methods reported by Chikako et al. [34].
pYL-CRISPR/Cas9 and pTG-CRISPR/Cas9 expression vector construction
pYL-CRISPR/Cas9 vector in which Cas9 is driven by the maize ubiquitin promoter(PUBI) contains hygromycin plant selectable marker gene and has two BsaI sites that flank a toxic ccdB gene for cloning of sgRNA expression cassettes. pTG-CRISPR/Cas9 vector in which Cas9 is driven by the cauliflower mosaic virus 35S promoter (P35S) contains NPTII (kanamycin resistance) plant selectable marker gene. Five sgRNAs targeting the different exons of the MsPDS gene were designed, termed sgRNA1 to sgRNA5. Each pair of targets was cloned into a single Cas9 binary vector to construct a paired-sgRNA/Cas9 binary. For the pYL-CRISPR/Cas9 system, sgRNA was first introduced into the region downstream of the U3d promoter and upstream of the sgRNA scaffold by overlapping PCR through the intermediate vector pYL-sgRNA-AtU3d. Then, 2 different sgRNA expression cassettes were constructed in the pYL-CRISPR/Cas9 vector by BsaI digestion and T4 ligation Golden Gate cloning [41]. For the pTG-CRISPR/Cas9 system, 2 different sgRNAs were first introduced downstream of the tRNA and upstream of the sgRNA scaffold by overlapping PCR through the intermediate vector pHLW-gRNA-tRNA, and then the sgRNA expression cassette was cloned into the pTG-CRISPR/Cas9 vector by BsaI digestion and T4 ligation Golden Gate Cloning [33]. The sgRNA oligos are shown in Additional file 2.
Tissue culture and Agrobacterium-mediated transformation
M. sieversii tissue culture was performed as previously described [48], and the leaf discs were used for Agrobacterium (EHA105)-mediated transformation. To obtain a stable transformation bud, we adopted two different genetic transformation methods owing to the extremely low genetic transformation efficiency. In the first method, after the leaf is infected by Agrobacterium, it grows on the screening medium, and a large number of resistant calli can be obtained after four months. The shortcoming of this method is obtaining fewer transformation buds. In the second method, the leaf discs are infected by Agrobacterium cultured in the bacteriostatic medium and given time for the adventitious buds to grow, and then the buds are transferred to the screening medium.
Mutant detection
Genomic DNA was extracted from resistant callus lines and regenerated buds. To validate the T-DNA insertion, Agrobacterium-specific primers (VCF/VCR) [49] and SP-L1/SP-R-L or SP-DL/SP-R-W of the T-DNA region were used. The mutation efficiencies were examined as the ratio of callus lines with a mutation. The fragments encompassing each target were separately amplified using the target-specific primers (Additional file 2). Approximately 500 ng of the respective PCR product was used for the T7E1 array with T7 endonuclease I according to the manufacturer’s instructions. After the T7E1 array, the reaction products were analysed by 1.5% agarose gel electrophoresis. Besides, to find out the specific type of mutant, the PCR products were cloned into the pMD20T vector. The ligated products were transformed into Escherichia coli strain DH5a cells, and clones were selected for Sanger sequencing.
Off-target analysis
The potential off target sites was predicted by reference Malus domestica genome(GDDH13, Version1.1) using the online tool CRISPR-GE (http://skl.scau.edu.cn/), and 9 potential off-target sites were retained. Site special primers were designed to clone these sequences in M.sieverii. 20 to 40 DNA with PDS edited were used for off-target analysis. PCR products 10 in a group were sequencing by Sanger sequence using sequencing primer (Additional file 3).
Supplementary Information
Additional file 1. MsPDS sequence, Supplement Table 1 Selection of target sites and off target possibility, Supplementary Fig 1 Identification of T-DNA insertion and indel detection by T7E1 assay.
Additional file 2. Primer used in this study.
Additional file 3. Predicted Off-target site and related primers.
Additional file 4. Off target site Sanger sequencing.
Additional file 5. Sanger sequencing of target site.
Acknowledgements
We thank Professor Yaoguan Liu at South China Agricultural University for providing the vector pYL-CRISPR/Cas9PUBI and Professor Hongwen Huang at Guangdong Provincial Key Laboratory of Applied Botany for providing the vector pTG-CRISPR/Cas9. We thank Dr. Dongchang Zeng and Dr. Zupeng Wang for their assistance during the molecular experiments.
Authors' contributions
DZ and YZ designed this study. YZ and PZ performed the experimental work and designed the figures. YZ wrote the manuscript. TAB provided comments on the manuscript. All authors read and approved the final manuscript.
Funding
The Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0502030403), West Light Cross Team Project of CAS (No. 2018-XBJCTD-001), and NSFC-Xinjiang Key Project (No. U1703233).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Zhang and Ping Zhou contributed equally to this work
References
- 1.Lin PLY. The survey of the distribution of Tianshan Mountain wild fruit forests and it distribution in Ili prefecture. Beijing: Springer; 2000. [Google Scholar]
- 2.Duan N, Bai Y, Sun H, Wang N, Ma Y, Li M, Wang X, Jiao C, Legall N, Mao L, et al. Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat Commun. 2017;8(1):249. doi: 10.1038/s41467-017-00336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards CM, Volk GM, Reilley AA, Henk AD, Lockwood DR, Reeves PA, Forsline PL. Genetic diversity and population structure in Malus sieversii, a wild progenitor species of domesticated apple. Tree Genet Genomes. 2009;5(2):339–347. [Google Scholar]
- 4.Harris SA, Robinson JP, Juniper BE. Genetic clues to the origin of the apple. Trends Genet. 2002;18(8):426–430. doi: 10.1016/s0168-9525(02)02689-6. [DOI] [PubMed] [Google Scholar]
- 5.Bozorov TA, Luo Z, Li X, Zhang D. Agrilus mali Matsumara (Coleoptera: Buprestidae), a new invasive pest of wild apple in western China: DNA barcoding and life cycle. Ecol Evol. 2019;9(3):1160–1172. doi: 10.1002/ece3.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu AH, Zhang XP. Study on the Wild Fruit Tree Diseases of Tianshan Mountains and Their Distribution in XinjiangPreliminary Research on the Composite Damage of Agrilus mali Matsumura and Valsa mali Miyabe et Yamada in Wild Apple Trees in Tianshan Mountain. Xinjiang Agric Sci. 2014;51(12):2240–2244. [Google Scholar]
- 7.Wang X, Zang R, Yin Z, Kang Z, Huang L. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecol Evol. 2014;4(8):1369–1380. doi: 10.1002/ece3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Li F, Long H, Yu W, Yan X, Liu B, Zhang Y, Yan G, Song W. Ecological distribution, reproductive characteristics, and in situ conservation of Malus sieversii in Xinjiang China. HortScience. 2016;51(9):1197–1201. [Google Scholar]
- 9.Dobranszki J, da Silva JA. Micropropagation of apple—a review. Biotechnol Adv. 2010;28(4):462–488. doi: 10.1016/j.biotechadv.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Gao C. Targeted genome modification technologies and their applications in crop improvements. Plant Cell Rep. 2014;33(4):575–583. doi: 10.1007/s00299-013-1539-6. [DOI] [PubMed] [Google Scholar]
- 11.Mohanraju Prarthana. Makarova, Kira S, Zetsche, Bernd, Zhang F, Koonin, Eugene V, Oost D: Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 2016;87:48. doi: 10.1126/science.aad5147. [DOI] [PubMed] [Google Scholar]
- 12.Le C, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA. Multiplex genome engineering using CRISPR/Cas systems. Trends Genetics Tig. 2013;32(12):815. [Google Scholar]
- 13.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowder L, Malzahn A, Qi Y. Rapid evolution of manifold CRISPR systems for plant genome editing. Front Plant Sci. 2016;7:1683. doi: 10.3389/fpls.2016.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bortesi Fischer. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol Adv. 2015;33(1):41–52. doi: 10.1016/j.biotechadv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Joseph W. Paul, III, Yiping, Qi: CRISPR/Cas9 for plant genome editing: accomplishments, problems and prospects. Plant Cell Rep. 2016;35(7):1417–1427. doi: 10.1007/s00299-016-1985-z. [DOI] [PubMed] [Google Scholar]
- 18.Schiml S, Fauser F, Puchta H. The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J. 2015;80(6):1139–1150. doi: 10.1111/tpj.12704. [DOI] [PubMed] [Google Scholar]
- 19.Schiml S, Puchta H. Revolutionizing plant biology: multiple ways of genome engineering by CRISPR/Cas. Plant Methods. 2016;12(1):8. doi: 10.1186/s13007-016-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mali P, Yang L, Esvelt KM, Aach J, Guell M, Dicarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015;16(1):144. doi: 10.1186/s13059-015-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao Y, Zhang Z, Feng Z, Wei P, Zhang H. Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications inArabidopsis. Plant Biotechnol J. 2015;14:2. doi: 10.1111/pbi.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyun Y, Kim J, Cho SW, Choi Y, Kim JS, Coupland G. Site-directed mutagenesis in Arabidopsis thaliana using dividing tissue-targeted RGEN of the CRISPR/Cas system to generate heritable null alleles. Planta. 2015;241(1):271–284. doi: 10.1007/s00425-014-2180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L, Wei S, Wu Y, Hu R, Li H, Yang W, Xie Q. High-efficiency genome editing in Arabidopsis using YAO promoter-driven CRISPR/Cas9 system. Mol Plant. 2015;12:1820–1823. doi: 10.1016/j.molp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Zhou H, Bi H, Michael F, Bing Y, Weeks DP. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nuclc Acids Res. 2013;20:20. doi: 10.1093/nar/gkt780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li JF, Norville JE, Aach J, Mccormack M. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol. 2013;31(8):688. doi: 10.1038/nbt.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nekrasov V, Staskawicz B, Weigel D, Jones J, Kamoun S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(8):691–693. doi: 10.1038/nbt.2655. [DOI] [PubMed] [Google Scholar]
- 28.Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu JL. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol. 2013;31(8):686. doi: 10.1038/nbt.2650. [DOI] [PubMed] [Google Scholar]
- 29.Lowder LG, Zhang D, Baltes NJ, Paul JW, Tang X, Zheng X, Voytas DF, Hsieh T-F, Zhang Y, Qi Y. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol. 2015;169(2):971–985. doi: 10.1104/pp.15.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing HL, Dong D, Wang ZP, Zhang HY, Han CY. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014;2014:14. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler C, Kandzia R, Marillonnet S, ElShemy HA. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE. 2008;3(11):e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie K, Minkenberg B, Yang Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci USA. 2015;112(11):3570–3575. doi: 10.1073/pnas.1420294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Wang S, Li D, Zhang Q, Li L, Zhong C, Liu Y, Huang H. Optimized paired-sgRNA/Cas9 cloning and expression cassette triggers high-efficiency multiplex genome editing in kiwifruit. Plant Biotechnol J. 2018;16:8. doi: 10.1111/pbi.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishitani C, Hirai N, Komori S, Wada M, Okada K, Osakabe K, Yamamoto T, Osakabe Y. Efficient genome editing in apple using a CRISPR/Cas9 system. Sci Rep. 2016;6:31481. doi: 10.1038/srep31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osakabe Y, Liang Z, Ren C, Nishitani C, Osakabe K, Wada M, Komori S, Malnoy M, Velasco R, Poli M. CRISPR–Cas9-mediated genome editing in apple and grapevine. Nat Protocols. 2018;2:578. doi: 10.1038/s41596-018-0067-9. [DOI] [PubMed] [Google Scholar]
- 36.Xingliang M, Qunyu Z, Qinlong Z, Wei L, Yan C. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant. 2015;8(8):1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Fan D, Liu T, Li C, Jiao B, Li S, Hou Y, Luo K. Efficient CRISPR/Cas9-mediated targeted Mutagenesis in Populus in the first generation. Sci Rep. 2015;5(12217):12217. doi: 10.1038/srep12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Z, Zhang B, Ding W, Liu X, Yang DL, Wei P, Cao F, Zhu S, Zhang F, Mao Y. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013;23(10):1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin G, Gu H, Ma L, Peng Y, Deng XW, Chen Z, Qu LJ. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 2007;17(5):471–482. doi: 10.1038/cr.2007.40. [DOI] [PubMed] [Google Scholar]
- 40.Zeng D, Ma X, Xie X, Zhu Q, Liu Y-G. A protocol for CRISPR/Cas9-based multi-gene editing and sequence decoding of mutant sites in plants. SCIENTIA SINICA Vitae. 2018;48(7):783–794. [Google Scholar]
- 41.Ma X. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant. 2015;8(8):1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Pribil M, Palmgren M, Gao C. A CRISPR way for accelerating improvement of food crops. Nature Food. 2020;1(4):200–205. [Google Scholar]
- 43.Fayu Y, Changbao L, Ding C, Mengjun T, Haihua X. CRISPR/Cas9-loxP-mediated gene editing as a novel site-specific genetic manipulation tool. Mol Ther Nucleic Acids. 2017 doi: 10.1016/j.omtn.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pompili V, Costa LD, Piazza S, Pindo M, Malnoy M. Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant Biotechnol J. 2020;18:845–858. doi: 10.1111/pbi.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang N, Qu C, Jiang S, Chen Z, Xu H, Fang H, Su M, Zhang J, Wang Y, Liu W, et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018;96(1):39–55. doi: 10.1111/tpj.14013. [DOI] [PubMed] [Google Scholar]
- 46.He X, Huo Y, Liu X, Zhou Q, Feng S, Shen X, Li B, Wu S, Chen X. Activation of disease resistance against Botryosphaeria dothidea by downregulating the expression of MdSYP121 in apple. Hortic Res. 2018;5:24. doi: 10.1038/s41438-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant ONA. Nucleic Acids Res. 1980;8:19. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Bozorov TA, Li DX, Zhou P, Wen XJ, Ding Y, Zhang DY. An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants. Plant Methods. 2020;16:56. doi: 10.1186/s13007-020-00599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawada H, Ieki H, Matsuda I. PCR detection of Ti and Ri plasmids from phytopathogenic Agrobacterium strains. Arch Microbiol. 1994;161(4):300–309. doi: 10.1128/aem.61.2.828-831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. MsPDS sequence, Supplement Table 1 Selection of target sites and off target possibility, Supplementary Fig 1 Identification of T-DNA insertion and indel detection by T7E1 assay.
Additional file 2. Primer used in this study.
Additional file 3. Predicted Off-target site and related primers.
Additional file 4. Off target site Sanger sequencing.
Additional file 5. Sanger sequencing of target site.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.