Abstract
Improving early cancer detection has the potential to significantly reduce cancer-related mortality. Cell-free methylated DNA immunoprecipitation and high-throughput sequencing is a highly sensitive assay capable of detecting early-stage tumors. We report accurate classification of patients across all stages of renal cell carcinoma (RCC) in plasma (AUROC 0.99) and demonstrate the validity of this assay to identify patients with RCC using urine cell-free DNA (AUROC 0.86).
Identifying tumor-specific alterations in cell-free DNA (cfDNA) presents a powerful opportunity to potentially reduce cancer-related mortality through early detection. Although the ability to identify tumor-derived cfDNA in patients with metastatic cancer is established, assays with sufficient sensitivity to detect localized disease are now emerging. With the development of these highly sensitive assays1–4, the field is at a tipping point of utilizing non-invasive biomarkers in clinical practice for early cancer detection. To ensure efficient and rapid translation to clinical practice, it is imperative that promising methods are independently validated to maximize clinical and analytical validity. Ideal tests will be sufficiently sensitive to detect localized disease, accurate, cost-effective, and easily implemented in clinical practice. We recently published a cell-free methylated DNA immunoprecipitation and high-throughput sequencing assay (cfMeDIP-seq) that satisfies these criteria1,5. cfMeDIP-seq is an enrichment-based method for comprehensive cfDNA methylation profiling, demonstrated to be highly sensitive for detecting and classifying several tumor types across localized and metastatic disease1,5. Additional benefits include low DNA input (≤ 10 ng) and balance of genome coverage, resolution, and cost1,5.
Despite the fact that approximately 35% of renal cell carcinoma (RCC) cases are diagnosed when the disease has spread beyond the kidney, no FDA-approved blood or radiologic screening test exists for RCC screening in the general population. Of all extra-cranial tumors, RCC sheds the least amount of cfDNA6; accordingly, two large studies of cfDNA in advanced RCC only identified genomic alterations in fewer than 80% of patients6,7. In contrast, two small studies focused on individual genes highlight the potential of cfDNA methylation analysis for detection of early-stage RCC8,9. In the first genome-wide cfDNA methylation analysis that included patients with early-stage RCC, cfMeDIP-seq achieved an area under the receiver operating characteristic (AUROC) curve of approximately 0.9 for detecting and classifying RCC patients from patients with other tumor types and healthy controls1.
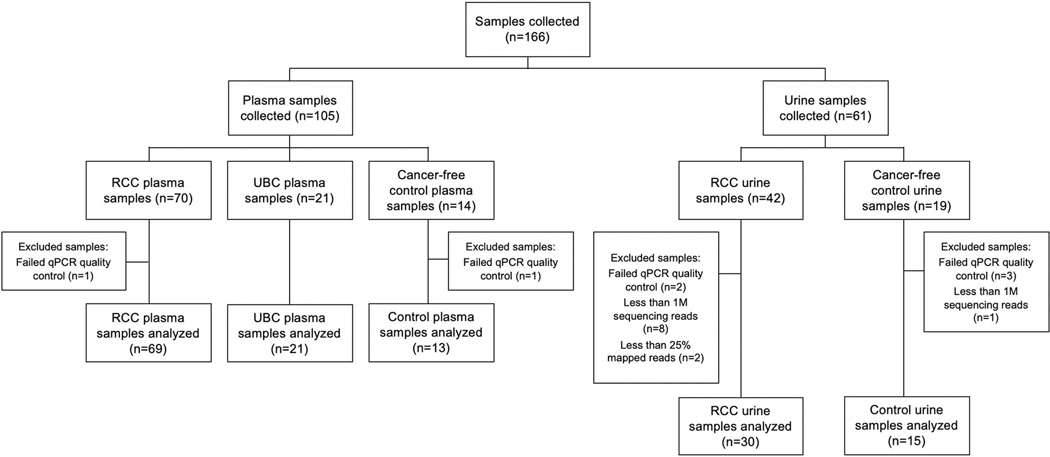
Herein, we report the first independent validation of cfMeDIP-seq for detection of RCC with an emphasis on early-stage disease. We also report the first application of cfMeDIP-seq on urine cfDNA for cancer detection. We performed cfMeDIP-seq on 148 samples: N=99 stage I-IV RCC cases, N=21 stage IV urothelial bladder cancer (UBC) samples from 15 patients, and N=28 healthy cancer-free controls. Notably, 33.3% of plasma samples and 66.7% of urine samples came from patients with TNM Stage I/II disease (Extended Data Fig. 1; Supplementary Tables 1–3).
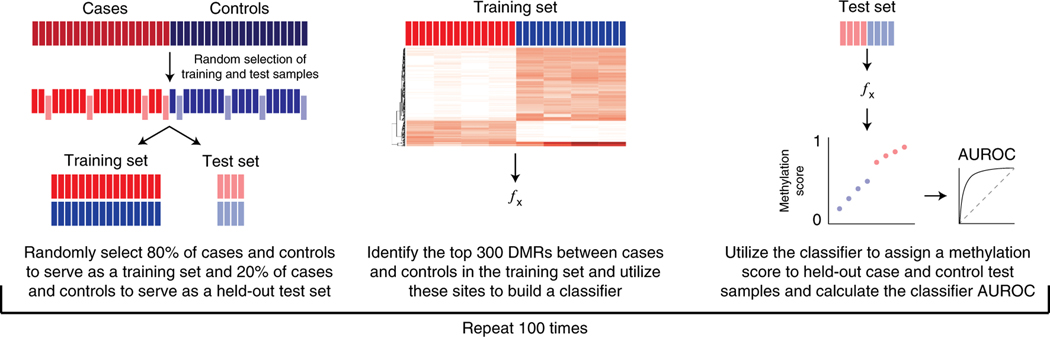
We first performed cfMeDIP-seq on plasma cfDNA and identified differentially methylated regions (DMRs) between patient groups following previously published methods1,5. To evaluate the ability of cfDNA DMRs to accurately classify patient samples, we first randomly selected 80% of case and control samples for a training set and selected the top 300 DMRs between case and control training samples. We then built a classifier based on the top 300 DMRs, which was used to assign a methylation score to the withheld samples. This process was repeated with 100 randomly-selected training-test sets (Fig.1).
Figure 1:
Summary figure describing the analytical methods used to classify samples based on differential methylation in cfDNA.
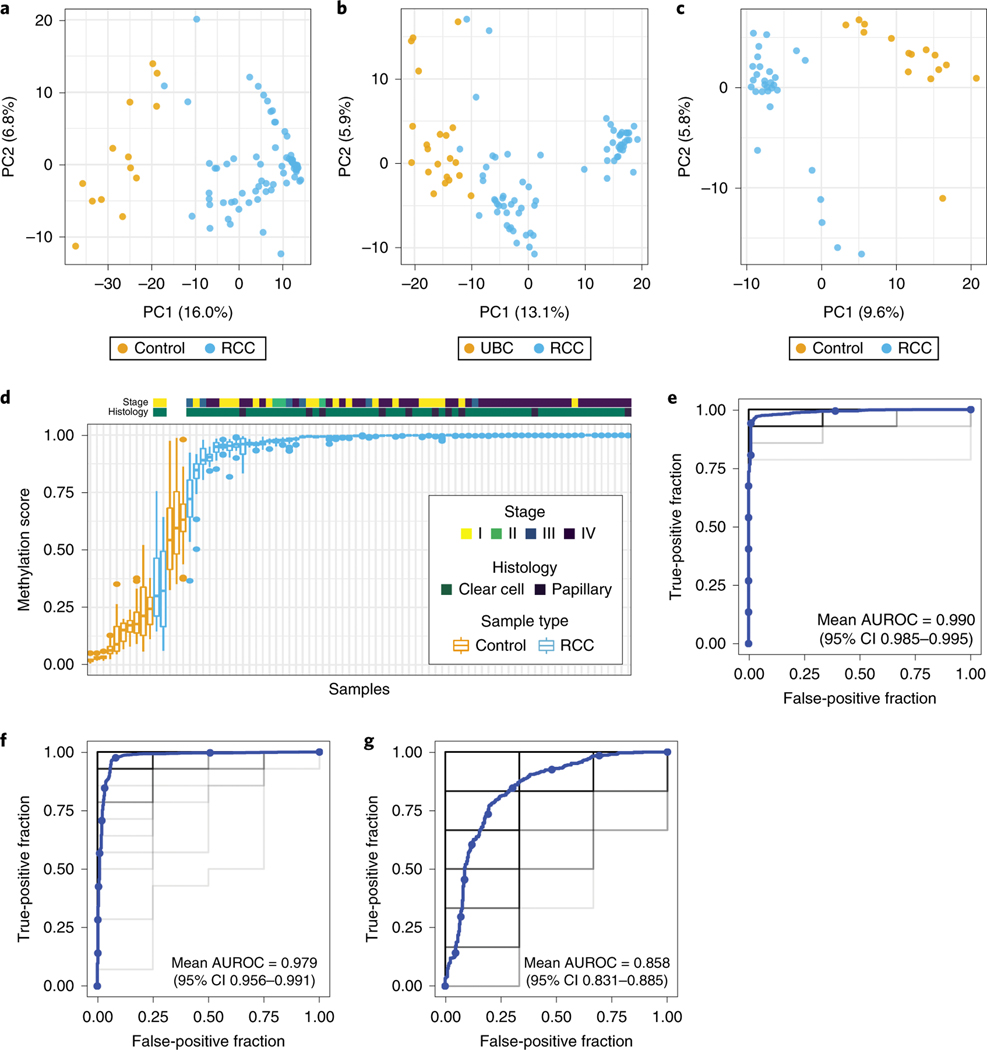
The top 300 DMRs cleanly partitioned plasma RCC and control samples (Fig. 2a; Extended Data Fig. 2–3), plasma RCC and UBC samples (Fig. 2b), and urine RCC and control samples (Fig. 2c). Across 100 training-test sets, 67/69 (97.1%) RCC samples were assigned a higher median methylation score than all control samples (Fig. 2d), culminating in a mean AUROC of 0.990 (95% confidence interval [CI] 0.985–0.995) (Fig. 2e). The two RCC cases with the lowest methylation score were both small (T1a) stage I clear cell tumors, however, there was no statistical association between RCC stage (p=0.09) or histology (p=0.38) and methylation score. To evaluate classification performance between different genitourinary tumors, we performed the same analysis comparing patients with RCC and UBC, resulting in a mean AUROC of 0.979 (95% CI 0.956–0.991) (Fig. 2f). To investigate the performance of cfMeDIP-seq on urine, we conducted the same analyses on urine cfDNA from patients with RCC and healthy controls. The mean AUROC of 100 training-test sets was 0.858 (95% CI 0.831–0.885) (Fig. 2g).
Figure 2:
Classification of RCC using cfDNA methylomes. The top 300 DMRs between groups were used to generate principal component (PC) plots for plasma RCC versus control samples (a), plasma RCC versus UBC samples (b), and urine RCC versus control samples (c). Boxplot of methylation scores of individual plasma RCC and control samples from n=100 training-test sets (d). Boxplots are displayed with a median center line, box range of 25th to 75th percentile, and whiskers extending to the most extreme observation within 1.5 times the interquartile range (IQR); points outside the IQR are shown individually. AUROC curves for classifiers generated from n=100 training-test sets comparing plasma RCC versus control samples (e), plasma RCC versus UBC samples (f), and urine RCC versus control samples (g). Individual AUROC curves are shown in light grey with more overlapping curves indicated by darker shades of grey/black along with a smoothed mean curve in blue.
Different approaches have been developed for non-invasive cancer detection1–4 and the optimal assay will depend on the clinical scenario under investigation. In RCC, the performance of genetic- and epigenetic-based cfDNA tests may reflect the prevalence of recurrently altered features. Comprehensive molecular characterization of RCC tumors in TCGA identified 289 genes with aberrant DNA methylation compared to only 19 recurrently mutated genes10. Accordingly, genomic alterations were observed in plasma cfDNA in fewer than 80% of patients with advanced RCC6,7. Further, these studies did not account for false positive results from clonal hematopoiesis or germline alterations, which likely inflates detection rates by up to 25%11. In comparison, we report near-perfect epigenetic classification of patients across all stages of RCC using cfMeDIP-seq.
Given the ease of routine clinical sampling, urinary cfDNA is an attractive source for non-invasive cancer detection. To our knowledge, no genome-wide analysis of urine cfDNA has been conducted to detect RCC. Herein, we report that cfMeDIP-seq has an AUROC of 0.858 for correctly classifying urine RCC and control samples in a cohort where two-thirds of RCC patients had localized disease. Notably, these data was generated using a protocol optimized for plasma. While urine-based classification was not as accurate as plasma, we believe that performance can be improved through technical and computational optimization, such as size selection of cfDNA to enrich for tumor-derived DNA and utilizing tumor methylation data to inform cfDNA methylation analysis. Nevertheless, these results highlight the potential value of urine cfMeDIP-seq for early detection of localized RCC. Studies to optimize urine cfDNA accuracy are ongoing.
RCC is a malignancy with cancer-related mortality of more than 15,000 lives per year in the US alone. There is no clinically-validated non-invasive biomarker for cancer detection. Imaging studies are expensive, expose patients to unnecessary radiation, and do not always accurately distinguish benign from malignant kidney tumors12. Screening protocols in patients with known heritable syndromes associated with development of RCC, such as Von Hippel-Lindau Syndrome, rely on imaging. Further, incidental renal masses are seen on up to 1 in 4 abdominal imaging studies13. An accurate, highly sensitive and specific non-invasive test – alone or in combination with imaging – could transform clinical management by enabling early detection and reducing unnecessary kidney biopsies and nephrectomies.
In summary, we performed the first independent validation of plasma cfMeDIP-seq, demonstrating very accurate classification of cancer-free controls and patients with RCC in a cohort containing patients with early-stage disease. We also extended the potential of cfDNA methylome-based biomarkers for cancer detection in urine. After training on larger datasets and performing prospective validation, this method could ultimately reduce morbidity and mortality through early and accurate detection of RCC and other cancers.
Methods
Patient selection
Patients with a histologically confirmed diagnosis of renal cell carcinoma (RCC) and urothelial bladder cancer (UBC) diagnosed and treated at the Dana-Farber Cancer Institute, Brigham and Women’s Hospital, or Beth Israel Deaconess Medical Center between February 2005 and November 2017 were selected for this study. All patients provided written informed consent, and the use of samples was approved by the Institutional Review Board (01–130/11–104/17–000), following all relevant ethical regulations. Stage was determined using the contemporaneous American Joint Committee on Cancer (AJCC) staging system. Urine samples from healthy individuals being considered for living kidney donation were obtained during a donor screening visit in the Nephrology Division at Brigham and Women’s Hospital. Individuals had no history of diabetes, cancer, or major medical illnesses that would preclude a living donation. All healthy donors provided written informed consent, and all samples were obtained with approval of the Brigham and Women’s Hospital Institutional Review Board 2010P00270, according to ethical regulations.
Sample processing and cfDNA isolation
Samples from all cases and controls were processed by the following method. Peripheral blood was collected in EDTA Vacutainer tubes (BD), and processed within 3 hours of collection. Plasma was separated by centrifugation at 2,500 × g for 10 minutes, transferred to microcentrifuge tubes, and centrifuged at 2,500 × g at room temperature for 10 minutes, to remove cellular debris. The supernatant was aliquoted into 1–2 mL aliquots and stored at −80°C until the time of DNA extraction. cfDNA was isolated from 1 mL of plasma, using the Qiagen Circulating Nucleic Acids Kit (Qiagen GmbH), then incubated with proteinase K for 30 minutes at 60°C. cfDNA was eluted in 50 μl AE buffer and stored at −80°C. DNA concentration was measured using the Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher).
Urine from RCC and healthy patients was collected in a sterile urine specimen container and processed within 3 hours of collection. Urine was centrifuged at 2,500 × g for 10 minutes, and the supernatant was aliquoted into 1–2 mL aliquots and stored at −80°C until the time of DNA extraction. cfDNA was isolated from 2 mL of urine using the Qiagen genomic DNA extraction Kit (Qiagen GmbH) and incubated with proteinase K for 2 hours at 56°C. cfDNA was eluted with AE buffer and stored at −80°C. DNA concentration was measured using the Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher).
cfMeDIP–seq protocol
cfMeDIP-seq was performed using previously published methods1,5. cfDNA library preparation was performed using KAPA HyperPrep Kit (KAPA Biosystems) according to the manufacturer’s protocol. In brief, after end-repair and A-tailing, samples were ligated to 18.1 nM per 1 ng of cfDNA of NEBNext adaptor (NEBNext Multiplex Oligos for Illumina kit, New England BioLabs) by incubating at 20°C for 20 minutes and were purified with AMPure XP beads (Beckman Coulter). Eluted libraries were digested using the USER enzyme (New England BioLabs), followed by purification with AMPure XP beads (Beckman Coulter). λ DNA was added to prepared libraries to achieve a total amount of 100 ng DNA. MeDIP was performed using the MagMeDIP kit (Diagenode). This DNA consists of a mixture of unmethylated and in vitro methylated λ amplicons of different CpG densities, similar in size to adaptor-ligated cfDNA libraries. 0.3 ng of methylated and unmethylated Arabidopsis thaliana DNA was added for quality control (Diagenode). DNA was heated to 95°C for 10 minutes, then incubated in an ice water bath for 10 minutes. Samples were partitioned into two 0.2 ml PCR tubes: 10% input control (7.5 μL) and 90% (75 μl) for immunoprecipitation, which was performed using the MagMeDIP kit (Diagenode) following the manufacturer’s protocol. Samples were purified using the iPure Kit v2 (Diagenode), and eluted in 50 μL of Buffer C. The success of the immunoprecipitation was confirmed using qPCR to detect recovery of the spiked-in methylated and unmethylated Arabidopsis thaliana DNA (Diagenode) per manufacturer’s instructions. Samples that did not pass the quality control threshold of <1% recovery of unmethylated control DNA and >99% recovery of methylated control DNA were excluded.
Next-generation sequencing library construction
KAPA HiFi Hotstart ReadyMix (KAPA Biosystems) and NEBNext Multiplex Oligos for Illumina (New England Biolabs) were added to a final concentration of 0.3 μM and libraries were amplified as follows: activation at 95°C for 3 minutes, amplification cycles of 98°C for 20 seconds, 65°C for 15 seconds, 72°C for 30 seconds, and a final extension of 72°C for 1 minute. Amplified libraries were purified using Solid Phase Reversible Immobilization magnetic bead purification protocol (Beckman Coulter). Final library size distribution was analyzed (BioAnalyzer2100, Agilent) and quantitated (KAPA Library Quantitation Kit) before pooling and sequencing (Novogene Corporation, CA) on Illumina HiSeq 4000 to generate 150 bp paired end reads. Libraries were multiplexed as twelve samples per lane.
Quality control and processing of sequencing reads
After sequencing, the quality and quantity of the raw reads were examined using FastQC version 0.11.5 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and MultiQC14 version 1.7. The total number of reads and percentage of duplicate reads are reported in Supplementary Figure 1A and B, respectively. Raw reads were quality and adapter trimmed using Trim Galore! version 0.6.0 (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) using default settings in paired-end mode. The trimmed reads then were aligned to hg19 using Bowtie215 version 2.3.5.1 in paired-end mode and all other settings default. The SAMtools16 version 1.10 software suite was used to convert SAM alignment files to BAM format, sort and index reads, and remove duplicates. The R package RSamtools version 2.2.1 was used to calculate the number of unique mapped reads.
Sample inclusion
Based on the quality control results, nine samples (8 RCC urine, 1 healthy urine) were removed due to having fewer than 1M reads. Samples with low mapping rate (<25%) were also removed. This resulted in the removal of two RCC urine sample. Five samples were also removed due to failure of qPCR quality control (1 control plasma, 1 RCC plasma, 3 healthy urine) (Extended Data Fig. 1). Saturation analyses to evaluate reproducibility of each library were carried out using the R Bioconductor package MEDIPS17 version 1.38.0. Based on visual inspection of the saturation curves, two RCC urine samples were removed due to low saturation
Detection of DMRs
Differentially Methylated Regions (DMRs) of size 300 base pairs were detected by first binning the genome into 300 base-pair windows, and then testing each for differential methylation between RCC and control (or UBC) using limma-voom18 (using R package limma version 3.42.0) on TMM-normalized counts19 (using R package edgeR version 3.28.0). Only bins with a total count above a fixed threshold were tested for differential methylation, where the threshold was set at 20% of the total number of samples across both groups. A set of 300 DMRs was obtained by selecting the top 150 DMRs with gain in RCC and the top 150 DMRs with loss in RCC relative to control (or UBC) samples. The same procedure was carried out on both plasma and urine samples.
Prediction of sample status using DMR signature
We randomly selected 80% of RCC and control samples for a training set with the remaining 20% of RCC and control samples to serve as held-out test samples. 300 DMRs between RCC and control training samples were then selected as described above. Region filtering and normalization were both carried out on the training set alone (with no input from the test set). Log-transformed TMM-normalized counts with a pseudocount of 1 in the training set were used to fit a GLMnet model on the 300 selected DMRs using the glmnet R package20 version 3.0–2. Three-fold cross-validation was used over a grid of lambda values ranging from 0.01 to 0.05 with optimization for AUC. The elastic net mixing parameter alpha was set at 0.5. The same procedure was carried out on both plasma and urine training samples. Each test set was TMM-normalized using the training set as a reference, and log-transformed normalized values with a pseudocount of 1 were fit to the GLMnet model trained on the training set to obtain prediction probabilities of belonging to the RCC group. These fitted probabilities were used to estimate AUC using the ROCR R package version 1.0–7 (Fig. 2e)21. Boxplots displayed in Fig. 2d are displayed with a median center line, box range of 25th to 75th percentile, and whiskers that extend to the most extreme observation within 1.5 times the interquartile range (IQR). Points outside the IQR are shown individually.
Differences in methylation score by stage (F=2.26; df=3 and 65) and histology (F=0.767; df=1 and 67) for plasma RCC and control samples were assessed by one-way ANOVA.
Overlap of plasma- versus tissue-derived RCC-specific DMRs
Raw IDAT files containing red and green channel intensities for 450K methylation arrays, along with sample meta data, were downloaded for the following datasets: 1) 93 PBMC samples from the pilot experiment in GEO accession number GSE67393 and 2) 324 TCGA-KIRC primary tumor samples from TCGAbiolinks22,23 version 2.14.1. Four samples from the TCGA-KIRC which were duplicates from two individuals were removed, leaving a total of 320 individuals. Samples were normalized using the Normal-exponential using out-of-band probes (noob) method as implemented in the minfi R package24,25 version 1.33.1. Probes on the X or Y chromosome, those with a detection probability of less than 0.01, and those methylated in healthy plasma were removed26. Delta beta values were computed for each probe by taking the difference of the average of the beta values (methylation levels) across all samples in each set. These values were plotted against the log2 fold change for the corresponding region in the plasma cfMeDIP data (Extended Data Fig. 3). Probes that did not overlap a region with sufficient coverage to be included for DMR testing, and regions that did not overlap any of the included probes, were excluded from this plot. Points were plotted using a hexbin density plot, where the color of each hexbin corresponds to the number of points in that bin. A simple linear regression line is shown in red. The spearman rank correlation was computed and a two-sided hypothesis of whether this correlation is equal to zero was carried out.
Reporting summary
Further information on research design is available in the Nature Research Life Sciences Reporting Summary linked to this article.
Data Availability
The cfMeDIP-seq NGS data for patient samples that support the findings of this study are available upon request from the corresponding author (M.L.F.) to comply with the Dana-Farber Cancer Institute ethics regulations to protect patient privacy. All requests for raw and analyzed data will be promptly reviewed by the Belfer Office for Dana-Farber Innovations to verify if the request is subject to any intellectual property or confidentiality obligations. Any data and materials that can be shared will be released via a Data Transfer Agreement.
Code Availability
All code used to process the data and carry out the analyses described in the methods is located in a publicly available GitHub repository at https://github.com/kdkorthauer/cfMeDIPseq-RCC/. This code is made available under an MIT license.
Extended Data
Extended Data Fig. 1. Flow chart of study samples.
Flow chart outlining the number of cfDNA samples collected, excluded per previously established pre-defined criteria, and included in the analytical cohorts.
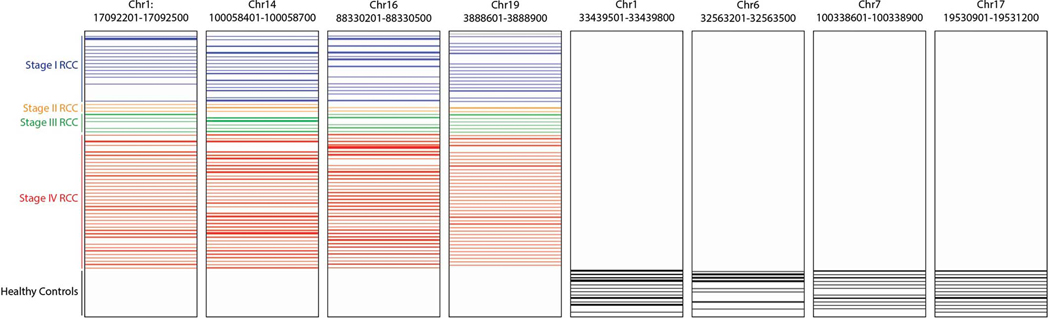
Extended Data Fig. 2. Differentially methylated regions in cfDNA between RCC and control samples.
Representative sites of plasma cfDNA methylation gain (left) and loss (right) in RCC relative to controls. Each panel is a 300 base pair window. Each row represents one sample. Row height represents amount of cfDNA methylation at that site.
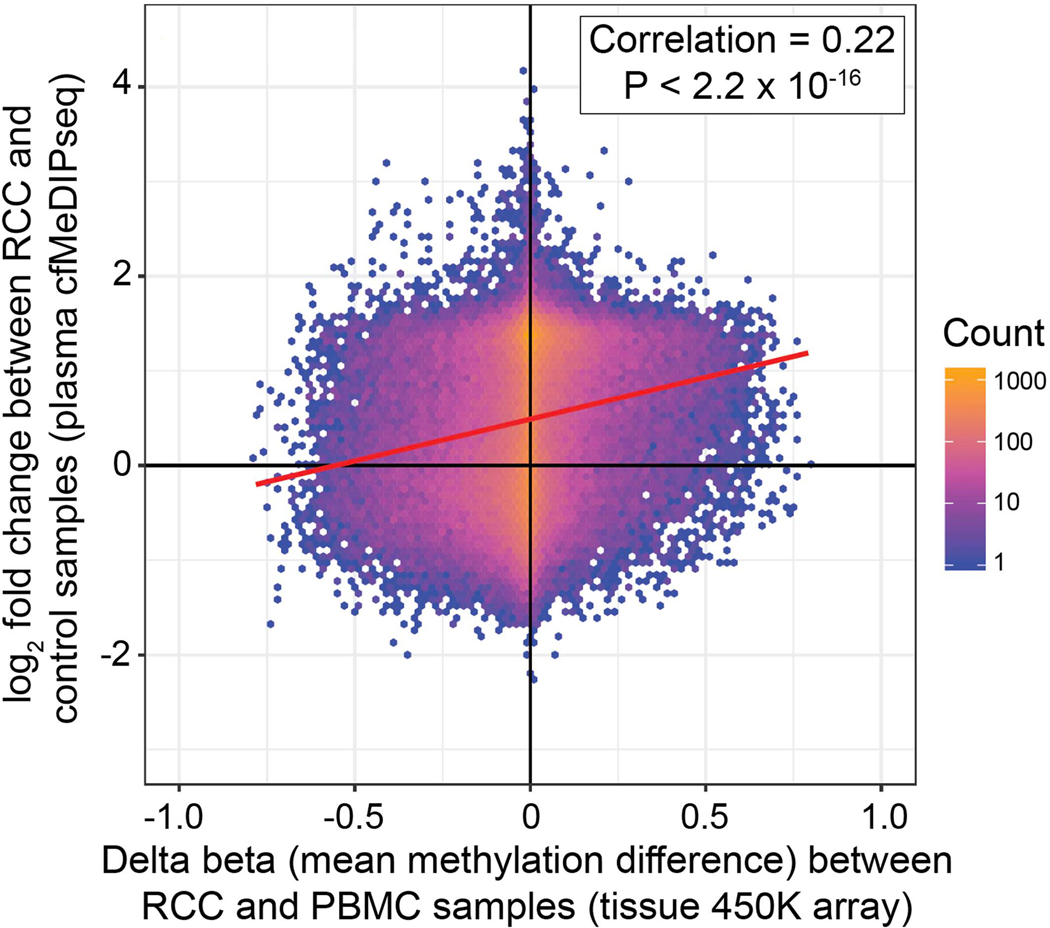
Extended Data Fig. 3. Correlation of differential methylation in plasma cfDNA and tumor DNA.
Density plot showing the overlap of plasma cfMeDIP-seq DMRs between 69 RCC and 13 control samples with differentially methylated CpGs in tissue from 450K methylation arrays between 93 peripheral blood mononuclear cell and 324 RCC tumor samples. Each observation (n=102,852) represents a 450K methylation array probe (x-axis) that overlaps a region covered by the cfMeDIP-seq assay (y-axis) where the color of each hex bin corresponds to the number of observations in that bin. A simple linear regression line is shown in red. The spearman rank correlation was computed and a 2-sided hypothesis of whether this correlation is equal to zero was carried out.
Supplementary Material
Acknowledgments:
This study was conducted with support from Rebecca and Nathan Milikowsky (M.P.), the Claudia Adams Barr Program for Innovative Cancer Research (M.L.F.) and the H.L. Snyder Medical Research Foundation (M.L.F.). K.K. is funded by the BC Children’s Hospital Research Institute (BCCHRI) Investigator Grant Award Program and the BCCHRI Establishment Award, and acknowledges support from Provincial Health Services Authority, the Children’s & Women’s Health Centre of BC, and the BC Children’s Hospital Foundation. A.C is supported by a Canadian Institutes of Health Research (CIHR) Banting Fellowship.
Disclosures (all outside the scope of the submitted manuscript):
• M.D.M.
○ Advisory Board: Pfizer, Exelixis, Eisai
• S.S.W.
○ S.S.W. has also received consulting fees from Public Health Advocacy Institute, GSK, CVS, Roth Capital Partners, Kantum, Mallinckrodt, Wolters Kluewer, GE Health Care, Allena Pharmaceuticals, Mass Medical International, JNJ, Venbio, Strataka, Takeda, Cerus, and Pfizer, all outside the submitted work.
• G.S.
○ Advisory Board: Pfizer, BMS, Genentech, EMD Serono, Novartis, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Amgen, Eisai, Bicycle Therapeutics
○ Research Support to Institution: Boehringer-Ingelheim, Bayer, Pfizer, Merck, Sanofi, Astrazeneca
○ Travel costs: BMS, Astrazeneca
○ Speaking fees: Physicians Education Resource (PER), Onclive, Research to Practice, Clinical Care Options
○ Writing fees: Uptodate
○ Steering committee of trials: BMS, Bavarian Nordic, Seattle Genetics, QED (all unpaid), and Astrazeneca and Debiopharm (both paid)
• D.D.D.C.
○ Research funding: Pfizer and Nektar therapeutics
• T.K.C.
○ Research (Institutional and personal): AstraZeneca, Alexion, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda
○ Honoraria: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), Research to Practice, L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology, Heron Therapeutics, Lilly
○ Consulting or Advisory Role: AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest.
○ Stock ownership: Pionyr, Tempest.
○ No leadership or employment in for-profit companies. Other present or past leadership roles: Director of GU Oncology Division at Dana-Farber and past President of medical Staff at Dana-Farber), member of NCCN Kidney panel and the GU Steering Committee, past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee)
○ Patents, royalties or other intellectual properties: Patents filed related to biomarkers related to response to immune checkpoint inhibitors
○ Travel, accommodations, expenses, in relation to consulting, advisory roles, or honoraria
○ Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies (ClinicalThinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, Oxford PharmaGenesis, and others).
○ The institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter.
○ CV provided upon request for scope of clinical practice and research
○ Mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components
Footnotes
Competing Interests Statement: A.C., S.Y.S., D.D.D.C. are listed as inventors on patents filed that are related to this method. T.K.C and M.L.F are listed as inventors on patents filed that are related to this method.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Shen SY et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 563, 579–583 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Cohen JD et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley JC et al. Detection and Surveillance of Bladder Cancer Using Urine Tumor DNA. Cancer Discov. 9, 500–509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sina AAI et al. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat. Commun. 9, 4915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen SY Burgener JM Bratman SV & De Carvalho DD Preparation of cfMeDIP-seq libraries for methylome profiling of plasma cell-free DNA. Nat. Protoc. 14, 2749–2780 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zill OA et al. The Landscape of Actionable Genomic Alterations in Cell-Free Circulating Tumor DNA from 21,807 Advanced Cancer Patients. Clin. Cancer Res. 24, 3528–3538 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Pal SK et al. Evolution of Circulating Tumor DNA Profile from First-line to Subsequent Therapy in Metastatic Renal Cell Carcinoma. Eur. Urol. 72, 557–564 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Hauser S Zahalka T Fechner G Müller SC & Ellinger J Serum DNA hypermethylation in patients with kidney cancer: results of a prospective study. Anticancer Res. 33, 4651–4656 (2013). [PubMed] [Google Scholar]
- 9.Skrypkina I. et al. Concentration and Methylation of Cell-Free DNA from Blood Plasma as Diagnostic Markers of Renal Cancer. Dis. Markers 2016, 3693096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TCGA Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasseter K, Nassar AH, Hamieh L. et al. Plasma cell-free DNA variant analysis compared with methylated DNA analysis in renal cell carcinoma. Genet Med (2020). 10.1038/s41436-020-0801-x [DOI] [PubMed] [Google Scholar]
- 12.Schoots IG Zaccai K Hunink MG & Verhagen PCMS Bosniak Classification for Complex Renal Cysts Reevaluated: A Systematic Review. Urol. 198, 12–21 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Gill IS Aron M Gervais DA Jewett MA Clinical practice. Small renal mass. N. Engl. J. Med. 362, 624–634 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Ewels P Magnusson M Lundin S & Käller M MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinforma. Oxf. Engl. 32, 3047–3048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmead B & Salzberg SL Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H. et al. The Sequence Alignment/Map Format and SAMtools. Bioinforma. Oxf. Engl. 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lienhard M Grimm C Morkel M Herwig R & Chavez L MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinformatics. 30, 284–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law CW Chen Y Shi W & Smyth GK voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson MD & Oshlack A A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman J Hastie T & Tibshirani R Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 33, 1–22 (2010). [PMC free article] [PubMed] [Google Scholar]
- 21.Sing T Sander O Beerenwinkel N & Lengauer T ROCR: visualizing classifier performance in R. Bioinformatics. 21, 3940–3941 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Inoshita M, et al. Sex differences of leukocytes DNA methylation adjusted for estimated cellular proportions. Biol Sex Differ. 25, 6–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colaprico A, et al. TCGAbiolinks: An R/Bioconductor Package for Integrative Analysis of TCGA Data. Nucleic Acids Res. 5, 44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triche TJ Weisenberger DJ Van Den Berg D Laird PW & Siegmund KD Low-level Processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 41, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aryee MJ, et al. Minfi: A Flexible and Comprehensive Bioconductor Package for the Analysis of Infinium DNA Methylation Microarrays. Bioinformatics. 30, 1363–1369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss J, et al. Comprehensive Human Cell-Type Methylation Atlas Reveals Origins of Circulating Cell-Free DNA in Health and Disease. Nat Commun. 29, 5068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cfMeDIP-seq NGS data for patient samples that support the findings of this study are available upon request from the corresponding author (M.L.F.) to comply with the Dana-Farber Cancer Institute ethics regulations to protect patient privacy. All requests for raw and analyzed data will be promptly reviewed by the Belfer Office for Dana-Farber Innovations to verify if the request is subject to any intellectual property or confidentiality obligations. Any data and materials that can be shared will be released via a Data Transfer Agreement.