Abstract
Background
The use of colonoscopy has increased and colorectal cancer (CRC) incidence has decreased after the introduction of screening colonoscopy in Germany. However, it remains unknown to what extent progress has been achieved in the prevention of cancer in the proximal colon, distal colon, and rectum.
Methods
We analyzed trends in CRC incidence (2000–2016) and mortality (2000–2018) in Germany by sex, age, and tumor location.
Results
The age-standardized incidence of CRC declined by 22.4% (from 65.3 to 50.7 per 100 000) in men and by 25.5% (from 42.7 to 31.8 per 100 000) in women. CRC mortality declined by 35.8% (from 29.6 to 19.0 per 100 000) in men and by 40.5% (19.0 to 11.3 per 100 000) in women. Despite demographic changes, the annual numbers of CRC cases and deaths still decreased from about 60 400 to 58 300 and from around 28 700 to 24 200, respectively. The decline in incidence was greatest in groups aged ≥ 55 years. While the incidence of cancer in the distal colon and rectum decreased by 34.5% and 26.2%, respectively, in men and by 41.0% and 27.9% in women, the incidence of proximal colon cancer remained stable in men and decreased by only 7.0% in women. However, a major shift towards earlier stages was observed for the proximal cancers.
Conclusion
The results support the assumption that the increased use of colonoscopy has contributed to substantial reductions in the incidence of distal CRC incidence and the mortality from cancers in the entire colon and rectum.
Colorectal cancer (CRC) is among the most commonly occurring cancers, with approximately 1.9 million new cases and 0.9 million deaths globally in 2018 (1). Despite a recent decline in both incidence and absolute case numbers, CRC still accounts for close to 60 000 cases and 25 000 deaths per year in Germany (2). In contrast to most other cancers, a number of screening examinations are available (including fecal occult blood tests, flexible sigmoidoscopy, and colonoscopy). Both randomized controlled trials (RCTs) and observational studies have shown that these procedures have the potential to substantially reduce CRC incidence and mortality (3– 6).
Table 1 shows the history of CRC screening options in Germany. Starting in October 2002, colonoscopy was offered as a primary screening examination covered by statutory health insurance. Up to 2019 screening was opportunistic, i.e., there was no organized invitation system. In the period from 2010 to 2016, more than 50% of the population in Germany aged 50 years or over reported having had a colonoscopy in the previous 10 years. Approximately half were primary screening examinations and the other half were carried out for diagnostic purposes (7).
Table 1. Colorectal cancer screening options in Germany before and during the study period.
| Period | Age | Test | Interval |
| 1977 to September 2002 | ≥ 45 | gFOBT | Annually |
| October 2002 to March 2017 *1 | 50–54 | gFOBT | Annually |
| ≥ 55 | Colonoscopy | Up to two colonoscopies ≥ 10 years apart*2 | |
| Alternatively: gFOBT | Biennially |
gFOBT, Guaiac-based fecal occult blood test
*1 Changes since March 2017:
- In April 2017 gFOBT was replaced by the immunological fecal immunochemical test (iFOBT).
- Since April 2019 colonoscopy is offered from age 50 years for men (unchanged for women).
- Since July 2019 personal invitation letters are sent to persons aged 50, 55, 60, and 65 years.
*2 Second screening colonoscopy offered only if first screening colonoscopy was done before age 65.
We have already shown that CRC incidence declined substantially between 2003 and 2012, the first decade during which screening colonoscopy was offered (8). In this article, we have updated our previous analysis of trends in CRC incidence (up to 2016) and mortality (up to 2018). We paid particular attention to trends in the incidence of cancer in different subsites of the colon and rectum, as there is uncertainty and ongoing debate whether colonoscopy is equally effective in preventing proximal and distal CRC. We additionally analyzed trends in stage distribution at the time of diagnosis since the introduction of screening colonoscopy.
Methods
Data sources
Estimates of the national incidence of CRC (ICD-10 codes C18, C19, and C20) in the period 2000 to 2016 were made on the basis of figures from the German Center for Cancer Registry Data (ZfKD) (2). The mortality rates for the years 2000 to 2018 were derived from data from the Federal Health Monitoring System based on cause-of-death statistics (9). Furthermore, we used anonymized data from the individual cancer registries (etable 1) to analyze trends in stage distribution between 2000 and 2016.
eTable 1. Characteristics of patients with colorectal cancer in Germany, 2000–2016*1.
| Characteristic | n (%) |
| Total | 872 318 |
| Sex | |
| Men | 468 451 (53.7) |
| Women | 403 867 (46.3) |
| Age at diagnosis | |
| < 55 years | 82 463 (9.5) |
| 55–64 years | 149 869 (17.2) |
| 65–74 years | 266 060 (30.5) |
| 75–84 years | 263 482 (30.2) |
| ≥ 85 years | 110 444 (12.7) |
| Tumor site (ICD-10 codes) | |
| Proximal colon (C18.0–C18.4) | 241 635 (27.7) |
| Distal colon (C18.5–C18.7) | 218 837 (25.1) |
| Rectum (C19–C20) | 301 052 (34.5) |
| Overlapping and unspecified sites in the colon (C18.8–C18.9)*2 | 110 794 (12.7) |
| Stage | |
| I | 82 536 (9.5) |
| II | 112 567 (12.9) |
| III | 119 235 (13.7) |
| IV | 128 926 (14.8) |
| No data*3 | 429 054 (49.2) |
*1 Divergent availability of data:
Baden–Württemberg 2009–2016, Bavaria 2002–2016, Berlin 2000–2015, Brandenburg 2000–2015, Mecklenburg–Western Pomerania 2000–2015, Saxony 2000–2015, Saxony–Anhalt 2000–2015, Thuringia 2000–2015. For Saarland, data on stage distribution were not available for 2015 and 2016.
*2 The proportion of these cases was lowest in 2014 (9.4%) and highest in 2000 (18.1%).
*3 The proportion of these cases was lowest in 2014 (40.8%) and highest in 2000 (59.5%).
Statistical analysis
We calculated age-standardized incidence and mortality rates among men and women on the basis of the European Standard Population for 1976 and estimated average annual percent changes (AAPCs) using Joinpoint regression.
We analyzed incidence rates and stage distribution for any site in the colon and rectum (C18–C20) and for the subsites proximal colon (proximal to the left flexure, C18.0–C18.4), distal colon (C18.5–C18.7), and rectum (C19–C20).
Analyses of CRC mortality were also conducted for all sites combined (C18–C20) and for colon cancer (C18) and rectal cancer (C19–C20) separately.
Furthermore, we calculated the cumulative CRC incidence and mortality (expressed in %) for specific age groups (<55, 55–64, 65–74, 75–84, and 0–84 years) in the years 2000 to 2002 and 2014 to 2016, together with the percentage changes between these two periods. The cumulative CRC incidence and mortality reflect the probability of developing or dying from CRC in the absence of another (competing) cause of death (10).
All statistical analyses were conducted using R 3.6.1, SAS 9.4 (SAS Institute Inc., Cary, NC, USA), and the Joinpoint regression software 4.7.0.0 provided by the US National Cancer Institute (11).
Further details of the evaluation techniques can be found in the eMethods.
Results
Trends in CRC incidence and stage distribution
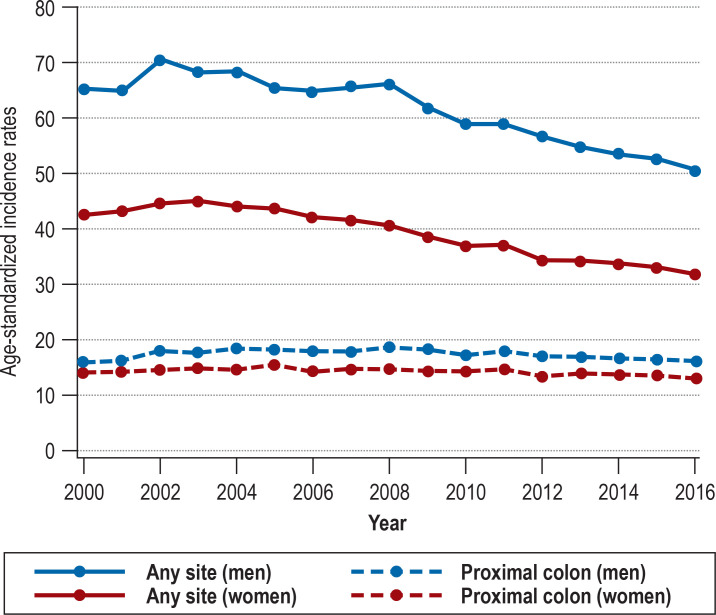
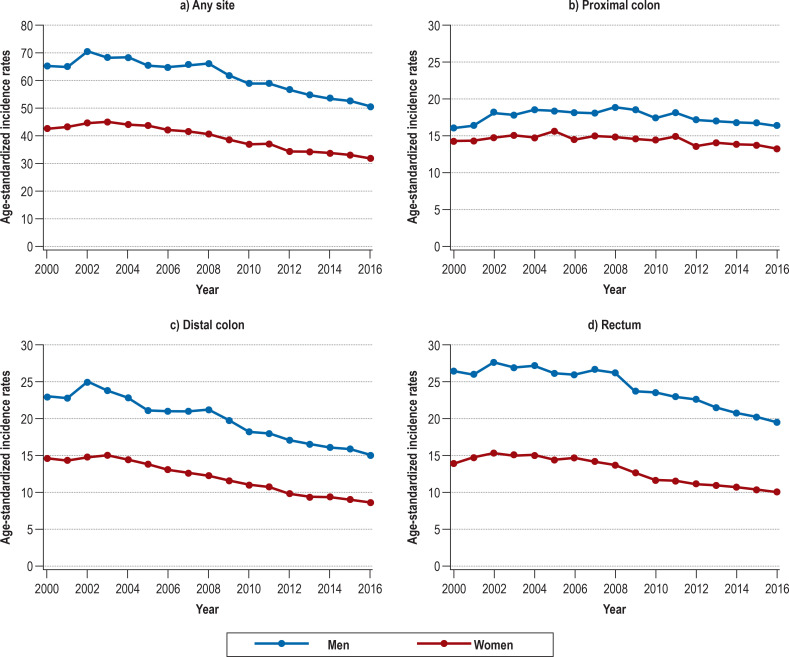
Between 2000 and 2016, the age-standardized incidence rates decreased from 65.3 to 50.7 per 100 000 among men [corresponding to -22.4%; AAPC –1.8; 95% confidence interval [–2.3; –1.4]) and from 42.7 to 31.8 per 100 000 among women [corresponding to -25.5%; AAPC –2.2 [–2.6; –1.8]) (Figure 1, eTable 2). Despite the demographic changes, the annual number of CRC cases decreased from about 60 400 to 58 300. Nevertheless, substantial differences were observed among the specific tumor sites (eTable 2, eFigure 1). The incidence rates of distal colon cancer and rectal cancer, respectively, declined by 34.5% (AAPC –3.0 [–3.4; –2.5]) and 26.2% (AAPC –2.1 [–2.5; –1.6]) among men and by 41.0% (AAPC –3.7 [–4.1; –3.3]) and 27.9% (AAPC –2.7 [–3.3; –2.1]) among women. By contrast, the incidence rates of proximal colon cancer remained stable (AAPC –0.2 [–0.7; 0.3]) among men and decreased by only 7.0% (AAPC –0.5 [–0.9; –0.2]) among women.
Figure 1.
Trends in age-standardized incidence rates (per 100 000 inhabitants) of colorectal cancer (C18–C20) and proximal colon cancer (C18.0–C18.4) in Germany over the period 2000 to 2016
eTable 2. Average annual percent change in incidence of and mortality from colorectal cancer in Germany, stratified by sex.
| AAPC [95% CI] | ||
| Men | Women | |
| Incidence (2000–2016) | ||
| Colorectal cancer | –1.8 [–2.3; –1.4] | –2.2 [–2.6; –1.8] |
| Proximal colon cancer | –0.2 [–0.7; 0.3] | –0.5 [–0.9; –0.2] |
| Distal colon cancer | –3.0 [–3.4; –2.5] | –3.7 [–4.1; –3.3] |
| Rectal cancer | –2.1 [–2.5; –1.6] | –2.7 [–3.3; –2.1] |
| Mortality (2000–2018) | ||
| Colorectal cancer | –2.5 [–2.7; –2.3] | –3.0 [–3.2; –2.8] |
| Colon cancer | –3.1 [–3.3; –2.9] | –3.3 [–3.6; –3.1] |
| Rectal cancer | –1.5 [–1.7; –1.2] | –2.1 [–2.3; –2.0] |
AAPC, Average annual percent change; CI, confidence interval
eFigure 1.
Trends in age-standardized incidence rates (per 100 000 inhabitants) of colorectal cancer (C18–C20), proximal colon cancer (C18.0–C18.4), distal colon cancer (C18.5–C18.7), and rectal cancer (C19–C20) in Germany from 2000 to 2016
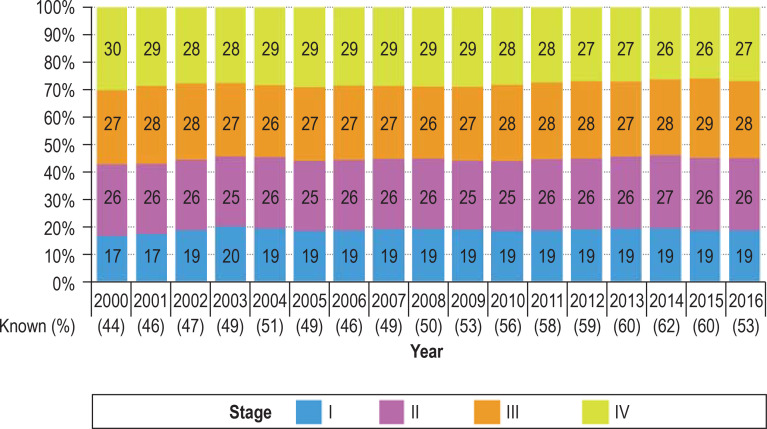
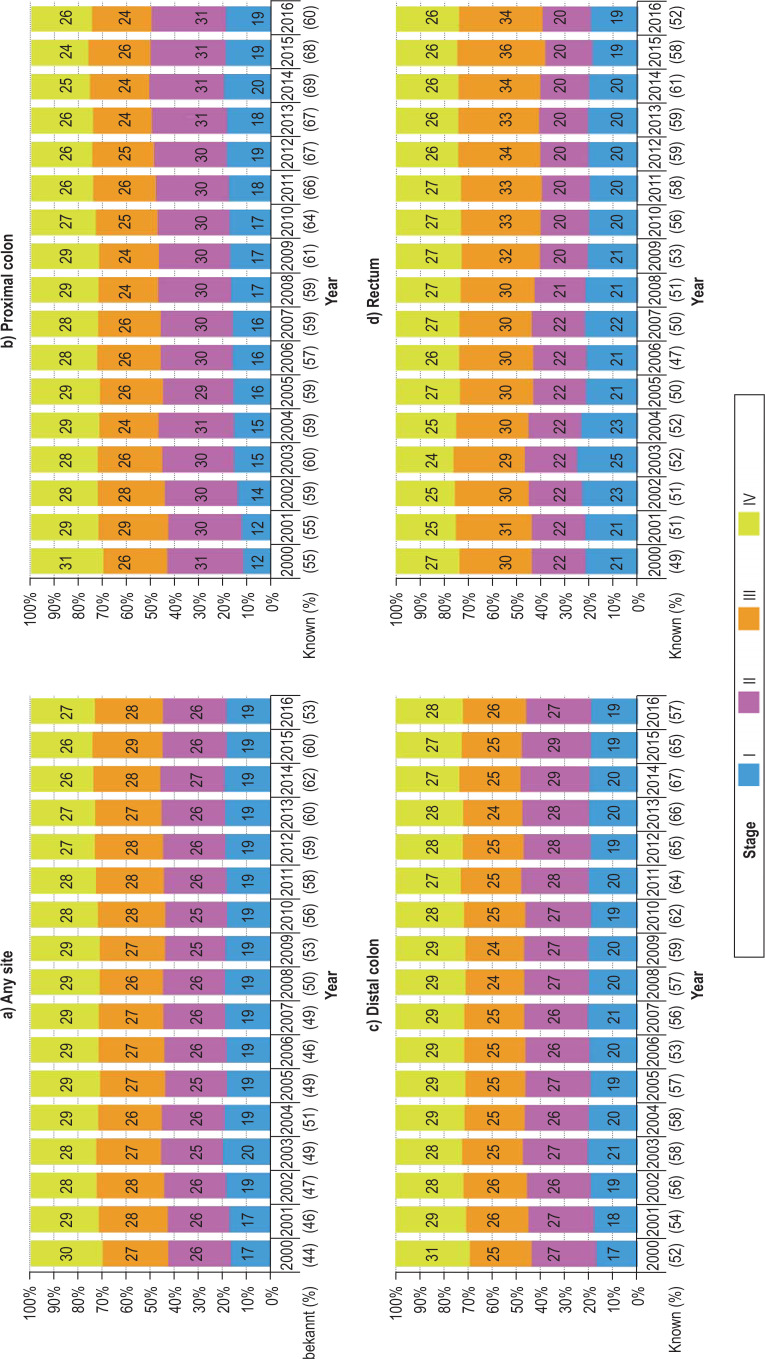
The proportion of tumors detected at stage I increased after the introduction of screening colonoscopy in 2002 and the proportion detected at stage IV decreased in later years (Figure 2, eFigure 2). These trends were found predominantly for proximal colon cancer (12% stage I and 31% stage IV cancers in 2000, 19% stage I and 26% stage IV cancers in 2016), but to a certain extent also for distal colon cancer (17% stage I and 31% stage IV cancers in 2000, 19% stage I and 28% stage IV cancers in 2016). For rectal cancer, we observed an increase in the proportion of stage I cancer at diagnosis in 2002–2004, but this did not persist in later years. There was also a substantial increase in the proportion of stage III rectal cancers over time.
Figure 2.
Trends in stage distribution of colorectal cancer (all sites, C18–C20) in Germany over the period 2000 to 2016. The proportion of cases in which the stage was known is given in parentheses for each calendar year.
eFigure 2.
Trends in stage distribution of colorectal cancer (C18–C20), proximal colon cancer (C18.0–C18.4), distal colon cancer (C18.5–C18.7), and rectal cancer (C19–C20) in Germany from 2000 to 2016. The proportion of cases in which the stage was known is given in parentheses for each calendar year.
Trends in CRC mortality
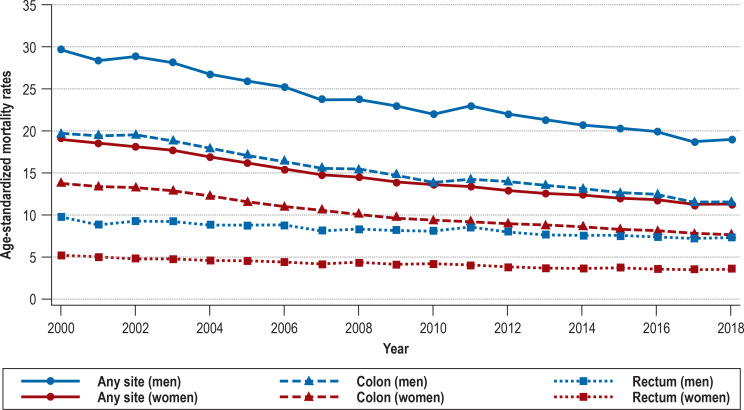
In line with the incidence trends, mortality rates were much higher for men than for women and decreased substantially between 2000 and 2018: for men from 29.6 to 19.0 per 100 000 (–35.8%; AAPC –2.5 [–2.7; –2.3]); for women from 19.0 to 11.3 per 100 000 (–40.5%; AAPC –3.0 [–3.2; –2.8]) (Figure 3, eTable 2). The annual number of deaths from CRC also decreased, from approximately 28 700 to 24 200. Colon cancer mortality was higher than rectal cancer mortality throughout the study period. Over the same time span, the decrease in mortality was greater for colon cancer (men: –41.4%, AAPC –3.1 [–3.3; –2.9]; women: –44.2%, AAPC –3.3 [–3.6; –3.1]) than for rectal cancer (men: –24.5%, AAPC –1.5 [–1.7; –1.2]; women: –30.8%, AAPC –2.1 [–2.3; –2.0]).
Figure 3.
Trends in age-standardized mortality rates (per 100 000) for colorectal cancer in Germany over the period 2000 to 2018
Age-specific trends in CRC incidence and mortality
Overall, the cumulative risk of developing CRC substantially declined from 2000–2002 to 2014–2016 in both men and women (table 2). The decreases were largest for the age groups 65–74 years (men –24.5%, women –25.9%) and 75–84 years (men –23.5%, women –30.2%) and smallest for the age group 0–54 years (men –15.3%, women –16.7%). For distal colon cancer the decline was most pronounced in persons over 55 years of age. In contrast, a much less pronounced decrease, or even an increase, was observed across the various age groups for proximal colon cancer.
Table 2. Changes in the cumulative risk of disease and mortality from colorectal cancer between 2000–2002 and 2014–2016 by tumor site, sex, and age.
| Tumor site | Age group (years) | Men | Women | ||||
| 2000–02 | 2014–16 | Change (%) | 2000–02 | 2014–16 | Change (%) | ||
| Cumulative incidence [%] | |||||||
| Any | < 55 | 0.59 | 0.50 | −15.3 | 0.48 | 0.40 | −16.7 |
| 55–64 | 1.48 | 1.17 | −20.9 | 0.84 | 0.68 | −19.0 | |
| 65–74 | 3.18 | 2.40 | −24.5 | 1.85 | 1.37 | −25.9 | |
| 75–84 | 4.89 | 3.74 | −23.5 | 3.44 | 2.40 | −30.2 | |
| 0–84 | 9.82 | 7.62 | −22.4 | 6.48 | 4.77 | −26.4 | |
| Proximal colon | < 55 | 0.14 | 0.15 | +7.1 | 0.14 | 0.13 | −7.1 |
| 55–64 | 0.30 | 0.30 | 0.0 | 0.22 | 0.24 | +9.1 | |
| 65–74 | 0.77 | 0.75 | −2.6 | 0.60 | 0.61 | +1.7 | |
| 75–84 | 1.60 | 1.44 | −10.0 | 1.40 | 1.16 | −17.1 | |
| 0–84 | 2.79 | 2.61 | −6.5 | 2.34 | 2.13 | −9.0 | |
| Distal colon | < 55 | 0.17 | 0.14 | −17.6 | 0.16 | 0.13 | −18.8 |
| 55–64 | 0.49 | 0.35 | −28.6 | 0.30 | 0.20 | −33.3 | |
| 65–74 | 1.19 | 0.73 | −38.7 | 0.65 | 0.35 | −46.2 | |
| 75–84 | 1.82 | 1.18 | −35.2 | 1.09 | 0.61 | −44.0 | |
| 0–84 | 3.62 | 2.37 | −34.5 | 2.18 | 1.28 | −41.3 | |
| Rectum | < 55 | 0.28 | 0.22 | −20.6 | 0.19 | 0.15 | −21.1 |
| 55–64 | 0.70 | 0.53 | −24.1 | 0.32 | 0.24 | −25.0 | |
| 65–74 | 1.25 | 0.94 | −24.8 | 0.62 | 0.42 | −32.3 | |
| 75–84 | 1.55 | 1.18 | −23.9 | 0.99 | 0.64 | −35.4 | |
| 0–84 | 3.74 | 2.84 | −24.1 | 2.11 | 1.45 | −31.3 | |
| Cumulative mortality [%] | |||||||
| Any | < 55 | 0.17 | 0.12 | −29.4 | 0.12 | 0.09 | −25.0 |
| 55–64 | 0.52 | 0.35 | −32.7 | 0.29 | 0.19 | −34.5 | |
| 65–74 | 1.33 | 0.89 | −33.1 | 0.73 | 0.46 | −37.0 | |
| 75–84 | 2.63 | 1.86 | −29.3 | 1.83 | 1.09 | −40.4 | |
| 0–84 | 4.59 | 3.19 | −30.5 | 2.95 | 1.83 | −38.0 | |
| Colon | < 55 | 0.11 | 0.07 | −36.4 | 0.08 | 0.06 | −25.0 |
| 55–64 | 0.32 | 0.2 | −37.5 | 0.2 | 0.13 | −35.0 | |
| 65–74 | 0.9 | 0.54 | −40.0 | 0.53 | 0.31 | −41.5 | |
| 75–84 | 1.92 | 1.24 | −35.4 | 1.37 | 0.78 | −43.1 | |
| 0–84 | 3.22 | 2.04 | −36.6 | 2.17 | 1.28 | −41.0 | |
| Rectum | < 55 | 0.06 | 0.05 | −16.7 | 0.04 | 0.03 | −25.0 |
| 55–64 | 0.2 | 0.15 | −25.0 | 0.09 | 0.07 | −22.2 | |
| 65–74 | 0.43 | 0.35 | −18.6 | 0.2 | 0.15 | −25.0 | |
| 75–84 | 0.72 | 0.63 | −12.5 | 0.46 | 0.31 | −32.6 | |
| 0–84 | 1.41 | 1.18 | −16.3 | 0.79 | 0.56 | −29.1 | |
In line with the incidence trends, we observed the greatest reductions in cumulative risk of CRC mortality for the age groups 55–64 years (men –32.7%, women –34.5%), 65–74 years (men –33.1%, women –37.0%), and, for women, 75–84 years (–40.4%).
Discussion
Principal findings
We found large declines in CRC incidence and mortality for the periods concerned, most pronounced in the age groups for which screening colonoscopy was offered. While the incidence of rectal cancer and in particular distal colon cancer decreased strongly, for cancers in the proximal colon no reduction in incidence was observed over time for men and only a moderate reduction for women. Nevertheless, a major shift toward detection at earlier stages was observed for proximal cancers.
Comparison with data from RCTs and observational studies
Several randomized intervention studies of screening colonoscopy are ongoing; however, final results are not expected before the late 2020s (12– 14). In randomized trials of flexible sigmoidoscopy from the UK (15) and the USA (16) with long-term (16–17 years) follow-up of the study participants, intention-to-screen analyses have shown reductions of about 30–40% in distal CRC incidence and 45–50% in distal CRC mortality. A Norwegian trial found no significant declines in women, but in men there were decreases of 41% in distal CRC incidence and 35% in distal CRC mortality after 15 years of follow-up (17). Colonoscopy ought to be at least as effective in reducing distal CRC incidence and mortality; owing to its visualization of the entire colon and rectum, it can be expected also to reduce the incidence and mortality of proximal cancers (18). However, the evidence on reduction of proximal colon cancer incidence by colonoscopy remains limited and inconsistent (6, 19, 20). A meta-analysis of observational studies estimated that screening colonoscopy decreases CRC incidence and mortality by about 70–80% (6). With sparse data from only three studies (21– 23), however, no statistically significant reduction of proximal colon cancer incidence was found. Further studies likewise found no significant decline in proximal colon cancer incidence (19) or a less pronounced decrease in proximal than in distal CRC incidence (20).
In Germany, the use of colonoscopy has increased considerably since the introduction of screening examinations in 2002 (7, 24, 25). The observed downward trends in incidence and mortality were particularly pronounced for those aged ≥ 55 years, to whom screening colonoscopy was offered. This finding is in line with evidence from randomized trials and observational studies of endoscopic screening. Moreover, we also found a reduction, albeit less pronounced, in cumulative CRC risk in persons < 55 years. This may be explained by increasing use of colonoscopy in the age group 40–54 years, particularly among those with a family history of CRC (26).
Furthermore, the substantial differences in incidence trends between cancers of the distal colon and proximal colon support suggestions of higher effectiveness of screening colonoscopy in preventing distal than proximal cancers. Several factors may contribute to the lower effectiveness of colonoscopy in the proximal colon. These include, for example, lower detection rates of proximal neoplasms, and a higher proportion of serrated lesions in the proximal colon; the latter are more difficult to detect and tend to develop more rapidly into CRC than adenomas, the most frequently occurring precursors of CRC (27, 28).
As for stage distribution, there was a trend towards an increase in the proportion of stage I cancers. At the same time, the proportion of stage IV cancers decreased. This stage migration was most pronounced for proximal cancers. Earlier detection may therefore have contributed to the reduction of mortality from proximal colon cancer, despite the absent or only modest reduction in the incidence of proximal colon cancer. Unfortunately, the available mortality data do not allow reliable distinction between proximal and distal CRC. However, the marked reduction in overall colon cancer mortality (ranging from 35% to 45% in all age groups between 55 and 84 years) is unlikely to have been achieved if mortality from proximal colon cancers (which accounted for the majority of colon cancers in 2016) had not declined as well. Nevertheless, given the high proportion of cases with missing or unknown stage (38–56%), the findings on trends in stage distribution need to be interpreted with caution. Furthermore, stage migration (particularly from stage II to stage III) may have played a part, due to a more extensive scrutiny of lymph nodes in recent years (29). In our study, a considerable increase in stage III rectal cancers was particularly striking.
The role of other factors
Along with screening colonoscopy, the increased use of diagnostic colonoscopies may have played a role in the observed trends. Equally, the guaiac-based fecal occult blood test (gFOBT) offered since 1977, may have contributed to the reduction in mortality. Furthermore, the trends in incidence may have been influenced by changes in the prevalence of protective factors such as regular intake of aspirin (30, 31) and physical activity (32) and risk factors such as smoking, alcohol consumption, and overweight (32). It is, however, highly unlikely that these factors have played a major role, as such a pronounced decrease in incidence would have required drastic positive shifts in these factors, for which there is no evidence (33, 34). On the other hand, advances in CRC treatment (35) may have contributed to the decline in CRC mortality, particularly in view of the fact that mortality decreased more sharply than incidence.
Strengths and limitations
To our knowledge, this study represents the first comprehensive nationwide analysis of trends in CRC incidence and mortality in Germany, as well as stage distribution by sex, age, and tumor site, since Germany became one of the first countries to introduce colonoscopy as a primary screening test in 2002.
In evaluating the study, however, some limitations need to be borne in mind. First, due to legal considerations the cancer registry data could not be linked with the data from the screening colonoscopy program. This precluded comparative analysis of CRC incidence, mortality, and stage distribution between those who underwent screening colonoscopy and those who did not. Second, since over 60% of deaths from colon cancer were registered as overlapping or unspecific neoplasms, we were unable to differentiate proximal from distal cancers when analyzing mortality. Finally, in a high proportion of cases—which steadily decreased over time—the stage was missing or unknown. Analyses of stage-specific incidence were therefore not conducted as they would have been highly biased.
Conclusion
Our study shows substantial decreases in CRC incidence (between 2000 and 2016) and CRC mortality (between 2000 and 2018) in Germany. Both detection and removal of precancerous lesions and early detection of cancers on either screening or diagnostic colonoscopy, which are now performed in a majority of older adults (36), are likely to have made major contributions to the observed trends. The different patterns in incidence between the different tumor sites suggest that colonoscopies have been more effective in detecting and removing precancerous lesions in the distal colon and rectum than in the proximal colon. Enhanced awareness on the part of endoscopists and further improvement of their training in the detection of precancerous lesions (36), including serrated lesions in the proximal colon, allied with advances in technology, may help to strengthen prevention of proximal cancers in the future. Even stronger reductions of CRC incidence and mortality could be achieved, however, if the existing screening options, i.e., fecal immunochemical tests or colonoscopy, were taken up by a larger proportion of the target population (25, 37, 38). This could be most effectively achieved by an organized screening program combining readily understandable information with low-threshold access to effective screening options (39, 40).
Supplementary Material
eMethods
Estimation of colorectal cancer incidence in Germany
In Germany, cancer registration is carried out at federal state level, and completeness varies across registries. Therefore, the incidence of colorectal cancer (CRC) cannot rely solely on registered cases. The CRC incidence rates provided by the German Center for Cancer Registry Data (ZfKD) are estimates derived using a mixed Poisson regression model accounting for cancer-specific mortality, population size, and year of diagnosis, stratified by sex and age group. For adjustment of the model, data from registries with < 15% of cases registered solely on the basis of the death certificate in each year of the study period were used (2).
Analyses of CRC incidence by tumor site
Separate estimates for proximal colon cancer (C18.0–C18.4), distal colon cancer (C18.5–C18.7), and unspecified or overlapping sites (C18.8–C18.9) were calculated by multiplying the official estimates for colon cancer (C18) with the proportion for each subsite (by age, sex, and year of diagnosis), using all regional registries that could contribute data for the respective year. Cancers of overlapping or unspecific sites of the colon (C18.8–C18.9), which comprised 15–24% of all CRC, were allocated proportionally (by year, sex, and 5-year age groups) to the groups of cases with proximal or distal colon cancer according to the size of those groups.
Analyses of stage distribution
Stages I, II, III, and IV were derived according to the Union for International Cancer Control (UICC) TNM classification valid at the time of diagnosis. Analogous to the analyses of incidence, analyses of stage distribution were also conducted for all CRC sites (C18-C20) as well as separately for cancers in the proximal colon, distal colon, and rectum. We used all registries that could contribute data for each year, with the exception of the Rhineland–Palatinate cancer registry, for which data on stage were absent or the stage was not known in more than 83% of cases each year.
Analyses of CRC mortality
For mortality, colon cancer (C18) and rectal cancer (C19-C20) were analyzed separately; no further stratification by proximal or distal colon was applied, however, as for over 60% of deaths from colon cancer no more specific differentiation of site was possible on the basis of the death certificate.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2019;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Robert Koch-Institut and Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (eds.) Krebs in Deutschland 2015/2016. Berlin, www.krebsdaten.de and www.gekid.(last accessed on 25 January 2021) 2018 [Google Scholar]
- 3.Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61:1036–1040. doi: 10.1136/gutjnl-2011-300774. [DOI] [PubMed] [Google Scholar]
- 4.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 5.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348 doi: 10.1136/bmj.g2467. g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Stock C, Jansen L, Chang-Claude J, Hoffmeister M, Brenner H. Trends in colonoscopy and fecal occult blood test use after the introduction of dual screening offers in Germany: results from a large population-based study, 2003-2016. Prev Med. 2019;123:333–340. doi: 10.1016/j.ypmed.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 8.Brenner H, Schrotz-King P, Holleczek B, Katalinic A, Hoffmeister M. Declining bowel cancer incidence and mortality in Germany: an analysis of time trends in the first ten years after the introduction of screening colonoscopy. Dtsch Arztebl Int. 2016;113:101–106. doi: 10.3238/arztebl.2016.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statistisches Bundesamt Federal Health Monitoring System. Das Informationssystem der Gesundheitsberichterstattung des Bundes. www.gbe-bund.de (last accessed on 21 October 2020) [Google Scholar]
- 10.Day NE. Cancer incidence in five continents. Cumulative rate and cumulative risk. IARC Sci Publ. 1992:862–864. [PubMed] [Google Scholar]
- 11.US National Cancer Institute. Joinpoint Regression Program, version 4.7.0.0 (February 2019) Statistical methodology and applications branch, surveillance research program [Google Scholar]
- 12.Kaminski MF, Bretthauer M, Zauber AG, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44:695–702. doi: 10.1055/s-0032-1306895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 14.Dominitz JA, Robertson DJ, Ahnen DJ, et al. Colonoscopy vs. fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM): rationale for study design. Am J Gastroenterol. 2017;112:1736–1746. doi: 10.1038/ajg.2017.286. [DOI] [PubMed] [Google Scholar]
- 15.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389:1299–1311. doi: 10.1016/S0140-6736(17)30396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol Hepatol. 2019;4:101–110. doi: 10.1016/S2468-1253(18)30358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holme Ø, Løberg M, Kalager M, et al. Long-term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Ann Intern Med. 2018;168:775–782. doi: 10.7326/M17-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378:1734–1740. doi: 10.1056/NEJMsr1714643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morois S, Cottet V, Racine A, et al. Colonoscopy reduced distal colorectal cancer risk and excess cancer risk associated with family history. Cancer Causes Control. 2014;25:1329–1336. doi: 10.1007/s10552-014-0438-7. [DOI] [PubMed] [Google Scholar]
- 20.Steffen A, Weber MF, Roder DM, Banks E. Colorectal cancer screening and subsequent incidence of colorectal cancer: results from the 45 and Up Study. Med J Aust. 2014;201:523–527. doi: 10.5694/mja14.00197. [DOI] [PubMed] [Google Scholar]
- 21.Cotterchio M, Manno M, Klar N, McLaughlin J, Gallinger S. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control. 2005;16:865–875. doi: 10.1007/s10552-005-2370-3. [DOI] [PubMed] [Google Scholar]
- 22.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med. 2013;158:312–320. doi: 10.7326/0003-4819-158-5-201303050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146:709–717. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Guo F, Chen C, Schöttker B, Holleczek B, Hoffmeister M, Brenner H. Changes in colorectal cancer screening use after introduction of alternative screening offer in Germany: prospective cohort study. Int J Cancer. 2020;146:2423–2432. doi: 10.1002/ijc.32566. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso R, Niedermaier T, Chen C, Hoffmeister M, Brenner H. Colonoscopy and sigmoidoscopy use among the average-risk population for colorectal cancer: a systematic review and trend analysis. Cancer Prev Res (Phila) 2019;12:617–630. doi: 10.1158/1940-6207.CAPR-19-0202. [DOI] [PubMed] [Google Scholar]
- 26.Weigl K, Tikk K, Hoffmeister M, et al. Prevalence of a first-degree relative with colorectal cancer and uptake of screening among persons 40 to 54 years old. Clin Gastroenterol Hepatol. 2020;18:2535–2543. doi: 10.1016/j.cgh.2019.11.044. e3. [DOI] [PubMed] [Google Scholar]
- 27.Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019;157:949–966. doi: 10.1053/j.gastro.2019.06.041. e4. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmeister M, Bläker H, Jansen L, et al. Colonoscopy and reduction of colorectal cancer risk by molecular tumor subtypes: a population-based case-control study. Am J Gastroenterol. 2020;115:2007–2016. doi: 10.14309/ajg.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 29.Bläker H, Hildebrandt B, Riess H, et al. Lymph node count and prognosis in colorectal cancer: the influence of examination quality. Int J Cancer. 2015;136:1957–1966. doi: 10.1002/ijc.29221. [DOI] [PubMed] [Google Scholar]
- 30.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–769. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Cancer Research Fund, American Institute for Cancer Research. Continuous update project expert report 2018. Diet, nutrition, physical activity and colorectal cancer. www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf (last accessed 10 June 2020) [Google Scholar]
- 33.Behrens G, Gredner T, Stock C, Leitzmann MF, Brenner H, Mons U. Cancers due to excess weight, low physical activity, and unhealthy diet. Dtsch Arztebl Int. 2018;115:578–585. doi: 10.3238/arztebl.2018.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mons U, Gredner T, Behrens G, Stock C, Brenner H. Cancers due to smoking and high alcohol consumption. Dtsch Arztebl Int. 2018;115:571–577. doi: 10.3238/arztebl.2018.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 36.Brenner H, Altenhofen L, Kretschmann J, et al. Trends in adenoma detection rates during the first 10 years of the German screening colonoscopy program. Gastroenterology. 2015;149:356–366. doi: 10.1053/j.gastro.2015.04.012. e1. [DOI] [PubMed] [Google Scholar]
- 37.Cardoso R, Guo F, Heisser T, Hoffmeister M, Brenner H. Utilisation of colorectal cancer screening tests in European countries by type of screening offer: results from the European Health Interview Survey. Cancers (Basel) 2020;12 doi: 10.3390/cancers12061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155:1383–1391. doi: 10.1053/j.gastro.2018.07.017. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruner LF, Hoffmeister M, Ludwig L, Brenner H. The effects of differing invitation models on the uptake of immunological fecal occult blood testing. Dtsch Arztebl Int. 2020;117:423–430. doi: 10.3238/arztebl.2020.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1645–1658. doi: 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Estimation of colorectal cancer incidence in Germany
In Germany, cancer registration is carried out at federal state level, and completeness varies across registries. Therefore, the incidence of colorectal cancer (CRC) cannot rely solely on registered cases. The CRC incidence rates provided by the German Center for Cancer Registry Data (ZfKD) are estimates derived using a mixed Poisson regression model accounting for cancer-specific mortality, population size, and year of diagnosis, stratified by sex and age group. For adjustment of the model, data from registries with < 15% of cases registered solely on the basis of the death certificate in each year of the study period were used (2).
Analyses of CRC incidence by tumor site
Separate estimates for proximal colon cancer (C18.0–C18.4), distal colon cancer (C18.5–C18.7), and unspecified or overlapping sites (C18.8–C18.9) were calculated by multiplying the official estimates for colon cancer (C18) with the proportion for each subsite (by age, sex, and year of diagnosis), using all regional registries that could contribute data for the respective year. Cancers of overlapping or unspecific sites of the colon (C18.8–C18.9), which comprised 15–24% of all CRC, were allocated proportionally (by year, sex, and 5-year age groups) to the groups of cases with proximal or distal colon cancer according to the size of those groups.
Analyses of stage distribution
Stages I, II, III, and IV were derived according to the Union for International Cancer Control (UICC) TNM classification valid at the time of diagnosis. Analogous to the analyses of incidence, analyses of stage distribution were also conducted for all CRC sites (C18-C20) as well as separately for cancers in the proximal colon, distal colon, and rectum. We used all registries that could contribute data for each year, with the exception of the Rhineland–Palatinate cancer registry, for which data on stage were absent or the stage was not known in more than 83% of cases each year.
Analyses of CRC mortality
For mortality, colon cancer (C18) and rectal cancer (C19-C20) were analyzed separately; no further stratification by proximal or distal colon was applied, however, as for over 60% of deaths from colon cancer no more specific differentiation of site was possible on the basis of the death certificate.