Abstract
Novel targeted agents used in therapy of lymphoid malignancies, such as inhibitors of B-cell receptor-associated kinases, are recognized to have complex immune-mediated effects. NEDD8-activating enzyme (NAE) has been identified as a tractable target in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma. We and others have shown that pevonedistat (TAK-924), a small-molecule inhibitor of NAE, abrogates NF-κB signaling in malignant B cells. However, NF-κB pathway activity is indispensable in immune response, and T-cell function is altered in patients with CLL. Using T cells derived from patients with CLL, we demonstrate that although targeting NAE results in markedly differential expression of NF-κB-regulated genes and downregulation of interleukin (IL)-2 signaling during T-cell activation, T cells evade apoptosis. Meanwhile, NAE inhibition favorably modulates polarization of T cells in vitro, with decreased Treg differentiation and a shift toward TH1 phenotype, accompanied by increased interferon-γ production. These findings were recapitulated in vivo in immunocompetent mouse models. T cells exposed to pevonedistat in washout experiments, informed by its human pharmacokinetic profile, recover NAE activity, and maintain their response to T-cell receptor stimulation and cytotoxic potential. Our data shed light on the potential immune implications of targeting neddylation in CLL and lymphoid malignancies.
Introduction
Introduction of targeted therapies into daily clinical practice, including inhibitors of B-cell receptor (BCR)-associated kinases and BH3-mimetics, led to a significant improvement in outcomes of patients with chronic lymphocytic leukemia (CLL), regardless of their genetic features [1, 2]. However, resistance to novel agents is inevitable due to target mutations and activation of compensatory pro-survival signaling pathways. Furthermore, such therapies demonstrate limited efficacy in other lymphoid malignancies, providing further justification for drug development. In contrast to the unequivocally immunosuppressive effect of chemotherapy, novel targeted agents influence the immune system in a manner that is often unpredictable, due to both on- and off-target effects. Immunomodulatory activities of Bruton tyrosine kinase (BTK) inhibitors (ibrutinib, acalabrutinib) and phosphoinotiside-3 kinase inhibitors (idelalisib, duvelisib) are now better characterized. While some are favorable in that they may enhance both antitumor and anti-infectious immune responses [3, 4], others underlie therapy complications [5, 6]. In this context, understanding the immune effects of targeted therapies is critical to predict and mitigate adverse events. Just as importantly, such knowledge will inform rational drug combinations, either with other targeted agents or immune checkpoint inhibitors.
The ubiquitin-proteasome system has emerged as a vulnerable target with demonstrated clinical efficacy of proteasome inhibitors (e.g., bortezomib) and E3 ligase (cereblon) modulators (e.g., lenalidomide) in lymphoid neoplasia [7]. Our group and others have identified NEDD8-activating enzyme (NAE) as a tractable target in CLL and non-Hodgkin lymphoma [8–10]. NAE is indispensable to ensure that neddylated cullin-RING E3 ligases (CRLs) facilitate the ubiquitination and subsequent degradation of their substrates. Pevonedistat (TAK-924, MLN4924), an investigational NAE inhibitor, prevents neddylation of CRLs, leading to the stabilization of substrates including phosphorylated inhibitor of NF-κB (pIκBα) [8, 11]. We have demonstrated that pevonedistat abrogates stromal-mediated NF-κB signaling and thereby induces apoptosis of CLL cells [10]. However, NF-κB pathway activity is instrumental in T-cell activation and CD4+ T-cell differentiation, including polarization toward TH2 and Treg subsets [12, 13]. Despite this, the data regarding the role of neddylation in regulation of immune cell differentiation and function are sparse. Here we evaluated the effects of pharmacologic inhibition of NAE on T-cell function in context of CLL in vitro and in vivo.
Materials and methods
Patient samples and cell culture
Following approval by the Institutional Review Board and provision of written consent, peripheral blood was obtained from CLL patients treated at Oregon Health and Science University. Peripheral blood mononuclear cells were isolated using standard Ficoll-Hypaque technique (Amersham). Red blood cells were lysed using ACK buffer (Thermo Fisher Scientific). Where indicated, T cells were enriched using Dynabeads FlowComp Human CD3 Kit (Thermo Fisher Scientific). Primary cells were cultured in RPMI-1640 medium supplemented with 15% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2mM L-glutamine, 25 mM HEPES, 100 μM nonessential amino acids, and 1 mM sodium pyruvate (Lonza). T cells were activated using 0.5 μg/mL plate-bound anti-CD3 (clone UCHT1) and 0.5 μg/mL soluble anti-CD28 (clone CD28.2) (BD Biosciences). Mouse fibroblast cell line engineered to express CD40L (L4.5), OCI-LY3, and OCI-LY19 cells were obtained from DSMZ. Pevonedistat (TAK-924) was provided by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. BMS-345541 was purchased from Sigma-Aldrich.
Statistical analysis
At least three biological replicates were used in all experiments shown throughout the manuscript, unless noted otherwise. Statistical analysis was performed with Student’s t test or two-way ANOVA with Tukey’s multiple comparisons test, when indicated, in GraphPad Prism software. *p < 0.05 and **p < 0.01 throughout the manuscript.
Please see Supplementary information for additional details (Supplemental methods; Supplementary Tables 1 and 2).
Results
Pharmacologic targeting of NAE attenuates T-cell receptor (TCR) signaling
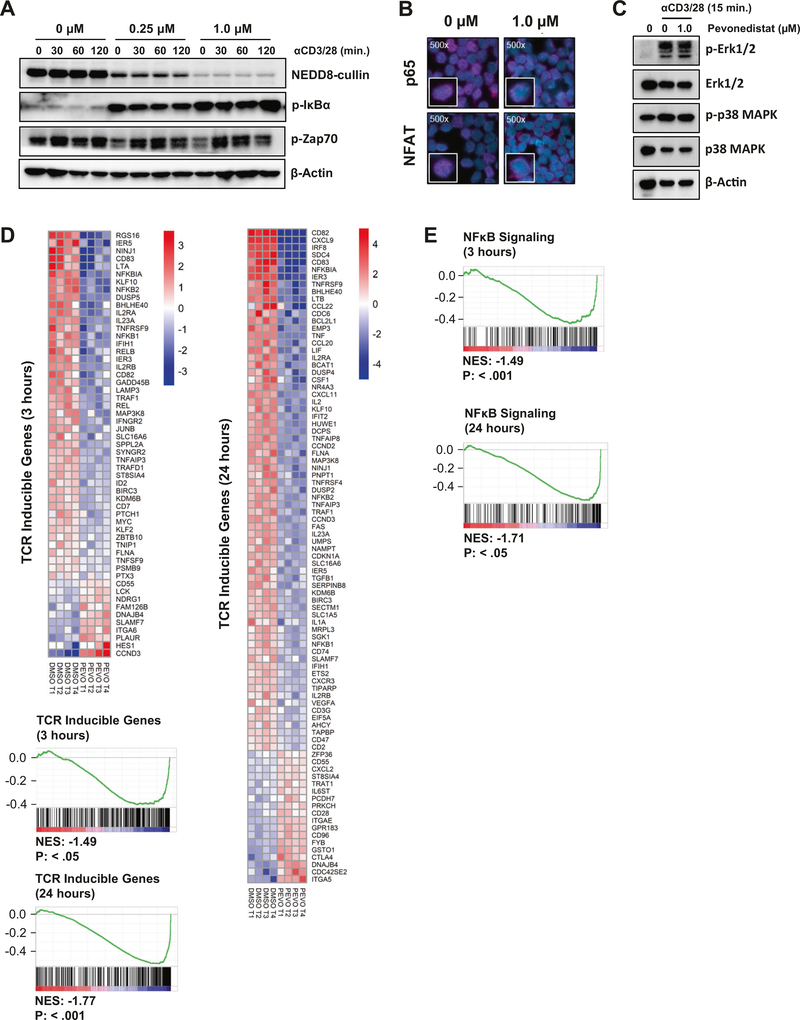
We first determined whether neddylation was involved in TCR signal transduction and T-cell activation. FACS-sorted naive CD4+ T cells from patients with CLL were preincubated with pevonedistat or vehicle control for 1 h and then stimulated with αCD3/αCD28 in the presence of the compound. TCR crosslinking for up to 2 h did not impact CRL neddylation (Fig. 1a). As expected, treatment with the NAE inhibitor pevonedistat led to dose- and time-dependent decrease in neddylated CRLs in the stimulated CD4+ T cells. NAE inhibition had no effect on activation of ZAP-70, a proximal TCR kinase (Fig. 1a and Supplementary Fig. 1). However, treatment of T cells with pevonedistat induced accumulation of pIκBα and thereby prevented nuclear translocation of the NF-κB transcription factor RelA/p65 (Fig. 1a, b). Interestingly, pevonedistat-treated T cells demonstrated reduced nuclear localization of NFAT (Fig. 1b), suggesting that neddylation may regulate additional events following TCR engagement. NAE inhibition led to no detectable change in the TCR-induced phosphorylation of ERK or p38 MAPK (Fig. 1c).
Fig. 1. Targeting NAE attenuates TCR signaling.
a T cells from patients with CLL were treated with pevonedistat at the indicated doses for 1 h, followed by TCR stimulation with 0.5 μg/mL aCD3/28. Cells were subjected to immunoblotting at the indicated timepoints. b CD3+ T cells were treated with 1 μM pevonedistat for 1 h, followed by TCR stimulation for 24 h. Protein expression was analyzed by immunocytochemistry. c–e Naive CD4+CD45RA+CD45RO− T cells from patients with CLL (four biological replicates) were preincubated with pevonedistat (1 μM) for 1 h and then subjected to TCR crosslinking. Cell lysate was harvested and subjected to immunoblotting (c). RNA was isolated after 3 (d) or 24h (e) and microarray analysis was performed as described in “Methods.” The heat maps represent a change in expression of the putative TCR target genes. Blue represents gene downregulation and red represents gene upregulation across the individual samples. GSEA demonstrate results for the hallmark TCR-inducible genes and NF-κB targets. NES normalized enrichment score.
We next employed gene expression profiling to evaluate the effect of NAE inhibition on TCR-regulated genes in naive CD4+ T cells (Supplementary Fig. 2a). Of the genes incorporated in the probe set, 21,448 were expressed. Using a cutoff of at least 1.5-fold change we determined that expression of genes known to be regulated by TCR signaling was significantly affected by pevonedistat at 3 h (p < 0.05; of the 55 genes, 37 [67%] were downregulated) as well as at 24 h (p < 0.01; of the 91 genes, 73 [80%] were downregulated; Fig. 1d). We then employed gene-set enrichment analysis (GSEA) to assess the distribution of a list of 252 TCR-regulated targets within our sequencing dataset (Supplementary Table 3). The majority of those genes were strongly underrepresented with a normalized enrichment score less than −1 at both studied timepoints (p < 0.05; Fig. 1d). Given the critical importance of NF-κB in mediating TCR effects [14], we conducted GSEA of the NF-κB signaling pathway in pevonedistat-treated cells. Similar to TCR-regulated genes, we saw a strong downregulation of the NF-κB transcriptional targets at both 3 and 24 h (p < 0.05; Fig. 1e). Several groups of NF-κB target genes were downregulated in our dataset, including antiapoptotic BCL2 family members (BCL2A1, BCL2L1), genes involved in cell cycle progression (CDC6, CDC25A, CCDN3, GADD45B, CDKN1A, MYC), and chemokines (CCL20, CCL22, CXCR3, CXCL9, CXCL11). Quantitative RT-PCR confirmed dose-dependent downmodulation of the NF-κB-regulated genes by pevonedistat (Supplementary Fig. 3a; BMS-345541, an IκB kinase inhibitor, was used as control). In addition, NF-κB was identified as the most enriched binding motif in the significantly downregulated genes at both 3 and 24 h (Supplementary Fig. 3b). Thus, NAE inhibition impeded downstream TCR signaling via interference with the NF-κB pathway.
Effect of NAE inhibition on T-cell activation
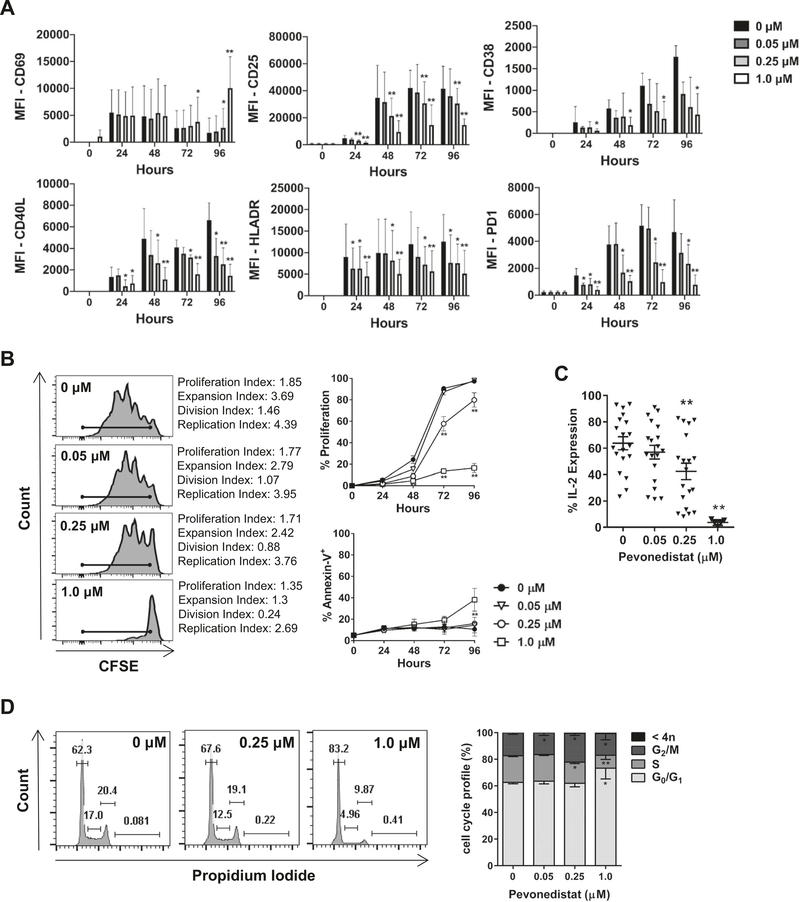
Given the above findings, we examined the effect of NAE inhibition on T-cell activation. We observed rapid upregulation of the early activation marker CD69 in CD4+ T cells despite NAE inhibition, which persisted for at least 96 h of stimulation (Fig. 2a and Supplementary Fig. 4). By contrast, treatment with pevonedistat led to a diminution of late T-cell activation in a dose-dependent manner. While low-to-intermediate doses of pevonedistat (0.05, 0.25 μM) led to a minimal reduction in activation markers CD25 (IL-2Rα), CD38, and CD40L over the course of 96 h, continuous exposure to 1 μM pevonedistat significantly reduced their expression by 48–72 h (p < 0.05; Fig. 2a and Supplementary Fig. 4). Furthermore, we observed dose-dependent downmodulation of HLA-DR, CD38, and T-cell exhaustion marker PD-1 at 48–96 h at all tested drug concentrations compared with vehicle control (Fig. 2a). Expansion of the CD3/28-stimulated T cells was mostly intact following exposure to low-to-intermediate concentrations of pevonedistat, but fully abrogated by 1 μM of the drug, accompanied by a significant reduction of interleukin (IL)-2 expressing cells. There was no increase in apoptosis of CD3/28-stimulated T cells under those conditions (Fig. 2b, c). We observed similar effects when T cells obtained from healthy donors were treated with pevonedistat (Supplementary Fig. 5).
Fig. 2. Pevonedistat influences T-cell activation and proliferation.
Magnetically enriched CD3+ T cells were activated with 0.5 μg/mL αCD3/28 in the presence of pevonedistat or vehicle control. Cells were analyzed in the CD4+ gate. Data are mean ± standard error (SE). a, b Surface expression of activation markers, cell proliferation (following CFSE staining), and apoptosis (annexin V) were analyzed by flow cytometry at the indicated timepoints as noted in “Methods” (N = 6). A representative image of CFSE distribution after 72 h of TCR engagement is shown. Proliferation was defined as the proportion of cells that have undergone at least one mitotic division as shown by CFSE peak dilutions. Two-way ANOVA and Tukey’s multiple comparison test were performed for statistical analysis. *p < 0.05, **p < 0.01 vs. control within each timepoint. c Following 72 h TCR engagement (in the presence or not of pevonedistat), T cells were restimulated for 5 h with PMA/ionomycin with monensin and cytokine expression was analyzed by flow cytometry. d Following 72 h TCR engagement (in the presence or not of pevonedistat), T cells were subjected to cell cycle analysis as described in “Methods.” *p < 0.05, **p < 0.01 vs. control.
We have previously observed that when CLL cells are induced to proliferate in the presence of pevonedistat, they sustained DNA rereplication, checkpoint activation, and cell cycle arrest in G2 phase due to accumulation of Cdt1 [15]. Interestingly, while targeting neddylation in T cells was accompanied by reduced S phase entry, we saw no evidence of G2/M arrest or rereplication (4N), indicating that reduced T-cell proliferation was not related to perturbations in cell cycle (Fig. 2d). In sum, NAE inhibition with pevonedistat resulted in reduced T-cell activation and was accompanied by reduced IL-2 secretion and cell proliferation in response to high concentrations of the drug, suggesting that neddylation may be involved in immune response.
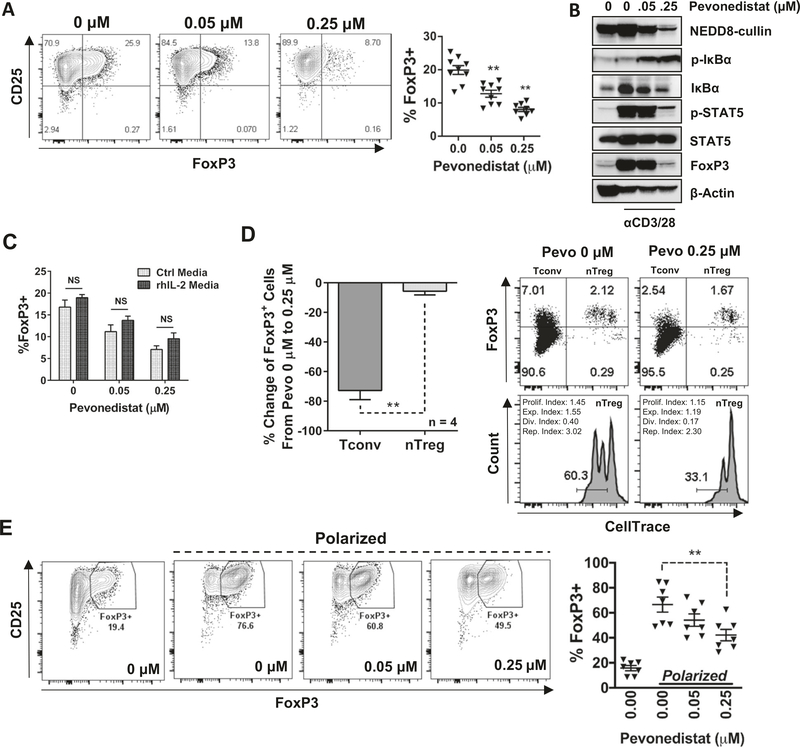
NAE inhibition downregulates inducible Tregs (iTregs)
NF-κB activation is indispensable in immune response. Among others, intact NF-κB signaling is required for the differentiation of Tregs and the stability of forkhead box P3 (FoxP3), the master regulator of Treg immunosuppressive function [16, 17]. Hence, we tested whether NAE inhibition interferes with expansion of Tregs. Magnetically enriched CD3+ T cells from patients with CLL were crosslinked with αCD3/28 for 24 h, followed by concurrent exposure to 0.05–0.25 μM pevonedistat for an additional 72 h. The drug concentrations and timing of exposure were chosen to minimize the impact on T-cell activation and proliferation. Targeting NAE led to a dose-dependent reduction of FoxP3-expressing CD4+ T cells (Fig. 3a). Reduced expression of FoxP3 was accompanied by phospho-IκBα induction and loss of phospho-STAT5 (Fig. 3b), a transcription factor crucial to FoxP3 stability in natural Tregs (nTregs) as well as FoxP3 induction in FoxP3-negative T cells [18]. Interestingly, recombinant IL-2 only partially rescued FoxP3 expression, indicating that the deregulated NF-κB/IL-2 axis did not fully account for the loss of iTregs (Fig. 3c).
Fig. 3. Pevonedistat prevents differentiation of iTregs.
Magnetically enriched CD3+ cells were activated with 0.5 μg/mL αCD3/28 for 24 h. Thereafter, stimulation continued in the presence of the indicated doses of pevonedistat or vehicle control for an additional 72 h. Data are mean ± standard error (SE). Student’s t test was performed for statistical analysis, *p < 0.05, **p < 0.01 vs. control. a FoxP3 expression was quantified in CD4+ T cells by flow cytometry. b Cells were collected after 72 h of drug exposure and whole-protein lysates were subjected to immunoblotting. c nTregs and Tconvs were separated by FACS. nTregs were then stained with CellTrace dye and remixed with Tconvs. After 72 h of TCR engagement, FoxP3 expression was quantified within both nTregs and Tconvs by flow cytometry. d Enriched CD3+ T cells were incubated with or without exogenous rh-IL-2 (20 ng/mL) for the entirety of activation and drug treatment. FoxP3 expression within CD4+ T cells was quantified by flow cytometry. e Sorted naive CD4+ T cells were subjected to Treg-polarizing conditions for 96 h, in the presence of the indicated concentrations of pevonedistat. FoxP3 expression was analyzed as previously.
Loss of FoxP3-expressing cells under the above circumstances could be attributed to either reduced iTregs differentiation or depletion of nTregs. To elucidate this, we flow-sorted nTregs (CD3+CD4+CD25highCD127low) and conventional T cells (Tconvs) (CD3+CD4+CD25lowCD127high) (Supplementary Fig. 2b). nTregs were labeled with CellTrace proliferation dye, and mixed back in with unlabeled Tconvs. This cell mixture was then stimulated (αCD3/28) for 96 h with or without pevonedistat. Contrary to nTreg population, Tconvs exhibited a 75% reduction of FoxP3 (Fig. 3d). Meanwhile, proliferation of nTregs was reduced in the presence of pevonedistat (Fig. 3d). In addition, we observed a significant reduction of Treg differentiation when naive CD4+ T cells were incubated under the Treg-polarizing conditions (Fig. 3e). Similar effects of pevonedistat were observed in healthy donor T cells (Supplementary Fig. 6). These data support a notion that targeting NAE thwarts FoxP3 in Tconvs, thereby impeding the formation of iTregs.
Neddylation modulates TH polarization
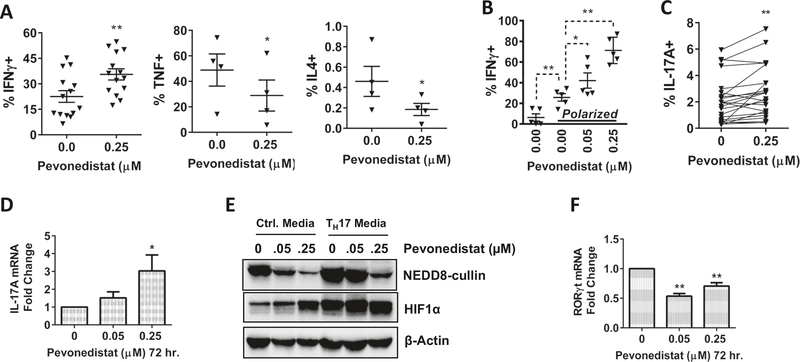
Following the reduction of Tregs, we sought to examine how pevonedistat can modulate the polarization of other T-cell subsets. Here, magnetically enriched CD3+ T cells were activated for 24 h prior to initiation of the concurrent pevonedistat exposure for an additional 48 h. We found that the proportion of TH1 IFNγ-producing cells moderately increased, whereas TH2 IL-4-producing cells decreased under these conditions (Fig. 4a). In addition, production of TNF-α, an NF-κB-regulated cytokine, was decreased. To examine if these changes to TH-phenotypes were due to modulated T-cell differentiation vs. selective expansion of pregenerated TH-cells, we incubated naive CD4+ T cells in TH1-polarizing conditions. The IFNγ-producing T-cell fraction significantly expanded under those conditions, followed by additional expansion upon exposure to the NAE inhibitor (Fig. 4b).
Fig. 4. Pevonedistat alters the polarization of CD4+ T cells.
Magnetically enriched CD3+ cells were activated with 0.5 μg/mL αCD3/28 for 24 h prior to exposure of pevonedistat. Cells resumed stimulation for 72 h in the presence of the drug. Data are mean ± standard error (SE). Student’s t test was performed for statistical analysis, *p < 0.05, **p < 0.01 vs. control. a CD3+ T cells were activated for 96 h. Thereafter, cells were restimulated with PMA/ionomycin in the presence of monensin for 5 h. Cytokine expression within CD4+ T cells was quantified by flow cytometry. b Sorted naive CD4+ T cells were incubated in TH1-polarizing conditions for 96 h, IFNγ cytokine expression was measured following the procedures described above. c–f CD3+ T cells were TCR-stimulated in the presence of pevonedistat for 10 days. IL-17A cytokine expression within CD4+ T cells was quantified as previously described above (c). Cells were harvested and mRNA expression was quantified by q-RT-PCR (d, f) or total lysate and subjected to immunoblotting (e).
The balance between the inhibitory Tregs and the proinflammatory TH17 cells is under the control of extracellular cytokine signaling and intracellular molecular mechanisms [19, 20]. We detected a small but statistically significant expansion of TH17 cells when TCR-stimulated CD4+ T cells were exposed to pevonedistat (Fig. 4c). Following treatment with 0.25 μM pevonedistat, the fraction of IL-17-expressing CD4+ T cells increased from 2.1 ± 1.7 to 2.7 ± 1.9% (p = 0.004). This was accompanied by upregulation of the IL-17 mRNA transcription in this cell population (Fig. 4d). The hypoxia-inducible factor 1α (HIF-1α) may contribute to the Treg/TH17 balance, where HIF-1α has previously been shown to enhance TH17 differentiation by directly activating transcription of the TH17 master regulator RORγt, followed by recruitment of p300 and RORγt to the IL-17 promoter [21]. Relevant to our work, HIF-1α is a CRL target [22]. We found that TCR-stimulated T cells exhibited stabilization of HIF-1α protein upon treatment with pevonedistat, yet RORγt mRNA transcript was not induced (Fig. 4e, f). Thus, targeting neddylation in vitro had minimal impact on TH17 cells in our study.
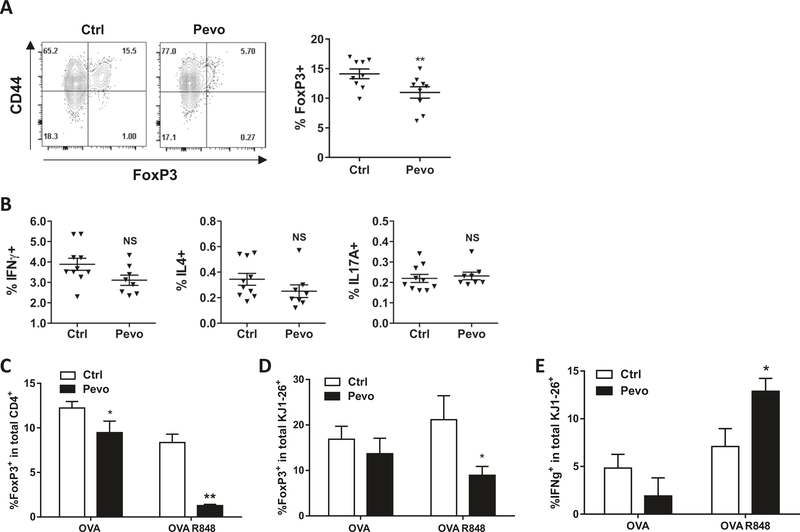
We next examined if the above modulations in TH subsets occurred in vivo. Since we were interested in the effects of pevonedistat on TH1/2 polarization and Treg differentiation, we used BALB/c mice, a prototypic TH2 mouse strain [23, 24]. Mice received either pevonedistat subcutaneously daily at 60 mg/kg or vehicle control for a total of 24 days. At the end of the experiment, splenocytes harvested from pevonedistat-treated mice showed a marked reduction in FoxP3-expressing Tregs within the CD3+/CD4+ population, compared with vehicle-treated mice (Fig. 5a). By contrast, we observed minimal changes in TH1, TH2, and TH17 subsets, and no changes in CD4/CD8 T-cell ratio (Fig. 5b, Supplementary Fig. 7a). We did not observe signs of toxicity or a significant change in mouse body weight during prolonged treatment with pevonedistat (Supplementary Fig. 7b).
Fig. 5. Pevonedistat modulates T-cell polarization in vivo.
a, b BALB/cJ mice were treated with pevonedistat (60 mg/kg) or vehicle control for 24 days (ten animals per condition). c, d Mice were immunized with ovalbumin and subjected to treatment with pevonedistat for 10 days as described in “Methods” At the end of each experiment, splenocytes were analyzed for FoxP3 expression (a, c, d) or re-activated with PMA/ionomycin with monensin to analyze cytokine expression (b, e). Cells were gated in the total CD4+ lymphocytesor CD4+KJ1–26+ (transplanted) lymphocytes as indicated. Two independent experiments were conducted, total of nine animals per condition. *p < 0.05, **p < 0.01 vs. control.
We further studied effects of pevonedistat in immunized mice. Splenocytes from mice carrying the MHC class II restricted rearranged TCR transgene (DO11.10) were transferred to recipient BALB/c mice. Mice were immunized with ovalbumin; R848 (a Toll-like receptor agonist) was used to enhance TH1 responses [25, 26]. After 10 days of treatment with pevonedistat, we again observed a reduction in FoxP3+ cells in the total CD4+ T-cell population as well as transplanted splenocytes (Fig. 5c, d). Consistent with our in vitro data, treatment with pevonedistat led to increased IFNγ production in the transplanted T-cell population subjected to Toll-like receptor agonist stimulation (Fig. 5e).
Taken together, our data suggest that targeting neddylation leads to a shift in T-cell subsets, including a significant reduction in Tregs, and polarization in favor of the TH1 phenotype.
Pevonedistat-treated T cells maintain antitumor potential
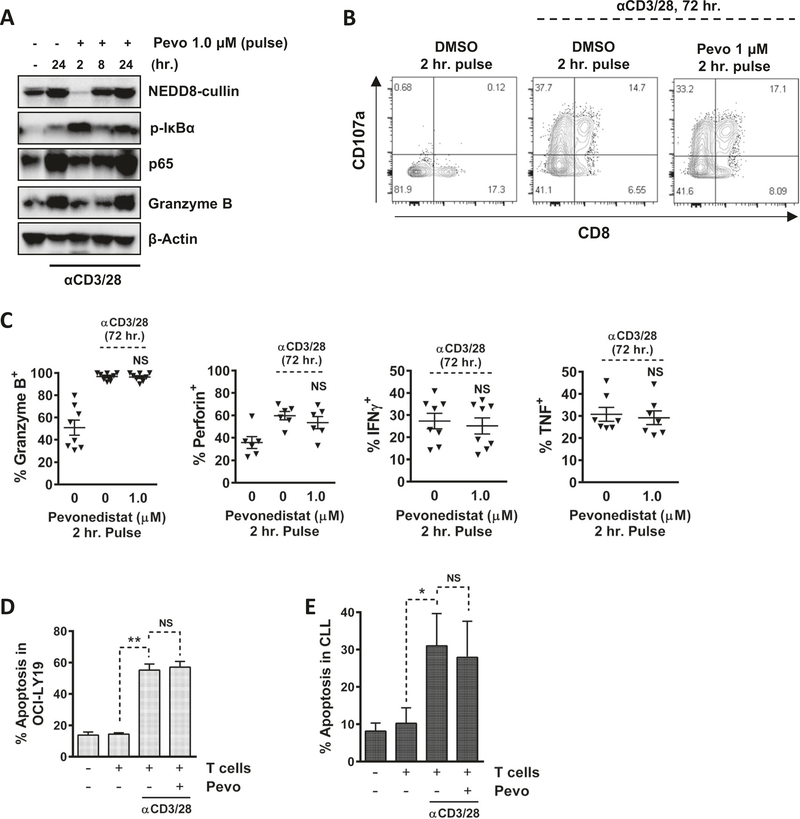
We next asked if T cells exposed to pevonedistat are able to recover NAE activity and exert antitumor immunity. Past human pharmacokinetic studies have shown that pevonedistat delivered intravenously exhibits a T1/2 of ~8 h [27]. The plasma drug concentration peaks at the end of 1 h infusion and then rapidly declines in a biphasic manner. In order to mimic this pharmacokinetic exposure in vitro, we conducted washout experiments. Resting CD3+ T cells were exposed to 1 μM pevonedistat for 2 h, drug was washed off, and cells were stimulated with αCD3/28. We observed an expected sharp decline in neddylated CRLs, followed by rapid accumulation of pIκBα (Fig. 6a). Cullin neddylation began to recover by 8 h of TCR stimulation (6 h after washout), accompanied by a decrease in pIkBα. A complete recovery was observed at 24 h. Notably, pIκBα was again upregulated at that timepoint, likely indicating a general activation of the NF-κB pathway, as confirmed by upregulation of p65 protein level (Fig. 6a). As pevonedistat forms an irreversible covalent bond with NEDD8 at the NAE active site, this recovery in neddylation activity is likely due to synthesis of new NAE in T cells.
Fig. 6. Pulse-exposure mimicking pevonedistat pharmacokinetics.
Magnetically enriched CD3+ T cells were pretreated with 1.0 μM pevonedistat for 2 h (pulse treatment). Drug was washed out and cells were crosslinked with 0.5 μg/mL αCD3/28. Data are mean ± standard error (SE). Student’s t test was performed for statistical analysis, *p < 0.05, **p < 0.01 vs. control. a Cells were harvested at the indicated timepoints and whole-protein lysates were subjected to immunoblotting. b Following pevonedistat pulse treatment (1.0 μM, 2 h) and then TCR stimulation (72 h), cells were stained with CD107a antibody for 1 h, followed by the addition of brefeldin A for 4 h. CD107A expression was analyzed by flow cytometry within the CD8+ T-cell population. c Following pevonedistat pulse treatment (1.0 μM, 2 h) and then TCR stimulation (72 h), cells were incubated with brefeldin A for 4 h. Cells were then harvested and analyzed by flow cytometry. Cytokine expression was measured within CD8+ population. d CD3+ T cells were pretreated with pevonedistat for 2 h and subjected to TCR stimulation for additional 24 h. T cells were incubated with allogeneic CFSE-stained OCI-LY19 cells at a 20:1 ratio for additional 48 h. OCI-LY19 cells were analyzed for apoptosis (annexin V). e Following isolation from blood, CD3+ T cells were subjected to drug treatment and CD3/28 crosslinking as above, while CLL cells were stimulated with CD40L-conditioned media for 24 h. Cells were then mixed at a 20:1 T-cell:CLL ratio for additional 48 h (without drug). Apoptosis was determined within the CD19+ population by flow cytometry (annexin V).
Cytotoxic CD8+ T cells expressed the degranulation marker (CD107a; Fig. 6b) and secreted cytotoxic cytokines following pulse treatment with pevonedistat (Fig. 6c). No change in IFNγ or TNF synthesis was observed. To test if T cells retained antitumor properties after pulse exposure to pevonedistat, T cells were incubated with OCI-LY19 cells, a diffuse-large B-cell lymphoma cell line, at a ratio of 20:1, respectively, for 48 h. Apoptosis of OCI-LY19 cells was significantly enhanced in the presence of activated allogeneic T cells, and pulse treatment of T cells with pevonedistat did not compromise cytotoxicity (Fig. 6d). We then conducted a similar experiment to evaluate the effect of NAE inhibition on the autologous T-cell-mediated cytotoxicity. Here, activated T cells pretreated with pevonedistat or not were incubated with CD40L-stimulated CLL cells from the same patient (CD40L stimulation has been previously shown to confer an antigen-presenting capacity to CLL cells) [28]. Under those conditions, pevonedistat-treated activated T cells continued to exhibit autologous cytotoxicity (Fig. 6e).
Discussion
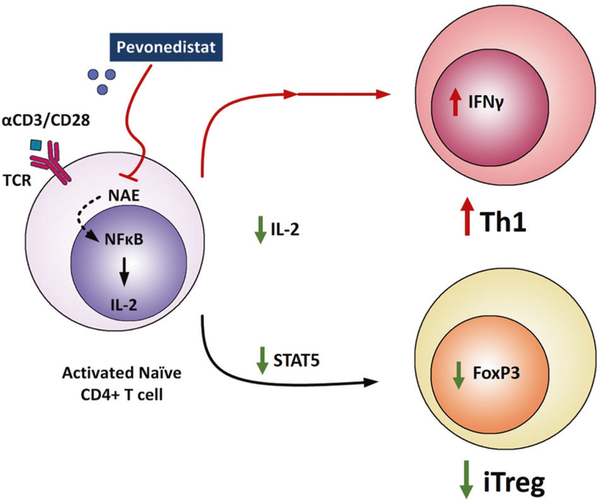
Novel agents used in therapy of CLL are recognized to have complex immune-mediated effects [29]. The BTK inhibitor ibrutinib may improve T-cell function through downmodulation of T-cell exhaustion markers, a shift in T-cell polarization in favor of the inflammatory TH1 cells, and a reduction of IL-10 secretion by CLL cells [3, 4]. In addition, ibrutinib may improve overall T-cell number and repertoire diversity as well as diminish the Treg/CD4+ T-cell ratio in patients with CLL [3, 30]. The immunomodulatory effects of drugs that interfere with protein degradation were recognized long before their effects on the ubiquitin pathway were elucidated. Lenalidomide, a cereblon/ubiquitin E3 ligase complex modulator, was shown to induce functional restoration of the lymphocyte function-associated antigen-1 in T cells, enhance formation of effective immunological synapse, and increase T-cell-mediated tumor killing, all thought to contribute to its efficacy in CLL [31, 32]. Here, we show that pevonedistat, an inhibitor of NAE, which prevents the specific activation of CRLs, has a distinct T-cell immunomodulatory effect in CLL (Fig. 7).
Fig. 7. Targeting NAE modulates T-cell subpopulations.
Pevonedistat inhibits NEDD8-activating enzyme (NAE) in T cells derived from patients with CLL, resulting in altered NF-κB-regulated gene expression and downregulation of interleukin (IL)-2 signaling during T-cell activation. Furthermore, NAE inhibition in CD4+ T cells resulted in reduced differentiation of inducible regulatory T-cells (iTregs) and polarization towards the Th1 phenotype.
Exposure of CLL patient-derived T cells to pevonedistat led to a decrease in neddylated Cullins, accumulation of pIκBα, a CRL substrate, and sequestration of p65, an NF-κB subunit, in the cytoplasm. These findings were similar to those previously reported by us in CLL cells [10, 33]. Concordant with the critical role of NF-κB in TCR signaling, we observed a significant decrease in the transcription of the NF-κB-regulated genes in TCR-stimulated T cells as early as 3 h after pevonedistat exposure. This was accompanied by a decreased expression of CD25/IL-2Rα and IL-2, an NF-κB-dependent cytokine receptor network that governs T-cell differentiation and expansion [34]. Despite this, we found that NAE inhibition did not fully abrogate T-cell activation. We observed unimpeded upregulation of the early activation marker CD69 and somewhat attenuated but robust upregulation of other markers (CD38, CD40L, HLA-DR) in TCR-stimulated T cells continuously treated with pevonedistat for up to 96 h. We have previously shown that CLL cells induced to proliferate undergo DNA rereplication, checkpoint activation, and G2 arrest when treated with pevonedistat [9]. This Cdt1-dependent phenomenon also occurs in other cancers and has been recognized as a key mechanism of NAE inhibition-mediated apoptosis [8, 35]. By contrast, cell cycle arrest was not observed in the proliferating T cells exposed to pevonedistat.
Jin et al. reported that CD4+ chimeric mouse T cells transduced with shUbc12 (an E2-conjugating enzyme of within the neddylation pathway) demonstrated reduced activation, proliferation, and IL-2 production in response to TCR stimulation with αCD3/28 [36]. This effect was partially recapitulated in murine CD4+ T cells treated with pevonedistat ex vivo and was proposed to be related to attenuated TCR-induced Erk activation [36]. By contrast, others observed that CRLs may in fact negatively regulate TCR signaling and IL-2 synthesis, and in vitro treatment of murine MA5.8ζ T-cell hybridomas with pevonedistat led to increased IL-2 production and cell proliferation [37]. Interestingly, we did not observe a reduction in TCR-induced ERK and p38 MAPK activation in response to pevonedistat. Our results align with data by Jin et al., given a significant decrease in transcription of TCR-driven genes in pevonedistat-treated T cells. However, the impact of pevonedistat on T-cell activation and proliferation was limited in human T cells derived from patients with CLL. Furthermore, drug washout experiments mimicking drug pharmacokinetics demonstrated that T cells recovered NAE activity. Under those conditions, CD8+ T cells maintained their cytotoxicity against both allogeneic and autologous neoplastic lymphoid cells. It is important to note that earlier experiments were conducted in immunocompetent mouse models, whereas our results were obtained in human T cells from patients with B-cell neoplasia, an important aspect of our study. Taken together, these data suggest that targeting NAE in the clinic is unlikely to be associated with impaired T-cell functionality.
By contrast, NAE inhibition altered T-cell polarization. Treatment of naive CD4+ T cells with pevonedistat suppressed induction of FoxP3+ Treg cells in vitro in both nonpolarizing and Treg-polarizing conditions. While NAE inhibition interfered with generation of new iTregs, it had only a minor impact on maintenance of FoxP3 expression by nTregs. One possibility was that a global reduction in T-cell activation contributed to the loss of Tregs. However, this explanation was ruled out because we observed a concomitant increase in the proportion of IFNγ-expressing TH1-like T cells, while the relative abundance of TH2 CD4+ cells was diminished in response to pevonedistat treatment. In addition, targeting NAE was accompanied by downmodulation of IL-2/STAT5 signaling, a critical pathway involved in Treg differentiation and expansion [38], and a likely explanation for iTreg diminution. Nevertheless, exogenous IL-2 only partially restored Treg polarization, suggesting that other mechanisms may also account for loss of Tregs in response to pevonedistat treatment, a subject of future studies. Our findings were confirmed in animal models where mice treated with pevonedistat exhibited a marked reduction in the proportion of FoxP3-expressing CD4+ T cells and an increase in IFNγ (TH1) production compared with vehicle-treated controls. contributes to regulation of TH17/Treg balance by the NAE will require further study.
How neddylation regulates TH1/TH2 balance remains unclear. It has been established that Tregs regulate immune response via suppression of T-cell IFNγ production and TH1 differentiation [39, 40]. Tregs also upregulate T-bet, a master transcriptional regulator of differentiation and inflammatory genes in TH1 cells, in response to IFNγ [41]. Such T-bet+ Tregs induce CXCR3 and selectively inhibit TH1 immunity. Furthermore, IL-2 is essential for the differentiation of TH2 cells [42]. Thus, pevonedistat-mediated reduction in IL-2 production and concomitant Treg suppression may foster expansion of the TH1 compartment by eliminating selective pressure toward TH2 polarization. Future experiments will be necessary to confirm this hypothesis.
Here it is critical to acknowledge that patients with CLL are immunocompromised. On one hand, CLL cells escape cytotoxic T cells through adaptive mechanisms such as upregulation of inhibitory checkpoint molecules (PD-L1, CTLA-4) and overexpression of the immunosuppressive cytokine IL-10 [43, 44]. On the other hand, deficient innate and adaptive immune responses contribute to disease progression, increased mortality from infections, and increased incidence of secondary malignancies in patients with CLL [45–47]. T cells from patients with CLL exhibit a terminally differentiated and exhausted phenotype, with an increased expression of PD-1 and CTLA-4 [46, 48], an imbalance in T-cell subpopulations in favor of Tregs and skewed polarization toward TH2 immune-suppressive phenotype [49]. Importantly, Tregs contribute to tumor progression not only in CLL but also in many other cancers. In this context, decreased induction of Tregs and a shift toward TH1 is a favorable outcome of NAE inhibition.
Tregs exist in balance with TH17 cells, which ensures a physiologic inflammatory response. Deregulated activation of TH17 cells occurs in a variety of autoimmune conditions (psoriasis, inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis) [50]. Here we report a statistically significant increase in TH17 differentiation in response to pevonedistat treatment of the TCR-stimulated T cells. However, this expansion was only minimal, consistent with clinical trial data where treatment with pevonedistat was not associated with autoimmune-mediated adverse toxicities [51]. HIF-1α, a CRL target, has been implicated in the dynamic control of TH17/Treg balance [21, 22]. We found that NAE inhibition led to the accumulation of HIF-1α in TCR-activated T cells. However, RORγt, the transcriptional target of HIF-1α responsible for the inflammatory activity of TH17 cells, was not induced in TCR-stimulated T cells following NAE inhibition. Thus, whether HIF-1α
While we have reported our results in primary T cells obtained from patients with CLL, it remains unknown whether neddylation regulates other immune cell types. We are hoping to shed additional light on the immune effects of targeting NAE in an ongoing analysis of T-cell functionality in patients with CLL and lymphoma who are treated with pevonedistat in combination with ibrutinib on a Phase I clinical trial (NCT03479268). Based on our findings, we predict that targeting neddylation will not impede T-cell immunity, but may lead to enhanced antitumor effects due to downmodulated differentiation of Tregs.
Supplementary Material
Acknowledgements
This study was supported by the Leukemia & Lymphoma Society Translational Research Program Award #6542-18 (to AVD) and by a grant from Takeda Oncology. AVD is a Leukemia and Lymphoma Society Scholar in Clinical Research (#2319-19).
Conflict of interest AVD has received research funding from Astra Zeneca, Gilead Sciences, Genentech, Aptose Biosciences, MEI Pharma, Takeda Oncology, Bayer Oncology, Verastem Oncology, and Bristol-Meyers-Squibb; honoraria from Astra Zeneca, Celgene, Curis, Genentech, Gilead Sciences, Janssen Oncology, Pharmacyclics, Seattle Genetics, TG Therapeutics, and Verastem Oncology. EFL has received research funding from Celgene, Amgen, Janssen Pharmaceuticals, Monojul, and Kyn Therapeutics. AB is employed by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Footnotes
Compliance with ethical standards
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information The online version of this article (https://doi.org/10.1038/s41375-020-0794-0) contains supplementary material, which is available to authorized users.
References
- 1.Kittai AS, Lunning M, Danilov AV. Relevance of prognostic factors in the era of targeted therapies in CLL. Curr Hematol Malig Rep. 2019;14:302–9. [DOI] [PubMed] [Google Scholar]
- 2.Shindiapina P, Brown JR, Danilov AV. A new hope: novel therapeutic approaches to treatment of chronic lymphocytic leukaemia with defects in TP53. Br J Haematol. 2014;167:149–61. [DOI] [PubMed] [Google Scholar]
- 3.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Investig. 2017;127:3052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132:1985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Dixit VM. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 2016;26:484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–23. [DOI] [PubMed] [Google Scholar]
- 9.Paiva C, Godbersen JC, Berger A, Brown JR, Danilov AV. Targeting neddylation induces DNA damage and checkpoint activation and sensitizes chronic lymphocytic leukemia B cells to alkylating agents. Cell Death Dis. 2015;6:e1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, Eastman A, et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-kappaB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20:1576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–6. [DOI] [PubMed] [Google Scholar]
- 12.Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, Eastman A, et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-κB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20:1576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh H, Ghosh S. NF-kappaB: roles and regulation in different CD4 (+) T-cell subsets. Immunol Rev. 2013;252:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul S, Schaefer BC. A new look at T cell receptor signaling to nuclear factor-kappaB. Trends Immunol. 2013;34:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Shi CC, Zhang HP, Li GQ, Li SS. MLN4924 suppresses neddylation and induces cell cycle arrest, senescence, and apoptosis in human osteosarcoma. Oncotarget. 2016;7:45263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q, Chen YH. Nuclear factor-kappaB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol. 2012;946:207–21. [DOI] [PubMed] [Google Scholar]
- 17.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–31. [DOI] [PubMed] [Google Scholar]
- 18.Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20:421–31. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. [DOI] [PubMed] [Google Scholar]
- 20.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. [DOI] [PubMed] [Google Scholar]
- 21.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. [DOI] [PubMed] [Google Scholar]
- 23.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, et al. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 1999;191:10–19. [DOI] [PubMed] [Google Scholar]
- 26.Vasilakos JP, Smith RM, Gibson SJ, Lindh JM, Pederson LK, Reiter MJ, et al. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 2000;204:64–74. [DOI] [PubMed] [Google Scholar]
- 27.Shah JJ, Jakubowiak AJ, O’Connor OA, Orlowski RZ, Harvey RD, Smith MR, et al. Phase I study of the novel investigational NEDD8-activating enzyme inhibitor pevonedistat (MLN4924) in patients with relapsed/refractory multiple myeloma or lymphoma. Clin Cancer Res. 2016;22:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krackhardt AM, Harig S, Witzens M, Broderick R, Barrett P, Gribben JG. T-cell responses against chronic lymphocytic leukemia cells: implications for immunotherapy. Blood. 2002;100:167–73. [DOI] [PubMed] [Google Scholar]
- 29.Maharaj K, Sahakian E, Pinilla-Ibarz J. Emerging role of BCR signaling inhibitors in immunomodulation of chronic lymphocytic leukemia. Blood Adv. 2017;1:1867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Q, Sivina M, Robins H, Yusko E, Vignali M, O’Brien S, et al. Ibrutinib therapy increases T cell repertoire diversity in patients with chronic lymphocytic leukemia. J Immunol. 2017;198:1740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Investig. 2008;118:2427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T-cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood. 2013;121:2704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godbersen JC, Paiva C, Danilova OV, Berger A, Brown JR, Danilov AV. Targeting neddylation effectively antagonizes nuclear factor-kappaB in chronic lymphocytic leukemia B-cells. Leuk Lymphoma. 2015;56:1566–9. [DOI] [PubMed] [Google Scholar]
- 34.Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol. 2018;36:411–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank JL, Liu XJ, Cosmopoulos K, Bouck DC, Garcia K, Bernard H, et al. Novel DNA damage checkpoints mediating cell death induced by the NEDD8-activating enzyme inhibitor MLN4924. Cancer Res. 2013;73:225–34. [DOI] [PubMed] [Google Scholar]
- 36.Jin HS, Liao L, Park Y, Liu YC. Neddylation pathway regulates T-cell function by targeting an adaptor protein Shc and a protein kinase Erk signaling. Proc Natl Acad Sci USA. 2013;110:624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friend SF, Peterson LK, Treacy E, Stefanski AL, Sosinowski T, Pennock ND, et al. The discovery of a reciprocal relationship between tyrosine-kinase signaling and cullin neddylation. PLoS ONE. 2013;8:e75200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan MY, Low JS, Tanimine N, Finn KK, Priyadharshini B, Germana SK, et al. Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep. 2018;25:1204–.e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitz A, Dominguez-Villar M. Molecular mechanisms underlying Th1-like Treg generation and function. Cell Mol Life Sci. 2017;74:4059–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen E, Zhao K, Wu C, Yang B. The suppressive effect of CD25 +Treg cells on Th1 differentiation requires cell-cell contact partially via TGF-beta production. Cell Biol Int. 2011;35:705–12. [DOI] [PubMed] [Google Scholar]
- 41.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci USA. 2004;101:3880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brusa D, Serra S, Coscia M, Rossi D, D’Arena G, Laurenti L, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27:170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francis S, Karanth M, Pratt G, Starczynski J, Hooper L, Fegan C, et al. The effect of immunoglobulin VH gene mutation status and other prognostic factors on the incidence of major infections in patients with chronic lymphocytic leukemia. Cancer. 2006;107:1023–33. [DOI] [PubMed] [Google Scholar]
- 46.Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin N Am. 2013;27:207–35. [DOI] [PubMed] [Google Scholar]
- 47.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–81. [DOI] [PubMed] [Google Scholar]
- 48.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Investig. 2005;115:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19:E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swords RT, Coutre S, Maris MB, Zeidner JF, Foran JM, Cruz J, et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood. 2018;131:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.