Abstract
In major depressive disorder (MDD), the anterior cingulate cortex (ACC) is widely related to depression impairment and antidepressant treatment response. The multiplicity of ACC subdivisions calls for a fine‐grained investigation of their functional impairment and recovery profiles. We recorded resting state fMRI signals from 59 MDD patients twice before and after 12‐week antidepressant treatment, as well as 59 healthy controls (HCs). With functional connectivity (FC) between each ACC voxel and four regions of interests (bilateral dorsolateral prefrontal cortex [DLPFC] and amygdalae), subdivisions with variable impairment were identified based on groups' dissimilarity values between MDD patients before treatment and HC. The ACC was subdivided into three impairment subdivisions named as MedialACC, DistalACC, and LateralACC according to their dominant locations. Furthermore, the impairment pattern and the recovery pattern were measured based on group statistical analyses. DistalACC impaired more on its FC with left DLPFC, whereas LateralACC showed more serious impairment on its FC with bilateral amygdalae. After treatment, FCs between DistalACC and left DLPFC, and between LateralACC and right amygdala were normalized while impaired FC between LateralACC and left amygdala kept dysfunctional. Subsequently, FC between DistalACC and left DLPFC might contribute to clinical outcome prediction. Our approach could provide an insight into how the ACC was impaired in depression and partly restored after antidepressant treatment, from the perspective of the interaction between ACC subregions and critical frontal and subcortical regions.
Keywords: anterior cingulate cortex, antidepressive agents, functional MRI, major depressive disorders, segmentation, unsupervised machine learning
This article identified the subregions of the anterior cingulate cortex with variable impairment in major depressive disorder patients and investigated the restoration pattern of subregions after antidepressant treatment.
1. INTRODUCTION
Major depressive disorder (MDD) is a notably prevalent mental health disorder, affecting estimated 264 million people worldwide (James et al., 2018). Antidepressants are widely used to treat MDD. However, their efficacy and effectiveness are debated and their mechanism of taking effect is imprecise (Cipriani et al., 2018). Therefore, it is urgent to address the functional impairment way of MDD and its functional recovery after antidepressants.
The anterior cingulate cortex (ACC) has been proven to have a critical impairment in MDD in recent research studies. Functional connectivity (FC) changes of the ACC (Rolls et al., 2018) and its altered white matter integrity have been reported in depression widely (Lichenstein, Verstynen, & Forbes, 2016). A genome‐wide study also identified different gene expression patterns in MDD at the ACC (Wray et al., 2018). Therefore, the ACC plays a vital role in addressing MDD. In addition, the ACC has some features worth exploring in terms of its connection with other brain regions. Previous research has shown that the ACC is involved in mediating both emotional and cognitive processing (Bush, Luu, & Posner, 2000). Meanwhile, the amygdala and dorsolateral prefrontal cortex (DLPFC) are thought to be the key regions in “bottom‐up” and “top‐down” brain circuitry separately, regulating emotion processing or cognitive control (Fales et al., 2008; Ongur, 2000). Thus, research on ACC connections to the amygdala and DLPFC can help to investigate the emotional and cognitive processing ability of the ACC. The DLPFC and amygdala are proven to have direct anatomical connections with ACC through cingulum bundles using diffusion tensor imaging (Lichenstein et al., 2016).
With regard to the functional impairment of the ACC in depression, functional imaging studies have demonstrated abnormal connections between the ACC and above‐mentioned regions, including the DLPFC and amygdala in depression. Decreased resting‐state FC between subgenual ACC (sgACC) and frontal cortex and hypoconnectivity between dACC and several frontal cortical regions have been observed (Cullen et al., 2009; Pannekoek et al., 2014). As for the amygdala and ACC, several studies also found abnormal connections between them. Pannekoek et al. (2014) found decreased negative connectivity between the rACC and bilateral amygdala. Another ACC subregion, pregenual ACC (pgACC), was also found to have abnormally weaker connections with limbic cortex, including the amygdala (Anand et al., 2005a). Overall, the DLPFC and amygdala were found to have impaired FCs with various ACC subregions in MDD patients.
Despite that the effect of antidepressant medications has complicated relationships with MDD pathophysiology, most studies suggested a normalized tendency of the ACC after antidepressant treatment (Posner et al., 2013). Delaveau et al. implied that ventral ACC had significantly increased activation after long‐term antidepressant brain effects (Delaveau et al., 2016). In addition, Ma (2015) added that core limbic parts of emotional network also showed a normalized tendency after antidepressant administration, where the activities of the ACC and amygdala inversely got decreased in response to negative stimuli while increased in response to positive emotions. Furthermore, major brain regions connecting to the ACC, including the DLPFC and amygdala also displayed a disrupted activation after antidepressant (Cullen et al., 2016; Ma, 2015). The amygdala was found to have a high density of 5‐HT transporters, which made it a prime site for SSRIs (Takahashi et al., 2005). It was reported that the mediating of the “fronto‐limbic” circuit that is mainly composed of the ACC, DLPFC, and amygdala is modulated by serotoninergic transmission (Vai et al., 2016).
Studies have shown that the ACC has sophisticated output and has been implicated in a wide range of diverse functions such as affective processing (Etkin, Egner, & Kalisch, 2011), conflict monitoring (Botvinick, Cohen, & Carter, 2004) and decision‐making (Haber & Behrens, 2014), which lead to the premise of segmenting the ACC into distinct subregions. In MDD, studies showed that the ACC can be segmented anatomically and functionally into distinct subregions such as sgACC, pgACC, and supragenual ACC (supACC) with each remains peculiar cytoarchitectural characteristics (Sambataro et al., 2018). Subregions can also be denominated as subgenual, rostral, and dorsal ACC, which is caused by inconsistent naming ways (Mohanty et al., 2010). Each part of ACC subregions is considered to possess a specific function and distinct impairment degree. ACC subregions have been reported suffering distinct impairment degrees in depression and inconsistent results were addressed. Cullen et al. (2009) reported decreased connection between sgACC and lateral frontal cortex while Liston et al. (2014) reported increased connection between sgACC to DMN including DLPFC here. Meanwhile, Pannekoek et al. (2014) noted reduced negative connection between the rACC and amygdala. Some questions have been raised about how impairment patterns distribute in distinct subregions and how distinct impairment patterns influence the balance between the mood regulation of “top‐down” and “bottom‐up.” In this article, we use “pattern” to represent a particular way in which multiple functional connectivities between ACC subregions and cortical–subcortical brain regions were impaired in MDD or changed after antidepressant.
A considerable amount of studies have explored depression impairment in a specific brain region by means of segmenting subregions, because segmentation has unique advantages to reflect more precise signals by dividing the specific region into parts with more consistent inner signals. It overcomed the problem that the heterogeneous signals can cover the realistic signals and cause the inconsistent results (Marusak et al., 2016). Sambataro et al. (2018) explored subregional impairment in MDD group and defined subregions by clusters where treatment effect scores correlated with local cortical volume. Ge et al. (2019) explored subregions both in MDD group and health group, and compared parcel size differences between corresponding subregions of two groups. These research studies contributed to a fine‐grained understanding of pathophysiology mechanisms exactly. However, they performed subdividing in MDD patients and healthy control (HC) separately and failed to compare their differences in a unified subregional zone, which was not intuitive enough and might miss some significant signals. Furthermore, most research studies considered impairment patterns only, without comparing them with the possible recovery patterns further.
Therefore, the present study aims to: (a) test if the human ACC can be segmented into diverse subregions based on FC dissimilarity in MDD patients compared to the healthy subjects; (b) investigate whether the brain function of MDD patients recovers in a pattern that is identical to the impairment pattern after treatment; and (c) explore the particular impaired ACC subregion that may be potential in depressive severity prediction.
2. MATERIALS AND METHODS
2.1. Participants
From December 2011 to May 2018, participants who underwent the first episode of depression were recruited from the department of psychiatry at the affiliated Brain Hospital of Nanjing Medical University.
By the Mini‐International Neuropsychiatric Interview (M.I.N.I, Chinese version, translated from English version 5.0.0) according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV), all patients were diagnosed as MDD at baseline by at least two attending doctors. The 17‐item Hamilton Rating Scale for Depression (HAMD) was performed to assess the depression severity. The inclusion criteria for depressed subjects were: (a) a 17‐item HAMD score at least 17; (b) being between the ages of 18 and 55 years and having ability to give voluntary informed consent; (c) current depressive episode having the duration longer than 1 month but shorter than 24 months; and (d) being right handed and Chinese Han. The criteria to exclude depressed subjects including: (a) having concurrent comorbidity with other DSM‐IV Axis‐I psychiatric disorders, including bipolar disorder, schizophrenia, schizoaffective disorder, or anxiety disorder; (b) having serious medical or neurological illness including severe somatic disease and organic brain disorders; (c) being acutely homicidal or suicidal; (d) matching DSM‐IV criteria for substance dependence in last year; and (e) being pregnant or breastfeeding or having metallic implants and other contraindications to MRI.
The criteria to include HC subjects comprised the following: aged 18–55 years; no family history of psychiatric disorder in their first‐degree relatives; no history of using psychotropic medications. Exclusion criteria for HCs were: substance dependence or abuse, neurological illness, and contraindication to MRI scans. All HCs were assessed with M.I.N.I to confirm the absence of a history of mental illness.
Depressed participants undertook the second MRI scan 12 weeks after the baseline scan. All patients received monotherapy with selective serotonin reuptake inhibitor (SSRIs). During the study period, no systematic psychological intervention was performed, such as cognitive behavior therapy. During the study period, the doses and types of SSRIs were judged by the attending physician, according to side effects and symptoms. The dose ranges were as follows: escitalopram 10–20 mg/day, sertraline 100–200 mg/day, and fluoxetine 20–60 mg/day. The average doses at the time of the second scans are shown in Table 1. After 12‐week treatment, people who achieved remission (defined as a HAMD score ≤7) without treatment therapy switching were enrolled for final analysis.
TABLE 1.
Demographic and clinical variables
| Subjects | Healthy controls | Pre‐scan MDD | Post‐scan MDD | p‐Value | |
|---|---|---|---|---|---|
| Number of subjects | 59 | 59 | 59 | ||
| Age (mean ± SD) | 32.20 ± 9.22 | 32.37 ± 8.96 | .92 | ||
| Gender (male/female) | 23/36 | 25/34 | .71 | ||
| Education level (mean ± SD) | 14.56 ± 2.00 | 13.98 ± 2.79 | .20 | ||
| Handedness (R/L) | 59/0 | 59/0 | |||
| HAMD17 score (mean ± SD) | 22.71 ± 4.60 | 5.32 ± 2.34 | <.01 | ||
| Pharmacological administration | Escitalopram | 41/16.14 ± 2.52 | |||
| Sertraline | 10/152.50 ± 26.10 | ||||
| Fluoxetine | 8/25.00 ± 8.66 | ||||
Note: A chi‐squared test was performed for gender comparison. Two‐sample t tests were used for age and education level. Paired t test was used for HAMD17 score. Pharmacological administration is shown as number of patients (n)/dosage (mg/day) and the dosage is presented as mean ± SD.
Abbreviations: HAMD17, 17‐item Hamilton Rating Scale for Depression; MDD, major depressive disorder; pre‐scan, before scan; post‐scan, after scan.
Of the initial 105 patients, 4 patients refused to participate in the second scan, 5 patients received an electroconvulsive therapy due to their illness condition, and 2 patients showed excessive head movement during scanning. Then, 15 patients were switched to SNRIs. In the remaining 79 patients, 64 patients achieved clinical response, defined as >50% reduction in HAMD scores and 59 patients achieved clinical remission. Finally, 59 remitted patients were recruited in further analysis. In addition, 59 HCs were enrolled with matched age, gender, and education. Therefore, a total of 118 participants were included for analysis.
This study was approved by the Research Ethics Review Board of Affiliated Brain Hospital of Nanjing Medical University. All participants provided written informed consent.
2.2. Data acquisition and preprocessing
All data were obtained using a 3 T Siemens Verio scanner (Erlangen, Germany) with an eight‐channel radio frequency coil. The resting‐state fMRI data were acquired by a standard echo planar imaging sequence. The scan parameters were as follows: repetition time (TR) = 3,000 ms, echo time (TE) = 40 ms, field of view (FOV) = 240 mm × 240 mm, flip angle = 90°, 32 slices with slice thickness = 4 mm without gap, matrix size = 64 × 64, and 133 volumes in a 6 min 45 s of resting‐state scanning. Next, the high‐resolution structural images were obtained by a T1‐weighted magnetization‐prepared rapidly acquisition gradient‐echo sequence with the parameters: TR = 1.9 s, TE = 2.48 ms, matrix = 256 × 256, FA = 9°, FOV = 250 × 250 mm, axial slices = 176, thickness = 1 mm, the scanning time = 4 min, and 18 s. Preprocessing of the resting‐state fMRI data was carried out using the SPM8 toolkit (www.fil.ion.ucl.ac.uk/spm/software/spm8) and DPARSF (www.restfmri.net). The first six volumes were discarded for subject orientation and T1 saturation effect. The slice timing for the remaining images was corrected, and images were realigned. Next, subjects would be excluded if the head motion exceeds 2 mm of translation or 2° of rotation during scan. High‐resolution T1 anatomical images were co‐registered and transformed into Montreal Neurological Institute (MNI) space by using the segmentation of white matter, gray matter and cerebrospinal fluid to calculate a transformation matrix. All fMRI images were normalized to the MNI space and resampled at 3 × 3 × 3 mm3. Then, spatial smoothing using a Gaussian kernel of 6 mm FWHM, linear detrending, temporal band‐pass filtering of 0.01–0.08 Hz was performed, followed by regressing out the nuisance signals related to head motion (Friston 24‐parameter), white matter, cerebrospinal fluid, and global signals.
2.3. First‐level statistics to detect group dissimilarity in voxel level
To find out the regions with different impairment trends, the ACC was subdivided based on functional dissimilarity between MDD patients before treatment and HC, with the pipeline shown in Figure 1. In order to represent functional profiles in voxel level, FCs between each voxel in the ACC and regions of interests (ROIs) were calculated. Considering the amygdala and DLPFC have multimodal connections with ACC and their specific role in depression and antidepressant, we selected bilateral amygdalae and DLPFCs as ROIs. Mean time series of these four ROIs were extracted based on masks by WFU_PickAtlas software package (http://fmri.wfubmc.edu/software/PickAtlas). Bilateral amygdalae were extracted from AAL template as label 41 and label 42, respectively. Bilateral DLPFCs were selected from BA template as labels 9 and 46 (Rajkowska & Goldman‐Rakic, 1995). Additionally, ACC was selected according to AAL template as the combination of atlas Cingulum_Ant_L and Cingulum_Ant_R (labels 31&32). For each subject, FCs between each voxel of ACC (838 voxels) and these four ROIs were calculated with Pearson's correlation coefficient. Fisher r‐to‐z transform was performed subsequently to stabilize the interindividual variance. Then we obtained an 838 × 4 FC matrix for each subject in HC and MDD groups.
FIGURE 1.
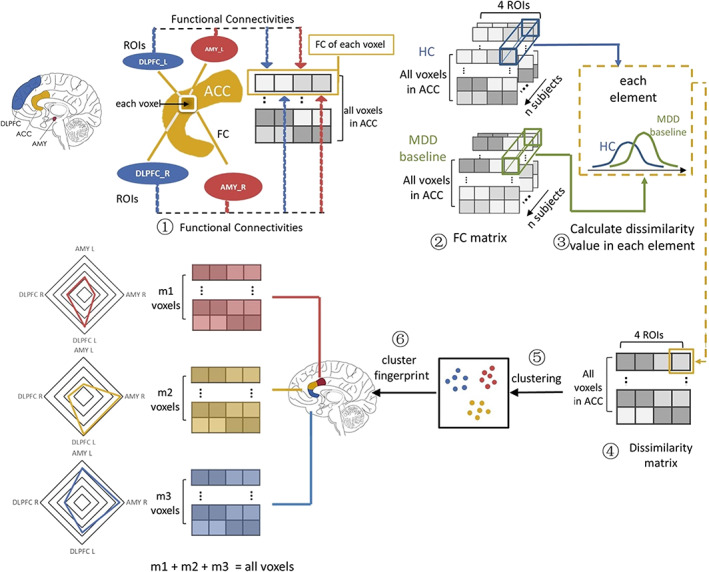
A pipeline of anterior cingulate cortex (ACC) subdivision analysis. ① For each voxel in ACC, functional connectivities (FCs) with left/right dorsolateral prefrontal cortex (DLPFC) and amygdala (AMY) were calculated. Therefore, each subject has a connection matrix of 838 × 4 where number 838 means voxel number in ACC and number 4 means amount of regions of interests (ROIs). ② Connection matrix was calculated individually in both healthy controls (HC) and major depressive disorder (MDD) patients before treatment. ③ The dissimilarity was calculated in each voxel. ④ A dissimilarity matrix in form of 838 × 4 dissimilarity value matrix was developed after group comparison. ⑤ Dissimilarity matrix was then clustered via a k‐means clustering method, after which 838 voxels were divided into three groups. ⑥ Each group has its own profile based on group functional dissimilarity. Finally, the clusters were mapped onto brain image, resulting the functional impairment‐based ACC subdivision
On each voxel, averaged FC was calculated in MDD patients and HCs separately. Then we took the difference of averaging FC between two groups. Larger difference corresponded to more severe impairment trend on brain function. Furthermore, the difference value was divided by the average SD for normalization, representing the dissimilarity between groups. Thus, we got an 838 × 4 dissimilarity matrix. Each row has four values, which denoted the group difference between MDD before treatment and HCs on the FCs between each ACC voxel and four ROIs (i.e., bilateral DLPFCs and amygdalae) respectively.
2.4. FC impairment‐based parcellation of the ACC
To explore the ACC subdivisions in relation to the functional impairment, a parcellation of ACC was performed via k‐means clustering approach over the dissimilarity matrix. The Euclidean distance between every two voxels in the form of their connectivity impairment to bilateral amygdalae and DLPFCs was used to measure the similarity between these two voxels. Here, clustering was repeated 100 times. During each iteration, voxels were reassigned and cluster centroids were recomputed. Finally, the clustering set with the minimum distance sum was chosen as an optimal solution.
The cluster number k was a freely chosen parameter and was chosen according to the elbow criterion of the cluster validity index, which was calculated by the ratio of within‐cluster distances to between‐cluster distances. The value k varied from 2 to 12. Increasing the number of clusters can reduce the sum of variance within each cluster and the most significant inflection point of the curve implies the correct number of clusters. Next, the isolated points in space were treated as outliers and eliminated based on DBSCAN clustering (Çelik, Dadaşer‐Çelik, & Dokuz, 2011) by scikit‐learn package (https://scikit-learn.org/stable). DBSCAN is a density‐based clustering algorithm that identifies clusters with similar density. Isolated points are the points fail to obey the local aggregation criterion. Two important parameters were needed for outlier detection: epsilon (“eps”) and minimum points (“MinPts”). The parameter eps was defined as the shortest distance within a cluster. The parameter MinPts was the minimum number of neighbors within “eps” radius.
To depict the best cluster solution, multidimensional scaling (MDS) was used to visualize the cluster separation, according to the dissimilarity in connectivity profiles with N‐dimensional “functional space” shown in 2D space. A distance matrix was calculated by one minus the pairwise correlation between individual voxels in the ACC. Next, we performed MDS on the eigenimage of the distance matrices. Furthermore, the locations of the three clusters were mapped back on the brain to visualize their anatomical location with REST toolbox and shown in three dimensions using ITK‐SNAP (Yushkevich et al., 2006). Then, ACC subdivisions' profile on impairment was measured by averaging all voxels' dissimilarity values within each subdivision.
2.5. Second‐level statistics to detect subdivisions' impairment and recovery pattern
First, Shapiro–Wilk test was performed to assess if FCs were normally distributed and the Levene's test was performed to determine whether the groups have equal variances. To statistically explore different impairment and recovery pattern within subdivisions, analysis of variance (ANOVA) was performed among three subject groups (HCs, MDD patients before treatment and MDD patients after treatment) on ACC subdivision's mean FC to four ROIs separately. The averaged FC z scores in each ACC subdivision were calculated. Post hoc analyses were then performed on FCs, which showed significant differences after ANOVA. We conducted a two‐sample t test on averaged FC z scores between HCs and MDD patients before treatment to explore the impairment pattern, and a two‐sample t test between HCs and MDD patients after treatment to explore the recovery pattern. A paired t test between MDD patients before and after treatment was also conducted. Statistics were considered significant at a false discovery rate (FDR) correction threshold of <0.05.
To explore the predictive ability on treatment effect of each ACC subdivision, single variable linear regression analyses were performed, where the HAMD reduction ratios were set as the outcome variable and FCs of each subdivision with four ROIs (bilateral DLPFCs and amygdalae) in baseline as the predictor variables. All analyses were conducted using SPSS version 26.0 (IBM Corp., 2019) and significant level was set at a Bonferroni correction threshold of <0.05.
3. RESULTS
3.1. Demographics and clinical characteristics
A chi‐squared test and two‐sample t test were used to determine differences in sex, age, and education years. There were no significant differences in sex (p = .71), age (p = .92), or education level (p = .20) between the MDD and HC groups (shown in Table 1). For the group of MDD patients, the depressive symptom assessed by the HAMD‐17 significantly decreased after treatment compared to those at baseline (p < .01).
3.2. FC impairment‐based parcellation of the ACC
The clustering performance was compared via the ratio of within‐cluster distances to between‐cluster distances when varying the clustering values in the range of [2, 12] (as shown in Figure 2d). According to the elbow criterion, optimal clustering performance was obtained when k = 3, indicating the most significant inflection point of the curve. Under the optimal cluster solution, the similarity matrix of ACC voxels was calculated with Pearson correlation coefficient and the distribution according to the MDS was displayed in Figure 2. The three subdivision clusters contained 281, 345, and 212 voxels, respectively, among which 18, 11, and 30 voxels were treated as outliers, respectively, by DBSCAN clustering, and then eliminated.
FIGURE 2.
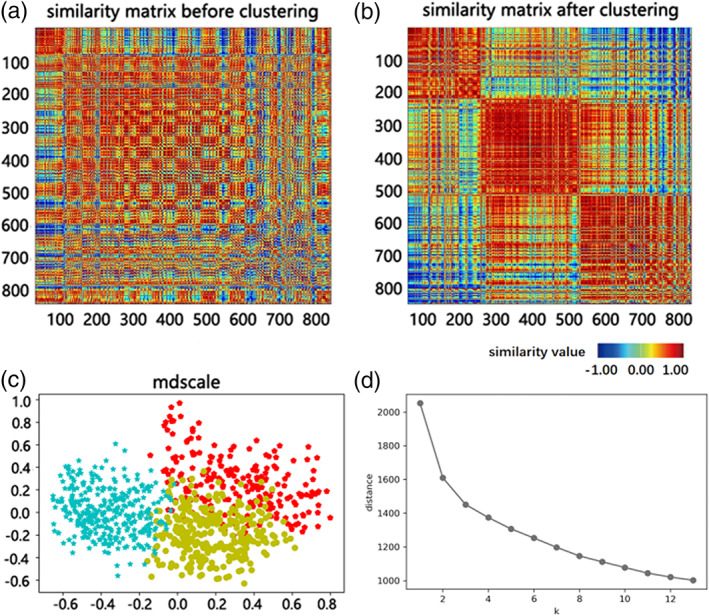
Visualization of the anterior cingulate cortex (ACC) voxels under the optimal cluster solution (k = 3) and clustering performance according to the cluster criteria. (a) Similarity matrix of the ACC voxels. (b) Similarity matrix of the ACC voxels reordered by their clustering affiliations. (c) Visualization of ACC voxels according to the three‐cluster solution by multidimensional scaling. Longer distance between points (denote ACC voxels) was associated with less similar impairment profile of these voxels in relation to their functional connectivity with bilateral dorsolateral prefrontal cortices and amygdalae. (d) According to elbow criteria, k = 3 was the most significant inflection point of the curve and was chosen as the optimal cluster number
The final ACC subdivision was determined and mapped back on brain imaging as shown in Figure 3a. Terminologia Anatomica is the official revision of anatomical terminology, which is used worldwide and accepted by the professional community (FCAT, 1998). With reference to TA, Subdivision 1 was named as MedialACC because most of the voxels of it were located in the medial of ACC. Similarly, Subdivision 2 was named as DistalACC while Subdivision 3 was named as LateralACC.
FIGURE 3.
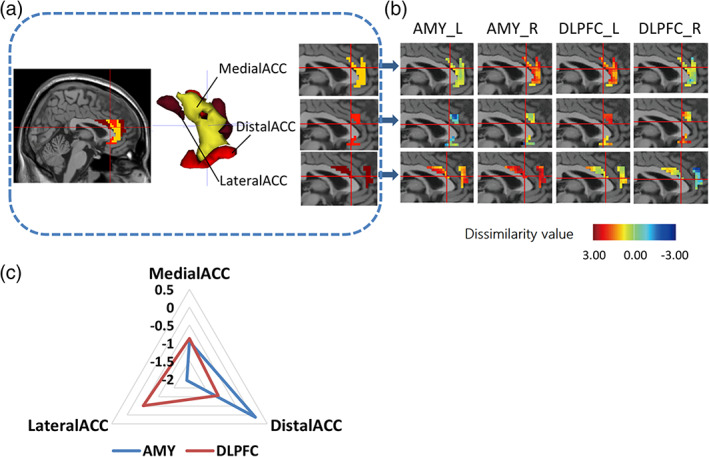
Visualization of anterior cingulate cortex (ACC) subdivisions and impairment functional profile across subdivisions. (a) Three ACC subdivisions were mapped back on brain which located in medial, distal, and lateral portion separately. Yellow: MedialACC, Red: DistalACC, Brown: LateralACC. (b) ACC subdivisions and their impaired functional pattern in relation to left/right amygdala and dorsolateral prefrontal cortex (DLPFC), illustrated via the dissimilarity value distribution over voxels after the comparison of healthy controls with pretreatment patients. (c) Radar map of dissimilarity value in each subdivisions. The values of ACC subdivisions were averaged in relation to their connectivities to amygdala (AMY) or DLPFC
3.3. Impaired functional profile of ACC subdivisions
The values from the dissimilarity matrix of each ACC voxel to four ROIs were presented in Figure 3b, suggesting dissimilarity between groups. Results showed that Subdivision 2 (DistalACC) has the highest dissimilarity value (shown as deeper color) with bilateral DLPFCs while Subdivision 3 (LateralACC) has the highest dissimilarity value with bilateral amygdalae, where the higher dissimilarity value represents more severe impairment on FCs.
Similar impairment profiles in relation to bilateral DLPFCs and amygdalae were found for the whole ACC, left ACC and right ACC. However, for the ACC subregions, distinct impairment profiles in relation to bilateral DLPFCs and amygdalae were suggested. It implied that subregion segmentation could provide an insight into how the ACC was impaired in depression (see Supplementary Material). To represent the impairment profiles of these three subdivisions more clearly, the dissimilarity values on the connection of each subdivision to bilateral DLPFCs and amygdalae are averaged and shown in Figure 3c. Overall, almost all the subdivisions have a downward tendency in MDD patients when comparing to HCs on FCs. Nevertheless, subdivisions have different impaired degrees in relation to different ROIs. DistalACC (Subregion 2) was suggested to have a more severe impairment on FC with DLPFC. On the contrary, LateralACC (Subregion 3) showed more serious impairment on FC with amygdala. MedialACC (Subregion 1) had relatively serious impairment on its connectivities to right amygdala and left DLPFC compared to other subdivisions.
3.4. Impairment and recovery pattern within subdivisions
To further verify the different impairment patterns of ACC subdivisions and quantify the corresponding recovery pattern of each subdivision, a further statistical analysis was performed based on the averaged FC values in subdivisions.
Based on the results of Shapiro–Wilk test, all the FCs were normally distributed (p > .05). The results of Levene's test indicated that the three groups had equal variances (p > .05). Then, we performed one‐way ANOVA on FCs connected to ACC subdivisions, and found significant differences on FCs between LateralACC and bilateral amygdalae, on FCs between DistalACC and left DLPFC (p < .05, FDR‐corrected for 12 tests). Then we performed post hoc analyses on these FCs. Compared patients before treatment to HC, results suggested that the LateralACC was found to have significantly different FC with bilateral amygdalae (p < .05, FDR‐corrected for nine tests), and the DistalACC was found to have significantly different FC with left DLPFC (as shown in Figure 4). After treatment, the corresponding impairments may be alleviated, in regard that there was no significant difference on FC between LateralACC and right amygdala, between DistalACC and left DLPFC, when the patients after treatment were compared to HC. Interestingly, the FC between LateralACC and left amygdala was still significantly lower in the patients after treatment compared that in HC. It implies that the FC between LateralACC and left amygdala may be a state feature that keeps dysfunctional over different states. Whereas the FC between LateralACC and right amygdala is suggested to be a disease related feature, the damaged function of which can be reversed along with the treatment of depression. The statistics above were considered significant at a FDR threshold of <0.05.
FIGURE 4.
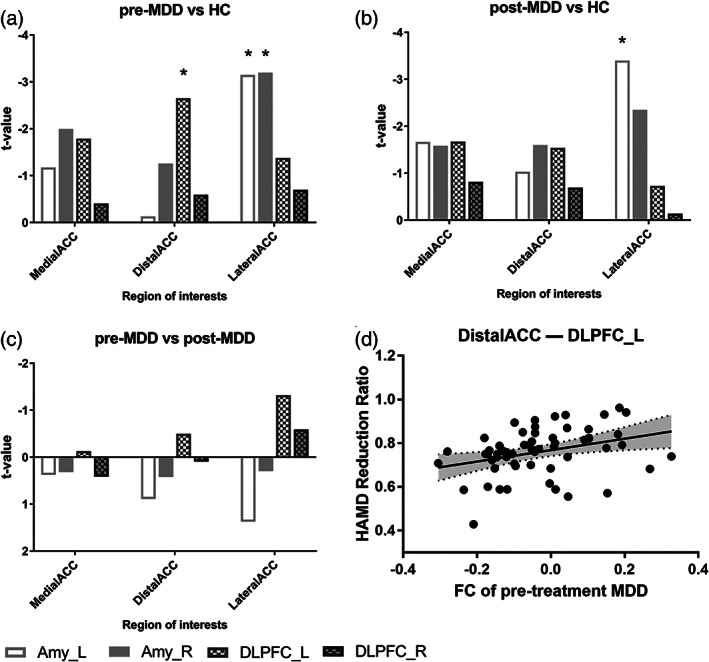
Impairment pattern and recovery pattern in subdivisions. (a) Histogram of t values compared between major depressive disorder (MDD) patients before treatment and healthy controls (HCs) shows impairment pattern across subdivisions. (b) Histogram of t values compared between MDD after treatment and HC across subdivisions shows recovery pattern. (c) Histogram of t values between MDD before and after treatment. *: significant after false‐discovery rate (FDR) correction for multiple comparisons. (d) Prediction effect of functional connectivity (FC) between DistalACC and left dorsolateral prefrontal cortex (DLPFC_L). Each regression line depicts the predicted model for Hamilton Depression Rating Scale (HAMD) reduction ratio and FC between DistalACC and left DLPFC. Confidence intervals are plotted in dotted black lines and filled with gray color
3.5. Treatment effect prediction from ACC subdivisions
Three FCs with significant group differences were used for further regression analyses, together with the individual HAMD reduction ratio. As shown in Figure 4d, only FC between DistalACC and left DLPFC significantly predicted HAMD reduction ratios (beta = .26, R 2 = .11, F = 6.96, p = .01) after strict Bonferroni corrections (corrected for three tests; p < .05/3). Therefore, ACC subdivisions exhibited different prediction capabilities of depression treatment effect.
4. DISCUSSION
In this study, the ACC was divided into three distinguished subdivisions according to different impairment patterns of their FC to the amygdalae and DLPFCs at voxel level. Each subdivision had a diverse impaired functional inclination. To the best of our knowledge, this was the first study to explore ACC functional impairment‐based subregions and its restoration pattern after antidepressant treatment.
Compared to healthy subjects, depressed patients had decreased FCs between LateralACC and bilateral amygdala, and between DistalACC and left DLPFC. After treatment, damaged FC between LateralACC and right amygdala, between DistalACC and left DLPFC got reversed while FC between LateralACC and left amygdala kept dysfunctional over different states.
Subdivisions of ACC distributed separately over brain space. The spatial distribution of MedialACC, DistalACC, and LateralACC were, respectively, overlapped with BA24 and BA32 mostly, BA25 and the top of BA24 at superior and inferior part, BA32 and BA33 at the anterior and posterior part, which had a partially space bias with anatomical ACC subregions (sgACC, pgACC, and supACC) (Sambataro et al., 2018). Depressed impairment had its unique distribution pattern, which may be caused by common influence of unstable and heterogeneous distribution of synaptic receptor subunit and long‐range connection synthetically (Phillips et al., 2015; Rive et al., 2013). Although each ACC subdivision had its own unique properties, all the subdivisions of ACC in this study showed hypoconnectivities with DLPFC to varying degrees in MDD patients. Similar findings were observed in many studies (Phillips, Drevets, Rauch, & Lane, 2003), which might contribute to a decreased support of cognitive functions, including selective attention and working memory in depression (Niendam et al., 2012). Moreover, our research implied that brain function of antidepressant treated patients might recover in a pattern that was not identical to the original impairment one.
4.1. Impairment pattern
MDD patients were found to have significantly impaired FCs between DistalACC (Subdivision 2) and left DLPFC, between LateralACC (Subdivision 3) and bilateral amygdalae.
DistalACC (Subdivision 2) was suggested to be functionally impaired more on the connection with DLPFC. This hypoconnectivity between ACC and DLPFC has been consistently observed in many research studies (Holmes & Pizzagalli, 2008; Vasic, Walter, Sambataro, & Wolf, 2009). DistalACC had its unique function in emotion regulation. Compared with other subregions, DistalACC had greater overlap with BA24 and BA25.
The location of superior part resembles that of BA a24', which is the caudal components of BA 24, while MedialACC is similar to the other parts of BA24 in position. Different cytoarchitectures and functions between MedialACC and DistalACC reported by Vogt (2005) may lay out the histological basis for the functional division of these two areas. The superior part had a high probability of interconnection with areas related to action control and decision‐making, including the DLPFC, parietal cortex and motor cortex (Heukelum et al., 2020). Task‐based studies on humans suggested that this region had cognitive‐related functions. This area was reported to have increased activity with intentional components in the initiation of movement (Felix, Christian, Karl, & Eickhoff, 2013). It had the functions to bias the selective attention by pre‐existing conditions (Paus, Petrides, Evans, & Meyer, 1993). The position of inferior part is similar to BA 25 in position. Previous studies had found similar receptor‐architecture between two parts of DistalACC (Palomero‐Gallagher, Vogt, Schleicher, Mayberg, & Zilles, 2010), suggesting a histological basis for them being subdivided into the same subregion. The two areas were included in a same neural circuitry that could be activated when processing negative valence information and inhibitory task demand (Goldstein et al., 2007) and this neural circuit had shown abnormality in MDD (Johansen‐Berg et al., 2008). Therefore, we can infer that DistalACC (Subdivision 2) may serve to deal with valence‐specific information processing.
With regard to the DLPFC, it is a key region mediating emotion regulation in both negative and positive emotion (Ma, 2015). Its connection with the ACC serves as a part of “top‐down” cortical control (Arnone, 2019). Decreased engagement of the DLPFC might be correlated with the decreased regulation and inhibition function of limbic circuit, which might cause the increased response to negative stimuli compared to positive stimuli. Furthermore, cognitive dysfunction was recognized as a core deficit in many psychotic disorders such as psychotic depression and negatively impact functional outcome (Sheffield, Karcher, & Barch, 2018). Thus, the DLPFC‐ACC hypoconnectivity might reflect a decreased support of cognitive functions to mediate emotion (Niendam et al., 2012). Overall, the abnormal FC between DistalACC and DLPFC might be associated with decreased “top‐down” emotion regulation to both positive and negative emotions.
LateralACC (Subdivision 3) was found to be functionally impaired more on the connection to amygdala. LateralACC had great spatially overlap with BA32 and BA33 at anterior and posterior parts. Anterior part of LateralACC was similar to BA32p in location (Vogt, 2016). BA32 forms an outer arc around the anterior cingulate gyrus (Brodmann, 2006). Compared to BA24, BA32 has a thin Layer VI and misses the large pyramidal neurons in Layer V, suggesting that LateralACC is different from other two ACC subregions in cytoarchitectonic architecture (Vogt, Pandya, & Rosene, 1987). Our results suggested that the anterior part of LateralACC mainly connected to amygdala, ventral and dorsal striatum, and orbitofrontal cortex. This area of ACC was associated with social interaction and mind tasks (Amodio & Frith, 2006). A meta‐analysis has reported activity during reward and reward‐expectation in this area (Beckmann, Johansen‐berg, & Rushworth, 2009). Previous research has found abnormal activation of ventral ACC that includes BA32 (Alexopoulos et al., 2012). The location of posterior part ensembles BA33, which was only found in humans. It is mainly located in the callosal sulcus and forms a belt around ACC. BA33 was relevant to sadness emotion control (Vogt, 2005) and the ACC has been found to control different emotions in separate.
The abnormal LateralACC‐amygdala connection was consistent with the previous study (Anand et al., 2005a) reporting an abnormal connection between limbic structures including amygdala and pgACC, which has a major overlapping area with LateralACC. This result was also consistent with task‐based studies under facial stimuli, where reduced connectivity in MDD was found between amygdala and regions located in LateralACC (Costafreda et al., 2013; Wackerhagen, Veer, Erk, Mohnke, & Walter, 2019). The amygdala can, through bottom‐up mechanisms, promoted perceptual processing by arousing or biasing attention (Comte et al., 2014; Phelps, 2005). The amygdala and ACC are key roles in the salience circuit, which is implicated in the detection of external environment including the demand of cognitive control (Seeley et al., 2007). The abnormal connection might suggest a decreased alertness of “bottom‐up” control to affection. Thus, the abnormal FC between LateralACC and amygdalae may be associated with “negative bias” in depression through bottom‐up mechanisms. Overall, impairment pattern may suggest impaired FC in both “top‐down” and “bottom‐up” emotion regulations, which were likely to contribute to abnormal emotional function in depression.
In a recent study, the ACC was subdivided based on FC with all other brain regions into two parts (Rolls et al., 2018). However, it can be observed in our study that there are several parts with different disrupted patterns in the same FC‐based subregion in ACC. This implies that the functional disrupted pattern may be heterogeneous in the same FC‐based ACC subregion. Specific impairment signals are likely to be masked by the averaged FC in each FC‐based ACC subregion. This possibly accounts for the intact functional connection with DLPFC and amygdala in the FC‐based ACC subregions. Thus, our method has the advantage to increase the sensitivity in detecting the impairment caused by MDD.
4.2. Recovery pattern
After treatment, MDD patients recovered in a pattern that was not identical to impairment pattern. Among them, FC between DistalACC and left DLPFC was normalized while FC between LateralACC and amygdalae only got partly normalized (only normalized in right amygdala). Based on the above analyses, this recovery pattern may suggest that although the functions in depression fail to get complete recovery in “bottom‐up” emotion regulation, they had an improvement in “top‐down” emotion regulation. Moreover, functions of MDD patients might get an incomplete recovery on negative emotion while a compensation on positive emotion regulation. We suspect the reasons for this as follows.
After treatment, the abnormally decreased FC between LateralACC and left amygdala was reversed. Most FCs had the tendency to reverse the hypoconnectivity pattern. The work of (Ma, 2015) reported that antidepressant treatment might engage to reverse the neural response pattern in MDD patients. This reversed pattern was consistent with the recent study showing the antidepressant effect of the mood‐regulating circuit (Anand et al., 2005b) and may relate to SSRI arm here (Williams, 2017). Serotonin can modulate implicit emotion regulation circuitry centered on the amygdala (Klucken, Wehrum, Schweckendiek, Merz, & Stark, 2013; Phillips et al., 2015), which can be modulated via serotonin reuptake inhibitors (SRIs) (Outhred et al., 2013; van Marle et al., 2011). Additionally, the normalized connection of DistalACC and DLPFC might enhance the ability to mediate limbic–cortical circuit and further contribute to the normalization of LateralACC to amygdala, which finally might be associated with the reverse of the negative bias pattern. Overall, recovery pattern may suggest that antidepressants would take effects mainly on “top‐down” emotion regulation.
Furthermore, higher DistalACC‐left DLPFC FC before treatment was found to be related to higher HAMD reduction ratio in this study, in line with previous report about a higher FC in cortico‐limbic networks that was predictive of antidepressant response in depressed patients (Andrew et al., 2011). It suggested the specific role of DistalACC in antidepressant again and implied that the particular subregion DistalACC and its functional link to the left DLPFC can be considered as a potential index for clinical outcome predication. Even if MDD patients reached remission finally, the remaining HAMD scores after treatment may suggest a potential functional impairment in MDD patients. They had different HAMD reduction ratios, which may imply different treatment effects.
Our methodology has some unique advantages worth mentioning. First, our study focused on the particular regions of the ACC only, so that the computation was greatly simplified, compared to the entire brain connection clustering (Zarei et al., 2013). It is also more suitable for the particular research that targets to discover the diverse impairment directions of the ACC in relation to the cognitive and emotion regulation. Second, most previous studies are based on univariate fMRI activity, which is robust but difficult to represent the whole activity of the brain region. Here we performed a more fine‐grained analysis and is more likely to reflect the neuron population codes of impairment pattern and recovery pattern in depression. Finally, in line with the fact that more and more scientists have risen up to against the dichotomous thinking of statistical values (Amrhein & Mcshane, 2019), our research makes an attempt of estimation thinking by clustering with dissimilarity value and explores the effect of subthreshold statistical powers, which is more intuitive and suggestive to observe the disease effect and the relation to ACC parcellation.
5. LIMITATIONS
The present study has limitations that should be addressed in future studies. First, our study focused more on depressed state between acute phase and remission state. Personalized treatment should be applied to patients with different inhibitors and different doses in order to promote the generalizability of the treatment results and enable the clustering power. Second, our study focused on functional impairment and recovery only in depression. It is still unclear how structural impairment patterns of ACC work and link to the functional impairment and recovery. Further studies are needed to explore their relationship with multimode neuroimaging. Third, the way to choose ACC mask is controversial. This article used the ACC mask defined in the AAL atlas, which may have overlap with midcingulate defined by Vogt (2016). Future studies on ACC mask defined by identified cytoarchitecture and receptor distribution are expected to extend our findings on impairment patterns of the ACC in MDD and their recovery after antidepressant. Fourth, in this study, the amygdala and DLPFC were chosen as ROIs to explore the emotional and cognitive processing ability of the ACC. However, it is still worth exploring the connection pattern generalized into other brain areas. Future studies will be conducted based on large‐scale circuit containing ACC, to explore the different disrupted pattern and restored pattern in ACC subregions on circuitry level.
6. CONCLUSIONS
We concluded that ACC consists of three parcellations having diverse functional impairment in relation to the DLPFC or amygdala. MDD patients were found to have significantly impaired FCs between DistalACC (our Subdivision) and left DLPFC, between LateralACC (our subdivision) and bilateral amygdalae. After treatment, FC between LateralACC and left amygdala failed to be recovered. In sum, this finding suggested that MDD might be associated with impaired emotion regulation circuit in both “top‐down” and “bottom‐up” pathway. However, MDD after treatment might mainly improve in “top‐down” emotion regulation. Connection between DistalACC and left DLPFC could predict HAMD treatment responses, suggesting the diverse prediction effect of ACC subregions. Furthermore, we suggested this subdivision could offer a more precise treatment target and promote personalized treatment in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This work was partly supported by National Natural Foundation of China (81871066); Jiangsu Provincial key research and development program (BE2018609 and BE2019675); Jiangsu Provincial Medical Innovation Team of the Project of Invigorating Health Care through Science, Technology and Education (CXTDC2016004); Key Project supported by Medical Science and Technology development Foundation, Jiangsu Commission of Health (K2019011); the Fundamental Research Funds for the Central Universities (2242021k30014 and 2242021k30059).
Zhang, Y. , Shao, J. , Wang, X. , Chen, Z. , Liu, H. , Pei, C. , Zhang, S. , Yao, Z. , & Lu, Q. (2021). Functional impairment‐based segmentation of anterior cingulate cortex in depression and its relationship with treatment effects. Human Brain Mapping, 42(12), 4035–4047. 10.1002/hbm.25537
Funding information Jiangsu Provincial Key Research and Development Program, Grant/Award Numbers: BE2018609, BE2019675; Jiangsu Provincial Medical Innovation Team of the Project of Invigorating Health Care through Science, Technology and Education, Grant/Award Number: CXTDC2016004; Medical Science and Technology development Foundation, Jiangsu Commission of Health, Grant/Award Number: K2019011; National Natural Science Foundation of China, Grant/Award Number: 81871066; the Fundamental Research Funds for the Central Universities:, Grant/Award Numbers: 2242021k30014, 2242021k30059
Contributor Information
Zhijian Yao, Email: zjyao@njmu.edu.cn.
Qing Lu, Email: luq@seu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Alexopoulos, G. S. , Hoptman, M. J. , Kanellopoulos, D. , Murphy, C. F. , Lim, K. O. , & Gunning, F. M. (2012). Functional connectivity in the cognitive control network and the default mode network in late‐life depression. Journal of Affective Disorders, 139(1), 56–65. 10.1016/j.jad.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio, D. M. , & Frith, C. D. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Amrhein, V. , & Mcshane, B. (2019). Scientists rise up against statistical significance. Nature, 567(7748), 305–307. 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
- Anand, A. , Li, Y. , Wang, Y. , Wu, J. , Gao, S. , Bukhari, L. , … Lowe, M. J. (2005a). Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biological Psychiatry, 57(10), 1079–1088. 10.1016/j.biopsych.2005.02.021 [DOI] [PubMed] [Google Scholar]
- Anand, A. , Li, Y. , Wang, Y. , Wu, J. , Gao, S. , Bukhari, L. , … Lowe, M. J. (2005b). Antidepressant effect on connectivity of the mood‐regulating circuit: An fMRI study. Neuropsychopharmacology, 30(7), 1334–1344. 10.1038/sj.npp.1300725 [DOI] [PubMed] [Google Scholar]
- Andrew, K. F. , Rao, U. , Lu, H. , Nakonezny, P. A. , Bruce, G. , Tamara, M. G. , … Trivedi, M. H. (2011). Functional connectivity of brain structures correlates with treatment outcome in major depressive disorder. Frontiers in Psychiatry, 2, 7. 10.3389/fpsyt.2011.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone, D. (2019). Functional MRI findings, pharmacological treatment in major depression and clinical response. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 91, 28–37. 10.1016/j.pnpbp.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Beckmann, M. , Johansen‐berg, H. , & Rushworth, M. F. S. (2009). Connectivity‐based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of Neuroscience, 29(4), 1175–1190. 10.1523/JNEUROSCI.3328-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick, M. M. , Cohen, J. D. , & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Brodmann, K. (2006). Description of individual brain maps. In Brodmann K. (Ed.), Brodmann's localisation in the cerebral cortex: The principles of comparative localisation in the cerebral cortex based on cytoarchitectonics (pp. 105–170). Boston, MA: Springer US. 10.1007/0-387-26919-3_5 [DOI] [Google Scholar]
- Bush, G. , Luu, P. , & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Çelik, M. , Dadaşer‐Çelik, F. , & Dokuz, A. Ş. (2011). Anomaly detection in temperature data using DBSCAN algorithm. In 2011 International Symposium on Innovations in Intelligent Systems and Applications (pp. 91–95). IEEE. 10.1109/INISTA.2011.5946052 [DOI]
- Cipriani, A. , Furukawa, T. A. , Salanti, G. , Chaimani, A. , Atkinson, L. Z. , Ogawa, Y. , … Higgins, J. P. T. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta‐analysis. Lancet, 391(10128), 1357–1366. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comte, M. , Schön, D. , Coull, J. T. , Reynaud, E. , Khalfa, S. , Belzeaux, R. , … Weinberger, D. R. (2014). Dissociating bottom‐up and top‐down mechanisms in the cortico‐limbic system during emotion processing. Cerebral Cortex, 26(1), 144–155. 10.1093/cercor/bhu185 [DOI] [PubMed] [Google Scholar]
- Costafreda, S. G. , Mccann, P. , Saker, P. , Cole, J. H. , Cohen‐Woods, S. , Farmer, A. E. , … Fu, C. (2013). Modulation of amygdala response and connectivity in depression by serotonin transporter polymorphism and diagnosis. Journal of Affective Disorders, 150(1), 96–103. 10.1016/j.jad.2013.02.028 [DOI] [PubMed] [Google Scholar]
- Cullen, K. R. , Gee, D. G. , Klimes‐Dougan, B. , Gabbay, V. , Hulvershorn, L. , Mueller, B. A. , … Kumra, S. (2009). A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters, 460(3), 227–231. 10.1016/j.neulet.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, K. R. , Klimes‐Dougan, B. , Pham Vu, D. , Schreiner, M. W. , Mueller, B. A. , Eberly, L. E. , … Lim, K. O. (2016). Neural correlates of antidepressant treatment response in adolescents with major depressive disorder. Journal of Child & Adolescent Psychopharmacology, 26(8), 705–712. 10.1089/cap.2015.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau, P. , Jabourian, M. , Lemogne, C. , Allaïli, N. , Choucha, W. , Girault, N. , … Fossati, P. (2016). Antidepressant short‐term and long‐term brain effects during self‐referential processing in major depression. Psychiatry Research, 247, 17–24. 10.1016/j.pscychresns.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales, C. L. , Barch, D. M. , Rundle, M. M. , Mintun, M. A. , Snyder, A. Z. , Cohen, J. D. , … Sheline, Y. I. (2008). Altered emotional interference processing in affective and cognitive‐control brain circuitry in major depression. Biological Psychiatry, 63(4), 377–384. 10.1016/j.biopsych.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FCAT . (1998). Terminologia Anatomica—International anatomical terminology. Stuttgart: Thieme Verlag. 10.1046/j.1469-7580.2001.199607412.x [DOI] [Google Scholar]
- Felix, H. , Christian, G. , Karl, Z. , & Eickhoff, S. B. (2013). The “what” and “when” of self‐initiated movements. Cerebral Cortex, 23(3), 520–530. 10.1093/cercor/bhr391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, R. , Torres, I. , Brown, J. J. , Gregory, E. , McLellan, E. , Downar, J. H. , … Vila‐Rodriguez, F. (2019). Functional disconnectivity of the hippocampal network and neural correlates of memory impairment in treatment‐resistant depression. Journal of Affective Disorders, 253, 248–256. 10.1016/j.jad.2019.04.096 [DOI] [PubMed] [Google Scholar]
- Goldstein, M. , Brendel, G. , Tuescher, O. , Pan, H. , Epstein, J. , Beutel, M. , … Silbersweig, D. (2007). Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: An emotional linguistic go/no‐go fMRI study. NeuroImage, 36(3), 1026–1040. 10.1016/j.neuroimage.2007.01.056 [DOI] [PubMed] [Google Scholar]
- Haber, S. , & Behrens, T. J. (2014). The neural network underlying incentive‐based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron, 83(5), 1019–1039. 10.1016/j.neuron.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukelum, S. V. , Mars, R. B. , Guthrie, M. , Buitelaar, J. K. , Beckmann, C. F. , Tiesinga, P. , … Havenith, M. N. (2020). Where is cingulate cortex? A Cross‐species view. Trends in Neurosciences, 43(5), 285–299. 10.1016/j.tins.2020.03.007 [DOI] [PubMed] [Google Scholar]
- Holmes, A. J. , & Pizzagalli, D. A. (2008). Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia, 46(12), 2904–2913. 10.1016/j.neuropsychologia.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp . (2019). IBM SPSS statistics for windows. Armonk, NY: IBM Corp; Retrieved from https://www.ibm.com/analytics/spss-statistics-software [Google Scholar]
- James, S. L. , Abate, D. , Abate, K. H. , Abay, S. M. , Abbafati, C. , Abbasi, N. , … Murray, C. J. L. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg, H. , Gutman, D. A. , Behrens, T. E. J. , Matthews, P. M. , Rushworth, M. F. S. , Katz, E. , … Mayberg, H. S. (2008). Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment‐resistant depression. Cerebral Cortex, 18(6), 1374–1383. 10.1093/cercor/bhm167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken, T. , Wehrum, S. , Schweckendiek, J. , Merz, C. J. , & Stark, R. (2013). The 5‐HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Human Brain Mapping, 34(10), 2549–2560. 10.1002/hbm.22085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein, S. D. , Verstynen, T. , & Forbes, E. E. (2016). Adolescent brain development and depression: A case for the importance of connectivity of the anterior cingulate cortex. Neuroscience & Biobehavioral Reviews, 70, 271–287. 10.1016/j.neubiorev.2016.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston, C. , Chen, A. C. , Zebley, B. D. , Drysdale, A. T. , Gordon, R. , Leuchter, B. , … Dubin, M. J. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry, 76(7), 517–526. 10.1016/j.biopsych.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. (2015). Neuropsychological mechanism underlying antidepressant effect: A systematic meta‐analysis. Molecular Psychiatry, 20(3), 311–319. 10.1038/mp.2014.24 [DOI] [PubMed] [Google Scholar]
- Marusak, H. A. , Thomason, M. E. , Peters, C. , Zundel, C. , Elrahal, F. , & Rabinak, C. A. (2016). You say ‘prefrontal cortex’ and I say ‘anterior cingulate’: Meta‐analysis of spatial overlap in amygdala‐to‐prefrontal connectivity and internalizing symptomology. Nature Publishing Group, 6(11), e944–e944. 10.1038/tp.2016.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, A. , Engels, A. S. , Herrington, J. D. , Heller, W. , Ho, M. R. , Banich, M. T. , … Miller, G. A. (2010). Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology, 44(3), 343–351. 10.1111/j.1469-8986.2007.00515.x [DOI] [PubMed] [Google Scholar]
- Niendam, T. A. , Laird, A. R. , Ray, K. L. , Dean, Y. M. , Glahn, D. C. , & Carter, C. S. (2012). Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur, D. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10(3), 206–219. 10.1093/cercor/10.3.206 [DOI] [PubMed] [Google Scholar]
- Outhred, T. , Hawkshead, B. E. , Wager, T. D. , Das, P. , Malhi, G. S. , & Kemp, A. H. (2013). Acute neural effects of selective serotonin reuptake inhibitors versus noradrenaline reuptake inhibitors on emotion processing: Implications for differential treatment efficacy. Neuroscience and Biobehavioral Reviews, 37(8), 1786–1800. 10.1016/j.neubiorev.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Palomero‐Gallagher, N. , Vogt, B. A. , Schleicher, A. , Mayberg, H. S. , & Zilles, K. (2010). Receptor architecture of human cingulate cortex: Evaluation of the four‐region neurobiological model. Human Brain Mapping, 30(8), 2336–2355. 10.1002/hbm.20667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek, J. N. , van der Werff, S. J. A. , Meens, P. H. F. , van den Bulk, B. G. , Jolles, D. D. , Veer, I. M. , … Vermeiren, R. R. J. M. (2014). Aberrant resting‐state functional connectivity in limbic and salience networks in treatment‐naive clinically depressed adolescents. Journal of Child Psychology & Psychiatry, 55(12), 1317–1327. 10.1111/jcpp.12266 [DOI] [PubMed] [Google Scholar]
- Paus, T. , Petrides, M. , Evans, A. C. , & Meyer, E. (1993). Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. Journal of Neurophysiology, 70(2), 453–469. 10.1152/jn.1993.70.2.453 [DOI] [PubMed] [Google Scholar]
- Phelps, E. A. (2005). Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology, 57(1), 27–53. 10.1146/annurev.psych.56.091103.070234 [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , Chase, H. W. , Ph, D. , Sheline, Y. I. , Etkin, A. , Ph, D. , … Ph, D. (2015). Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: Neuroimaging approaches. American Journal of Psychiatry, 172(2), 124–138. 10.1176/appi.ajp.2014.14010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. L. , Drevets, W. C. , Rauch, S. L. , & Lane, R. (2003). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54(5), 515–528. 10.1016/s0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- Posner, J. , Hellerstein, D. J. , Gat, I. , Mechling, A. , Klahr, K. , Wang, Z. , … Peterson, B. S. (2013). Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry, 70(4), 373–382. 10.1001/jamapsychiatry.2013.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska, G. , & Goldman‐Rakic, P. S. (1995). Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cerebral Cortex, 5(4), 323. 10.1093/cercor/5.4.323 [DOI] [PubMed] [Google Scholar]
- Rive, M. M. , van Rooijen, G. , Veltman, D. J. , Phillips, M. L. , Schene, A. H. , & Ruhé, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(10), 2529–2553. 10.1016/j.neubiorev.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Rolls, E. , Cheng, W. , Gong, W. , Qiu, J. , Zhou, C. , Zhang, J. , … Feng, J. (2018). Functional connectivity of the anterior cingulate cortex in depression and in health. Cerebral Cortex, 29(8), 3617–3630. 10.1093/cercor/bhy236 [DOI] [PubMed] [Google Scholar]
- Sambataro, F. , Doerig, N. , Hänggi, J. , Christian, R. , Brakowski, J. , Holtforth, G. , … Spinelli, S. (2018). Anterior cingulate volume predicts response to psychotherapy and functional connectivity with the inferior parietal cortex in major depressive disorder. European Neuropsychopharmacology, 28(1), 138–148. 10.1016/j.euroneuro.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, J. M. , Karcher, N. R. , & Barch, D. M. (2018). Cognitive deficits in psychotic disorders: A lifespan perspective. Neuropsychology Review, 28(4), 509–533. 10.1007/s11065-018-9388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Yahata, N. , Koeda, M. , Takano, A. , Asai, K. , Suhara, T. , & Okubo, Y. (2005). Effects of dopaminergic and serotonergic manipulation on emotional processing: A pharmacological fMRI study. NeuroImage, 27(4), 991–1001. 10.1016/j.neuroimage.2005.05.039 [DOI] [PubMed] [Google Scholar]
- Vai, B. , Bulgarelli, C. , Godlewska, B. R. , Cowen, P. J. , Benedetti, F. , & Harmer, C. J. (2016). Fronto‐limbic effective connectivity as possible predictor of antidepressant response to SSRI administration. European Neuropsychopharmacology, 26(12), 2000–2010. 10.1016/j.euroneuro.2016.09.640 [DOI] [PubMed] [Google Scholar]
- van Marle, H. J. F. , Tendolkar, I. , Urner, M. , Verkes, R. J. , Fernández, G. , & van Wingen, G. (2011). Subchronic duloxetine administration alters the extended amygdala circuitry in healthy individuals. NeuroImage, 55(2), 825–831. 10.1016/j.neuroimage.2010.12.051 [DOI] [PubMed] [Google Scholar]
- Vasic, N. , Walter, H. , Sambataro, F. , & Wolf, R. C. (2009). Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychological Medicine, 39(6), 977–987. 10.1017/S0033291708004443 [DOI] [PubMed] [Google Scholar]
- Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience, 6(7), 533–544. 10.1038/nrn1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, B. A. (2016). Midcingulate cortex: Structure, connections, homologies, functions and diseases. Journal of Chemical Neuroanatomy, 74, 28–46. 10.1016/j.jchemneu.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Vogt, B. A. , Pandya, D. N. , & Rosene, D. L. (1987). Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. Journal of Comparative Neurology, 262(2), 256–270. 10.1002/cne.902620207 [DOI] [PubMed] [Google Scholar]
- Wackerhagen, C. , Veer, I. M. , Erk, S. , Mohnke, S. , & Walter, H. (2019). Amygdala functional connectivity in major depression—Disentangling markers of pathology, risk and resilience. Psychological Medicine, 50(16), 2740–2750. 10.1017/S0033291719002885 [DOI] [PubMed] [Google Scholar]
- Williams, L. M. (2017). Getting personalized: Brain scan biomarkers for guiding depression interventions. American Journal of Psychiatry, 174(6), 503–505. 10.1176/appi.ajp.2017.17030314 [DOI] [PubMed] [Google Scholar]
- Wray, N. R. , Eley, T. C. , Marchini, J. , Dolan, C. V. , Penninx, B. W. J. H. , Cai, N. , … Tian, C. (2018). Genome‐wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 200(1), 61–77. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich, P. A. , Piven, J. , Hazlett, H. C. , Smith, R. G. , Ho, S. , Gee, J. C. , & Gerig, G. (2006). User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage, 31(3), 1116–1128. 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Zarei, M. , Beckmann, C. F. , Binnewijzend, M. A. A. , Schoonheim, M. M. , Ali, M. , Sanz‐Arigita, E. J. , … Barkhof, F. (2013). Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer's disease. NeuroImage, 66, 28–35. 10.1016/j.neuroimage.2012.10.071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


