Abstract
BACKGROUND
Men with nonmetastatic, castration-resistant prostate cancer and a rapidly rising prostate-specific antigen (PSA) level are at high risk for metastasis. We hypothesized that enzalutamide, which prolongs overall survival among patients with metastatic, castration-resistant prostate cancer, would delay metastasis in men with nonmetastatic, castration-resistant prostate cancer and a rapidly rising PSA level.
METHODS
In this double-blind, phase 3 trial, we randomly assigned, in a 2:1 ratio, men with non-metastatic, castration-resistant prostate cancer and a PSA doubling time of 10 months or less who were continuing androgen-deprivation therapy to receive enzalutamide (at a dose of 160 mg) or placebo once daily. The primary end point was metastasis-free survival (defined as the time from randomization to radiographic progression or as the time to death without radiographic progression).
RESULTS
A total of 1401 patients (median PSA doubling time, 3.7 months) underwent randomization. As of June 28, 2017, a total of 219 of 933 patients (23%) in the enzalutamide group had metastasis or had died, as compared with 228 of 468 (49%) in the placebo group. The median metastasis-free survival was 36.6 months in the enzalutamide group versus 14.7 months in the placebo group (hazard ratio for metastasis or death, 0.29; 95% confidence interval, 0.24 to 0.35; P<0.001). The time to the first use of a subsequent antineoplastic therapy was longer with enzalutamide treatment than with placebo (39.6 vs. 17.7 months; hazard ratio, 0.21; P<0.001; such therapy was used in 15% vs. 48% of patients) as was the time to PSA progression (37.2 vs. 3.9 months; hazard ratio, 0.07; P<0.001; progression occurred in 22% vs. 69% of patients). At the first interim analysis of overall survival, 103 patients (11%) receiving enzalutamide and 62 (13%) receiving placebo had died. Adverse events of grade 3 or higher occurred in 31% of the patients receiving enzalutamide, as compared with 23% of those receiving placebo.
CONCLUSIONS
Among men with nonmetastatic, castration-resistant prostate cancer with a rapidly rising PSA level, enzalutamide treatment led to a clinically meaningful and significant 71% lower risk of metastasis or death than placebo. Adverse events were consistent with the established safety profile of enzalutamide. (Funded by Pfizer and Astellas Pharma; PROSPER ClinicalTrials.gov number, NCT02003924.)
THE MEDIAN BONE METASTASIS–FREE survival among men with nonmetastatic, castration-resistant prostate cancer ranges from 25 to 30 months.1,2 The risk of metastases is associated with an increasing prostate-specific antigen (PSA) level and a PSA doubling time (the estimated time required for the PSA level to double) of 10 months or less.1–3 Metastatic, castration-resistant prostate cancer is fatal, with a median survival of approximately 3 years.4,5 Delaying the time to metastasis is clinically relevant and may delay cancer-related complications and prolong survival.6 In the United States, the current National Comprehensive Cancer Network guidelines for prostate cancer suggest observation if the PSA doubling time is 10 months or greater and treatment with additional lines of hormonal therapy if it is less than 10 months7; however, the clinical evidence for prolonging metastasis-free survival and overall survival with additional lines of hormonal therapy is limited.8–10 In Europe, observation plus continuous androgen-deprivation therapy is considered to be the standard of care for patients with nonmetastatic, castration-resistant prostate cancer, regardless of the PSA doubling time.11
Enzalutamide binds directly to the androgen receptor and inhibits the binding of androgens, androgen-receptor nuclear translocation, and androgen-receptor–mediated DNA binding.12 Its effect on overall survival has been shown in two international, phase 3 trials involving patients with metastatic, castration-resistant prostate cancer before and after the receipt of docetaxel.4,13 In addition, in a randomized, bicalutamide-controlled, phase 2 trial involving patients who had not received chemotherapy previously (STRIVE), treatment with enzalutamide prolonged radiographic progression–free survival in the subgroup of patients with nonmetastatic, castration-resistant prostate cancer (hazard ratio for radiographic progression or death, 0.24; 95% confidence interval [CI], 0.14 to 0.42).14 We hypothesized that enzalutamide treatment would delay the development of metastases in men with nonmetastatic, castration-resistant prostate cancer and a rapid PSA doubling time.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted PROSPER, an international, double-blind, randomized, placebo-controlled, phase 3 trial, which was approved by the independent review board at more than 300 sites in 32 countries. The trial was conducted according to the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation. All the patients provided written informed consent before participating. An independent data and safety monitoring committee reviewed safety data in an unblinded fashion at regular intervals.
The trial was designed and the protocol (available with the full text of this article at NEJM.org) was written by the first and last authors along with employees of the sponsors — Medivation (a Pfizer company) and Astellas Pharma, the co-developers of enzalutamide. Local site investigators administered the trial regimen, followed the patients, and collected the data. The data analyses reported here were performed by the sponsors and were provided to all the authors, who wrote the manuscript and made the decision to submit it for publication. A professional medical writer was paid by the sponsors and assisted in the preparation of the manuscript. The authors assume responsibility for the accuracy and completeness of the data and for the adherence of the trial to the protocol. All the authors and participating institutions have agreements with the sponsors regarding confidentiality of the data.
TRIAL PARTICIPANTS
Eligible patients had pathologically confirmed prostate adenocarcinoma without neuroendocrine differentiation, signet-cell features, or small-cell features and with a rising PSA level despite castration-associated testosterone levels (serum testosterone level, ≤1.73 nmol per liter [0.50 ng per milliliter]). Patients had to have been receiving androgen-deprivation therapy with a gonadotropin-releasing hormone agonist or antagonist or to have undergone bilateral orchiectomy. Patients also had to have a minimum of three rising PSA values at an interval of at least 1 week apart, a baseline PSA level of 2 ng per milliliter or greater, and a PSA doubling time of 10 months or less, as calculated by the sponsor with the use of the method of Pound et al.15 Patients had no previous or current evidence of metastatic disease as assessed by computed tomography or magnetic resonance imaging for soft-tissue disease and by whole-body radionuclide bone scanning. Radiographic assessments were conducted at trial sites and confirmed by means of central, blinded, independent radiologic review by Parexel International. All the patients had to have an Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a scale from 0 to 5, with higher scores indicating greater disability and a score of 5 indicating death). Patients with suspected brain metastases or active leptomeningeal disease or with a history of seizure or a condition that may confer a predisposition to seizure were excluded. More details regarding eligibility are provided in the Supplementary Appendix (available at NEJM.org) and in the protocol.
Patients were stratified according to the PSA doubling time (<6 months vs. ≥6 months) and previous or current use of a bone-targeting agent at baseline (yes vs. no) and were randomly assigned in a 2:1 ratio to receive enzalutamide (at a dose of 160 mg) or placebo once daily. The trial regimen was continued until radiographic progression, as assessed by central independent blinded radiographic review. Discontinuation of the trial regimen solely because of an increase in the PSA level was discouraged. However, discontinuation of the trial regimen on the basis of clinical progression or toxic effects was allowed.
TRIAL END POINTS
The primary end point was metastasis-free survival, defined as the time from randomization to radiographic progression, as determined by central review at any time, or as the time to death from any cause during the period from randomization to 112 days after the discontinuation of the trial regimen without evidence of radiographic progression, whichever occurred first. Secondary end points included the time to PSA progression, the PSA response rate (on the basis of a decrease from baseline of ≥50%), the time to the first use of a subsequent antineoplastic therapy, quality-of-life assessments, overall survival, and safety.
Radiographic imaging was performed every 16 weeks until radiographic progression was confirmed. Independent radiologists who were unaware of the trial-group assignments determined the status of progressive disease according to the Response Evaluation Criteria in Solid Tumors, version 1.1, for soft tissue and the appearance of one or more lesions for bone (bone lesions that were found in a single region necessitated confirmation with a second imaging method). The PSA level was assessed at a central laboratory; investigators and patients were unaware of the PSA values.
STATISTICAL ANALYSIS
The trial was originally designed to enroll approximately 1560 patients in order to detect at least 574 primary end-point events and at least 480 deaths. On the basis of efficacy results in the subgroup of patients with nonmetastatic disease in the STRIVE trial,14 the planned enrollment was reduced to 1440 patients in order to detect at least 440 primary end-point events and to uncouple the final analysis of the primary end point from the final analysis of overall survival.14 This change provided the trial with 90% power to detect a target hazard ratio of 0.72.
The primary end point was analyzed in the intention-to-treat population at a type I error rate of 0.05 (two-sided). Key secondary end points of the time to PSA progression and the time to the first use of a subsequent antineo-plastic therapy and the first interim analysis of overall survival were evaluated at the time of the primary analysis. To maintain the family-wise two-sided type I error rate at 0.05, a parallel testing strategy between overall survival (with an allocated type I error rate of 0.03) and the remaining key secondary end points (with an allocated type I error rate of 0.02 with sequential testing in hierarchical order) was used. If the differences in the remaining key secondary end points were significant at the 0.02 level, overall survival was to be allocated an overall type I error rate of 0.05. The first interim analysis for overall survival, the results of which are presented in this article, was conducted at the 0.001 significance level. The final analysis of overall survival has not yet been performed (see the protocol).
The trial groups were compared with the use of a log-rank test with stratification according to the same factors that were used in randomization. The Kaplan–Meier method was used to estimate medians. A stratified Cox regression model was used to estimate hazard ratios and 95% confidence intervals.
RESULTS
TRIAL PATIENTS
From November 26, 2013, to June 28, 2017, a total of 2874 patients underwent screening; 1401 eligible patients were enrolled and underwent randomization (933 patients to the enzalutamide group and 468 to the placebo group). Enrollment was halted after 447 primary end-point events occurred. The demographic and clinical characteristics of the patients at baseline were well balanced (Table 1). The median PSA doubling time among all the patients was 3.7 months. A total of 1395 patients received at least one dose of enzalutamide or placebo (Fig. S1 in the Supplementary Appendix). The median duration of the trial regimen was 18.4 months in the enzalutamide group and 11.1 months in the placebo group. At the data-cutoff date, 810 patients were receiving the trial regimen (634 patients in the enzalutamide group and 176 in the placebo group). The most common reason for discontinuation was disease progression (in 15% of the patients in the enzalutamide group and 44% of those in the placebo group), followed by adverse events (in 10% and 6%, respectively) (Fig. S1 in the Supplementary Appendix).
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Enzalutamide Group (N = 933) | Placebo Group (N = 468) |
|---|---|---|
| Age — yr | ||
| Median | 74 | 73 |
| Range | 50–95 | 53–92 |
| ECOG performance-status score — no. (%)† | ||
| 0 | 747 (80) | 382 (82) |
| 1 | 185 (20) | 85 (18) |
| Missing data | 1 (<1) | 1 (<1) |
| Serum PSA value — ng/ml | ||
| Median | 11.1 | 10.2 |
| Range | 0.8–1071.1 | 0.2–467.5 |
| PSA doubling time | ||
| Median — mo | 3.8 | 3.6 |
| Range — mo | 0.4–37.4 | 0.5–71.8 |
| Distribution — no. (%) | ||
| <6 mo | 715 (77) | 361 (77) |
| ≥6 mo | 217 (23) | 107 (23) |
| Missing data | 1 (<1) | 0 |
| Use of bone-targeting agent — no. (%) | ||
| No | 828 (89) | 420 (90) |
| Yes | 105 (11) | 48 (10) |
There were no significant between-group differences in these characteristics at baseline. Percentages may not total 100 because of rounding. PSA denotes prostate-specific antigen.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are on a scale from 0 to 5, with higher scores indicating greater disability and a score of 5 indicating death.
PRIMARY END POINT
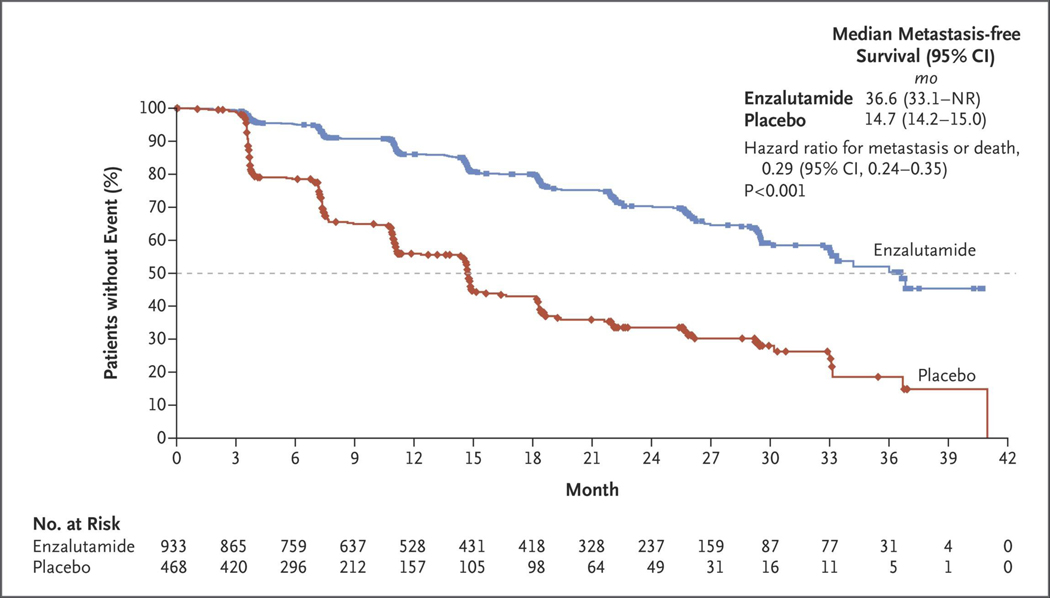
At the time of data cutoff, 219 patients (23%) in the enzalutamide group and 228 (49%) in the placebo group had had a primary end-point event. The median metastasis-free survival was 36.6 months in the enzalutamide group versus 14.7 months in the placebo group, with a median follow-up of 18.5 months and 15.1 months, respectively. Enzalutamide treatment resulted in a 71% lower risk of radiographic progression or death than did placebo (hazard ratio, 0.29; 95% CI, 0.24 to 0.35; P<0.001) (Fig. 1 and Table 2). The results of a prespecified sensitivity analysis in which deaths without radiographic progression were included regardless of timing were consistent with the results of the primary analysis of metastasis-free survival (hazard ratio for radiographic progression or death, 0.30; 95% CI, 0.25 to 0.36) (Table S1 in the Supplementary Appendix).
Figure 1. Kaplan–Meier Estimate of Metastasis-free Survival.
Shown are data for the primary end point of metastasis-free survival. The dashed line indicates the median. The hazard ratio was based on a Cox regression model that was stratified according to the prostate-specific antigen (PSA) doubling time (<6 months vs. ≥6 months) and previous or current use of a bone-targeting agent (yes vs. no), with trial group as the only covariate and a value less than 1.00 favoring enzalutamide treatment. Symbols indicate censored data. NR denotes not reached.
Table 2.
Primary and Secondary End Points.*
| End Point | Enzalutamide Group (N = 933) | Placebo Group (N = 468) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Primary end point | ||||
| Median metastasis-free survival — mo | 36.6 | 14.7 | 0.29 (0.24–0.35) | <0.001 |
| Metastasis or death — no. (%)† | 219 (23) | 228 (49) | — | — |
| Radiographic progression — no./total no. (%) | 187/219 (85) | 224/228 (98) | — | — |
| New bone metastases | 71/219 (32) | 79/228 (35) | — | — |
| New soft-tissue metastases | 109/219 (50) | 132/228 (58) | — | — |
| Metastases to lymph node | 79/219 (36) | 116/228 (51) | — | — |
| Visceral metastases | 34/219 (16) | 27/228 (12) | — | — |
| Concurrent new bone and soft-tissue metastases | 7/219 (3) | 13/228 (6) | — | — |
| Metastases to lymph node | 7/219 (3) | 12/228 (5) | — | — |
| Visceral metastases | 3/219 (1) | 1/228 (<1) | — | — |
| Death — no./total no (%)† | 32/219 (15) | 4/228 (2) | — | — |
| Secondary end points | ||||
| PSA progression | ||||
| Median time to progression — mo | 37.2 | 3.9 | 0.07 (0.05–0.08) | <0.001 |
| Patients with progression — no. (%) | 208 (22) | 324 (69) | — | — |
| Use of subsequent antineoplastic therapy | ||||
| Median time to first use — mo | 39.6 | 17.7 | 0.21 (0.17–0.26) | <0.001 |
| Patients with use — no. (%) | 142 (15) | 226 (48) | — | — |
| Overall survival | ||||
| Median survival — mo | NR | NR | 0.80 (0.58–1.09) | 0.15 |
| Patients who died — no. (%) | 103 (11) | 62 (13) | — | — |
| Confirmed PSA response ≥50% — no. (%) | 712 (76) | 11 (2) | — | — |
| FACT-P score degradation‡ | ||||
| Median time to score degradation — mo | 11.1 | 11.1 | 0.92 (0.79–1.08) | — |
| Patients with score degradation — no. (%) | 506 (54) | 239 (51) | — | — |
In the analysis of metastasis-free survival, the hazard ratio is for metastasis or death. In the analysis of overall survival, the hazard ratio is for death. NR denotes not reached.
Death was defined as death without evidence of radiographic progression that occurred in the period from randomization to 112 days after the discontinuation of the trial regimen. Causes of death are presented in Table S2 in the Supplementary Appendix.
Scores on the Functional Assessment of Cancer Therapy–Prostate (FACT-P) scale range from 0 to 156, with higher scores indicating more favorable health-related quality of life. Degradation in the FACT-P score was defined as a decrease of at least 10 points from baseline in the global score for each patient.
Of the 219 patients in the enzalutamide group who had a primary end-point event, 187 (85%) had radiographic progression and 32 (15%) died without radiographic progression. Of the 228 patients in the placebo group who had a primary end-point event, 224 (98%) had radiographic progression and 4 (2%) died without radiographic progression (Table 2). Of the 32 deaths without radiographic progression that occurred in the enzalutamide group, 2 were considered by the investigator to be related to the trial drug; there was no trend with respect to cause (Table S2 in the Supplementary Appendix). The median age of the patients who died without radiographic progression was 80 years in the enzalutamide group and 81 years in the placebo group.
More than half the cases of radiographic progression were in soft tissue (in 109 of 187 patients [58%] in the enzalutamide group and in 132 of 224 [59%] in the placebo group). The treatment effect of enzalutamide was consistent across all the prespecified subgroups (Fig. S2 in the Supplementary Appendix).
SECONDARY END POINTS
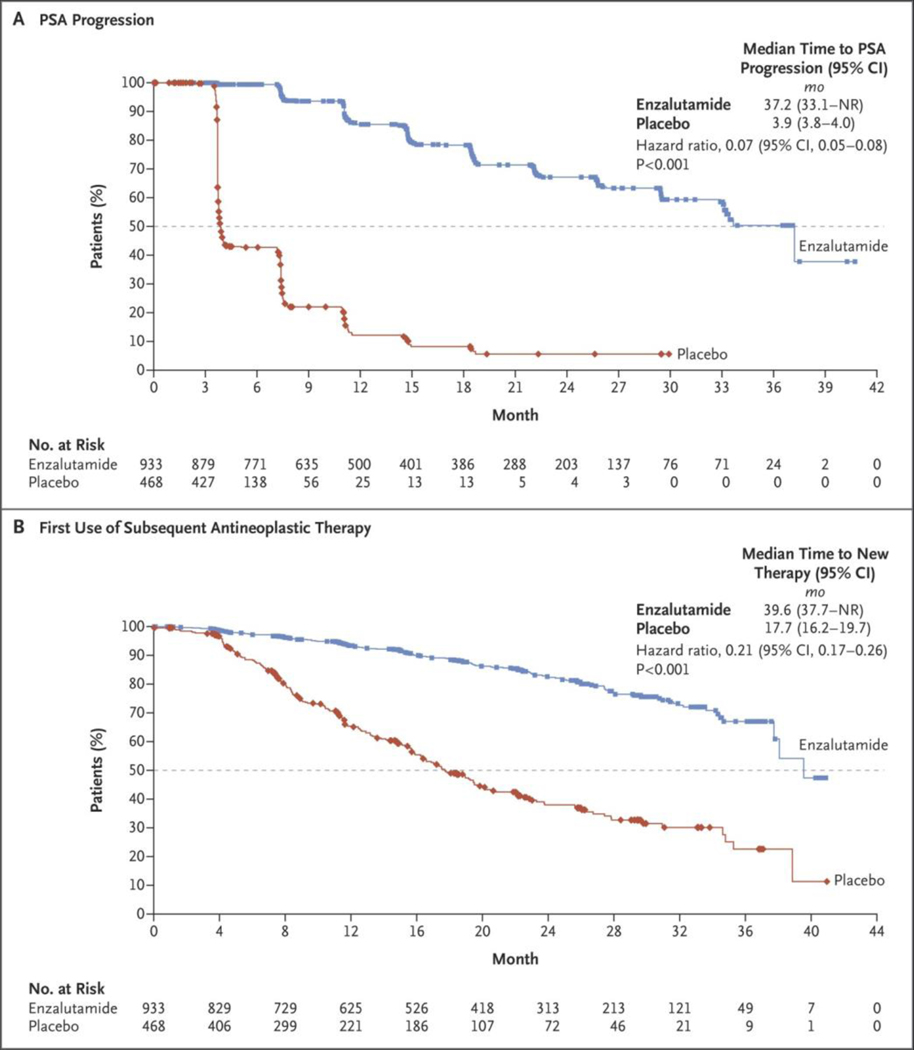
Enzalutamide treatment was superior to placebo with regard to the key secondary end points of time to PSA progression and time to the first use of a subsequent antineoplastic therapy (Table 2 and Fig. 2). The median interval between the discontinuation of the trial regimen and subsequent antineoplastic therapy was 25 days in the enzalutamide group and 18 days in the placebo group. A total of 138 patients (15%) in the enzalutamide group and 222 (48%) in the placebo group discontinued the trial regimen and received subsequent antineoplastic therapy. The most common subsequent therapy was abiraterone acetate (in 52 of 138 patients [38%] in the enzalutamide group and in 81 of 222 [36%] in the placebo group) (Table S3 in the Supplementary Appendix).
Figure 2. Kaplan–Meier Estimates of the Time to PSA Progression and the Time to the First Use of Subsequent Antineoplastic Therapy.
Shown are data for the secondary efficacy end points: the time to PSA progression (Panel A) and the time to the first use of a subsequent antineoplastic therapy (Panel B). The dashed line in each panel indicates the median. Hazard ratios were based on Cox regression models that were stratified according to the PSA doubling time (<6 months vs. ≥6 months) and previous or current use of a bone-targeting agent (yes vs. no), with trial group as the only covariate and values less than 1.00 favoring enzalutamide treatment. Symbols indicate censored data.
At the first interim analysis of overall survival, 103 patients (11%) in the enzalutamide group and 62 (13%) in the placebo group had died (Table 2). The median overall survival was not reached in either group (Table 2, and Fig. S3 in the Supplementary Appendix). The rate of PSA response of 50% or more was higher in the enzalutamide group than in the placebo group. The median time to degradation in the Functional Assessment of Cancer Therapy–Prostate score, indicating a clinically meaningful decrease in health-related quality of life, was the same in the two groups.
SAFETY
Adverse events regardless of relatedness to the trial regimen are reported in Table 3. The median reporting period for adverse events was 18.0 months in the enzalutamide group and 11.1 months in the placebo group. Adverse events of grade 3 or higher were reported in a higher percentage of patients in the enzalutamide group than in the placebo group. Discontinuations of the trial regimen due to adverse events were more frequent in the enzalutamide group than in the placebo group.
Table 3.
Adverse Events.
| Event | Enzalutamide Group (N = 930) | Placebo Group (N = 465) | ||
|---|---|---|---|---|
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| number of patients (percent) | ||||
| Any adverse event | 808 (87) | 292 (31) | 360 (77) | 109 (23) |
| Any serious adverse event* | 226 (24) | — | 85 (18) | — |
| Adverse event leading to discontinuation of trial regimen | 87 (9) | — | 28 (6) | — |
| Adverse event leading to death | 32 (3) | — | 3 (1) | — |
| Most common adverse events, occurring in ≥5% of patients† | ||||
| Fatigue | 303 (33) | 27 (3) | 64 (14) | 3 (1) |
| Hot flush | 121 (13) | 1 (<1) | 36 (8) | 0 |
| Nausea | 106 (11) | 3 (<1) | 40 (9) | 0 |
| Diarrhea | 91 (10) | 3 (<1) | 45 (10) | 2 (<1) |
| Hypertension | 111 (12) | 43 (5) | 24 (5) | 10 (2) |
| Fall | 106 (11) | 12 (1) | 19 (4) | 3 (1) |
| Constipation | 85 (9) | 2 (<1) | 32 (7) | 2 (<1) |
| Dizziness | 91 (10) | 4 (<1) | 20 (4) | 0 |
| Arthralgia | 78 (8) | 1 (<1) | 32 (7) | 1 (<1) |
| Asthenia | 82 (9) | 11 (1) | 28 (6) | 1 (<1) |
| Decreased appetite | 89 (10) | 2 (<1) | 18 (4) | 1 (<1) |
| Back pain | 73 (8) | 2 (<1) | 33 (7) | 1 (<1) |
| Headache | 85 (9) | 2 (<1) | 21 (5) | 0 |
| Hematuria | 62 (7) | 16 (2) | 36 (8) | 13 (3) |
| Urinary tract infection | 38 (4) | 7 (1) | 30 (6) | 3 (1) |
| Weight loss | 55 (6) | 2 (<1) | 7 (2) | 0 |
| Urinary retention | 20 (2) | 4 (<1) | 28 (6) | 5 (1) |
| Adverse events of special interest | ||||
| Hypertension‡ | 114 (12) | 43 (5) | 25 (5) | 11 (2) |
| Major adverse cardiovascular event§ | 48 (5) | 34 (4) | 13 (3) | 8 (2) |
| Mental impairment disorders¶ | 48 (5) | 1 (<1) | 9 (2) | 0 |
| Hepatic impairment | 11 (1) | 5 (1) | 9 (2) | 2 (<1) |
| Neutropenia | 9 (1) | 5 (1) | 1 (<1) | 1 (<1) |
| Convulsion | 3 (<1) | 2 (<1) | 0 | 0 |
| Posterior reversible encephalopathy syndrome | 0 | 0 | 0 | 0 |
Serious adverse events were events that resulted in death, were life-threatening, resulted in or prolonged hospitalization, resulted in inability to conduct normal life functions, or led to a congenital anomaly or birth defect. A full definition is provided in the protocol.
Listed in descending order are the adverse events that were reported in at least 5% of the patients in either group.
This adverse event includes increased blood pressure.
This adverse event includes acute myocardial infarction, hemorrhagic cerebrovascular conditions, ischemic cerebrovascular conditions, and heart failure.
This adverse event includes memory impairment, disturbance in attention, cognitive disorders, amnesia, Alzheimer’s disease, senile dementia, mental impairment, and vascular dementia.
The most common adverse event in patients receiving enzalutamide was fatigue (Table 3). Adverse events of special interest that occurred more frequently (by ≥2 percentage points) in the enzalutamide group than the placebo group, regardless of relatedness to the trial regimen, were hypertension (in 12% vs. 5%), major adverse cardiovascular events (in 5% vs. 3%), and mental impairment disorders (in 5% vs. 2%). Although no events of the posterior reversible encephalopathy syndrome were reported during the trial (Table 3), five patients receiving enzalutamide were identified as having “noninfectious encephalopathy or delirium” (three patients with delirium, one with encephalopathy, and one with leukoencephalopathy). Three patients in the enzalutamide group had convulsions, all of which were considered to be serious and drug-related and occurred within 180 days after the initiation of the trial drug. One patient with convulsions discontinued enzalutamide treatment. Another patient with convulsions had a complication that led to death. A higher percentage of patients receiving enzalutamide reported falls and nonpathologic fractures than did those receiving placebo (17% vs. 8%).
The most common adverse events leading to death were cardiac events (in nine patients [1%] receiving enzalutamide and two [<1%] receiving placebo). In the enzalutamide group, acute myocardial infarction occurred in six patients, and cardiac failure, cardiorespiratory arrest, and ventricular arrhythmia occurred in one patient each. In the placebo group, cardiac arrest and left ventricular failure occurred in one patient each. In each trial group, the incidence of major adverse cardiac events was higher among patients who had a history of cardiovascular disease, hypertension, diabetes mellitus, or hyperlipidemia at baseline or who were 75 years of age or older than among patients without those characteristics.
DISCUSSION
Among men with nonmetastatic, castration-resistant prostate cancer and a rapidly rising PSA value, the end points of the time to metastasis-free survival, the time to PSA progression, and the time to the first use of a subsequent antineoplastic therapy were significantly longer with enzalutamide treatment than with placebo (P<0.001 for all comparisons). Enzalutamide treatment was associated with a 71% lower risk of metastasis or death than placebo. A consistent benefit with regard to metastasis-free survival in favor of enzalutamide treatment was observed in all the prespecified subgroups. There was no decrease in quality of life associated with enzalutamide treatment.
Although the median overall survival was not reached in either group, in the first of several prespecified interim analyses, the risk of death was 20% lower with enzalutamide treatment than with placebo. This result did not reach statistical significance.
The median metastasis-free survival in the placebo group in our trial was more than 10 months shorter than in placebo groups of previous clinical trials involving men with progressive, nonmetastatic, castration-resistant prostate cancer. However, previous trials had no restriction regarding the PSA doubling time at baseline and only bone metastases were evaluated.1,2 In our trial, the median baseline PSA level was greater than 10 ng per milliliter, and the median PSA doubling time was 3.7 months, with 77% of the patients having a PSA doubling time of less than 6 months — all of which are factors that have been associated with a worse prognosis.2,3 In addition, all the metastases were evaluated.
The overall rate of death from any cause was lower in the enzalutamide group than in the placebo group (103 of 933 patients [11%] vs. 62 of 468 [13%] died). Among patients who died without evidence of radiographic progression, there was no trend regarding the cause of death (Table S2 in the Supplementary Appendix); only two deaths were considered to be related to enzalutamide treatment. Most of the deaths followed an acute event that was considered by the investigators to be unrelated to the trial regimen and that occurred in elderly patients with a high burden of coexisting conditions.
The safety profile of enzalutamide was consistent with that reported in previous clinical trials involving men with castration-resistant prostate cancer.4,13,14,16 Adverse events of grade 3 or higher were reported at a higher rate among patients in the enzalutamide group than among those in the placebo group. Cardiovascular events were reported more frequently in the enzalutamide group than in the placebo group. Hypertension, myocardial infarction, fatigue, falls, and fractures were more common in patients who received enzalutamide than in those who received placebo. Further safety analysis is ongoing to assess whether a subgroup of patients may be at higher risk for these adverse events.
The management of nonmetastatic, castration-resistant prostate cancer has historically involved watchful waiting or the use of agents that have shown modest activity and have not shown benefits regarding overall survival. The goal of treatment is to delay the progression to metastatic disease meaningfully, to mitigate cancer-related symptoms, and to prolong overall survival. Three previous phase 3 trials have evaluated experimental agents using bone metastasis–free survival or overall metastasis-free survival as the primary end point, with little delay in progression.3,17,18
In conclusion, in men with nonmetastatic, castration-resistant prostate cancer and a rapidly rising PSA value, enzalutamide treatment resulted in a significant delay in the development of metastases, in the time to the first use of a subsequent antineoplastic therapy, and in the time to PSA progression, with no difference in quality of life between the enzalutamide group and the placebo group. Adverse events were more common with enzalutamide treatment than with placebo.
Supplementary Material
Acknowledgments
Supported by Pfizer and Astellas Pharma.
Dr. Hussain reports receiving honoraria and travel fees from Sanofi, honoraria from OncLive and Physicians’ Education Resource, and grant support from AstraZeneca, Pfizer, Bayer, and Genentech; Dr. Fizazi, receiving honoraria and consulting fees from Amgen, Astellas Pharma, Bayer, Clovis Oncology, CureVac, Janssen Oncology, Orion, and Sanofi; Dr. Saad, receiving grant support, honoraria, and consulting fees from Janssen Oncology, Sanofi, Astellas Pharma, Bayer, and Pfizer; Dr. Shore, receiving grant support and consulting fees from AbbVie, Amgen, Astellas Pharma, Bayer, Dendreon Pharmaceuticals, Ferring Pharmaceuticals, Janssen Oncology, Pfizer, Sanofi–Genzyme and Tolmar Pharmaceuticals; Drs. Demirhan and Modelska, being employed by Medivation (a Pfizer company); Mr. Phung, being employed by Astellas Pharma; Dr. Krivoshik, being employed by Astellas Pharma, owning stock in AbbVie and Abbott, and holding a patent (U.S. patent number, 20090149461), licensed to Abbott–AbbVie, on a method of treating cancer and a patent (European patent number, 08856828.2–2123; U.S. patent number, 2008085628), licensed to Abbott–AbbVie, on oral compositions of ABT-263 for treating cancer; and Dr. Sternberg, receiving honoraria from Janssen Oncology, Astellas Pharma, and AstraZeneca, consulting fees from Clovis Oncology, Bayer, Ferring Pharmaceuticals, and Pfizer, honoraria and consulting fees from Sanofi, and grant support from Medivation.
We thank the patients, their caregivers and families, the investigators, and the trial personnel; Mohammad Hirmand, M.D., a former employee of Medivation, for contributions to the trial design; and Stephanie Vadasz, Ph.D., and Shannon Davis, B.A., of Ashfield Healthcare Communications for medical writing and editorial assistance with an earlier version of the manuscript.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Maha Hussain, Comprehensive Cancer Center, Feinberg School of Medicine, Northwestern University, Chicago
Karim Fizazi, Institut Gustave Roussy, University of Paris Sud, Villejuif, France
Fred Saad, University of Montreal Hospital Center, Montreal
Per Rathenborg, Herlev Hospital, Herlev, Denmark
Neal Shore, Carolina Urologic Research Center, Myrtle Beach, SC
Ubirajara Ferreira, State University of Campinas (Unicamp), Campinas, Brazil
Petro Ivashchenko, Kiev City Clinical Hospital 3, Kiev, Ukraine
Eren Demirhan, Pfizer, San Francisco
Katharina Modelska, Pfizer, San Francisco
De Phung, Astellas Pharma, Northbrook — both in Illinois
Andrew Krivoshik, Astellas Pharma, Northbrook — both in Illinois
Cora N. Sternberg, Department of Medical Oncology, San Camillo Forlanini Hospital, Rome
REFERENCES
- 1.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate non-metastatic prostate cancer. J Clin Oncol 2005; 23: 2918–25. [DOI] [PubMed] [Google Scholar]
- 2.Smith MR, Cook R, Lee KA, Nelson JB. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer 2011; 117: 2077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MR, Saad F, Oudard S, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol 2013; 31: 3800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371: 424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshar M, Evison F, James ND, Patel P. Shifting paradigms in the estimation of survival for castration-resistant prostate cancer: a tertiary academic center experience. Urol Oncol 2015; 33(8): 338.e1–7. [DOI] [PubMed] [Google Scholar]
- 6.Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017; 35: 3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: prostate cancer. Fort Washington, PA: NCCN, 2016. (https://www.tri-kobe.org/nccn/guideline/urological/english/prostate.pdf). [Google Scholar]
- 8.Scher HI, Liebertz C, Kelly WK, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol 1997; 15: 2928–38. [DOI] [PubMed] [Google Scholar]
- 9.Lodde M, Lacombe L, Fradet Y. Salvage therapy with bicalutamide 150 mg in nonmetastatic castration-resistant prostate cancer. Urology 2010; 76: 1189–93. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Okihara K, Miyake H, et al. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol 2008; 180: 921–7. [DOI] [PubMed] [Google Scholar]
- 11.Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V. Prostate cancer: ESMO Consensus Conference guidelines 2012. Ann Oncol 2013; 24: 1141–62. [DOI] [PubMed] [Google Scholar]
- 12.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324:787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367: 1187–97. [DOI] [PubMed] [Google Scholar]
- 14.Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol 2016; 34: 2098–106. [DOI] [PubMed] [Google Scholar]
- 15.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281: 1591–7. [DOI] [PubMed] [Google Scholar]
- 16.Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 2016; 17: 153–63. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 2008; 113: 2478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller K, Moul JW, Gleave M, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2013; 16: 187–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.