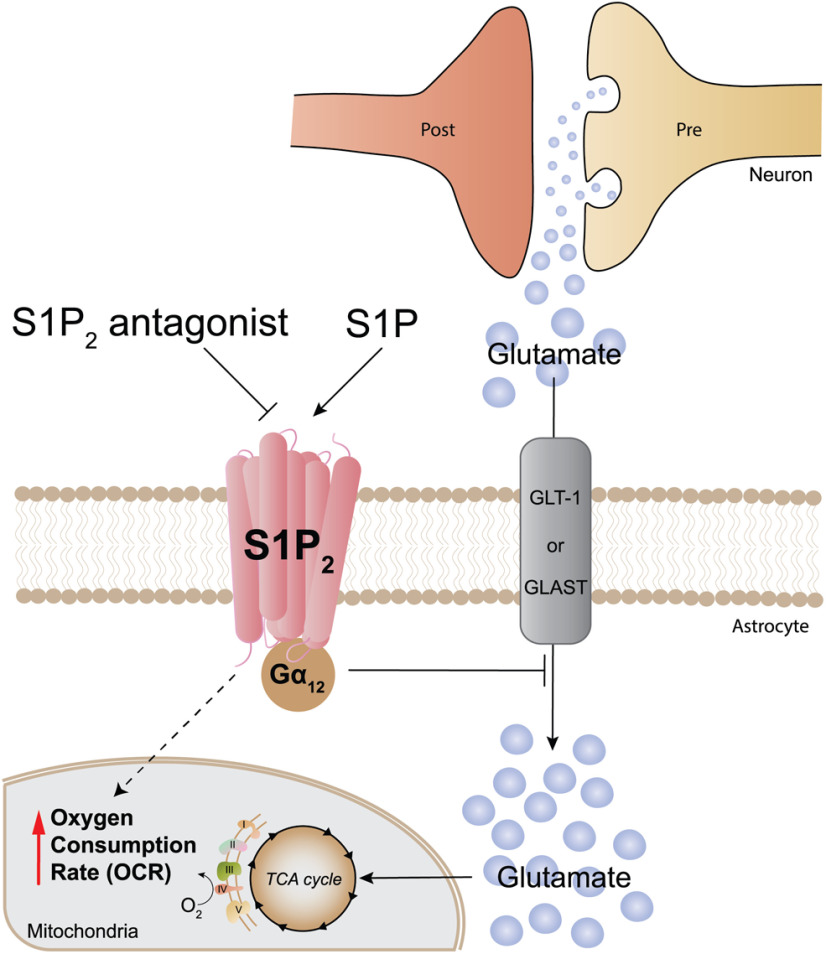
Visual Abstract
Keywords: ponesimod, sphingolipids, lysophospholipids, fingolimod, siponimod, ozanimod
Abstract
Glutamate is the principal excitatory neurotransmitter in the human brain. Following neurotransmission, astrocytes remove excess extracellular glutamate to prevent neurotoxicity. Glutamate neurotoxicity has been reported in multiple neurologic diseases including multiple sclerosis (MS), representing a shared neurodegenerative mechanism. A potential modulator of glutamate neurotoxicity is the bioactive lysophospholipid sphingosine 1-phosphate (S1P) that signals through five cognate G-protein-coupled receptors, S1P1–S1P5; however, a clear link between glutamate homeostasis and S1P signaling has not been established. Here, S1P receptor knock-out mice, primary astrocyte cultures, and receptor-selective chemical tools were used to examine the effects of S1P on glutamate uptake. S1P inhibited astrocytic glutamate uptake in a dose-dependent manner and increased mitochondrial oxygen consumption, primarily through S1P2. Primary cultures of wild-type mouse astrocytes expressed S1P1,2,3 transcripts, and selective deletion of S1P1 and/or S1P3 in cerebral cortical astrocytes, did not alter S1P-mediated, dose-dependent inhibition of glutamate uptake. Pharmacological antagonists, S1P2-null astrocytes, and Gα12 hemizygous-null astrocytes indicated that S1P2-Gα12-Rho/ROCK signaling was primarily responsible for the S1P-dependent inhibition of glutamate uptake. In addition, S1P exposure increased mitochondrial oxygen consumption rates (OCRs) in wild-type astrocytes and reduced OCRs in S1P2-null astrocytes, implicating receptor selective metabolic consequences of S1P-mediated glutamate uptake inhibition. Astrocytic S1P-S1P2 signaling increased extracellular glutamate, which could contribute to neurotoxicity. This effect was not observed with the FDA-approved S1P receptor modulators, siponimod and fingolimod. Development and use of S1P2-selective antagonists may provide a new approach to reduce glutamate neurotoxicity in neurologic diseases.
Significance Statement
Extracellular glutamate is excitotoxic and its levels are controlled by astrocyte uptake. Sphingosine 1-phosphate (S1P) is a bioactive lipid originating from cell membrane sphingolipids and associates with carrier molecules like albumin, ApoM, and ApoA4 to produce cellular effects. S1P signals extracellularly through five G-protein-coupled receptors and it is found in higher concentrations in neurologic diseases like multiple sclerosis (MS) where excitotoxic neurodegeneration has been implicated. Here, we show that astrocytic S1P2 activation by S1P results in glutamate uptake inhibition to promote excitotoxic damage. S1P receptor modulators, including approved drugs for treating MS, e.g., fingolimod (FTY720) and siponimod (BAF312), do not engage S1P2, thus avoiding glutamate uptake inhibition. S1P2 antagonists may provide a means to reduce S1P-induced glutamate neurotoxicity and ameliorate neurologic diseases.
Introduction
L-glutamate is the most abundant free amino acid in the brain, and it is the primary excitatory neurotransmitter (Lipton and Rosenberg, 1994; Vandenberg and Ryan, 2013; Lewerenz and Maher, 2015). Glutamate dysregulation has been associated with neurologic conditions such as epilepsy (Mathern et al., 1999; Barker-Haliski and White, 2015), Alzheimer’s disease (Masliah et al., 1996; Li et al., 1997; Scott et al., 2011), Huntington’s disease (André et al., 2010), amyotrophic lateral sclerosis (Bruijn et al., 1997; Ilieva et al., 2009), and multiple sclerosis (MS; Srinivasan et al., 2005; Baranzini et al., 2010; Muhlert et al., 2014; MacMillan et al., 2016; Macrez et al., 2016). The involvement of glutamate dysregulation in these neurologic diseases makes pharmacological modulation of glutamate uptake an attractive therapeutic target (Anderson and Swanson, 2000).
Astrocytes are the predominant cell type involved in the maintenance of glutamate homeostasis, which can be altered in disease states (Mahmoud et al., 2019). Astrocytes take up excess glutamate via the transmembrane expression of glutamate transporter-1 (GLT-1; Pines et al., 1992) and L-glutamate/L-aspartate transporter (GLAST; Storck et al., 1992) and then glutamate is converted to non-toxic glutamine (Henn et al., 1974; Su et al., 2003). Glutamate uptake is an ATP-driven process (McKenna, 2013), where the glutamate taken up by astrocytes enters the TCA cycle (McKenna et al., 1996) and aids in mitochondrial respiration (Sonnewald et al., 1993; Hertz and Hertz, 2003). Activated astrocytes have also been implicated in several disease states (Sofroniew, 2014; Groves et al., 2018; Ponath et al., 2018; Yamanaka and Komine, 2018; Siracusa et al., 2019), and in a recent report using a mouse model of MS, astrocytes were predominantly activated during the disease development phase and were suppressed by fingolimod (FTY720) treatment (ieAstrocytes; Groves et al., 2018). FTY720 is a prodrug approved as a medicine for MS patients that must be phosphorylated to become an active agent (FTY720-P), where it acts a structural analog of sphingosine 1-phosphate (S1P; Blaho and Hla, 2014) to target S1P1,3,4,5 (Brinkmann et al., 2002; Mandala et al., 2002). Additional S1P receptor modulators have recently become available for MS treatment that do not require phosphorylation, including the S1P1,5-selective modulators, ozanimod and siponimod (BAF312; Chun et al., 2021). As additional S1P receptor modulators are developed for central nervous system disease treatment, an understanding of how S1P signaling functions within the brain, including the receptors mediating these functions, becomes increasingly important. In the present study, primary astrocytes from wild-type and S1P receptor-null mouse cerebral cortices were combined with pharmacological assessments employing S1P receptor modulators to identify S1P receptor-mediated effects on glutamate uptake and mitochondrial respiration.
Materials and Methods
Mice
All animal procedures were conducted in accordance with Institutional Care and Use Committee guidelines of Sanford Burnham Prebys Medical Discovery Institute. Postnatal day (P)0–1 C57BL/6J pups were collected for primary astrocyte cultures. S1P1 astrocyte conditional (GFAP Cre) null As-S1P1−/− mice, S1P3 null mice, As-S1P1/S1P3 double-null mice, and Gα12 heterozygous-null mice were on a C57BL/6J background. S1P2−/− mice were on a BALB/c background. All the experiments were performed regardless of sex of the mouse.
Primary astrocyte culture
Cerebral hemispheres were harvested from P0–P1 C57BL/6J pups and the meninges were removed and placed in DMEM/F12 (Life Technologies) containing penicillin and streptomycin (PS; Life Technologies), 50 μg/ml DNase I (Sigma-Aldrich), and 0.05% trypsin (Sigma-Aldrich). After gentle dissociation, the tissue was incubated for 30 min at 37°C. Trypsin was inactivated with DMEM/F12 containing 10% FBS (Gemini), and the cell suspension was centrifuged at 800 rpm for 5 min, resuspended in DMEM/F12 containing 10% FBS, and filtered through a 40 μm cell strainer (BD Biosciences). The cells were cultured in DMEM/F12 containing 10% FBS and passaged once. On their second passage, the cells were distributed into 24-well plates. After 24 h, the cells were serum-starved overnight, and experiments were conducted unless otherwise noted. FTY720-P-containing medium was replenished every 24 h over 3.5 d of exposure to ensure functional antagonism activity.
Reagents
L-[3,4-3H]-glutamic acid was obtained from PerkinElmer. S1P and VPC23019 were obtained from Avanti Polar Lipids. FTY720-phosphate was obtained from Novartis Pharma, AG and Cayman Chemical. RP001, Y27632, and JTE013 were from Tocris Bioscience. BAF312 (Siponimod) was obtained from Novartis Pharma AG and Apexbio. Pertussis toxin (PTX) from Bordetella pertussis was obtained from List Biological Laboratories.
Glutamate uptake assay
Serum-starved primary astrocytes (∼105 cells per sample) were incubated with 2 μM L-sodium glutamate (Sigma) and 20 nM 3H-Glutamic acid. Cells were immediately stimulated with agonist or treated with vehicle for 1 h at 37°C. The cell supernatant was then aspirated, the primary astrocytes were washed thoroughly with cold HBSS containing Ca2+/Mg2+ ions, and then incubated with 0.25 mL of 0.2 N NaOH for 2 h at room temperature. EcoLume scintillation cocktail (MP Biomedicals) was added to the samples and radioactivity [counts per minute (cpm)] was measured with a scintillation counter (Beckman). The percentage (%) of glutamate uptake by astrocytes was calculated as the (cpm in the test sample × 100) / (cpm in the vehicle sample).
Gene expression analysis
Gene expression was measured using TaqMan fast advance master mix and TaqMan probes for β-actin and S1P1-5 (ThermoFisher Scientific) and analyses were conducted on a Bio-Rad CFX 384 Touch Real-Time PCR Detection System. All reactions were performed in triplicate and the average Ct was used to calculate the receptor expression relative to β-actin.
Seahorse analysis
An Agilent Seahorse XF Cell Mito Stress Kit was used to measure oxygen consumption rate (OCR). Briefly, primary astrocytes (7 × 104/well) were plated on the Seahorse 24-well culture plate. After allowing the cells to acclimate in a 37°C non-CO2 incubator, the analyzer was calibrated, and the OCR was measured through sequential injection of electron transport chain inhibitors: (1) 1 μM oligomycin (ATP synthase inhibitor); (2) 1 μM FCCP (uncoupling agent); and (3) a combination of 0.5 μM rotenone (complex I inhibitor) and 0.5 μM antimycin A (complex III inhibitor). The non-mitochondrial respiratory rate (NMRR) corresponds to the OCR measurement after injection of rotenone and antimycin A. Basal OCR was calculated by subtracting NMRR from the initial recorded OCR (endogenous OCR). Proton leak was calculated by subtracting the NMRR from the OCR following oligomycin injection. Maximal OCR was calculated by subtracting the NMRR from the OCR following FCCP injection. Spare respiratory capacity is the difference between the maximal and the basal OCR.
Statistical analysis
Statistics were analyzed using GraphPad Prism 8. All dose response curves are an average of two to three independent experiments with two technical replicates each. The data were fitted by performing nonlinear regression. All data are represented as mean ± SEM unless otherwise noted. A summary of the statistical analyses is presented in Table 1.
Table 1.
Statistical table
| Figure | Method performed |
|---|---|
| 1A,C,D | Non-linear regression |
| 1E | Two-way ANOVA/Sidak’s multiple comparisons test |
| 2A–C | Non-linear regression |
| 3A–D | Two-way ANOVA/Sidak’s multiple comparisons test |
| 4A,B | Non-linear regression |
| 5C | Unpaired t test |
| 5D–H | Ordinary one-way ANOVA/Tukey’s multiple comparisons test |
| 5I | Two-way ANOVA/Tukey’s multiple comparisons test |
Results
S1P-mediated inhibition of glutamate uptake by astrocytes is dose-dependent and distinct from S1P receptor modulators
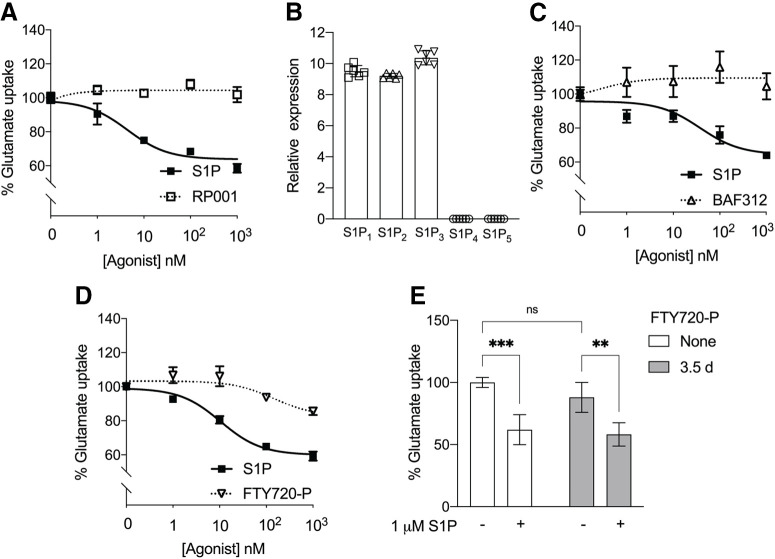
To understand how S1P affects astrocyte glutamate uptake, mouse primary astrocytes were cultured in the presence of radiolabeled glutamate and treated with increasing doses of S1P for 1 h. The percent of astrocytic glutamate uptake decreased with an increasing dose of S1P, resulting in a ∼40% reduction in glutamate uptake at the highest doses (Fig. 1A). An S1P1-selective agonist, RP001, did not inhibit glutamate uptake (Fig. 1A), indicating that S1P1 is not the primary receptor responsible for inhibiting glutamate uptake. Quantitative RT-PCR revealed that mouse primary astrocytes express mRNAs for S1P1,2,3 but not S1P4,5 (Fig. 1B). To determine whether S1P receptor modulators with distinct receptor subtype engagement affect astrocyte glutamate uptake, BAF312 and FTY720-P were tested on primary astrocytes. The S1P1,5 modulator, BAF312 (Chun et al., 2021), did not alter glutamate uptake at any dose tested under agonist conditions (Fig. 1C). FTY720-P, a high-affinity S1P receptor modulator for S1P1,3,4,5, did produce a 10% reduction of glutamate uptake at 1 μM, the highest concentration examined (Fig. 1D). A 3.5 d pretreatment with FTY720-P (functional antagonist conditions) did not produce an observable change in S1P-mediated astrocyte glutamate uptake inhibition compared with non-treated controls (Fig. 1E). These data show that astrocyte-mediated control of extracellular glutamate occurs through an S1P dose-dependent mechanism and is likely mediated through S1P2.
Figure 1.
Inhibition of glutamate uptake by S1P contrasts with S1P receptor modulator effects. Primary astrocytes were incubated with labeled glutamate, S1P, or respective agonists for 1 h at 37°C. The glutamate uptake assay was performed as described. A, S1P inhibited glutamate uptake by astrocytes in a dose-dependent manner, while the S1P1-specific agonist, RP001, did not. B, The relative expression of S1P1-5 (normalized to -actin) in wild-type primary astrocytes derived from cerebral cortices of P0 pups. C, The S1P1,5 modulator, BAF312, did not induce glutamate uptake inhibition at the indicated doses. D, The S1P1,3,4,5 modulator, FTY720-P, induced partial inhibition. A, C, D, Data are from two independent experiments and curve fitting was performed by nonlinear regression. E, Incubation with FTY720-P for 3.5 d, followed by stimulation with S1P, induced glutamate uptake inhibition similar to no FTY720-P pretreatment controls. Data are representative of three independent experiments. Error bars indicate (mean ± SD). Statistics are calculated by two-way ANOVA performing Sidak’s multiple comparisons test (***p < 0.0005, **p < 0.005; ns, not significant).
Receptor-null astrocytes show that glutamate uptake inhibition is not mediated through S1P1 or S1P3
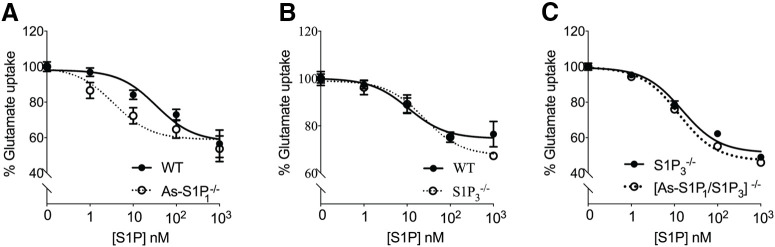
To understand how S1P1 and/or S1P3 influence astrocyte glutamate uptake, primary astrocytes derived from mice lacking these receptors were incubated with increasing doses of S1P (Fig. 2). A moderate decrease (left shift of curve) in glutamate uptake was observed in the absence of S1P1 on astrocytes compared with wild-type astrocytes (Fig. 2A), indicating that S1P1 might aid in glutamate uptake homeostasis. On the other hand, in the absence of S1P3, glutamate uptake was inhibited in a manner similar to that of wild-type astrocytes (Fig. 2B). To confirm the role of S1P1 on astrocyte glutamate uptake, As-S1P1-null and S1P3-null mice were crossed to obtain As-S1P1/S1P3-null mice. Primary astrocytes cultured from As-S1P1/S1P3-null mice exhibited a similar trend to that of S1P3-null with increasing doses of S1P, indicating that neither S1P1 nor S1P3 are primarily involved in astrocyte glutamate uptake (Fig. 2C).
Figure 2.
Glutamate uptake inhibition is not eliminated in S1P3-null and astrocyte-specific (As)-S1P1/S1P3-double-null astrocytes. Primary astrocytes from As-S1P1, S1P3, and As-S1P1/S1P3 receptor-null mice were incubated with labeled glutamate and S1P for 1 h at 37°C. A, Glutamate uptake in As-S1P1−/− astrocytes exhibited a slight left shift (decrease in glutamate uptake) compared with littermate controls. S1P3−/− (B) and As-S1P1/S1P3−/− (C) astrocytes exhibited control levels of glutamate uptake. Data in each panel are from three independent experiments. Curve fitting was performed by nonlinear regression.
S1P2 mediates S1P-induced astrocyte-mediated glutamate uptake inhibition
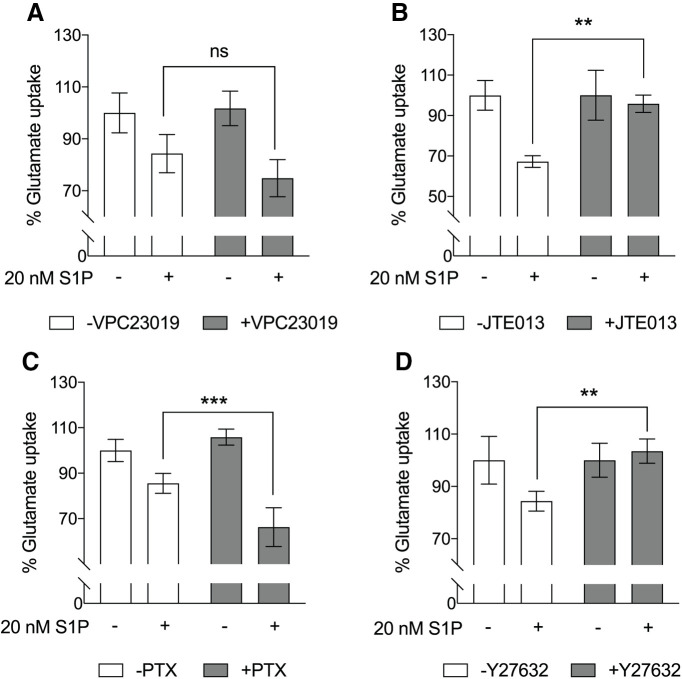
Pharmacological antagonists were employed to test whether S1P2 plays a role in astrocyte glutamate uptake inhibition. Primary astrocytes were preincubated with VPC23019, an antagonist for S1P1 and S1P3, and then stimulated with S1P for 1 h (Fig. 3A). No significant differences in glutamate uptake were observed compared with non-treated controls. Combined with prior results (Figs. 1, 2), these data indicated that S1P2 is primarily involved in inhibiting glutamate uptake. Indeed, the S1P2 antagonist, JTE013, rescued the inhibitory effect of S1P on astrocyte glutamate uptake (Fig. 3B). S1P2 couples with Gi, G12/13, or Gq proteins to initiate downstream signaling pathways. Therefore, astrocytes were pretreated with PTX (an inhibitor of the Gi proteins by ADP-ribosylation) and Y27632 (an inhibitor of Rho kinase downstream of G12/13) to deermine the pathways involved in S1P2-mediated glutamate uptake inhibition. Pretreatment with PTX did not alter S1P-induced glutamate uptake inhibition (Fig. 3C). Conversely, Y27632 completely rescued the S1P-induced inhibition of astrocyte glutamate uptake (Fig. 3D). These results indicated that Gα12/13 signaling downstream of S1P2 inhibits glutamate uptake in astrocytes.
Figure 3.
Pharmacological inhibition of S1P2, but not S1P1 or S1P3, eliminates astrocytic inhibition of glutamate uptake by S1P. Primary astrocytes were preincubated (A) with or without 10 μM VPC23019, an antagonist for S1P1 and S1P3, for 45 min, (B) 10 μM JTE 013, an S1P2 antagonist, for 30 min, (C) 200 ng/mL PTX overnight, and (D) 10 μM Y27632 for 30 min at 37°C followed by incubation with or without 20 nM S1P for 1 h. Glutamate uptake assays were performed as described. Data in each panel are representative of two independent experiments (n = 3 or 4). Error bars represent mean ± SD; ns, not significant; **p < 0.005, ***p < 0.001, two-way ANOVA with Sidak’s multiple comparisons test.
In addition to pharmacological studies, glutamate uptake in astrocytes genetically-null for S1P2 or hemizygously null for Gα12 was tested. Astrocytes null for S1P2 (Fig. 4A) or hemizygously null for Gα12 (Fig. 4B) exhibited an increase (right shift of curve) in glutamate uptake when compared with controls which supports S1P2 and Gα12 playing a role in inhibiting glutamate uptake. The possible involvement of additional S1P receptors or non-receptor mechanisms (e.g., non-cell autonomous effects) in S1P-induced glutamate uptake inhibition could not be excluded since glutamate uptake was not completely restored by genetic ablation of S1P2 or reduction of Gα12.
Figure 4.
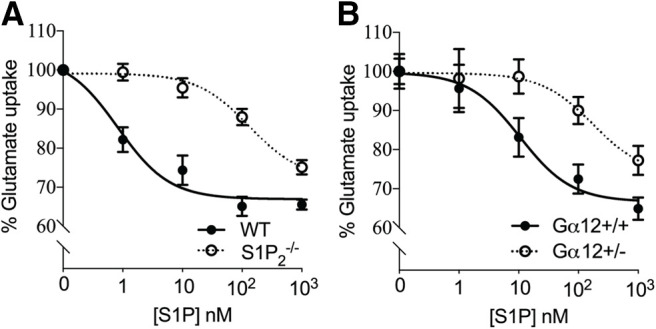
Genetic removal of S1P2 or Gα12 downstream signaling reduces astrocytic glutamate uptake inhibition by S1P. Primary astrocytes from S1P2-null or Gα12-hemizygous-null mice were incubated with labeled glutamate and S1P for 1 h at 37°C. Glutamate uptake in S1P2−/− (A) and Gα12+/− (B) astrocytes exhibited a right shift (increase in glutamate uptake) when compared with controls. Data in each panel are from three independent experiments. Curve fitting was performed by nonlinear regression.
S1P affects mitochondrial respiration through S1P2
Astrocytic glutamate uptake is an ATP-driven process known to affect mitochondrial function and health (Sonnewald et al., 1993; Hertz and Hertz, 2003). To understand how exposure to glutamate and S1P affects mitochondrial health, Seahorse analyses were used to measure the rate of oxygen consumption (Fig. 5A). Wild-type primary astrocytes were exposed to vehicle, glutamate, S1P, or a combination of glutamate and S1P for 1 h, then washed and incubated with Seahorse assay media containing glucose and pyruvate. The OCR was measured through the sequential addition of oligomycin, FCCP, and rotenone/antimycin A (Fig. 5B). S1P increased the endogenous OCR when compared with vehicle (Fig. 5C), indicating an increased energy demand. In all employed conditions, no significant difference in the NMRR was observed (Fig. 5D). Glutamate did not change basal OCR (Fig. 5E) or proton leak (Fig. 5F), whereas S1P increased basal OCR and proton leak regardless of the presence or absence of glutamate. These data suggest that S1P signaling directly increases mitochondrial OCR, which may be independent from intracellular glutamate pools (Hamilton and Attwell, 2010). However, the maximal OCR (Fig. 5G) and the spare respiratory capacity (Fig. 5H) were significantly elevated by glutamate exposure, but were not affected by S1P, indicating that a glutamate pool is crucial for responding to increased energy demand. The observed elevation in spare respiratory capacity on glutamate exposure was diminished by the addition of S1P (Fig. 5H), indicating that S1P inhibition of glutamate uptake might be responsible for this decrease.
Figure 5.
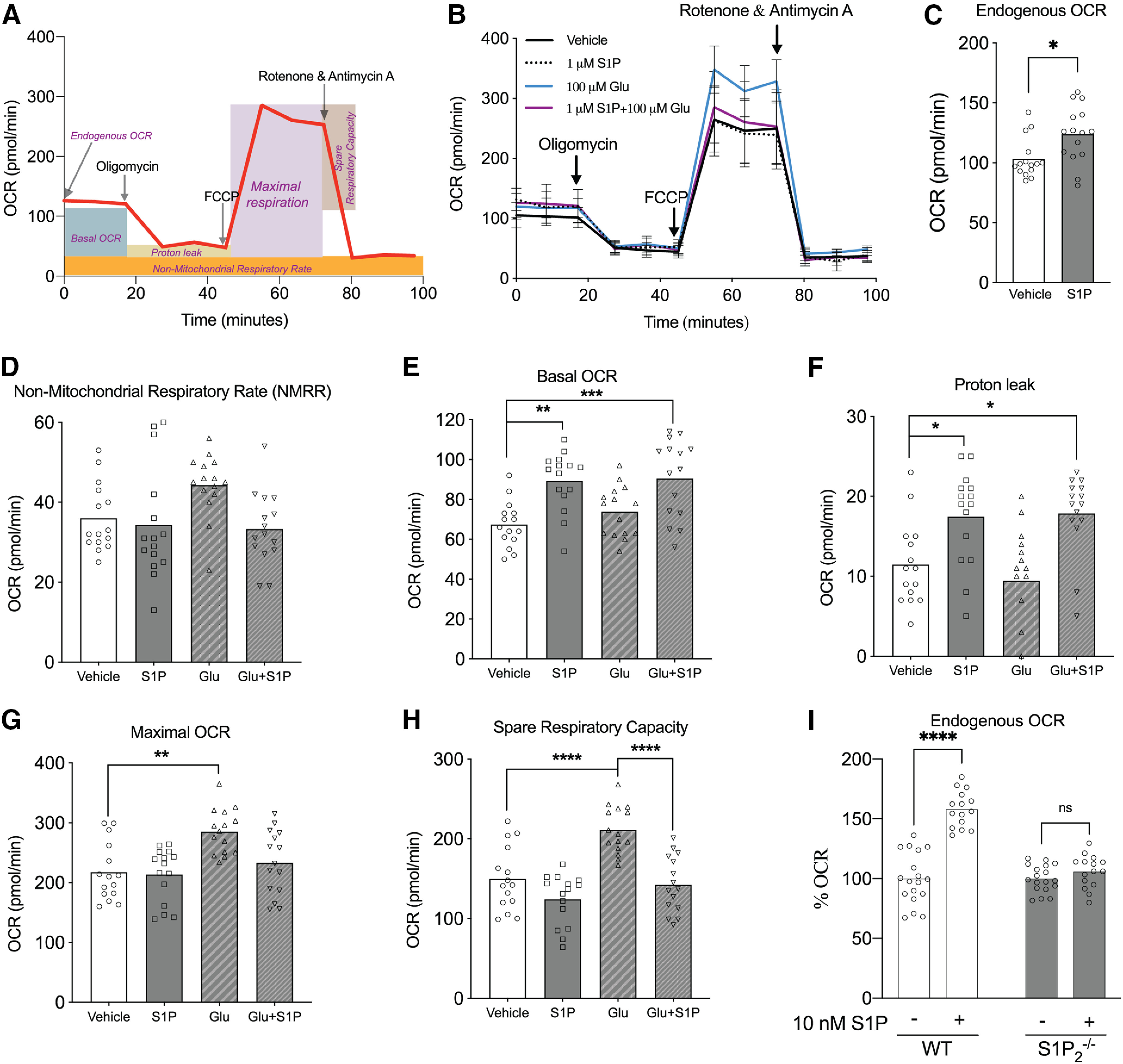
S1P increases astrocyte mitochondrial oxygen consumption rate (OCR) that is S1P2 dependent. A, Schematic of Seahorse Mito Stress analysis. B, Primary astrocytes were exposed to four conditions: vehicle, 100 μM glutamate, 1 μM S1P, or a combination of glutamate and S1P for 1 h at 37°C. After washing, Seahorse assay medium containing 1 mM pyruvate and 10 mM glucose was added and the OCR measured with an Agilent Seahorse XF24 Cell Mito Stress kit. OCR was measured three times per injection and was read in triplicate. C, S1P increased endogenous OCR when compared with vehicle *p < 0.05 unpaired t test. Non-mitochondrial respiratory rate (NMMR = OCR after rotenone/antimycin A addition; D), basal OCR (= endogenous OCR – NMRR; E), maximal OCR (= OCR after FCCP addition – NMRR; F), proton leak (= OCR after oligomycin injection – NMRR; G), and spare respiratory capacity (= maximal OCR – basal OCR; H) is represented under different conditions as indicated, n = 5 with OCR measured in triplicate at each step. Data are representative of two independent experiments with similar results. D–H, Ordinary one-way ANOVA with Tukey’s multiple comparisons test (*p < 0.05, **p < 0.005, ***p ≤ 0.0005, ****p < 0.0001). I, Wild-type and S1P2 null primary astrocytes were incubated with Seahorse assay medium containing 1 mM pyruvate and 10 mM glucose followed by treatment with 10 nM S1P for 1 h. OCR was then measured using the Agilent Seahorse XF24 analyzer. % OCR in the presence of S1P was calculated with respect to vehicle as indicated, ****p < 0.0001, two-way ANOVA with Tukey’s multiple comparisons test. ns, not significant.
Lastly, to test whether the endogenous astrocyte OCR is altered in an S1P2-dependent manner, astrocytes derived from wild-type and S1P2-null mutant mice were exposed to S1P for 1 h, and the OCR was measured. Exposure of wild-type astrocytes to S1P produced a statistically significant increase in OCR that was not observed in S1P2 null astrocytes (Fig. 5I), indicating that S1P-S1P2 signaling increases mitochondrial respiration.
Discussion
This is the first study to report that astrocyte S1P-S1P2 signaling affects glutamate uptake. S1P concentration gradients are perturbed in neurologic diseases (Cartier and Hla, 2019) and could contribute to glutamate neurotoxicity by disrupting glutamate homeostasis to produce increased extracellular glutamate (Mahmoud et al., 2019). Glutamate levels are controlled in part by astrocytes that express glutamate transporters capable of removing excess glutamate following neurotransmission (Anderson and Swanson, 2000; Mahmoud et al., 2019). S1P was found to inhibit glutamate uptake in a dose-dependent manner that was primarily because of the activation of S1P2 through a Gα12 pathway. Glutamate also affects astrocyte mitochondrial metabolism via entry into the TCA cycle (Jackson and Robinson, 2018), which was reported to be important for the maintenance of surrounding neurons in a stroke model (Fiebig et al., 2019). Seahorse analyses indicated that inhibition of glutamate uptake through S1P2 may affect mitochondrial health, implicating a change in the metabolic state of the astrocytes that can be abrogated by the loss of S1P2.
The results from the present study were obtained using primary cultures of astrocytes from newborn S1P2-null mice on a BALB/c background. Previous research reported seizure activity in S1P2-null mice which was attributed to background strain differences and possible glutamate excitotoxicity during a developmentally discrete period of young adulthood (between P25 and P45; MacLennan et al., 2001; Ishii et al., 2002; Akahoshi et al., 2011). S1P2 is also required for auditory and vestibular function wherein its constitutive loss results in early postnatal cochlear and vestibular degeneration (MacLennan et al., 2006; Herr et al., 2007; Kono et al., 2007). This involvement of S1P-S1P2 is consistent with the rare occurrence of autosomal recessive hearing impairment from S1P2 missense variants (Santos-Cortez et al., 2016). This further underscores links between S1P-S1P2, where modification by developmental age and genetic background could result in additional pathology beyond glutamate neurotoxicity.
Hypothetically, S1P receptor modulators could also increase extracellular glutamate levels because they engage S1P receptors. However, none of the assayed S1P receptor modulators inhibited glutamate uptake, which is consistent with their avoidance of S1P2 engagement. RP001 (S1P1-selective), BAF312 (siponimod, Mayzent; S1P1/5 selective; Jackson et al., 2011; Gergely et al., 2012; Al-Salama, 2019; Fig. 1C), and phosphorylated FTY720 (fingolimod/Gilenya; S1P1,3,4,5 selective; Brinkmann et al., 2002; Mandala et al., 2002) did not substantively inhibit glutamate uptake at 10 nM, with FTY720-P showing only a 10% reduction in glutamate uptake at 1 μM, which may reflect partial engagement of S1P2 at this high concentration (Sobel et al., 2015). These results are consistent with the lack of high-affinity S1P2 engagement by these modulators as compared with S1P for both agonist and functional antagonist properties. These results support the development of S1P2 antagonists to restore astrocytic glutamate uptake, which adds to the list of widely diverse activities of S1P2 (Adada et al., 2013), including the known effects on CCL5 expression that reduce neuroinflammation (Yester et al., 2015). The prevention of glutamate uptake inhibition by potential S1P2 antagonists currently under development could be beneficial in preventing glutamate neurotoxicity for a range of neurologic diseases with associated high brain levels of S1P. In summary, S1P-S1P2-Gα12-Rho/ROCK signaling controls astrocytic glutamate uptake and mitochondrial oxygen consumption, representing another important role for S1P signaling in central nervous system health and disease.
Acknowledgments
Acknowledgements: We thank the SBPMDI Animal Resources Core for maintaining the animals, Dr. Jing Yong and Dr. David Scott at SBPMDI and all laboratory members for helpful discussions, and Dr. Gwendolyn Kaeser and Danielle Jones for editorial assistance.
Synthesis
Reviewing Editor: Karen Szumlinski, University of California at Santa Barbara
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Anthony Reeder, Tomomi Furihata.
Upon consultation with two expert reviewers, we have reached the decision of “Revise and Re-review” as some concerns were raised by both reviewers, but the amount of work required to revise the study should take less than 2 months to complete. Both reviewers considered the data to be potentially impactful. Reviewer 1 has provided a list of relatively minor revisions and would like to see the implications of the findings expanded. Reviewer 2 was more critical of the report, particularly with respect to its descriptive nature. That being said, Reviewer 2 acknowledges that some important new leads are generated by the report and they provide a number of suggestions for improvement. Please find the reviewers’ comments below. I look forward to receiving your revised report. Given the pandemic, should you require additional time to complete your revision, please simply notify the eNeuro team.
Reviewer 1
Demonstrates that S1P2 is important in astrocyte uptake of toxic glutamate.
Is it possible that S1P2 blockade would inhibit learning and memory, especially if dose were too high?
Mention potential benefit in neurodegenerative CNS diseases at end of Discussion.
Grammar changes will enhance impact and clarity.
Abstract:
By combining... This key sentence of abstract is awkward, passive tense, and does not agree in verb usage.
S1P1,2 & 3. Modulation
Taken together... Awkward: two clauses, and the receptor targets of FTY and SIP should be mentioned.
Page 2, top
S1P is a bioactive “cell membrane” lipid
is found in higher concentrations
SIP increases glutamate uptake inhibition
Key word S1P2 astrocyte are most important facets
Introduction:
Page 3, middle
including the S1P1 & 5-selective
Methods:
Page 5
Glutamate uptake assay. Serum-starved
Bottom
Is this antimycin A?
Briefly describe the role of each of these inhibitors.
Results:
Page 8
(Fig. 2). A moderate left shift in glutamate uptake, i.e., less uptake in k/o
3B). Since S1P2 couples with Gi, G12/13, or Gq proteins, pertussis toxin (PTX
Bottom
3B). S1P2 couples with Gi, G12/13, or Gq proteins. Therefore, pertussis toxin (PTX
did not block S1P-induced GUI (Fig. 3C).
page 9
(Fig. 4B) exhibited a right shift, i.e., more uptake in k/o
To understand how S1P-dependent
Delete: using a Seahorse XF24 Analyzer per the manufacturer’s instructions
Page 10
S1P2-dependent manner
Discussion
intermediate35 and astrocytic
rewrite: Previous research reported S1P2...
page 11
tenses do not agree: of S1P-S1P2 relevant to glutamate homeostasis, which could counter
Figures:
Fine The WT vs. experimental conditions are consistently labeled.
Reviewer 2
The authors have identified involvement of S1P2 receptor in astrocyte glutamate homeostasis through regulation of the glutamate uptake activity. The paper may contain new findings that contribute to the field of astrocyte physiology and neurochemistry. However, in my impression, the study is incomplete, and thus is difficult to consider for the publication as it is.
1. First, it is unclear why the authors focused on mitochondrial functions in this study. No clear explanation is provided. Then, while S1P2 could be involved in regulation of mitochondrial functions, the data directly linking S1P2-mediated GUI to the functional alteration of mitochondria are missing. Two evens guided by S1P2 signaling might be independent. It is also difficult to understand how the partial glutamate uptake inhibition leads to complete inhibition of glutamate-mediated OCR increase.
2. Why is the treatment period of the antagonist so long? The 3.5-day exposure seems unusual.
3. The uptake activity is better to be normalized by mg protein, because the cell number affects the cpm value unless the cell density is confluent.
4. The writing organization is premature, especially in the discussion section. In addition, several errors can be found: e.g., the last sentence of the introduction - “GUI” is inappropriate; in the method section - concentration of antinomycin A is missing.
5. In the title, what does “differentially reduced by S1P receptor modulators” mean? GUI is rescued only by JTE013 in Fig. 3, but it is not surprising given Fig 1 and Fig 2.
References
- Adada M, Canals D, Hannun YA, Obeid LM (2013) Sphingosine-1-phosphate receptor 2. FEBS J 280:6354–6366. 10.1111/febs.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahoshi N, Ishizaki Y, Yasuda H, Murashima YL, Shinba T, Goto K, Himi T, Chun J, Ishii I (2011) Frequent spontaneous seizures followed by spatial working memory/anxiety deficits in mice lacking sphingosine 1-phosphate receptor 2. Epilepsy Behav 22:659–665. 10.1016/j.yebeh.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Al-Salama ZT (2019) Siponimod: first global approval. Drugs 79:1009–1015. 10.1007/s40265-019-01140-x [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14. [DOI] [PubMed] [Google Scholar]
- André VM, Cepeda C, Levine MS (2010) Dopamine and glutamate in Huntington’s disease: a balancing act. CNS Neurosci Ther 16:163–178. 10.1111/j.1755-5949.2010.00134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranzini SE, Srinivasan R, Khankhanian P, Okuda DT, Nelson SJ, Matthews PM, Hauser SL, Oksenberg JR, Pelletier D (2010) Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain 133:2603–2611. 10.1093/brain/awq192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Haliski M, White HS (2015) Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med 5:a022863. 10.1101/cshperspect.a022863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho VA, Hla T (2014) An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res 55:1596–1608. 10.1194/jlr.R046300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277:21453–21457. 10.1074/jbc.C200176200 [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18:327–338. 10.1016/s0896-6273(00)80272-x [DOI] [PubMed] [Google Scholar]
- Cartier A, Hla T (2019) Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366:eaar5551. 10.1126/science.aar5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Giovannoni G, Hunter SF (2021) Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: differential downstream receptor signalling and clinical profile effects. Drugs 81:207–231. 10.1007/s40265-020-01431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig C, Keiner S, Ebert B, Schäffner I, Jagasia R, Lie DC, Beckervordersandforth R (2019) Mitochondrial dysfunction in astrocytes impairs the generation of reactive astrocytes and enhances neuronal cell death in the cortex upon photothrombotic lesion. Front Mol Neurosci 12:40–40. 10.3389/fnmol.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, Pan S, Gray NS, Hinterding K, Cooke NG, Groenewegen A, Vitaliti A, Sing T, Luttringer O, Yang J, Gardin A, Wang N, Crumb WJ Jr, Saltzman M, Rosenberg M, et al. (2012) The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol 167:1035–1047. 10.1111/j.1476-5381.2012.02061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A, Kihara Y, Jonnalagadda D, Rivera R, Kennedy G, Mayford M, Chun J (2018) A functionally defined in vivo astrocyte population identified by c-Fos activation in a mouse model of multiple sclerosis modulated by S1P signaling: immediate-early astrocytes (ieAstrocytes). eNeuro 5:ENEURO.0239-18.2018. 10.1523/ENEURO.0239-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D (2010) Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11:227–238. 10.1038/nrn2803 [DOI] [PubMed] [Google Scholar]
- Henn FA, Goldstein MN, Hamberger A (1974) Uptake of the neurotransmitter candidate glutamate by glia. Nature 249:663. 10.1038/249663a0 [DOI] [PubMed] [Google Scholar]
- Herr DR, Grillet N, Schwander M, Rivera R, Müller U, Chun J (2007) Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci 27:1474. 10.1523/JNEUROSCI.4245-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Hertz E (2003) Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochem Int 43:355–361. 10.1016/s0197-0186(03)00022-6 [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW (2009) Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187:761. 10.1083/jcb.200908164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J (2002) Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem 277:25152–25159. 10.1074/jbc.M200137200 [DOI] [PubMed] [Google Scholar]
- Jackson JG, Robinson MB (2018) Regulation of mitochondrial dynamics in astrocytes: mechanisms, consequences, and unknowns. Glia 66:1213–1234. 10.1002/glia.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SJ, Giovannoni G, Baker D (2011) Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation 8:76. 10.1186/1742-2094-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL (2007) Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem 282:10690–10696. 10.1074/jbc.M700370200 [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Maher P (2015) Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front Neurosci 9:469. 10.3389/fnins.2015.00469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E (1997) Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol 56:901–911. 10.1097/00005072-199708000-00008 [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622. 10.1056/NEJM199403033300907 [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, Anderson KJ, Roper SN, Lee N (2001) An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci 14:203–209. 10.1046/j.0953-816x.2001.01634.x [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R, Karpman AM, Cronier SA, Lee N, Erway LC, Miller ML (2006) The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res 220:38–48. 10.1016/j.heares.2006.06.016 [DOI] [PubMed] [Google Scholar]
- MacMillan EL, Tam R, Zhao Y, Vavasour IM, Li DKB, Oger J, Freedman MS, Kolind SH, Traboulsee AL (2016) Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult Scler 22:112–116. 10.1177/1352458515586086 [DOI] [PubMed] [Google Scholar]
- Macrez R, Stys PK, Vivien D, Lipton SA, Docagne F (2016) Mechanisms of glutamate toxicity in multiple sclerosis: biomarker and therapeutic opportunities. Lancet Neurol 15:1089–1102. 10.1016/S1474-4422(16)30165-X [DOI] [PubMed] [Google Scholar]
- Mahmoud S, Gharagozloo M, Simard C, Gris D (2019) Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8:184. 10.3390/cells8020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346–349. 10.1126/science.1070238 [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Alford M, Deteresa R, Mallory M (1996) Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol 40:759–766. 10.1002/ana.410400512 [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD (1999) Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology 52:453. 10.1212/wnl.52.3.453 [DOI] [PubMed] [Google Scholar]
- McKenna MC (2013) Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 4:191–191. 10.3389/fendo.2013.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393. 10.1046/j.1471-4159.1996.66010386.x [DOI] [PubMed] [Google Scholar]
- Muhlert N, Atzori M, De Vita E, Thomas DL, Samson RS, Wheeler-Kingshott CAM, Geurts JJG, Miller DH, Thompson AJ, Ciccarelli O (2014) Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J Neurol Neurosurg Psychiatry 85:833. 10.1136/jnnp-2013-306662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI (1992) Cloning and expression of a rat brain L-glutamate transporter. Nature 360:464. 10.1038/360464a0 [DOI] [PubMed] [Google Scholar]
- Ponath G, Park C, Pitt D (2018) The role of astrocytes in multiple sclerosis. Front Immunol 9:217–217. 10.3389/fimmu.2018.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Cortez RL, Faridi R, Rehman AU, Lee K, Ansar M, Wang X, Morell RJ, Isaacson R, Belyantseva IA, Dai H, Acharya A, Qaiser TA, Muhammad D, Ali RA, Shams S, Hassan MJ, Shahzad S, Raza SI, Bashir ZE, Smith JD, et al. (2016) Autosomal-recessive hearing impairment due to rare missense variants within S1PR2. Am J Hum Genet 98:331–338. 10.1016/j.ajhg.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR (2011) Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol Aging 32:553.e1–11. 10.1016/j.neurobiolaging.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Siracusa R, Fusco R, Cuzzocrea S (2019) Astrocytes: role and functions in brain pathologies. Front Pharmacol 10:1114. 10.3389/fphar.2019.01114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel K, Monnier L, Menyhart K, Bolinger M, Studer R, Nayler O, Gatfield J (2015) FTY720 phosphate activates sphingosine-1-phosphate receptor 2 and selectively couples to Gα12/13/Rho/ROCK to induce myofibroblast contraction. Mol Pharmacol 87:916–927. 10.1124/mol.114.097261 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. 10.1101/cshperspect.a020420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Petersen SB, Unsgård G, Schousboe A (1993) Metabolism of [U-13C]glutamate in astrocytes studied by 13C NMR spectroscopy: incorporation of more label into lactate than into glutamine demonstrates the importance of the tricarboxylic acid cycle. J Neurochem 61:1179–1182. 10.1111/j.1471-4159.1993.tb03641.x [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D (2005) Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 128:1016–1025. 10.1093/brain/awh467 [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W (1992) Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA 89:10955. 10.1073/pnas.89.22.10955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-z, Leszczyniecka M, Kang D-c, Sarkar D, Chao W, Volsky DJ, Fisher PB (2003) Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2). Proc Natl Acad Sci USA 100:1955. 10.1073/pnas.0136555100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg RJ, Ryan RM (2013) Mechanisms of glutamate transport. Physiol Rev 93:1621–1657. 10.1152/physrev.00007.2013 [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Komine O (2018) The multi-dimensional roles of astrocytes in ALS. Neurosci Res 126:31–38. 10.1016/j.neures.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Yester JW, Bryan L, Waters MR, Mierzenski B, Biswas DD, Gupta AS, Bhardwaj R, Surace MJ, Eltit JM, Milstien S, Spiegel S, Kordula T (2015) Sphingosine-1-phosphate inhibits IL-1-induced expression of C-C motif ligand 5 via c-Fos-dependent suppression of IFN-β amplification loop. FASEB J 29:4853–4865. 10.1096/fj.15-275180 [DOI] [PMC free article] [PubMed] [Google Scholar]