Abstract
Purpose
Nonarteritic anterior ischemic optic neuropathy (NAION) is a common acute optic neuropathy in those older than 50 years. There is no blood diagnostic test or efficient treatment for NAION. We investigated the suitability of blood inflammatory proteins as biomarkers and therapeutic targets of NAION.
Methods
We conducted an exploratory, cross-sectional case-control study including 18 patients with NAION (n = 5 acute, and n = 13 chronic) and 9 controls. NAION was confirmed by clinical examination and optical coherence tomography. Subjects underwent peripheral blood collection; plasma was isolated within 2 hours and analyzed using a 76-plex array of cytokines, chemokines, and growth factors.
Results
In acute NAION, there was increased peripapillary retinal thickness on optical coherence tomography consistent with optic disc edema. Plasma profiling revealed dramatic changes in inflammatory proteins in NAION. Statistical analysis generated a list of 20 top-ranked molecules in NAION, with 15% overlap in acute and chronic NAION. Principal component analysis, hierarchical clustering, and Spearman correlation generally segregated controls, acute and chronic NAION, with some overlap. Longitudinal data from one patient demonstrated an evolving inflammatory pattern from acute to chronic NAION. In acute NAION, Eotaxin-3, MCP-2, TPO, and TRAIL were the top biomarker candidates. In chronic NAION, IL-1α and CXCL10 emerged as the strongest therapeutic targets.
Conclusions
Post-NAION inflammation occurs in both acute and chronic NAION. Statistical analysis of plasma profile changes generated a list of 20 potential biomarker and therapeutic targets of NAION.
Translational Relevance
We identified blood molecular targets to improve NAION diagnosis and treatment.
Keywords: ischemic optic neuropathy, optic nerve, inflammation, Luminex, blood biomarkers
Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) is a common acute optic neuropathy in those older than 50 years of age, with an annual incidence of 2.3 to 10.2 cases per 100,000 persons.1,2 In NAION, ischemia of the anterior, unmyelinated, optic nerve leads to optic disc edema and irreversible vision loss due to progressive damage to retinal ganglion cells. Although the exact pathogenesis is unclear, studies have demonstrated that NAION results from hypoperfusion of small vessels supplying the anterior optic nerve with a crowded optic nerve head. Hypertension, hypotension, diabetes, dyslipidemia, ischemic cerebral, or cardiac vascular disease, anemia, obstructive sleep apnea (OSA), and smoking have been identified as risk factors of NAION.3
NAION is primarily a clinical diagnosis based on the manifestations of acute onset, painless, monocular vision or visual field loss with a relative afferent pupillary defect and optic nerve head swelling as well as “disc at risk” in the contralateral eye. The optic disc edema usually resolves after 6 to 8 weeks and sectoral disc atrophy develops. Twelve to 15% of patients have second eye involvement at 5 years.4,5 Although the levels of erythrocyte sedimentation rate (ESR) and high sensitivity C-reactive protein are diagnostic blood tests with 97% specificity for the arteritic AION (AAION),6 there is no diagnostic laboratory test for NAION.3
NAION biomarkers are critically needed to differentiate NAION from other acute optic nerve head pathologies, including AAION, neuroretinitis, a very anterior optic neuritis and optic nerve head granuloma, and to help understand the pathogenesis of NAION and justify further clinical trials targeting inflammatory signaling. There is evidence of increased peripheral circulating RNAs7 and of post-ischemic inflammation in NAION, including elevated interleukin-8 in the plasma,8 vascular endothelial growth factor in the aqueous humor,9 and increased interleukin-6, tumor necrosis factor-α, and macrophage inflammatory protein-2 in the plasma and optic nerve in a rodent NAION model.10 In this study, we performed a comprehensive profiling of cytokines, chemokines, and growth factors in the plasma from patients with NAION compared with controls. Although the ultimate goal is to develop a quick diagnostic test to differentiate NAION from other similar pathologies in order to identify who might benefit from urgent treatment, this exploratory approach comparing NAION and controls is the first step to understand the plasma changes in NAION and can suggest candidate biomarkers and therapeutic targets for future studies and trials.
Methods
We performed a cross-sectional case-control study of patients with NAION and age-matched controls. This study was approved by the Institutional Review Board of Stanford University and adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act Subjects were evaluated and recruited at Byers Eye Institute at Stanford University Medical Center between April and October 2019.
Participants and Clinical Evaluation
Eighteen patients with NAION and 9 controls were enrolled. NAION subjects had comprehensive neuro-ophthalmic examination and confirmation by color and autofluorescence funduscopic photography and optical coherence tomography (OCT). Acute NAION included patients with the last attack within 3 months prior to the blood collection. Chronic NAION included patients with the last attack more than 3 months prior to the blood collection and was further divided into unilateral or bilateral groups. Control individuals had normal optic nerves and retina per fundus examination, no uncontrolled severe systemic diseases, and included the candidates for cataract or pterygium surgery, with best corrected visual acuity between 20/20 to 20/40 after surgery. We included samples from fasting and non-fasting patients in our study (fasting controls: n = 7, fasting NAION: n = 3; all other samples were from non-fasting patients). For the exclusion criteria, we excluded all subjects with intraocular pressure greater than 21 mm Hg or history of any other optic neuropathies, including glaucoma. We also excluded drug-induced NAION.
Blood Collection and Plasma Separation
Blood was collected into EDTA-coated tubes, placed on ice, and processed for plasma separation within 2 hours, using a Ficoll density gradient centrifugation at 800 xg for 20 minutes at 4°C. Plasma was stored at −80°C. To minimize variability due to changes in the circadian rhythm that may alter blood protein levels, we performed blood collection consistently at mornings (between 8 AM and 12 PM).
Immunoprofiling
Plasma proteins were measured using Luminex xMAP Technology using microbeads (Luminex, Austin, TX, USA), at the Stanford University Human Immune Monitoring Center. We used human 76-plex Procarta kits custom-built by eBioscience (eBiosciences/Affymetrix/Thermo Fisher, Santa Clara, CA, USA) and measured mean fluorescence intensity to compare the amount of protein in each sample. All samples were run at the same time, and average fluorescence intensity was calculated for each molecule and sample. Each well included 4 Chex internal control beads (Radix Biosolutions, Georgetown, TX, USA). Briefly, microbeads containing antibodies against 76 molecules (one antibody type per bead, 50–100 beads per antibody) were added to a 96-well plate and incubated with plasma samples (room temperature for 1 hour then overnight at 4°C with orbital shaking at 500–600 rpm). The plate was washed with a Biotek ELx405 washer and biotinylated detection antibody was added and incubated for 75 minutes at room temperature with shaking. The plate was washed again and streptavidin-phycoerythrin (fluorescence label) was added and incubated for 30 minutes at room temperature. Finally, the plate was washed, reading buffer was added to each well, and fluorescence intensity was measured using a FM3D FlexMap instrument. Results are shown as mean fluorescence intensity averaged between duplicates for each sample and normalized against Chex #4 values.
Data Analysis
The expression levels for most of the proteins had long right tails, so we log-transformed them; we then scaled each protein to have mean zero and standard deviation one. We compared the mean values between control and chronic NAION groups with t-tests and tested the significance of the coefficient of group in multiple linear regressions of protein measurements when sex and age were also covariates. Because there were only five individuals with acute NAION cases, we used a permutation test11,12 to compare control and acute NAION groups. We checked whether the resulting P values were significant after Bonferroni correction for testing 76 proteins (P < 0.1/76 = 0.0013). We then performed principal component analysis (PCA) with subjectively chosen clusters, hierarchical clustering and Spearman correlations of the molecules that had P < 0.05 for status effect in multiple linear regression or P < 0.05 in the permutation test.
Results
Immunoprofiling of Plasma Shows a Distinct Pattern of Inflammation in Acute and Chronic NAION
We profiled plasma immune molecules in 5 patients with acute NAION, 13 with chronic NAION, and 9 controls (Table 1). Gender was balanced in controls, but 78% of NAION group was male patients. The median time between the last attack and blood collection was 1.0 ± 0.8 month for acute, 39.5 ± 28.1 months for chronic unilateral, and 54.0 ± 37.5 months for chronic bilateral NAION. The most common systemic conditions were hypertension (40% of patients with acute and 69% of patients with chronic NAION) and hyperlipidemia (40% of acute and 54% of chronic patients), which were also common in the control group (33% and 44%, respectively). Obstructive sleep apnea was the most common systemic condition in patients with chronic NAION (20% of patients with acute and 77% of patients with chronic NAION). The age range in patients with NAION was from 46 to 86 years old (60.5 ± 10.4 years, median ± standard deviation) and in control patients was 57 to 80 (69.0 ± 7.3) years. Acute NAION median age was 52 ± 9.76 years, chronic unilateral NAION was 60.0 ± 14.0 years and chronic bilateral NAION was 64.0 ± 4.2 years.
Table 1.
Demographics and Clinical Characteristics of Controls and Patients With Ischemic Optic Neuropathy
| Control (n = 9 Subjects) | NAION Acute (n = 5 Subjects) | NAION Chronic Unilateral (n = 6) | NAION Chronic Bilateral (n = 7) | |
|---|---|---|---|---|
| Age (y)a | 69 ± 7.3 | 52 ± 9.7 | 60 ± 14 | 64 ± 4.2 |
| Female, n (%) | 4 (44.4) | 0 (0) | 2 (33.3) | 2 (20) |
| Male, n (%) | 5 (55.6) | 5 (100) | 4 (66.7) | 8 (80) |
| Plasma immune profiling, n (%) | 9 (100) | 5 (100) | 6 (100) | 7 (100) |
| VF MD (dB), n (%)a | NA | −15.4 ± 9.0, 5 (100) | −26.1 ± 3.0, 6 (100) | −15.5 ± 11, 2 (28.6) |
| Hypertension, n (%) | 3 (33.3) | 2 (40) | 4 (66.7) | 5 (71.4) |
| Diabetes, n (%) | 1 (11.1) | 1 (20) | 2 (33.3) | 1 (14.3) |
| Hyperlipidemia, n (%) | 4 (44.4) | 2 (40) | 2 (33.3) | 5 (71.4) |
| Anemia, n (%) | 1 (11.1) | 0 (0) | 0 (0) | 1 (14.3) |
| Smoking, n (%) | 0 (0) | 0 (0) | 1 (16.7) | 1 (14.3) |
| OSA, n (%) | 0 (0) | 1 (20) | 5 (83.3) | 5 (71.4) |
| Hight altitude, n (%) | 0 (0) | 2 (40) | 1 (16.7) | 1 (14.3) |
| Had steroids, n (%) | 0 (0) | 3 (60) | 1 (16.7) | 0 (0) |
| Time between the first attack and blood collection (months)a | NA | 3.5 ± 11 | 39.5 ± 28.1 | 57.0 ± 82.3 |
| Time between the last attack and blood collection (months)a | NA | 1.0 ± 0.8 | 39.5 ± 28.1 | 54.0 ± 37.5 |
In total, 18 patients with NAION and 9 controls were enrolled.
Median ± standard deviation.
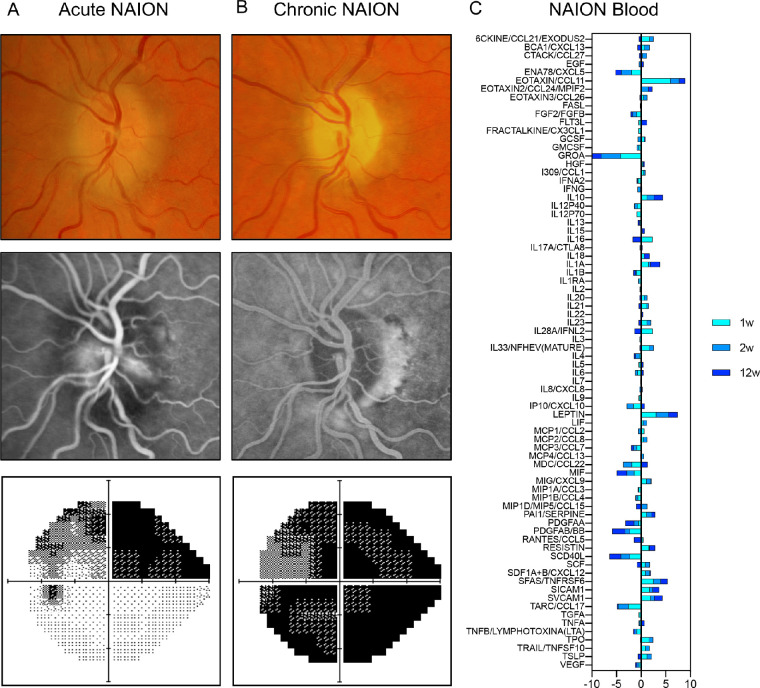
We assessed a 70-year-old male Caucasian presenting with second eye NAION over 3 time points. He had a history of NAION in the right eye 8 months before. At the first visit, his corrected visual acuity was counting fingers in the right eye and 20/30 in the left eye. Fundus examination revealed a pale optic disc in the right eye and a hyperemic swollen disc with absence of cup in the left eye. Humphrey visual field showed superior visual field defect in the left eye. Fundus fluorescein angiography (FFA) identified a delayed filling of peripapillary choroid involving the left optic nerve head and optic disc leakage (Fig. 1A).
Figure 1.
NAION description and blood profiling over time. A presentative right eye with non-arteritic ischemic optic neuropathy (NAION) in a 70-year-old male patient baseline, acute NAION (A), and chronic NAION (B). From the top row to the bottom row, the figure shows the fundus photo, optical coherence tomography and angiography, and grayscale displays of Humphrey visual field. (C) Blood multiplex immunoprofiling at 1 (turquoise), 2 (sky blue), and 12 (dark blue) weeks after NAION onset. Ratio to 9 controls was log2-scaled.
During the first 2 weeks after NAION onset in this patient, immunoprofiling of the plasma showed that the levels of several inflammatory proteins had dramatically changed compared to 9 control samples (Fig. 1C, turquoise and blue-sky bars). After 12 weeks, optic disc edema was resolved and repeat FFA showed hypofluorescence of prelaminar disc without any delayed perfusion of peripapillary choroid. Unfortunately, the patient developed worsening vision loss with a final acuity of 20/100 and severe visual field loss in the left eye (Fig. 1B). Despite resolution of optic disc edema, plasma immunoprofiling still showed some proteins with distinct levels from controls (Fig. 1C, dark-blue bars), revealing that post-ischemic inflammation is present early after NAION and does not completely resolve over time.
To further investigate this inflammatory pattern, we performed immunoprofiling of 5 patients with acute and 13 patients with chronic NAION compared with 9 controls. In acute NAION, there were 8 molecules in the plasma whose levels were distinct from controls (unadjusted P < 0.05, permutation test; Table 2). In chronic NAION, despite the complete resolution of optic disc edema, there was evidence of persistent inflammation and 15 molecules were distinct from controls (P < 0.05 for status effect in multiple linear regression; see Table 2). In fact, C-X-C motif chemokine ligand 10 (CXCL10) and interleukin-1α (IL-1α) were significant even after Bonferroni correction for 76 tests (P < 0.1/76 = 0.0013) and are the strongest biomarker candidates of chronic NAION. When comparing the list of molecules in acute and chronic NAION, three proteins were common to both groups (see Table 2, italicized). Analysis of chronic NAION did not indicate a difference between unilateral and bilateral. The measurements of all 76 proteins across all patients can be found in Supplementary Table S1 and the minimum and maximum values detected per sample can be found in Supplementary Table S2. In brief, statistical analysis of acute and chronic immunoprofiling changes revealed a list of 20 molecules that have unadjusted P < 0.05 and are potential biomarkers of NAION, with 15% overlap in acute and chronic NAION. Although only 2 molecules had significance level 0.1 with Bonferroni correction in the preceding tests, both sets of P values have additional excess of P values less than 0.05. Further exploratory analysis might suggest whether some of these suggestive molecules hold promise for future research.
Table 2.
List of Top-Ranked Molecules
| Control vs. Acute NAION | |||||
|---|---|---|---|---|---|
| Protein | Delta | P | |||
| MCP1/CCL2 | −1.342 | 0.003 | |||
| MCP2/CCL8 | 1.383 | 0.0145 | |||
| SFAS/TNFRSF6 | 1.271 | 0.0205 | |||
| TPO | 1.132 | 0.021 | |||
| SICAM1 | 1.061 | 0.024 | |||
| EOTAXIN3/CCL26 | 1.216 | 0.0255 | |||
| TRAIL/TNFSF10 | 1.105 | 0.0275 | |||
| RESISTIN | 0.822 | 0.043 | |||
| Control vs. chronic NAION | |||||
| Protein | Estimate | Se | t | p | p. bonf. |
| IL1A | 1.41 | 0.37 | 3.8 | 0.0012 | 0.094 |
| IP10/CXCL10 | 1.16 | 0.3 | 3.8 | 0.0012 | 0.095 |
| SFAS/TNFRSF6 | 1.12 | 0.37 | 3 | 0.007 | 0.529 |
| TNFA | 1.24 | 0.41 | 3 | 0.008 | 0.609 |
| IL18 | 1.22 | 0.41 | 3 | 0.0084 | 0.64 |
| IFNG | 1.08 | 0.38 | 2.9 | 0.0101 | 0.771 |
| RESISTIN | 1.22 | 0.45 | 2.7 | 0.0134 | 1 |
| SVCAM1 | 1.15 | 0.42 | 2.7 | 0.0141 | 1 |
| PDGFAB/BB | −1.11 | 0.41 | −2.7 | 0.0144 | 1 |
| IL22 | 0.99 | 0.41 | 2.4 | 0.0256 | 1 |
| IL17A/CTLA8 | 1.03 | 0.44 | 2.3 | 0.0307 | 1 |
| PDGFAA | −0.94 | 0.4 | −2.3 | 0.0326 | 1 |
| IL12P40 | 0.86 | 0.38 | 2.3 | 0.0349 | 1 |
| TPO | 0.93 | 0.43 | 2.2 | 0.0433 | 1 |
| PAI1/SERPINE | 0.85 | 0.4 | 2.1 | 0.0491 | 1 |
In acute NAION, there were eight molecules that were distinct from age-matched controls (unadjusted P < 0.05, permutation test). In chronic NAION, there were 15 molecules that were distinct from controls (P < 0.05 for status effect in multiple linear regression. C-X-C motif chemokine ligand 10 (CXCL10) and interleukin-1α (IL-1α) had the smallest P values in Bonferroni test (top of the chronic list). Three proteins were common to acute and chronic NAION (italicized).
Delta, average over controls subtracted from the average over chronic cases; P, fraction of permutation tests that have delta at least as large as the observed delta; Estimate, linear regression coefficient; SE, standard error of the estimate; T, estimate/SE; p: P value of linear regression; p. bonf: P value of Bonferroni test.
Principal Component Analysis
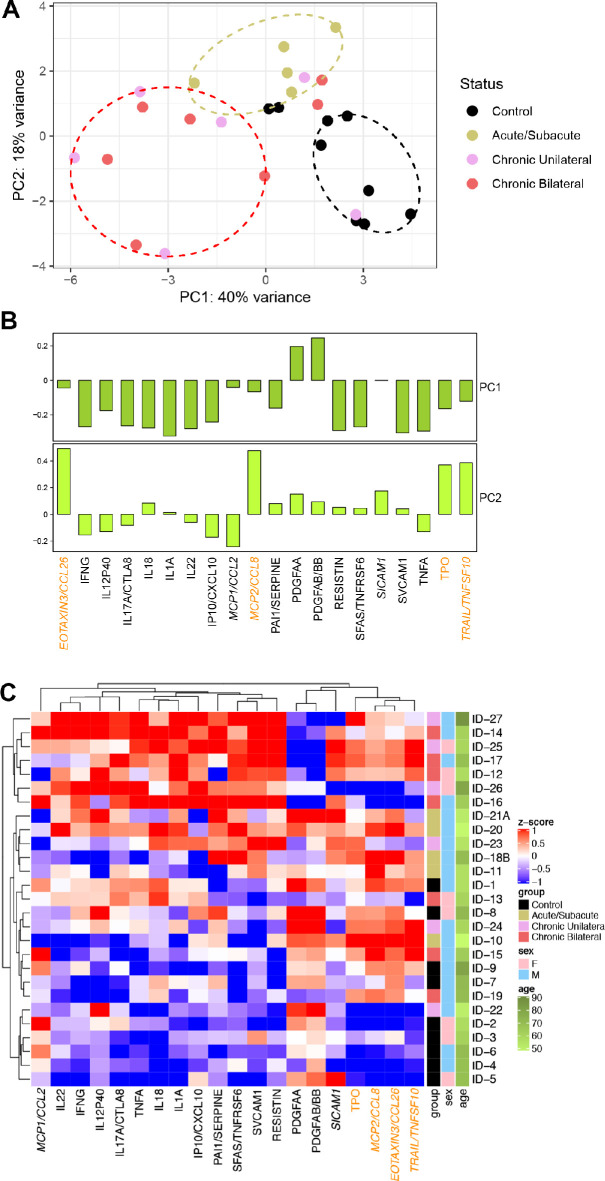
The PCA of the 20 molecules in Table 2 showed that individuals generally clustered by disease status, separating controls (black ellipse), acute (khaki ellipse), and chronic (red ellipse) NAION, with some overlap, in the plot of the first two PCs (Fig. 2A). PC1 and PC2 were not associated with age or gender (P > 0.1 for each of 4 univariate regression tests). PC1 generally distinguishes chronic NAION individuals (more to the left) from control (more to the right), with acute NAION between chronic and controls. PC2 generally distinguishes acute from both controls and chronic NAION. The 4 proteins with higher loadings in PC2 were Eotaxin-3, monocyte chemotactic protein-2 (MCP2), Thrombopoietin (TPO), and TNF-related apoptosis-inducing ligand (TRAIL; Fig. 2B, orange font), which are therefore potential biomarkers of acute AION. The PCA plot in Figure 2A was created using one time point per patient. To investigate whether the longitudinal data from the example patient with NAION shown in Figure 1 would fit the pattern, we applied the same rotation to the 2 additional time points and added them to the PCA plot (Supplementary Fig. S1). The 1- and 2-week time points are located close to the acute cases, whereas the 12-week time point is close to the chronic cases. This segregation of data from controls, and acute and chronic NAION is consistent with an inflammatory pattern that evolves over time after NAION onset.
Figure 2.
Potential biomarkers have a segregated distribution in controls and NAION subjects. (A) Principal component analysis (PCA) of the log-transformed levels of the 20 molecules in the biomarker candidate list. Individuals are organized into three distinguishable clusters separating controls (black ellipse), acute (khaki ellipse), and chronic (red ellipse) NAION, with some overlap. (B) PC1 and PC2 loadings. The 5 proteins that are only in the acute NAION list are italicized, and those with higher loadings in PC2 are in orange font; chronic NAION molecules have higher loadings in PC1. (C) Hierarchical clustering generally ordered the individuals from controls at the bottom to acute in the middle to chronic at the top, with some overlap.
Hierarchical Clustering and Spearman Correlation Matrix
Hierarchical clustering of the molecules in Table 2 generally ordered the controls at the bottom, acute in the middle, and chronic at the top, although there was overlap (Fig. 2C). The controls had relatively low levels of these molecules whereas the chronic cases had relatively high levels, especially for molecules to the left of PDGFAA in this heatmap. The 4 molecules farthest to the right in this heatmap also have the heaviest loadings in PC2. Age was not strongly associated with the ordering of the individuals.
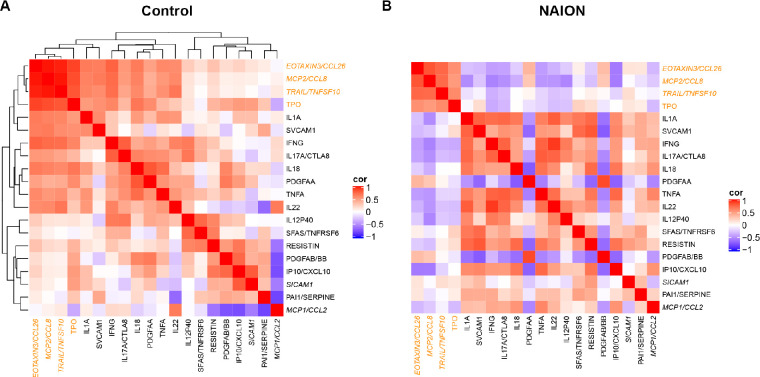
Spearman correlation matrix for these molecules shows different patterns for control (Fig. 3A) and NAION (Fig. 3B). To simplify comparison, NAION heatmap has the same molecule ordering determined by agglomerative hierarchical clustering in the control. Some molecules that are strongly (either positively or negatively) correlated in controls seem to be uncorrelated in NAION cases, whereas a few pairs of molecules that are uncorrelated in controls become strongly correlated in NAION cases. A few pairs that were strongly positively correlated in controls were actually strongly negatively correlated in NAION cases.
Figure 3.
Spearman correlation matrix of the biomarker candidate list for control and NAION. Differential patterns for control (A) and NAION (B). To simplify comparison, NAION heatmap has the same molecule ordering determined by clustering in the control. The 5 proteins that are only in the acute NAION list are italicized, and those with higher loadings in PC2 are in orange font.
Discussion
Immunoprofiling of NAION
We showed for the first time an immunoprofiling of patients with NAION and identified a list of molecules that generally segregated NAION and control individuals according to their protein levels. Out of 76 analytes, we provide a list of 20 proteins for further consideration in NAION studies, many of them for the first time in NAION. Data analysis using PCA, hierarchical clustering, and Spearman correlation revealed that NAION plasma were mostly distinguishable from controls by their levels of 20 (or fewer) inflammatory proteins. Of these, 10 molecules generally segregate chronic NAION from controls (and 2 of them were even significant after Bonferroni correction) whereas 4 distinguished acute NAION from controls and chronic NAION. Although no definitive molecule emerged as diagnostic biomarker for acute NAION, like ESR or CRP for arteritic AION in giant cell arteritis,6 IL-1α and CXCL10 are most promising in distinguishing chronic NAION to controls. Interestingly, the longitudinal data of one patient positioned acute time points next to the other acute patient samples, and a more chronic time point was situated near other chronic patient samples, consistent with an evolving immune profiling as disease progresses. Given the heterogeneity of these patients, future longitudinal, natural history studies should use proteomics approaches to identify molecules that correlate with disease stage and prognostics. The investigation of inflammatory changes after NAION and specific profiling of acute and chronic NAION may lead to novel diagnostics and therapeutic tools.
Inflammation in NAION: Chicken or Egg
While postischemic edema is the hallmark of acute NAION, which exhibits prominent edema and inflammation,8,9 chronic NAION plasma had more inflammatory molecules that helped distinguish them from that of controls. This may be related to persistent inflammation following NAION, but we cannot rule out that it may be caused by the systemic factors that led to NAION. We wonder about the contribution of risk factors such as hypertension and hyperlipidemia,13 which promote local vascular inflammation, although the control group also had the same risk factors. Certainly, systemic inflammation after NAION may play an important role in the development of secondary neuronal loss. Because we could not distinguish unilateral from bilateral patients, we cannot assume that changes in systemic inflammatory proteins increase the risk for developing a second attack, and larger studies are needed to clarify that. Although no other studies have described blood biomarkers of chronic NAION, a proteomics study identified 22 proteins unique to glaucomatous samples, including immune mediators and cell death signaling proteins,14 but there was no overlap with our biomarker candidate list for chronic NAION, supporting the hypothesis that our findings are specific to NAION.
Most Promising Diagnostic Biomarkers for Acute NAION
In acute NAION, we found that Eotaxin-3, MCP2, TPO, and TRAIL are the most prominent biomarker candidates out of 76 analytes and within our list of 20 top-ranked molecules. Eotaxin-3 or CCL26 is a member of the eotaxins family of C-C motif chemokines that bind the chemokine receptor CCR3.15 Although eotaxins have been identified by their role in eosinophil recruitment in parasitic infections and allergy, Eotaxin-1/CCL11 plays an important role in age-related neurodegeneration.16 MCP-2/CCL8 is a member of the monocyte chemotactic protein family that binds to the CCR1, CCR2, and CCR5 receptors and is increased in multiple sclerosis lesions17 and modulated by HIV-1 infection in the brain.18 TPO regulates platelet production and maturation and is increased in patients with acute myocardial infarction19 and ischemic stroke,20 which is thought to contribute to disease development and progression. TRAIL is a member of the TNF superfamily that is associated to induction of cell death after ischemic stroke.21,22 The changes in these four molecules in NAION and their association with central nervous system (CNS) disease supports their further investigation as NAION biomarkers. Although a previous study has described increased IL-8 in patients with acute NAION,8 we did not find significant changes in the levels of IL-8 in our study, which may reflect patient variability and small sample size of acute NAION. None of the 20 top-ranked molecules has been measured in human NAION, with the exception of TNFα, which has been previously associated with NAION animal models but not with human NAION.8,9
Most Promising Therapeutic Targets for Chronic NAION
The most robust protein changes in chronic NAION were IL-1α and CXCL10, both of which were significantly increased when compared to control, revealing potential therapeutic targets for chronic NAION. IL-1α is a member of the IL-1 family of cytokines, which have been described for their pro-inflammatory role in cardiovascular diseases such as atherosclerosis, myocardial infarction, aneurysm, and stroke.23 IL-1α has been attributed a triggering role in acute myocardial infarction24 and, curiously, both IL-1α and CXCL10 are increased in the aqueous humor in patients with retinal vein occlusion.25 CXCL10, or interferon-gamma-induced protein 10 (IP-10), belongs to the CXC chemokine subfamily and acts by binding to the CXCR3 receptors.26 CXCL10 has been studied in CNS diseases due to its role on neuroinflammation, such as in inducing leukocyte migration to sites of axonal injury27 and in astrocyte reactivity after cerebral ischemia.28 The top-ranked molecules in chronic NAION, CXL10 and IL-1α, have been associated with ischemic diseases and are strong targets for future investigation in NAION.
Existing Therapeutic Approaches Targeting Inflammation in NAION
Although there is no effective treatment for NAION, several studies have discussed the role of inflammatory pathways in the pathogenesis of NAION. For instance, NAION can be induced after treatment for hepatitis C with interferon alpha,29 which is a master regulator of cytokines. Treatment with steroids has not proven effective in ameliorating vision loss in NAION,30 but animal studies suggested that methyl prednisone improved retinal ganglion cell survival if treatment were given in a very short window of 1 day after injury.31 Inefficacious treatment with steroids reveals a need for more specific drug targeting, consistent with the dynamic, evolving inflammatory pattern that we found when comparing acute and chronic NAION profiles.
Limitations and Future Directions
The relatively small sample size used in this study limited the power and robustness of the statistical analysis and larger studies should be performed to overcome patient variability and to enable analysis of potential classification algorithms, including selecting the most effective combination of biomarkers. We also acknowledge cytokine measurements are subject to significant variability.32,33 To minimize this, we kept samples in storage for no more than 7 months prior to processing; only used EDTA plasma, which is a more sensitive matrix to detect low-abundance proteins than serum; and we reported mean fluorescence intensity instead of a converted concentration, because the former is considered more accurate than the latter.34
This study provides novel evidence of systemic inflammation in patients with NAION and opens a new avenue for investigation of inflammatory biomarkers and therapeutic targets of this disease. Future studies should include larger sample sizes, especially of patients with acute NAION, and patients with other acute optic nerve head pathologies in order to investigate whether blood inflammatory changes are specific of NAION and therefore can be used to develop an accurate diagnostic tool. Because inhibition of specific cytokine-receptor binding in the eye has proven beneficial in an animal model of retina-ischemic reperfusion,35 drug targeting of selected molecules in animal models of NAION and the study of their neuroprotective effects will contribute to the development of new treatment opportunities.
Conclusions
We found evidence of increased inflammation in both acute and chronic human NAION. Using immune profiling and an unbiased statistical approach, we generated a list of 20 biomarker candidates for further investigation. Ten molecules generally distinguished chronic NAION from controls and acute NAION, whereas four different molecules distinguished acute NAION from both controls and chronic NAION. Although the small sample sizes precluded attempting to test classification algorithms, these results encourage larger studies focusing on the 20 candidates to understand the relation of NAION and inflammation, including on the risk of second eye involvement. These cytokines or their receptors are potential candidates for biomarkers for diagnostics and monitoring of NAION.
Supplementary Material
Acknowledgments
The authors thank Ali Shariati, Sangeetha Pugazhendhi and Mariana Nunez for help with sample collection, Shailja Patel and Joanna Liliental for blood processing, and Yael Rosenberg-Hasson and Jing Liang for immunoprofiling.
Supported by the Stanford Translational Research and Applied Medicine Pilot Grant, the National Eye Institute (P30-026877), Research to Prevent Blindness, Inc., and the National Institutes of Health (R01 HL134776 and R01 HL139664).
Disclosure: L.A. Mesentier-Louro, None; L. Stell, None; Y. Yan, None; A.A. Montague, None; V. de Jesus Perez, None; Y.J. Liao, None
References
- 1. Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT.. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol . 1997; 123(1): 103–107. [DOI] [PubMed] [Google Scholar]
- 2. Johnson LN, Arnold AC.. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol . 1994; 14(1): 38–44. [PubMed] [Google Scholar]
- 3. Biousse V, Newman NJ.. Ischemic optic neuropathies. N Engl J Med . 2015; 373(17): 1677. [DOI] [PubMed] [Google Scholar]
- 4. Beck RW, Hayreh SS, Podhajsky PA, Tan ES, Moke PS.. Aspirin therapy in nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol . 1997; 123(2): 212–217. [DOI] [PubMed] [Google Scholar]
- 5. Newman NJ, Scherer R, Langenberg P, et al.. The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol . 2002; 134(3): 317–328. [DOI] [PubMed] [Google Scholar]
- 6. Hayreh SS, Podhajsky PA, Raman R, Zimmerman B.. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol . 1997; 123(3): 285–296. [DOI] [PubMed] [Google Scholar]
- 7. Suo J, Xu X, Xu H, et al.. Transcriptomic analysis of circRNAs in the peripheral blood of nonarteritic anterior ischemic optic neuropathy. Biomed Res Int . 2020; 2020: 5732124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldenberg-Cohen N, Kramer M, Bahar I, Monselise Y, Weinberger D.. Elevated plasma levels of interleukin 8 in patients with acute anterior ischaemic optic neuropathy. Br J Ophthalmol . 2004; 88(12): 1538–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Micieli JA, Lam C, Najem K, Margolin EA.. Aqueous humor cytokines in patients with acute nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol . 2017; 177: 175–181. [DOI] [PubMed] [Google Scholar]
- 10. Avraham BC, Dotan G, Hasanreisoglu M, et al.. Increased plasma and optic nerve levels of IL-6, TNF-alpha, and MIP-2 following induction of ischemic optic neuropathy in mice. Curr Eye Res . 2008; 33(4): 395–401. [DOI] [PubMed] [Google Scholar]
- 11. Browning BL. PRESTO: rapid calculation of order statistic distributions and multiple-testing adjusted P-values via permutation for one and two-stage genetic association studies. BMC Bioinformatics . 2008; 9: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leem S, Huh I, Park T.. Enhanced permutation tests via multiple pruning. Front Genet . 2020; 11: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayreh SS, Joos KM, Podhajsky PA, Long CR.. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol . 1994; 118(6): 766–780. [DOI] [PubMed] [Google Scholar]
- 14. Tezel G. A proteomics view of the molecular mechanisms and biomarkers of glaucomatous neurodegeneration. Prog Retin Eye Res . 2013; 35: 18–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huber AK, Giles DA, Segal BM, Irani DN.. An emerging role for eotaxins in neurodegenerative disease. Clin Immunol . 2018; 189: 29–33. [DOI] [PubMed] [Google Scholar]
- 16. Villeda SA, Luo J, Mosher KI, et al.. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature . 2011; 477(7362): 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF.. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol . 1998; 86(1): 20–29. [DOI] [PubMed] [Google Scholar]
- 18. Rom S, Rom I, Passiatore G, et al.. CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J . 2010; 24(7): 2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Senaran H, Ileri M, Altinbas A, et al.. Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol . 2001; 24(5): 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balcik OS, Bilen S, Ulusoy EK, et al.. Thrombopoietin and mean platelet volume in patients with ischemic stroke. Clin Appl Thromb Hemost . 2013; 19(1): 92–95. [DOI] [PubMed] [Google Scholar]
- 21. Tisato V, Gonelli A, Voltan R, Secchiero P, Zauli G.. Clinical perspectives of TRAIL: insights into central nervous system disorders. Cell Mol Life Sci . 2016; 73(10): 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui M, Wang L, Liang X, et al.. Blocking TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal damage after transient global cerebral ischemia. Neurobiol Dis . 2010; 39(2): 138–147. [DOI] [PubMed] [Google Scholar]
- 23. Pfeiler S, Winkels H, Kelm M, Gerdes N.. IL-1 family cytokines in cardiovascular disease. Cytokine . 2019; 122: 154215. [DOI] [PubMed] [Google Scholar]
- 24. Lugrin J, Parapanov R, Rosenblatt-Velin N, et al.. Cutting edge: IL-1alpha is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol . 2015; 194(2): 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung SH, Kim KA, Sohn SW, Yang SJ.. Association of aqueous humor cytokines with the development of retinal ischemia and recurrent macular edema in retinal vein occlusion. Invest Ophthalmol Vis Sci . 2014; 55(4): 2290–2296. [DOI] [PubMed] [Google Scholar]
- 26. Koper OM, Kaminska J, Sawicki K, Kemona H.. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med . 2018; 27(6): 849–856. [DOI] [PubMed] [Google Scholar]
- 27. Babcock AA, Kuziel WA, Rivest S, Owens T.. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci . 2003; 23(21): 7922–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zamanian JL, Xu L, Foo LC, et al.. Genomic analysis of reactive astrogliosis. J Neurosci . 2012; 32(18): 6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vardizer Y, Linhart Y, Loewenstein A, Garzozi H, Mazawi N, Kesler A.. Interferon-alpha-associated bilateral simultaneous ischemic optic neuropathy. J Neuroophthalmol . 2003; 23(4): 256–259. [DOI] [PubMed] [Google Scholar]
- 30. Pakravan M, Esfandiari H, Hassanpour K, Razavi S, Pakravan P.. The effect of combined systemic erythropoietin and steroid on non-arteritic anterior ischemic optic neuropathy: a prospective study. Curr Eye Res . 2017; 42(7): 1079–1084. [DOI] [PubMed] [Google Scholar]
- 31. Huang TL, Wen YT, Chang CH, Chang SW, Lin KH, Tsai RK.. Early methylprednisolone treatment can stabilize the blood-optic nerve barrier in a rat model of anterior ischemic optic neuropathy (rAION). Invest Ophthalmol Vis Sci . 2017; 58(3): 1628–1636. [DOI] [PubMed] [Google Scholar]
- 32. Banks RE. Measurement of cytokines in clinical samples using immunoassays: problems and pitfalls. Crit Rev Clin Lab Sci . 2000; 37(2): 131–182. [DOI] [PubMed] [Google Scholar]
- 33. de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol . 2009; 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg-Hasson Y, Hansmann L, Liedtke M, Herschmann I, Maecker HT.. Effects of serum and plasma matrices on multiplex immunoassays. Immunol Res . 2014; 58(2–3): 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang Y, Xiao Z, Pan L, et al.. Therapeutic targeting of retinal immune microenvironment with CSF-1 receptor antibody promotes visual function recovery after ischemic optic neuropathy. Front Immunol . 2020; 11: 585918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.