Abstract
Background:
Discontinuation of bisphosphonates (BP) or a “drug holiday” after several years of treatment is increasingly common. However, the association of drug holiday duration with future fracture risk is unclear.
Objectives:
We evaluated the rate of fracture in relation to various lengths of drug holidays among women receiving long-term BP therapy.
Research Design:
Observational cohort study using US Medicare data 2006–2016. Incidence rates (IRs) and Cox proportional hazards models were used to evaluate the rate and adjusted hazard ratios (aHRs) controlling for potential confounders.
Subjects:
Women aged 65 years and above enrolled in fee-for-service Medicare who had been adherent (≥ 80%) to alendronate, risedronate, or zoledronate for ≥ 3 years.
Measures:
Hip, humerus, distal forearm, and clinical vertebral fracture.
Results:
Among 81,427 eligible women observed for a median (interquartile range) of 4.0 (2.5, 5.3) years, 28% of women underwent a drug holiday. In the alendronate cohort (73% overall), the IR of hip fracture among women who discontinued BP for > 2 years was 13.2 per 1000 person-years. Risk was increased (aHR =1.3, 1.1–1.4) versus continuing therapy (IR =8.8, referent). Rates were elevated for humerus fracture with discontinuation >2 years (aHR =1.3, 1.1–1.66) and for clinical vertebral fracture with discontinuation > 2 years (aHR =1.2, 1.1–1.4). Results were similar for risedronate, zoledronate, and ibandronate for hip and clinical vertebral fracture.
Conclusion:
Discontinuing alendronate beyond 2 years was associated with increased risk of hip, humerus, and clinical vertebral fractures.
Keywords: bisphosphonate, osteoporosis, fractures, drug holiday
Bisphosphonates (BPs) reduce both vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis and in other groups at higher risk for fractures.1 Osteoporosis is a chronic condition that is typically not cured or even sufficiently improved after only 3 years of BP treatment (the typical length of most phase 3 trials) to allow for cessation of therapy. Thus, patients may receive osteoporosis treatments for many years.
Since BPs are intercalated into the hydroxyapatite bone matrix leading to prolonged skeletal retention, a variety of safety concerns have been raised related to long-term BP use including osteonecrosis of the jaw and atypical femur fractures.2–4 Although rare, these potential adverse effects numerous case reports and case series, FDA warnings,5 and position papers from specialty societies such as the American Society for Bone and Mineral Research (ASBMR).4 Given the unique properties of these drugs, coupled with these safety concerns, clinicians may recommend to their patients a “drug holiday”; a strategy of temporary or permanent cessation of all potent antiresorptive bone-protective therapies such as BPs.4
However, results from the 1099-person Fracture Intervention Long Term Extension (FLEX) trial, showed that continued alendronate treatment was associated with significant, albeit small, absolute risk reduction in clinical vertebral fracture risk but not nonvertebral fractures, and a significant increase in bone mineral density (BMD).6 In a subgroup analysis, women who had osteoporosis at the time of re-randomization, achieved a significant benefit from continued alendronate to reduce hip fracture risk.7 Likewise, a protective but nonsignificant trend was observed in women with hip T-scores between −2.5 and −2.0. However, neither change in BMD, nor bone turnover markers, was shown to be helpful to risk stratify patients with respect to the benefits of continuing therapy.8,9 FLEX was limited by the overall poor generalizability of clinical trials, relatively small numbers of fractures (n = 33 total hip fractures), and small number of outcomes in key subgroups such as women with prevalent vertebral fractures. Similar conclusions and limitations were observed from the extension trial of the once yearly intravenous (IV) BP zoledronate.10 Both studies suggest that the protracted skeletal effects of long-term BPs may differ by patient subgroup, fracture site, and even by specific BP.
Moreover, there is a disappointing inability to accurately predict fracture risk during a drug holiday or to effectively monitor patients with tools such as change in BMD or bone turnover markers. In addition, evidence from observational studies is scant, leaving clinicians with uncertainty regarding the optimal duration of a BP drug holiday. Expert guidance from the ASBMR suggested that after 3–5 years of IV and oral BP use, reassessment of risk should be considered.4 Key factors proffered to help risk-stratify patients include a prior major osteoporotic fracture and low hip T-score. A suggestion was made, based on the limited data described above, that a drug holiday of 2–3 years could be considered, recognizing that, “It is unlikely that future trials will provide data for formulating definitive recommendations.” The European Menopause Society has offered suggestions for when it may be appropriate to restart therapy after a BP drug holiday, but this was not evidence based, as the group noted.11
Given the expectation that there will be few, if any, future clinical trials to inform the optimal duration of a drug holiday, we conducted an observational cohort study of older women adherent with oral or IV BP therapy for at least 3 years. We evaluated the hypothesis that fracture risk was associated with a drug holiday, relative to continued use.
METHODS
Overview
We used data from the US Medicare program (January 1, 2006–December 31, 2016) to assemble a cohort of women who were new users of alendronate, risedronate, oral ibandronate, and zoledronate, and who subsequently received therapy for at least 3 years. Four separate, BP-specific exposure cohorts were constructed (alendronate, risedronate, ibandronate, and zoledronate) because of potential differences in the relationship between the duration of a drug holiday with each medication, given their different properties including different binding affinities to bone and pharmacodynamic effects.12 There were not enough IV ibandronate users to form a separate IV ibandronate exposure cohort. Cumulative adherence of ≥80% as estimated by the proportion of days covered was required to be eligible for analysis. Key time intervals related to the study are shown in Figure 1.
FIGURE 1.
Study design. Baseline refers to the 12 months before the index date. BP indicates bisphosphonate.
Cohort Eligibility
Women were potentially eligible for analysis if they were aged 65 years and above, had continuous traditional fee-for-service Medicare enrollment with Part A (hospital coverage), Part B (outpatient), and Part D (pharmacy) coverage. They were required to be new users of 1 of the 4 BPs, with at least a 12 month “clean” period free of all BP therapy before first use (but all available data was used, if > 12 mo was available), and to have received BP treatment for a minimum of 3 years. Consistent with both the FLEX trial design and other conventions in the BP adherence literature,13 women had to have at least 80% cumulative adherence (from first BP exposure to the start of follow-up). To maintain the homogeneity of the drug-specific exposure cohorts, women were not permitted to switch between BPs and were censored if they started a different BP. The start of follow-up, defined as the “index date,” was anchored to the time when women met all eligibility criteria.
The 12 months before the index date was defined as “baseline” and used for covariate assessment. Women were excluded if they had prior malignancy (ignoring nonmelanoma skin cancer) or Paget’s disease of bone in the baseline period or earlier. The malignancy exclusion required 2 paired diagnoses for malignancy with the first 3 digits of the diagnosis code in common, separated by at least 30 days and occurring within 365 days. Women were also excluded if they used denosumab, teriparatide, calcitonin, hormone therapy, or raloxifene in the 12-month baseline; had discontinued BPs for > 30 days at the index date; or used any BP not indicated for the treatment of osteoporosis (eg, pamidronate) during baseline or earlier. Women were excluded if they had an inconsistent date of birth, death, or sex in the data.
Definition of BP Drug Holiday
BP exposure was based on the number of days supplied by filled prescriptions and the usual dosing interval of 365 days for zoledronate. Exposure was updated for each person-day of the analysis, and women could move between current exposure and recent exposure (BP exposure within last 12 mo) dynamically. A drug holiday was defined as no exposure to any BP for at least 24 months, to minimize short-term variability in adherence that would not reflect the intentional cessation of treatment. Drug holiday durations of > 2 year (731+ d), for all 4 therapies were constructed in a time-dependent manner, so that women could contribute person-time to each of these categories if she stayed on a drug holiday long enough. These durations were constructed to align with the ASBMR recommendations suggesting a drug holiday of 2–3 years may be reasonable and prudent for most patients, as well as the sufficiency of the available data.
Fracture Outcomes
Clinical fractures were ascertained using a validated algorithm that has been shown to have high validity (positive predictive value > 85% for all fracture sites) compared with the gold standard of medical record review.14 In keeping with the goal of optimizing specificity, fractures required hospitalization or surgical repair, except for clinical vertebral fractures, where there had to be evidence of spine imaging, vertebroplasty, or kyphoplasty. Separate outcomes for hip, humerus, distal forearm, and clinical vertebral fractures were considered consistent with the “major osteoporotic fracture” grouping that comprises FRAX.15 Because of the potential for misclassification of an incident vertebral fracture in a patient with prevalent vertebral fracture,16 women with baseline vertebral fracture were excluded in the analysis of clinical vertebral fracture but not in the other 3 fracture outcomes.
Statistical Analysis
Descriptive characteristics were reported as of the index date, stratifying women into mutually exclusive groups based on whether they had a drug holiday. Standardized mean differences were used to compare groups, with standardized mean differences ≥ 0.10 used as a threshold for differences that might be clinically relevant.17 The proportion of women who had a drug holiday for the first time was reported descriptively by calendar time, in 6-month intervals. Incidence rates (IRs) per 1000 person-years (py) of each fracture type were estimated with Poisson regression, and examined by duration of BP drug holiday, referent to continued BP use.
Extended Cox proportional hazards models18 with age as the time axis were used to characterize the crude and adjusted hazard ratio (aHR) of fracture associated with a drug holiday, controlling for potentially confounding factors selected based upon subject matter expertise. Covariates included in the model were race, year of cohort entry, cumulative proportion of days covered (80%–90% vs. 90%–100%), receipt of dual-energy x-ray absorptiometry (DXA), emergency department visit, inpatient hospitalization, skilled nursing facility use, prior fragility fracture (hip, humerus, distal forearm, clinical vertebral, femur, pelvis), oral glucocorticoid use, and proton-pump inhibitor use. Using the SAS PHREG option, we stratified all models by baseline Charlson score,19 birth year and geographic region, which allowed the baseline hazard to vary by these factors. In addition, the competing risk of death was adjusted for using methods described by Fine and Grey, but these were not presented given that results were minimally different than in our main analyses.18,20 Women were censored for all conditions that were exclusionary at baseline (Paget’s, malignancy, use of non-BP bone-protective therapies).
As DXA results were not available in the data source, a subgroup analysis was conducted among women in the alendronate cohort who had prior fragility fractures, in the baseline, to identify a high-risk group.
RESULTS
After applying selection criteria (Fig. 2), we identified a total of 81,427 eligible women. A total of 22,846 (28%) underwent a drug holiday (Table 1). The proportion of women who initiated a drug holiday peaked at 5.8% in 2014 (Fig. 3). Approximately 73% used alendronate, and mean age on the index date was 79 years. Compared with women who did not undergo a drug holiday, those who did were more likely to be white and have lower Charlson comorbidity scores. Otherwise, there were few differences between women who underwent a drug holiday and those who did not, although women with prior fracture were somewhat less likely to take a drug holiday (20.3% for those without fracture vs. 16.0% with fracture). Corresponding data for each of the exposure cohorts are shown in Supplementary Tables 1a–1d (Supplemental Digital Content 1, http://links.lww.com/MLR/B947).
FIGURE 2.
Cohort eligibility. BP indicates bisphosphonate; DOB, date of birth; DOD, date of death; NB, number of Medicare beneficiaries; OP, osteoporosis; PDC, proportion of days covered (a measure of adherence). Start date = initiation date of first BP.
TABLE 1.
Baseline* Characteristics of Women Who Underwent a Bisphosphonate Drug Holiday* Versus Those Who Did Not
| Exposure Group, n (%) |
|||
|---|---|---|---|
| Drug Holiday† | No Drug Holiday | SMD | |
| Beneficiaries | 22,846 (28.06) | 58,581 (71.94) | |
| Alendronate cohort | 16,309 (71.4) | 42,965 (73.3) | 0.04 |
| Risedronate cohort | 1881 (8.2) | 4927 (8.4) | 0.01 |
| Zoledronate cohort | 2584 (11.3) | 5438 (9.3) | 0.07 |
| Ibandronate cohort | 2072 (9.1) | 5251 (9.0) | 0.00 |
| Demographics | |||
| Age at index date | 78.76 (6.82) | 79.12 (7.38) | 0.05 |
| Age category at index date | |||
| 65–69 | 1918 (8.4) | 5670 (9.7) | 0.04 |
| 70–74 | 5953 (26.1) | 14,413 (24.6) | 0.03 |
| 75–79 | 5445 (23.8) | 12,893 (22.0) | 0.04 |
| 80–84 | 4904 (21.5) | 11,948 (20.4) | 0.03 |
| 85+ | 4626 (20.2) | 13,657 (23.3) | 0.07 |
| Race | |||
| White | 20,311 (88.9) | 48,710 (83.1) | 0.17 |
| Region | |||
| Midwest | 7020 (30.7) | 17,532 (29.9) | 0.02 |
| Northeast | 4414 (19.3) | 9883 (16.9) | 0.06 |
| South | 7742 (33.9) | 20,416 (34.9) | 0.02 |
| West | 3670 (16.1) | 10,750 (18.4) | 0.06 |
| Comorbidities | |||
| Charlson score‡ | 1.36 (1.95) | 1.79 (2.26) | 0.20 |
| Charlson score | |||
| Category‡ | |||
| 0 | 11,750 (51.4) | 25,050 (42.8) | 0.17 |
| 1–2 | 5987 (26.2) | 15,958 (27.2) | 0.02 |
| 3+ | 5109 (22.4) | 17,573 (30.0) | 0.17 |
| Prior fragility fracture | |||
| Major fragility fracture baseline§ | 3648 (16.0) | 11,868 (20.3) | 0.11 |
| Hip fracture§ | 933 (4.1) | 3369 (5.8) | 0.08 |
| Pelvis fracture§ | 215 (0.9) | 806 (1.4) | 0.04 |
| Humerus fracture § | 411 (1.8) | 1393 (2.4) | 0.04 |
| Distal forearm fracture§ | 864 (3.8) | 2625 (4.5) | 0.04 |
| Clinical vertebral fracture§ | 1708 (7.5) | 5678 (9.7) | 0.08 |
| Medication use | |||
| Oral glucocorticoids‡∥ | 803 (3.5) | 2835 (4.8) | 0.07 |
| Proton-pump inhibitor‡ | 6057 (26.5) | 18,334 (31.3) | 0.11 |
| Healthcare utilization | |||
| Any ED visit* | 11,035 (48.3) | 31,793 (54.3) | 0.12 |
| No. ED visits* | 1.14 (2.54) | 1.43 (2.36) | 0.12 |
| Inpatient hospitalization* | 7152 (31.3) | 21,051 (35.9) | 0.10 |
| No. inpatient days* | 2.93 (10.31) | 3.89 (11.83) | 0.09 |
| Any SNF use* | 1888 (8.3) | 6251 (10.7) | 0.08 |
| SNF days* | 3.95 (37.42) | 6.82 (54.42) | 0.06 |
| Receipt of DXA§ | 20,135 (88.1) | 49,476 (84.5) | 0.11 |
| BP use history | |||
| Time since first BP exposure (d)* | 1113.64 (92.79) | 1122.82 (124.52) | 0.08 |
| Cumulative PDC since first BP* | 91.95 (6.17) | 91.82 (6.28) | 0.02 |
| Cumulative PDC since first BP* | |||
| 80%–< 90% | 8041 (35.2) | 20,906 (35.7) | 0.01 |
| 90%–100% | 14,805 (64.8) | 37,675 (64.3) | 0.01 |
| Alendronate equivalent cumulative dose (mg)* | 10,223.36 (873.56) | 10,284.69 | 0.06 |
| Year of index date | |||
| 2010 or before | 6781 (29.7) | 11,696 (20.0) | 0.23 |
| 2011 | 7987 (35.0) | 14,635 (25.0) | 0.22 |
| 2012 | 5009 (21.9) | 12,534 (21.4) | 0.01 |
| 2013 | 2325 (10.2) | 10,555 (18.0) | 0.23 |
| 2014 | 744 (3.3) | 9161 (15.6) | 0.43 |
Bold values indicates SMD > 0.10.
Mean (SD) for continuous variables.
N (%) for categorical variables.
Alendronate, risedronate, ibandronate, and zoledronate cohorts pooled together. Detail for each drug-specific cohort shown in Supplementary Tables 1a–1d (Supplemental Digital Content 1, http://links.lww.com/MLR/B947).
Baseline variables measured from first BP administration date.
Defined as > 12-month continuous gap in BP exposure after the start of follow-up.
Baseline variables measured during 365 days before index date.
Baseline variables measured since first eligible coverage start date.
Defined as ≥ 675 mg of prednisone-equivalent medication.
BP indicates bisphosphonate; ED, emergency department; IQR, interquartile range; OP, osteoporosis; PDC, proportion of days covered; SMD, standardized mean difference; SNF, skilled nursing facility.
FIGURE 3.
Drug holiday trends over time.
For the alendronate cohort (n = 59,254), median [interquartile range (IQR)] follow-up was 3.9 (2.5, 5.3) years. Among women who discontinued, and measured at time of discontinuation, median (IQR) time from first exposure to alendronate was 5.2 (4.3, 6.0) years, and they had received a cumulative mean (SD) of 10,210 (892) mg of alendronate. The IRs and aHRs for hip fracture in relation to various drug holiday durations are shown in Figure 4A. The crude IR of hip fracture among women who continued therapy was 8.8 per 1000py and was 13.2 per 1000py among those with drug holiday > 2 years, yielding an aHR of 1.27 (95% confidence interval, 1.12–1.44). Risk was similarly elevated beyond 2 years for humerus fracture (aHR = 1.34, 1.07–1.66) although not significant for distal forearm fractures (aHR = 1.04, 0.88–1.24). Risk for clinical vertebral fracture also was elevated starting at >2 years (aHR = 1.22, 1.10–1.40). Results were highly consistent in the subgroup of women using alendronate with prior fragility fracture (Supplementary Fig., Supplemental Digital Content 2, http://links.lww.com/MLR/B948). In these women with prior fracture, all the hazard ratios were numerically higher, and the absolute IRs of fracture were ~1.5–2.0-fold higher compared with the main alendronate cohort.
FIGURE 4.
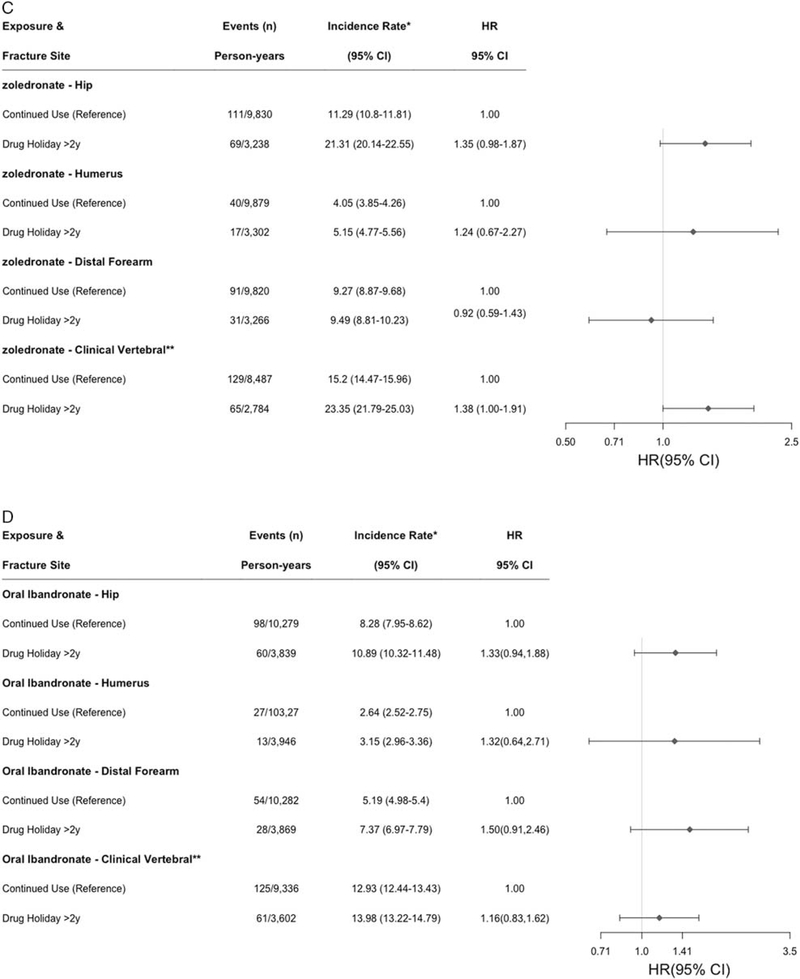
Number of fracture events, person-years, incidence rate per 1000 person-years, and adjusted* hazard ratio (HR) [95% confidence interval (CI)], for alendronate (A), risedronate (B), zoledronate (C), and Ibandronate (D). *Controlling for race, year of cohort entry, cumulative proportion of days covered (80%–90% vs. 90%–100%), receipt of DXA, emergency department visit, inpatient hospitalization, skilled nursing facility use, prior fragility fracture, oral glucocorticoid use, and proton-pump inhibitor use, Charlson score, birth year, and geographic region.
In the risedronate cohort (n = 6,808 women), median (IQR) follow-up time was somewhat shorter at 4.4 (2.6, 5.8) years. Risk for hip fracture shown in Figure 4B was significantly elevated with drug holidays > 2 years (aHR = 1.54, 1.06–2.26), as were clinical vertebral fractures (aHR = 1.59, 1.09–2.33). Rates were not elevated for humerus or distal forearm fractures, although based on <20 fracture events in the drug holiday group. In the zoledronate cohort (n = 8022) with median (IQR) follow-up of 3.7 (2.6, 4.7) years, there was numerically elevated risk of hip fracture (aHR = 1.35, 0.98–1.87) and a marginally increased risk for clinical vertebral fractures (aHR = 1.38, 1.00–1.91) observed in association with drug holidays of > 2 years (Fig. 4C). Risk was numerically elevated in the ibandronate cohort for all fracture types (HRs ranging from 1.16 to 1.50), although given the sample size, none were significant.
DISCUSSION
In this large study of long-term BP users, we found hip fracture risk after alendronate discontinuation was increased by 27%, and rates for humerus and clinical vertebral fractures were similarly increased. We found a similar association with risedronate holidays for hip and clinical vertebral fracture, with the magnitude of risk as large, if not larger, as that for alendronate holidays. In contrast, we did not find a significantly increased fracture risk with drug holidays of any duration with zoledronate or ibandronate, although the smaller sample sizes for these treatments may have contributed to the lack of statistical significance, given that the hip fracture effect estimates were numerically elevated. We also observed that BP drug holidays began to plateau in 2012, a time frame expected given the safety concerns and FDA warning in 2010 regarding risks for atypical femur fracture with prolonged BP use.
Aside from the extensions to the 2 alendronate and zoledronate trials previously mentioned,6,10 there is scant additional literature with which to compare our results. We previously showed that there was evidence for increased hip fracture rate in women who had been very adherent (≥ 80%) with BPs (predominantly alendronate) with a drug holiday.21 However, the nonsignificant result observed (aHR = 2.0; 95% confidence interval, 0.9–9.3) was based only on 23 hip fracture events and could not quantify any time-dependence in the duration of a drug holiday at which risk might increase. The absolute rates of hip fracture we observed (8.8/1000py) are comparable to those observed in the FLEX trial (7/1000py),6 particularly when considering mean age of the 2 studies (FLEX: 73 y; current: 79 y).
In a recent study conducted in Kaiser Permanente patients, Adams et al,22 found no increased risk for fracture in relation to a drug holiday (aHR = 0.95, 0.83–1.10) for any length holiday compared with continued BP use. Although the absolute fracture rates among women persistent with BPs are consistent with ours (eg, hip fractures: 8.8/1000 in our study, 8.3/1000 in the Kaiser study), several methodological differences between their study and ours may explain these discordant results. The Kaiser study included a somewhat younger cohort of women (mean age, 70 vs. 79 y in our study). Women had to be only ≥ 50% or more adherent, unlike our ≥ 80% adherence requirement. Their adherence and drug holiday exposure status were assessed annually, which may have misclassified whether people were on persistent or on a drug holiday by up to 1 year. Women were not censored if they restarted BP therapy but continued to be classified in the BP drug holiday group (8.5% of the overall drug holiday subgroup in our analysis), which also could misclassify BP exposure. The size of the Kaiser study’s drug holiday cohort (n = 11,497) was approximately half the size of ours, and all oral and IV BPs were mixed together as a single exposure. The mean follow-up time was approximately the same in both studies (~4.0 y). Although the Kaiser study had BMD by DXA results available, stratification by BMD results had no meaningful impact on their findings. Surprisingly, among women with a history of prior fracture, they found a counterintuitively reduced rate of osteoporotic fracture among women undergoing a drug holiday (aHR = 0.79, 0.67–0.94). Finally, the factors associated with women stopping, and thus channeling bias (or lack thereof), may have changed over time, and our study included data through the end of 2016, whereas the Kaiser study used data only through 2012.
However, our study must be interpreted considering its design. Women were not randomized to discontinue BP therapy, and our ability to control for possible confounding was limited to factors that could be measured in administrative data. Notably, we lacked information regarding BMD, although analyses conducted in the subgroup of women with prior fragility fracture yielded fundamentally the same results as our main analysis. We did not confirm fractures with medical record adjudication, although the positive predict value of the algorithms that we relied upon have been shown to exceed 90% in a large validation study,14 particularly for hip fractures. Given the nature of the data source, we studied clinical vertebral fractures as an outcome, but not all vertebral fractures such as might be captured in an osteoporosis clinical trial where serial spine x-rays are systematically taken at the beginning and end of the trial; only about one-third of radiographic vertebral fractures are clinically recognized, although these may be considered more medically relevant. We did not have information regarding the reasons that women stopped BP therapy nor whether it was intentional. Although we defined cessation as a drug holiday, we nevertheless recognize that women may have discontinued therapy for other reasons. In particular, we recognize the potential for confounding due to healthy behaviors associated with medication adherence,23–25 or conversely, a “sick stopper” effect, whereby fracture rates may be higher immediately following discontinuation due to confounding from comorbidities leading to abrupt drug discontinuation. For that reason, we defined a drug holiday as starting > 24 months since cessation of BP therapy to lessen this concern. We did not study some of the safety-related outcomes, such as osteonecrosis of the jaw and atypical femoral fracture, which sometimes motivate clinicians to recommend a holiday. Indeed, given the limited ability of administrative claims data to accurately detect these conditions, we were unable to determine whether these adverse outcomes occur at a lower frequency with BP discontinuation and thus were unable to put our findings into a full risk-benefit perspective. Results from our analysis conducted in women enrolled in Medicare with a mean age 79 years may not generalize to younger women at lower fracture risk based on age, nor necessarily to women with a more prolonged duration of prior BP exposure (eg, > 5 or > 10 y).
CONCLUSIONS
A drug holiday from alendronate for > 2 years is likely to adversely impact fracture risk, at least for hip and clinical vertebral fracture, the sites at which BPs have been shown to be most effective for fracture prevention. Similar patterns were observed for risedronate with at least as strong if not a stronger effect. Although shared decision making regarding the risks and benefits of a drug holiday should be individualized, these results may inform such decisions and future clinical guidelines in the optimal duration of drug holidays for long-term BP users.
Supplementary Material
Acknowledgments
Supported in part by NIAMS (1U34AR062891). NIAMS had no role in the writing of or decision to publish the manuscript.
J.R.C.: research grants, consulting (Amgen, Lilly, Radius). K.G.S.: research grant, consulting (Amgen, Mereo, Radius, Roche). N.C.W.: research grants (Amgen), consulting/expert witness (Pfizer). T.A., H.Y., S.D., R.M., and E.D.: research grants (Amgen).
Footnotes
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.lwwmedicalcare.com.
REFERENCES
- 1.Byun JH, Jang S, Lee S, et al. The efficacy of bisphosphonates for prevention of osteoporotic fracture: an update meta-analysis. J Bone Metab. 2017;24:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. [DOI] [PubMed] [Google Scholar]
- 3.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29: 1–23. [DOI] [PubMed] [Google Scholar]
- 4.Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:1910. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast- track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1166–1172. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Longterm Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz AV, Bauer DC, Cummings SR, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: The FLEX trial. J Bone Miner Res. 2010;5:976–982. [DOI] [PubMed] [Google Scholar]
- 8.Bauer DC, Schwartz A, Palermo L, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med. 2014;174:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNabb BL, Vittinghoff E, Schwartz AV, et al. BMD changes and predictors of increased bone loss in postmenopausal women after a 5-year course of alendronate. J Bone Miner Res. 2013;28:1319–1327. [DOI] [PubMed] [Google Scholar]
- 10.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2011;2: 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anagnostis P, Paschou SA, Mintziori G, et al. Drug holidays from bisphosphonates and denosumab in postmenopausal osteoporosis: EMAS position statement. Maturitas. 2017;101:23–30. [DOI] [PubMed] [Google Scholar]
- 12.Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–627. [DOI] [PubMed] [Google Scholar]
- 13.Curtis JR, Westfall AO, Cheng H, et al. The benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008; 23:1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright NC, Daigle SG, Melton ME, et al. The design and validation of a new algorithm to identify incident fractures in administrative claims data. J Bone Miner Res. 2019;10:1798–1807. [DOI] [PubMed] [Google Scholar]
- 15.Fracture Risk Assessment Tool. Availble at: http://shef.ac.uk/FRAX. Accessed May 31, 2018.
- 16.Curtis JR, Mudano AS, Solomon DH, et al. Identification and validation of vertebral compression fractures using administrative claims data. Med Care. 2009;47:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 18.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis JR, Westfall AO, Cheng H, et al. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams AL, Adams JL, Raebel MA, et al. Bisphosphonate drug holiday and fracture risk: a population-based cohort study. J Bone Miner Res. 2018;33:1252–1259. [DOI] [PubMed] [Google Scholar]
- 23.Curtis JR, Yun H, Lange JL, et al. Does medication adherence itself confer fracture protection? An investigation of the healthy adherer effect in observational data. Arthritis Care Res (Hoboken). 2012;64:1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis JR, Larson JC, Delzell E, et al. Placebo adherence, clinical outcomes, and mortality in the women’s health initiative randomized hormone therapy trials. Med Care. 2011;49:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis JR, Delzell E, Chen L, et al. The relationship between bisphosphonate adherence and fracture: is it the behavior or the medication? Results from the placebo arm of the fracture intervention trial. J Bone Miner Res. 2011;26:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.