Abstract
APE2 is a rising vital player in the maintenance of genome and epigenome integrity. In the past several years, a series of studies have shown the critical roles and functions of APE2. We seek to provide the first comprehensive review on several aspects of APE2 in genome and epigenome integrity. We first summarize the distinct functional domains or motifs within APE2 including EEP (endonuclease/exonuclease/phosphatase) domain, PIP box and Zf-GRF motifs from eight species (i.e., Homo sapiens, Mus musculus, Xenopus laevis, Ciona intestinalis, Arabidopsis thaliana, Schizosaccharomyces pombe, Saccharomyces cerevisiae, and Trypanosoma cruzi). Then we analyze various APE2 nuclease activities and associated DNA substrates, including AP endonuclease, 3'-phosphodiesterase, 3'-phosphatase, and 3'-5' exonuclease activities. We also examine several APE2 interaction proteins, including PCNA, Chk1, APE1, Myh1, and homologous recombination (HR) factors such as Rad51, Rad52, BRCA1, BRCA2, and BARD1. Furthermore, we provide insights into the roles of APE2 in various DNA repair pathways (base excision repair, single-strand break repair, and double-strand break repair), DNA damage response (DDR) pathways (ATR-Chk1 and p53-dependent), immunoglobulin class switch recombination and somatic hypermutation, as well as active DNA demethylation. Lastly, we summarize critical functions of APE2 in growth, development, and diseases. In this review, we provide the first comprehensive perspective which dissects all aspects of the multiple-function protein APE2 in genome and epigenome integrity.
Keywords: APE2, ATR-Chk1 pathway, DNA repair, DNA demethylation, immune response, genome and epigenome integrity
1. Introduction
Apurinic/apyrimidinic (AP) sites are derived from spontaneous base loss or enzymatic removal of damaged bases during base excision repair (BER) [1-3]. In response to DNA damage from endogenous or exogenous sources including AP sites, cells have evolved various DNA repair and DNA damage response (DDR) pathways to maintain genome integrity [1, 4]. When there are deficiencies in these pathways, it often leads to mutagenesis and genome instability known to be linked to human diseases such as cancer and neurodegenerative disorders [5, 6]. E. coli have been found to deal with AP sites via two major class II AP endonucleases: Exonuclease III (ExoIII) and Endonuclease IV (EndoIV) [7]. AP endonuclease 1 (APE1, also known as HAP1, REF-1 or APEX1) was cloned in early 1990s and characterized as the first Class II AP endonuclease [8-11]. AP endonuclease 2 (APE2, also known as APN2 or APEX2) was cloned in budding yeast in 1998 and characterized as the second Class II AP endonuclease to repair AP sites [12]. Human APE2 was identified and characterized in early 2000s, showing sequence homology to E. coli ExoIII/EndoIV and budding yeast APN2 [13, 14]. These earlier studies suggest that APE2 is an evolutionarily conserved nuclease from bacteria to human cells.
A series of recent studies have revealed the critical roles and functions of APE2 in DNA repair and DDR pathways. It has been demonstrated that oxidative stress triggers an APE2-dependent ATR-Chk1 DDR pathway in Xenopus egg extracts [15, 16]. Furthermore, a site-specific plasmid-based single-strand break (SSB) structure triggers the ATR-Chk1 DDR pathway in a replication-independent manner and APE2 is critical for the activation of the defined SSB-induced DDR pathway [17]. Interestingly, an APE1/APE2-mediated two-step process was determined to be the underlying mechanism for the SSB-induced ATR DDR pathway [18]. In addition, APE2 is vital for SSB repair in the Xenopus system and homologous recombination (HR) in myeloma [19, 20]. Importantly, a CRISPR-based genetic screening method identified APE2 as a synthetic lethal target of BRCA2 in mammalian cells [21]. Intriguingly, APE2 has also been revealed as an essential regulator for active DNA demethylation and DNA repair in Arabidopsis thaliana [22]. The accumulating evidence has shown that APE2 is an arising vital player in the maintenance of genome and epigenome integrity.
Recent evidence also suggests the pathophysiological implications of APE2 in health and disease. Genomic alterations and abnormal expression of APE2 are present in multiple types of cancer, including kidney, breast, lung, liver, and uterus cancers [23]. This finding is consistent with APE2 upregulation found in multiple myeloma (MM) patients [20]. In addition, yeast APE2 was shown to be critical for the suppression of Topoisomerase 1-induced mutagenesis at the sites of mis-incorporated ribonucleotide monophosphates (rNMPs) [24, 25]. Conversely, in mice it was found that APE2 is upregulated in germinal center B cells in order to promote error-prone repair in somatic hypermutation [26].
This is the first comprehensive review on the functions and mechanisms of APE2 in the genome and epigenome integrity. In this review, we summarize several aspects of APE2 in genome/epigenome integrity and potential implications in health and diseases, including but not limited to: (I) a comparison of the structure and functional domains of APE2 proteins in different species; (II) a summary of the distinct roles of APE2 in the maintenance of genome and epigenome integrity; (III) a description of different DNA substrates of APE2 and associated enzymatic activities; (IV) a summary and review of the interaction proteins of APE2; and (V) suggestions for future directions of APE2 studies in genome integrity.
2. Structure and functional domains of APE2 protein
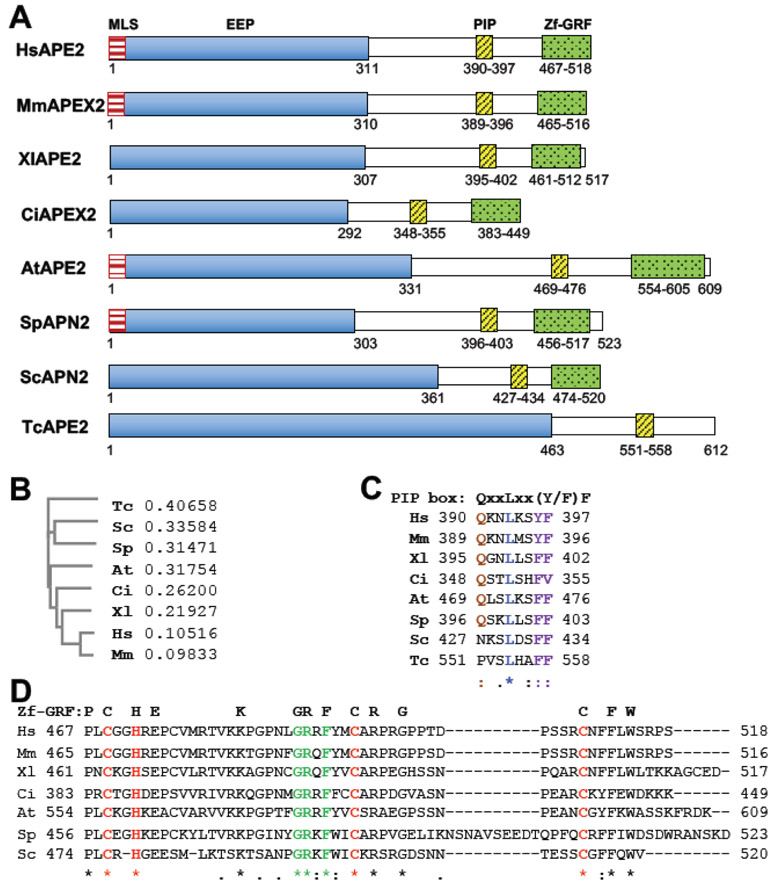
To compare the structure and functional domains within APE2, we focused on APE2 from eight eukaryotic species including Homo sapiens (HsAPE2), Mus musculus (MmAPEX2), Xenopus laevis (XlAPE2), Ciona intestinalis (CiAPEX2), Arabidopsis thaliana (AtAPE2), Schizosaccharomyces pombe (SpAPN2), Saccharomyces cerevisiae (ScAPN2), and Trypanosoma cruzi (TcAPE2) (Fig. 1A) [12, 14, 15, 22, 27-30]. Phylogenic tree analysis of the eight APE2 proteins using Clustal Omega software indicates that HsAPE2 is closer to MmAPEX2 and XlAPE2 but far away from SpAPN2, ScAPN2 and TcAPE2 (Fig. 1B). Furthermore, the relatedness of AtAPE2 and CiAPEX2 are between APE2 from vertebrates (i.e., HsAPE2, MmAPEX2, and XlAPE2) and that from single-cell eukaryotes (i.e., SpAPN2, ScAPN2 and TcAPE2) (Fig. 1B). APE2 protein is composed of an N-terminal exonuclease/endonuclease/phosphatase (EEP) catalytic domain, a C-terminal zinc finger domain with a conserved GRxF motif (termed Zf-GRF) and a flexible region that includes a PCNA-interacting protein (PIP) box motif in between the C- and N-termini (Fig. 1A). Due to nuclear and mitochondrial localization [14], HsAPE2 is supposed to contain putative mitochondria/nuclear localization signal (MLS/NLS) motifs; however, the MLS/NLS motif is less characterized in literature compared to other functional domains. Although the EEP domain, PIP box and Zf-GRF domain within APE2 contain slightly variable residues in different species, their functions are relatively conserved. For example, the coding sequence of HsAPE2 is ~83% identical to MmAPEX2, while HsAPE2 and MmAPEX2 share ~80% amino acids (aa) homologue (Fig. 1A) [31].
Fig. 1. Comparison of APE2 and its functional domains in eight different species.
(A) Schematic diagram shows structures of APE2/APN2 family proteins (Homo sapiens (HsAPE2, NP_055296.2), Mus musculus (MmAPEX2, Q68G58.1), Xenopus laevis (XlAPE2, AAH77433.1), Ciona intestinalis (CiAPEX2, ENSCINP00000010808.2), Arabidopsis thaliana (AtAPN2, NP_001328802), Schizosaccharomyces pombe (SpAPN2, AAR83752.1), Saccharomyces cerevisiae (ScAPN2, NP_009534.1), and Trypanosoma cruzi (TcAPE2, AGT41677.1). The functional domains (MLS, mitochondria localization sequence; EEP exonuclease/endonuclease/phosphatase; PIP, PCNA-interacting protein box; Zf-GRF, zinc finger motif containing conserved GRxF) and their associated amino acid locations are indicated by numbers below each protein map. (B) Phylogenetic analysis of the APE2 superfamily from Clustal Omega. The numbers after each of the eight species represents branch length of different APE2. Branch lengths are calculated from the multiple sequence alignment using Clustal Omega software and are proportional to the number of mutations from each of the sequences examined. (C-D) Alignment of amino acid sequences of PIP box or Zf-GRF motifs of APE2 in different species using Clustal Omega. *, identical residues; :, highly conserved residues; ., moderately conserved residues.
2.1. EEP domain
All eight APE2 proteins contain an EEP domain. Of note, the length of the EEP domain among different species ranges from 292 aa (CiAPN2) to 463 (TcAPE2) (Fig. 1A). HsAPE2 protein consists of 518 amino acids with a N-terminal EEP domain (aa 1–311) that is homologous to E. coli XTH and human APE1 [13, 14]. The N-terminal EEP region (aa 1-310) of MmAPEX2 is highly homologous to that of HsAPE2 (86% identity) [31]. Furthermore, SpAPN2 contains 523 residues in which the N-terminal 303 residues are highly homologous (with ~57% conserved residues) to human APE1 [32]. AtAPE2 has two isoforms that are likely generated by alternative splicing [22]. The longer isoform of AtAPE2 harbors an intact EEP domain, whereas the shorter form of AtAPE2 lacks almost one-third of the EEP domain [22]. For simplicity, we only included the longer isoform of AtAPE2 in our sequence alignment analysis (Fig. 1).
The EEP domain is within APE2’s catalytic domain, displaying AP endonuclease, 3'-phosphodiesterase, 3'-phosphatase, and/or 3'-5' exonuclease activities (Fig. 2A). Several EEP domain mutants have been characterized as having deficient enzymatic activity (Fig. 3A and 3B). For example, the E59A mutant of ScAPN2 is defective for 3'-phosphodiesterase or 3'-exonuclease activity [33]. Furthermore, the D277A mutant in HsAPE2 inactivates all strong 3'-5' exonuclease and 3' phosphodiesterase activities, in addition to weak AP endonuclease activity [34].
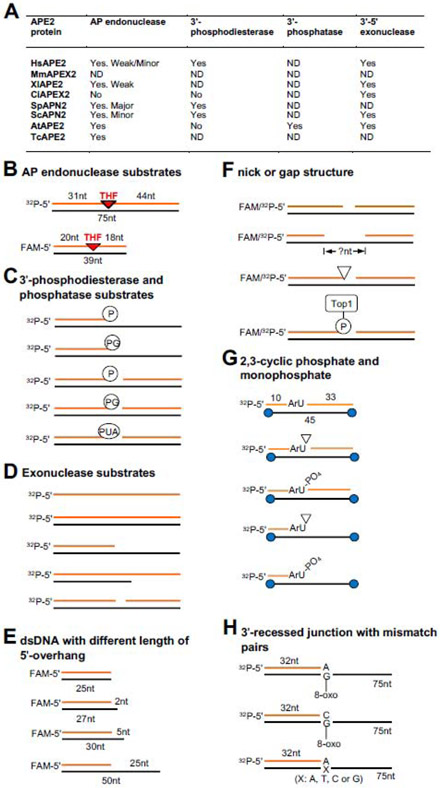
Fig. 2. APE2 enzymatic activities and associated DNA substrates.
(A) A summary of APE2’s AP endonuclease, 3'-phosphodiesterase, 3'-phosphatase, and 3'-5' exonuclease activities from different species. “ND” indicates not determined. (B-H) Various different substrates of APE2 enzymatic activities. (B) AP endonuclease substrates; (C) 3'-phosphodiesterase and phosphatase substrates; (D) Exonuclease substrates; (E) dsDNA with different length of 5' overhang; (F) Nick or gap structure; (G) 2,3-cyclic phosphate and monophosphate; (H) 3'-recessed junction with mismatch pairs or opposite to 8-oxoG.
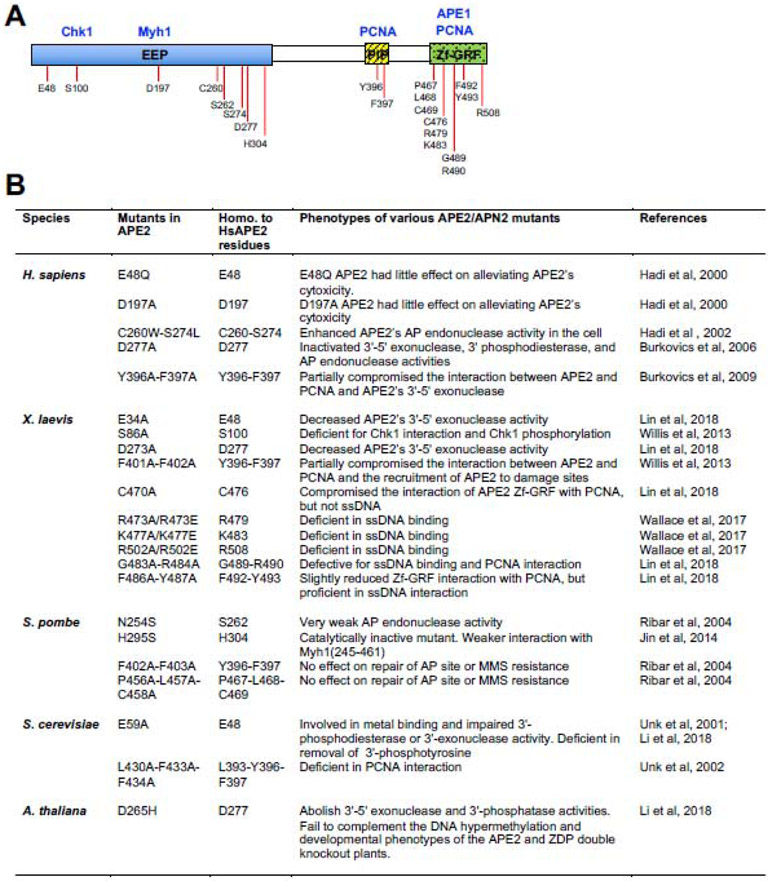
Fig. 3. Current knowledge of the known APE2 interaction proteins and phenotypes of its various mutants.
(A) APE2 interaction proteins and their binding domains or sites within APE2. The residues of human APE2 that have been mutated and characterized in panel (B) are also labeled. (B) Summary of phenotypes from various APE2 mutants from different species and the homologous residues to HsAPE2 in these mutants.
2.2. PIP box
The C-terminal region (aa 312-518) of HsAPE2 is not homologous to XTH or APE1, but is partially homologous to the corresponding regions of ScAPN2, SpAPN2, and AtAPE2 [14]. A PCNA-interaction PIP box is highly conserved in APE2 across all eight species (Fig. 1A and 1C) and shares an 8-aa consensus sequence of QxxLxx(Y/F)F (x indicates any aa) [35]. The PIP box of MmAPEX2 is almost 90% identical to that of HsAPE2 [16]. The glutamine residue (Q) in the PIP box is substituted by an asparagine residue (N) in ScAPN2 and a proline residue (P) in TcAPE2 [14] (Fig. 1C). The leucine residue (L) is identical in all eight APE2s. The last two residues are either tyrosine (Y) or phenylalanine (F), although CiAPEX2 contains a valine residue (V) at the last position in the PIP box (Fig. 1C).
Research suggests that the major role of the PIP box within APE2 is to associate with PCNA. For example, studies of ScAPN2 have shown PCNA interaction via its PIP box, which in turn, promotes ScAPN2 3'-5' exonuclease and 3'-phosphodiesterase activities. However, PCNA interaction is completely dispensable for ScAPE2 AP endonuclease activity measured in vitro [36, 37]. Biochemical evidence also shows that the HsAPE2 Y396A-F397A mutant in the PIP box compromises the interaction between APE2 and PCNA and the PCNA-dependent stimulation of APE2 exonuclease activity [35]. Similarly, the Y396A-F397A mutant in XlAPE2 is deficient for PCNA interaction, impairing its 3'-5' exonuclease activity [15, 17].
In addition to its exonuclease activity, PCNA interaction via APE2 PIP box is important for the recruitment of APE2 to damage sites such as AP sites for appropriate repair and DDR pathways. It has been demonstrated that the ScAPN2-PCNA interaction is important for repairing AP sites in vivo in yeast [33, 36, 37]. XlAPE2 mutant in the PIP box is deficient for its recruitment to sites of oxidative DNA damage [15].
2.3. Zf-GRF motif
APE2 in nearly all species except Trypanosoma contains a conserved Zf-GRF in its C-terminal region (Fig. 1D). Zf-GRF domains are ~50-aa domains widely distributed throughout eukaryotes in >100 proteins that are involved in DDR, transcriptional regulation, and RNA metabolism [16]. The elucidated structure of the XlAPE2 Zf-GRF motif shows a three-stranded anti-parallel β-sheet (β1–β3) that folds into a crescent-shaped, claw-like structure. A single bound Zn2+ ion plays a central structural role in this domain and is coordinated with tetrahedral geometry by a “CHCC” sequence motif [16]. The first two Zn2+ ligands (C463 and H466) are found in a loop preceding β1, whereas the second half of the motif (C489 and C503) maps to the β2-β3 connecting loop. The identity of these Zn2+ ligands in APE2 Zf-GRF (i.e., CHCC, highlighted in red in Fig. 1D) is highly conserved across almost all species, while the GRxF motif (highlighted in green in Fig. 1D) is identical in APE2 Zf-GRF motifs in all seven species examined.
The APE2 Zf-GRF motif appears to have two functions: single-strand DNA (ssDNA) interaction and PCNA association [16, 17]. XlAPE2 Zf-GRF bears a distinctive electropositive groove on its concave surface and is bound with a sulfate (SO42−) molecule salt-bridged to the conserved basic residues R473 and K476 [16]. Other positively charged residues within Zf-GRF (i.e., K477, R484, R491, and R502) are combined into an adjacent extended basic surface that appears appropriate for binding nucleic acid. It has been characterized that the XlAPE2 Zf-GRF motif associates with ssDNA and an R502E mutant of APE2 is deficient for ssDNA interaction but proficient for PCNA association [16]. Mutation analysis also identified that the C470A mutant of XlAPE2 is defective for PCNA interaction but proficient for the ssDNA association [17]. Thus, the C-terminal Zf-GRF motif is a defining feature of APE2 that distinguishes it from other EEP containing enzymes such as APE1.
A BLASTP search of human APE2 Zf-GRF (aa 467–518) revealed high similarity to putative Zf-GRF motifs in NEIL3 and topoisomerase 3 alpha. Recent studies have showed that the two Zf-GRF motifs in Xenopus NEIL3 interact with ssDNA in vitro [38, 39]. Therefore, investigation of APE2 Zf-GRF may provide new insight into mechanistic studies of other Zf-GRF-containing proteins.
2.4. Mitochondria/nuclear localization signal (MLS/NLS)
HsAPE2 protein is mostly localized in the nuclei and to some extent in the mitochondria [14]. Hadi et al. demonstrated that the C-terminal domain of APE2 is essential for nuclear localization by showing that a C-terminal fragment (i.e., aa 312-518) of HsAPE2 remains localized to the nucleus similar to the wild type counterpart [13]. However, the exact NLS of APE2 has not been identified. A MLS in HsAPE2 consists of N-terminal 15 aa and may not be processed after being translocated into mitochondria [14]. Electron microscopic immunocytochemistry analysis showed that HsAPE2 is localized in the inner membrane of mitochondria [14]. Considering the sequence similarity to HsAPE2, MmAPEX2, SpAPN2 and AtAPE2 may possess a putative MLS motif at their N-termini. Thus, subcellular localization of APE2 in nuclei and mitochondria supports its functions in both subcellular compartments.
3. Enzymatic activities and DNA substrates of APE2
As expected for an EEP family protein, APE2 exhibits AP endonuclease, 3'-phosphodiesterase, 3'-phosphatase, and 3'-5' exonuclease activities. APE2 was considered as a back-up DNA repair mechanism via its AP endonuclease activity in the absence of APE1 [12, 14, 36]. However, recent several lines of evidence suggest that APE2 is a key enzyme to process a broad blocking DNA 3'-termini via its 3'-end processing functionalities from different species [16, 17, 22, 24, 25, 40]. Here, we summarize enzymatic activities of APE2 and its various DNA substrates in different species (Fig. 2A).
3.1. APE2 endonuclease activity and substrates
It is well understood that APE2 displays AP endonuclease activity in most species (Fig. 2A). Regarding AP endonuclease in budding yeast, APN2 only accounts for less than 10% AP endonuclease activity, whereas APN1 accounts for more than 90% of the total AP endonuclease activity [12, 36, 41]. In contrast, APN2 plays a significant role in repairing AP sites via AP endonuclease in fission yeast, and APN1 functions as a secondary mechanism for the repair of AP sites [29]. HsAPE2 displays weak AP endonuclease activity as a relatively minor AP endonuclease [2], while human APE1 is the major AP endonuclease accounting for >95% of the total AP endonuclease activity in human cells [34, 42]. Furthermore, recombinant TcAPE2 displays AP endonuclease activity in vitro using double-strand DNA (dsDNA) containing an AP site, which is inhibited when such substrate is pretreated with a chemical compound called methoxyamine [30]. In Arabidopsis thaliana, one study has demonstrated AP endonuclease activity of AtAPE2 in vitro [43]. In addition, CiAPEX2 had no detectable AP endonuclease activity in vitro [28]. At the current time, we can conclude that APE2 displays AP endonuclease activity in vitro in most tested species (Fig. 2A).
AP endonuclease assays have been reconstituted in vitro using different substrates containing AP sites. Burkovics et al. have shown that a 75-bp 5'-32P-labeled dsDNA containing an AP-mimic tetrahydrofuran (THF) residue can be incised into a 30-nt product by recombinant wild type (WT) HsAPE2, suggesting the 5'-side of the AP site is incised by APE2’s endonuclease activity (Fig. 2B) [34]. The D277A mutant version of HsAPE2 failed to incise the AP site in vitro, confirming that the D277A mutation indeed rendered APE2 catalytically inactive and that the observed AP endonuclease activity is intrinsic to HsAPE2 [34]. However, D277A is not a separation-of-function mutant of APE2 endonuclease activity as D277A also impairs other nuclease activities of APE2. Similarly, Wallace et al. developed an endonuclease assay using a 39-bp 5'-FAM-labeled dsDNA containing an AP-mimicking THF as a substrate which can be incised by recombinant XlAPE2 (Fig. 2B) [16].
The endonuclease activity of HsAPE2 is most active at 150 mM of NaCl concentration, which is close to physiological condition [34]. However, the AP endonuclease activity of APE1 was reduced at 150 mM NaCl compared to the absence of NaCl. The AP endonuclease activity of ScAPN2 is not affected in the absence of magnesium or other metals [33]. Although PCNA interacts with APE2, PCNA doesn’t affect the AP endonuclease activity of APE2, possibly because the AP-containing substrate is not a preferred structure for PCNA binding and stimulation [35]. Interestingly, a C260W/S274L mutant HsAPE2 introducing an APE1-like hydrophobic pocket significantly enhanced AP endonuclease activity, suggesting residues in the active site of APE2 are critical for its endonuclease activity [44].
3.2. APE2 3'-phosphodiesterase and 3'-phosphatase activities and substrates
3'-phosphate (3'-P), 3'-phosphoglycolate (3'-PG), 3'-phospho-α,β-unsaturated aldehyde (3'-PUA), 2'-3' cyclic phosphate, and 3'-Top1 adduct are often found at the 3'-side of DNA strand breaks or intermediate repair products [45]. Due to the deficiency in strand synthesis, these heterogenous 3'-blocking termini must be processed to generate the typical 3'-hydroxyl group (3'-OH) for appropriate repair synthesis and ligation. To process these 3'-blocking termini, APE2 displays differential 3'-phosphodiesterase activity and sometimes 3'-phosphatase activity among various species (Fig. 2A).
To test whether APE2 3'-phosphodiesterase activity can remove 3'-blocking termini, Burkovics et al. showed that a 3'-recessed ds/ssDNA junction containing a 3'-PG terminus is processed into a 3'-OH by recombinant WT but not D277A HsAPE2, indicating the 3'-phosphodiesterase activity of HsAPE2 in the 3'-recessed structure (Fig. 2C) [34]. The 3'-phosphodiesterase activity targeting a 3'-recessed dsDNA with a 3'-PG terminus is conserved in ScAPN2 and can be stimulated by the presence of PCNA [37]. Furthermore, a gapped dsDNA containing a 3'-PG in the middle was processed by ScAPN2 into a 3'-OH, suggesting APE2 3'-phosphodiesterase activity in the gapped dsDNA structure (Fig. 2C) [33]. Biochemical and genetic evidence infers that SpAPN2 is responsible for removing a 3'-PUA in a nicked structure derived from Nth1 lyase-mediated incision of an AP site [46]. It was also reported that SpAPN2 has 3'-phosphodiesterase activity in vitro, and such nuclease activity can be stimulated by DNA glycosylase Myh1 [32].
In addition, a 3'-recessed ds/ssDNA junction containing a 3'-P terminus was processed by WT but not D265H mutant AtAPE2, suggesting 3'-phosphatase activity of AtAPE2 (Fig. 2C) [22]. However, the 3'-phosphatase activity of APE2 has not yet been established in other species (Fig. 2A). Intriguingly, another Arabidopsis thaliana endonucleases APE1L can also convert the 3'-P to 3'-OH in vitro [43].
3.3. APE2 3'-5' exonuclease and substrates
To determine the 3'-5' exonuclease activity of APE2, different groups of substrates have been utilized to characterize APE2 exonuclease preference and activity in in vitro reconstitution systems. What has been established is that an intrinsic 3'-5' exonuclease activity of APE2 was present in almost all species tested so far (Fig. 2A) [17, 22, 28, 33, 34]. Furthermore, the 3'-5' exonuclease activity of APE2 can be stimulated by PCNA interaction.
3.3.1. ssDNA and dsDNA containing blunt end or 3'/5'-recessed junctions
In budding yeast, ScAPE2 can resect dsDNA with a blunt end and 3’-recessed dsDNA with ~3-6nt, but only incise the ssDNA and 5’-recessed DNA structures by ~1-2nt (Fig. 2D), suggesting that dsDNA and 3’-recessed dsDNA/ssDNA structures are the preferred substrates for 3'-5' exonuclease activity [33]. Notably, the 3'-5' exonuclease activity using dsDNA with a blunt end and 3'-recessed dsDNA was completely compromised in the E59A mutant of ScAPN2 [33]. In vitro exonuclease assay showed that a 5-nt overhang in a 3'-recessed dsDNA structure is sufficient for XlAPE2 to display its increased 3'-5' exonuclease activity compared with the 2-nt and 25-nt overhangs (Fig. 2E) [16]. HsAPE2 also showed strong 3'-5' exonuclease activity on a 3'-recessed dsDNA, but reduced activity on dsDNA with a blunt end and residual activity on ssDNA or 5'-recessed dsDNA [34]. The 3'-5' exonuclease activity on a 3'-recessed dsDNA substrate is conserved in CiAPEX2 and AtAPE2 (Fig. 2A) [22, 28].
To determine APE2 exonuclease activity, Burkovics et al. have optimized the reaction conditions including pH range of 6.0-8.0, no or low salt (NaCl), and manganese other than magnesium ion [34]. The presence of PCNA significantly stimulates the 3'-5' exonuclease activity of HsAPE2, XlAPE2Sc, and ScPN2 in in vitro exonuclease assays with similar 3'-recessed dsDNA structures as substrates [16, 35, 37]. Since PCNA stimulates APE2 3'-5' exonuclease activity but not AP endonuclease activity, it is suggestive that PCNA may affect APE2 only when it encircles DNA at a primer-template junction such as a 3'-recessed dsDNA structures. This idea was confirmed when the PCNA-mediated stimulation of HsAPE2 3'-5' exonuclease activity was shown to be compromised when PCNA could not be loaded on a 3'-recessed dsDNA substrate containing biotin-streptavidin complex at both ends [35]. In addition, RFC, PCNA, and RPA cooperatively stimulate ScAPN2 3'-5' exonuclease activity at NaCl’s physiological concentration [37]. While APE2 alone only degrades a couple of nucleotides on 3'-recessed dsDNA structures via 3'-5' exonuclease activity, the PCNA-stimulated APE2 3'-5' exonuclease activity can remove almost all nucleotides on 3'-recessed dsDNA structures in the 3'-5' direction [16, 35].
3.3.2. dsDNA containing a single-strand break (nick) or a gap structure
DNA SSBs or nicks are estimated to occur ~10,000 times per mammalian cell each day, while gap structures within dsDNA are often intermediate products of DNA repair pathways [47, 48]. HsAPE2 has about 2-fold more robust 3'-5' exonuclease activity on a dsDNA structure containing 1-nt gap than on a nick-containing dsDNA, and that increasing gap size from 1nt to 4nt does not make a significant difference for HsAPE2 3'-5' exonuclease activity (Fig. 2F) [34]. Furthermore, XlAPE2 prefers a dsDNA containing ~1-3nt gap structures than a dsDNA with an SSB/nick structure evaluated by in vitro 3'-5' exonuclease assays with the presence of PCNA [17]. In the Xenopus system, the PCNA-mediated XlAPE2 3'-5' exonuclease activity is faster than APE1 and can continue more prolonged end resection while APE1 prefers SSB for the initiation of the SSB end resection in the 3'-5' direction [17, 18]. Consistent with these findings in Xenopus, CiAPEX2 can resect nicked/gapped dsDNA substrates derived from an AP-site-containing dsDNA after HsAPE1 incision in vitro [28].
3.3.3. Heterogeneous 3'-blocking termini
rNMPs are often misincorporated in the genome and are removed via RNase H2 mediated excision repair or Topoisomerase 1 (Top1)-catalyzed cleavage [49]. The latter process generates SSB or nick structures containing 3'-blocking termini such as 2', 3'-cyclic phosphate, or a Top1 cleavage complex (Top1cc) (Fig. 2E), which leads to mutagenesis if not properly repaired [50]. A recent study demonstrated that ScAPN2 resects two nucleotides at a 2',3'-cyclic phosphate or monophosphate in a 3'-recessed structure or a nicked dsDNA structure in vitro (Fig. 2G) and that PCNA stimulates APE2’s exonuclease activity to further resect 8-10 nucleotides in the 3'-5' direction [24, 25]. In addition to the Tdp1-Tpp1 pathway, ScAPN2 also functions to remove Top1cc in vitro and in vivo [25]. After APE2 treatment and processing, Pol δ can continue to repair DNA synthesis at 3'-recessed or nicked dsDNA structures containing 3'-blocking termini [25]. These studies demonstrate the 3'-5' exonuclease activity of APE2 on dsDNA containing heterogeneous 3'-blocking termini.
3.3.4. Heterogeneous 3' mismatch pairs
Biochemical analysis and quantification have revealed that HsAPE2 removed 3'-mismatched nucleotides 2- to 40-fold more efficiently than those matched pairs at the 3'-recessed dsDNA structures in vitro [34]. For example, APE2 had a very limited 3'-5' exonuclease on a 3'-recessed dsDNA containing a matched A opposite from a template T; however, it exhibited a more robust exonuclease activity on unmatched A opposite from G, A or C. APE2 exonuclease has a significant preference for dsDNA structures containing heterogeneous 3'-mismatch pairs.
Oxidative stress such as reactive oxygen species (ROS) can results in DNA lesions in the genome such as 8-oxo-7,8-dihydroguanine (8-oxoG). During DNA replication, an adenine (A) is incorporated by DNA polymerase delta more frequently than a cytosine (C) opposite 8-oxoG, leading to GC to TA transversions. In addition to direct removal of 8-oxoG and associated repair pathway, cells have evolved several nucleases to remove mismatch pairs. It was shown that HsAPE2 removed mismatched A more efficiently than C opposite from an 8-oxoG residue on a 3'-recessed dsDNA structure (Fig. 2H) [35]. In a more quantitative approach, the steady-state kinetic parameters Vmax and Km of the 3'-5' exonuclease assay showed that HsAPE2 removed A 8.5-fold more efficiently than it removed C from opposite the 8-oxoG, and removed A opposite from 8-oxoG more efficiently than opposite from undamaged T. Furthermore, PCNA stimulated the removal of A and C opposite from 8-oxoG but did not change the preference of mismatched A [35]. Thus, APE2 plays a proofreading role via its 3'-5' exonuclease in PCNA-dependent bypass synthesis opposite 8-oxoG.
4. APE2 interaction proteins
To better understand APE2 functions, we summarize several APE2 interaction proteins including PCNA, Chk1, APE1, Myh1, and HR factors such as Rad52, BRCA1, BRCA2, and BARD1 (Fig. 3A). Biochemical and molecular approaches have been utilized to characterize these APE2 interaction proteins.
4.1. PCNA
PCNA is the most characterized APE2 interacting protein, and the interaction between APE2 and PCNA is rather stable even in the presence of 500 mM NaCl in vitro (Fig. 3A) [14]. There are two modes for the APE2-PCNA interaction: APE2’s PIP box interacts with PCNA’s interdomain connector loop (IDCL) (Mode I), while APE2’s Zf-GRF motif interacts with PCNA’s C-terminus (Mode II) [17]. The Mode I interaction of APE2-PCNA is well characterized biochemically in budding yeast, Xenopus laevis, and humans [15, 35, 37]. Various protein-protein interaction approaches, including pulldown binding assays with purified recombinant proteins, have indicated that APE2 directly binds PCNA in vitro, and their association is mediated by APE2’s PIP box [17, 35, 37]. The Mode II interaction of APE2-PCNA is distinguished from APE2 Zf-GRF association with ssDNA as a C470A mutant in APE2 Zf-GRF is deficient for PCNA interaction while still being proficient for ssDNA interaction [17].
What are the roles of PCNA in APE2 functions? PCNA forms a homotrimer with a ring structure and functions as a DNA sliding clamp for many proteins involved in DNA replication. DNA repair, DDR, and DNA damage tolerance pathways [51]. First, PCNA is essential for the recruitment of APE2 to damage sites. It is reported that the APE2-PCNA interaction is increased upon more misincorporation of dUMP into DNA in human cells, suggesting a role of PCNA for the recruitment of APE2 to damage sites [14]. Consistent with this concept, a PIP box mutant of XlAPE2 is deficient for recruiting to oxidative stress-damaged chromatin DNA in the Xenopus system [15]. Second, PCNA interaction promotes APE2’s 3′-Phosphoesterase and 3′-5′ exonuclease activities but not AP endonuclease activity [35]. Recent studies also indicate the stimulation of PCNA on APE2’s nucleolytic activity to resolve 3′-blocking termini such as 2′,3′-cyclic-phosphate, and 3′-Top1 [22, 40]. Third, the two modes of APE2-PCNA interaction may fine-tune APE2’s nuclease activity with or without the presence of DNA [14, 37]. Although PCNA mono-ubiquitination or sumoylation is important for recruiting its interacting proteins to damage site, there are currently no reports on the identification of possible ubiquitin or sumoylation interacting motifs within APE2.
4.2. Chk1
Claspin interacts with Chk1 and has been implicated in the ATR-Chk1 DDR pathway in response to different types of stressful conditions [52]. Two conserved repeats within Claspin were identified as Chk1-binding (CKB) motifs, which is important for Claspin-mediated Chk1 phosphorylation by activated ATR [53]. Interestingly, APE2 associates with Chk1 via a similar Claspin CKB-like motif in its N-terminus as a Ser86 residue mutation fails to interact with Chk1 (Fig. 3A) [15]. Importantly, the Claspin-like Chk1 binding is required for Chk1 phosphorylation by activated ATR kinase following oxidative stress [15]. Future studies are needed to test whether the Chk1 interaction with APE2 is conserved during evolution. Bioinformatics analysis from cancer patients using available data from The Cancer Genome Atlas (TCGA) suggests that the mRNA expression of APE2 is positively correlated with that of Chk1 [23]. Although the potential biological significance of the APE2-Chk1 interaction remains to be determined, we speculate that APE2 may regulate cell cycle progression via its interaction with Chk1.
4.3. APE1
APE1 is a multiple-function protein in genome integrity, including redox regulation of transcription, BER, and NIR repair pathways [8, 54]. APE1 interacts with XlAPE2 via in vitro biochemical protein-protein interaction approaches [18]. Domain dissection experiments revealed that APE2’s Zf-GRF motif is required and sufficient for interacting with APE1 and that the catalytic domain of APE1 may mediate APE2 interaction (Fig. 3A) [18]. Of note, APE2 Zf-GRF defective mutants for its interaction with ssDNA and PCNA (i.e., R502E and C470A) are proficient for APE1 interaction, suggesting that APE1 interaction is another distinct feature of the APE2 Zf-GRF motif [18]. Although it is proposed that APE1 is recruited to the SSB site first to create a small ssDNA gap (~1-3nt) for APE2 recruitment and activation, it remains unclear whether the APE1-APE2 interaction plays a direct role in the recruitment of APE2 to SSB sites [18]. In addition, the APE1-APE2 interaction is further validated by a genetic interaction approach in human cells [40], suggesting the evolutionary conservation of the APE1-APE2 interaction. TCGA data analysis from cancer patients reveals that APE2 mRNA expression is positively correlated with APE1 mRNA expression [23].
Given their similar but distinct endonuclease and exonuclease activities, why does APE2 interact and work together with APE1 in common pathways? It is well known that DNA polymerase α synthesizes a RNA primer and a short DNA strand in replication initiation, followed by replication elongation by DNA polymerases δ and/or ε, although they all interact with PCNA [55]. We speculate that APE1 and APE2 may coordinate their recruitment and dissociation at SSB sites for DNA degradation via their exonuclease activity in SSB end resection. Furthermore, APE1 and APE2 may work redundantly to recognize and process AP sites via their endonuclease activity.
4.4. Myh1
Myh1 (also known as MutY homolog, MYH, or MUTYH) is a DNA glycosylase which excises adenine paired with 8-oxoG, generating an AP site. Interestingly, SpAPN2 was shown to interact with Myh1, promoting the glycosylase activity of Myh1 and the 3′-phosphodiesterase activity of SpAPN2 [32]. Domain dissection analysis shows that SpAPN2 interacts with the interdomain connector motif of Myh1 and that the EEP domain of SpAPN2 associates with Myh1 (Fig. 3A). Thus, the APE2-Myh1 interaction forms the initial BER complex to reduce G:C to T:A mutations at sites of 8-oxoG in the genome.
4.5. HR factors including Rad52, BRCA1, BRCA2, and BARD1
APE2 knockdown reduced the Rad51 focus formation and HR-mediated double-strand break (DSB) repair in MM cells [20]. Although APE1 is shown to regulate the transcription of Rad51, it remains unclear whether APE2 directly regulates Rad51 expression. At least, Rad51 was not one of those genes downregulated in APE2-knockout(KO) mice [56]. Consistent with the role of APE2 in HR, observations from co-immunoprecipitation experiments show that APE2 interacts with HR factors including Rad52, BRCA1, BRCA2, and BARD1 [20]. Nevertheless, it remains unclear which domain within APE2 is critical for interacting with HR factors. Two recent studies indicate that APE2 is a synthetical lethality target of BRCA1 and BRCA2, suggesting that APE2 and BRCA1/2 may be in different pathways [21, 40]. Thus, it seems difficult to reconcile the discrepancy between different studies. Future studies are needed to determine the exact role of APE2 in HR via biochemical and/or genetic interactions with HR factors.
5. Distinct roles of APE2 in the maintenance of genome and epigenome integrity
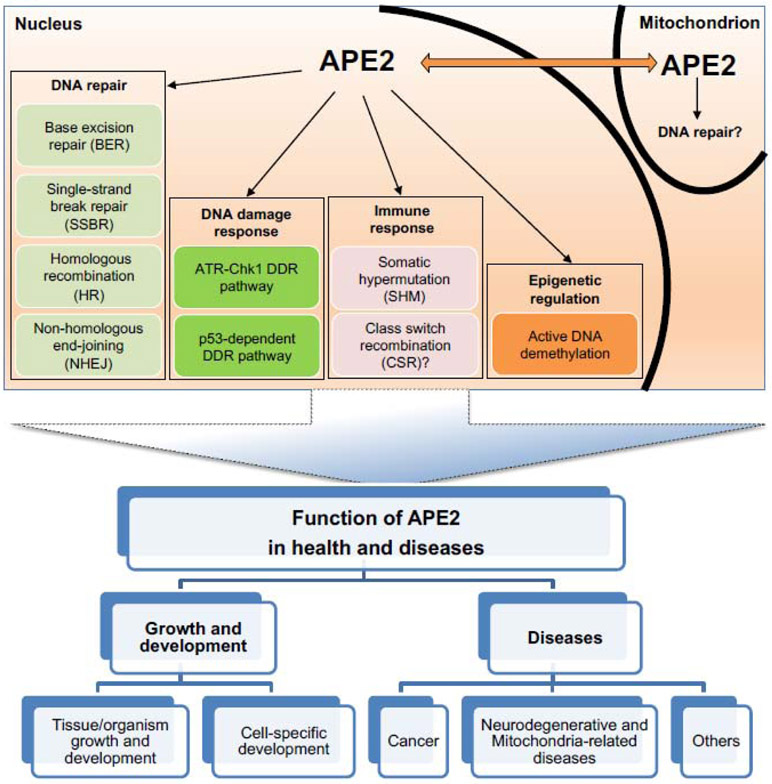
Compared with APE1 [54, 57], above analyses have shown that APE2 exhibits AP endonuclease activity, 3'-phosphodiesterase, and 3'-phosphatase activity, as well as PCNA-dependent and -independent 3'-5' exonuclease activity. To understand the distinct roles of APE2 in the maintenance of genome integrity and epigenetic changes, various experimental systems have been utilized to dissect different aspects of APE2 functionalities in: (I) DNA repair pathways including BER pathway, SSB repair pathway, HR, and nonhomologous end joining (NHEJ), (II) DDR pathways including ATR-Chk1-mediated DDR pathway and p53-dependent DDR pathway, (III) immune response including class switch recombination (CSR) and somatic hypermutation (SHM), and (IV) epigenetic regulation including active DNA demethylation (Fig. 4). In addition, APE2 is localized to the mitochondrion and may be involved in DNA repair in the mitochondrion as well (Fig. 4).
Fig. 4. A diagram of the known and predicted APE2 functions and our understanding of its biological significance.
(top) APE2 functions in DNA repair, DNA damage response, immune response, and epigenetic regulation in nucleus and possible DNA repair in mitochondrion. It is not clear whether APE2 communicates with each other between nucleus and mitochondrion. “?” indicates the undefined or controversial role of APE2. (bottom) An overview of the current significance that APE2 has concerning health and diseases.
5.1. DNA repair pathways
5.1.1. Role of APE2 in BER pathway
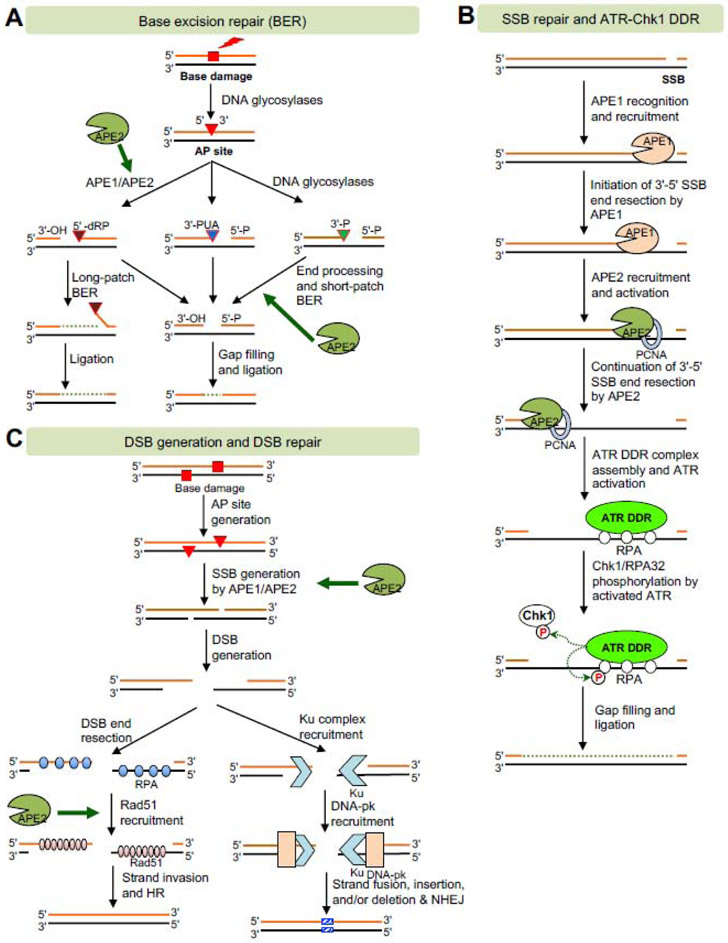
The BER pathway is an evolutionarily conserved process for repairing base damages from oxidative stress such as ROS and alkylation agents, which is necessary to maintain genomic integrity [5, 58, 59]. There are several steps involved in the BER pathway (Fig. 5A) [59, 60]: (I) The first step is the removal of damaged bases by DNA glycosylases such as uracil DNA glycosylase (UNG) and Myh1 to create an AP site. (II) The 5'-side of AP site is incised by APE1 and/or APE2 via their AP endonuclease activity to create 3′-OH and 5′-deoxyribose phosphate (5'-dRP). Alternatively, the 3′-side of AP site is cleaved by bifunctional DNA glycosylases such as OGG1 via their lyase activity and NTH1 via β-elimination to generate 3'-PUA. The 3′-side of AP site can also be cleaved by NEIL1 or NEIL2 via β,δ-elimination to generate 3′-P. (III) While 5'-dRP is removed by DNA polymerase β, the 3'-PUA and 3′-P are further processed by end processing enzymes APE1/APE2 and PNKP (polynucleotide kinase-phosphatase), respectively, to generate the usual 3'-OH for continuing the short-patch BER. Alternatively, long-batch BER utilizes DNA polymerase δ or ε with PCNA to extend longer nascent DNA strand, replacing a 5'-dRP-containing flap structure followed by FEN1-mediated cleavage. (IV) While the subsequent nick in long-patch BER is ligated by DNA Ligase I, the resulting 1-nt gap in short-patch BER is filled by Polymerase β and ligated by DNA ligase I/III. BER is responsible for the repair of 10,000 lesions per cell per day. BER is also mostly responsible for repairing oxidative stress-induced DNA damage and DNA demethylation in mammals and plants [5].
Fig. 5. Molecular mechanisms of APE2 in DNA repair and DDR pathways.
(A) Base excision repair (BER) pathway. APE2 may contribute to the generation of SSB from AP site via its endonuclease activity or the end processing of SSB with heterogenous termini after bifunctional DNA glycosylases via its 3'-phosphodiesterase or 3'-phosphatase activities. (B) SSB repair and ATR-Chk1 DDR pathways. APE2 promotes the continuation of 3'-5' SSB end resection via its exonuclease activity to generate longer ssDNA for ATR-Chk1 DDR pathway activation and subsequent SSB repair. (C) DSB generation and DSB repair including HR and NHEJ sub-pathways. APE2 may promotes DSB generation when the two AP sites are close to each other. APE2 may also promote Rad51 recruitment to ssDNA for strand invasion for HR.
Budding yeast studies have shown that ScAPN2-deletion mutant enhances the sensitivity to alkylation damage induced by methyl methanesulfonate (MMS) in the APN1-deletion background, suggesting that APE2/APN2 is essential for the repair of alkylation damage via its AP endonuclease activity as a back-up mechanism in the absence of APN1 [12, 36]. An E59A mutant in ScAPN2’s active site is deficient for its 3'-phosphodiesterase and 3'-5' exonuclease activities and fails to rescue the resistance to hydrogen peroxide, suggesting the significance of APN2-mediated BER in the repair of oxidative DNA damage [33]. HsAPE2 is a crucial player in the PCNA-dependent repair of hydrogen peroxide-induced oxidative DNA damage likely through its AP endonuclease and 3'-5' exonuclease activities [13, 35].
Another mechanism of APE2 involved in BER is its strong 3'-5' exonuclease activity and its preference for removing mismatched nucleotides at the 3'-junctions or 3'-side of gapped structures [33, 34]. During short-patch BER, Pol β carries out the DNA repair synthesis. However, Pol β lacks proofreading exonuclease activity, making an average one mistake per 4000 base insertions, leading to higher mutagenesis. It has been suggested that APE2 may function as an alternative proofreader for Pol β to improve the fidelity of BER, in addition to APE1 [34].
HsAPE2 is mostly localized to the nucleus and also in mitochondria to some extent [14]. A BER complex, including DNA glycosylases (OGG1 and MUTYH), APE2, DNA Polymerase γ, and DNA ligase III, is associated with the inner membrane and likely the mitochondria DNA, suggesting that APE2 may participate in the BER pathway in mitochondria in addition to nuclei [61]. In summary, the role of APE2 in BER pathway is likely at the step of 5'-cleavage of AP site via its AP endonuclease in the absence of APE1, and/or at the end processing step of SSBs containing complexed 3'-termini via its 3'-phosphodiesterase and 3'-5' exonuclease activities (Fig. 5A).
5.1.2. Role of APE2 in SSBR pathway
SSBs have been found in approximatively 10,000 times per mammalian cell each day. PARP1 and XRCC1 have been demonstrated in canonical SSB repair pathway and homology-directed recombination or repair pathways [48, 62-64]. New lines of evidence have revealed that XlAPE2 is vital for repairing a plasmid-based defined SSB structure in the Xenopus egg extract system [19, 65]. A series of mechanistic studies in the Xenopus further show that an SSB structure is recognized first by XlAPE1 to create a 1-3nt gap, leading to the initiation of 3'-5' SSB end resection (Fig. 5B). Then XlAPE2 is recruited and activated to the gapped structure for the continuation stage of the 3'-5' SSB end resection via its PCNA-mediated 3'-5' exonuclease activity, generating a longer track of ssDNA (~18-26nt) (Fig. 5B) [17, 18]. Consistent with the mechanism of APE2 in the SSBR pathway in Xenopus, ScAPN2 also resolves different types of 3'-termini including 2',3'-cyclic phosphate or monophosphate and resects several nucleotides in the 3'-5' direction for subsequent proper repair synthesis [24, 25]. In addition, the role of APE2 in SSB repair is supported by the observation that levels of SSBs increased in MmAPEX2-KO thymocytes compared to wild-type thymocytes [56]. Thus, compelling evidence supports the role of APE2 in the newly defined 3'-5' end-resection-mediated SSB repair pathway and highlights the significance of APE2’s 3'-5' exonuclease activity in genome integrity (Fig. 5B).
5.1.3. Role of APE2 in DSB generation and DSB repair pathway
Two AP sites in opposite DNA strands situated 5nt apart in mouse cells can be cleaved by APE1 or APE2 into two SSBs, which results in a DSB structure [66]. These AP-derived DSBs can be repaired via NHEJ in a Ku80-dependent and -independent manner [66]. These observations provide evidence that APE2 is essential for DSB generation at clustered lesions via its AP endonuclease activity especially in the absence of APE1 (Fig. 5C).
Conversely, more DSBs are generated in mouse cells in the absence of APE2, suggesting that APE2 is a critical factor for promoting DSB repair or suppressing DSB generation [56]. One study demonstrated that APE2 is important for HR in MM cells [20]. To repair DSBs, HR uses homologous chromosomes or DNA for error-free repair, while NHEJ is more error-prone since repairing a DSB by NHEJ often leads to small insertions or deletions at the lesion site. During HR, the DSB end is resected for RPA recruitment followed by Rad51 replacement and strand invasion (Fig. 5C). Notably, knock-down or chemical inhibition of APE2 impairs HR activity while APE2 overexpression enhances HR activity [20]. The underlying mechanism of APE2 in HR regulation is likely at the step of Rad51 expression and recruitment, although biochemical evidence also shows APE2 interacts with HR factors including BRCA1, BRCA2, BARD1, and Rad52 [20]. In addition, a recent genome-wide screen of genetic interactions has revealed that APE2 is synthetic lethality of BRCA1 and BRCA2 deficiency [21].
5.2. DNA damage response pathways
5.2.1. ATR-Chk1-mediated DDR pathway
Hydrogen peroxide-induced oxidative DNA damage triggers the ATR-Chk1 DDR pathway in Xenopus egg extracts [15]. Evidence from immunodepletion and rescue experiments supports the conclusion that APE2 is critical for the hydrogen peroxide-induced ATR DDR pathway [15]. XlAPE2 associates with Chk1, and the S86A mutant XlAPE2 is defective for Chk1 interaction and hydrogen peroxide-induced ATR DDR pathway, indicating a Claspin-like but distinct role for APE2 in ATR-Chk1 signaling [15]. Further structure and function analyses indicate that XlAPE2 recruitment to the site of oxidative DNA damage via its interaction with PCNA, and XlAPE2 3′-5′ exonuclease activity are important for APE2-mediated ATR DDR pathway activation in oxidative stress [15]. Interestingly, the C-terminal Zf-GRF motif of XlAPE2 interacts with ssDNA and promotes APE2 3′-5′ exonuclease activity, shedding new light on the critical Zf-GRF motif in the regulation of APE2 function in ATR-Chk1 DDR pathway [16].
DNA lesions induced by hydrogen peroxide include damaged bases, AP sites, SSBs, and DSBs. To further test the potential role of APE2 in the SSB-induced DDR pathway, newly developed SSB signaling technology has elucidated that APE2 is important for ATR-Chk1 DDR pathway activation induced by a plasmid-based defined SSB structure in the Xenopus system [17, 67]. A C470A mutation in the Zf-GRF motif affects APE2’s interaction with PCNA and impairs the strong 3'-5' exonuclease activity and the defined SSB-induced ATR-Chk1 DDR pathway [17].
How is APE2 recruited to the SSB sites for the ATR-Chk1 DDR pathway? A recent study demonstrates that APE1 is required for APE2 recruitment to the SSB site, but APE2 is dispensable for APE1 recruitment to the SSB site (Fig. 5B) [18]. Further investigation suggests that APE1 initiates the end resection of the SSB structure in the 3'-5' direction, leading to a small gap (~1nt–3nt), which is the preferred substrate for the recognition and association of APE2 [18]. Then APE2 is recruited by PCNA via its PIP box and activated by Zf-GRF interaction with ssDNA and PCNA C-terminus for the continuation of the 3'-5' SSB end resection [38]. After the initiation and continuation of SSB end resection, a longer stretch of ssDNA(~18-26nt) is generated for appropriate assembly of ATR DDR complex, including ATR, ATRIP, TopBP1, and the 9-1-1 complex(Rad9-Rad1-Hus1) (Fig. 5B). The activated ATR phosphorylates its substrate proteins including Chk1 and RPA32 (Fig. 5B). Thus, an APE1/APE2-mediated two-step SSB end resection mechanism is proposed for the SSB-induced ATR-Chk1 DDR pathway [31].
5.2.2. p53-dependent DDR pathway
APE2-KO mice are viable but display moderate growth retardation and severe deficiency in lymphopoiesis 27]. Gene expression profiling in APE2-KO has revealed that various genes involved in DNA replication, recombination, repair, and DNA damage response, as well as apoptosis, are altered significantly [56]. Furthermore, more γH2AX foci and increased phosphorylation of p53 at Ser23, and to a lesser extent at Ser 16, are found in most APE2-KO thymocytes than wild-type thymocytes [56]. However, it has not been tested whether the increased γH2AX is dependent on ATM or ATR.
A recently published paper reports that APE2 loss was lethal in p53-proficient RPE1-hTERT cells but viable in their p53-mutated counterparts, suggesting that APE2 deficiency in RPE1 cells leads to p53-dependent cell death [40]. It remains unknown whether the observed p53-dependent cell death in the absence of APE2 can be applied to a more general DDR or only to RPE1 cell-type-specific phenotype.
5.3. Immunoglobulin somatic hypermutation (SHM) and class switch recombination (CSR)
Immunoglobulin (Ig) CSR and SHM are two critical immune responses in B cells [68]. CSR is an intrachromosomal recombination process at DSB sites in the switch (S) regions located 5' of each constant region gene. SHM is due to point mutations in the recombined V(D)J and Ig switch regions. The deamination of cytosines initiates CSR and SHM by the activation-induced cytidine deaminase (AID), generating uracils in S regions. The BER enzyme UNG excises uracils, resulting in AP sites [69]. AP sites will then be cleaved by endonuclease activity of AP endonucleases and/or AP lyase activity of bifunctional DNA glycosylases.
Compared to its wild type counterpart, CSR is reduced to about 65% in APE2-deficient splenic B cells isolated from male APE2Y/− mice, suggesting that APE2 plays a role in CSR [70]. Switching to IgG1, IgG2a, IgG2b, but not IgA, were reduced in APE2-null B cells, likely due to reduced DSB formation from opposing AP sites during Ig CSR in the absence of APE2 [70]. Also, the production of IgG and IgM in APE2-null mice after ovalbumin immunization was attenuated in comparison with WT mice, suggesting a minor role of APE2 in Ig CSR [27]. However, data from another group showed that switching to IgG1 was not reduced in APE2-deficient B cells isolated from male APE2Y/− mice, suggesting a conflicting result [71]. Besides, gene targeting-derived APE2 deficiency has no effect on CSR for switching to IgA with the presence or absence of APE1 in mouse B cell line CH12F3 cells [72]. The discrepancy in the requirement of APE2 for CSR may be due to differences in vitro culture and stimulation conditions or the detailed Ig isotypes [73].
The role of APE2 in SHM was also investigated. APE2 deficiency leads to a drastic reduction of SHM and the number of mutations per mutated clone without affecting the pattern of base substitution, suggesting that APE2 is required for efficient SHM [71]. Another study shows that APE2 expression is upregulated in the germinal center (GC) B cells where APE1 is down-regulated [26]. Notably, up-regulated APE2 promotes error-prone repair and high mutations at A-T base pairs, insertions, and deletions, suggesting a critical role of APE2 in SHM in GC [26]. Overall, APE2 plays a convincing role in SHM in immune response, although the role of APE2 in CSR remains to be further validated or clarified.
5.4. Epigenetic regulation – Active DNA demethylation
The level and pattern of 5-methyldeoxycytidine (5mC) in the genome is established by the combined actions of DNA methyltransferases and demethylases [74]. DNA demethylation is composed of two mechanisms: active DNA demethylation (active removal or modification of the methyl group from 5mC by enzymatic processes) and passive DNA demethylation (passive loss or dilution of 5mC during DNA replication in the absence of DNA methyltransferases) [74, 75]. In Arabidopsis thaliana, DNA glycosylases such as ROS1, DME, DML2, and DML3 can directly excise 5mC to generate an AP site via their glycosylase activity, initiating active DNA demethylation [74]. Because these enzymes also have AP lyase activity, the 3′ side of the phosphodiester backbone of the AP site can be cleaved via β-elimination reaction or successive β, δ-elimination reactions, producing a gap with a 3′-PUA or a 3′-P, respectively [74]. Either 3′-PUA or 3′-P precludes repair DNA synthesis; therefore, it must be further processed to produce normal 3′-OH.
APE2, either alone or working together with ZDP (Zinc finger DNA 3′-Phosphoesterase), functions downstream in ROS1-mediated active DNA demethylation in Arabidopsis [22]. WT but not D265H mutant AtAPE2 can cleave the 3′-P group within a 3′-recessed junction structure, suggesting that both the EEP domain are essential for its 3′-phophatase activity [22]. Whole-genome bisulfite sequencing analysis has revealed that APE2 deficiency causes DNA hypermethylation and hypomethylation at hundreds of loci and that the DNA demethylation function of AtAPE2 and ZDP are overlapping at some genomic loci [22]. Furthermore, such hypermethylation in APE2 and ZDP double mutants is partially reversed by the absence of DNA glycosylase ROS-1, suggesting that APE2 functions in ROS1-mediated active DNA demethylation [22]. How does APE2 absence leads to more DNA methylation? Li et al. speculated three possible mechanisms: (1) AtAPE2 may be important for displacing ROS1 and increasing turnover and catalytic efficiency of ROS1. Release of ROS1 from its 5mC and turnover rate of ROS1 is decreased in the absence of AtAPE2, leading to DNA hypermethylation. (2) Some AP sites remain unrepaired in the absence of AtAPE2, which may inhibit the binding and activity of ROS1 toward 5mC on the opposite strand. (3) It is interesting to note that AtAPE2 and ZDP double mutants increase the expression of DNA methylation maintenance proteins including MET1, CMT3, VIM1, and VIM2. Future studies are warranted to reveal the exact molecular mechanisms of how APE2 contributes to active DNA demethylation. Since PNKP is the mammalian homolog to ZDP, future studies are needed to examine the possible overlapping role of APE2 and PNKP in active DNA demethylation and DNA repair in animals. Overall, current studies of APE2 in Arabidopsis suggest its critical role in active DNA demethylation in epigenome regulation.
6. Multiple functions of APE2 in health and diseases
The full extent of APE2 dysfunction in human health and diseases is still poorly understood; however, there are still multiple functions of APE2 in growth, development, and diseases under various physiological and pathological conditions that can be highlighted (Fig. 4).
6.1. Growth and development
6.1.1. Function of APE2 in growth and development at the level of tissue/organism
The first set of observations on APE2 functions in growth and development came from studies of APE2-KO mice [27]. Although APE1 is essential for early embryonic development in mice [76]. APE2-KO mice are viable, suggesting that APE2 is not required for embryogenesis [27]. However, as APE2 is localized on the X chromosome in mice [31], the APE2−/Y male mice displayed moderate growth retardation with about 20% reduced body weight compared with WT male littermates at birth, childhood, and adulthood [27]. Of note, the thymus in APE2-KO mice exhibited significant atrophy compared with WT mice, and the total number of thymocytes in APE2-KO thymus was also decreased significantly compared with WT thymus [27].
APE2’s role in development was further investigated through studies of AtAPN2 in plants. Although a single knock-out of APE2, APE1L, or ARP shows no apparent phenotype, a double knock-out of APE2 and APE1L is lethal and leads to deficiency in seed development in Arabidopsis thaliana [77]. Interestingly, a double mutant of APE1L and ZDP is also embryonic lethal [43]. Whereas APE2 and ZDP in plants share overlapping 3′-Phosphatase activity [22], embryonic lethality by APE2 or ZDP deficiency in APE1L-KO background suggests that other unidentified non-overlapping enzymatic activities in APE2 and ZDP are essential for their critical role in development.
6.1.2. Role of APE2 in cell-specific growth and development
G2/M arrest was found in thymocytes and mitogen-stimulated splenocytes from APE2-KO mice, suggesting that APE2 is required for proper cell cycle progression in proliferating lymphocytes [21]. MmAPEX2 is also necessary for normal B-cell development [78]. In particular, APE2 deficiency leads to defects in the transition from pro-B cells to pre-B cells. It was recently demonstrated that differentially expressed APE2 in germinal centers promotes error-prone repair and mutations during somatic hypermutation [26]. APE2 can resolve these endogenously-generated DNA 3′-termini, including 2',3'-cyclic phosphate, and 3′-Top1, in budding yeast and human cultured cells [24, 25, 40]. These studies suggest that APE2 plays an important role in cell growth and development in a cell-type specific manner.
6.2. Diseases
6.2.1. Cancer etiology and therapeutics
Based on the critical roles of APE2 in genomic and epigenomic integrity, it is plausible to expect that APE2 is involved in cancer etiology. Indeed, four recent studies have shown genetic alterations and overexpression of APE2 in various cancer patients. First, Kumar et al. reported in 2018 that APE2 mRNA expression was upregulated in cultured MM cells and samples from MM patients (n=112) compared with control groups [20]. Second, Jensen et al. demonstrated in 2020 that genomic alterations of APE2 including amplification, gain, homozygous and heterozygous deletion as well as mutations were found in cancer patients at ~17% frequency across 14 different cancer types (n=21,769), and that APE2 mRNA expression was significantly upregulated in tumor tissues in kidneys, breast, lung, liver, and uterine cancers compared with non-malignant tissues [23]. Third, Omeroglu Simsek et al. reported in 2020 that ~5-fold higher mRNA expression of APE2 was found in patients with malignant pleural mesothelioma (MPM) (n=12), a common cancer type caused by environmental exposure to asbestos [79, 80]. Last, APE2 is proposed as an oncogene in liver cancer [81]. These studies suggest a possible role of APE2 in cancer development, consistent with the role of APE2 in SSB repair and HR-mediated DSB repair [17, 19, 20]. However, the exact underlying mechanism of APE2 in cancer etiology remains to be investigated.
From a therapeutic perspective, screening and identifying target molecules that will provide synergetic effects to ATR or PARP1 inhibitors are urgently needed. Accumulating evidence suggests that APE2 may be an excellent target for such efforts. It was reported that suppression of APE2 in a MM cell line leads to susceptibility to a PARP inhibitor PJ34 [20]. CRISPR-mediated KO of APE2 increased sensitivity of cancer cells including HCT116 to ATR inhibitor AZD6738 [82]. Future studies are needed to dissect the exact mechanisms for the synergy phenotype of APE2 suspension or inhibition for ATR/PARP1 inhibitors.
A recent CRISPR-mediated screen identified APE2 and FEN1 as synthetic lethal targets in BRCA2-deficient cells [21]. Genetic interaction approaches further validated APE2 as a synthetic lethal target of both BRCA1 and BRCA2 deficiency [40]. Mechanistically, APE2 can reverse the endogenous complex DNA 3′-termini derived from AP sites or mis-incorporated dUMP that block further repair synthesis, due to the vulnerabilities of BRCA1 and BRCA2 deficiency [40]. Because of the broad spectrum of functions of BRCA1 and BRCA2 in genome integrity including DSB repair and hereditary mutation of BRCA1/2 in cancer patients [83], it is urgently needed to determine the exact mechanisms of how APE2 contributes to cancer development in BRCA1/2-proficient or -deficient background.
6.2.2. Neurodegenerative and mitochondria-related diseases
ROS-induced cellular changes have been implicated in a multitude of diseases, such as arthritis, aging, and neurodegenerative disorders [24]. In response to ROS-induced DNA damage, cells have evolved several defense mechanisms that include numerous DNA repair and DDR pathways [58]. ROS can cause DNA base lesions and strand breaks and if left unrepaired may result in mutations and instability in nuclei and mitochondria. Somatic and hereditary mutations of mitochondria DNA (mtDNA) are associated with a variety of diseases including neurodegenerative disorders [84].
It is conceivable that APE2 plays a role in the repair of oxidatively damaged mtDNA due to its mitochondria localization. HsAPE2 possesses a MLS and was found to colocalize in both mitochondria and nuclei, with possible BER function in both subcellular compartments [14]. MmAPEX2 was found to be located in the inner mitochondrial membrane through the detection of immunogold signals for APE2 by electron microscopic immunocytochemistry [31]. This implies that proper mitochondrial function relies, in part, on proper APE2 function. Consistent with this concept, Wang et al. developed a novel statistical method of sequence robust multi-array analysis and found a link between mitochondrial DNA maintenance dysfunction and APE2 mutation [85]. Therefore, the role of APE2 in the mitochondria might be connected to both neurodegenerative disorders and mitochondria-related diseases.
6.2.3. Others
Although the role of APE2 in various diseases remains to be fully elucidated, accumulating evidence suggests that APE2 may be involved in other disease such as immune response deficiency and osteoarthritis. APE2-KO mice exhibited thymus atrophy and an attenuated immune response with severely reduced B lymphocytes [27]. Conversely, upregulation of APE2 in germinal center (GC) B cells with downregulation of APE1 may promote error-prone DNA repair for somatic hypermutation [26]. Evidence also suggests a role of APE2 for Ig CSR which improves effector function of the humoral immune response [70]. APE2 was also shown to be required for proper chondrocyte production of proteoglycan, a major component of articular cartilage [86]. This suggests downregulation of APE2 in chondrocytes may cause osteoarthritis.
6. Summary and future directions
In this review, we sought to provide the first comprehensive perspectives on APE2 in genome and epigenome integrity. We summarized the structure and functional domains of APE2 in eight species. We also focused on various DNA substrates and APE2 nuclease activities including AP endonuclease, 3'-phosphodiesterase, 3'-phosphatase, and 3'-5' exonuclease activities. We further summarized five APE2 interaction proteins with available characteristics. Lastly, we provided insights into critical roles of APE2 in DNA repair, DDR pathways, immune response, and active DNA demethylation and proposed APE2 as a multiple function protein in growth, development, and diseases.
With accumulating evidence showing APE2’s critical roles in genome and epigenome integrity, APE2 has been a rising star molecule with a high potential to be a target for pharmaceutical, pre-clinical and clinical studies. For example, (a) is APE2 a driver or passenger molecule for cancer development? (b) is APE2 a biomarker for cancer or at least for some certain types of cancer? (c) how is APE2 expression and function regulated at the levels of transcription, translation, and post-translation modifications? (d) what is the underlying mechanism for APE2 in SSB repair and/or DSB repair?
APE2 is an evolutionarily conserved protein with multiple nuclease activities and involved in many diseases. Further investigation is needed to elucidate the molecular details of APE2’s involvement in genome stability and is necessary to determine the potential relevance of APE2 function to human disease pathology, which will benefit public health.
Highlights:
Distinct functional domains of APE2 from eight species
Various DNA substrates and enzymatic activities of APE2
APE2 interaction proteins and phenotypes of APE2 mutants
Functions of APE2 in DNA repair, DNA damage response, and DNA demethylation
Biological significance of APE2 in growth, development, and diseases
Acknowledgements
The Yan lab was supported, in part, by grants from the National Cancer Institute (R01CA225637) and the National Institute of General Medical Sciences (R15GM114713) in the National Institutes of Health, and funds from University of North Carolina at Charlotte.
Abbreviations
- 3'-OH
3'-hydroxyl termini
- 3'-P
3'-phosphate
- 3'-PG
3'-phosphoglycolate
- 3'-PUA
3'-phospho-α, β-unsaturated aldehyde
- 5'-dRP
5′-deoxyribose phosphate
- 5mC
5-methylcytosine
- 8-oxoG
8-oxo-7,8-dihydroguanine
- 9-1-1 complex
Rad9-Rad1-Hus1 complex
- AP
apurinic/apyrimidinic
- APE1
AP endonuclease 1
- APE2
AP endonuclease 2
- BER
Base excision repair
- CKB motif
Chk1-binding motif
- CSR
class switch recombination
- DDR
DNA damage response
- DSB
double-strand break
- dsDNA
double-strand DNA
- EEP
Exonuclease/endonuclease/phosphatase catalytic domain
- EndoIV
Endonuclease IV
- ExoIII
Exonuclease III
- HR
homologous recombination
- IDCL motif
Interdomain connector loop motif
- Ig
immunoglobulin
- MM
multiple myeloma
- MMS
Methyl methanesulfonate
- MLS
mitochondria localization signal
- Myh1
MutY homolog
- NHEJ
nonhomologous end joining
- NLS
nuclease localization signal
- PIP box
PCNA-interacting protein box
- PNKP
polynucleotide kinase-phosphatase
- rNMPs
Ribonucleotide monophosphates
- ROS
reactive oxygen species
- SSB
single-strand break
- ssDNA
single-strand DNA
- SHM
somatic hypermutation
- TCGA
The Cancer Genome Atlas
- Top1
Topoisomerase I
- THF
tetrahydrofuran
- UNG
uracil DNA glycosylase
- WT
wild type
- ZDP
Zinc finger DNA 3′-Phosphoesterase
- Zf-GRF
zinc finger with a conserved GRxF motif
Footnotes
Declaration of Competing Interest
The authors declare non conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lindahl T, Instability and decay of the primary structure of DNA, Nature, 362 (1993) 709–715. [DOI] [PubMed] [Google Scholar]
- [2].Boiteux S, Guillet M, Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae, DNA Repair (Amst), 3 (2004) 1–12. [DOI] [PubMed] [Google Scholar]
- [3].Thompson PS, Cortez D, New insights into abasic site repair and tolerance, DNA Repair (Amst), 90 (2020) 102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ciccia A, Elledge SJ, The DNA damage response: making it safe to play with knives, Mol Cell, 40 (2010) 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yan S, Sorrell M, Berman Z, Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress, Cell Mol Life Sci, 71 (2014) 3951–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jackson SP, Bartek J, The DNA-damage response in human biology and disease, Nature, 461 (2009) 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Demple B, Johnson A, Fung D, Exonuclease III and endonuclease IV remove 3' blocks from DNA synthesis primers in H2O2-damaged Escherichia coli, Proc Natl Acad Sci USA, 83 (1986) 7731–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li M, Wilson DM 3rd, Human apurinic/apyrimidinic endonuclease 1, Antioxid Redox Signal, 20 (2014) 678–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Demple B, Herman T, Chen DS, Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes, Proc Natl Acad Sci USA, 88 (1991) 11450–11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Robson CN, Hickson ID, Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants, Nucleic Acids Res, 19 (1991) 5519–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xanthoudakis S, Curran T, Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity, EMBO J, 11 (1992) 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson RE, Torres-Ramos CA, Izumi T, Mitra S, Prakash S, Prakash L, Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites, Genes Dev, 12 (1998) 3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hadi MZ, Wilson DM 3rd, Second human protein with homology to the Escherichia coli abasic endonuclease exonuclease III, Environ Mol Mutagen, 36 (2000) 312–324. [PubMed] [Google Scholar]
- [14].Tsuchimoto D, Sakai Y, Sakumi K, Nishioka K, Sasaki M, Fujiwara T, Nakabeppu Y, Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen, Nucleic Acids Res, 29 (2001) 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Willis J, Patel Y, Lentz BL, Yan S, APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress, Proc Natl Acad Sci USA, 110 (2013) 10592–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wallace BD, Berman Z, Mueller GA, Lin Y, Chang T, Andres SN, Wojtaszek JL, DeRose EF, Appel CD, London RE, Yan S, Williams RS, APE2 Zf-GRF facilitates 3'-5' resection of DNA damage following oxidative stress, Proc Natl Acad Sci USA, 114 (2017) 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin Y, Bai L, Cupello S, Hossain MA, Deem B, McLeod M, Raj J, Yan S, APE2 promotes DNA damage response pathway from a single-strand break, Nucleic Acids Res, 46 (2018) 2479–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin Y, Raj J, Li J, Ha A, Hossain MA, Richardson C, Mukherjee P, Yan S, APE1 senses DNA single-strand breaks for repair and signaling, Nucleic Acids Res, 48 (2020) 1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cupello S, Lin Y, Yan S, Distinct roles of XRCC1 in genome integrity in Xenopus egg extracts, Biochem J, 476 (2019) 3791–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kumar S, Talluri S, Pal J, Yuan X, Lu R, Nanjappa P, Samur MK, Munshi NC, Shammas MA, Role of apurinic/apyrimidinic nucleases in the regulation of homologous recombination in myeloma: mechanisms and translational significance, Blood Cancer J, 8 (2018) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mengwasser KE, Adeyemi RO, Leng Y, Choi MY, Clairmont C, D'Andrea AD, Elledge SJ, Genetic screens reveal FEN1 and APEX2 as BrCa2 synthetic lethal targets, Mol Cell, 73 (2019) 885–899 e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li J, Liang W, Li Y, Qian W, Apurinic/apyrimidinic endonuclease 2 and Zinc finger DNA 3'-phosphoesterase play overlapping roles in the maintenance of epigenome and genome stability, Plant Cell, 30 (2018) 1954–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jensen KA, Shi X, Yan S, Genomic alterations and abnormal expression of APE2 in multiple cancers, Sci Rep, 10 (2020) 3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yan S, Resolution of a complex crisis at DNA 3' termini, Nat Struct Mol Biol, 26 (2019) 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li F, Wang Q, Seol JH, Che J, Lu X, Shim EY, Lee SE, Niu H, Apn2 resolves blocked 3' ends and suppresses Top1-induced mutagenesis at genomic rNMP sites, Nat Struct Mol Biol, 26 (2019) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stavnezer J, Linehan EK, Thompson MR, Habboub G, Ucher AJ, Kadungure T, Tsuchimoto D, Nakabeppu Y, Schrader CE, Differential expression of APE1 and APE2 in germinal centers promotes error-prone repair and A:T mutations during somatic hypermutation, Proc Natl Acad Sci USA, 111 (2014) 9217–9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ide Y, Tsuchimoto D, Tominaga Y, Nakashima M, Watanabe T, Sakumi K, Ohno M, Nakabeppu Y, Growth retardation and dyslymphopoiesis accompanied by G2/M arrest in APEX2-null mice, Blood, 104 (2004) 4097–4103. [DOI] [PubMed] [Google Scholar]
- [28].Funakoshi M, Nambara D, Hayashi Y, Zhang-Akiyama QM, CiAPEX2 and CiP0, candidates of AP endonucleases in Ciona intestinalis, have 3'-5' exonuclease activity and contribute to protection against oxidative stress, Genes Environ, 39 (2017) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ribar B, Izumi T, Mitra S, The major role of human AP-endonuclease homolog Apn2 in repair of abasic sites in Schizosaccharomyces pombe, Nucleic Acids Res, 32 (2004) 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sepulveda S, Valenzuela L, Ponce I, Sierra S, Bahamondes P, Ramirez S, Rojas V, Kemmerling U, Galanti N, Cabrera G, Expression, functionality, and localization of apurinic/apyrimidinic endonucleases in replicative and non-replicative forms of Trypanosoma cruzi, J Cell Biochem, 115 (2014) 397–409. [DOI] [PubMed] [Google Scholar]
- [31].Ide Y, Tsuchimoto D, Tominaga Y, Iwamoto Y, Nakabeppu Y, Characterization of the genomic structure and expression of the mouse Apex2 gene, Genomics, 81 (2003) 47–57. [DOI] [PubMed] [Google Scholar]
- [32].Jin J, Hwang BJ, Chang PW, Toth EA, Lu AL, Interaction of apurinic/apyrimidinic endonuclease 2 (Apn2) with Myh1 DNA glycosylase in fission yeast, DNA Repair (Amst), 15 (2014) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Unk I, Haracska L, Prakash S, Prakash L, 3'-phosphodiesterase and 3'-->5' exonuclease activities of yeast Apn2 protein and requirement of these activities for repair of oxidative DNA damage, Mol Cell Biol, 21 (2001) 1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burkovics P, Szukacsov V, Unk I, Haracska L, Human Ape2 protein has a 3'-5' exonuclease activity that acts preferentially on mismatched base pairs, Nucleic Acids Res, 34 (2006) 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Burkovics P, Hajdu I, Szukacsov V, Unk I, Haracska L, Role of PCNA-dependent stimulation of 3'-phosphodiesterase and 3'-5' exonuclease activities of human Ape2 in repair of oxidative DNA damage, Nucleic Acids Res, 37 (2009) 4247–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Unk I, Haracska L, Johnson RE, Prakash S, Prakash L, Apurinic endonuclease activity of yeast Apn2 protein, J Biol Chem, 275 (2000) 22427–22434. [DOI] [PubMed] [Google Scholar]
- [37].Unk I, Haracska L, Gomes XV, Burgers PM, Prakash L, Prakash S, Stimulation of 3'->5' exonuclease and 3'-phosphodiesterase activities of yeast apn2 by proliferating cell nuclear antigen, Mol Cell Biol, 22 (2002) 6480–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu RA, Semlow DR, Kamimae-Lanning AN, Kochenova OV, Chistol G, Hodskinson MR, Amunugama R, Sparks JL, Wang M, Deng L, Mimoso CA, Low E, Patel KJ, Walter JC, TRAIP is a master regulator of DNA interstrand crosslink repair, Nature, 567 (2019) 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ha A, Lin Y, Yan S, A non-canonical role for the DNA glycosylase NEIL3 in suppressing APE1 endonuclease-mediated ssDNA damage, J Biol Chem, 295 (2020) 14222–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alvarez-Quilon A, Wojtaszek JL, Mathieu MC, Patel T, Appel CD, Hustedt N, Rossi SE, Wallace BD, Setiaputra D, Adam S, Ohashi Y, Melo H, Cho T, Gervais C, Munoz IM, Grazzini E, Young JTF, Rouse J, Zinda M, Williams RS, Durocher D, Endogenous DNA 3' blocks are vulnerabilities for BRCA1 and BRCA2 deficiency and are reversed by the APE2 nuclease, Mol Cell, 78 (2020) 1152–1165 e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Johnson AW, Demple B, Yeast DNA 3'-repair diesterase is the major cellular apurinic/apyrimidinic endonuclease: substrate specificity and kinetics, J Biol Chem, 263 (1988) 18017–18022. [PubMed] [Google Scholar]
- [42].Wilson DM 3rd, Takeshita M, Grollman AP, Demple B, Incision activity of human apurinic endonuclease (Ape) at abasic site analogs in DNA, J Biol Chem, 270 (1995) 16002–16007. [DOI] [PubMed] [Google Scholar]
- [43].Li Y, Cordoba-Canero D, Qian W, Zhu X, Tang K, Zhang H, Ariza RR, Roldan-Arjona T, Zhu JK, An AP endonuclease functions in active DNA demethylation and gene imprinting in Arabidopsis [corrected], PLoS Genet, 11 (2015) e1004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hadi MZ, Ginalski K, Nguyen LH, Wilson DM 3rd, Determinants in nuclease specificity of Ape1 and Ape2, human homologues of Escherichia coli exonuclease III, J Mol Biol, 316 (2002) 853–866. [DOI] [PubMed] [Google Scholar]
- [45].Andres SN, Schellenberg MJ, Wallace BD, Tumbale P, Williams RS, Recognition and repair of chemically heterogeneous structures at DNA ends, Environ Mol Mutagen, 56 (2015) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sugimoto T, Igawa E, Tanihigashi H, Matsubara M, Ide H, Ikeda S, Roles of base excision repair enzymes Nth1p and Apn2p from Schizosaccharomyces pombe in processing alkylation and oxidative DNA damage, DNA Repair (Amst), 4 (2005) 1270–1280. [DOI] [PubMed] [Google Scholar]
- [47].Hossain MA, Lin Y, Yan S, Single-strand break end resection in genome integrity: mechanism and regulation by APE2, Int J Mol Sci, 19 (2018) 2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Caldecott KW, Single-strand break repair and genetic disease, Nat Rev Genet, 9 (2008) 619–631. [DOI] [PubMed] [Google Scholar]
- [49].Kim N, Huang SN, Williams JS, Li YC, Clark AB, Cho JE, Kunkel TA, Pommier Y, Jinks-Robertson S, Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I, Science, 332 (2011) 1561–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sekiguchi J, Shuman S, Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I, Mol Cell, 1 (1997) 89–97. [DOI] [PubMed] [Google Scholar]
- [51].Mailand N, Gibbs-Seymour I, Bekker-Jensen S, Regulation of PCNA-protein interactions for genome stability, Nat Rev Mol Cell Biol, 14 (2013) 269–282. [DOI] [PubMed] [Google Scholar]
- [52].Kumagai A, Dunphy WG, Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts, Mol Cell, 6 (2000) 839–849. [DOI] [PubMed] [Google Scholar]
- [53].Kumagai A, Dunphy WG, Repeated phosphopeptide motifs in Claspin mediate the regulated binding of Chk1, Nat Cell Biol, 5 (2003) 161–165. [DOI] [PubMed] [Google Scholar]
- [54].Tell G, Quadrifoglio F, Tiribelli C, Kelley MR, The many functions of APE1/Ref-1: not only a DNA repair enzyme, Antioxid Redox Signal, 11 (2009) 601–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hoitsma NM, Whitaker AM, Schaich MA, Smith MR, Fairlamb MS, Freudenthal BD, Structure and function relationships in mammalian DNA polymerases, Cell Mol Life Sci, 77 (2020) 35–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dan Y, Ohta Y, Tsuchimoto D, Ohno M, Ide Y, Sami M, Kanda T, Sakumi K, Nakabeppu Y, Altered gene expression profiles and higher frequency of spontaneous DNA strand breaks in APEX2-null thymus, DNA Repair (Amst), 7 (2008) 1437–1454. [DOI] [PubMed] [Google Scholar]
- [57].Mol CD, Hosfield DJ, Tainer JA, Abasic site recognition by two apurinic/apyrimidinic endonuclease families in DNA base excision repair: the 3' ends justify the means, Mutat Res, 460 (2000) 211–229. [DOI] [PubMed] [Google Scholar]
- [58].Hegde ML, Hazra TK, Mitra S, Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells, Cell Res, 18 (2008) 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Krokan HE, Bjoras M, Base excision repair, Cold Spring Harbor perspectives in biology, 5 (2013) a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Svilar D, Goellner EM, Almeida KH, Sobol RW, Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage, Antioxid Redox Signal, 14 (2011) 2491–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nakabeppu Y, Tsuchimoto D, Ichinoe A, Ohno M, Ide Y, Hirano S, Yoshimura D, Tominaga Y, Furuichi M, Sakumi K, Biological significance of the defense mechanisms against oxidative damage in nucleic acids caused by reactive oxygen species: from mitochondria to nuclei, Ann N Y Acad Sci, 1011 (2004) 101–111. [DOI] [PubMed] [Google Scholar]
- [62].Davis L, Maizels N, Homology-directed repair of DNA nicks via pathways distinct from canonical double-strand break repair, Proc Natl Acad Sci USA, 111 (2014) E924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Brem R, Hall J, XRCC1 is required for DNA single-strand break repair in human cells, Nucleic Acids Res, 33 (2005) 2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Eustermann S, Wu WF, Langelier MF, Yang JC, Easton LE, Riccio AA, Pascal JM, Neuhaus D, Structural basis of detection and signaling of DNA single-strand breaks by human PARP-1, Mol Cell, 60 (2015) 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cupello S, Richardson C, Yan S, Cell-free Xenopus egg extracts for studying DNA damage response pathways, Int J Dev Biol, 60 (2016) 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Malyarchuk S, Castore R, Harrison L, DNA repair of clustered lesions in mammalian cells: involvement of non-homologous end-joining, Nucleic Acids Res, 36 (2008) 4872–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lin Y, Ha A, Yan S, Methods for studying DNA single-strand break repair and signaling in Xenopus laevis egg extracts, Methods Mol Biol, 1999 (2019) 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stratigopoulou M, van Dam TP, Guikema JEJ, Base excision repair in the immune system: small DNA lesions with big consequences, Front Immunol, 11 (2020) 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schrader CE, Guikema JE, Wu X, Stavnezer J, The roles of APE1, APE2, DNA polymerase beta and mismatch repair in creating S region DNA breaks during antibody class switch, Philos Trans R Soc Lond B Biol Sci, 364 (2009) 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Guikema JE, Linehan EK, Tsuchimoto D, Nakabeppu Y, Strauss PR, Stavnezer J, Schrader CE, APE1- and APE2-dependent DNA breaks in immunoglobulin class switch recombination, J Exp Med, 204 (2007) 3017–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sabouri Z, Okazaki IM, Shinkura R, Begum N, Nagaoka H, Tsuchimoto D, Nakabeppu Y, Honjo T, Apex2 is required for efficient somatic hypermutation but not for class switch recombination of immunoglobulin genes, Int Immunol, 21 (2009) 947–955. [DOI] [PubMed] [Google Scholar]
- [72].Masani S, Han L, Yu K, Apurinic/apyrimidinic endonuclease 1 is the essential nuclease during immunoglobulin class switch recombination, Mol Cell Biol, 33 (2013) 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Guikema JE, Stavnezer J, Schrader CE, The role of Apex2 in class-switch recombination of immunoglobulin genes, Int Immunol, 22 (2010) 213; author reply 213-214. [DOI] [PubMed] [Google Scholar]
- [74].Zhu JK, Active DNA demethylation mediated by DNA glycosylases, Annu Rev Genet, 43 (2009) 143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kohli RM, Zhang Y, TET enzymes, TDG and the dynamics of DNA demethylation, Nature, 502 (2013) 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T, The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice, Proc Natl Acad Sci USA, 93 (1996) 8919–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Murphy TM, Belmonte M, Shu S, Britt AB, Hatteroth J, Requirement for abasic endonuclease gene homologues in Arabidopsis seed development, PLoS One, 4 (2009) e4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Guikema JE, Gerstein RM, Linehan EK, Cloherty EK, Evan-Browning E, Tsuchimoto D, Nakabeppu Y, Schrader CE, Apurinic/apyrimidinic endonuclease 2 is necessary for normal B cell development and recovery of lymphoid progenitors after chemotherapeutic challenge, J Immunol, 186 (2011) 1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Omeroglu Simsek G, Agababaoglu I, Dursun D, Ozekinci S, Ercetin P, Ellidokuz H, Aktas S, Gurel D, Oztop I, Akkoclu A, Evaluation of gene expression levels in the diagnosis of lung adenocarcinoma and malignant pleural mesothelioma, Turk Gogus Kalp Damar Cerrahisi Derg, 28 (2020) 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Metintas M, Ozdemir N, Hillerdal G, Ucgun I, Metintas S, Baykul C, Elbek O, Mutlu S, Kolsuz M, Environmental asbestos exposure and malignant pleural mesothelioma, Respir Med, 93 (1999) 349–355. [DOI] [PubMed] [Google Scholar]
- [81].Zheng R, Zhu HL, Hu B, Ruan X, Cai H, Identification of APEX2 as an oncogene in liver cancer, World J Clin Cases, 8 (2020) 2917–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hustedt N, Alvarez-Quilon A, McEwan A, Yuan JY, Cho T, Koob L, Hart T, Durocher D, A consensus set of genetic vulnerabilities to ATR inhibition, Open Biol, 9 (2019) 190156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Petrucelli N, Daly MB, Feldman GL, Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2, Genet Med, 12 (2010) 245–259. [DOI] [PubMed] [Google Scholar]
- [84].Akbari M, Visnes T, Krokan HE, Otterlei M, Mitochondrial base excision repair of uracil and AP sites takes place by single-nucleotide insertion and long-patch DNA synthesis, DNA Repair (Amst), 7 (2008) 605–616. [DOI] [PubMed] [Google Scholar]
- [85].Wang W, Shen P, Thiyagarajan S, Lin S, Palm C, Horvath R, Klopstock T, Cutler D, Pique L, Schrijver I, Davis RW, Mindrinos M, Speed TP, Scharfe C, Identification of rare DNA variants in mitochondrial disorders with improved array-based sequencing, Nucleic Acids Res, 39 (2011) 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yui N, Yoshioka H, Fujiya H, Musha H, Beppu M, Karasawa R, Yudoh K, The DNA repair enzyme Apurinic/Apyrimidinic Endonuclease (Apex Nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis, Int J Mol Sci, 15 (2014) 14921–14934. [DOI] [PMC free article] [PubMed] [Google Scholar]