Abstract
Background:
Cytomegalovirus (CMV) serostatus of recipient (R) and donor (D) influences hematopoietic stem cell transplant (HSCT) outcome. However, it is not a reliable indicator of CMV infection in primary immunodeficiency disorder (PIDD) recipients who are unable to make adequate antigen-specific immunoglobulin (Ig) or who receive intravenous Ig (IVIg) prior to testing.
Objective:
Since no data exist on PIDD with unknown CMV serostatus, we aimed to evaluate the relationship between pre-HSCT recipient and donor serostatus and incidence of CMV infection in recipients with unknown serostatus.
Methods:
A retrospective analysis of all pediatric PIDD HSCTs (2007–2018) was performed at University of California San Francisco. Recipients were separated into categories based on pre-transplant serostatus: 1) seropositive (R(+)), 2) seronegative (R(−)), and 3) unknown serostatus (R(x)). Patients with pre-HSCT active CMV viremia were excluded.
Results:
A total of 90 patients were included, 69% male. The overall incidence of CMV infection was 20%, but varied in R(+), R(−), and R(x) at 80%, 0%, and 14%, (P-value = .0001). Similarly, 5-year survival differed among groups, 60% R(+), 100% R(−), and 90% of R(x) (P-value = .0045). There was no difference in CMV reactivation by donor serostatus (P-value = .29), however, faster time to clearance of CMV was observed for R(x)/D(+) group (median 9.5 days (IQR 2.5–18), P-value = .024).
Conclusion:
We identify a novel group of recipients, R(x), with an intermediate level of survival and CMV incidence post-HSCT, when compared to seropositive and seronegative recipients. No evidence of CMV transmission from D(+) in R(−) and R(x) was observed. We believe R(x) should be considered as a separate category in future studies to better delineate recipient risk status.
Keywords: Cytomegalovirus, hematopoietic stem cell transplant, infection, primary immunodeficiency, serostatus
1 |. INTRODUCTION
Primary Immunodeficiency Disorders (PIDD) represent a heterogeneous group of inborn errors of immunity, with susceptibility to opportunistic infections and/or autoimmunity. While outcomes for hematopoietic stem cell transplant (HSCT) in PIDD continue to improve, Cytomegalovirus (CMV) remains an important complication.1–3 The incidence of CMV infection increases with the severity and the duration of the immunosuppression and without prophylaxis reaches 70%−80% in allogeneic bone marrow transplant seropositive recipients.4–7 CMV disease can manifest clinically with different organ involvement, of which pneumonitis is the predominant manifestation, and can lead to significant morbidity and mortality.8 CMV infections can occur as a result of reactivation of latent virus or, less commonly, new infection. The cytomegalovirus status of recipient (R) and donor (D) influences the outcome of HSCT.9 Recipients who are CMV naïve are potentially at risk of contracting CMV from a CMV seropositive donor, through reactivation of latent virus transferred at the time of HSCT.
The current guidelines for CMV management in patients with hematological malignancies after HSCT from the 2017 European Conference on Infections in Leukemia (ECIL 7) as well as the guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients of the American Society for Transplantation and Cellular Therapy recommend a CMV-seronegative donor for a CMV-seronegative recipient and a CMV-seropositive donor for a CMV-seropositive recipient, especially in allogeneic HSCT with myeloablative conditioning.9,10 Furthermore, ECIL7 recommends CMV IgG pre-transplant screening for all patients near the time of HSCT. Other guidelines, including the British Committee for Standards in Haematology, emphasize the importance of testing all potential HSCT recipients for the presence of CMV IgG status prior to starting therapies, to avoid potential for inaccurate testing caused by passive transmission with transfusion of CMV IgG-positive blood products.4 However, CMV serostatus is not always reliable in PIDD HSCT recipients, especially in patients whose antigen-specific immunoglobulin responses are impaired (eg, severe combined immunodeficiency (SCID), or X-linked hyper-IgM syndrome), and is uninterpretable in those who receive intravenous or subcutaneous infusions of IgG prior to testing of serostatus. In these patients, in the absence of a known prior CMV infection, the categorization as seropositive or seronegative is unachievable and the selection of the ideal donor becomes challenging. CMV is a recognized risk factor in allogeneic transplant and identifying the appropriate complement of recipient-donor CMV status is essential for an optimal outcome. Here, we aim to evaluate the relationship between R/D serostatus pre-HSCT and incidence of CMV infection post-HSCT, focusing specifically on recipients with uncertain prior CMV exposure, R(x).
2 |. MATERIALS AND METHODS
This is a retrospective analysis of all allogeneic HSCT performed at UCSF Benioff Children’s Hospital, San Francisco, from 2007 to 2018 for the treatment of PIDD, including primary immune regulatory disorders (PIRD).11 PIDD classification is in accordance with the 2019 IUIS phenotypical classification of inborn errors of immunity.12 This study was approved by the UCSF Institutional Review Board in accordance with the Declaration of Helsinki.
Data recorded by individual chart review include patient characteristics, donor type (matched sibling donors, haploidentical donors, and unrelated donors), HSCT characteristics, conditioning, graft-versus-host disease (GVHD), use of IgG infusions, antiviral prophylaxis, history of CMV infection pre- and post-HSCT, and outcome. During the period of this study, CD34-selected peripheral blood stem cell (PBSC) was used when donors were haploidentical.13,14
Recipients were separated into categories based on their pre-transplant CMV serostatus: 1) seropositive (R(+)) or known resolved CMV infection, 2) seronegative (R(−)), and 3) unknown (R(x)). Nine patients with pre-HSCT active CMV infection were excluded from this analysis. Patients included in the category of unknown serostatus were those who had an immune defect resulting in impaired antibody production or those with positive CMV IgG within 3 months after receipt of Ig infusion, with negative quantitative real-time polymerase chain reaction (RT-PCR) for CMV. Patients classified as seronegative had negative CMV serologies and a PIDD not associated with a defect in antibody production (IgG, IgA, and IgM) or one that, despite the underlying defect, demonstrated adequate specific antibody response to tetanus, diphtheria, and pneumococcal vaccines. IgG, IgA, and IgM levels were assessed with respect to age-matched reference intervals. Protective titers to tetanus, diphtheria, or pneumococcal vaccines were determined by UCSF Clinical Immunology Laboratory with a standardized cut-off.15 Pre-transplant testing for recipient included CMV IgG and RT-PCR within 30 days prior to HSCT.
RT-PCR with a lower limit of detection of 137 IU/mL was sent weekly after admission for HSCT until day + 100 or until T-cell reconstitution, whichever came later. Indications for therapy included detection of PCR copy number above the UCSF Laboratory cut-off, early reactivations (<30 days post-HSCT) below the cut-off, and reactivations below the cut-off in high-risk patients (defined as patients who received a T-cell depleted transplant or an umbilical cord blood transplant, conditioning with alemtuzumab, previous CMV reactivations, GVHD, on steroids, and/or in the R(+)/D(−) group). Strategies for therapy were applied as per UCSF protocol and current guidelines. CMV infection was considered as CMV viremia without any systemic or organ involvement, while CMV disease comprised both symptoms of infection and documented viral culture, PCR, or direct antigenemia assay, testing from the infected site or blood.
Survival was evaluated at 100 days, 1 year, and 5 years or last follow-up post-transplant. When patients required > 1 HSCT, the initial one was considered for evaluation. All transplant risk factors for CMV infection were evaluated using a χ2 test or a Fisher exact test for categorical variables, and ANOVA test or a Wilcoxon Rank Sum test for continuous variables. The incidence of CMV infection or reactivation was assessed using a Kaplan-Meier graph and log-rank test. Overall survival was estimated from the date of the first infusion to the date of death owing to any cause or to the date of last follow up, with a Kaplan-Meier and log-rank test. Incidence of post-transplant complications, such as GVHD, was estimated by cumulative incidence function for competing risk events. Statistical significance was set at P < .05. Statistical analysis was performed with STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
3 |. RESULTS
3.1 |. Patient characteristics
Ninety PIDD patients without active CMV infection at time of HSCT were included in this study, of whom 62 (69%) male and 28 (31%) females. Donors included 42 (47%) matched unrelated donor and 48 (53%) related donors (34% matched related siblings and 66% haploidentical donor). Patients’ characteristics and general complications/outcomes are summarized in Tables 1 and 2. The specific diagnoses of all patients in the three recipient serostatus categories are reported in Table 3. Median follow-up was 6 years (IQR 4–9 years).
TABLE 1.
Overall cohort characteristics and subset at risk for CMV infection post-HSCT
| Categories | All patients | Subset at risk for CMV, R(+) and R(x), with CMV post-HSCT | Subset at risk for CMV, R(+) and R(x), without CMV post-HSCT | P-value |
|---|---|---|---|---|
| N | 90 | 18 | 62 | |
| Age at transplant, median months (IQR) | 10 (3,43) | 42 (8,97) | 6 (3,17) | |
| Biological sex | ||||
| Male | 62 (69%) | 12 (67%) | 43 (69%) | .83 |
| Female | 28 (31%) | 6 (33%) | 19 (31%) | |
| Diagnosis† | ||||
| TINF2 mutation | 1 (1%) | 0 (0%) | 1 (2%) | .11 |
| CID | 9 (10%) | 4 (22%) | 4 (6%) | |
| CID with syndromic features | 15 (17%) | 2 (11%) | 12 (19%) | |
| Congenital defects of phagocytes | 3 (3%) | 1 (6%) | 0 (0%) | |
| PIRD | 21 (23%) | 5 (28%) | 10 (17%) | |
| SCID | 41 (46%) | 6 (33%) | 35 (56%) | |
| Donor relation | ||||
| Matched unrelated | 42 (47%) | 7 (39%) | 29 (47%) | .55 |
| Matched related | 48 (53%) | 11 (61%) | 33 (53%) | |
| Source of cells | ||||
| BM | 44 (49%) | 8 (44%) | 28 (45%) | .99 |
| Cord Blood | 5 (6%) | 1 (6%) | 4 (7%) | |
| PBSC | 41 (45%) | 9 (50%) | 30 (48%) | |
| ABO Incompatibility | ||||
| No | 52 (58%) | 11 (61%) | 37 (60%) | .85 |
| Major | 16 (18%) | 3 (17%) | 12 (19%) | |
| Bidirectional | 2 (2%) | 0 (0%) | 2 (3%) | |
| Minor | 20 (22%) | 4 (22%) | 11 (18%) | |
| Alemtuzumab | 50 (56%) | 7 (39%) | 33 (53%) | .28 |
| Conditioning | ||||
| None | 15 (17%) | 3 (17%) | 12 (19%) | .94 |
| NMAC | 39 (43%) | 9 (50%) | 28 (45%) | |
| MAC | 36 (40%) | 6 (33%) | 22 (36%) | |
| GVHD prophylaxis | ||||
| None | 29 (32%) | 5 (28%) | 24 (39%) | .34 |
| CNI + MTX | 39 (43%) | 8 (44%) | 25 (40%) | |
| CNI + MMF | 7 (8%) | 3 (17%) | 3 (5%) | |
| CNI + Others | 15 (17%) | 2 (11%) | 10 (16%) | |
| T-cell depletion | 33 (37%) | 6 (33%) | 27 (44%) | .44 |
T-cell depletion 33 (37%) 6 (33%) 27 (4 4%) .44
Note: Patients characteristics and risk for CMV infection post-HSCT: overall column with characteristics of all 90 patients: other columns with univariate analysis performed only for subset of recipients at risk of CMV infection, R(+) and R(x).
Abbreviations: BM, bone marrow; CID, combined immunodeficiency; CMV, cy tomegalovirus; CsA, cyclosporin A; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; IQR, interquar tile range;MAC, myeloablative conditioning; MMF, mycophenolate mofetil; MT X, methotrexate, CNI, calcineurin inhibitors; NMAC, non-myeloablative conditioning; PBSC, peripheral blood stem cells; PIRD, primar y immune regulatory disorder; R(+), CMV seropositive; R(x) unknown CMV serostatus; SCID, severe combined immunodeficiency.
Based on IUIS categories of PIDD.
TABLE 2.
Complications post-HSCT and risk for CMV infection post-HSCT
| Variables | All patients | Subset at risk for CMV, R(+), and R(x), with CMV post-HSCT | Subset at risk for CMV, R(+), and R(x), without CMV post-HSCT | P-value |
|---|---|---|---|---|
| N | 90 | 18 | 62 | |
| Acute GVHD | ||||
| 100-day CI of grade II-IV | 20 (18%) | 3 (17%) | 12 (20%) | .42 |
| 100-day CI of grade III-IV | 7 (8%) | 1 (6%) | 6 (10%) | |
| aGVHD, median days after (IQR) | 22 (15–63) | 20 (16–34) | 25 (13.5–105) | .82 |
| Chronic GVHD | ||||
| 5-year CI of any cGVHD | 5 (22%) | 1 (6%) | 4 (22%) | .89 |
| 5-year CI of severe cGVHD | 3 (20%) | 0 (0%) | 3 (21%) | |
| cGVHD, median days after (IQR) | 75 (50–203) | 48 (48–48)† | 187 (169–1426.5) | .16 |
| Use of Systemic Steroids | 40 (44%) | 12 (67%) | 23 (37%) | .026 |
| Graft Failure | ||||
| None | 71 (79%) | 14 (78%) | 47 (76%) | .98 |
| Primary | 5 (6%) | 1 (6%) | 4 (6%) | |
| Secondary | 14 (16%) | 3 (17%) | 11 (18%) |
Note: Complications post-HSCT and outcome: overall column with characteristics of all 90 patients; univariate analysis performed only for subset of recipients at risk of CMV infection, R(+) and R(x).
Abbreviations: aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; CI, cumulative incidence; CMV, cy tomegalovirus; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; IQR, interquar tile range.
This patient developed an atypical cGVHD at 48 days after the transplant, affecting skin, eyes, mouth, and vaginal mucosa.
TABLE 3.
Distribution of PIDDs by serostatus
| Diagnosis | R(x): N = 70 | R(+): N = 10 | R(−): N = 10 |
|---|---|---|---|
| Defects with poor production of specific IgG | |||
| ZAP70 mutation | 2 | 1§ | |
| CD40 ligand deficiency | 4 | 1‡ | |
| IKBa deficiency | 1 | ||
| PIK3CD mutation | 1 | ||
| STAT3 GOF mutation | 1 | ||
| IPEX syndrome | 1 | 1‡ | |
| XLP | 3 | 1§ | |
| SCID | 41 | ||
| WAS | 11 | ||
| Cartilage Hair Hypoplasia | 2 | 1§ | |
| CID (not known gene defect) | 1‡ | ||
| Defects with intact production of specific IgG | |||
| Chediak-Higashi syndrome | 1 | ||
| Dyskeratosis congenita | 1† | ||
| C1q deficiency | 1† | ||
| HLH | 1† | 5 | 3 |
| CGD | 1 | 1 | |
| Congenital neutropenia | 1 | ||
| IL-10R deficiency | 1 | 1 |
Note: Distribution of PIDDs by serostatus: PIDDs has been divided into two none: defects in production of specific IgG and capable of production of specific IgG.
Abbreviations: CGD, chronic granulomatous diseasCID, combined immunodeficiency; HLH, hemophagocytic lymphohistiocytosis; IPEX, Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome; PIDDs, primar y immunodeficiency disorders; R(−), seronegative; R(+), seropositive; R(x), unknown CMV serostatus; SCID, Severe combined Immunodeficiency; WAS, Wiskott-Aldrich syndrome; XLP, X-linked lymphoproliferative syndrome.
CMV IgG + and no passive immunity.
CMV IgG + while on IVIg.
Despite the underlying defect, these patients demonstrated specific antibodies to tetanus, diphtheria, and pneumococcal vaccines.
3.2 |. CMV infection: incidence and risk factors
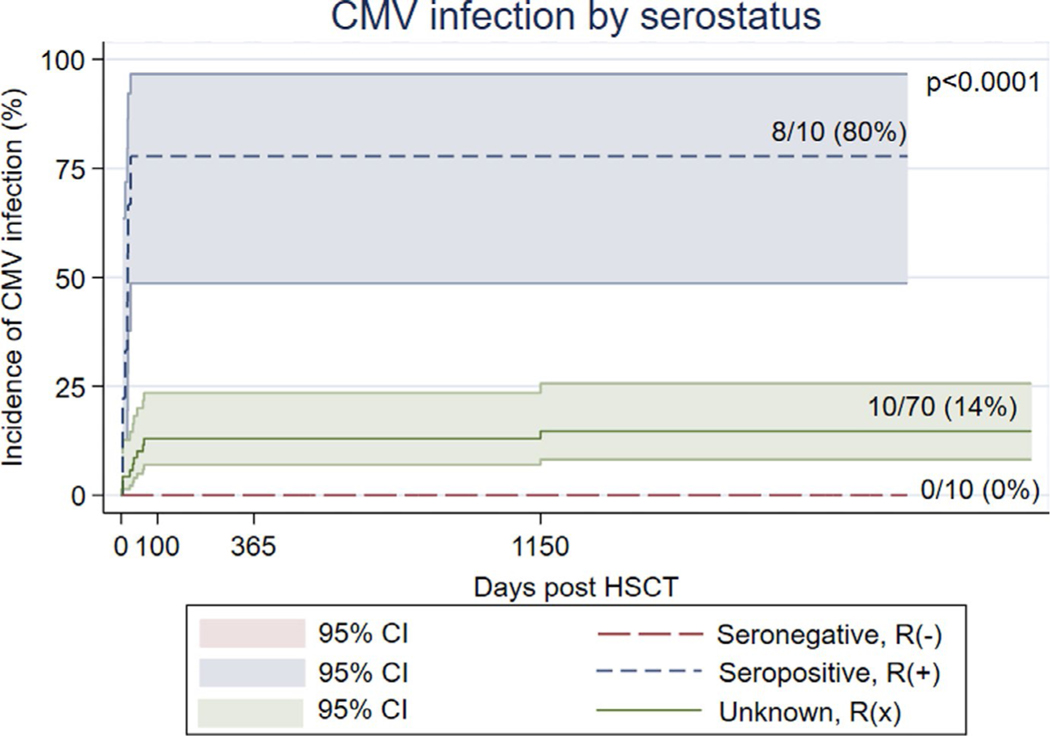
Our analysis showed a strong association between recipient category and CMV infection post-HSCT: the overall occurrence of CMV in this PIDD cohort was 20%, but varied dramatically in those who were R(+), R(−), and R(x) at 80% (95% confidence interval (CI), 0.44–0.97), 0% (95% CI, 0–0.30), and 14% (95% CI, 0.07–0.24), respectively (P-value < .001) (Table 4; Figure 1). Of the R(x) patients, 34 (48.6%) received a graft from D(+), while 36 (51.4%) from a D(−).
TABLE 4.
CMV Reactivation
| Donor (+) | Donor (−) | Total | |
|---|---|---|---|
| Recipient (+) | 78% (7/9) | 10 0% (1/1) | 80% (8/10) |
| Recipient (−) | 0% (0/5) | 0% (0/5) | 0% (0/10) |
| Recipient (x) | 15% (5/34) | 14% (5/36) | 14% (10/70) |
| Total | 25% (12/48) | 14% (6/42) | 20% (18/90) |
Note: CMV Reactivation: distribution into three categories R(+), R(−), and R(X) and correspondent matching with D(+) and D(−).
FIGURE 1.
Incidence of CMV infection by recipient serostatus: Kaplan-Meier graph showing the incidence of CMV infection (y) after HSCT (x) in seropositive category R(+), unknown serostatus R(x), and seronegative R(−)
In most cases, CMV presented as viremia alone (78%). The characteristics, treatments, and outcomes of patients with post-HSCT CMV infection are shown in Table 5. CMV post-HSCT was seen in 18/90 (20%) at a median time of 18.5 days post-HSCT (IQR 4–35). The median peak of CMV viral load was 1726.5 UI/mL (IQR 728–14,412). Virus clearance was observed in 16/18 (89%) patients, with a median time of clearance of 40 days (IQR 31–83). Two patients died with active CMV viremia, one for respiratory failure and one for infection of Human parainfluenza virus, although both with no present CMV disease and negative bronchoalveolar lavage (BAL) for CMV.
TABLE 5.
Characteristics, Treatments, and Outcomes of the 18 CMV infected patients
| CMV Recipient category | |
| Seropositive | 8 (44%) |
| Unknown | 10 (56%) |
| Infection, median days post-HSCT (IQR) | 18.5 (4, 35) |
| CMV maximum viral load, median (IQR) | 1726.5 (728–14412) |
| Clinical findings | |
| Viremia | 14 (78%) |
| Pneumonia | 3 (16) |
| Retinitis & Enteritis | 1 (6%) |
| Detection of CMV in BAL | 2 (11%) |
| Genotype UL97 | |
| Positive/tested | 0/7 (0%) |
| Genotype UL54 | |
| Positive/tested | 1/7 (25%) |
| Positive overall incidence | 1 (6%) |
| First-Line CMV therapy | |
| Foscarnet | 7 (40%) |
| Ganciclovir | 7 (40%) |
| Cidofovir | 1 (5%) |
| Ganciclovir + Foscarnet | 1 (5%) |
| Foscarnet + Leflunomide | 1 (5%) |
| None | 1 (5%) |
| Second-Line CMV therapy | |
| Valganciclovir | 2 (22%) |
| Foscarnet | 2 (22%) |
| Cidofovir | 4 (45) |
| Coartem + Leflunomide† | 1 (11%) |
| Intraocular Foscarnet Administered | 1 (6%) |
| CMV-Enriched IVIg Administered | 5 (28%) |
| Unmodified DLI Administered | 1 (6%) |
| Duration of total therapy, median days (IQR) | 40 (31.5–82.7) |
| Clearance | 16 (89%) |
| Time of Clearance, median days (IQR) | 31.5 (11–169) |
| > 1 reactivation post-HSCT | 4 (22%) |
Abbreviations: BAL, bronchoalveolar lavage; CMV, cytomegalovirus; DLI, Donor Lymphocy te Infusions; IVIg, Intravenous immune globulin.
This patient, who received an atypical therapy, is the one who had UL54 mutation.
Tables 1 and 2 show variables associated with CMV infection for recipients at risk, R(+) and R(x), in univariate analysis, including: age at transplant (42 months (IQR 8–97) for those who had CMV infection versus 6 months (IQR 3–17), p-value = 0.008) and use of systemic steroid post-HSCT (p-value = 0.026).
In univariate analysis, there were no correlations between CMV infection and donor relation (p-value = 0.55), T-cell depleted transplants (p-value = 0.44), or use of alemtuzumab in the conditioning regimen (p-value = 0.28). Four (22%) recipients experienced > 1 reactivation. Of those with aGVHD, 3 (15%) experienced > 1 CMV reactivation post-HSCTs, versus 1 (1.4%) of those without aGVHD (p-value = 0.008).
Regarding other concomitant infections (see Table S1 in the Online Repository), 6/18 (33%) patients with CMV infection post-HSCT had also HHV6 infection, (P-value = .006). There were no correlations between CMV infection and occurrence of other dsDNA virus (P-value > .05) or other virus infection (p-value > 0.05), while there was a positive correlation with bacterial infection (P-value = .043).
3.3 |. CMV infection and donor serostatus
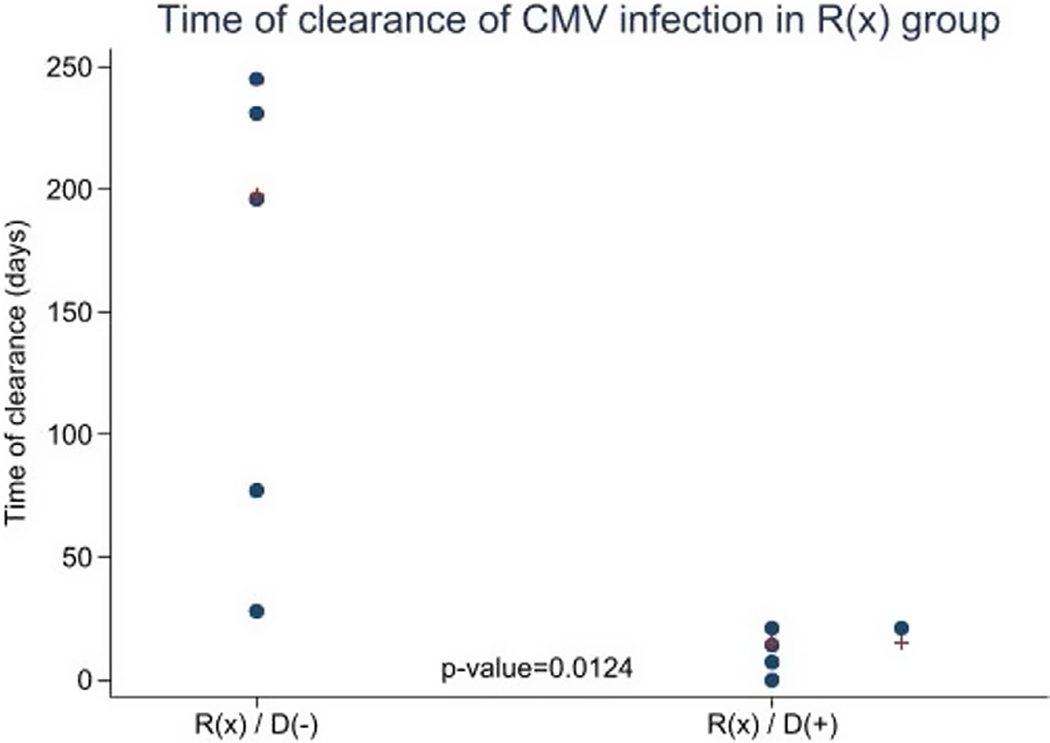
Overall, there was no difference in CMV infection by donor serostatus, 25% for D(+) versus 14% for D(−) (P-value = .29) (Table 4); this was confounded by preferential selection of seropositive donors for seropositive recipients. When considering R(x), there was no difference in subsequent reactivation by donor serostatus (P-value = .92). However, as shown in Figure 2 there was a significant association with time to clearance, with a median time to clearance for R(x)/D(+) group of 15 days (IQR 4–20) versus 198 days (IQR 74–231) for R(x)/D(−) group (p-value = 0.0124). Moreover, there was a significant association with the total duration of therapy in treated infected R(x), with a median duration of therapy for R(x)/D(−) group of 137 days (IQR 87–201) versus R(x)/D(+) group of 21 days (IQR 0–48), (p-value = 0.032). There were no statistically significant differences in peak viral load for infected R(x) patients among D(+) and (D-) (P = .23), with a median of 600 UI/mL (IQR 600–1000) for R(x)/D(+) versus 7500 (IQR 1000–55058) for R(x)/D(−) group. Likewise, for R(x) patients with subsequent CMV infection, there were no differences based on donor CMV serostatus either in onset of CMV infection post-HSCT (p-value = 0.26) or in severity of CMV presentation (P-value = .44).
FIGURE 2.
Time to clearance of CMV infection: time in days to clearance of CMV infection in the unknown serostatus category R(x), compared between the two donor groups, D(+) and D(−)
3.4 |. CMV infection and outcome
The estimated survival at 100 days, 1 year, and 5 years or last follow-up post-HSCT was 97% (95% CI, 0.91–0.99), 93% (95% CI, 0.86–0.98), and 88% (95% CI, 0.81–0.95), respectively. Our analysis showed a significant correlation between post-HSCT CMV infection and survival at 100 days (P-value = .04), with 89% (95% CI, 0.65–0.99) of patients alive; however, we found no significant findings at 1-year (P-value = .057) and at 5-years post-HSCT (P-value = .4), with 83% (95% CI, 0.59–0.96) of patients alive at both 1-year and 5-years post.
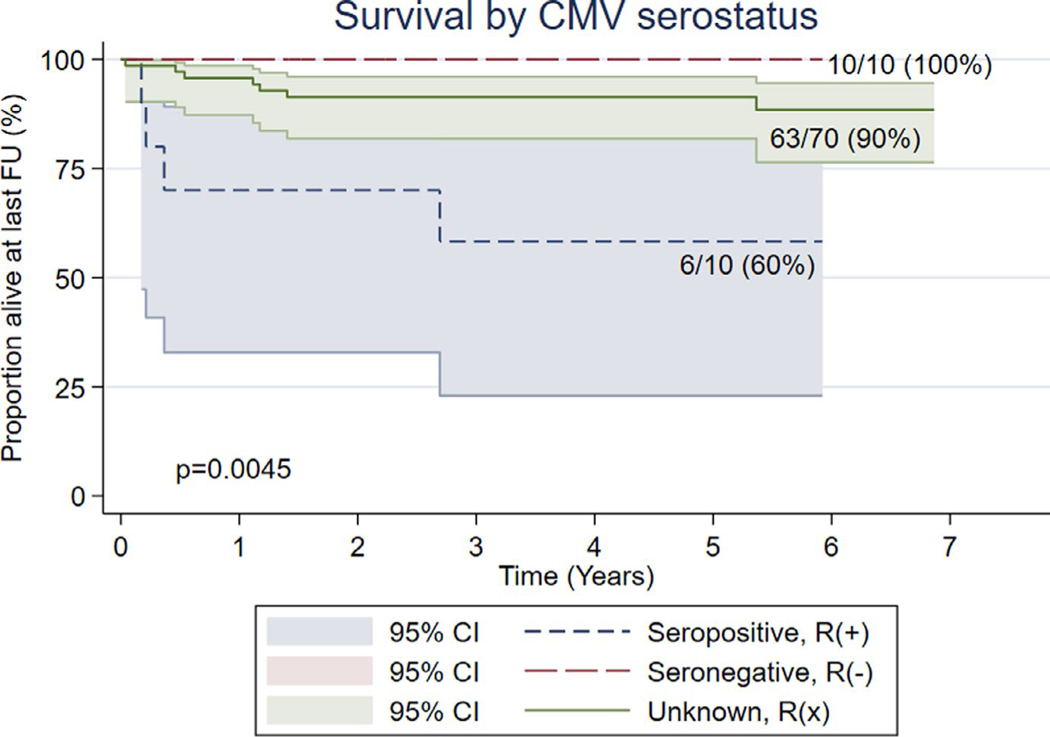
As shown in Figure 3, there was a significant difference in 5-year overall survival in those who were R(+) versus R(−) and R(x), at 60% (95% CI, 0.26–0.88), versus 100% (95% CI, 0.70–1.0) and 90% (95% CI, 0.80–0.96), respectively (P = .0045). The 5-year overall survival for the R(x) group by donor status was 92% (95% CI, 0.77–0.98) for D(−) versus 88% (95% CI, 0.73–0.97) for D(+) (P-value = .63).
FIGURE 3.
Survival by CMV serostatus: Kaplan-Meier graph showing survival (y) at 5 years post-HSCT or at last follow-up (x) in seropositive R(+), seronegative R(−), and unknown serostatus R(x)
4 |. DISCUSSION
Despite advances in prophylaxis and treatment, CMV infection remains a significant complication for patients undergoing allogeneic HSCT. The majority of data on CMV infection after allogeneic hematopoietic stem cell transplantation is derived from patients with hematological malignancies.16,17 CMV serology in many PIDD patients is unreliable as a result of impaired antigen-specific antibody production. Therefore, patients with negative PCR and past primary CMV infection may be incorrectly classified as ‘CMV seronegative’. Instead, we propose a new category of ‘CMV unknown’/ R(x), which demonstrates a post-HSCT CMV infection rate well below that of CMV seropositive recipients, but above that of ‘true’ CMV seronegative recipients. To our knowledge, this is the first study that investigates the CMV unknown serostatus recipient in PIDD patients.
Patients, during pre-transplant evaluation, are usually classified into three categories of risk based upon their CMV serostatus: low (R−/D−), intermediate (R−/D+ < R+/D+), and high risk (R+/D−).4,18 CMV infection is rare in the low-risk group, reported between 5% and 7%; in the intermediate group for R(−)/D(+) it is approximately 20% to 30%, while for R(+) is 70 to 80%, described by some authors as independent from donor serostatus, whereas by others slightly higher in R(+)/D(+) matching.4,6,19–21 In our PIDD cohort, the incidence of CMV varied between categories, with an infection rate of 80% in R(+), 0% in R(−), and 14% in the new category R(x).
Additionally, we found that there is no correlation between incidence of CMV infection and donor serostatus. Thus, it may be appropriate to consider the potential benefits of choosing a CMV-seropositive donor. The ability to control CMV infection in the setting of HSCT is mediated by CMV-specific memory T lymphocytes passively transferred with the graft.22–24 Rapid reconstitution of CMV-specific CD8+ T cells and CD4+ T cells in R(+)/D(+), compared to R(+)/D(−), has been highlighted in several studies, with R(+)/D(+) group producing a higher amount of multifunctional CD8+ T cells secreting significant quantities of IFNγ and TNFα.2,25–29 Our data confirm that of other studies, showing the negative effects of serological matching R(+)/D(−) transplants: immunological anti-CMV reconstitution is prolonged because of the lack of CMV-specific memory T cells and this leads to increased risk of CMV disease, occurrence of late reactivation of CMV, repeated reactivations with longer episodes, and decreased survival.25,30,31 Furthermore, we observed a longer duration of therapy in group R(x)/D(−), which is consistent with published data.2 This prolonged exposure to antiviral drugs could potentially lead to increased side effects, and the use of antiviral therapy for longer than 4 weeks has been reported as a risk factor for late CMV disease.32,33 Finally, in the event of CMV infection, patients could benefit from transfer of donor immune effectors and potentially receive donor-derived CMV-specific T cells.34
Our data suggest that pre-transplant seropositivity of recipients is one of the most important predictors of both poor survival and CMV reactivation, with 80% reactivation rate in our population. These data are consistent with previous studies, where CMV seropositivity is important not only for predicting CMV reactivation but also as a marker of poor outcome of HSCTs.2,7,17,35 This specific group of patients may benefit from novel prophylaxis strategies and measures. Our analysis also shows some indirect effects of CMV. We noted a significant correlation between CMV infection and incidence of HHV6 infection, as well as with bacterial infections, similar to what is reported in patients undergoing solid organ transplant.32,36 Concurrent infection with more than one dsDNA virus is not uncommon following cord blood transplant and T-cell depleted transplant, and appears to be associated with adverse outcomes.23,37 Additionally, our study supports previous observations demonstrating significant correlations between CMV infection and age at transplant and use of systemic steroids, in fact a significantly higher percentage of subjects who received systemic steroids developed CMV infection post-HSCT.5,6,30,32 Finally, aGVHD is associated with more than one CMV reactivation post-HSCT, as has been shown elsewhere.5,38,39
Key limitations of this analysis are a relatively small sample size and the retrospective nature of the study.
Here, we identify a novel risk group of patients with ‘unknown’ CMV serostatus, where the risk of CMV reactivation is estimated to be 14% (95% CI, 95% CI, 0.07–0.24). We identified no increase in post-HSCT CMV infections for any recipient CMV serostatus category when using a CMV-seropositive donor, suggesting that transfer of infection from CMV-seropositive donors is a rare event. Instead, there appears to be some benefits also of viral clearance when a R(+) donor was utilized. Therefore, in the era of readily available donor-derived anti-CMV CTLs, it may be prudent to purposefully select CMV-seropositive donors for recipients with unknown CMV serostatus. Future studies including PIDD patients should utilize the CMV serostatus-unknown status, R(x), to better delineate recipients risk status.
Supplementary Material
Acknowledgments
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Chemaly RF, Chou S, Einsele H, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2018;68 (8):1420 −1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou W, Longmate J, Lacey SF, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Heiden P, Marijt E, Falkenburg F, Jedema I. Control of cytomegalovirus viremia after allogeneic stem cell transplantation: a review on CMV-Specific T cell reconstitution. Biol Blood Marrow Transplant. 2018;24(9):1776–1782. [DOI] [PubMed] [Google Scholar]
- 4.Emery V, Zuckerman M, Jackson G, et al. Management of cytomegalovirus infection in haemopoietic stem cell transplantation. Br J Haematol. 2013;162(1):25–39. [DOI] [PubMed] [Google Scholar]
- 5.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV ) serostatus remains the most impor tant determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12(4):322–329. [DOI] [PubMed] [Google Scholar]
- 6.Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett. 2014;342(1):1–8. [DOI] [PubMed] [Google Scholar]
- 7.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25(1):151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo JF, Komanduri K V. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematology/Oncology and Stem Cell Therapy. 2017;10 (4):233–238. [DOI] [PubMed] [Google Scholar]
- 9.Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infectious Dis. 2019;19(8):e260–e272. [DOI] [PubMed] [Google Scholar]
- 10.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan AY, Leiding JW, Liu X, et al. Hematopoietic cell transplantation in patients with primar y immune regulator y disorders (PIRD): a primar y immune deficiency treatment consor tium (PIDTC) sur vey. Front Immunol. 2020;21(11):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousfiha A, Jeddane L, Picard C, et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J Clin Immunol. 2020;40 (1):66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvorak CC, Gilman AL, Horn B, et al. Haploidentical related-donor hematopoietic cell transplantation in children using megadoses of CliniMACs-selected CD34+ cells and a fixed CD3+ dose. Bone Marrow Transplant. 2013;48(4):508–513. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak CC, Long-Boyle J, Dara J, et al. Low exposure busulfan conditioning to achieve sufficient multilineage chimerism in patients with severe combined immunodeficiency. Biol Blood Marrow Transplant. 2019;25(7):1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh R A, Orange JS. Antibody deficiency testing for primar y immunodeficiency: a practical review for the clinician. Ann Allergy Asthma Immunol. 2019;123(5):444–453. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359–3364. [DOI] [PubMed] [Google Scholar]
- 17.Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganepola S, Gentilini C, Hilbers U, et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(5):293–299. [DOI] [PubMed] [Google Scholar]
- 19.Kotton C, Kumar D, Caliendo A, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2010;1(89):779–795. [DOI] [PubMed] [Google Scholar]
- 20.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemaly RF, El Haddad L, Winston DJ, et al. Cytomegalovirus (CMV ) Cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: The REACT Study. Clin Infect Dis. 2020; 10.1093/cid/ciz1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winston DJ, Huang E-S, Miller MJ, et al. Molecular epidemiology of cytomegalovirus infections associated with bone marrow transplantation. Ann Intern Med. 1985;102(1):16–20. [DOI] [PubMed] [Google Scholar]
- 23.Blyth E, Withers B, Clancy L, Gottlieb D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence. 2016;7(8):967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gratama JW, van Esser JWJ, Lamers CHJ, et al. Tetramer-based quantification of cy tomegalovirus (CMV )–specific CD8+ T lymphocytes in T-cell–depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–1364. [DOI] [PubMed] [Google Scholar]
- 25.Pietersma FL, van Dorp S, Minnema MC, et al. Influence of donor cy tomegalovirus (CMV ) status on severity of viral reactivation after allogeneic stem cell transplantation in CMV-seropositive recipients. Clin Infect Dis. 2011;52(7):e14 4-e148. [DOI] [PubMed] [Google Scholar]
- 26.Foster AE, Gottlieb DJ, Sartor M, Hertzberg MS, Bradstock KF. Cy tomegalovirus-specific CD4+ and CD8+ T-cells follow a similar reconstitution pattern after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(9):501–511. [DOI] [PubMed] [Google Scholar]
- 27.Ugar te-Torres A, Hoegh-Petersen M, Liu Y, et al. Donor serostatus has an impact on cytomegalovirus-specific immunity, cytomegaloviral disease incidence, and survival in seropositive hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011;17(4):574–585. [DOI] [PubMed] [Google Scholar]
- 28.Zdziarski P. CMV-specific immune response-new patients, new insight: central role of specific IgG during infancy and long-lasting immune deficiency after allogenic stem cell transplantation. Int J Mol Sci. 2019;20 (2):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheinberg P, Melenhorst JJ, Brenchley JM, et al. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 20 09;114(24):5071–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Özdemir E, Saliba RM, Champlin RE, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40 (2):125–136. [DOI] [PubMed] [Google Scholar]
- 31.Beck JC, Wagner JE, DeFor TE, et al. Impact of cy tomegalovirus (CMV ) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzannou I, Leen AM. Accelerating immune reconstitution after hematopoietic stem cell transplantation. Clin Transl Immunology. 2014;3(2):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–558. [DOI] [PubMed] [Google Scholar]
- 34.Cho S-Y, Lee D-G, Kim H-J. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci. 2019;20 (11):2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003–20 08. [DOI] [PubMed] [Google Scholar]
- 36.Schachtner T, Stein M, Reinke P. Sepsis after renal transplantation: clinical, immunological, and microbiological risk factors. Transpl Infect Dis. 2017;19(3):e12695. [DOI] [PubMed] [Google Scholar]
- 37.Schaber t VF III, Mozaffari E, Lee Y-C, Casciano R. Double-stranded DNA (dsDNA) viral infections among allogeneic hematopoietic cell transplant (HCT) recipients in the first year after transplant. Blood. 2015;126(23):3296. [Google Scholar]
- 38.Boeckh M, Fries B, Garrett NW. Recent advances in the prevention of CMV infection and disease after hematopoietic stem cell transplantation. Pediatr Transplant. 20 04;8(s5):19–27. [DOI] [PubMed] [Google Scholar]
- 39.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 20 06;91(1):78. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.