Abstract
Purpose
The purpose of this study was to compare the clinical outcomes and corneal biomechanical changes between 120-µm and 140-µm cap thickness after small incision lenticule extraction (SMILE).
Methods
This prospective study included 150 eyes (150 patients: 91 eyes in the 120-µm group, and 59 eyes in the 140-µm group) who underwent SMILE. Enhanced correction nomograms were applied for patients according to cap thickness. Clinical outcomes, including visual acuity, refraction, and corneal wavefront aberrations, were compared between the two groups. Corneal biomechanics were evaluated using the Corvis ST (Oculus, Wetzlar, Germany).
Results
The mean uncorrected-distance visual acuity, safety and efficacy indices, and refractive predictability were comparable in the 120-µm and 140-µm groups after SMILE. The postoperative total corneal root mean square higher-order aberrations (HOAs) and spherical aberrations was 0.48 ± 0.31 and 0.26 ± 0.10 in the 120-µm group, and 0.53 ± 0.16 and 0.34 ± 0.13 in the 140-µm group, which showed significant differences between the two groups (P = 0.027, and <0.001, respectively). Although corneal stiffness decreased after SMILE in both groups, the changes in the deformation amplitude ratio were significantly higher in the 140-µm group than in the 120-µm group (P = 0.022).
Conclusions
SMILE with 120-µm and 140-µm cap thickness provided excellent predictable outcomes according to our enhanced correction nomogram. The amount of tissue removal required to achieve the same amount of refractive correction was greater in the thicker cap group. The induction of corneal HOAs and weakening of corneal biomechanics were less pronounced in the thin-cap group, which may be associated with the thinner cap, lesser lenticule thickness, or thicker residual stromal bed.
Translational Relevance
Although SMILE with different cap thickness was effective, thicker lenticule thickness in the thick-cap group may be associated with induction of HOAs, and corneal stiffness changes.
Keywords: corneal biomechanics, SMILE, cap thickness, visual outcome
Introduction
The introduction of femtosecond laser (FSL) technology in corneal refractive surgery over the past 2 decades has resulted in remarkable innovations in this field.1,2 FSL has enabled tissue dissection via photo disruption and plasma cavitation.3 Initially, the FSL was predominantly used to make a corneal flap during laser in situ keratomileusis (LASIK), followed by stromal ablation using an excimer laser.4 A new surgical technique called femtosecond lenticule extraction was developed that uses only FSL to dissect two interfaces to create a refractive lenticule and subsequently remove it, which is very similar to LASIK.5 Small incision lenticule extraction (SMILE), which is an advanced version of the all-in-one FSL refractive technique, does not require a corneal flap, but requires a small incision through which the separated refractive lenticule is removed; the corneal tissue above the lenticule is known as the cap.6–11 SMILE has been widely used globally for correcting myopia or myopic astigmatism, since its clinical outcomes were first published in 2011.12,13 SMILE provides excellent visual outcomes and its advantages include a less pronounced reduction in corneal sensitivity and lack of flap-related complications, compared to LASIK.8
Because corneal biomechanics is associated with various corneal diseases, it is attracting attention from many researchers and clinicians. Corneal ectasia is one of the most severe complications after refractive surgery, hence corneal biomechanics is also an important topic in the field of laser vision correction.14 Theoretically, SMILE may preserve corneal biomechanics better than LASIK, because the anterior stroma, which is stiffer than the posterior stroma, remains intact in SMILE.14–16 However, some controversies exist, because previous studies that investigated corneal biomechanics have reported inconsistent outcomes, although SMILE is generally considered equal to or better than LASIK.17–21 Biomechanical weakening of the cornea and iatrogenic corneal ectasia have also been reported after SMILE.22–24 Moreover, creating a deeper refractive lenticule may result in a stronger cornea due to greater preservation of the anterior lamellae of the cornea, because the tensile strength progressively decreases from the anterior cornea to posterior cornea.15,25 On the contrary, retaining a sufficient residual stromal bed is essential for preventing iatrogenic corneal ectasia. Hence, creating a thin cap may be effective and desirable.26 Although several studies have investigated the difference between the biomechanical responses of SMILE and LASIK, few studies have evaluated the influence of cap thickness on the biomechanical strength after SMILE.3,17,27–30 El-Massry et al. used the Ocular Response Analyzer (ORA; Reichert Ophthalmic Instruments, Depew, NY, USA) and reported that a greater cap thickness resulted in higher postoperative corneal hysteresis (CH) and a corneal resistance factor (CRF).3 However, a rabbit study reported no significant difference in the corneal biomechanics with caps of different thicknesses.30 Recently, Wu et al. reported that the postoperative second applanation time, deformation amplitude (DA), and integrated radius were significantly lower with thinner caps, indicating a stiffer response.31
The aim of the current study was to investigate and compare the clinical outcomes including visual acuity, refractive error, and corneal aberrometric changes, along with the corneal biomechanical outcomes after SMILE using the Corvis ST (Oculus, Wetzlar, Germany) based on two different cap thicknesses.
Patients and Methods
Participants
This prospective comparative study was approved by the institutional review board of Yonsei University College of Medicine (Seoul, South Korea) and registered at ClinicalTrials.gov. The study was conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided informed consent after a detailed explanation of the possible risks and benefits of the study. Patients were recruited from Severance hospital and Eyereum Eye Clinic between May 2017 and June 2018. The inclusion criteria for this study were as follows: patients aged between 20 and 45 years, myopia <8.00 diopters (D), stable refraction for at least 1 year, and corrected-distance visual acuity (CDVA) of 0.8 Snellen fraction or better. The exclusion criteria for this study included the presence of severe ocular surface diseases, keratoconus, or cataract, and/or a history of intraocular or corneal surgery. A total of 150 eyes of 150 patients were enrolled (91 eyes with 120-µm cap thickness were enrolled between May 2017 and December 2017; 59 eyes with 140-µm cap thickness were enrolled between January 2018 and June 2018). If both eyes of a patient were eligible for enrollment, data from the right or left eye were randomly chosen using randomization tables, irrespective of ocular dominance, refraction, or the presence of aberrations.
Preoperative and Postoperative Assessment
All patients underwent detailed ophthalmological examinations before surgery that included evaluation of the logarithm of the minimum angle of resolution uncorrected-distance visual acuity (UDVA) and CDVA, manifest refraction, slit-lamp examination (Haag-Streit, Köniz, Switzerland), keratometry, and Scheimpflug-based corneal tomography (Pentacam HR, Oculus). Dynamic corneal response (DCR) parameters were examined using the Corvis ST. Corneal wavefront aberrations were measured using the Keratron Scout (Optikon 2000, Rome, Italy) for 6 mm zone. All examinations were repeated 1, 3, and 6 months after surgery.
Surgical Technique
The surgical procedure conformed to a previously described method.10 Surgery was performed with standardized techniques with the triple centration technique using the 500-KHz VisuMax system (Carl Zeiss Meditec AG, Jena, Germany). The superior cap depth was set at 120 or 140 µm, and the depth of the side cut was set at 2 mm. Different nomograms were applied according to the cap thickness based on the authors’ previous analyses32: amount of spherical equivalent (SE) correction (D) = −0.588 + (1.019 * pre-operative sphere) + (0.003 * age) for 120 µm cap thickness, and the amount of SE correction (D) = −0.986 + (1.015 * pre-operative sphere) for 140 µm cap thickness. The anterior (upper) and posterior (lower) planes of the lenticule were defined first, and the anterior and posterior interfaces were dissected using a microspatula with a blunt circular tip and extracted with microforceps. The integrity of the lenticule was subsequently assessed. A 0.5% topical levofloxacin solution (Cravit; Santen Pharmaceutical Co., Ltd., Osaka, Japan) and 0.1% fluorometholone (FML; Allergan, NJ, USA) were applied every 2 hours for 1 day postoperatively, followed by application 4 times a day for 1 month. The dosage was gradually reduced over 3 months.
Statistical Analysis
The sample size was calculated using the postoperative DA ratio values of our previous study that investigated corneal biomechanics with Corvis ST.33 The calculated sample size was 56 or more in each group, when the power was set to 0.80 and alpha to 0.05. Data are expressed as mean values ± standard deviation/standard error. Student's t-test was used to determine the (significant) differences between the continuous variables of the two groups, and the χ2 test was used for the categorical variables. Continuous variables were compared using linear regression analysis. The analysis of covariance (ANCOVA) was conducted to compare changes in the DCR parameters and bIOP of the two groups, using the Δmanifest refraction spherical equivalent (MRSE) or Δcentral corneal thickness (CCT) as a covariate. Statistical analyses were performed with SPSS statistics software (version 23; IBM Corporation, Armonk, NY, USA). P values ≤0.05 were considered statistically significant.
Results
This study included 91 eyes in the 120-µm group and 59 eyes in the 140-µm group. The baseline characteristics of the both groups are depicted in Table 1. Age, sex, pre-operative refractive errors, pre-operative visual acuity, or optical zone did not differ significantly between the two groups (see Table 1). The nomograms (created according to cap thickness) revealed that lenticule thickness was significantly lesser in the 120-µm group by about 8 µm. Therefore, residual stromal thickness was thicker in the thin-cap group (see Table 1). Significant differences were not observed between the two groups in pre-operative and postoperative CCT.
Table 1.
Characteristics of Eyes That Underwent SMILE With Either 120 µm or 140 µm Cap Thickness
| Characteristics | 120 µm | 140 µm | P Value |
|---|---|---|---|
| Number of eyes | 91 (R: L = 49: 42) | 59 (R: L = 30: 29) | 0.719 |
| Sex | M: F = 41: 50 | M: F = 36: 23 | 0.056 |
| Age, years old | 27.79 ± 5.95 (20 to 44) | 27.32 ± 7.04 (20 to 43) | 0.682 |
| Refractive errors (D) | |||
| Sphere | −3.18 ± 1.28 (−6.25 to −0.75) | −3.26 ± 1.49 (−6.12 to −0.50) | 0.741 |
| Cylindrical | −0.93 ± 0.69 (−3.12 to 0.00) | −0.97 ± 0.94 (−3.12 to 0.00) | 0.761 |
| SE | −3.65 ± 1.42 (−7.00 to −1.13) | −3.74 ± 1.58 (−7.00 to −1.00) | 0.697 |
| logMAR CDVA | −0.12 ± 0.06 (−0.18 to 0.00) | −0.11 ± 0.07 (−0.18 to 0.05) | 0.592 |
| logMAR UDVA | 1.11 ± 0.29 (0.52 to 2.00) | 1.11 ± 0.37 (0.22 to 2.00) | 0.976 |
| Preoperative CCT (µm) | 565.18 ± 24.44 (517 to 618) | 563.88 ± 21.95 (520 to 603) | 0.843 |
| Optical zone (mm) | 6.80 ± 0.19 (6.30 to 7.20) | 6.79 ± 0.19 (6.50 to 7.10) | 0.819 |
| Lenticule thickness (µm) | 95.27 ± 23.32 (49 to 141) | 103.29 ± 25.27 (54 to 154) | 0.049* |
| RST (µm) | 349.90 ± 31.20 (277 to 425) | 320.59 ± 30.60 (268 to 393) | <0.001* |
Results are expressed as means ± standard deviation (range).
SMILE = small incision lenticule extraction; D = diopters; SE = spherical equivalent; logMAR = logarithm of the minimum angle of resolution; CDVA = corrected distance visual acuity; UDVA = uncorrected distance visual acuity; CCT = central corneal thickness; RST = residual stromal thickness; * significantly different between 120 µm and 140 µm groups using t-test.
Visual Acuity, Efficacy, and Safety
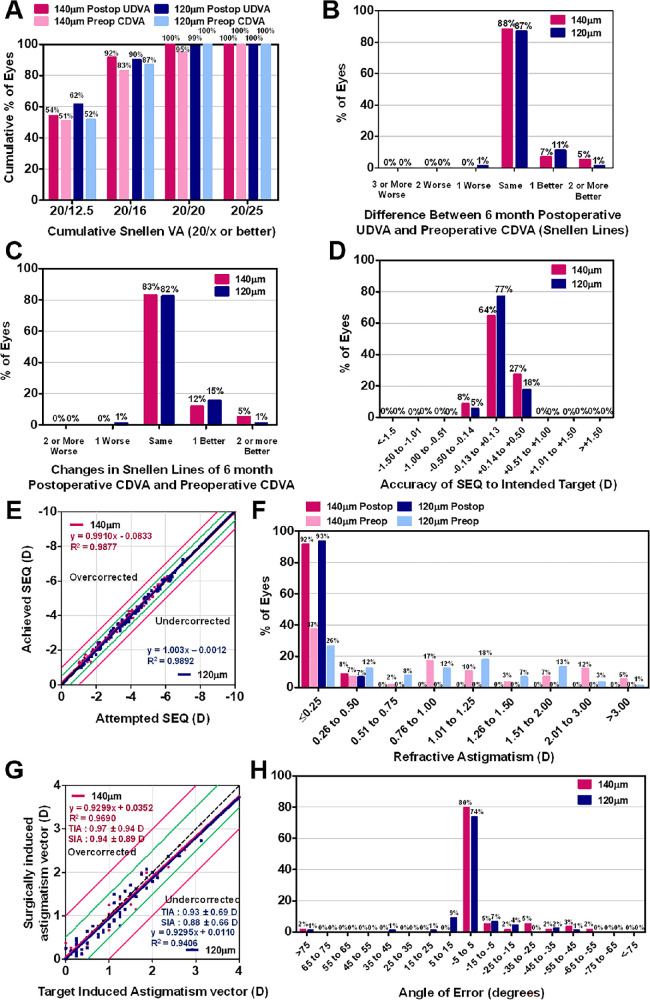
The mean UDVA improved significantly after SMILE in both groups (from 1.11 ± 0.29 to −0.13 ± 0.06 in the 120-µm group, and from 1.11 ± 0.37 to −0.13 ± 0.06 in the 140-µm group; P < 0.001 in both groups; Tables 1, 2). Ninety eyes from the 120-µm group (99%) and all eyes from the 140-µm group (100%) demonstrated 20/20 or better UDVA 6 months after surgery (Fig. 1). No significant differences were observed between the mean efficacy index (ratio of postoperative UDVA to pre-operative CDVA; 1.03 ± 0.09, and 1.03 ± 0.10, respectively; P = 0.853), and mean safety index (ratio of postoperative CDVA to pre-operative CDVA; 1.04 ± 0.10, and 1.05 ± 0.13, respectively; P = 0.621) of the 2 groups 6 months after surgery.
Table 2.
Comparison of Postoperative Visual Acuity and Refractive Errors of The Patients Who Underwent SMILE
| 120 µm | 140 µm | P Value | |
|---|---|---|---|
| logMAR UDVA | −0.13 ± 0.06 (−0.18 to 0.10) | −0.13 ± 0.06 (−0.18 to 0.00) | 0.641 |
| logMAR CDVA | −0.13 ± 0.06 (−0.18 to 0.05) | −0.13 ± 0.06 (−0.18 to 0.00) | 0.742 |
| Refractive errors (D) | |||
| Sphere | 0.10 ± 0.14 (−0.25 to 0.37) | 0.12 ± 0.17 (−0.25 to 0.50) | 0.400 |
| Cylindrical | −0.18 ± 0.14 (−0.50 to 0.00) | −0.15 ± 0.15 (−0.50 to 0.00) | 0.283 |
| SE | 0.01 ± 0.15 (−0.25 to 0.25) | 0.05 ± 0.18 (−0.38 to 0.38) | 0.209 |
| Postoperative CCT (µm) | 480.01 ± 32.04 (403 to 554) | 481.07 ± 27.90 (427 to 548) | 0.466 |
Results are expressed as means ± standard deviation (range).
logMAR = logarithm of the minimum angle of resolution; UDVA = uncorrected distance visual acuity; D = diopters; SE = spherical equivalent; SMILE = small incision lenticule extraction.
Figure 1.
Visual outcomes after small incision lenticule extraction (SMILE) according to cap thickness (120 µm and 140 µm). (A) Cumulative 6-month postoperative uncorrected-distance visual acuity (UDVA) and pre-operative corrected-distance visual acuity (CDVA) Changes in Snellen lines of postoperative UDVA (B) and CDVA (C), relative to the preoperative CDVA, are shown. The accuracy of spherical equivalent refraction (SEQ) with respect to the intended target (D) and attempted versus achieved changes in SEQ (E) 6 months after surgery. The comparative distribution of the preoperative and 6-month postoperative cylinder (F) and target-induced versus surgically induced astigmatism vectors (G) 6 months after surgery can be seen. (H) Refractive astigmatism angle of error distribution is shown 6 months after surgery. D = diopter; SD = standard deviation.
Refraction
The outcomes of refractive error correction were excellent and the mean MRSE improved significantly after treatment in both groups (see Tables 1, 2). The postoperative MRSE was within 0.5 D and the postoperative cylinder was less than 0.5 D in all operated eyes in both groups. Linear regression between the attempted and achieved SE in the 120-µm group and 140-µm group revealed slopes of 1.003 and 0.9910, respectively, and coefficients (R2) of 0.9892 and 0.9877, respectively (see Fig. 1). Linear regression analysis between the target induced astigmatism vector and surgically induced astigmatism vector for each technique revealed a slope and coefficient (R2) of 0.9295 and 0.9406, respectively, in the 120-µm group, and 0.9299 and 0.9690, respectively, in the 140-µm group. The angle of error was within 5 degrees for 67 eyes of the 120-µm group (74%) and 47 eyes of the 140-µm group (80%) 6 months after surgery (see Fig. 1).
Higher-Order Aberrations
The total corneal root mean square (RMS) higher-order aberrations (HOAs) increased significantly after surgery in the 140-µm group, whereas no difference was observed in the RMS HOAs in the 120-µm group (Table 3, Fig. 2). The postoperative RMS HOAs of the 120-µm group were smaller than those of the 140-µm group. Notably, the corneal spherical aberration decreased significantly in the 120-µm group after surgery, but increased significantly in the 140-µm group. Moreover, the postoperative spherical aberration values were significantly lower in the 120-µm group (see Table 3, Fig. 2). The corneal coma did not change after surgery in both groups, and did not differ between them. Corneal trefoil decreased significantly only in the 140-µm group, but did not differ significantly between the 2 groups (see Table 3, Fig. 2).
Table 3.
Comparison of Corneal Aberrations of Eyes With High Astigmatism That Underwent SMILE
| RMS HOA | Spherical Aberration | Coma | Trefoil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 120 µm | 140 µm | P Value | 120 µm | 140 µm | P Value | 120 µm | 140 µm | P Value | 120 µm | 140 µm | P Value | |
| Pre-operative | 0.49 ± 0.10(0.27 to 0.82) | 0.48 ± 0.13(0.23 to 0.89) | 0.701 | 0.29 ± 0.09(0.06 to 0.50) | 0.28 ± 0.11(0.00 to 0.51) | 0.518 | 0.26 ± 0.13(0.02 to 0.63) | 0.26 ± 0.15(0.02 to 0.67) | 0.851 | 0.20 ± 0.11(0.01 to 0.42) | 0.18 ± 0.11(0.01 to 0.55) | 0.265 |
| 6 month | 0.48 ± 0.13(0.22 to 0.86) | 0.53 ± 0.16(0.26 to 1.14) | 0.027 | 0.26 ± 0.10(0.04 to 0.46) | 0.34 ± 0.13(0.08 to 0.70) | <0.001* | 0.26 ± 0.14(0.04 to 0.62) | 0.29 ± 0.17(0.06 to 0.90) | 0.290 | 0.17 ± 0.09(0.03 to 0.38) | 0.15 ± 0.09(0.01 to 0.41) | 0.239 |
| P value (versus preop.) | 0.717 | 0.016* | 0.006* | <0.001* | 0.431 | 0.262 | 0.179 | 0.029* | ||||
| Δ (Pre versus 6 month) | −0.01 ± 0.14(−0.27 to 0.41) | 0.06 ± 0.17(−0.36 to 0.54) | 0.018* | −0.03 ± 0.09(−0.28 to 0.15) | 0.06 ± 0.10(−0.12 to 0.39) | <0.001* | 0.01 ± 0.14(0.27 to 0.46) | 0.03 ± 0.20(−0.44 to 0.65) | 0.448 | −0.03 ± 0.11(−0.27 to 0.26) | −0.03 ± 0.09(−0.27 to 0.15) | 0.860 |
Results are expressed as means ± standard deviation (range).
SMILE = small incision lenticule extraction; RMS = Root mean square; HOA = higher-order aberrations; Δ = change; * P value < 0.05.
Figure 2.
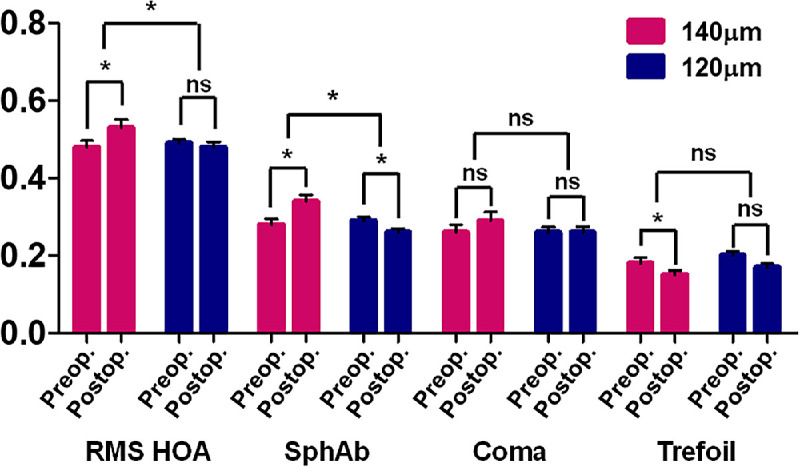
Changes in higher order aberrations (HOAs) 6 months after small incision lenticule extraction (SMILE) according to cap thickness (120 µm and 140 µm). Data are presented as the mean ± standard error of the mean (SEM). RMS = root mean square; SphAb = spherical aberration; ns = not significant; * significant difference.
Biomechanical Outcomes
Table 4 summarizes the values of the DCR parameters before and after SMILE. The pre-operative DCR values were comparable between the two groups, and all the parameters analyzed, except bIOP, showed significant changes after surgery (all P < 0.001). The DA ratio and integrated inverse radius increased significantly, whereas the stiffness parameter at the first applanation (SP-A1), Ambrósio's relational thickness through the horizontal meridian (ARTh), and stress strain index (SSI) decreased significantly, indicating that the stiffness or resistance to deformation decreased after surgery. Interestingly, significant differences were observed between the changes in the pre- and postoperative values of the DA ratio and integrated inverse radius of the two groups, which implies a lower degree of corneal weakening in the 120-µm group (see Table 4; P = 0.022 for DA ratio; and P = 0.011 for the integrated inverse radius). Furthermore, the comparison of the changes in the DCR parameters using ANCOVA (with the ΔMRSE or ΔCCT as a covariate) revealed that significant differences in the DA ratio and integrated inverse radius were maintained between the two groups (see Table 4). SP-A1, ARTh, and SSI did not show statistical significance between cap thickness groups.
Table 4.
Comparison of Dynamic Corneal Response (DCR) Parameters Between the 120 µm and 140 µm Groups
| 120 µm | 140 µm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | P a | Pre | Post | Δ | P a | P b | P c | P d | P e | |
| DA ratio | 4.30 ± 0.37 | 5.48 ± 0.51 | 1.18 ± 0.40 | <0.001* | 4.24 ± 0.33 | 5.57 ± 0.45 | 1.33± 0.36 | <0.001* | 0.315 | 0.022* | 0.022* | 0.034* |
| SP-A1 | 115.17 ± 10.95 | 93.02 ± 10.74 | −22.35 ± 9.60 | <0.001* | 117.99 ± 11.14 | 93.67 ± 10.87 | −24.32± 11.08 | <0.001* | 0.129 | 0.248 | 0.308 | 0.407 |
| Integrated inverse radius | 8.17 ± 0.79 | 10.53 ± 1.02 | 2.35 ± 0.75 | <0.001* | 8.11 ± 0.90 | 10.81 ± 1.18 | 2.71 ± 0.93 | <0.001* | 0.634 | 0.011* | 0.009* | 0.013* |
| ARTh | 462.00 ± 93.82 | 209.98 ± 54.87 | −252.32 ± 86.54 | <0.001* | 487.12 ± 107.85 | 208.16 ± 64.01 | −278.97 ± 92.77 | <0.001* | 0.133 | 0.075 | 0.087 | 0.151 |
| SSI | 1.02 ± 0.13 | 0.88 ± 0.14 | −0.14 ±0.15 | <0.001* | 1.03 ± 0.15 | 0.86 ± 0.13 | −0.17 ±0.10 | <0.001* | 0.502 | 0.184 | 0.158 | 0.139 |
| bIOP (mm Hg) | 15.89 ± 1.19 | 15.77 ± 1.17 | −0.13 ± 0.85 | 0.162 | 16.25 ± 1.33 | 16.06 ± 1.20 | −0.19 ± 0.75 | 0.059 | 0.083 | 0.643 | 0.600 | 0.524 |
Results are expressed as means ± standard deviation.
Δ = change (Post – Pre); DA = deformation amplitude; SP-A1 = stiffness parameter at first applanation; ARTh = Ambrósio Relational Thickness through the horizontal meridian; SSI = stress strain index; bIOP = biomechanically-corrected intraocular pressure.
P a value between preoperative and postoperative DCR parameters.
P b value between the two groups’ preoperative DCR parameters
P c value between the two groups regarding changes in DCR parameters.
P d value between the two groups regarding changes in DCR parameters with ANCOVA with the refractive error change as a covariate.
P e value between the two groups regarding changes in DCR parameters with ANCOVA with the corneal thickness change as a covariate.
Discussion
We investigated the surgical outcomes of SMILE in myopic eyes using different cap thicknesses. The visual outcomes achieved with SMILE in this study were in line with those of previous studies and suggested that SMILE is an excellent and safe treatment modality, irrespective of the cap thickness.8–10 The postoperative visual acuity and refractive outcomes were comparable in both cap thickness groups. Moreover, the safety and efficacy indices did not differ between the 120-µm and 140-µm groups.
The influence of cap thickness on the correction of refractive error was previously investigated in patients who underwent SMILE.3,27,28 Guell et al.27 evaluated the clinical outcomes, including the visual acuity and refraction correction, in patients who underwent SMILE with cap thicknesses of 130 µm, 140 µm, 150 µm, and 160 µm. They applied different nomograms according to the cap thickness to overcome any possible energy loss. They increased the SE correction by 3% for every 10 µm of increase in cap thickness compared to the 130 µm cap, which means that a 10% overcorrection was applied to the 160-µm cap. The postoperative SE was −0.10 ± 0.60, −0.15 ± 0.27, −0.12 ± 0.23, and −0.17 ± 0.25 for the 130-µm, 140-µm, 150-µm, and 160-µm caps, respectively, and no significant differences were observed among the various groups. On the contrary, two studies reported comparable postoperative SE outcomes without any correction according to cap thickness. However, they may have been underpowered, because the sample size of both studies was smaller than that of the current study.3,28 An ex vivo study17 showed that the average undercorrection was 3.5% in the 110-µm cap, and 11% in the 160-µm cap after −4 D SMILE; and (surprisingly) that after −8 D SMILE was 21% in the 110-µm cap, and 43% in the 160-µm cap. Because the corneal epithelium was removed before SMILE, the lenticule was created at a much deeper part of the corneal stroma compared to (clinical) lenticule formation in actual patients treated with 110 or 160-µm cap, which is one of the reasons for significant undercorrection after SMILE. We generated different nomograms based on cap thickness, because we had previously formulated the regression equation according to cap thickness.32 The amount of SE correction was (D) = −0.588 + (1.019 × pre-operative sphere) + (0.003 × age) for 120-µm cap thickness, and (D) = −0.986 + (1.015 × pre-operative sphere) for 140-µm cap thickness. Therefore, the lenticule was slightly but significantly thicker in the 140-µm group than that in the 120-µm group, even though their respective pre-operative SEs were comparable. However, the postoperative SE was also comparable between the two groups. The postoperative CCT did not differ between the two groups, despite the difference in lenticule thickness. We suggest that different nomograms need to be created according to the cap thickness in SMILE.
The results of aberrometric analysis in the current study were interesting. Significant differences were observed between the postoperative corneal RMS HOAs and spherical aberrations of the two groups. The corneal RMS HOAs remained stable in the 120-µm group; however, those of the 140-µm group increased significantly. Furthermore, the corneal spherical aberrations decreased significantly after SMILE in the 120-µm group, whereas there was a significant induction of spherical aberrations in the 140-µm group. There were no significant differences between the corneal coma or trefoil of the two groups. This is concurrent with the findings of a previous study,10 which reported that no changes were observed in the aberrometric values after SMILE. A previous study3 that compared SMILE with 100-µm and 160-µm cap thicknesses in contralateral eyes (30 patients with 30 eyes in each group) found that the HOAs were comparable for both groups. Another study28 also reported a lack of difference between the HOAs for the 120-µm and 140-µm cap thickness, although it included 40 participants (with 40 eyes in each group). Both studies may have lacked sufficient power to detect a difference. In addition, several studies have reported no significant differences among the HOAs with flaps of different thicknesses in LASIK.34,35 The significant induction of corneal RMS HOAs and spherical aberrations in the 140-µm group in the current study may be associated with greater lenticule thickness and lower residual stromal bed thickness compared to the 120-µm group. The post hoc power of postoperative corneal RMS HOAs and spherical aberration was 0.51, and 0.98, respectively.
SMILE may facilitate better corneal biomechanical preservation than that with LASIK, because flap creation during LASIK cleaves a biomechanically stronger part of cornea including Bowman's layer and the anterior stroma.14,15 A study that investigated the biomechanical effect of SMLE and LASIK using finite element analysis showed that the stress distributions between the SMILE and LASIK simulations were distinct with reduced stress in the flap region compared to the cap region and increased stress in the residual stromal bed in LASIK compared to SMILE, despite the same volume of tissue removed in both.16 This indicates that the flap region can no longer bear the same stress as pre-operatively, so more of the load is transferred to the residual stromal bed in LASIK than in SMILE. This is consistent with a contralateral clinical study comparing FLEx (lenticule under a flap) versus SMILE with subject specific finite element models to evaluate changes in stiffness and stress distribution.36 The flap procedure produced a 49% greater mean reduction in stromal collagen fiber stiffness within the flap region versus the fellow eye cap region with SMILE, and lower stresses in the residual stromal bed in the SMILE eye. Several previous studies19,20 have reported that SMILE conserves corneal stiffness better than LASIK, although other studies could not find any significant difference.18,21 Nevertheless, postoperative corneal weakening and corneal ectasia still occur after SMILE.22–24 Many surgeons suggest that the use of a thick cap with lenticule creation in the deeper layer can better preserve the stiffness of the treated cornea, which may improve corneal strength after SMILE.17 On the contrary, deep lenticule creation results in a thinner residual stromal bed and requires a deeper side incision for lenticule removal, which may have negative ramifications on corneal biomechanics, as a thin flap results in a stronger cornea in LASIK.37 The current study demonstrated corneal weakening after SMILE, as reported by previous studies. This finding is to be expected since the primary biomechanical alteration in SMILE or LASIK is tissue removal, which creates a refractive effect. Both procedures produce a reduction in stiffness, which reflects the availability of lesser tissue that can resist deformation. SSI is a recently developed indicator of corneal stiffness and has been shown to not correlate with IOP and CCT.38 In this study, the value of SSI was also measured and it was confirmed that it decreased significantly after surgery.
We also noted that the degree of biomechanical weakening of the cornea was greater in the 140-µm cap group compared to the 120-µm cap group, which is reflected by the greater differences in the sensitive corneal (shape) deformation DCR parameters (i.e. the DA ratio and integrated inverse radius) in the 140-µm cap group. As mentioned earlier, the greater lenticule thickness needed to provide comparable refractive outcomes in the 140-µm group results in a thinner residual stromal bed post-operatively, leading to the difference in the changes of the DA ratio and integrated inverse radius between the two groups. The post hoc power of changes in DA ratio and integrated inverse radius was 0.67 and 0.70, respectively. Surprisingly, the differences retained statistical significance even after adjusting for CCT, and SE changes before and after surgery (using the ANCOVA). A previous study that evaluated corneal biomechanics after SMILE in rabbit eyes showed that the second applanation time was shorter in the 100-µm cap than that in the 160-µm cap 4 months after surgery: the context of these findings is the same as that of our results, because an earlier recovery time is consistent with a stronger cornea.30 Recently, Wu et al. reported that the changes in DA, second applanation time, and integrated radius were less in the 110-µm cap than that in the 140-µm cap with a contralateral study design, indicating less biomechanical weakening with the thinner flap, consistent with the current study, but without a nomogram adjustment.31 In this case, the thinner cap was associated with a significantly thicker residual stromal bed, also consistent with the current study. Another study showed that SMILE with a 160-µm cap had a less pronounced effect on the CH and CRF measured using the ORA than that with a 100-µm cap.3 However, CH and CRF represent ability to dissipate energy, rather than a change in stiffness. An ex vivo study17 that used inflation testing on human donor corneas revealed that the reduction in the biomechanical strength was not significantly different in the 110-µm cap than that in the 160-µm cap. However, there were only 8 donor eyes in each group, and the study also reported greater myopic correction with 110-µm cap, consistent with our nomogram for greater lenticule thickness with thicker caps, as well as reporting less posterior steepening with the 110-µm cap, which is indicative of less biomechanical response, also consistent with the current study. Studies on the corneal wound healing process have shown that significant stromal remodeling does not occur except at the flap margin, which is beneficial in reducing corneal scarring or haze, but may have a detrimental effect on corneal stiffness (i.e. decrease it).39 The complete reconstruction of the interlinks across an interface requires a considerably long period of time, and may perhaps, never occur. Furthermore, the vertical corneal wound also remains weak, even though it appears fully healed.40,41 Hence, the strength of the cap may be weaker than that of the residual corneal bed after SMILE. Liu et al.28 reported that the corneal wound healing response lower in the 140-µm cap compared to the 120-µm cap. However, the tensile strength of anterior stroma is higher than posterior, and mathematical model predicted that the thicker the cap thickness, the greater postoperative total tensile strength, although it does not consider the effect of the side incision or the mismatch between the arclength of the posterior cap versus the anterior residual stromal bed.15 Further research is continually needed to draw conclusions in this regard.
The limitations of this study include the lack of evaluation of optical quality of the interface and functional visual quality. Nevertheless, this study is valuable owing to its prospective design and large sample size. Moreover, it investigated the corneal biomechanics using Corvis ST after correcting covariates for the two different cap thicknesses. Furthermore, these outcomes may provide a new perspective for selecting the appropriate cap thickness for SMILE. Nevertheless, the old question of whether a thicker anterior cap may be beneficial for biomechanics after SMILE still remains unclear due to the differences in lenticule thickness, that is the amount of tissue removed, in the two groups.
In conclusion, SMILE using different cap thicknesses resulted in effective and safe clinical outcomes for correction of myopic astigmatism. However, creating and applying different nomograms according to cap thickness is essential to elicit excellent outcomes. The thicker lenticule required for a thick cap may be associated with increased induction of corneal HOAs and greater alteration in corneal biomechanics, associated with a thinner residual stromal bed. Moreover, future studies are necessary to comprehensively understand the effects of cap thickness on clinical outcomes, corneal biomechanics, and the cap–stromal bed interface, in order to identify the best parameters, including cap thickness, based on the patient's ocular condition.
Acknowledgments
Supported by the Basic Science Research Program (NRF-2021R1I1A1A01047951) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT, and Future Planning. The funding organization had no role in the design or conduct of this study.
Disclosure: I. Jun, None; D.S.Y. Kang, Advisor for SCWIND eye-tech-solutions (Kleinostheim, Germany) and Carl Zeiss Meditech (Jena, Germany); C.J. Roberts, Consultant for Oculus Optikgeräte GmbH (Wetzlar, Germany), Ziemer Ophthalmic Systems AG (Port, Switzerland), and Optimo Medical AG (Biel, Switzerland); H. Lee, None; S.K. Jean, None; E.K. Kim, None; K.Y. Seo, None; Tae-im Kim, None
References
- 1. Nordan LT, Slade SG, Baker RN, Suarez C, Juhasz T, Kurtz R.. Femtosecond laser flap creation for laser in situ keratomileusis: six-month follow-up of initial U.S. clinical series. J Refract Surg. 2003; 19(1): 8–14. [DOI] [PubMed] [Google Scholar]
- 2. Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002; 13(4): 246–249. [DOI] [PubMed] [Google Scholar]
- 3. El-Massry AA, Goweida MB, Shama Ael S, Elkhawaga MH, Abdalla MF. Contralateral eye comparison between femtosecond small incision intrastromal lenticule extraction at depths of 100 and 160 mum. Cornea. 2015; 34(10): 1272–1275. [DOI] [PubMed] [Google Scholar]
- 4. Ratkay-Traub I, Ferincz IE, Juhasz T, Kurtz RM, Krueger RR.. First clinical results with the femtosecond neodynium-glass laser in refractive surgery. J Refract Surg. 2003; 19(2): 94–103. [DOI] [PubMed] [Google Scholar]
- 5. Sekundo W, Kunert K, Russmann C, et al.. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg. 2008; 34(9): 1513–1520. [DOI] [PubMed] [Google Scholar]
- 6. Liu YC, Rosman M, Mehta JS.. Enhancement after small-incision lenticule extraction: incidence, risk factors, and outcomes. Ophthalmology. 2017; 124(6): 813–821. [DOI] [PubMed] [Google Scholar]
- 7. Moshirfar M, McCaughey MV, Reinstein DZ, Shah R, Santiago-Caban L, Fenzl CR.. Small-incision lenticule extraction. J Cataract Refract Surg. 2015; 41(3): 652–665. [DOI] [PubMed] [Google Scholar]
- 8. Chan C, Lawless M, Sutton G, Versace P, Hodge C.. Small incision lenticule extraction (SMILE) in 2015. Clin Exp Optom. 2016; 99(3): 204–212. [DOI] [PubMed] [Google Scholar]
- 9. Kamiya K, Shimizu K, Igarashi A, Kobashi H.. Visual and refractive outcomes of femtosecond lenticule extraction and small-incision lenticule extraction for myopia. Am J Ophthalmol. 2014; 157(1): 128–134.e122. [DOI] [PubMed] [Google Scholar]
- 10. Jun I, Kang DSY, Reinstein DZ, et al.. Clinical outcomes of SMILE with a triple centration technique and corneal wavefront-guided transepithelial PRK in high astigmatism. J Refract Surg. 2018; 34(3): 156–163. [DOI] [PubMed] [Google Scholar]
- 11. Jun I, Kang DSY, Arba-Mosquera S, et al.. Comparison of clinical outcomes between vector planning and manifest refraction planning in SMILE for myopic astigmatism. J Cataract Refract Surg. 2020; 46(8): 1149–1158. [DOI] [PubMed] [Google Scholar]
- 12. Shah R, Shah S, Sengupta S.. Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery. J Cataract Refract Surg. 2011; 37(1): 127–137. [DOI] [PubMed] [Google Scholar]
- 13. Sekundo W, Kunert KS, Blum M.. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol. 2011; 95(3): 335–339. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez J, Rodriguez-Vallejo M, Martinez J, Tauste A, Pinero DP.. Corneal biomechanics after laser refractive surgery: Unmasking differences between techniques. J Cataract Refract Surg. 2018; 44(3): 390–398. [DOI] [PubMed] [Google Scholar]
- 15. Reinstein DZ, Archer TJ, Randleman JB.. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg. 2013; 29(7): 454–460. [DOI] [PubMed] [Google Scholar]
- 16. Sinha Roy A, Dupps WJ Jr., Roberts CJ. Comparison of biomechanical effects of small-incision lenticule extraction and laser in situ keratomileusis: finite-element analysis. J Cataract Refract Surg. 2014; 40(6): 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damgaard IB, Ivarsen A, Hjortdal J.. Refractive correction and biomechanical strength following SMILE with a 110- or 160-mum cap thickness, evaluated ex vivo by inflation test. Invest Ophthalmol Vis Sci. 2018; 59(5): 1836–1843. [DOI] [PubMed] [Google Scholar]
- 18. Shen Y, Chen Z, Knorz MC, Li M, Zhao J, Zhou X.. Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J Refract Surg. 2014; 30(5): 310–318. [DOI] [PubMed] [Google Scholar]
- 19. Wang D, Liu M, Chen Y, et al.. Differences in the corneal biomechanical changes after SMILE and LASIK. J Refract Surg. 2014; 30(10): 702–707. [DOI] [PubMed] [Google Scholar]
- 20. Wu D, Wang Y, Zhang L, Wei S, Tang X.. Corneal biomechanical effects: small-incision lenticule extraction versus femtosecond laser-assisted laser in situ keratomileusis. J Cataract Refract Surg. 2014; 40(6): 954–962. [DOI] [PubMed] [Google Scholar]
- 21. Sefat SM, Wiltfang R, Bechmann M, Mayer WJ, Kampik A, Kook D.. Evaluation of changes in human corneas after femtosecond laser-assisted LASIK and small-incision lenticule extraction (SMILE) using non-contact tonometry and ultra-high-speed camera (Corvis ST). Curr Eye Res. 2016; 41(7): 917–922. [DOI] [PubMed] [Google Scholar]
- 22. Mattila JS, Holopainen JM.. Bilateral ectasia after femtosecond laser-assisted small incision lenticule extraction (SMILE). J Refract Surg. 2016; 32(7): 497–500. [DOI] [PubMed] [Google Scholar]
- 23. El-Naggar MT. Bilateral ectasia after femtosecond laser-assisted small-incision lenticule extraction. J Cataract Refract Surg. 2015; 41(4): 884–888. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Cui C, Li Z, et al.. Corneal ectasia 6.5 months after small-incision lenticule extraction. J Cataract Refract Surg. 2015; 41(5): 1100–1106. [DOI] [PubMed] [Google Scholar]
- 25. Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF.. Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008; 24(1): S85–89. [DOI] [PubMed] [Google Scholar]
- 26. Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD.. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003; 110(2): 267–275. [DOI] [PubMed] [Google Scholar]
- 27. Guell JL, Verdaguer P, Mateu-Figueras G, et al.. SMILE procedures with four different cap thicknesses for the correction of myopia and myopic astigmatism. J Refract Surg. 2015; 31(9): 580–585. [DOI] [PubMed] [Google Scholar]
- 28. Liu M, Zhou Y, Wu X, Ye T, Liu Q.. Comparison of 120- and 140-mum SMILE cap thickness results in eyes with thick corneas. Cornea. 2016; 35(10): 1308–1314. [DOI] [PubMed] [Google Scholar]
- 29. Weng S, Liu M, Yang X, et al.. Evaluation of human corneal lenticule quality after SMILE with different cap thicknesses using scanning electron microscopy. Cornea. 2018; 37(1): 59–65. [DOI] [PubMed] [Google Scholar]
- 30. He M, Wang W, Ding H, Zhong X.. Comparison of two cap thickness in small incision lenticule extraction: 100mum versus 160mum. PLoS One. 2016; 11(9): e0163259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu D, Liu C, Li B, Wang D, Fang X.. Influence of cap thickness on corneal curvature and corneal biomechanics after SMILE: A prospective, contralateral eye study. J Refract Surg. 2020; 36(2): 82–88. [DOI] [PubMed] [Google Scholar]
- 32. Lee H, Kang DSY, Reinstein DZ, et al.. Adjustment of spherical equivalent correction according to cap thickness for myopic small incision lenticule extraction. J Refract Surg. 2019; 35(3): 153–160. [DOI] [PubMed] [Google Scholar]
- 33. Lee H, Roberts CJ, Ambrósio R Jr., Elsheikh A, Kang DSY, Kim TI. Effect of accelerated corneal crosslinking combined with transepithelial photorefractive keratectomy on dynamic corneal response parameters and biomechanically corrected intraocular pressure measured with a dynamic Scheimpflug analyzer in healthy myopic patients. J Cataract Refract Surg. 2017; 43(7): 937–945. [DOI] [PubMed] [Google Scholar]
- 34. Cheng ZY, He JC, Zhou XT, Chu RY.. Effect of flap thickness on higher order wavefront aberrations induced by LASIK: a bilateral study. J Refract Surg. 2008; 24(5): 524–529. [DOI] [PubMed] [Google Scholar]
- 35. Lim DH, Keum JE, Ju WK, Lee JH, Chung TY, Chung ES.. Prospective contralateral eye study to compare 80- and 120-mum flap LASIK using the VisuMax femtosecond laser. J Refract Surg. 2013; 29(7): 462–468. [DOI] [PubMed] [Google Scholar]
- 36. Seven I, Vahdati A, Pedersen IB, et al.. Contralateral eye comparison of SMILE and flap-based corneal refractive surgery: Computational analysis of biomechanical impact. J Refract Surg. 2017; 33(7): 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slade SG. Thin-flap laser-assisted in situ keratomileusis. Curr Opin Ophthalmol. 2008; 19(4): 325–329. [DOI] [PubMed] [Google Scholar]
- 38. Eliasy A, Chen KJ, Vinciguerra R, et al.. Determination of corneal biomechanical behavior in-vivo for healthy eyes using CorVis ST tonometry: stress-strain index. Front Bioeng Biotechnol. 2019; 7: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ljubimov AV, Saghizadeh M.. Progress in corneal wound healing. Prog Retin Eye Res. 2015; 49: 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Calkins JL, Hochheimer BF, Stark WJ.. Corneal wound healing: holographic stress-test analysis. Invest Ophthalmol Vis Sci. 1981; 21(2): 322–334. [PubMed] [Google Scholar]
- 41. Bryant MR, Szerenyi K, Schmotzer H, McDonnell PJ.. Corneal tensile strength in fully healed radial keratotomy wounds. Invest Ophthalmol Vis Sci. 1994; 35(7): 3022–3031. [PubMed] [Google Scholar]