Abstract
Background
Some 5–10% of the German population are affected by Raynaud’s phenomenon (RP). In around 10–20% of cases RP arises from an underlying disease, most commonly a connective tissue disease. This review encompasses the diagnosis and differential diagnosis of RP and examines the efficacy of the currently available pharmaceutical and non-pharmaceutical treatment options.
Methods
We conducted a selective literature search in PubMed using the search terms “Raynaud‘s phenomenon”, “Raynaud‘s syndrome,” “vasospasm,” “vascular acrosyndrome,” and “systemic sclerosis,” together with a search of the Cochrane Database of Systematic Reviews up to April 2020.
Results
Raynaud’s phenomenon mainly affects the fingers or toes and is typically triggered by cold or emotional stressors. The most important diagnostic steps are demonstration of a tendency towards digital vasospasm, exclusion of occlusions in the afferent arteries and acral vessels, nail-fold capillaroscopy, and determination of autoantibody status. Tumor screening should be arranged in the presence of B symptoms or first manifestation of RP in old age. The onset of RP in childhood is a rare occurrence and points to a secondary origin. The principal options for treatment are protection against cold and administration of calcium antagonists, which reduces the occurrence of RP by around 20–40 %. The treatment of RP in patients with systemic sclerosis is described in the recommendations of the European League Against Rheumatism (EULAR).
Conclusion
At onset or after years of latency, patients with Raynaud phenomenon may have an underlying disease (most commonly a connective tissue disease). Long-term specialist care is necessary for asymptomatic patients with risk factors and for those with clinically manifest symptoms of an underlying condition alike.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submissions is 22 April 2022.
Raynaud’s phenomenon (RP) describes an intermittent change in color in affected acra from white to bluish-cyanotic and finally to red, caused by vasospasm (ischemic phase) and subsequent vasodilation (hyperemic phase) of digital arteries (1) (Figure 1). It is not always the case that all three color changes occur. According to the European Society of Vascular Medicine (ESVM) guideline, a phase of white discoloration must, by definition, occur (1), while other authors also consider intermittent cyanosis to be sufficient (“blue Raynaud’s phenomenon”) (2). RP manifests primarily on the fingers and toes, and less frequently on other acra (1, 2). The duration of individual attacks varies, lasting on average 20 min (2). RP occurs at least once a day in 60% of patients, while 10% report between one and three attacks per month and 1% report occasional attacks (2).
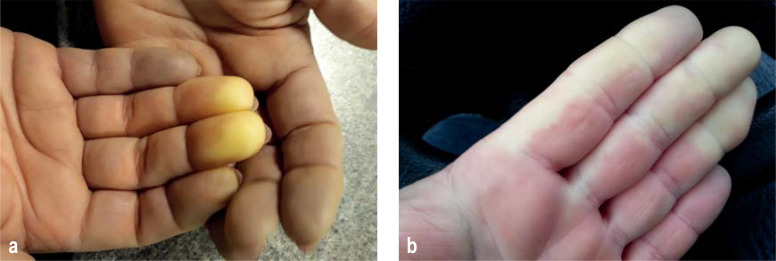
Figure 1 (a, b).
Typical “white phase” of Raynaud’s phenomenon in the fingers
Cold and emotional stressors act as triggers of the vasospasm (1, 2). It is extremely rare for RP to occur in the absence of these triggers. Since the color changes are regularly accompanied by pain, dysesthesia, motor impairment, and general cold intolerance, the term “Raynaud’s syndrome” is also commonly used in German-speaking countries. A distinction is made between primary Raynaud’s phenomenon (without underlying disease) and secondary Raynaud’s phenomenon (with underlying disease), with the most common being systemic sclerosis (1). The initial manifestation of RP can precede the onset of a connective tissue disease by years to decades (1, 2). The rate of transition from seemingly primary RP to secondary RP is approximately 10% over 10 years (3).
In addition to connective tissue diseases and vasculitis, other causal factors include arterial occlusion of other etiologies in the hand or arm, malignancies, hand arm vibration syndrome, and occupational exposure, for example to vinyl chloride (1). There is also an association with upper limb compression syndromes, the causality of which has not been elucidated (1). Raynaud’s phenomenon can also be triggered or exacerbated by medications and drugs (4) (box). In primary FP, an increased incidence of affective and anxiety disorders has also been reported (5).
BOX. Medications/drugs that can trigger or exacerbate Raynaud’s phenomenon.
ß-Blockers
Clonidine
Anti-migraine drugs including monoclonal CGRP antibodies
Ergotamine derivatives including bromocriptine
Interferon-α and -ß
Catecholamines
Cyclosporine
Cisplatin and derivatives
Bleomycin
Tyrosine kinase inhibitors
Selective serotonin-reuptake inhibitors
Estrogens
Amphetamine and derivatives
Ribavirin
Gemcitabine
Secukinumab
Cocaine and derivatives
Nicotine
Proton pump inhibitors?
CGRP, calcitonin gene-related peptide
The frequency of the presence of primary and secondary RP at the time of initial diagnosis varies depending on age (high probability of secondary RP in children and older patients). As a result of selection mechanisms in the referral process, greater familiarity with the differential diagnosis, and due to the fact that discriminatory testing methods such as capillaroscopy are virtually only available in specialized medical centers, secondary RP is diagnosed earlier and more frequently in such centers.
In the Framingham study, secondary RP was found in about 20% of cases, based on the general population (6). In a meta-analysis including 639 patients and a prospective study with 586 patients, 12.6 and 13.6%, respectively, of patients that first presented to a physician for RP developed a manifest connective tissue disease within 10–20 years (7, 8). Therefore, in the primary care setting, one can assume a low probability (10–20%) of secondary RP at the time of initial diagnosis.
Vasospastic syndrome describes the overlapping of RP with other disorders caused by vasospasm. Migraine is the most frequent concomitant disorder. Conversely, RP is found in every third to fifth migraine patient asked in a targeted manner (9), which is relevant in terms of differential therapy, since medications for the treatment and prevention of migraine can exacerbate RP (box).
Prevalence in the population
The Framingham study found a prevalence of Raynaud’s phenomenon of 9.6% in women and 8.1% in men (6). For Germany, a prevalence of 5.8% has been calculated on the basis of more recent data (10). The frequency of RP depends on geographical latitude (being more frequent in the north than in the south) and altitude (being more frequent in mountainous regions than in lowland areas), and also varies according to season (occurring more often in the winter than in the summer) (1). Women tend to be more frequently affected compared to men, whereby the sex ratio varies in the literature (1, 2, 6).
Freedman et al. found a familial aggregation of RP in approximately 25% of first-degree relatives (11), while studies on twins demonstrated a higher heritability rate of 55% (12). A recent study identified an association with a variant in the NOS1 gene, the precise functional characterization of which is still awaited (13).
Pathophysiology
There are numerous reports of impaired temperature regulation and vascular responsiveness to cold stimuli in patients with primary Raynaud’s phenomenon. According to Flavahan, RP occurs primarily in skin areas with a high density of AV anastomoses, which are equipped with two different sympathetic transmission systems: norepinephrine (vasoconstrictive) and acetylcholine (vasodilatory) (14). Cold physiologically results in vasoconstriction of the AV shunts. Local cold exposure (hands, fingers) appears to be more important in this process than does the central sympathetic outflow, as induced by a drop in ambient temperature (14). In contrast to healthy individuals, patients with RP respond to local cold stimuli at finger temperatures of <25 °C with vasospasm, which advances to complete temporary vascular occlusion if the temperature is further reduced to an average of 19 °C (15). It is still largely unclear what role thermosensitive transient receptor potentials (TRPs) play in this process and how they interact with endothelial vasoactive peptides and neuronal effects (13). In systemic sclerosis, vasospasm encounters vessels that are already structurally compromised and stenosed by subintimal fibrosis, making it easier for RP to manifest (16).
Diagnostic approach
On the part of the primary care provider, it is important to take a detailed medical history, measure the patient’s pulse, and perform a functional test to evaluate blood flow to the hand via the radial and ulnar arteries (Allen’s test). The demonstration of a tendency towards digital vasospasm, the identification of brachial and acral artery stenosis or occlusion, the performance of nailfold capillaroscopy, as well as the determination of antinuclear antibodies (ANA) are specialist tasks primarily reserved for rheumatologists, angiologists, and dermatologists (17) (table 1). If the patient exhibits B symptoms or new-onset RP in old age—the literature does not permit an exact cut-off value to be derived—tumor screening should also be initiated, since RP can also manifest as a paraneoplastic acrosyndrome in rare cases (18). There is no evidence of an association with specific malignancies (18). Differences between primary and secondary RP are listed in Table 2.
Table 1. Workflow on the differential diagnostic investigation of Raynaud’s phenomenon.
| Family physician | Specialist physician | |
| General history-taking including family, medication, and drug history | + | |
| Occupational history and leisure behavior | + | |
| Pulse | + | + |
| Allen test | + | + |
| Measurement of acral skin blood flow using digital photoplethysmography (D-PPG) and/or other methods involving thermal and/or pharmaceutical provocation | + | |
| Color duplex sonography | + | |
| Antibody testing (ANA, ENA) | + | |
| Nailfold capillaroscopy | + | |
| General and gender-specific tumor screening in the case of late onset and/or B symptoms | + |
ANA, antinuclear antibodies; ENA, extractable nuclear antigens
Table 2. Differentiation between primary and secondary Raynaud’s phenomenon (RP).
| Primary RP | Secondary RP | |
| Associated disorder (underlying disease) | No | Yes |
| Age at onset | Adolescence, young adulthood | Childhood, old age |
| Pattern of clinical manifestation | Seasonally variable severity; typical three color change | Variable; often tends towards permanent acral ischemia |
| Arterial stenosis/ occlusion | No | Frequent |
| Digital ulcers | No | Frequent |
| Nailfold capillaroscopy | Normal findings or nonspecific morphological changes | Depending on underlying disease, nonspecific morphological changes or typical disease pattern (e.g., scleroderma pattern) |
| Autoantibodies | Negative or low-titer without detection of specific antigens (nonspecific antibodies) | Positive, with antigen detection (specific antibodies) |
In the case of suspected occupational disease (for example, hand arm vibration syndrome), it is important to report this to the responsible employers’ liability insurance association in order to initiate an investigation procedure, and to seek the advice of a rheumatologist in the case of suspected connective tissue disease or vasculitis.
Looking out for “red flags” is crucial to the early detection of systemic sclerosis:
Raynaud’s phenomenon
Puffy fingers
Positive specific autoantibodies and/or
Abnormal capillaroscopy (8, 19). Advanced scleroderma-like disorders exhibit typical skin and hand changes, often accompanied by permanent acral ischemia (Figure 2).
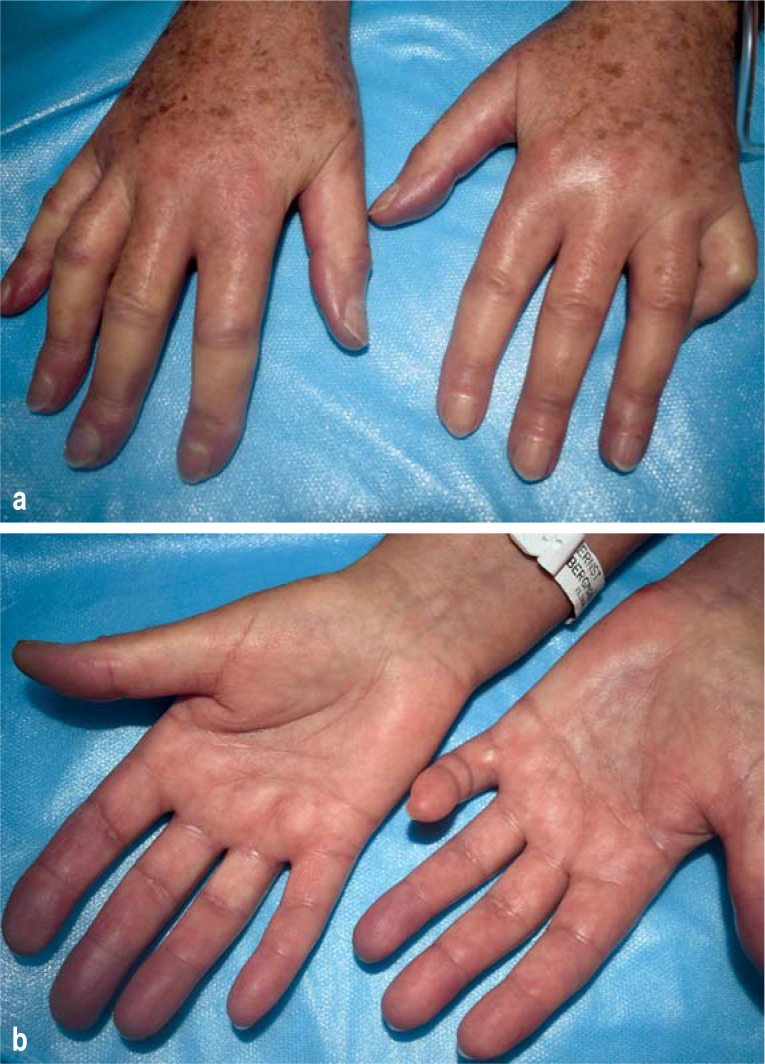
Figure 2 (a, b).
Systemic sclerosis with permanent digital ischemia in hand/digital artery occlusion and early flexion contracture of the fingers
Autoantibody determination
The determination of antinuclear antibodies (ANA) functions as a screening test for the presence or identification of an increased risk for the development of a connective tissue disease (1, 7, 8, 19). In a prospective study of 1039 patients, of which >10% were followed-up for more than 10 years, only 2% of patients with RP, no evidence of digital/hand artery occlusion, and negative ANA developed a connective tissue disease (20). However, the positive predictive value of ANA screening alone for the development of a connective tissue disease is low at approximately 30% (7). Therefore, in the case of a positive test, screening for extractable nuclear antigens (ENA) should always be additionally performed in order to detect specific disease-related antibodies (for example, anti-double-stranded DNA antibodies [anti-dsDNA ab] in lupus erythematosus [SLE], anti-topoisomerase antibodies in diffuse systemic sclerosis, anti-centromere antibodies in limited systemic sclerosis, and anti-Ro/SSA or anti-La/SSB antibodies in Sjögren’s syndrome) (21).
Nailfold capillaroscopy
This examination serves the same purpose as ANA screening (1, 7, 8, 19). At 47%, its positive predictive value is better than that of ANA screening (7). Capillaroscopy is able to clearly distinguish between “normal findings” and “abnormal findings” (interobserver agreement for “normal findings,” 0.95–0.98) (22). A nailfold capillary density of = 7/mm, as well as hairpin-shaped and/or tortuous capillaries with a narrow apex speak strongly against the presence of a connective tissue disease (figure 3). On the other hand, the practiced capillaroscopist is able to identify patterns of findings that permit their classification as a connective tissue disease with a high degree of probability and enable early detection of risk (interobserver agreement, 0.90–0.96) (22, 23). Apical ectasia, giant capillaries (apical diameter > 50 µm), as well as capillary loss and hemorrhage are the most readily reproducible morphological changes (24) (figure 4).
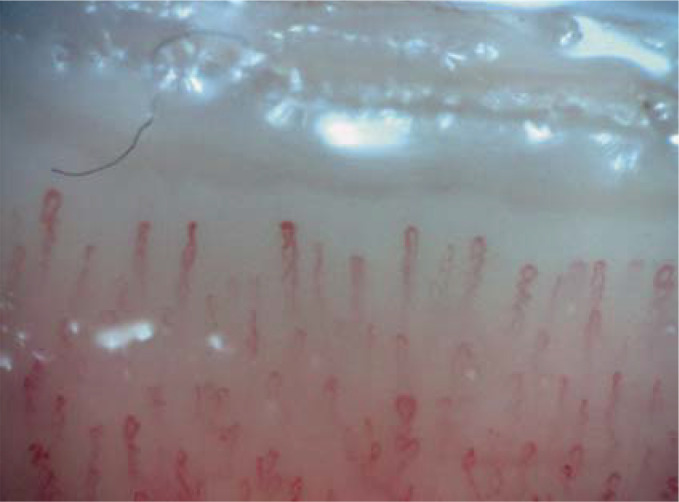
Figure 3.
Example of normal findings in nailfold capillaroscopy showing normal capillary density and normal capillary morphology
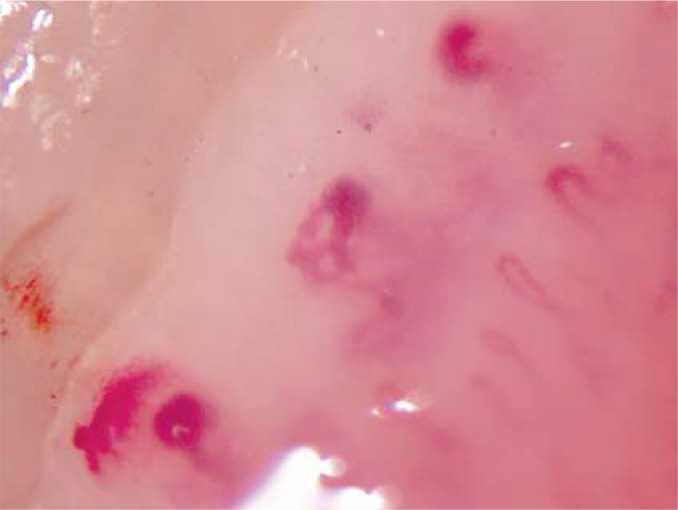
Figure 4.
Nailfold capillary morphological findings in systemic sclerosis
Relevance of combining antibody determination and capillaroscopy
By combining specific autoantibody diagnostic tests and nailfold capillaroscopy, it is possible to identify significantly more at-risk patients compared to individual investigations (8). If, at the time of initial presentation due to Raynaud’s phenomenon, scleroderma-specific autoantibodies were positive and abnormal capillaroscopy showing capillary loss and capillary telangiectasia was also present, the adjusted hazard ratio for the development of a connective tissue disease within an observation period of up to 20 years was 60.08; 79.5% of the study patients developed systemic sclerosis. In contrast, isolated abnormal capillaroscopy or positive antibody detection alone were significantly less predictive, with adjusted hazard ratios of 5.03 and 8.5, respectively. If both investigations were normal, only 1.8% of patients developed systemic sclerosis (8). The present study supports the guideline recommendation to always perform both investigations at initial diagnosis of RP (1).
Specialist medical follow-up
There are no evidence-based recommendations to date on the duration or time intervals of follow-up. Due to limited angiology and rheumatology resources in Germany, specialist medical follow-up at 2- to 3-year intervals in clinically asymptomatic patients in whom specific autoantibodies have been detected and/or abnormal nailfold capillaroscopy has been recorded seems judicious to us. In our opinion, patients with a very high probability of primary RP (young women, positive family history, as well as no clinical, serological, or capillaroscopic abnormalities) do not require specialist follow-up. These patients should be advised to return for a specialist consultation if the RP worsens and/or hand/finger edema develops.
Raynaud’s diary
In order to better assess subjectively perceived impairment as well as the response to treatment, a Raynaud’s diary should be kept, at least temporarily (initial diagnosis, change of medication, phases of worsening), recording the frequency, duration, and severity of attacks (visual analog scale 1–10). A simplification and updating of data collection and transmission by means of a digital health application would be desirable in the future.
Treatment of Raynaud’s phenomenon
Drug treatment is not indicated in all cases of RP. In the case of infrequent attacks or mild subjective impairment, general measures to protect against cold, as well as avoidance of specific trigger situations, are sufficient (1). Table 3 summarizes the effects of non-pharmaceutical and pharmaceutical treatment interventions in RP.
Table 3. Effects of non-pharmaceutical and pharmaceutical treatment of Raynaud’s phenomenon (RP).
| Treatment |
Type of study Patient number |
Raynaud’s attacks | ||
| Publication | Number of attacks | Duration | Severity of attacks | |
| Form of RP | ||||
| Low-level laser | Placebo-controlled, double-blind crossover study; n = 48 patients |
Laser treatment: reduction in RP from 2.5 ± 1.5 attacks per day to 1.6 ± 1.0; (−36% compared to baseline); sham treatment: reduction from 2.1 ± 1.2 to 2.0 ± 1.2; (−4.8 % compared to baseline); ARR: 0.31; NNT: 4 |
Not determined | Reduction on VAS (10 cm) from 3.2 ± 1.2 to 2.3 ± 1.0 (−28 % compared to baseline); control treatment: reduction from 2.9 ± 1.2 to 2.8 ± 1.1; (−3.4% compared to baseline); ARR: 0.25; NNT: 4 |
| Hirschl, 2004 (25) | ||||
| Primary | ||||
| Complementary. Use of galvanic current |
Single-blinded randomized controlled study with parallel design; n = 34 patients |
Reduction from 31.29 ± 21.02 attacks per week to 11.53 ± 8.55 following active treatment in week 7 (−63% compared to baseline) and further to 8.23 ± 9.97 at the end of the observation phase in week 15 (−74 % compared to baseline); increase in attacks in the control group from 25.11 ± 9.9 to 27.17 ± 8.16 in week 7 (+8% compared to baseline) and to 28.00 ± 7.83 in week 15 (+12% compared to baseline) ARR: 0.71; NNT: 2 (week 7) ARR: 0.86; NNT: 2 (week 15) |
Not determined | Reduction on the VAS from 2.64 ± 2.22 to 1.66 ± 1.92 in week 7 (−37 % compared to baseline) and further to 2.59 ± 2.64 in week 15 (−2 % compared to baseline); increase in the control group from 3.60 ± 2.58 to 4.4 ± 2.66 in week 7 (+22 % compared to baseline) and further to 4.79 ± 2.33 in week 15 (+33 % compared to baseline) ARR: 0.59; NNT: 2 (week 7) ARR: 0.35; NNT: 3 (week 15) |
| Tapia-Haro, 2020 (26) | ||||
| Primary and secondary | ||||
| Calcium alcium channel blockers (primarily nifedipine) | Meta-analysis of 23 randomized controlled studies; n = 528 patients |
MWD –6.13 (−6.60 to –5.67) attacks per week (−45 % compared to control group); after exclusion of an older study that showed an overproportionately positive effect, the MWD was only –2.93 (−3.44 to –2.43) attacks per week (−21 % compared to control group); mean value for the control group (placebo) 13.7 attacks per week (no confidence interval given) |
MWD –1.67 (−3.29 to 0) min (−9% compared to control group) mean value for the control group (placebo) 18.8 min (no confidence interval given) | MWD –0.62 (−0.72 to –0.51) on the VAS (10 cm); −9 % compared to the control group;mean value for the control group (placebo) 6.7 cm (no confidence interval given) |
| Rirash, 2017 (28) | ||||
| Primary and secondary | ||||
| Selective serotonin reuptake inhibitor fluoxetine | Prospective randomized crossover study over 16 weeks during the winter period with two 14-day washout periods;fluoxetine 20 mg/day versus nifedipine 40 mg/day; n = 53 patients |
Cannot be reliably determined, since no numerical treatment effects and confidence intervals were given for the two treatment groups compared to the respective baseline value; the figures point to a decrease of around 1.3 attacks per day with fluoxetine from a baseline of 2.98 ± 0.31 attacks (–44%) and a decrease of around 0.5 attacks per day with nifedipine from a baseline of 2.72 ± 0.26 attacks per day (around –18%). |
Not investigated | Cannot be reliably determined, since no numerical treatment effects and confidence intervals were given for the two treatment groups compared to the respective baseline value; the figures point to a difference of around –2 cm on VAS under fluoxetine from a baseline value of 4.35 ± 0.39 (about –46%) and a difference of about –0.8 cm under nifedipine from a baseline value of 3.82 ± 0.36 (about –21%). |
| Coleiro, 2001 (29) | ||||
| Primary (n = 26) and secondary (n = 27) | ||||
| Sartans (losartan) | Prospective controlled randomized parallel-group study over 15 weeks. Losartan 50 mg/day versus nifedipine 2 x 20 mg/day retarded with an initial 3-week washout period; n = 52 patients |
Losartan: reduction from 3.52 ± 2.16 attacks to 1.96 ± 1.90 attacks per day (−44 % compared to baseline); nifedipine: increase from 3.65 ± 3.01 attacks to 4.40 ± 4.17 attacks per day (+21 % compared to baseline); ARR: 0.65; NNT: 2 |
Not determined | Losartan: reduction on VAS from 5.50 ± 2.46 to 2.84 ± 2.40 cm (−48% compared to baseline);nifedipine: reduction on VAS from 4.48 ± 2.40 to 3.90 ± 2.77 cm (−13 % compared to baseline); AAR: 0.35; NNT: 3 |
| Dziadzio, 1999 (31) | ||||
| Primary (n = 25) and secondary (n = 27) (systemic sclerosis) | ||||
| Phosphodiesterase-5 inhibitors (sildenafil, vardenafil, tadalafin) | Systematic review and meta-analysis of 6 RCT; n = 244 patients |
MWD –0.49 (–0.71 to –0.28) attacks per day (−19 % compared to baseline); baseline in the control groups (placebo) 2.64 ± 2.0 attacks per day |
MWD –14.62 (–20.25 to –9.0) min per day (−28 % compared to baseline); baseline in the control groups (placebo) 51.87 ± 68.64 min per day | MWD –0.46 (–0.74 to –0.17) on the Raynaud Condition Score; (−13 % compared to baseline); baseline in the control group (placebo) 3.51 ± 2.34 |
| Roustit, 2013 (32) | ||||
| Secondary (91.8 % systemic sclerosis) | ||||
| Prostanoids (iloprost)* | Meta-analysis of 7 RCT; n = 332 patients |
Undeterminable; baseline in the control group not reported |
MWD 0.0 (−7.28 to –7.28) min;baseline in the control group not reported | MWD –0.69 (−1.12 to –0.26) on the Raynaud Condition Score (when including a study of 11 patients and nine controls with an exceptionally strong treatment effect); baseline in the control group not reported |
| Pope 2000 (34) | ||||
| Secondary (systemic sclerosis) | ||||
| Prostanoids (cyclic iloprost) | 12-Month prospective randomized single-blinded parallel-group study, 46 patients, Iloprost i. v. 2 μg/kg BW, min for 8 h on 5 consecutive days, followed by 1 infusion day every 6 weeks versus oral nifedipine retard 2 x 20 mg/day; n = 46 patients |
Reduction in RP with iloprost from initially 2.17 ± 0.2 to 1.22 ± 0.13 attacks per day (−44% compared to baseline; under nifedipine, reduction from 2.08 ± 0.34 attacks per day to 1.33 ± 0.22 (−36% compared to baseline, ARR: 0.08; NNT: 13 | Not determined | Not determined |
| Scorza 2001 (35) | ||||
| Secondary (systemic sclerosis) | ||||
* The review included one study with cisaprost. Only results for iloprost compared to placebo are given, since cisaprost is not available.
MWD (mean weighted difference): effect measure for continuous endpoints. The MWD is pooled to describe the overall effect in meta-analyses.
In this context, individual studies are weighted differently, for example, due to the size of the study or other quality characteristics.
ARR, absolute risk reduction, and NNT, number needed to treat (1/ARR), were calculated from the published data; i. v., intravenous; BW, body weight; VAS, visual analog scale
Non-pharmaceutical treatment
Protection from the cold, wet, and wind represents the overriding recommendation in terms of RP prevention (1). The scope of possibilities ranges from conventional gloves, heating pads, and pocket warmers to heated gloves, shoe inserts, and socks. To the best of our knowledge, the quantitative effects of these measures on the frequency of RP and quality of life of patients have not been investigated in a targeted manner as yet.
A controlled clinical study on the efficacy of low-level laser therapy showed favorable treatment effects in primary RP (25). RP was reduced on average by about one attack per day, while sham treatment only prevented one attack every 10 days. In addition, RP was somewhat less severe in the laser group. There were no differences in average outdoor temperature during treatment phases. The low number of cases limits the validity of these results. Data on quality of life were not recorded.
In a small controlled study of patients, a Spanish working group described positive effects for the use of galvanic current (20 sessions over 7 weeks), in addition to unspecified baseline pharmaceutical treatment, on the number and severity of RP attacks (26). The treatment effects were unusually pronounced, given that the frequency and intensity of RP in the control group consistently increased over the duration of the study. Since the outdoor temperature was not recorded as part of the study, these effects could be due to climatic differences. Data on quality of life were not recorded.
Relaxation exercises and biofeedback have been investigated primarily in smaller studies, which have been summarized in a meta-analysis (27). Due to a lack of control groups, it was not possible to quantify the treatment effects, meaning that no evidence-based recommendation could be made for or against these therapies (27).
In view of the disorders of emotionality and anxiety described in primary RP, psychotherapeutic treatment approaches are conceivable (5). To our knowledge, no interventional studies in this regard are available to date.
Pharmaceutical treatment
Drugs that induce vasospasm should generally be discontinued or switched (box).
Dihydropyridine-type calcium antagonists are predominantly used to treat RP. A systematic Cochrane review showed an effect for calcium channel blockers primarily on the frequency of RP attacks (28). In untreated patients, an RP attack occurred approximately once per day. At best, calcium channel blockers reduced the frequency of attacks to around one every 2 days. Data on quality of life were not analyzed. Calcium channel blockers often cause adverse drug reactions that limit their use at the high doses tested (for example, 2–3 × 20 mg nifedipine) (hypotension, orthostasis, palpitations, peripheral edema, constipation).
The selective serotonin re-uptake inhibitor (SSRI) fluoxetine was tested versus nifedipine in a controlled study (29). The frequency and severity of RP was reduced. The extremely low number of cases limits the validity of these results. In addition, the publication suffers from severe formal deficiencies. Fluoxetine–like other SSRIs–can trigger vasospasm (30). It has the potential to cause central nervous and cardiac side effects, thereby making patient monitoring necessary. It is not officially approved in Germany for the treatment of RP.
Losartan reduced the frequency and severity of RP compared to nifedipine in a prospective randomized pilot study (31). The small number of cases limits the validity of these results. The observed increase in RP attacks under nifedipine is not explained. No outdoor temperature monitoring was carried out, meaning that temperature differences over the course of the study could have contributed to this increase. Data on quality of life were not recorded. Typical adverse drug reactions (ADRs) to losarten include hypotension, dizziness, fatigue, weakness, and headache. Losarten is not approved in Germany for the treatment of RP.
Controlled studies on the efficacy of phosphodiesterase-5 inhibitors (sildenafil, tadalafin, vardenafil) in primary RP have not been published. A meta-analysis of six randomized controlled trials in systemic sclerosis showed small to moderate positive effects on the frequency, duration, and severity of RP (32). At present, the European League Against Rheumatism (EULAR) makes a Grade A recommendation only for patients at high risk for digital ulcers and florid digital ulcers in the setting of systemic sclerosis (33). ADRs to phosphodiesterase-5 inhibitors include hypotension, cephalalgia, visual disturbances, myalgia, chest pain, dyspepsia, and nasal congestion. Simultaneous use of nitro-compounds must be avoided. PDE-5 inhibitors are not approved in Germany for the treatment of RP.
In a systematic Cochrane review, iloprost was able to reduce only the severity of RP (34). This effect is based on a single older study. Therefore, the authors conclude that it is difficult to find robust evidence for the effective treatment of RP with iloprost in systemic sclerosis (34). A 12-month treatment study with cyclical intravenous iloprost infusion resulted in an absolute risk reduction of 8% compared to nifedipine, albeit with disproportionately higher treatment complexity (35). For this reason, the EULAR recommends intravenous iloprost infusion only when oral treatment options have been exhausted or are not tolerated (33). Randomized clinical studies on the use of iloprost in primary Raynaud’s phenomenon have not been published. Common ADRs include headaches, flushing symptoms, hypotension, nausea, and vomiting.
Riociguat, a soluble guanylate cyclase stimulator, is considered to be a promising candidate for the treatment of RP, but is still in an early phase of clinical trials for this indication (36).
Botulinum toxins are potent neurotoxins produced by clostridia as exotoxins (BTX). In 2004, the first pilot study was published reporting the treatment of RP using perivascular BTX-A injections in the hand (37). BTX-B is now also used in this indication (38). Clinically, highly heterogeneous treatment effects were observed (38– 40). Therefore, at present, the use of BTX cannot be recommended outside controlled clinical trials.
In summary, the efficacy of current drug treatment options for RP is limited and often only supported by low-level evidence.
Questions on the article in issue 16/2021:
Raynaud’s Phenomenon: A Vascular Acrosyndrome That Requires Long-Term Care
The submission deadline is 22 April 2022. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the approximate prevalence of Raynaud’s phenomenon in Germany?
Around 2%
Around 6%
Around 15%
Around 18%
Around 23%
Question 2
Which examination should already be performed by the general practitioner to investigate differential diagnoses of Raynaud’s phenomenon?
Color duplex sonography
Antibody testing
Nailfold capillaroscopy
Pulse measurement and Allen test
Digital photoplethysmography to measure acral skin blood flow
Question 3
Approximately how high is the probability that secondary RP is present when Raynaud’s phenomenon (RP) is first diagnosed in the primary care setting?
10–20%
30–40%
50–60%
70–80%
80–90%
Question 4
Which factors are more suggestive of primary Raynaud’s phenomenon (RP) in the differentiation between primary and secondary RP?
Frequent digital ulcers, starting in childhood
Pre-existing arterial stenosis, positive specific antibody detection
Seasonally variable severity, starting in adolescence/early adulthood
Scleroderma pattern in nailfold capillaroscopy, old age
Old age, relatively frequent/constant acral ischemia
Question 5
What does the abbreviation ANA stand for in the context of a screening test for the risk of developing a connective tissue disease?
Autoneuronal antibodies
Antineuronal antibodies
Antinuclear antigens
Antigen-neutralizing antibodies
Antinuclear antibodies
Question 6
Which two investigations should be performed in combination in order to identify patients at risk for developing a connective tissue disease?
ANA screening and ultrasound
Allen test and tumor screening
ANA screening and nailfold capillaroscopy
Color duplex sonography and pulse measurement
Allen test and drug screening
Question 7
Which drugs are used for the pharmaceutical treatment of Raynaud’s phenomenon?
Beta-blockers
Calcium channel blockers
Estrogens
Cisplatin and derivatives
Clonidine
Question 8
What is the positive predictive value of ANA screening alone in relation to the development of a connective tissue disease?
Around 5%
Around 10%
Around 25%
Around 30%
Around 50%
Question 9
Patients with Raynaud’s phenomenon respond to local cold stimuli on the fingers with vasospasm; a further drop in temperature poses a risk of complete temporary vascular occlusion. Vasospasm or temporary vascular occlusion occur from which approximate temperatures?
<20 °C and 17 °C, respectively
<25 °C and 19 °C, respectively
<28 °C and 22 °C, respectively
<30 °C and 20 °C, respectively
<32 °C and 25 °C, respectively
Question 10
A small randomized controlled study investigated the efficacy of losartan over a 15-week period in relation to Raynaud’s attacks per day. What was the relative reduction in attacks per day compared to baseline?
11%
21%
32%
44%
50%
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interest statement
Dr. Sander received speaker’s fees from Actelion.
The remaining authors declare that no conflict of interests exists.
References
- 1.Belch J, Carlizza A, Carpentier PH, et al. ESVM guidelines - the diagnosis and management of Raynaud‘s phenomenon. Vasa. 2017;46:413–423. doi: 10.1024/0301-1526/a000661. [DOI] [PubMed] [Google Scholar]
- 2.Heidrich H, Helmis J, Fahrig C, et al. Clinical characteristics of primary, secondary and suspected secondary Raynaud’s syndrome and diagnostic transition in the long-term follow-up. A retrospective study in900 patients. VASA. 2008;37:S2–S25. [Google Scholar]
- 3.Hirschl M, Hirschl K, Lenz M, et al. Transition from primary Raynaud‘s phenomenon to secondary Raynaud‘s phenomenon identified by diagnosis of an associated disease: results of ten years of prospective surveillance. Arthritis Rheum. 2006;54:1974–1981. doi: 10.1002/art.21912. [DOI] [PubMed] [Google Scholar]
- 4.Khouri C, Blaise S, Carpentier P, et al. Drug-induced Raynaud‘s phenomenon: beyond ß-adrenoceptor blockers. Br J Clin Pharmacol. 2016;82:6–16. doi: 10.1111/bcp.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigel I, Klein-Weigel P, Kinzl J, et al. Psychometrische Untersuchungen an Tiroler Patienten mit Raynaud Phänomen. Wien Med Wochenschr. 2006;156:574–582. doi: 10.1007/s10354-006-0338-x. [DOI] [PubMed] [Google Scholar]
- 6.Brand FN, Larson MG, Kannel WB, et al. The occurrence of Raynaud‘s phenomenon in a general population: the Framingham Study. Vasc Med. 1997;2:296–301. doi: 10.1177/1358863X9700200404. [DOI] [PubMed] [Google Scholar]
- 7.Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med. 1998;158:595–600. doi: 10.1001/archinte.158.6.595. [DOI] [PubMed] [Google Scholar]
- 8.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud‘s phenomenon to systemic sclerosis. A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902–3912. doi: 10.1002/art.24038. [DOI] [PubMed] [Google Scholar]
- 9.Zahavi I, Chagnac A, Hering R, et al. Prevalence of Raynaud‘s phenomenon in patients with migraine. Arch Intern Med. 1984;144:742–744. [PubMed] [Google Scholar]
- 10.Sander O, Schroeder M, Ostendorf B, et al. Capillary microscopy - a crosssectional study in the population. Arthritis Rheum. 2010;62:S671–S672. [Google Scholar]
- 11.Freedman RR, Mayes MD. Familial aggregation of primary Raynaud‘s disease. Arthritis Rheum. 1996;39:1189–1191. doi: 10.1002/art.1780390717. [DOI] [PubMed] [Google Scholar]
- 12.Cherkas LF, Williams FM, Carter L, et al. Heritability of Raynaud‘s phenomenon and vascular responsiveness to cold: a study of adult female twins. Arthritis Rheum. 2007;57:524–528. doi: 10.1002/art.22626. [DOI] [PubMed] [Google Scholar]
- 13.Munir S, Freidin MB, Brain S, et al. Association of Raynaud‘s phenomenon with a polymorphism in the NOS1 gene. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196279. e0196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flavahan NA. A vascular mechanistic approach to understanding Raynaud phenomenon. Nat Rev Rheumatol. 2015;11:146–158. doi: 10.1038/nrrheum.2014.195. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, de Trafford JC, Baskerville PA, et al. Digital artery calibre measurement—a ew technique of assessing Raynaud‘s phenomenon. Eur J Vasc Surg. 1991;5:199–203. doi: 10.1016/s0950-821x(05)80688-7. [DOI] [PubMed] [Google Scholar]
- 16.Klein-Weigel P, Opitz C, Riemekasten G. Systemic sclerosis - a systematic overview: part 1 - disease characteristics and classification, pathophysiologic concepts, and recommendations for diagnosis and surveillance. Vasa. 2011;40:6–19. doi: 10.1024/0301-1526/a000065. [DOI] [PubMed] [Google Scholar]
- 17.Marcoccia A, Klein-Weigel PF, Gschwandtner ME, et al. Microcirculatory assessment of vascular diseases. Vasa. 2020;49:175–186. doi: 10.1024/0301-1526/a000851. [DOI] [PubMed] [Google Scholar]
- 18.Poszepczynska-Guigné E, Viguier C, Orcel B, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. JAAD. 2002;47:47–52. doi: 10.1067/mjd.2002.120474. [DOI] [PubMed] [Google Scholar]
- 19.Ingegnoli F, Boracchi P, Gualtierotti R, et al. Improving outcome prediction of systemic sclerosis from isolated Raynaud‘s phenomenon: role of autoantibodies and nail-fold capillaroscopy. Rheumatology (Oxford) 2010;49:797–805. doi: 10.1093/rheumatology/kep447. [DOI] [PubMed] [Google Scholar]
- 20.Landry GJ, Edwards JM, McLafferty RB, et al. Long-term outcome of Raynaud‘s syndrome in a prospectively analyzed patient cohort. J Vasc Surg. 1996;23:76–85. doi: 10.1016/s0741-5214(05)80037-0. [DOI] [PubMed] [Google Scholar]
- 21.Kallenberg CG, Wouda AA, Hoet MH, et al. Development of connective tissue disease in patients presenting with Raynaud‘s phenomenon: a six year follow up with emphasis on the predictive value of antinuclear antibodies as detected by immunoblotting. Ann Rheum Dis. 1988;47:634–641. doi: 10.1136/ard.47.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein-Weigel PF, Sunderkötter C, Sander O. Nailfold capillaroscopy microscopy— an interdisciplinary appraisal. Vasa. 2016;45:353–364. doi: 10.1024/0301-1526/a000553. [DOI] [PubMed] [Google Scholar]
- 23.Cutolo M, Pizzorni C, Sulli A, et al. Early diagnostic and predictive value of capillaroscopy in systemic sclerosis. Curr Rheumatol Rev. 2013;9:249–253. doi: 10.2174/157339710904140417125010. [DOI] [PubMed] [Google Scholar]
- 24.Smith V, Herrick AL, Ingegnoli F, et al. EULAR study group on microcirculation in rheumatic diseases and the scleroderma clinical trials consortium group on capillaroscopy. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud‘s phenomenon and systemic sclerosis. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102458. 102458. [DOI] [PubMed] [Google Scholar]
- 25.Hirschl M, Katzenschlager R, Francesconi C, et al. Low level laser therapy in primary Raynaud‘s phenomenon-results of a placebo controlled, double blind intervention study. J Rheumatol. 2004;31:2408–2412. [PubMed] [Google Scholar]
- 26.Tapia-Haro RM, García-Ríos MC, Toledano-Moreno S, et al. The complementary effects of galvanic current electrical stimulation associated with conservative treatment to increase vasodilation in patients with Raynaud‘s phenomenon: a randomized trial. Clin Rehabil. 2020 May;34:595–606. doi: 10.1177/0269215520907652. [DOI] [PubMed] [Google Scholar]
- 27.Daniels J, Pauling JD, Eccleston C. Behaviour change interventions for the management of Raynaud‘s phenomenon: a systematic literature review. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-024528. e024528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rirash F, Tingey PC, Harding SE, et al. Calcium channel blockers for primary and secondary Raynaud‘s phenomenon. Cochrane Database Syst Rev. 2017;12 doi: 10.1002/14651858.CD000467.pub2. CD000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleiro B, Marshall SE, Denton CP, et al. Treatment of Raynaud‘s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford) 2001;40:1038–1043. doi: 10.1093/rheumatology/40.9.1038. [DOI] [PubMed] [Google Scholar]
- 30.Khouri C, Gailland T, Lepelley M, Roustit M, Cracowski JL. Fluoxetine and Raynaud‘s phenomenon: friend or foe? Br J Clin Pharmacol. 2017;83:2307–2309. doi: 10.1111/bcp.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dziadzio M, Denton CP, Smith R, et al. Losartan therapy for Raynaud‘s phenomenon and scleroderma: clinical and biochemical findings in a fifteen-week, randomized, parallel-group, controlled trial. Arthritis Rheum. 1999;42:2646–2655. doi: 10.1002/1529-0131(199912)42:12<2646::AID-ANR21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Roustit M, Blaise S, Allanore Y, et al. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud‘s phenomenon: systematic review and meta-analysis of randomised trials. Ann Rheum Dis. 2013;72:1696–1699. doi: 10.1136/annrheumdis-2012-202836. [DOI] [PubMed] [Google Scholar]
- 33.Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327–1339. doi: 10.1136/annrheumdis-2016-209909. [DOI] [PubMed] [Google Scholar]
- 34.Pope J, Fenlon D, Thompson A, et al. Iloprost and cisaprost for Raynaud‘s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 2000;1998 doi: 10.1002/14651858.CD000953. CD000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scorza R, Caronni M, Mascagni B, et al. Effects of long-term cyclic iloprost therapy in systemic sclerosis with Raynaud‘s phenomenon. A randomized, controlled study. Clin Exp Rheumatol. 2001;19:503–508. [PubMed] [Google Scholar]
- 36.Huntgeburth M, Kießling J, Weimann G, et al. Riociguat for the treatment of Raynaud‘s phenomenon: a single-dose, double-blind, randomized, placebo controlled cross-over pilot study (DIGIT) Clin Drug Investig. 2018;38:1061–1069. doi: 10.1007/s40261-018-0698-1. [DOI] [PubMed] [Google Scholar]
- 37.Sycha T, Graninger M, Auff E, et al. Botulinum toxin in the treatment of Raynaud‘s´phenomenon: a pilot study. Eur J Clin Invest. 2004;34:312–313. doi: 10.1111/j.1365-2362.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- 38.Motegi SI, Sekiguchi A, Saito S, et al. Successful treatment of Raynaud‘s phenomenon and digital ulcers in systemic sclerosis patients with botulinum toxin B injection: assessment of peripheral vascular disorder by angiography and dermoscopic image of nail fold capillary. J Dermatol. 2018;45:349–352. doi: 10.1111/1346-8138.14140. [DOI] [PubMed] [Google Scholar]
- 39.Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud‘s phenomenon: a review. Semin Arthritis Rheum. 2012;41:599–603. doi: 10.1016/j.semarthrit.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Bello RJ, Cooney CM, Melamed E, et al. The therapeutic efficacy of botulinum toxin in treating scleroderma-associated Raynaud‘s phenomenon: a randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:1661–1669. doi: 10.1002/art.40123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belch J, Carlizza A, Carpentier PH, et al. ESVM guidelines - the diagnosis and management of Raynaud‘s phenomenon. Vasa. 2017;46:413–423. doi: 10.1024/0301-1526/a000661. [DOI] [PubMed] [Google Scholar]