Abstract
Background
Emodin has been widely used in traditional Chinese medicine, but few studies have tried to understand the mechanism of its anti-hypercholesterolemic effect.
Material/Methods
To delineate the underlying pathways, high-cholesterol diet (HCD)-fed Sprague-Dawley rats were orally administrated emodin or the lipid-lowering medicine simvastatin. Emodin was administered at 10, 30, or 100 mg/kg, while simvastatin was administered at 10 mg/kg. Parameters measured included lipid profiles (serum total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, aorta endothelium-dependent vasorelaxation in response to acetylcholine, and nitric oxide (NO) production. RT-qPCR and western blotting were performed to evaluate aortic endothelial nitric oxide synthase (eNOS), phosphorylated eNOS (p-eNOS), and hepatic LDL receptor (LDLR). Indices of liver and serum oxidation were also measured.
Results
The atherogenic index was increased by the HCD but significantly reduced in all treatment groups. The HCD-fed experimental group treated with emodin at 10 mg/kg had significantly lower serum total-C and LDL-C and improved aorta vasorelaxation and enhanced NO production. Also, emodin significantly attenuated the lipid profiles and restored endothelial function, as reflected by upregulated expression of hepatic LDLR and p-eNOS, respectively. Furthermore, emodin at 10 mg/kg significantly enhanced superoxide dismutase activity, lowered the malondialdehyde level in both liver and serum, and enhanced catalase activity in serum.
Conclusions
The ability of emodin to inhibit hypercholesterolemia in HCD-fed rats was associated with lower serum total-C and LDL-C, restoration of aortic endothelial function, and improved antioxidant capacity. Low-dose emodin showed better protection of aortic endothelium and better antioxidant activity than did higher doses.
Keywords: Antioxidants, Emodin, Hypercholesterolemia
Background
Cardiovascular disease is the leading cause of mortality worldwide [1]. The frequent combination of an unhealthy high-fat and high-salt diet and a sedentary lifestyle increases the prevalence of hypercholesterolemia (high total blood cholesterol), thereby raising the morbidity of cardiovascular disease. Endothelial dysfunction [2] and hypercholesterolemia [3,4] are considered to be the dominant risk factors for cardiovascular diseases, and treatment of hyperlipidemia through the reduction of total cholesterol (total-C) and low-density lipoprotein cholesterol (LDL-C) have been shown to be effective at reducing cardiovascular disease in animal models and clinical studies [3]. Protecting endothelial integrity with lipid-lowering agents such as simvastatin has become standard therapy for the treatment and prevention of cardiovascular disease [5].
Emodin, 1,3,8-trihydroxy-6-methylanthraquinone (C15H10O5, mol wt. 270.23), is an active component of the root of Rheum palmatum Linn. (Polygonaceae) that has been reported to possess several biological activities including antiinflammation [6], antiviral [7], antibacterial [8], vasorelaxation [9], immunosuppression [10], hepatoprotection, [11] anticancer [12], antioxidation [13], and antiatherogenic [14]. However, no study of emodin has addressed its lipid lowering effect, and the mechanisms underlying its anti-hypercholesterolemic activity remain unclear. We hypothesized that the ability of emodin to inhibit hypercholesterolemia was likely associated with a reduction in serum cholesterol and the restoration of aortic endothelial function.
To test our hypothesis, we administered emodin (10, 30, 100 mg/kg) by oral gavage to Sprague-Dawley rats fed a diet high in cholesterol and compared these to rats fed a diet high in cholesterol and administered simvastatin (10 mg/kg, per os [p.o.]). The study also included a group of normally fed rats and a group of control rats fed a diet of high cholesterol that received no treatment. The 3 doses of emodin were chosen based on reports that 30 mg/kg p.o. showed optimal hepatoprotective ability against acetaminophen-induced toxicity in rats [15].
Material and Methods
Chemicals
Emodin, as a fine red powder (>98% purity by the high-performance liquid chromatography [HPLC] method), was purchased from Nanjing Qingze (Nanjing, China), and simvastatin tablets (20 mg, 10% purity by HPLC) were provided by Hangzhou MSD (Hangzhou, China). Acetylcholine, indomethacin, neostigmine, and phenylephrine were supplied by Sigma-Aldrich, and were of analytical grade.
Experimental Design and Tissue Collection
Forty-eight male Sprague-Dawley rats (170±10 g) were purchased from the Guangdong Provincial Medical Laboratory Animal Center (Guangzhou, China). Four animals were housed in each cage at a temperature of 25±2°C with a 12-h light/dark cycle. After a 7-day quarantine period, the rats were randomly assigned to 1 of 6 groups (n = 8 per group): standard diet and no treatment (control); high-cholesterol diet and no treatment (HCD group); high-cholesterol diet and simvastatin (10 mg/kg) treatment (SIM group); high-cholesterol diet and low-dose emodin (10 mg/kg) treatment (EL group); high-cholesterol diet and medium-dose emodin (30 mg/kg) treatment (EM group); and high-cholesterol diet and high-dose emodin (100 mg/kg) treatment (EH group). The control group was fed with a standard rat chow diet containing protein (~14%), fat (~10%), and carbohydrate (~76%), while the high-cholesterol groups were fed the standard diet supplemented with 1% cholic acid, 2% pure cholesterol, and 5.5% edible peanut oil, as described in our previous studies [16,17]. All rats could freely consume water and their corresponding diet. The emodin was dissolved in distilled water and mixed thoroughly to make a uniform suspension before administration. The rats were administered distilled water alone or their corresponding drug by oral gavage (20 mL/kg body weight) between 9: 30 and 10: 00 AM daily for 38 consecutive days. At the end of the study, the animals were fasted for 12 h and then euthanized by cervical dislocation. Blood was immediately collected in 10-mL tubes by cardiac puncture and then centrifuged at 800 g for 15 min at 4°C. The serum was stored at −80°C for subsequent analysis. The aortas were then isolated rapidly and immersed in ice-cold Tyrode’s buffer composed of NaCl, 118 mM; KCl, 4.7 mM; KH2PO4, 1.2 mM; NaHCO3, 25 mM; glucose, 11 mM; CaCl2, 2.5 mM; and MgSO4, 1.2 mM. After removing adhered fat and connective tissue, the isolated aortas were dissected into 3 sections. The thoracic aortic ring (~3 mm) was used for measuring isometric tension, a second section (~15 mm) was used to test for NO production in vitro and western blotting, and the remainder was used for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis. Two pieces of hepatic tissues were obtained: a fragment of ~100 mg was used for RT-qPCR analysis and a larger fragment of ~1 g was used for the antioxidant assay. The study was approved by the Ethics Committee of the Hong Kong Polytechnic University (approval No: 180905, approval date: December 10, 2018).
Measurement of Serum Lipid Profiles
Serum total-C and triglycerides were measured via the enzymatic-colorimetric method, and LDL-C and high-density lipoprotein cholesterol (HDL-C) were measured via the direct method [18,19]. The atherogenic index was calculated as [(total-C)–(HDL-C)]/(HDL-C). Total-C, triglycerides, LDL-C, and HDL-C were measured with a Beckman Coulter AU680 analyzer using a Beckman kit.
Evaluation of the Isometric Tension of the Thoracic Aorta
A segment of the thoracic aortic vessel (~3 mm) from each animal was kept at a passive tension of 1.2 g for 60 min in a tissue bath containing 5 mL Tyrode’s buffer at 37°C, injected with a mixture of 95% oxygen and 5% carbon dioxide. After the 60-min equilibration period, 2 doses of 60 mM KCl were added. To measure the artery vasodilation in response to acetylcholine, the Tyrode’s buffer was supplemented with 1 μM neostigmine (an anticholinesterase) and 1 μM indomethacin (a nonselective cyclo-oxygenase inhibitor), and then 1 μM phenylephrine was added to allow the aortic ring to pre-contract and establish a baseline contraction. After a steady-state contraction was established, increasing concentrations of acetylcholine ranging from 10 nM to 10 μM were added to the tissue bath. The contractile response (isometric tension, in g) of each aortic ring was measured and recorded.
Nitric Oxide Production
To assess the damage to the vascular endothelium induced by the HCD, nitric oxide (NO) production of the blood vessels was measured in vitro, as described previously [16,20]. The isolated aortic rings (~15 mm) were incubated in culture dishes containing 37°C Tyrode’s solution in the presence of 1 μM neostigmine and 1 μM acetylcholine in a thermostatic incubator. After 2 h of incubation, each aortic ring was weighed, and the medium was collected. The collected medium was vacuum freeze-dried and the pellets re-dissolved with 300 μL of distilled water. Nitrate/nitrite concentrations were analyzed with a nitric oxide kit (KeyGen, Nanjing, China), and absorbance was read by spectrophotometry at 540 nm. The NO levels were calculated according to the kit instructions.
RT-qPCR Analysis
The aortas and hepatic tissues were homogenized, and RNA was extracted with the TRIzol reagent kit, according to the manufacturer’s instructions (Life Technologies). Samples were used for cDNA synthesis with a cDNA synthesis kit (Fermentas) as follows: 65°C for 5 min, 42°C for 60 min, and 70°C for 5 min. The levels of endothelial NO synthase (eNOS), LDL receptor (LDLR), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were measured using a LightCycler 480 and SYBR Green 1 Master Mix (Roche). Amplification was performed in a reaction volume of 20 μL as follows: 5 min at 95°C; followed by 42 cycles at 95°C for 10 s, 60°C for 30 s, and 72°C for 20 s; with a final extension at 72°C for 10 min. Primer sequences are listed in Table 1. The housekeeping gene GAPDH was used as an internal control to calibrate expression levels across specimens. The expression levels of the mRNAs in the experimental animals were calculated as a percentage of the expression in the control group. The experiment was repeated 3 times independently.
Table 1.
Primer sequences.
| Gene | Orientation | Sequence (5′ to 3′) |
|---|---|---|
| eNOS | Forward | GGATTCTGGCAAGACCGATTAC |
| Reverse | GGTGAGGACTTGTCCAAACACT | |
| LDLR | Forward | TGGCTATGAGTGCCTATGTC |
| Reverse | GGTGAAGAGCAGAAACCCTA | |
| GAPDH | Forward | TGCACCACCAACTGCTTAG |
| Reverse | AGTGGATGCAGGGATGATGT |
eNOS – endothelial nitric oxide synthase; LDLR – low density lipoprotein receptor; GAPDH – glyceraldehyde-3-phosphate dehydrogenase.
Antioxidant Assay
To evaluate the activities of antioxidative enzymes in the liver, hepatic tissues (~1 g) from each rat were weighed and stored immediately at −80°C until assayed. The samples were homogenized in precooled saline (9 mL saline was added to 1 g tissue) using an ultra-Turax T-25 homogenizer. The homogenates were centrifuged at 10 000 g for 5 min (4°C), and antioxidant indices were measured on the supernatant. Malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were measured using Nanjing KeyGen kits. The activities of SOD and CAT were measured in U/mg protein, and the MDA level was expressed as nmol/mg protein. The protein concentration was measured with a bicinchoninic acid assay kit (Shenergy, China). Serum SOD and CAT activities and MDA concentrations were determined with the same assays.
Western Blotting
Aortic total eNOS (T-eNOS), phosphorylated eNOS (p-eNOS), and hepatic LDLR protein expression levels were quantified by western blotting using the housekeeping protein GAPDH as an internal standard. Protein extracts (20 μg) were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Life Sciences). Blots were incubated overnight at 4°C with the appropriate antibodies: anti-eNOS (diluted at 1: 500); anti-phospho-eNOSSer1177 (p-eNOS, diluted at 1: 500); anti-LDLR (diluted at 1: 200); and anti-GAPDH (diluted at 1: 1000). Subsequently, the membrane was probed with a secondary goat anti-rabbit IgG conjugated horseradish peroxidase (dilution at 1: 10,000). Anti-p-eNOS antibody was purchased from Upstate, and the other antibodies were purchased from Abcam. Immunoreactive bands were visualized using the Pierce ECL detection system and documented with the Bio-Rad Chemi DOC XRS. All images were analyzed by Bio-Rad Quantity One software for Windows. Specific protein expression levels were normalized to the respective GAPDH bands and expressed as a percentage of the control group. This experiment was repeated independently 3 times.
Statistical Analysis
The data are represented as mean±standard deviation (SD) and n represents the number of animals used. Maximal relaxation is denoted as Emax. pEC50 values are defined as the negative logarithm of the concentration of acetylcholine producing 50% of Emax. Differences between groups were assessed with one-way analysis of variance followed by the LSD post hoc test using SPSS version 23.0. Statistical significance was defined as P<0.05.
Results
Determination of Lipid Profiles
The serum lipid profiles including total-C, triglycerides, LDL-C, HDL-C, and the atherogenic index are shown in Table 2. Total-C was significantly decreased in all treated groups (SIM, EH, EM, and EL) compared with that of the untreated HCD rats (P<0.05), but only the lowest dose of emodin (10 mg/kg) significantly reduced LDL-C, with an effect close to that of simvastatin. Emodin at 10 and 30 mg/kg also significantly reduced serum triglycerides by 27.40% and 26.03%, respectively, compared to that of the untreated HCD control group.
Table 2.
Serum lipid profiles of rats in each group.
| Group | Control | HCD | SIM (10 mg/kg) | EH (100 mg/kg) | EM (30 mg/kg) | EL (10 mg/kg) |
|---|---|---|---|---|---|---|
| Total-Cholesterol | 2.36±0.13 | 5.73±0.89### | 3.77±0.98##,*** | 4.42±0.87###,* | 4.63±0.92##,* | 4.53±0.56###,* |
| Triglyceride | 0.70±0.04 | 0.73±0.09 | 0.68±0.08 | 0.71±0.19 | 0.54±0.10#,* | 0.53±0.09#,* |
| LDL-C | 0.35±0.13 | 1.00±0.09### | 0.77±0.25###,* | 0.95±0.14### | 0.83±0.18### | 0.59±0.22#,*** |
| HDL-C | 0.63±0.14 | 1.34±0.15### | 1.41±0.15### | 1.34±0.35### | 1.40±0.17### | 1.43±0.31### |
| Atherogenic index | 2.21±0.30 | 3.54±0.66## | 2.38±0.47** | 2.64±0.68* | 2.77±0.85* | 2.59±0.23* |
The values represent the average of 6 to 8 separate experiments standard deviation and expressed in mmol/L. HCD – high-cholesterol diet group; SIM – simvastatin group; EH – high-dose emodin group; EM – medium-dose emodin group; EL – low-dose emodin group; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol. Atherogenic index=[(total-C)–(HDL-C)]/(HDL-C).
P<0.05,
P<0.01,
P<0.001 vs control group.
P<0.05,
P<0.01,
P<0.001 vs HCD group.
An elevated atherogenic index is a sign of coronary heart disease. Although it was increased in all groups fed the HCD, all treatment groups (SIM, EH, EM, and EL) showed an atherogenic index that was significantly lower than that of the untreated HCD group (Table 2), and not significantly different from the control group on the standard diet.
Comparison of Acetylcholine-Induced Aortic Relaxation, In Vitro
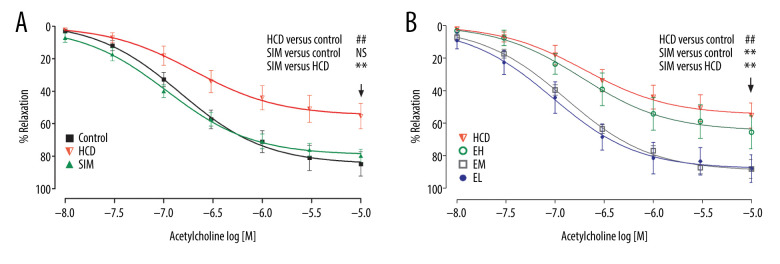
To test endothelial relaxation, we allowed phenylephrine (1 μM)-induced contraction to reach a steady-state in Tyrode’s solution, and then added acetylcholine with 1 μM neostigmine and 1 μM indomethacin. This resulted in an acetylcholine concentration-dependent (10 nM to 10 μM) vasorelaxation in all HCD groups, with ~50% to 90% Emax at 10 μM (Figure 1). As shown in Table 3, the Emax and pEC50 in the HCD group were 49.83±15.60% and 6.55±0.31, respectively (P<0.05 vs control). Compared to the HCD group, the Emax was significantly higher in the groups administered simvastatin or the lower doses of emodin (30 and 10 mg/kg) (P<0.01), and simvastatin and the lowest dose of emodin (10 mg/kg) produced the highest vasorelaxation with pEC50 of 7.03±0.21 and 7.03±0.33, respectively (P<0.01 vs HCD).
Figure 1.
The acetylcholine concentration-response curves of the thoracic aortic rings are presented as decreases in the percentage of the steady-state precontraction tension obtained with 1 μM phenylephrine in (A) control, HCD, and SIM groups and (B) HCD, EH, EM, and EL groups. Data shown are the mean±SD, n=6–8. HCD – high-cholesterol diet group; SIM – simvastatin (10 mg/kg) treatment group; EH – high-dose emodin (100 mg/kg) treatment group; EM – medium-dose emodin (30 mg/kg) treatment group; EL – low-dose emodin (10 mg/kg) treatment group. ## P<0.01 vs control group; ** P<0.01 vs HCD group; NS – not significant.
Table 3.
The maximum relaxation (%) and pEC50 values of each group. Data are expressed as mean±standard deviation. Emax represents the maximal relaxation (%) with 10 μM of acetylcholine; the pEC50 (the negative logarithm of the concentration of acetylcholine giving 50% relaxation) values were obtained directly from individual log concentration-response curves; n represents the number of animals.
| Group | Emax (%) | pEC50 (M) | n |
|---|---|---|---|
| Control | 88.91±21.14 | 6.89±0.13 | 6 |
| HCD | 49.83±15.60## | 6.55±0.31# | 6 |
| SIM (10 mg/kg) | 84.70±6.19** | 7.03±0.21** | 8 |
| EH (100 mg/kg) | 71.26±19.14 | 6.67±0.36 | 7 |
| EM (30 mg/kg) | 88.25±14.20** | 6.87±0.20 | 6 |
| EL (10 mg/kg) | 88.02±20.84** | 7.03±0.33** | 6 |
HCD – high-cholesterol diet group; SIM – simvastatin group; EH – high-dose emodin group; EM – medium-dose emodin group; EL – low-dose emodin group.
P<0.05,
P<0.01 vs control group.
P<0.01 vs untreated HCD group.
NO Production
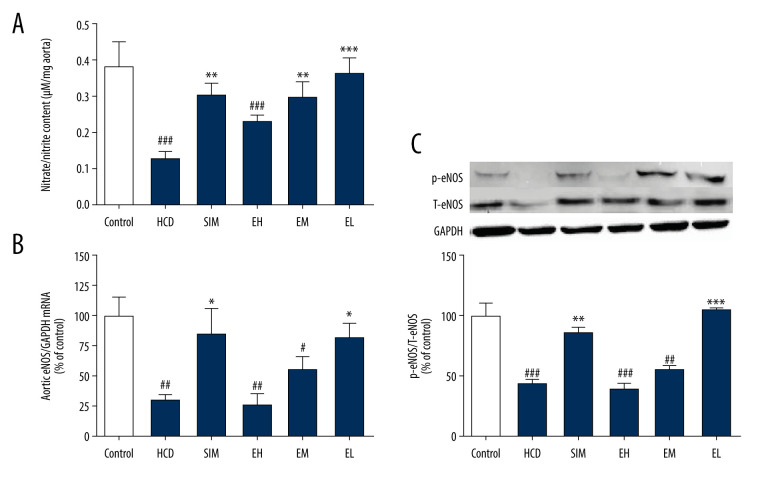
HCD-induced damage of aortic endothelial cells was measured as decreased NO production in the aortic rings after 2 h of pre-incubation. As shown in Figure 2A, NO production was attenuated in the HCD group compared to the non-HCD controls, but it was significantly restored in all treatment groups, except the EH group (100 mg/kg). As measured by RT-qPCR (Figure 2B), the expression level of eNOS mRNA in the HCD group was significantly lower than that of the standard diet group (P<0.01), but was restored in the SIM and EL (10 mg/kg) treatment groups to 85.06±20.68% and 82.40±11.90% respectively, of the levels in the standard diet controls. Notably, the eNOS mRNA levels of animals treated with the higher doses of emodin (30 and 100 mg/kg) were not significantly higher than those in the untreated HCD group.
Figure 2.
Emodin increased nitrate/nitrite production and upregulated aortic eNOS. (A) In vitro nitrate/nitrite production from isolated aortas challenged with acetylcholine (1 μM). (B) Emodin upregulated aortic eNOS mRNA expression and (C) p-eNOS protein expression. Aortic eNOS mRNA and p-eNOS protein levels were determined by RT-qPCR and western blotting, respectively. Data are expressed as means±SD, n=6–8. HCD – high-cholesterol diet group; SIM – simvastatin (10 mg/kg) treatment group; EH – high-dose emodin (100 mg/kg) treatment group; EM – medium-dose emodin (30 mg/kg) treatment group; EL – low-dose emodin (10 mg/kg) treatment group; T-eNOS – total endothelial nitric oxide synthase; p-eNOS – phosphorylated/activated form of eNOS. # P<0.05, ## P<0.01, ### P<0.001 vs standard diet control group; * P<0.05, ** P<0.01, *** P<0.001 vs untreated HCD group.
Measurement of Aortic T-eNOS and p-eNOS Expression
We next investigated the protein expression levels of T-eNOS and p-eNOSSer1177 (p-eNOS, the phosphorylated/activated form of eNOS) by western blotting (Figure 2C). Consistent with the RT-qPCR results, the levels of phosphorylated eNOS were lower in HCD groups compared to that in the standard diet control group, but were dramatically increased in the SIM and the EL (10 mg/kg) groups, but not with the higher doses in the EM and EH (30 and 100 mg/kg) groups.
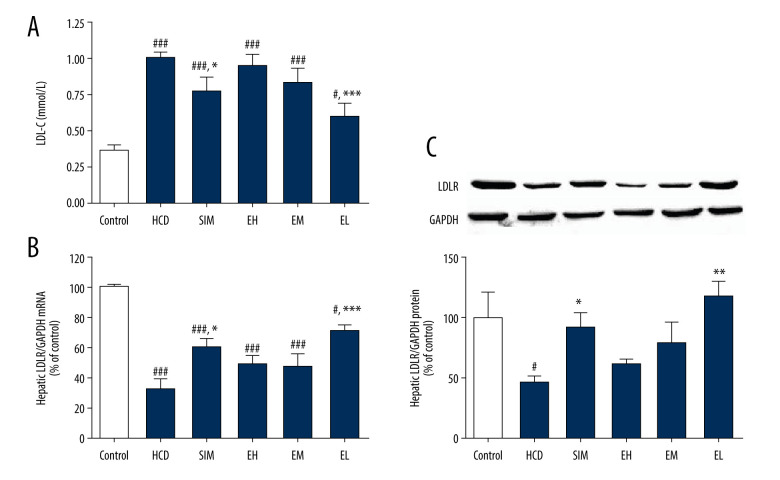
Measurement of LDLR Expression
To explore the molecular pathway involved in the cholesterol-lowering action of emodin, LDL consumption was assessed by measuring hepatic LDLR gene and protein expression. Changes in LDL-C concentration for each group are shown in Figure 3A. The mRNA levels of LDLR in the HCD control group were reduced compared with the standard diet controls, but increased in all treatment groups, although the difference was statistically significant only in the SIM and EL groups (Figure 3B). Similarly, protein levels of LDLR, as measured by western blotting, were also higher in all treatment groups than in HCD controls, but the increase was significant only in the SIM and EL groups (Figure 3C).
Figure 3.
(A) Emodin reduced LDL-C concentration in serum and upregulated (B) hepatic LDLR mRNA expression and (C) LDLR protein expression. Hepatic LDLR mRNA and protein levels were determined by RT-qPCR and western blotting, respectively. Data are expressed as mean±SD, n=6–8. HCD – high-cholesterol diet group; SIM – simvastatin (10 mg/kg) treatment group; EH – high-dose emodin (100 mg/kg) treatment group; EM – medium-dose emodin (30 mg/kg) treatment group; EL – low-dose emodin (10 mg/kg) treatment group. # P<0.05, ## P<0.01, ### P<0.001 vs standard diet control group; * P<0.05, ** P<0.01, *** P<0.001 vs untreated HCD group.
Measurement of SOD, CAT, and MDA in Liver and Serum
To identify the antioxidant mechanism of emodin, we determined the oxidation-related indices of SOD, CAT, and MDA in liver and serum. Compared to the standard diet group, the HCD groups had a dramatic reduction in liver SOD (−26.86%) and CAT (−20.39%) activities as well as a significant increase in MDA (43.46%). Only low-dose emodin (EL,10 mg/kg) preserved the activities of SOD (84.50% of levels in standard diet control group) (Table 4). In addition, compared to the HCD control group, the increase in liver MDA was significantly attenuated in all groups given simvastatin or emodin (100, 30, 10 mg/kg), with levels that were just slightly above (SIM, 14.75%; EH, 3.75%) or even below (EM, −16.36%; EL,−13.55%) the MDA levels in the standard diet control group. The liver CAT levels were decreased in the HCD group and not significantly restored in any of the treatment groups, but were significantly lower in the EH and EM groups. In the serum, compared with controls on a standard diet, the HCD also reduced SOD (−17.12%) and CAT (−43.97%) activities and showed a significant increase in MDA (92.21%) production. Only the lower doses of emodin (30 and 10 mg/kg) preserved the serum levels of SOD (EM, 99.46% and EL, 102.93% of standard diet controls), while these 2 groups also showed more CAT activity (EM, 83.30% and EL, 95.18% of standard diet controls). The increase in serum MDA production was also significantly attenuated with simvastatin and all doses of emodin (SIM, −33.12%; EH, 28.72%; EM,−3.54%; EL, −39.65% of standard diet controls).
Table 4.
Effect of emodin on parameters of oxidative stress.
| Group | Control | HCD | SIM (10 mg/kg) | EH (100 mg/kg) | EM (30 mg/kg) | EL (10 mg/kg) |
|---|---|---|---|---|---|---|
| SOD-liver (U/mg protein) | 38.98±2.45 | 28.51±2.04### | 30.49±2.40### | 22.12±3.70###,** | 22.84±5.48###,** | 32.94±6.36##,* |
| CAT-liver (U/mg protein) | 22.27±2.24 | 17.73±1.94## | 18.08±0.86# | 12.608±3.55###,** | 14.76±3.63###,* | 16.16±5.38## |
| MDA-liver (nmol/mg protein) | 2.14±0.35 | 3.07±0.23### | 2.37±0.25** | 2.22±0.73*** | 1.79±0.24*** | 1.85±0.34*** |
| SOD-serum (U/mL) | 163.37±4.43 | 135.41±4.92### | 135.55±7.52### | 113.26±12.58###,*** | 162.48±5.47*** | 168.16±4.39*** |
| CAT-serum (U/mL) | 5.39±1.42 | 3.02±0.81## | 3.82±1.93# | 3.37±0.92## | 4.49±1.21* | 5.13±1.63** |
| MDA-serum (nmol/mL) | 12.71±1.76 | 24.43±4.04### | 8.50±1.67##,*** | 16.36±2.94##,*** | 12.26±2.47*** | 7.67±0.98###,*** |
The values represent the average of 6 to 8 separate experiments±standard deviation. HCD – high-cholesterol diet group; SIM – simvastatin group; EH – high-dose emodin group; EM – medium-dose emodin group; EL – low-dose emodin group; SOD-liver – superoxide dismutase activity in liver tissue; CAT-liver – catalase activity in liver tissue; MDA-liver – concentration of malondialdehyde in liver tissue; SOD-serum – superoxide dismutase activity in serum; CAT-serum – catalase activity in serum; MDA-serum – concentration of malondialdehyde in serum.
P<0.05,
P<0.01,
P<0.001 vs control group.
P<0.05,
P<0.01,
P<0.001 vs untreated HCD group.
Discussion
In this study, we demonstrated that the oral administration of emodin effectively improved the serum lipid profiles in animals given a diet consisting of high cholesterol, including reductions in the serum total-C, LDL-C, and the atherogenic index. The HCD, enriched in cholic acid, cholesterol, and oil, was used because supplementation of these components in a standard diet has been successfully used to induce aortic atherosclerotic lesions in rats and mice [21,22]. The addition of cholic acid to a cholesterol-containing diet has been shown to increase plasma total-C levels [23]. In our study, the rats fed with HCD had abnormal lipid profiles with significantly increased total-C, LDL-C, and HDL-C levels, and emodin produced no significant change in HDL-C when compared with the HCD group. Therefore, the observed that the beneficial activity of emodin on the atherogenic index is mainly due to decreased total-C, which together with LDL-C, plays an important role in the development of coronary heart disease [24]. The improved lipid profiles suggest that emodin has anti-hypercholesterolemic activity similar to that of simvastatin, which served as the positive control in this study and is often used in the efficacious treatment of hyperlipidemia [25,26].
According to Jin et al, during hypercholesterolemia and early stages of atherosclerosis, the most common vascular dysfunction is a reduction of endothelium-dependent vasorelaxation [27]. We found that aortic ring relaxation in response to acetylcholine, an endothelium-dependent vasodilator, was markedly blunted in untreated animals given the HCD. We found that the lowest dose of emodin (EL, 10 mg/kg) showed an increase in vasorelaxation similar to that seen with simvastatin, including significantly elevated values for Emax and pEC50. EM (30 mg/kg) significantly increased Emax but had no effect on pEC50. EH (100 mg/kg) had no effect on either Emax or pEC50. To explore the discrepancy between the effects of the low dose of emodin compared to the higher doses, we measured gene expression of aortic eNOS and hepatic LDLR as well as the oxidation related indices.
Kawashima et al showed that the atherosclerotic impairment of endothelium-dependent vasodilation is related to the reduced bioavailability of NO produced from eNOS [28]. The low dose of emodin (10 mg/kg) significantly increased the aortic eNOS mRNA, protein levels of p-eNOSSer1177, and activated form of eNOS and it also upregulated the phosphorylation of eNOS, which is essential for maintaining adequate NO production in the endothelium. Like simvastatin, EL significantly improved acetylcholine-induced aortic relaxation, thus demonstrating a protective effect on the endothelium [29]. The molecular mechanism of p-eNOS upregulation in the treatment groups is unknown, but the promising ability of emodin to beneficially affect the eNOS signaling pathways is currently under investigation in our laboratory.
Plasma LDL enters cells via endocytosis mediated by cell-surface LDLRs that remove cholesterol-rich lipoprotein particles from the circulation, to be metabolized principally by the liver [30,31]. Accordingly, the activity of the LDLR in the liver is a major determinant of plasma LDL-C levels [32]. We found that animals fed the HCD had a significant decrease in hepatic LDLR mRNA and protein, along with an increase in serum LDL-C. A successful strategy to reduce circulating LDL would be through the upregulation of LDLR on the surface of hepatocytes [33]. Consistent with previous reports [17,34], we found that hepatic LDLR mRNA and protein were significantly increased with simvastatin but were also significantly increased with low-dose emodin (10 mg/kg). All 4 treatment groups showed lower total-C and had significantly reduced atherogenic indices, but only the SIM and EL groups showed increases in hepatic LDLR mRNA and protein. Although these results need to be confirmed, the apparent contradiction may indicate that emodin lowers cholesterol by acting through more than a single pathway.
Vascular risk factors such as dyslipidemia and atherosclerosis are associated with a marked increase in the production of vascular reactive oxygen species (ROS) [35]. When the production of free oxygen radicals exceeds the threshold of the antioxidant defense systems, there is successive oxidation of macromolecules such as DNA, protein, and lipids [36]. MDA is a product of lipid peroxidation and is one of the most frequently measured biomarkers of oxidative stress [37]. SOD and CAT are important antioxidant enzymes, which can scavenge ROS and protect cells against ROS-induced toxicity. In our present study, the HCD animals had lower SOD and CAT activities and higher MDA levels in the liver. All 4 treatment groups had significantly reduced MDA levels compared with the HCD group, but only low-dose emodin (10 mg/kg) significantly increased hepatic SOD activity, while treatment with the higher doses of emodin (30 and 100 mg/kg) significantly inhibited hepatic SOD and CAT activity.
In the serum, the 2 lower doses of emodin (10 and 30 mg/kg) significantly increased SOD and CAT activity, while high-dose emodin (100 mg/kg) decreased SOD activity and showed only a slight increase in CAT activity. Although in vitro [38] and in vivo [11] studies have demonstrated the hepatoprotective effect of emodin, an increasing number of studies have described its hepatotoxicity [39], and most of these studies were in vitro. Our in vivo study showed that the hepatoxicity of emodin may be related to the oxidative hepatic damage due to a reduction in the activity of SOD and CAT enzymes, as we observed in animals given the high dose of emodin. The molecular mechanism for the upregulation of SOD and CAT enzyme activity induced by low doses of emodin (10 mg/kg) is unknown, but warrants further study to minimize its potential toxicity.
Interestingly, recent studies have found that emodin has the ability to directly scavenge hydroxyl [40] and superoxide radicals [13]. Our results suggest that low-dose emodin (10 mg/kg) can increase serum and hepatic SOD and CAT activities and decrease the levels of hepatic and serum MDA in rats given the HCD. Taken together, we believe that emodin could be a strong non-enzymatic antioxidant that can scavenge free radicals, lower MDA levels in the liver and serum, and improve the activities of enzymatic antioxidants such as SOD (in liver and serum) and CAT (in serum).
Conclusions
Oral administration of emodin ameliorated serum lipid profiles and protected endothelium function in HCD-fed hypercholesterolemic rats by enhancing antioxidant systems and upregulating the expression of aortic p-eNOS and hepatic LDLR, leading to increased NO production and lower serum total-C and LDL-C concentrations. Low-dose emodin (10 mg/kg) showed better protection of aortic endothelium and a greater antioxidant effect than did higher doses (30 and 100 mg/kg). Although the precise mechanism through which emodin exerts its anti-hypercholesterolemic and antioxidant effects is currently unclear, our results suggest that emodin may be a promising candidate to aid in the management of hypercholesterolemia in people consuming a diet consisting of high cholesterol.
Acknowledgements
The authors thank Prof. Howard Takiff for proofreading the manuscript and Xu Huang for valuable suggestions.
Footnotes
Conflicts of Interest
None.
Source of support: This study was supported in part by the Grant for Scientific and Technology Research of the Bureau of Science and Technology Innovation of Nanshan (Grant No. 2019085) and the Sanming Project of Medicine in Shenzhen China (Grant No. SZSM201603029)
References
- 1.World Health Organization. World health statistics 2020: Monitoring health for the SDGs, sustainable development goals. Geneva, Switzerland: 2020. [Google Scholar]
- 2.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–36. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care. 2013;40:195–211. doi: 10.1016/j.pop.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J Lipids. 2015;2015:1–50. doi: 10.1155/2015/971453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KH, Park WJ. Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30:1213–25. doi: 10.3346/jkms.2015.30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia S, Ni Y, Zhou Q, et al. Emodin attenuates severe acute pancreatitis via antioxidant and anti-inflammatory activity. Inflammation. 2019;42:2129–38. doi: 10.1007/s10753-019-01077-z. [DOI] [PubMed] [Google Scholar]
- 7.Koyama J, Inoue M, Morita I, et al. Correlation between reduction potentials and inhibitory effects on Epstein-Barr virus activation by emodin derivatives. Cancer Lett. 2006;241:263–67. doi: 10.1016/j.canlet.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Song X, Yin Z, et al. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol Res. 2016;186–187:139–45. doi: 10.1016/j.micres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Li WY. Hong Kong: The Hong Kong Polytechnic University; 2006. Pharmacological studies on Radix et Rhizoma Rhei (Da Huang) and its active component Emodin [Dissertation] [Google Scholar]
- 10.Qiu FF, Liu HZ, Liang CL, et al. A new immunosuppressive molecule emodin induces both CD4+FoxP3+ and CD8+CD122+ regulatory T cells and suppresses murine allograft rejection. Front Immunol. 2017;8:1519–33. doi: 10.3389/fimmu.2017.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BH, Huang YY, Duh PD, et al. Hepatoprotection of emodin and Polygonum multiflorum against CCl(4)-induced liver injury. Pharm Biol. 2012;50:351–59. doi: 10.3109/13880209.2011.604335. [DOI] [PubMed] [Google Scholar]
- 12.Cha TL, Chuang MJ, Tang SH, et al. Emodin modulates epigenetic modifications and suppresses bladder carcinoma cell growth. Mol Carcinog. 2015;54:167–77. doi: 10.1002/mc.22084. [DOI] [PubMed] [Google Scholar]
- 13.Rossi M, Wen K, Caruso F, et al. Emodin scavenging of superoxide radical includes π–π interaction. X-Ray crystal structure, hydrodynamic voltammetry and theoretical studies. Antioxidants. 2020;9:194. doi: 10.3390/antiox9030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heo SK, Yun HJ, Park WH, et al. Emodin inhibits TNF-alpha-induced human aortic smooth-muscle cell proliferation via caspase- and mitochondrial-dependent apoptosis. J Cell Biochem. 2008;105:70–80. doi: 10.1002/jcb.21805. [DOI] [PubMed] [Google Scholar]
- 15.Bhadauria M. Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Exp Toxicol Pathol. 2010;62:627–35. doi: 10.1016/j.etp.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Wu JH, Guo DJ, et al. Suppression of diet-induced hypercholesterolemia by scutellarin in rats. Planta Med. 2009;75:1203–8. doi: 10.1055/s-0029-1185539. [DOI] [PubMed] [Google Scholar]
- 17.Wu JH, Wang QH, Li F, et al. Suppression of diet-induced hypercholesterolemia by turtle jelly, a traditional Chinese functional food, in rats. Evid Based Complement Alternat Med. 2012;2012:320304. doi: 10.1155/2012/320304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riesen WF. Lipid Metabolism. In: Thomas L, editor. Clinical laboratory diagnostics. Use and assessemnt of clinical laboratory results. Frankfurt: TH-Books Verlagsgesellschaft; 1998. pp. 167–73. [Google Scholar]
- 19.World Health Organization. Use of anticoagulants in diagnostic laboratory investigations: Stability of blood, plasma and serum samples. 2002:1–64. [Google Scholar]
- 20.Wu JH, Li Q, Wu MY, et al. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J Nutr Biochem. 2010;21:613–20. doi: 10.1016/j.jnutbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, Izumiyama N, Nakamura K, et al. Age-associated ultrastructural changes in the aortic intima of rats with diet-induced hypercholesterolemia. Atherosclerosis. 1989;79:101–11. doi: 10.1016/0021-9150(89)90114-7. [DOI] [PubMed] [Google Scholar]
- 22.Nishina PM, Verstuyft J, Paigen B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res. 1990;31:859–69. [PubMed] [Google Scholar]
- 23.Chiang MT, Chen YC, Huang AL. Plasma lipoprotein cholesterol levels in rats fed a diet enriched in cholesterol and cholic acid. Int J Vitam Nutr Res. 1998;68:328–34. [PubMed] [Google Scholar]
- 24.Li S, Guo YL, Zhao X, et al. Novel and traditional lipid-related biomarkers and their combinations in predicting coronary severity. Sci Rep. 2017;7:360. doi: 10.1038/s41598-017-00499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies JT, Delfino SF, Feinberg CE, et al. Current and emerging uses of statins in clinical therapeutics: A review. Lipid Insights. 2016;9:13–29. doi: 10.4137/LPI.S37450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meor Anuar Shuhaili MFR, Samsudin IN, Stanslas J, et al. Effects of different types of statins on lipid profile: A perspective on Asians. Int J Endocrinol Metab. 2017;15:e43319. doi: 10.5812/ijem.43319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin L, Caldwell RB, Li-Masters T, et al. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol. 2007;58:191–206. [PubMed] [Google Scholar]
- 28.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 29.Carneado J, Alvarez de Sotomayor M, Perez-Guerrero C, et al. Simvastatin improves endothelial function in spontaneously hypertensive rats through a superoxide dismutase mediated antioxidant effect. J Hypertens. 2002;20:429–37. doi: 10.1097/00004872-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–72. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Beglova N, Blacklow SC. The LDL receptor: How acid pulls the trigger. Trends Biochem Sci. 2005;30:309–17. doi: 10.1016/j.tibs.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Scharnagl H, März W. New lipid-lowering agents acting on LDL receptors. Curr Top Med Chem. 2005;5:233–42. doi: 10.2174/1568026053544524. [DOI] [PubMed] [Google Scholar]
- 34.Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007;193:86–93. doi: 10.1016/j.atherosclerosis.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Vogiatzi G, Tousoulis D, Stefanadis C. The role of oxidative stress in atherosclerosis hellenic. J Cardiol. 2009;50:402–9. [PubMed] [Google Scholar]
- 36.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 2nd edition. London: Oxford University Press (Clarendon); 2007. [Google Scholar]
- 37.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Yin XR, Gong X, Jiang R, et al. Emodin ameliorated lipopolysaccharide-induced fulminant hepatic failure by blockade of TLR4/MD2 complex expression in D-galactosamine-sensitized mice. Int Immunopharmacol. 2014;23:66–72. doi: 10.1016/j.intimp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Liu XY, Liu YQ, Qu Y, et al. Metabolomic profiling of emodin-induced cytotoxicity in human liver cells and mechanistic study. Toxicol Res. 2015;4:948–55. [Google Scholar]
- 40.Yen GC, Duh PD, Chuang DY. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000;70:437–41. [Google Scholar]