Abstract
Objective:
To examine the association between initial patterns of prescription opioid supply (POS) and risk of all-cause mortality among an insured opioid-naïve patient population in the United States (US).
Methods:
This retrospective observational cohort study used de-identified, administrative health care claims data from a large national insurer (Optum Clinformatics® Data Mart) from 2010 to 2015. Participants included insured, cancer-free adults prescribed opioid analgesics. Prescription opioids received during the first 6 months of therapy were used to categorize initial patterns of POS as daily or non-daily. Cox regression was used to estimate the association of initial patterns of POS with all-cause mortality within one year of follow-up, adjusting for baseline covariates to control for confounding.
Results:
A total of 4,054,417 patients were included, of which 2.75% had incident daily POS; 54.8% were female; median age was 50 years; mean Charlson comorbidity index (CCI) was 0.21 (standard deviation [SD] = 0.77); and mean daily morphine milligram equivalent (MME) was 34.61 (95% CI: 34.59, 34.63). There were 2,068 more deaths per 100,000 person-years among patients who were prescribed opioids daily than non-daily. After adjusting for baseline covariates, the hazard of all-cause mortality among patients with incident daily POS was nearly twice that among those prescribed nondaily (hazard ratio [HR] = 1.94; 95% CI: 1.84, 2.04).
Conclusion:
Among insured adult patients with noncancer pain, incident chronic POS was associated with a significantly increased risk of all-cause mortality over at most one year of follow-up. Because these results may be susceptible to bias, more research is needed to establish causality.
Keywords: prescription opioids, initial pattern of use, opioid-naive, all-cause mortality, claims data
INTRODUCTION
Several studies have reported a connection between opioid prescribing practices and opioid-related adverse health outcomes, including opioid use disorder (OUD), overdose, and death.1–3 Death from opioid-related overdose is usually due to respiratory depression induced by opioids, especially at doses that the individual cannot tolerate. Compared with the general population, increased mortality among patients with OUD has been attributed to opioid overdose and traumatic injury.4,5 In addition, higher rates of hospitalization for localized and systemic infections due to injection drug use have been reported among patients with OUD.6,7 Other studies have linked long-term opioid therapy with adverse effects on the immune system, leading to increased risk of certain serious infections.8–11 Indeed, over the last decade, deaths due to opioid overdose have tripled in the US. In 2016, opioid overdoses accounted for more than 42,000 deaths with about 40% of cases involving prescription opioids.12 Recent studies suggest that there may be an association between receipt of prescription opioids and mortality.1,13,14 Among patients ages 50 and older who visited participating general practices in the United Kingdom with osteoarthritis, initial prescription of opioid analgesics was associated with increased mortality within one year of follow-up compared with some commonly prescribed non-steroidal anti-inflammatory drugs.13
The initial pattern of prescription opioid supply (POS) and utilization varies among patients depending on the indications, severity of pain, comorbidities and local prescribing norms. Often initial opioid prescriptions intended for short term use may result in long-term or incident chronic use with possible adverse health outcomes.15,16 Studies have found that the likelihood of persistent opioid exposure may be associated with the dose and/or duration of the first opioid prescription.17,18 However, one case-control study of US veterans that examined the relationship between patterns of POS and health outcomes found that receiving as-needed opioids in addition to regularly scheduled opioid therapy was not associated with an increased risk of overdose.1
To date, most state and federal efforts aimed at curbing the opioid crisis have primarily focused on restricting the supply of prescription opioids through policies such as prescription drug monitoring programs, opioid prescribing guidelines, quantity-limit regulations, and law enforcement activities. Current opioid prescribing guidelines recommend weighing likely risks and benefits of prescription opioids prior to initiating opioid therapy; and reassessing this risk-benefit balance frequently during therapy.19 Despite these guidelines, initial opioid prescriptions intended for short term use often lead to incident chronic POS and little is known about the effects of initial patterns of POS on mortality. A recent analysis of changes in opioid prescribing after the CDC guidelines were released suggests some effects of duration of opioid therapy on mortality.20 However, no studies have examined the role of initial patterns of POS in all-cause mortality among opioid-naïve patients. In this study, we sought to define clinically meaningful and relevant types of initial patterns of POS and to determine how these initial patterns are associated with all-cause mortality among commercially insured opioid naive adult patients in the US. Assessing the mortality risks associated with incident patterns of POS may inform efforts to address the current opioid crisis in the US.
METHODS
Study design and data source:
This was a retrospective cohort study using de-identified administrative claims data (Optum Clinformatics® Data Mart) from 2010 to 2015. The database contains only enrollees with both medical and prescription coverage and provides information on member demographics and eligibility history, inpatient and outpatient medical services, and outpatient pharmacy claims. Clinical procedures and professional services are recorded using the Current Procedural Terminology (CPT) codes while associated diagnoses are recorded using the International Classification of Diseases, 9th and 10th Revisions, Clinical Modification (ICD-9 and ICD-10) codes. The dataset was linked to mortality information on all enrollees before it was made available for research. The study protocol was approved by the University of Rhode Island Institutional Review Board.,
Study population:
The American Hospital Formulary Service classification codes were used to identify records of all patients in the database who filled at least one opioid prescription (Figure 1). Akin to the design of a randomized trial,21 we focused on opioid-naïve patients, defined as having no opioid prescription or OUD claim during the first observed 6 months of continuous enrollment prior to the index date.22 The index date was defined as the date of the first dispensed opioid prescription during the study period (eFigure 1). To allow for adequate ascertainment of baseline characteristics and initial patterns of POS, we required patients to be continuously enrolled (allowing gaps in enrollment of up to 90 days) for at least 6 months before (i.e., baseline period) and 6 months after the index date. Like the design of a randomized trial, we applied inclusion and exclusion criteria (eTable 1). Patients were excluded if they were younger than 18 years old as of the index date, or if their first opioid prescription was for a medication for OUD, implying prior exposure to prescription opioids was more likely.23 The study sample was further restricted to patients with no claims for any cancer diagnosis, or use of palliative or hospice care during the index or baseline period.24 Hospice and palliative care services were identified with ICD-9 and CPT codes. We excluded individuals who were unlikely to have enough data to establish sufficient follow-up, specifically those with less than 6 months of enrolment after the index date. The final study sample consisted of 4,054,417 patients.
Figure 1.
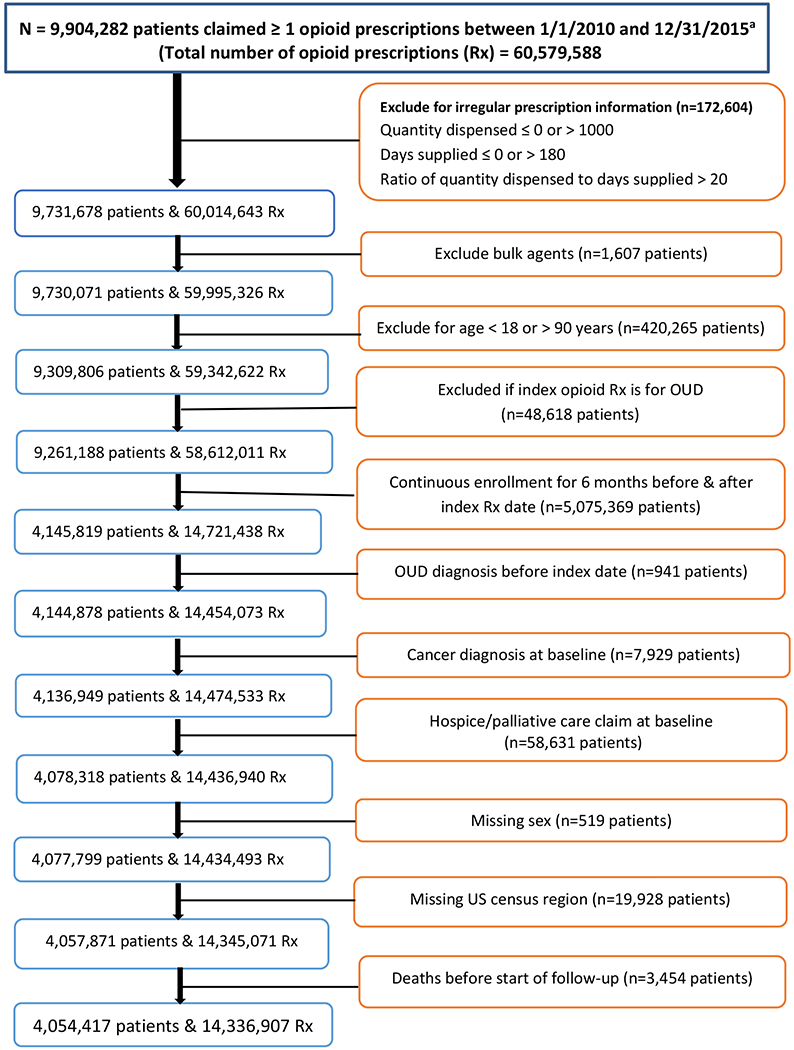
Study population and sample selection from all patients who received at least one opioid prescription in the database, 2010-2015
aAmerican Hospital Formulary Service class code was used to identify opioid prescription claims
Exposure definition:
Exposure was defined as receipt of a prescription opioid analgesic between 2010 and 2015. Based on the Consortium to Study Opioid Use and Trends criteria,25,26 we summed all the days’ supply for all opioid prescriptions filled by each patient during the first 6 months of opioid therapy (i.e., index period). This information was used to assign each patient to a treatment strategy categorized either as daily POS (i.e., long-term or chronic use) if a patient had more than 90 days’ supply of prescription opioids during the index period or as non-daily POS (i.e., short term use) otherwise.27 This strategy assumed that overlapping opioid prescriptions were taken sequentially until all opioid medications were consumed. We disregarded treatment changes that occurred during the follow-up period as patients were assumed to remain on their defined treatment strategy. We computed the average daily MME, and the number of providers and pharmacies used during the index period to fulfill opioid prescription requirements. Opioid prescription dosing information was converted to daily morphine milligram equivalent (MME) by multiplying the quantity of each prescription by the strength of the prescription, divided by the days’ supply received, and multiplying this total by published conversion factors.28 For patients who received more than one opioid prescription on any given day, the MME of all prescriptions were added together. Although there is no known ceiling effect for most opioid analgesics, based on recent studies and CDC prescribing guidelines, the average daily MME was categorized as < 50, 50-90, or >90 MME daily for descriptive purposes.9,19,29
Covariate information:
Confounding factors needed to ensure comparability of exposure groups were specified a priori. The dataset contained information on the patient’s sex, type of insurance, type of health plan, and US states grouped into five census regions. Age in years, was calculated as of the index opioid prescription date. Clinical characteristics that may influence both the initial pattern of POS and mortality, such as Charlson comorbidity index, common pain conditions, mental health disorders, pregnancy-related conditions and surgical procedures; occurrence of sprains and fractures, and substance use disorders including alcohol, tobacco, opioids and other substances were identified during the baseline and index periods using ICD-9 and ICD-10 codes.30 All clinical diagnosis variables were used to indicate if the patient had been diagnosed with or treated for the condition during the baseline period. Use of psychiatric medications such as benzodiazepines, antidepressants, antipsychotics, and gabapentin was defined as having one or more prescription claims during the index period, irrespective of whether that use overlapped with use of an opioid prescription.26 We used prescription information to identify psychiatric conditions on the assumption that this approach is likely to define the presence of clinically significant psychiatric comorbidity with greater specificity than diagnostic codes. A listing of ICD-9, ICD-10 and CPT codes used to identify various conditions is provided in the Online Appendix (eTable 2–7).
Outcome measurement:
The study outcome was all-cause mortality independently ascertained by the data vendor through linkage of the database with the Social Security Administration Death Master file.31 The specificity of this master file for detecting mortality status has been estimated to range from 97% to 100%.32–35 Our database contained no information on the underlying causes of death and since only the month and year of death are recorded, the date of death was imputed to be the last calendar day of each month. For each patient, person-time of follow-up was measured from the end of index period to death, disenrollment, the end of a 1-year follow-up period or end of the study (i.e., December 31, 2015), whichever came first. Because mortality was captured in the master file and not measured, individuals were censored 12 months after the last medical encounter.36
Statistical Analysis:
Descriptive statistics were computed for demographic, clinical, and prescription characteristics at baseline and presented by initial pattern of POS. We estimated overall median follow-up time and crude death rate for the study sample. Overall survival among the two exposure groups (daily versus non-daily) was compared using unadjusted and inverse-probability-weighted (IPW) Kaplan-Meier survival curves.37 We assumed that conditional on measured baseline covariates, those exposed to daily and nondaily POS are exchangeable. We fit unadjusted and adjusted multivariable Cox regression model to estimate hazard ratios (HR) with 95% confidence intervals (CI) used to determine the association of initial pattern of POS with the rate of all-cause mortality, adjusting for patient-level baseline covariates to control for confounding. Restricted quadratic splines were used to finely control for continuous confounders (age and average MME) allowing for a non-linear relationship with time-to-death.38 Based on the graphical visual inspection of the log-log survival curves the assumption of proportional hazards for initial pattern of POS was satisfied (Appendix eFigure 2).
We performed two additional analyses to assess effect modification by opioid dose and age. To assess whether the effect of the initial patterns of POS on all-cause mortality was modified by MME, we added a cross-product term between initial patterns of POS and a binary MME variable (low dose, ≤90 versus high dose, >90). The definition of high daily opioid dose was based on the Department of Veterans’ Affairs (VA) and CDC opioid prescribing guidelines.19,39 These opioid prescribing guidelines recommend weighing likely risks and benefits of prescription opioids prior to initiating opioid therapy; reassessing the risk-benefit balance when exceeding 50 MME daily, and avoiding daily doses in excess of 90 MME daily. Because the interaction term was significant (p < 0.0001), a stratified analysis was performed. Effect modification by age was evaluated similarly and a stratified analysis conducted. Furthermore, in a subgroup analysis restricted to a full sample of 15,630 patients who died during follow-up, we described health conditions with claims in the primary or secondary position and use of health services during a 6-month period preceding death stratified by the initial pattern of POS.
All data manipulations and statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) or R statistical software, version 3.2.3 (R Core Team 2016).40 All tests of statistical significance were two-sided and performed at the 0.05 significance level.
RESULTS
A total of 4,054,417 patients were included in the study, of which 2.75% were patients who were prescribed opioids daily; 54.8% were female; median age was 50 years; mean Charlson comorbidity index (CCI) was 0.21 (standard deviation [SD] = 0.77); and mean daily MME was 34.61 (95% confidence interval [CI]: 34.59, 34.63). Table 1 shows baseline characteristics of patients included in the study; overall and by incident POS during the index period. The average age of patients who were prescribed daily opioids was 62.2 years (SD = 15.9) compared with 49.7 (18.2) years among patients who were prescribed non-daily. Overall, the proportion of patients who were prescribed opioids daily increased progressively with age while the proportion of patients who were prescribed non-daily appeared to plateau after age 25 years. Almost 57% of patients prescribed opioids daily received Medicare coverage compared to only 23% of patients who were prescribed non-daily opioid supply.
Table 1.
Characteristics of patients exposed to prescription opioid analgesics by initial pattern of prescription opioid supply for non-cancer pain and non-palliative care among insured adults in the United States, 2010-2015 (N=4,054,417)
| Initial pattern of prescription opioid supply | |||
|---|---|---|---|
| Overall | Non-daily supply | Daily supply | |
| Patients characteristics | n = 4,054,417 | n=3,943,034 | n=111,383 |
| Observation time in months, mean (SD) | 9.74 (3.66) | 9.74 (3.66) | 9.76 (3.67) |
| Charlson comorbidity index, mean (SD) | 0.21 (0.77) | 0.20 (0.75) | 0.467(1.22) |
| Female, n (%) | 2,221,906 (54.80) | 2,160,536 (54.79) | 61,370 (55.10)a |
| Age in years, mean (SD) | 50.01 (18.26) | 49.67 (18.20) | 62.15 (15.92) |
| Age group in years, n (%) | |||
| 18-24 | 393,954 (9.72) | 392,228 (9.95) | 1,726 (1.55) |
| 25-34 | 573,268 (14.14) | 567,892 (14.40) | 5,376 (4.83) |
| 35-44 | 666,217 (16.43) | 656,876 (16.66) | 9,341 (8.39) |
| 45-54 | 744,309 (18.36) | 726,993 (18.44) | 17,316 (15.55) |
| 55-64 | 664,687 (16.39) | 642,355 (16.29) | 22,332 (20.05) |
| 65+ | 1,011,982 (24.96) | 956,690 (24.26) | 55,292 (49.64) |
| Insurance type, n (%) | |||
| Commercial | 3,078,129 (75.92) | 3,029,814 (76.84) | 48,315 (43.38) |
| Medicare | 976,288 (24.08) | 913,220 (23.16) | 63,068 (56.62) |
| Health plan type | |||
| Exclusive provider organization | 364,973 (9.00) | 359,516 (9.12) | 5,457 (4.90) |
| Health maintenance organization | 757,067 (18.67) | 717,885 (18.21) | 39,182 (35.18) |
| Indemnity | 39,895 (0.98) | 38,088 (0.97) | 1,807 (1.62) |
| Others | 448,367 (11.06) | 424,561 (10.77) | 23,806 (21.37) |
| Point of service | 2,278,627 (56.20) | 2,244,141 (56.91) | 34,486 (30.96) |
| Preferred-provider organization | 165,448 (4.08) | 158,843 (4.03) | 6,645 (5.97) |
| US census region, n (%) | |||
| Midwest | 943,302 (23.27) | 922,117 (23.39) | 21,185 (19.02) |
| Northeast | 461,554 (11.38) | 449,788 (11.41) | 11,766 (10.56) |
| South | 1,722,371 (42.48) | 1,676,020 (42.51) | 46,351 (41.61) |
| West | 927,190 (22.87) | 895,109 (22.70) | 32,081 (28.80) |
| Substance use and misuse, n (%) | |||
| Drug use | 3,143 (0.08) | 2,637 (0.07) | 506 (0.45) |
| OUD | 1,608 (0.04) | 1,306 (0.03) | 302 (0.27) |
| Overdose | 1,799 (0.04) | 1,633 (0.04) | 166 (0.15) |
| Opioid overdose | 317 (0.01) | 270 (0.01) | 47 (0.04) |
| Alcohol use | 14,411 (0.36) | 13,585 (0.34) | 826 (0.74) |
| Tobacco use | 88,338 (2.18) | 84,405 (2.14) | 3,933 (3.53) |
| Cocaine | 750 (0.02) | 699 (0.02) | 51 (0.05) |
| Marijuana | 717 (0.02) | 701 (0.02) | 16 (0.01)b |
| Other substance usec | 1,669 (0.04) | 1,534 (0.04) | 135 (0.12) |
| Pain-related diagnosis, n (%) | |||
| Headaches/migraines | 119,534 (2.95) | 115,654 (2.93) | 3,880 (3.48) |
| Neck pain | 134,293 (3.31) | 128,314 (3.25) | 5,979 (5.37) |
| Back pain | 299,055 (7.38) | 283,046 (7.18) | 16,009 (14.37) |
| Abdominal pain | 220,857 (5.45) | 214,574 (5.44) | 6,283 (5.64) |
| Joint pain/arthritis | 609,390 (15.03) | 583,732 (14.80) | 25,658 (23.04) |
| Fibromyalgia/CFS | 59,497 (1.47) | 55,838 (1.42) | 3,659 (3.29) |
| Chronic pain syndromes | 171,277 (4.22) | 158,164 (4.01) | 13,113 (11.77) |
| Other pains | 131,099 (3.23) | 125,427 (3.18) | 5,672 (5.09) |
| Comorbid conditions, n (%) | |||
| Fractures/strains | 235,215 (5.80) | 227,255 (5.76) | 7,960 (7.15) |
| Surgical diagnosis | 358,187 (8.83) | 348,817 (9.85) | 9,370 (8.41) |
| Mental health disordersd | 253,245 (6.25) | 241,244 (6.12) | 12,001 (10.77) |
| Pregnancy-related claim | 739,434 (18.24) | 713,180 (18.09) | 26,254 (23.57) |
| Use of Psychotropic medications, n (%) | |||
| Benzodiazepines | 290,368 (7.16) | 275,858 (7.00) | 14,510 (13.03) |
| Antidepressant | 536,975 (13.24) | 505,982 (12.83) | 30,993 (27.83) |
| Antipsychotics | 45,521 (1.12) | 41,542 (1.05) | 3,979 (3.57) |
| Gabapentin | 42,908 (1.06) | 37,154 (0.94) | 5,754 (5.17) |
| Characteristics of opioid Rx during index period, mean (SD) | |||
| Number of prescriptions | 1.74 (1.63) | 1.58 (1.18) | 7.13 (4.14) |
| Number of prescription types | 1.17 (0.44) | 1.16 (0.41) | 1.65 (0.84) |
| Number of providers involved | 1.29 (0.65) | 1.26 (0.60) | 2.12 (1.41) |
| Number of pharmacies used | 1.13 (0.42) | 1.12 (0.38) | 1.59 (0.99) |
| Average daily dose, MME | 34.61 (20.42) | 34.47 (18.74) | 39.44 (52.13) |
| Average daily MME, n (%) | |||
| <50 | 3,365,583 (83.01) | 3,275,993 (83.08) | 89,590 (80.43) |
| 50-90 | 633,853 (15.63) | 619,626 (15.71) | 14,227 (12.77) |
| >90 | 54,981 (1.36) | 47,415 (1.29) | 7,566 (6.79) |
| Charlson comorbidity index, n (%) | |||
| 0 | 3,626,558 (89.45) | 3,537,215 (89.71) | 89,343 (80.21) |
| 1+ | 427,859 (10.55) | 405,819 (10.29) | 22,040 (19.79) |
Abbreviations: OUD=Opioid use disorder, SD=standard deviation, CI=confidence interval, MME=morphine milligram equivalents.
p-value = 0.03;
p-value > 0.05; all other p-values < 0.0001.
Includes anxiolytics, stimulants, hallucinogenic drugs, or use of unspecified drugs.
Includes adjustment disorders, anxiety disorders, conduct disorders, cognitive disorders, mood disorders, schizophrenia and psychotic disorders, alcohol-related disorders, substance-related disorders, and miscellaneous mental health disorders.
Tobacco and alcohol were the most commonly used substances and were observed in 2.2% and 0.36% of the study cohort, respectively. Joint and arthritis pain (15%) were the most common pain syndromes with medical claims during the index period followed by back pain (7.4%). Almost 9% of patients had a surgery-related claim during the baseline period and 6% had a claim for sprains and fractures. The proportion of patients who filled at least one antidepressant (13.2%) or a benzodiazepine (7.2%) during the index period was much higher than the proportion of patients with claims for mental health disorders during the baseline period (6%). Overall, almost 90% of patients had no comorbid physical condition. However, 20% of patients on incident daily POS had one or more comorbid physical conditions compared to 10% among patients who were prescribed opioids non-daily. During the index period 1,608 (0.04%) patients were diagnosed with OUD. The incidence of OUD during the index period was 0.27% among patients with daily POS compared with only 0.03% among patients with non-daily POS. Similarly, on average, the number of opioid prescriptions, number of different opioid types used, mean daily MME, and number of providers and pharmacies involved were higher among patients who were prescribed opioids daily than non-daily.
Patients were followed for up to one year (mean of 9.7 months) with a total of 3,289,758 person-years during which 15,630 (0.39%) died (Table 2). Overall crude death rate was 475 deaths per 100,000 person-years. Among patients on incident daily POS, the crude incidence rate of all-cause mortality was 2,486 per 100,000 person-years compared to 418 per 100,000 person-years among patients with non-daily POS. The incidence rate difference (IRD) of 2,068 per 100,000 person-years represents the mortality rate among patients who were prescribed opioids that is attributable to a pattern of incident daily use, assuming patients who were prescribed opioids daily (i.e., exposed) would have had a mortality rate equal to patients who were prescribed opioids non-daily (i.e., unexposed) had they not been given prescription opioids daily. The crude rate of mortality among patients on incident daily POS was almost six times that of the rate among patients with non-daily POS (crude incident rate ratio [IRR] = 5.95; 95% CI: 5.69, 6.22). The survival probabilities of patients with incident daily and non-daily exposure to prescription opioids are shown in Figure 2 and 3. Overall, survival was associated with initial pattern of POS (p-value < 0.0001). For the duration of follow-up, patients with incident daily POS had lower survival rates than patients with non-daily POS with this difference becoming attenuated over time. The differences persisted even after adjusting for potential confounders (i.e., age, average MME/day, gender, calendar year, region, insurance type, health plan type, use of tobacco and alcohol, opioid overdose, pain conditions, surgery, pregnancy-related conditions, and CCI) using inverse probability of exposure weights.
Table 2.
Number of deaths and person-years of observation among cohort of patients initially exposed to prescription opioid daily and non-daily for non-cancer, non-palliative care conditions among insured adults in the United States, 2010-2015 (N=4,054,417)
| Initial pattern of prescription opioid exposure | |||
|---|---|---|---|
| Daily opioid supply | Non-daily opioid supply | Total | |
| Number of deaths, n (%) | 2,253 (2.02) | 13,377 (0.34) | 15,630 (0.39) |
| Person-years of observation | 90,624.40 | 3,199,133.00 | 3,289,758.00 |
| Crude mortality matea | 2486.09 | 418.14 | 475.11 |
| Crude IRR (95% CI) | 5.95 (5.69, 6.22) | ||
| Crude RD (95% CI)a | 2067.94 (1965.04, 2170.84) | ||
Abbreviations: IRR=incidence rate ratio, IRD=incidence rate difference, CI=confidence interval
Cases/100,000 person-years
Figure 2.
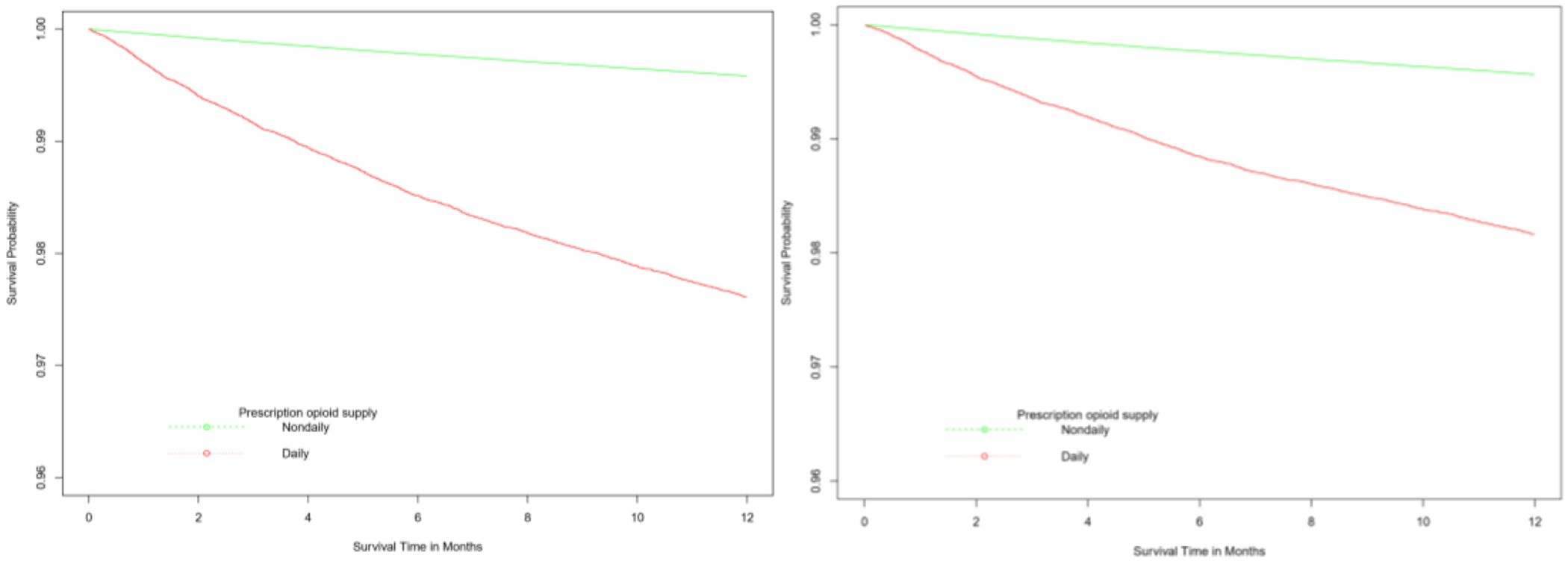
Unweighted (left panel) and inverse probability of exposure weighteda (right panel) Kaplan-Meier curves showing the survival experience of patients with receipt of daily and non-daily prescription opioids, 2010-2015.
aIP-weighted curve was adjusted for baseline covariates: age, sex, US census region, index year, Charlson comorbidity index, substance use disorders (alcohol, smoking, opioid overdose), surgical procedure, fracture and strains, pain conditions (headache, neck and jaw pain, back pain, abdominal pain, fibromyalgia), measured at baseline and index periods, and opioid use disorder diagnosis, psychiatric medications (benzodiazepines, antidepressants, gabapentin), number of opioid providers and average daily prescription opioid dose during the index period.
Figure 3.
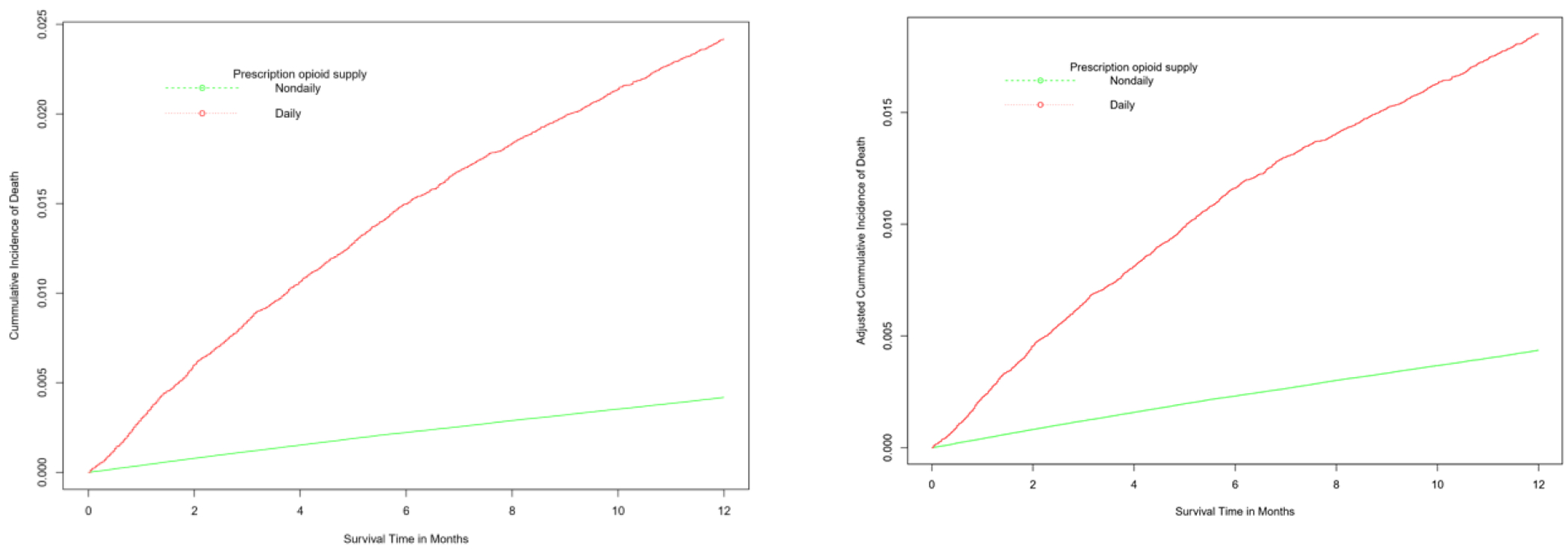
Unweighted (left panel) and IP-weighteda (right panel) cumulative incidence of mortality for patients with receipt of daily and non-daily prescription opioid, 2010-2015.
aIP-weighted curve was adjusted for baseline covariates: age, sex, US census region, index year, Charlson comorbidity index, substance use disorders (alcohol, smoking, opioid overdose), surgical procedure, fracture and strains, pain conditions (headache, neck and jaw pain, back pain, abdominal pain, fibromyalgia), measured at baseline and index periods, and opioid use disorder diagnosis, psychiatric medications (benzodiazepines, antidepressants, gabapentin), number of opioid providers and average daily prescription opioid dose during the index period.
Table 3 shows the results from the unadjusted and covariate adjusted Cox regression models. In the unadjusted model, the hazard of all-cause mortality among patients on incident daily POS was almost six times the hazard among patients who were prescribed opioids non-daily (Hazard ratio [HR] = 5.95; 95% CI: 5.69, 6.22). After adjusting for baseline demographic and clinical variables, the hazard of death among patients with incident daily POS was almost two times the hazard of all-cause mortality among patients with non-daily POS (HR = 1.94; 95% CI: 1.84, 2.04).
Table 3.
Unadjusted and adjusted hazard ratios for all-cause mortality associated with exposure to incident daily prescription opioid supply versus non-daily incident prescription opioid supply among insured adults in the United States, 2010-2015 (N=4,054,417)
| Daily opioid supply | Non-daily opioid supply | Overall | |
|---|---|---|---|
| n=111,383 | n=3,943,034 | n=4,054,417 | |
| Deaths, n (%) | 2,253 (2.02) | 13,377 (0.34) | 15,630 (0.39) |
| Person-years of observation | 90,624.40 | 3,199,133.00 | 3,289,758.00 |
| Unadjusted hazard ratio (95% CI) | 5.95 (5.69, 6.22) | Reference | |
| Adjusted hazard ratio (95% CI)a | |||
| Overall | 1.94 (1.84, 2.04) | Reference | |
| MME category (mg/day) | |||
| Low dose (< 90) | 1.88 (1.78, 1.98) | Reference | |
| High dose (90+) | 2.37 (1.92, 2.94) | Reference | |
| Age category (years) | |||
| < 65 years | 1.86 (1.78, 1.96) | Reference | |
| 65+ | 1.70 (1.60, 1.80) | Reference | |
Abbreviations: CI=confidence interval; MME=morphine milligram equivalent
Model adjusted for differences in age, sex, US census region, index year, type of insurance, type of health plan, Charlson comorbidity index, substance use disorders (alcohol, smoking, opioid overdose), surgical procedure, pregnancy-related conditions, fracture and strains, pain conditions (headache, neck and jaw pain, back pain, abdominal pain, fibromyalgia), measured at baseline and index periods, and opioid use disorder diagnosis, psychiatric medications (benzodiazepines, antidepressants, gabapentin), number of opioid providers and average daily prescription opioid dose during the index period.
About 54,981 (1.36%) received high dose during the first 6 months of therapy. The estimated association between incident daily POS and all-cause mortality was stronger among patients receiving a high dose compared to low dose opioid prescriptions, while among patients aged 65 and younger, the estimated association was slightly stronger than among patients 65 years and older.
Among 15,630 decedents during follow-up, there were 13,377 (85.6%) patients who had a medical diagnosis claim during the 6 months prior to death. There were more claims for drug use (p=0.0288), opioid overdose (p < 0.0001) and cancer (p=0.0156) among patients on incident daily POS compared with patients who used prescription opioids non-daily but there was no difference in the use of medications for OUD and hospice care services (eTable 8). We examined 212 distinct diagnosis codes with frequencies of ≥ 10 among 8,840 (56.6%) decedents; 17.4% were for cardiovascular disorders, 17.2% for cancer-related diagnoses, 16.4% for respiratory disorders, 12.9% for septicemia and other infections, and 11.2% for cerebrovascular accidents and other CNS conditions. About 4.2% had a claim for fractures and other noncancer-related pain conditions (eTable 9).
DISCUSSION
We found that the initial patterns of incident chronic POS was associated with increased hazard of all-cause mortality, even after adjusting for confounding factors measured at baseline. A stratified analysis suggests that the association may be modified, at least in part, by the dose of the opioid prescription. The association was slightly stronger among patients younger than 65 years old than among patients aged 65 years and older. Almost 3% of our study population was exposed to incident chronic POS which was comparable to rates reported in previous studies.41 The likelihood of chronic opioid exposure has been associated with the dose and duration of initial opioid prescription.17,18 Overall, about 28% of insured individuals were exposed to at least one opioid prescription during a six-year period for which data was available. This is remarkably similar to findings of a 2015 study which estimated that 92 million US adults used at least one opioid prescription, corresponding to about 30% of the adult population.42 Although incident chronic POS accounted for a relatively small proportion of the study population, it was associated with a significantly increased risk of all-cause mortality within one year of follow-up.
All observational studies using claims data face several challenges, including residual and/or unmeasured confounding because patients are not randomly assigned to treatment and several confounders may not be well-captured, and our study is no exception. We acknowledge that patients who received daily prescription opioids were, on average, older and had poorer health status and more comorbidities than those who received non-daily prescription opioids. However, among patients younger than 65 years old, the association between the receipt of incident chronic opioid therapy and all-cause mortality was slightly stronger than that observed among older patients, but the difference was not statistically significant. Given our inability to adequately measure disease burden and severity of medical conditions including frailty, debility and severity of pain using claims data, our findings should be interpreted with these limitations in mind. In addition, POS was based on prescription fills and adherence was not measured. We attempted to minimize exposure misclassification by defining the exposure window over a 6-month period. Because we did not account for the use of multiple types of opioids, it is possible that the observed association may be related to the type of opioid utilized. For example, use of long-acting prescription opioids for chronic noncancer pain has been associated with an increased risk of mortality compared with anticonvulsants and cyclic antidepressants.14 We also attempted to reduce imbalance between the exposure groups by including several potential confounders, specified a priori and measured at baseline and during the index period, in our multivariable Cox regression model. Restricted quadratic splines were used to finely control for continuous confounders.38
Because analgesic medications, including prescription opioids, are often used to alleviate pain and discomfort during terminal care,43 we excluded patients with any cancer diagnoses or hospice care services claims during the baseline or index periods. However, this restriction may not have eliminated all confounding by indication. A subgroup analysis of patients who died within one year of follow-up showed that cardiac conditions, cancer, respiratory disorders and infections were the most common terminal medical diagnoses recorded and patients who were given daily opioids had significantly higher rates of substance use, overdose and cancer diagnosis within 6 months of death, although use of hospice care services was remarkably similar. Both overdose and cancer diagnoses have been associated with an increased risk of death.
Of over 70,000 drug overdose deaths in 2017 in the US, about two-thirds were related to opioid exposure.44 In most cases, patients who die from overdose do not live long enough to benefit from hospice care. While it is possible that respiratory disorders are not necessarily due to opioid-related overdose, several studies have found a relationship between POS and serious human infections including pneumonia.10,45,46 It is possible that some patients may have initiated prescription opioids in response to early signs and symptoms of undiagnosed cancer. Although we excluded patients diagnosed with cancer during the baseline and index periods, this, or other forms of protopathic bias cannot be excluded as a likely explanation for our findings.47,48 Such patients may have died from an unrelated condition such as myocardial infarction, opioid overdose or the underlying cancer - the second leading cause of death in the US overall, after heart disease.49 Furthermore, the risk of developing OUD during the index period was 9 times higher among patients with daily POS compared with non-daily use suggesting that some of the patients who were prescribed opioids as chronic therapy may have developed OUD early with subsequent death from opioid-related overdose.26 OUD has been associated with a 20-fold higher risk of premature death due to overdose, infectious diseases, trauma, and suicide.50 Prior studies have found that individuals, especially older ones, with an OUD are also more likely to die from chronic diseases than drug-related causes such as overdose, suicide and other unnatural death.51,52 However, during the last 6 months of observation before death, claims for opioid-related overdose were significantly more common among patient who received incident chronic therapy (p < 0.0001). For many of the observed causes of death (e.g., cancer), there may be no plausible causal link between the initial pattern of opioid exposure and the outcome, whereas other causes of mortality may be related directly or indirectly to opioid exposure or consequences of OUD (e.g., overdose death, infections).
Our findings are consistent with several prior studies that have reported significant risks associated with chronic opioid therapy.53 Prescription opioid-related deaths have been associated with the use of long-acting agents,14,54 high daily dose therapy,55 concurrent use of benzodiazepines,2,55,56 sedatives, antidepressants and/or gabapentin,57 and use of opioids in high risk patients such as those with sleep disorder,58 chronic obstructive pulmonary disease, renal and hepatic failure,59 and patients 65 years of age and older.60,61 These studies suggest that there may be several pathways to increased risk of mortality among patients on prescription opioids, especially incident chronic POS. We did not find a statistically significant higher risk of all-cause mortality associated with POS among patients older than 65 years compared to younger patients in this study.
LIMITATIONS
Our study had some important limitations. First, we could not rule out unmeasured confounding by indication and protopathic bias as a likely explanation of the association between incident chronic receipt of prescription opioids and increased risk of all-cause mortality within the first year of follow-up. Patients with cancer and hospice claims at baseline and index period were excluded from our analysis, and several potential confounders, identified a priori, were included in the adjusted model. Second, we assumed that patients were opioid-naive if they had not filled any opioid prescription for six months although this may not imply that they were never exposed to opioids previously. Patients were assumed to take overlapping opioid prescriptions sequentially until all medications were consumed,26 and we were unable to determine the specific dosing instructions provided to the patient by the provider. Although outpatient pharmacy data reflect POS, we could not account for prescriptions paid for in cash that may not be captured in the database. Lack of information on POS at other health plans or prior to start of the database may have led to misclassification of some patients who were already receiving prescription opioids as opioid-naive. Such misclassification was minimized by requiring at least 6 months of enrollment with medical and pharmacy benefits prior to the index date and use of the index period to estimate initial patterns of POS. Third, using ICD-9 and ICD-10 codes in administrative data may underestimate certain medical and lifestyle conditions such as OUD, tobacco and alcohol use disorders leading to potential confounder misclassification. We also lacked access to data on socioeconomic status likely limiting our ability to include a sufficient set of covariates to adjust for confounding.62 Finally, we could not rule out unmeasured confounding by indication and protopathic bias as a likely explanation of the association between incident chronic receipt of prescription opioids and increased risk of all-cause mortality within the first year of follow-up.
CONCLUSIONS
This study is a large longitudinal observational study of the association between initial patterns of POS and all-cause mortality analyzed with an intent-to-treat analytical approach. Incident chronic POS was associated with a 1.9-fold increased risk of all-cause mortality over one year of follow-up. The greatest impact was seen among patients who received high daily dose of prescription opioids. These findings are consistent with current CDC treatment guidelines that recommend the use the lowest effective prescription opioid dose for the shortest duration of treatment possible, especially among opioid-naïve patients. Because the results of this study may be susceptible to bias more research is needed to establish causality. Such analyses may use structural models to replicate and extend our findings by accounting for time-varying confounding and possible informative loss to follow-up as POS is inherently time-varying.
Supplementary Material
Grant Support:
Dr Rich was supported by grant number P20GM125507 and Drs. Buchanan and Kogut were partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Prior submission: Earlier versions of this study were accepted for poster presentations at:
1. The 34th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Prague, Czech Republic; August 22-26, 2018
2. The Society for Epidemiologic Research (SER) Conference, Baltimore, MD; June 19-22, 2018
REFERENCES
- 1.Bohnert AS, Valenstein M, Bair MJ, et al. Association between Opioid Prescribing Patterns and Opioid Overdose-Related Deaths. JAMA. 2011;305(13):1315–1321. [DOI] [PubMed] [Google Scholar]
- 2.Gomes T, Mamdani MM, Dhalla IA, et al. Opioid Dose and Drug-Related Mortality in Patients with Nonmalignant Pain. Arch Intern Med. 2011;171(7):686–691. [DOI] [PubMed] [Google Scholar]
- 3.Dunn KM, Saunders KW, Rutter CM, et al. Opioid Prescriptions for Chronic Pain and Overdose: A Cohort Study. Ann Intern Med. 2010;152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degenhardt L, Randall D, Hall W, et al. Mortality among Clients of a State-Wide Opioid Pharmacotherapy Program over 20 Years: Risk Factors and Lives Saved. Drug Alcohol Depend. 2009;105(1-2):9–15. [DOI] [PubMed] [Google Scholar]
- 5.Gronbladh L, Ohlund LS and Gunne LM. Mortality in Heroin Addiction: Impact of Methadone Treatment. Acta Psychiatr Scand. 1990;82(3):223–227. [DOI] [PubMed] [Google Scholar]
- 6.Ronan MV and Herzig SJ. Hospitalizations Related to Opioid Abuse/Dependence and Associated Serious Infections Increased Sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad C, Bradley HM, Broz D, et al. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman EJ, Gordon KS, Crothers K, et al. Association of Prescribed Opioids with Increased Risk of Community-Acquired Pneumonia among Patients with and without HIV. JAMA Internal Medicine. 2019;179(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiese AD, Griffin MR, Schaffner W, et al. Opioid Analgesic Use and Risk for Invasive Pneumococcal Diseases: A Nested Case-Control Study. Annals of Internal medicine. 2018;168(6):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiese AD, Griffin MR, Stein CM, et al. Opioid Analgesics and the Risk of Serious Infections among Patients with Rheumatoid Arthritis: A Self-Controlled Case Series Study. Arthritis & Rheumatology. 2017;68(2):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dublin S, Walker RL, Jackson ML, et al. Use of Opioids or Benzodiazepines and Risk of Pneumonia in Older Adults: A Population-Based Case–Control Study. Journal of the American Geriatrics Society. 2011;59(10):1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedegaard H WM, Miniño AM. Drug Overdose Deaths in the United States, 1999–2016. Hyattsville, Md: National Center for Health Statistics; 2017. NCHS Data Brief, No. 294. 2017. [Google Scholar]
- 13.Zeng C, Dubreuil M, LaRochelle MR, et al. Association of Tramadol with All-Cause Mortality among Patients with Osteoarthritis. JAMA. 2019;321(10):969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray WA, Chung CP, Murray KT, et al. Prescription of Long-Acting Opioids and Mortality in Patients with Chronic Noncancer Pain. JAMA. 2016;315(22):2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stumbo SP, Yarborough BJ, McCarty D, et al. Patient-Reported Pathways to Opioid Use Disorders and Pain-Related Barriers to Treatment Engagement. J Subst Abuse Treat. 2017;73:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Hallvik SE, Hildebran C, et al. Association between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah A, Hayes CJ and Martin BC . Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piper BJ, Shah DT, Simoyan OM, et al. Trends in Medical Use of Opioids in the U.S., 2006-2016. Am J Prev Med. 2018;54(5):652–660. [DOI] [PubMed] [Google Scholar]
- 19.Dowell D, Haegerich TM and Chou R. Cdc Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 20.Bohnert ASB, Guy GP Jr. and Losby JL. Opioid Prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 Opioid Guideline. Ann Intern Med. 2018;169(6):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernan MA and Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016;183(8):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulozzi LJ, Strickler GK, Kreiner PW, et al. Controlled Substance Prescribing Patterns--Prescription Behavior Surveillance System, Eight States, 2013. MMWR Surveill Summ. 2015;64(9):1–14. [DOI] [PubMed] [Google Scholar]
- 23.Clemans-Cope L EM, Kenney GM. Rapid Growth In Medicaid Spending on Medications to Treat Opioid Use Disorder and Overdose. Washington, DC: Urban Institute Health Policy Center;2017. [Google Scholar]
- 24.Chastek B, Harley C, Kallich J, et al. Health Care Costs for Patients with Cancer at the End of Life. J Oncol Pract. 2012;8(6):75s–80s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin BC, Fan MY, Edlund MJ, et al. Long-Term Chronic Opioid Therapy Discontinuation Rates from the Troup Study. J Gen Intern Med. 2011;26(12):1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulozzi LJ, Zhang K, Jones CM, et al. Risk of Adverse Health Outcomes with Increasing Duration and Regularity of Opioid Therapy. J Am Board Fam Med. 2014;27(3):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Korff M, Saunders K, Thomas Ray G, et al. De Facto Long-Term Opioid Therapy for Noncancer Pain. Clin J Pain. 2008;24(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC. National Center for Injury Prevention and Control. Cdc Compilation of Benzodiazepines, Muscle Relaxants, Stimulants, Zolpidem, and Opioid Analgesics with Oral Morphine Milligram Equivalent Conversion Factors, 2015 Version. . Centers for Disease Control and Prevention. Web site. http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Published 2015. Accessed October 26, 2017. [Google Scholar]
- 29.Banerjee G, Edelman EJ, Barry DT, et al. High-Dose Prescribed Opioids Are Associated with Increased Risk of Heroin Use among United States Military Veterans. Pain. 2019;160(9):2126–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in Icd-9-Cm and Icd-10 Administrative Data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 31.SSA/DMF. Social Security Administration. Social Security’s Death Master File. 2018. Https://Www.Ssa.Gov/Dataexchange/Request_Dmf.Html Accessed December 18, 2018.
- 32.Schisterman EF and Whitcomb BW. Use of the Social Security Administration Death Master File for Ascertainment of Mortality Status. Popul Health Metr. 2004;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermansen SW, Leitzmann MF and Schatzkin A. The Impact on National Death Index Ascertainment of Limiting Submissions to Social Security Administration Death Master File Matches in Epidemiologic Studies of Mortality. Am J Epidemiol. 2009;169(7):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wentworth DN, Neaton JD and Rasmussen WL. An Evaluation of the Social Security Administration Master Beneficiary Record File and the National Death Index in the Ascertainment of Vital Status. Am J Public Health. 1983;73(11):1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauser TH and Ho KK. Accuracy of on-Line Databases in Determining Vital Status. J Clin Epidemiol. 2001;54(12):1267–1270. [DOI] [PubMed] [Google Scholar]
- 36.Lesko CR, Edwards JK, Cole SR, et al. When to Censor? Am J Epidemiol. 2018;187(3):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole SR and Hernan MA. Adjusted Survival Curves with Inverse Probability Weights. Comput Methods Programs Biomed. 2004;75(1):45–49. [DOI] [PubMed] [Google Scholar]
- 38.Howe CJ, Cole SR, Westreich DJ, et al. Splines for Trend Analysis and Continuous Confounder Control. Epidemiology. 2011;22(6):874–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Department of Veterans Affairs/Department of Defense (VADoD). Clinical Practice Guidelines for Management of Opioid Therapy for Chronic Pain, 2017. Availavle at Https://Www.Healthquality.Va.Gov/Guidelines/Pain/Cot/Vadodotcpg022717.Pdf. 2017. Accessed December 20, 2018.
- 40.Team RC. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2019. URL: https://wwwR-projectorg. 2019. [Google Scholar]
- 41.Boudreau D, Von Korff M, Rutter CM, et al. Trends in Long-Term Opioid Therapy for Chronic Non-Cancer Pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han B, Compton WM, Blanco C, et al. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Annals of Internal Medicine. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 43.Caraceni A, Hanks G, Kaasa S, et al. Use of Opioid Analgesics in the Treatment of Cancer Pain: Evidence-Based Recommendations from the Eapc. Lancet Oncol. 2012;13(2):e58–68. [DOI] [PubMed] [Google Scholar]
- 44.Scholl L, Seth P, Kariisa M, et al. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiese AD, Griffin MR, Schaffner W, et al. Opioid Analgesic Use and Risk for Invasive Pneumococcal Diseases: A Nested Case-Control Study. Ann Intern Med. 2018;168(6):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dublin S, Walker RL, Jackson ML, et al. Use of Opioids or Benzodiazepines and Risk of Pneumonia in Older Adults: A Population-Based Case-Control Study. J Am Geriatr Soc. 2011;59(10):1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaye JA, Margulis AV, Fortuny J, et al. Cancer Incidence after Initiation of Antimuscarinic Medications for Overactive Bladder in the United Kingdom: Evidence for Protopathic Bias. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2017;37(6):673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faillie J-L. Indication Bias or Protopathic Bias? British Journal of Clinical Pharmacology. 2015;80(4):779–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy S, Xu J, Kochanek K, et al. Mortality in the United States, 2017. NCHS Data Brief, No 328. Hyattsville, Md: National Center for Health Statistics. 2018. 2018. [PubMed] [Google Scholar]
- 50.Schuckit MA. Treatment of Opioid-Use Disorders. New England Journal of Medicine. 2016;375(4):357–368. [DOI] [PubMed] [Google Scholar]
- 51.Odegard E, Amundsen EJ and Kielland KB. Fatal Overdoses and Deaths by Other Causes in a Cohort of Norwegian Drug Abusers--a Competing Risk Approach. Drug Alcohol Depend. 2007;89(2-3): 176–182. [DOI] [PubMed] [Google Scholar]
- 52.Larney S, Bohnert ASB, Ganoczy D, et al. Mortality among Older Adults with Opioid Use Disorders in the Veteran’s Health Administration, 2000–2011(). Drug and alcohol dependence. 2015;147:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park TW, Lin LA, Hosanagar A, et al. Understanding Risk Factors for Opioid Overdose in Clinical Populations to Inform Treatment and Policy. J Addict Med. 2016;10(6):369–381. [DOI] [PubMed] [Google Scholar]
- 54.Chou R Long-Acting Opioids for Chronic Noncancer Pain Were Linked to Mortality. Ann Intern Med. 2016;165(6):JC34. [DOI] [PubMed] [Google Scholar]
- 55.Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort Study of the Impact of High-Dose Opioid Analgesics on Overdose Mortality. Pain Medicine. 2016;17(1):85–98. [DOI] [PubMed] [Google Scholar]
- 56.Jones CM and McAninch JK. Emergency Department Visits and Overdose Deaths from Combined Use of Opioids and Benzodiazepines. Am J Prev Med. 2015;49(4):493–501. [DOI] [PubMed] [Google Scholar]
- 57.Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, Opioids, and the Risk of Opioid-Related Death: A Population-Based Nested Case-Control Study. PLoS Med. 2017;14(10):e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster LR, Choi Y, Desai H, et al. Sleep-Disordered Breathing and Chronic Opioid Therapy. Pain Med. 2008;9(4):425–432. [DOI] [PubMed] [Google Scholar]
- 59.Rowe JW, Andres R, Tobin JD, et al. The Effect of Age on Creatinine Clearance in Men: A Cross-Sectional and Longitudinal Study. J Gerontol. 1976;31(2):155–163. [DOI] [PubMed] [Google Scholar]
- 60.Vozoris NT, Wang X, Fischer HD, et al. Incident Opioid Drug Use and Adverse Respiratory Outcomes among Older Adults with Copd. Eur Respir J. 2016;48(3):683–693. [DOI] [PubMed] [Google Scholar]
- 61.Vozoris NT, Wang X, Austin PC, et al. Adverse Cardiac Events Associated with Incident Opioid Drug Use among Older Adults with Copd. Eur J Clin Pharmacol. 2017;73(10):1287–1295. [DOI] [PubMed] [Google Scholar]
- 62.Xu J MS, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief, No 267. Hyattsville, Maryland, 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


