Abstract
Background
Schizophrenia has been associated with pregnancy and birth complications, and fetal exposure to inflammation is thought to be a common underlying mechanism. However, it is unclear whether risk is specific to particular phases of pregnancy, as no prior studies have examined maternal serum samples across assessments from the first trimester onward. The aim of this study was to characterize and compare longitudinal patterns of maternal serum levels of cytokines across pregnancy between offspring who were later ascertained as having a psychotic disorder diagnosis, non-psychotic siblings of these cases, and unrelated, non-psychotic individuals who served as controls.
Methods
Participants included 90 cases, 79 siblings, and 273 matched controls identified from the Philadelphia cohort of pregnant women followed by the National Collaborative Perinatal Project [1959–1965]. Psychotic disorder diagnoses in adulthood were assessed with review of medical records and were confirmed with a validation study. Cytokine levels [TNFα, IL-1β, IL-5, IL-6, IL-8, IL-10, and IL-17a] were assessed using a multiplex bead assay in archived maternal serum samples collected across prenatal visits and birth. Cytokine patterns were characterized with linear mixed models.
Outcomes
Levels of pro-inflammatory TNFα, IL-1β, and IL-6 were significantly higher in maternal serum of offspring who later developed psychosis relative to maternal serum of matched controls. These differences were maximal in first half of pregnancy (7–20 weeks), tapering to non-significant during the second half of pregnancy.
Interpretation
These findings suggest that exposure to elevated maternal pro-inflammatory cytokine levels in early pregnancy may play a role in the etiology of psychosis.
Introduction
Schizophrenia has been linked with a variety of adverse prenatal events, including maternal infection with certain pathogens,1 hypoxic birth complications,2 and maternal stress.3 Fetal exposure to maternal inflammation is one hypothesized mechanism that may underlie the association of these risk factors with schizophrenia and other psychotic disorders.1,4 One candidate inflammatory pathway is the cytokine system, which consists of regulatory pro- and anti-inflammatory proteins secreted by immune and other cell types in response to immune challenges.4 In addition to their role in inflammatory signaling, cytokines actively contribute to a variety of prenatal neurodevelopmental processes including neuronal and glial migration and differentiation, synaptic maturation, and myelination.5 Rodent studies have demonstrated that maternal immune challenge produces alterations in cytokine levels throughout maternal and fetal compartments,6 can have lasting effects on cytokine levels in the fetal brain,7 and leads to neurological and behavioral changes in the adult offspring that mimic schizophrenia.1 The long-term effects of prenatal immune exposure are influenced by the timing of the insult during gestation8 and can be affected by the balance between pro and anti-inflammatory cytokines.9 These findings suggest that understanding the timing and nature of prenatal cytokine perturbations associated with risk for psychosis could provide greater understanding of the neurodevelopmental origins of the disorder.
In human studies, risk for psychosis has been associated with perturbations in mean pro-inflammatory cytokine concentrations in maternal serum during the second and third trimester, including higher IL-8 in the second trimester,10 higher IL-6 (for male offspring) and lower TNFα levels (for female offspring) in the third trimester,11 and elevated TNFα at delivery.12 A protective effect has been observed for higher levels of a composite score of anti-inflammatory IL-4, IL-5, and IL-13 at delivery.13 These findings provide intriguing hints that alterations in maternal levels of specific cytokines during pregnancy may influence the development of psychosis. However, studies to date have included only one cytokine sample from pregnancy and have characterized gestation by trimester rather than by week, which limits the ability to differentiate sensitive periods within pregnancy and identify specific neurodevelopmental processes that may be particularly impacted by immune disruptions. Maternal cytokine levels in the first trimester have not been examined in association with psychosis risk, which may be a particularly vulnerable period for neurodevelopmental disruption.8 Additionally, it remains unknown if observed differences in maternal cytokine levels relative to control pregnancies are specific to pregnancies with offspring that ultimately develop psychosis or if pregnancies with unaffected siblings exhibit similar patterns. Longitudinal studies of maternal cytokine signaling across gestation would provide needed insight into specific periods of prenatal development that may be particularly sensitive to disruption and result in increased risk for psychosis among offspring relative to siblings and controls.
The objectives of the present study were: to assess the reliability of measurement of cytokines in maternal serum samples that underwent extended storage, investigate differences in prenatal cytokine levels between offspring who later developed psychosis, their siblings, and controls, and determine if there is a specific window of gestation in which exposure to inflammation particularly influences risk for psychosis.
Methods
Study Population
The National Collaborative Perinatal Project (NCPP) was a large-scale prospective longitudinal study that assessed the effects of perinatal factors on infant and child development. At sites across the United States, over 50,000 pregnant women were enrolled during prenatal clinical visits between 1959 and 1965.14 The present study draws from the Philadelphia cohort of the NCPP, which includes 9236 surviving offspring of 6753 pregnant women.15 Interview and examination data were collected via standardized protocols and were recorded by trained staff during maternal registration for prenatal care and at subsequent visits. Maternal blood samples were collected at each prenatal visit and at delivery, and were stored at −20 °C in a National Institutes of Health (NIH) repository.14
Diagnostic Screening
Diagnostic assessment procedures were used to identify offspring from the Philadelphia cohort who had developed psychotic disorders by adulthood. The details of these procedures, and human subjects approval from affiliated institutions, have been described elsewhere.15 A subset of these offspring “cases”, siblings of cases, and matched controls were previously selected for case-control studies and are described in Results. As the study is now solely in data analysis phase, it is considered IRB exempt at Yale University.
Cytokine Measurement
Cytokines were selected for analysis based on existing evidence indicating association with risk for psychosis and/or to signaling in pregnancy. Cytokines were measured in maternal serum samples using the Mesoscale V-PLEX Proinflammatory Panel 1 multiplex bead assay (MSD, Rockville MD, USA), a kit that has exhibited high sensitivity cytokine detection. Assays were run in duplicate and according to manufacturer’s instructions,17 All samples were run on the same plate for 75% of the pregnancies, and plate number was assessed as a random effect in analyses (see below). 7% of samples (86/1214, from 14 cases and 41 controls) had undergone at least one previous thaw cycle. Values were expressed as pg/mL concentrations relative to standards containing known amounts of target cytokines. Values below the threshold of detection (LOD) were rescored as LOD/2. Cytokine measurements were log-transformed to promote normality, and normality was assessed using qq plots and histograms. A subset of 46 duplicate samples, collected during the same 23 prenatal visits but stored in separate vials, were also analyzed to further examine reliability of cytokine measurement. Assays were conducted in the Stanley Laboratory of Developmental Neurovirology at Johns Hopkins University Medical Center under the supervision of Dr. Robert Yolken (lab protocol: https://www.stanleylab.org/laboratory/). Samples were handled and analyzed consistently, without knowledge of case status.
Statistical Analyses
Cases, siblings, and controls were compared on covariates using ANOVAs and chi-square tests. Linear mixed models were fit to each cytokine, using lowess plots to inform whether quadratic or linear terms for gestational age were tested. Gestational week was used as a continuous variable for all analyses, and was tested as both linear and quadratic terms. Though individuals with two serum samples (n=176, 40%) could not contribute to estimation of quadratic terms, their data supported estimation of linear and intercept terms and maximized power. Random intercepts for pregnancy ID were included in all models. Random slopes for pregnancy ID either did not converge or improve model fit, and were not included in final models. Assessment of maternal ID as a second-level effect and assay plate number and previous thaw cycles as additional first-level effects revealed that these parameters did not account for additional variance beyond pregnancy ID and/or exhibited ICCs below the suggested threshold of ·118 for inclusion of random effects, and were not included in final models. Covariates (Table 1) were tested simultaneously for significant prediction of each cytokine to control for overlap with other covariates. To avoid multicollinearity, pairs of covariates with substantial correlations (>·4) were run in separate regressions. Covariates that significantly (p < ·05) predicted levels of a cytokine were then confirmed in individual regressions and added to the final model. Interactions between gestational week (linear term) and case status was tested as predictors for each of the seven cytokines, and p-values were FDR-corrected.19 Mean levels of each cytokine in the first half of pregnancy and at delivery were compared across case status groups using ANOVAs. All significance tests were two-tailed at α = ·05 (FDR-corrected). Analyses did not change substantially when outliers above or below 3 SDs were excluded.
Table 1.
Demographic and obstetric characteristics by adult psychiatric outcome
| Psychosis cases (n = 90) | Siblings of cases (n = 79) | Controls (n = 273) | X2/F | p | |
|---|---|---|---|---|---|
| Female, n (%) | 42 (46·7%) | 42 (53·2%) | 121 (44·3%) | 1·93 | ·38 |
| African-American | 84 (93·3%) | 74 (96·1%) | 250 (91·6%) | 1·89 | ·39 |
| SES (0=low, 99=high) | 37·64 (19·51) | 35·53 (16·46) | 41·03 (16·90) | 2·96 | ·053 |
| Maternal Education (years completed) | 39·80 (24·11) | 37·82 (21·94) | 43·82 (21·32) | 2·28 | ·10 |
| Maternal Age (years) | 23·99 (7·13) | 23·53 (5·32) | 24·42 (6·23) | ·65 | ·52 |
| Maternal Parity | 2·56 (2·59) | 3·25 (2·56) | 2·50 (2·55) | 2·25 | ·11 |
| First Born | 24 (26·7%) | 8 (10·4%) | 55 (20·1%) | 7·0 | ·030 |
| Overweight/Obese (Pre-Pregnancy) | 21 (27·3%) | 26 (35·1%) | 80 (32·0%) | 1·13 | ·89 |
| Smoked During Pregnancy | 50 (56·8%) | 47 (61·0%) | 128 (47·8%) | 5·27 | ·072 |
| Antibiotics during pregnancy | 34 (37·8%) | 21 (26·6%) | 73 (26·7%) | 4·15 | ·13 |
| Maternal Health Condition During Pregnancy15 | 54 (60·7%) | 45 (58·4%) | 161 (59·6%) | ·08 | ·96 |
| Hypoxic Complications15 | 56 (62·2%) | 38 (49·4%) | 160 (58·6%) | 3·05 | ·22 |
| Preterm Birth | 18 (20·0%) | 14 (17·8%) | 55 (20·1%) | ·24 | ·89 |
| Number of Samples | 2·63 (0·68) | 2·76 (·80) | 2·78 (·72) | 1·053 | ·35 |
SES, socio-economic status; BMI, body mass index
Data cleaning and univariate analyses were run in SAS 9.4. Multivariate analyses and graphing were conducted in R using the lme4,20 heatmap.2,21 and ggplot222 packages.
Role of the Funding Source
Funders were not involved in any aspect of the study. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
The sample consists of a previously identified subset of 111 offspring who received a diagnosis of a major psychotic disorder (confirmed by chart review) and 333 controls matched to the affected offspring by gender, race, and birth date.16 91 siblings of affected offspring were also examined in the current study. All available maternal serum samples from pregnancy and delivery for the 535 subjects were acquired from the NIH repository, and were collected between 7 and 48 weeks gestation (see SI) at prenatal visits between 1959 and 1965. These serum samples were acquired and analyzed in 2019 and do not overlap with previous cytokine analyses by our group. As we were not able to acquire at least two usable serum samples for some individuals, the final sample consisted of 90 cases, 79 siblings (of 40 cases), and 273 controls (N = 442). 39.8% (176/442) of individuals had two available samples, and 60.2% of individuals had three or more samples (3 samples: n = 202/442, 45.7%; 4 samples, n = 57/442, 12.9%; 5 or 6 samples, n = 7/442, 1.5%). The majority of cases were diagnosed with schizophrenia or schizoaffective disorder (n = 53) or major depressive disorder with psychosis (n = 29), with a few individuals with bipolar disorder with psychosis (n = 8).16 Demographic characteristics of the cases, siblings, and controls are given in Table 1. Notably, hypoxia rates did not differ significantly by case status.
Our selection of cytokines for analysis was informed by reliability assessments. We had the opportunity to examine the reliability of cytokine measurement in 46 duplicate serum samples, in which maternal serum from the same blood draw was stored in two separate vials. Intra-class correlations (ICCs) for each cytokine in the two samples ranged from ·13 (IL-17a) to ·91 (IL-6), with a mean ICC of ·49 (Table 2). ICCs were highly influenced by the frequency of cytokines below the limits of detection (LOD), which had been rescaled to half of the lowest detected value during data cleaning. When samples under the LOD were dropped, ICCs improved to a range from ·43 (IL-12p70) to ·94 (IL-6), with an average of ·73 (Table 2). These results indicate that factors related to serum handling and storage for over 60 years likely introduced mild to moderate noise in cytokine measurements, and that noise is particularly influenced by rates of values below the LOD.
Table 2.
Beta estimates and significance tests (FDR-corrected) for interactions between case status and gestational week. Significant covariates that were included in final models are noted with superscripts.
| Cytokine | Case*time | Sib *time | ||||
|---|---|---|---|---|---|---|
| B (SE) | T | p | B/SE | T | p | |
| TNFαa,b | −·022 (·006) | −3·418 | ·0046 | −·007 (·007) | −·974 | ·96 |
| IL-1βa | −·026 (·011) | −2·453 | ·033 | ·002 (·011) | ·136 | ·96 |
| IL-8a,c,e | −·017 (·013) | −1·351 | ·31 | −·008 (·014) | −·569 | ·96 |
| IL-6a,b,c | −·027 (·010) | −2·560 | ·033 | ·003 (·011) | ·246 | ·96 |
| IL-10a,b,c | ·002 (·009) | ·179 | ·86 | −·007 (·009) | −·695 | ·96 |
| IL-5d | ·010 (·013) | −·706 | ·67 | ·005 (·014) | −·158 | ·96 |
| IL-17a | ·003 (·014) | ·188 | ·86 | −·026 (·016) | −1·692 | ·64 |
quadratic week term
first-born child
preterm
maternal health conditions
year of pregnancy
We used this information about the influence of LOD values to inform our selection of cytokines for further analysis. Across the 12 examined cytokines, rates of LOD ranged from 0·1% (1/1587; IL-8) to 46% (737/1587; IL-2) (SI Table 1). In addition to concerns about measurement reliability stemming from the analysis of duplicate samples, assumptions about normality of distributions were also violated when LOD rates exceeded 6% of the observed data. To avoid the introduction of bias into analyses, cytokines with LODs above this threshold (IFNg, IL-4, IL-12, IL-12p70, IL-2) were excluded from further analysis.
Relationships between the seven selected cytokines within each sample were examined visually using heat maps and statistically using within-sample ICCs, and indicated minimal consistency (within-sample ICC: ·137) of cytokine concentrations within a given sample (see SI). The strength of association between cytokines across time was assessed with Spearman rank correlations (collapsed across gestational age) and through unsupervised hierarchical clustering with Manhattan distance and Ward’s D agglomeration method. Relations of moderate strength (correlations ranging from ·46 to ·66) were observed between IL-6 and IL-10 as well as between IL-8, IL-1β, and TNFα (see SI). Due to the large number of serum samples (N =1214), consistency of longitudinal cytokine measurements within a given pregnancy could not be effectively clustered. Instead, within-pregnancy consistency was assessed using ICCs calculated using null linear mixed models with only time parameters and random intercepts for individuals and families. Cytokine ICCs within pregnancy ranged from ·10 to ·25, indicating sufficient consistency to include pregnancy ID as a random effect in linear mixed models.18 Due to limited sample size, within-mother ICCs (i.e., consistency of maternal cytokine levels between pregnancies of cases and siblings) are not reported.
To assess patterns of cytokine concentration across gestation, linear mixed models were fit to each cytokine (Figure 1). Gestational week was coded as a continuous variable, and a quadratic term best described longitudinal patterns for 5 of the 7 cytokines. An inverted U shape was observed for TNFα, IL-1β, and IL-8, with concentrations peaking around 25–30 weeks gestation. In contrast, IL-6 and IL-10 exhibited increasing concentrations beginning around 25–30 weeks and continuing until delivery, with particularly rapid rates observed for IL-6. Cytokine concentration patterns were best described by linear trends for IL-5 and IL-17a, and exhibited decreasing concentrations across gestation. Though some differences in magnitude of change were observed (Figure 1, SI Table 3), the common shapes and trends observed in linear mixed models aligned with the clusters and correlations observed within samples and across gestation for TNFα, IL-1β, and IL-8 and for IL-6 and IL-10.
Figure 1.
Linear mixed models for change in log-transformed cytokine concentration across gestation (coded continuously).
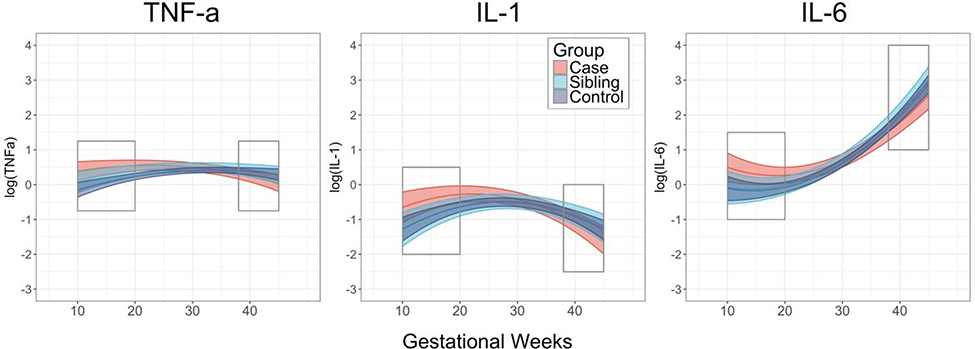
Potential differences in cytokine signaling across gestation between cases, siblings, and controls were assessed with interaction effects between case status and linear gestational week parameters in linear mixed models (see SI). Following FDR correction, cytokine patterns differed significantly between cases and controls for three cytokines: TNFα, IL-1β, and IL-6 (Figure 2, Table 2), and did not differ significantly for four cytokines: IL-5, IL-8, IL-10, IL-17a (Table 4). Siblings and controls did not significantly differ for any of the cytokines. Findings were robust to all covariates listed in Table 1, and significant covariates included in final models are noted in Table 4. Results did not change if cases with bipolar or major depression with psychosis were excluded (see SI). Follow-up assessment of main effects for case status confirmed that no case differences were observed for IL-8, IL-5, IL-10, and IL-17a.
Figure 2.
Cytokine levels by case status for TNFα, IL-1β, and IL-6 levels across pregnancy. Boxes denote early pregnancy (prior to 20 weeks) and near delivery (38+ weeks).
Figure 2 suggests that differences in cytokine concentration by case status may be particularly pronounced at the beginning of pregnancy and around delivery. Post-hoc ANOVAs were conducted to compare cytokine concentration by case status in the first half of pregnancy (10–20 weeks, mean=16·0 weeks, SD=3·3) and around delivery (38+ weeks, mean=40·1 weeks, SD=1·8). In the first half of pregnancy, cases exhibited significantly higher levels than controls for all three cytokines (Table 3). In contrast, differences by case status at the end of pregnancy were not significant. Exclusion of individuals exposed to hypoxia did not change results (see SI). These findings highlight the potential importance of exposure to inflammation prior to 20 weeks of pregnancy in the etiology of psychosis.
Table 3.
Mean cytokine concentration levels [log(pg/mL)] in early pregnancy (10–20 weeks) and near delivery (38+ weeks) by case status.
| Early Pregnancy | Delivery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Status (# samples) | Case (n=44) | Sibling (n=32) | Control (n=117) | F | p | Case (n=54) | Sibling (n=49) | Control (n=161) | F | p |
| TNFα | 4·27 | ·015 | ·48 | ·62 | ||||||
| M (SD) | ·49 (·76) | ·28 (·54) | ·12 (·70) | ·20 (·80) | ·27 (·90) | ·31 (·73) | ||||
| IL-1β | 3·40 | ·036 | ·32 | ·73 | ||||||
| M (SD) | −·41 (1·02) | −·96 (·77) | −·81 (1·07) | −1·13 (1·45) | −1·00 (1·77) | −·94 (1·68) | ||||
| IL-6 | 5·26 | ·0060 | 2·43 | ·090 | ||||||
| M (SD) | ·28 (·96) | −·08 (·55) | −·12 (·63) | 1·95 (1·17) | 1·94 (·90) | 2·23 (1·08) | ||||
Discussion
In this study, offspring who later developed psychosis exhibited distinct patterns of maternal cytokine levels during pregnancy for three pro-inflammatory cytokines (TNFα, IL-1β, and IL-6) relative to controls, with significant elevations during specifically the first half of pregnancy. The study design allowed for monitoring maternal prenatal serum cytokine levels earlier in pregnancy than in prior studies and for examining trajectories in cytokine levels across pregnancy at both the individual and group levels. Although interest in neurodevelopmental processes in the etiology of schizophrenia and related disorders has a long history, the current findings place the timing of risk associated with maternal inflammation much earlier in embryologic development than previously documented in humans.
Neurodevelopmental events during early pregnancy (10–20 weeks) may be particularly sensitive to cytokine alterations in the pathogenesis of schizophrenia. Pyramidal neuron and interneuron proliferation and migration predominate in this period23,24, and initial connections between cortical and subcortical neurons form in the subplate.24 Previous findings in human studies identified inflammation in mid to late pregnancy with risk for psychosis and did not implicate these early developmental events, highlighting a novel area for investigations of inflammation in the etiology of psychosis. Importantly, early exposure to inflammation in pregnancy may also be influential because disruptions to neurodevelopment can accumulate and compound.8 Further investigations in animal models that clarify the specific effects of exposure to maternal inflammation in early gestation on neurodevelopment would further elucidate the nature of the early neurodevelopmental roots of psychosis.
As the vast majority of offspring exposed to maternal inflammation during prenatal development do not develop schizophrenia,25 it is likely that maternal cytokine signaling interacts with factors such as fetal genetic liability, maternal genetic factors, and environmental influences to affect neurodevelopment. Parsing the relationships between maternal and fetal genetic and environmental factors is challenging, and ongoing work in the field implicates both interactions and covariation. For example, a recent study identified that the variance in risk for schizophrenia explained by genome-wide polygenic risk scores increases by five-fold for individuals who experienced early-life complications. The genes implicated in this gene-environment interaction are highly expressed in the placenta and are differentially expressed in complicated pregnancies and in pregnancies with male fetuses.26 A different study examining the relationship between familial history of schizophrenia, pregnancy complications, and environmental stressors on risk for psychosis among offspring found substantial covariation between family history of schizophrenia and environmental stressors, but not with pregnancy complications.27 Though we attempted to account for maternal obstetric complications and environmental factors in our analyses, we were not able to analyze genetic data due to the age of our samples and cannot rule in or out the relevance of our finding to these debates.
Siblings of offspring who later developed psychosis did not differ from controls on levels of pro-inflammatory cytokines, indicating that the observed differences are specific to psychosis cases relative to controls and are likely not solely attributable to any maternal factors that were shared across pregnancies. Though we did not have sufficient power to directly compare cytokine levels of cases and siblings, sibling levels of TNFα, IL-1β, and IL-6 during the first half of pregnancy appeared intermediate to psychosis case and control levels. Further studies with larger numbers of offspring who develop psychosis and their siblings are needed to clarify the relationship between maternal serum cytokine levels during pregnancies with eventual cases and their siblings.
Our results implicate elevated maternal levels of TNFα, IL-1β, and IL-6 as risk factors for psychosis among offspring. These three cytokines are potent pro-inflammatory proteins that play a critical role in the initial response to infection and in initiating and sustaining inflammatory responses.28 Notably, associations between maternal serum TNFα11,12 and IL-611 levels during pregnancy and offspring psychosis have also been identified in previous studies. Though these markers have been implicated in a variety of neurodevelopmental processes, including neurogenesis (IL-6) and neuronal migration (IL-1β),29 further work is needed to clarify the relationship between maternal and fetal cytokine signaling across gestation before specific inferences can be made. Rodent studies suggest that cytokines are transduced from maternal serum to the fetus by directly crossing the placenta or through signal transduction that evokes cytokine signaling on the fetal side of the placenta.1 Though maternal immune activation affects cytokine levels across maternal and fetal tissues, the specific cytokines do not always correspond between maternal serum and the fetal brain.6 Further work that clarifies patterns of cytokine disruption across maternal serum and the fetal brain would provide insight into the specific effects of maternal cytokine disruptions on fetal development, and could ultimately provide a platform for preventative interventions during the prenatal and neonatal periods.
Most previous studies investigating associations between maternal cytokine levels and risk for schizophrenia spectrum disorders have identified late pregnancy as a risk period,10–13 aside from one study that examined mean IL-8 levels collapsing across the second trimester. These results contrast with our findings that are specific to the first half of pregnancy, with particularly pronounced differences at the end of the first trimester. These differences may be attributable to variation in study design, as previous studies included only one cytokine measurement per individual, collapsed across trimesters, consisted of smaller sample sizes, included different racial and socioeconomic compositions exposed to varying levels of stress, and utilized other cytokine assays. Studies of maternal cytokine levels in pregnancy also identified inconsistent findings for cytokine patterns across pregnancy, and similarly exhibit a variety of study designs.30 However, IL-6 and TNFα were identified by previous studies11,12 and by our current work, which could indicate particular relevance of these markers to the neurodevelopment of schizophrenia. Additional longitudinal studies of maternal cytokine levels that assess cytokine levels by gestational week (rather than by trimester), utilize a range of assays, and have large sample sizes are needed to clarify both normative cytokine patterns during pregnancy and patterns associated with risk for schizophrenia among offspring.
Though the prospective design of the study eliminates the concern of recall bias, the long length of serum storage introduces limitations. Though the ~60 year storage time of the blood samples likely contributed to noise in cytokine measurement, a number of precautions were taken to minimize the effect of noise on results (i.e., selecting sera with no thaw cycles when possible, use of assays with high sensitivity, assessment of reliability within duplicate samples stored in separate vials, exclusion of cytokines with poor measurement reliability that interfered with statistical assumptions). Furthermore, we would expect the effect of noise to affect all seven cytokines similarly, and to bias results towards null findings.
It is important to note that early immunologic disruption is only one possible etiology for psychosis. Other pathways that may influence the development of psychosis include the HPA axis, oxidative stress, maternal nutrient deficiency, or maternal autoimmune disorders.30 This study highlights the relevance of early immunological disruptions to the development of psychosis, yielding insight into a potential developmental pathway to the disorder.
Supplementary Material
Research In Context.
Evidence before this study
We searched Google Scholar for articles from database inception to June 15, 2019, with the terms “cytokine” AND “prenatal” AND “psychosis” or “schizophrenia.” This search was augmented by the reference list of a recent review paper on this topic by the first author. In a small number of prospective studies of prenatal exposure to maternal cytokines in association with risk for psychosis among offspring, there was some evidence for risk associated with altered cytokine levels in the second half of pregnancy and at birth. However, these studies included only one cytokine measurement per pregnancy and collapsed across trimester, and none had specifically investigated maternal cytokine levels in the first trimester.
Added value of this study
This is the first study, to our knowledge, to examine longitudinal patterns of maternal cytokine levels during pregnancy that encompass the first trimester to birth. We found that higher levels of pro-inflammatory cytokines in specifically the first half of pregnancy were associated with risk for psychosis among offspring, implicating an earlier timepoint in gestation than previously understood in the etiology of psychosis.
Implications of all the available evidence
A more nuanced understanding of the effects of maternal cytokine exposure during specific timepoints of gestation will further our understanding of the prenatal origins of psychosis and could ultimately provide a platform for early preventative treatment.
Acknowledgements
This study was supported by funding from the NIMH (P50 Silvio O. Conte Center at Johns Hopkins (MH-94268), the Stanley Foundation, the March of Dimes, and Yale University. Ms. Allswede’s work was funded by an NSF Graduate Research Fellowship (DGE-1122492). A proposal for access to the prenatal serum samples was approved by the NICHD/DIPHR Biospecimen Respository Access and Data Sharing Committee.
Funding: NIMH (P50 Silvio O. Conte Center at Johns Hopkins (MH-94268), Stanley Foundation, March of Dimes, Yale University, National Science Foundation GRFP, NICHD/DIPHR BRADS committee
Footnotes
Declaration of Interests
Dr. Cannon reports that he is a consultant to Boehringer Ingelheim Pharmaceuticals and to Lundbeck A/S. Dr. Yolken reports that he is a Scientific Advisor to the Stanley Medical Research Institute. Dr. Buka and Ms. Allswede do not report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyer U Developmental neuroinflammation and schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry 2013; 42: 20–34. [DOI] [PubMed] [Google Scholar]
- 2.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: The role of obstetric complications. Schizophr Bull 2008; 34: 1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Os J, Selten J-P. Prenatal exposure to maternal stress and subsequent schizophrenia: The May 1940 invasion of the Netherlands. Br J Psychiatry 1998; 172: 324–6. [DOI] [PubMed] [Google Scholar]
- 4.Miller BJ, Culpepper N, Rapaport MH, Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: A review of human studies. Prog Neuro-Psychopharmacology Biol Psychiatry 2013; 42: 92–100. [DOI] [PubMed] [Google Scholar]
- 5.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron 2009; 64: 61–78. [DOI] [PubMed] [Google Scholar]
- 6.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 2001; 47: 27–36. [DOI] [PubMed] [Google Scholar]
- 7.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 2013; 31: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: The earlier the worse? Neurosci 2007; 13: 241–56. [DOI] [PubMed] [Google Scholar]
- 9.Girard S, Kadhim H, Larouche A, Roy M, Gobeil F, Sébire G. Pro-inflammatory disequilibrium of the IL-1β/IL-1ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine 2008; 43: 54–62. [DOI] [PubMed] [Google Scholar]
- 10.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry 2004; 161: 889–95. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli J-AL, Remington AG, Tsuang MT, et al. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol Med 2014; 44: 3249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun 2001; 15: 411–20. [DOI] [PubMed] [Google Scholar]
- 13.Allswede DM, Buka SL, Yolken RH, Torrey EF, Cannon TD. Elevated maternal cytokines at birth and risk for psychosis in adult offspring. Schizophr Res 2016; 172: 41–5. [DOI] [PubMed] [Google Scholar]
- 14.Niswader KR, Gordon M. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The Women and Their Pregnancies. Philadelphia, PA: W.B. Saunders; 1972. [Google Scholar]
- 15.Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. Prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000; 26: 351–66. [DOI] [PubMed] [Google Scholar]
- 16.Cannon TD, Yolken R, Buka S, Torrey EF. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry 2008; 64:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MesoScaleDiscovery. MSD MULTI-SPOT assay system - V-PLEX proinflammatory panel 1 (human) kits. 2018. lk 1–34.
- 18.Vajargah KF, Nikbakht M. Application REML model and determining cut off of ICC by multi-level model based on Markov chains. Indian J Fundam Appl Life Sci 2015; 5(S2):1432–48. [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. R Stat Soc 1995; 57: 289–300. [Google Scholar]
- 20.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. [Google Scholar]
- 21.Warnes G, Bolker B, Bonebakker R, Gentleman R, Huber W, Liaw A, et al. gplots: Various R programming tools for plotting data. R package version 3.0.1. 2016. [Google Scholar]
- 22.Wickham H ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 23.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: What is happening when? Early Hum Dev 2006; 82: 257–66. [DOI] [PubMed] [Google Scholar]
- 24.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology 2010; 35: 147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: Historical and meta-analytic review. Am J Psychiatry 2002; 159: 1080–92. [DOI] [PubMed] [Google Scholar]
- 26.Ursini G, Punzi G, Chen Q, Marenco S, Robinson J, Porcelli A, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med 2018; 24: 792–801. [DOI] [PubMed] [Google Scholar]
- 27.Morgan VA, Di Prinzio P, Valuri G, Croft M, McNeil T, Jablensky A. Are familial liability for schizophrenia and obstetric complications independently associated with risk of psychotic illness, after adjusting for other environmental stressors in childhood? Austrailian New Zeal J Psychiatry 2019; (in press). [DOI] [PubMed] [Google Scholar]
- 28.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 2009; 35: 959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013; 106–107: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allswede DM, Cannon TD. Prenatal inflammation and risk for schizophrenia: A role for immune proteins in neurodevelopment. Dev Psychopathol 2018; 30: 1157–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.