Abstract
Objective: This study aimed to compare the effectiveness of titanium mesh cages (TMCs) with autogenous iliac bone grafts (AIBG) in posterior-only surgery for thoracic and lumbar spinal tuberculosis.
Design: Retrospective investigative design.
Setting: The First Affiliated Hospital of Fujian Medical University, Fuzhou, China.
Participants: A total of 146 patients with thoracic or lumbar tuberculosis.
Interventions: All patients underwent a posterior-only approach with either a TMC (86 cases) or AIBG (60 cases).
Outcomes measures: Operation duration, intraoperative blood loss, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), visual analogue scale (VAS), and related complications were used to compare the effectiveness and feasibility of the two techniques. Frankel grading system, Cobb angle, and loss of angular correction were employed to assess neurological and kyphotic improvements.
Results: There were significant improvements in ESR, CRP, VAS, Frankel grade, and Cobb angle at the last follow-up (P < 0.05) when compared with the preoperative state. The TMC group was superior in operation duration (P < 0.001), intraoperative blood loss (P = 0.007), VAS (P < 0.001), loss of angular correction (P < 0.001), and surgical complications as compared with the AIBG group. There were no significant differences in the improvement of the Frankel grade and Cobb angle between the TMC and AIBG groups (P > 0.05). A recurrence of tuberculosis was not found in either of the groups.
Conclusion: Compared to autogenous iliac bone grafts, titanium mesh cages could serve as a superior material in posterior-only operative therapy for thoracic and lumbar spinal tuberculosis.
Keywords: Spinal tuberculosis, Titanium mesh cages, Autogenous iliac bone graft, Posterior-only approach
Introduction
The number of patients with tuberculosis (TB) is increasing, especially in developing countries. As a frequently seen form of extrapulmonary TB, spinal TB is the most severe form of skeletal TB.1 The thoracic and lumbar spine are the most commonly affected sites, with the anterior and middle columns often being involved, resulting in kyphosis and even paraplegia.2 Therapies for thoracic and lumbar spinal TB include conservative and surgical treatments. With the development of radiological technology, such as computed tomography (CT) and magnetic resonance image (MRI) scanning, anti-TB chemotherapy remains the mainstay treatment for early stage thoracic and lumbar spinal TB.3 Nevertheless, for patients who have developed a progressive neurological deficit, abscess, refractory infection, kyphosis, or segmental instability, surgery is still required.4 Various surgical treatments have been performed on patients with thoracic and lumbar spinal TB,5,6 and a posterior-only debridement followed by an interbody fusion and instrumentation has been proven to be an effective approach.7 Titanium mesh cages (TMCs) and autogenous iliac bone grafts (AIBG) have both been applied in posterior-only surgery to restore vertebral height. However, their clinical effectiveness has not, as yet, been well compared. Therefore, this study aims to review and compare the clinical efficacy of TMCs and AIBGs in posterior-only surgery for patients with TB of the thoracic and lumbar spine.
Materials and methods
Patients
A total of 146 patients with thoracic or lumbar TB who underwent a posterior-only approach in our hospital from September 2009 to August 2016 were retrospectively reviewed. A diagnosis of TB was confirmed by clinical manifestation, laboratory and radiological results, and pathological examination. All patients included in the study complained of typical symptoms of neck pain, low-grade fever, night sweats, or weight loss. Laboratory examination included the positive results of tuberculin test and T-SPOT test, along with increased levels of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Radiological imaging included the identification of spinal destruction or abscess through plain radiographs, computed tomography (CT), or magnetic resonance imaging (MRI). Pathological examination observed the sequestrum and granulation tissues. Back pain was rated using a visual analogue scale (VAS), and neurological status was evaluated according to the Frankel grading system. To restore vertebral height after debridement, 86 patients received instrumentation with a TMC and the other 60 patients received instrumentation with an AIBG. The Cobb angle was the angle between the first normal vertebrae above and below the lesion on lateral radiographs.8 Bony fusion was assessed according to Lee’s standard: Adjacent surface without gaps, obvious trabecular bone through the graft surface, and displacement of less than 3° on dynamic X-ray films.9 Indicators including operation duration, intraoperative blood loss, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), VAS, and related complications were used to assess the clinical effectiveness and feasibility of the two surgeries.
Preoperative procedure
At least two weeks prior to surgery, all of the patients received anti-TB chemotherapy with isoniazid (5–10 mg/kg•day, with maximal dose of 300 mg/day), rifampicin (5–10 mg/kg•day, with maximal dose of 450 mg/day), ethambutol (15 mg/kg•day, with maximal dose of 750 mg/day), and pyrazinamide (25 mg/kg•day, with maximal dose of 750 mg/day). Surgical treatment was performed upon relief of typical symptoms; when the ESR, CRP, and temperature had significantly decreased; and when hypoproteinemia had recovered.
Operative procedures
After general anesthesia the patients were positioned in a prone position, and an incision was made at the posterior midline. Both sides of the facet joints, spinous processes, lamina, and nerve roots of the diseased vertebrae were exposed, extending as far as one vertebra above and below. Before debridement and decompression, a temporary rod on the mild side of the abscess was employed to stabilize the spine and avoid any spinal cord injury. Next, any pus, the affected vertebral bodies, any necrotic disc tissue, and the sequestrum and granulation tissues were entirely cleared. After washing the surgical site with hydrogen peroxide and saline, an AIBG (Fig. 1) or TMC (Fig. 2) was used to recreate the intervertebral height. For the AIBG technique, an autogenous tricortical bone graft was taken from the anterior superior iliac crest. The internal fixation instrumentation was then compressed and stretched to correct the kyphosis. Lastly, streptomycin (1 g) and isoniazid (0.3 g) were infused onto the operation site. To achieve an improved and focused debridement, two drainage tubes were inserted into the abscess cavity before the incision was closed.
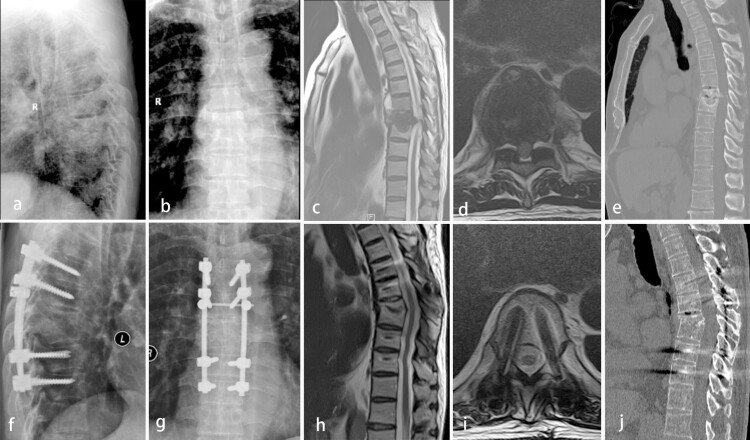
Figure 1.
A 73-year-old male with thoracic tuberculosis (TB) (T7–8) treated via a posterior-only debridement, autogenous iliac bone graft, internal fusion, and instrumentation. Preoperative radiography (a and b), MRI (c and d), and CT (e) showed the destruction of T7–8 anterior and middle columns and the development of an abscess with marked bony destruction in a lesion around the vertebral body of T7. Radiography (f and g) at the final follow-up showed the achievement of definitive bony fusion. Postoperative MRI (h and i) and CT (j) showed complete resolution of the epidural abscess and decompression of the neural component.
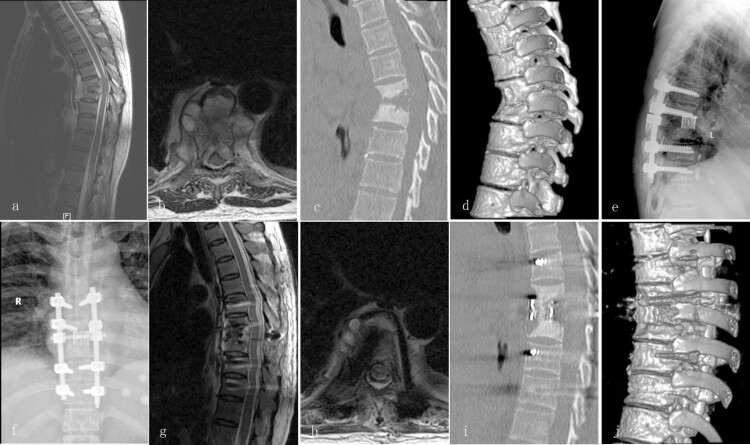
Figure 2.
A 48-year-old female with thoracic TB (T7–8) treated via a posterior-only debridement, titanium mesh cage, internal fusion, and instrumentation. Preoperative MRI (a and b) and CT (e and d) showed destruction of the T7–8 spinal column with kyphosis. Radiography (e and f) showed definitive bony fusion and maintenance of the correction at the final follow-up. Postoperative MRI (g and h) and CT (i and j) showed complete decompression of the neural component and good bony fusion.
Postoperative management
When the volume loss for the surgical drains was under 30 ml/24 h, the drainage tubes were removed. Postoperative patients received treatments of mannitol dehydration (375 ml/day) and conventional nerve nutrition (mecobalamin, 1.5 mg/day) to reduce any postoperative edema of the incision and nerves, and to accelerate neurological recovery. Patients were instructed to exercise their limb muscles in bed for two weeks after surgery, and wear an orthosis until bony fusion had been achieved. Anti-TB chemotherapy, along with the preoperative regimen, was administrated for six months after surgery, followed by another 9–12 months of isoniazid, rifampicin, and ethambutol. All patients underwent periodical radiological and laboratory examinations at one week, and three, six, and twelve months after surgery, and annually thereafter.
Statistical analysis
All continuous data were presented as mean ± standard deviation (SD). All data were statistically analyzed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA). Pre and postoperative comparisons of the continuous parameters were carried out by a paired-sample t-test. Comparisons of continuous parameters between the TMC and AIBG groups were carried out with an independent-sample t-test. A P value <0.05 was considered statistically significant.
Results
Baseline
There were 55 (64.0%) males and 31 (36.0%) females in the TMC group with an average age of 53.1 ± 18.8 years old, and 33 (55.0%) males and 27 (45.0%) females in the AIBG group with an average age of 48.0 ± 16.5 years old. The affected thoracic and lumbar vertebrae were 2.5 ± 1.5 and 2.1 ± 0.4 in the TMC group, respectively, and were 2.3 ± 0.7 and 2.1 ± 0.7 in the AIBG group, respectively. Patients in the TMC and AIBG groups had suffered with TB for an average duration of 11.3 ± 17.6 and 12.6 ± 17.9 months, respectively. Before surgery, patients received chemotherapy for an average duration of 19.8 ± 7.0 days in the TMC group and 21.3 ± 13.6 days in the AIBG group. There were no significant differences in age, sex, affected vertebrae number, disease duration, and chemotherapy duration between the TMC and AIBG groups (P > 0.05, Table 1). Preoperative VAS scores were 3.6 ± 1.2 in the TMC group and 3.8 ± 1.1 in the AIBG group. Preoperative ESR levels were 84.4 ± 24.3 mm/h in the TMC group and 91.5 ± 29.3 mm/h in the AIBG group. Preoperative CRP levels were 56.3 ± 14.4 mg/l in the TMC group and 51.7 ± 18.1 mg/l in the AIBG group (Table 2). In the TMC group, there was one patient in each of the Frankel grades A and B, five with grade C, 34 with grade D, and 45 patients with grade E. In the AIBG group, there was one patient with grade A, four with grade B, two with grade C, 22 with grade D, and 31 patients with grade E (Table 3). There were no significant differences in the preoperative VAS scores, ESR and CRP levels, and the Frankel grading between the TMC and AIBG groups (P > 0.05, Tables 2 and 3). The average follow-up time was 59.2 ± 17.4 months in the TMC group and 68.0 ± 23.4 months in the AIBG group, with no significant difference between the two groups (P > 0.05, Table 1).
Table 1. Baseline characteristics of the 146 spinal tuberculosis patients.
| Total | TMC group | AIBG group | P | |
|---|---|---|---|---|
| Number of patients | 146 | 86 | 60 | |
| Age (years) | 51.0 ± 18.0 | 53.1 ± 18.8 | 48.0 ± 16.5 | 0.92 |
| Sex | ||||
| Male | 88 | 55 | 33 | 0.20 |
| Female | 58 | 31 | 27 | |
| Number of vertebra affected | ||||
| Thoracic | 2.5 ± 1.3 | 2.5 ± 1.5 | 2.3 ± 0.7 | 0.56 |
| Lumbar | 2.1 ± 0.6 | 2.1 ± 0.4 | 2.1 ± 0.7 | 0.80 |
| Duration of disease (months) | 11.9 ± 17.7 | 11.3 ± 17.6 | 12.6 ± 17.9 | 0.67 |
| Chemotherapy duration (days) | 20.5 ± 10.2 | 19.8 ± 7.0 | 21.3 ± 13.6 | 0.39 |
| Follow-up (months) | 58.5 ± 16.0 | 59.2 ± 17.4 | 57.5 ± 13.8 | 0.53 |
Note: TMC, Titanium mesh cages; AIBG, Autogenous iliac bone graft.
Table 2. Comparison of clinical and radiologic parameters between the two groups.
| TMC group | AIBG group | P | |
|---|---|---|---|
| Operation duration (min) | 191.8 ± 68.0 | 273.2 ± 82.8 | <0.001 |
| Blood loss (ml) | 617.4 ± 499.6 | 946.0 ± 948.4 | 0.007 |
| VAS (score) | |||
| Preoperative | 3.6 ± 1.2 | 3.8 ± 1.1 | 0.28 |
| Final follow-up | 0.7 ± 0.9 | 1.4 ± 0.6 | <0.001 |
| ESR (mm/h) | |||
| Preoperative | 84.4 ± 24.3 | 91.5 ± 29.3 | 0.11 |
| Final follow-up | 15.5 ± 4.3* | 16.0 ± 5.6* | 0.57 |
| CRP (mg/l) | |||
| Preoperative | 56.3 ± 14.4 | 51.7 ± 18.1 | 0.09 |
| Final follow-up | 11.5 ± 7.1* | 10.8 ± 6.8* | 0.55 |
| Preoperative Cobb angle (°) | |||
| Thoracic | 18.4 ± 13.1 | 14.3 ± 8.3 | 0.14 |
| Lumbar | −6.2 ± 13.7 | −6.6 ± 9.0 | 0.87 |
| Postoperative Cobb angle (°) | |||
| Thoracic | 10.1 ± 7.1* | 10.6 ± 4.7* | 0.77 |
| Lumbar | −14.2 ± 12.7* | −13.7 ± 13.1* | 0.80 |
| Loss of angular correction (°) | |||
| Thoracic | 1.3 ± 0.2 | 2.4 ± 0.5 | <0.001 |
| Lumbar | 1.4 ± 0.3 | 2.2 ± 0.4 | <0.001 |
Note: TMC, titanium mesh cages; AIBG, autogenous iliac bone graft; VAS, visual analogue scale; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; *Significant difference compared with pre-operation.
Table 3. Neurologic status evaluated by Frankel grade.
| Frankel grade (%) | TMC group (n = 86) | AIBG group (n = 60) | ||
|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | |
| A | 1 (1.2) | 0 (0) | 1 (1.7) | 0 (0) |
| B | 1 (1.2) | 1 (1.2) | 4 (6.7) | 0 (0) |
| C | 5 (5.8) | 1 (1.2) | 2 (3.2) | 2 (3.3) |
| D | 34 (39.5) | 8 (9.3) | 22 (36.7) | 7 (11.7) |
| E | 45 (52.3) | 76 (88.3) | 31 (51.7) | 51 (85.0) |
Note: TMC, titanium mesh cages; AIBG, autogenous iliac bone graft.
Clinical efficacy comparison
The average operation duration in the TMC group was 191.8 ± 68.0 min, with a mean intraoperative blood loss of 617.4 ± 499.6 ml. Whereas, patients in the AIBG group underwent a significantly longer surgical duration (P < 0.001) and had a higher intraoperative blood loss (P = 0.007), with an average duration of 273.2 ± 82.8 min and blood loss of 946.0 ± 948.4 ml. Patients in the TMC group achieved a more significant level of pain relief (P < 0.001), with a postoperative VAS score of 0.7 ± 0.9 as compared with 1.4 ± 0.6 in the AIBG group ( Table 2). After surgery, ESR and CRP levels decreased to 15.5 ± 4.3 mm/h and 11.5 ± 7.1 mg/l, respectively, in the TMC group, and to 16.0 ± 5.6 mm/h and 10.8 ± 6.8 mg/l, respectively, in the AIBG group. Although there were significant differences between the pre and postoperative ESR and CPR levels (P < 0.05), there were no significant differences in the postoperative ESR and CPR levels between the TMC and AIBG groups (P > 0.05, Table 2). Postoperative Frankel grading in the TMC group achieved improvements for eight (9.3%) patients in grade D and 76 (88.3%) patients in grade E. Similarly, the AIBG group also achieved improvements for seven (11.7%) patients in grade D, and 51 (85.0%) patients in grade E. No significant difference was observed in postoperative neurological function between the two groups (P = 0.77, Table 3).
Radiological outcome comparison
There was no difference in the preoperative Cobb angles of the thoracic (P = 0.14) and lumbar (P = 0.87) vertebrae between the TMC and AIBG groups (Table 2). After surgery, the thoracic Cobb angle corrected to 10.1° ± 7.1° in the TMC group and 10.6° ± 4.7° in the AIBG group. The lumbar Cobb angle corrected to −14.2° ± 12.7° in the TMC group and −13.7° ± 13.1° in the AIBG group. Meaning that there were significant differences in thoracic and lumbar Cobb angles between the pre and postoperative states of the two groups (P < 0.05). However, no statistically significant difference was found in the postoperative thoracic (P = 0.77) and lumbar (P = 0.80) Cobb angles. A lower level in the loss of angular correction was observed in the TMC group as compared with the AIBG group for both the thoracic (P < 0.001) and lumbar (P < 0.001) vertebrae (Table 2).
Postoperative complications
Four cases in the TMC group and two cases in the AIBG group suffered from cerebrospinal fluid leakage, and two cases in the TMC group and three cases in the AIBG group suffered from an infection at the incision site. Twenty-three patients (38.3%) in the AIBG group suffered from donor site complications, including persistent pain in 19 patients, a large hematoma in three patients, and infection in one patient, which were cured by symptomatic treatment, antibiotics, and debridement. Twenty-one cases (24.4%) suffered from subsidence in the TMC group. No instrumentation related complications were found in any patient at the time of the last follow-up (Figs. 3 and 4). Recurrence of tuberculosis was not found in any of the patients.
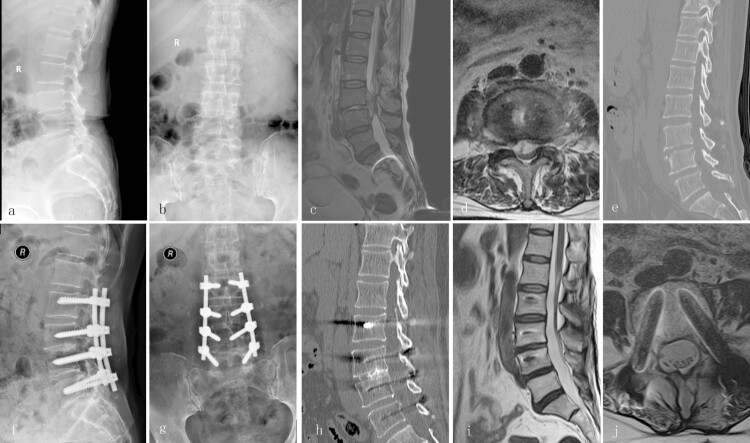
Figure 3.
A 63-year-old female with lumbar TB (L3–4) treated via a posterior-only debridement, autogenous iliac bone graft, internal fusion, and instrumentation. Preoperative radiography (a and b), MRI (c and d), and CT (e) showed the development of an abscess around the vertebral body of L3–4 with neurological deficit. Radiography (f and g) and CT (h) at the final follow-up showed the achievement of definitive bony fusion. Postoperative MRI (i and j) showed complete resolution of the epidural abscess and decompression of the neural component.
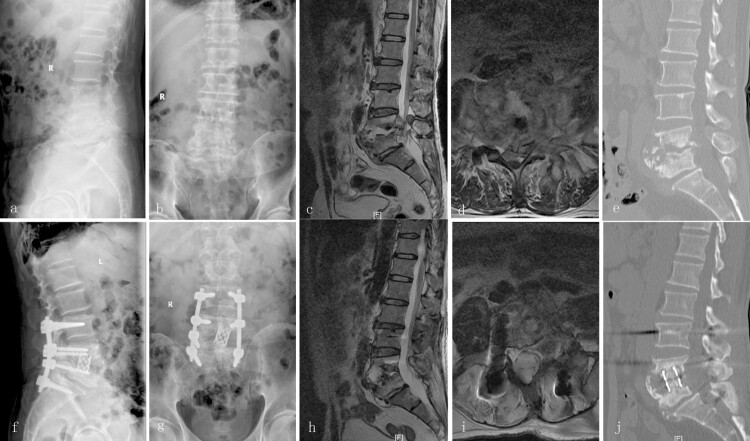
Figure 4.
A 56-year-old male with lumbar TB (L4–5) treated via a posterior-only debridement, titanium mesh cage, internal fusion, and instrumentation. Preoperative radiography (a and b), MRI (c and d), and CT (e) showed destruction of the L4–5 anterior and middle columns with neurological deficit. Radiography (f and g) at the final follow-up showed the achievement of definitive bony fusion. Postoperative MRI (h and i) and CT (j) showed complete resolution of the epidural abscess and decompression of the neural component.
Discussion
The spine is the most common extrapulmonary site of TB. Most patients with spinal TB can be treated with conservative chemotherapy. However, surgical treatments are required for those patients who develop kyphotic deformities or neurological deficits in order to debride the infection focus, recover nerve function, and reconstruct the spinal intervertebral height.10,11 Although different approaches have been reported to be employed in the treatment of spinal TB, a preferred approach has yet to be established.
During the late 19th and early 20th centuries, surgical options for the treatment of spinal tuberculosis were limited. Capener pioneered a procedure termed a “lateral rhachotomy”, providing the surgeon with a more ventral exposure from a more lateral trajectory.12 However, the approach was not popularized because of its limitation. In the 1960s, Hodgson et al. reported the anterior debridement and interbody fusion approach for the treatment of spinal tuberculosis.13 Since TB lesions frequently involve the anterior and middle columns of spine,14,15 an anterior-only approach could thoroughly remove the TB focus and achieve spinal cord decompression.16,17 However, the anterior-only approach suffered disadvantages in the form of ineffective correction of kyphosis and complications related to the thoracic and abdominal cavities.18 In 1976, Larson and his team developed the “lateral extracavitary approach (LECA)”, which allowed the surgeon access to both the posterior and lateral aspects of the spinal canal through the same incision in order to achieve posterior fusion and instrumentation without any complications related to the thoracic and abdominal cavities.19 However, disadvantages, including the degree of operational difficulty and procedure-related complications such as excessive blood loss, could not be ignored. With the development of pedicle screws, posterior-only and combined anterior-posterior approaches subsequently became more widespread.20,21
Recently, the surgical treatment of spinal TB tends to be undertaken with a smaller incision and static interbody fixation through only one approach.22,23 A single-stage approach also minimizes the risk of tuberculosis spread. Zhang et al.7 indicated that a posterior-only approach achieved a more satisfactory outcome when compared to a combined anterior-posterior approach, due to the advantages of minor surgical invasion and less procedure-related complications. Moreover, the posterior-only approach was demonstrated to be effective for those patients with obvious kyphosis and neurological deficits, owing to the persistent spinal cord compression.7 Kyphosis correction was obviously improved for all patients at the final follow-up in the present study, which confirmed that a posterior-only approach could serve as an effective therapy in the management of thoracic and lumbar spinal tuberculosis.
In 1982, Larson firstly used an AIBG to recreate intervertebral height, although this was for odontoid fractures.24 Subsequently, Maiman and others began using TMCs in the LECA for thoracic and lumbar spinal tuberculosis.25 Nowadays, TMCs and AIBGs are widely used for interbody grafts to reconstruct spinal intervertebral height after a posterior-only debridement. However, few studies have compared the efficacy of the two materials. In the present study, we quantitatively assessed the clinical and radiological parameters, as well as postoperative complications, to compare the clinical effectiveness of TMCs and AIBGs in posterior-only surgical therapy for thoracic and lumbar spinal tuberculosis.
AIBGs are regarded as gold standard treatment for bone defect repairs, with the advantages of good osteogenesis, bone induction, bone conductibility, and biocompatibility, which bring an optimal fusion rate.11 However, the limitations of AIBGs are also apparent. Kemp et al.26 reported postoperative correction loss and progressive kyphosis development as a consequence of the autografts failure to maintain sagittal plane alignment. In the present study, patients in the AIBG group demonstrated a more severe loss of angular correction. Moreover, the AIBGs demonstrated a risk of fixation failure and stress fractures.16 Furthermore, nonunion, graft collapse or dislodgement, and donor site complications, such as chronic pain and infection, can also not be ignored.27 Iliac crest donor site complications have been a serious postoperative concern for both patients and surgeons in AIBGs. In the AIBG group of the present study, 19 patients with persistent pain, three with a large hematoma, and one patient with an infection requiring drainage were observed. The donor site complication rate was 38.3% (23/60), which is consistent with one previous study.28
Many studies have reported that TMCs hold large advantages in reliable spinal reconstruction, stable bony fusion, and low implant-related problems.27 For example, TMCs are filled with excised vertebral lamina and articular processes, avoiding donor site related complications. Additionally, posterior instrumentation and TMCs enforce the stability of operative segments, correction and maintenance of the deformity, and bony fusion. Moreover, the Cobb angle loss in the TMC group was smaller than that in the AIBG group for the present study, which may be attributed to a reduction of bone resorption. However, TMCs continue to have some limitations. In the present study, the TMC group had a subsidence rate of 24.4%, which is consistent with one previous study.29 In our opinion, the subsidence behavior of TMCs may be attributed to factors including three-dimensional segmental stability, TMC design, contact area of the implant–bone interface, and the bone quality of the vertebral end plates. If the upper part of the vertebra is not severely destroyed by infection, transpedicular screws can be placed into the affected vertebra to decrease the settling. In the current study, solid bony fusion, improvement of neurological function, and good radiographic outcomes were achieved in the TMC group, although with an acceptable loss of kyphosis.
There are several limitations to our study. Firstly, it was a single-centered retrospective study rather than a prospective study, which could cause selection bias. Secondly, our study included a relatively limited number of patients, which may also have attenuated the outcome comparison.
Conclusion
Compared to autogenous iliac bone grafts, titanium mesh cages could serve as a superior material for the posterior debridement, internal fusion and instrumentation of thoracic and lumbar spinal tuberculosis.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest Authors have no conflict of interests.
References
- 1.Dunn R, Zondagh I, Candy S.. Spinal tuberculosis: magnetic resonance imaging and neurological impairment. Spine. 2011;36(6):469–73. doi: 10.1097/BRS.0b013e3181d265c0 [DOI] [PubMed] [Google Scholar]
- 2.Yin XH, Zhou ZH, Yu HG, Hu XK, Guo Q, Zhang HQ.. Comparison between the antero-posterior and posterior only approaches for treating thoracolumbar tuberculosis (T10-L2) with kyphosis in children: a minimum 3-year follow-up. Childs Nerv Syst. 2016;32(1):127–33. doi: 10.1007/s00381-015-2935-8 [DOI] [PubMed] [Google Scholar]
- 3.Lee TC, Lu K, Yang LC, Huang HY, Liang CL.. Transpedicular instrumentation as an adjunct in the treatment of thoracolumbar and lumbar spine tuberculosis with early stage bone destruction. J Neurosurg. 1999;91(2 Suppl):163–9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HQ, Li JS, Zhao SS, Shao YX, Liu SH, Gao Q, et al. . Surgical management for thoracic spinal tuberculosis in the elderly: posterior only versus combined posterior and anterior approaches. Arch Orthop Trauma Surg. 2012;132(12):1717–23. doi: 10.1007/s00402-012-1618-0 [DOI] [PubMed] [Google Scholar]
- 5.Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M.. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363–6. doi: 10.1007/s00264-006-0166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q, Xu J.. Comparison between the antero-posterior and anterior approaches for treating L5-S1 vertebral tuberculosis. Int Orthop. 2012;36(2):345–51. doi: 10.1007/s00264-011-1307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HQ, Lin MZ, Li JS, Tang MX, Guo CF, Wu JH, et al. . One-stage posterior debridement, transforaminal lumbar interbody fusion and instrumentation in treatment of lumbar spinal tuberculosis: a retrospective case series. Arch Orthop Trauma Surg. 2013;133(3):333–41. doi: 10.1007/s00402-012-1669-2 [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran S. The natural history of post-tubercular kyphosis in children. Radiological signs which predict late increase in deformity. J Bone Joint Surg Br. 2001;83-B(7):954–62. doi: 10.1302/0301-620X.83B7.0830954 [DOI] [PubMed] [Google Scholar]
- 9.Lee CK, Vessa P, Lee JK.. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20(3):356–61. doi: 10.1097/00007632-199502000-00018 [DOI] [PubMed] [Google Scholar]
- 10.Gong K, Wang Z, Luo Z.. Single-stage posterior debridement and transforaminal lumbar interbody fusion with autogenous bone grafting and posterior instrumentation in the surgical management of lumbar tuberculosis. Arch Orthop Trauma Surg. 2011;131(2):217–23. doi: 10.1007/s00402-010-1138-8 [DOI] [PubMed] [Google Scholar]
- 11.Sundararaj GD, Amritanand R, Venkatesh K, Arockiaraj J.. The use of titanium mesh cages in the reconstruction of anterior column defects in active spinal infections: can we rest the crest? Asian Spine J. 2011;5(3):155–61. doi: 10.4184/asj.2011.5.3.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capener N. The evolution of lateral rhachotomy. J Bone Joint Surg Br. 1954;36-b(2):173–9. doi: 10.1302/0301-620X.36B2.173 [DOI] [PubMed] [Google Scholar]
- 13.Hodgson AR, Stock FE, Fang HS, Ong GB.. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott's disease of the spine. Br J Surg. 1960;48:172–8. doi: 10.1002/bjs.18004820819 [DOI] [PubMed] [Google Scholar]
- 14.Talu U, Gogus A, Ozturk C, Hamzaoglu A, Domanic U.. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech. 2006;19(8):554–9. doi: 10.1097/01.bsd.0000211202.93125.c7 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Ji J, Liu B.. Management of spinal tuberculosis: a systematic review and meta-analysis. J Int Med Res. 2013;41(5):1395–407. doi: 10.1177/0300060513498023 [DOI] [PubMed] [Google Scholar]
- 16.Qureshi MA, Khalique AB, Afzal W, Pasha IF, Aebi M.. Surgical management of contiguous multilevel thoracolumbar tuberculous spondylitis. Eur Spine J. 2013;22(Suppl 4):618–23. doi: 10.1007/s00586-012-2459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan K, Elmorshidy E.. Anterior versus posterior approach in surgical treatment of tuberculous spondylodiscitis of thoracic and lumbar spine. Eur Spine J. 2016;25(4):1056–63. doi: 10.1007/s00586-016-4451-2 [DOI] [PubMed] [Google Scholar]
- 18.Zeng H, Zhang P, Shen X, Luo C, Xu Z, Zhang Y, et al. . One-stage posterior-only approach in surgical treatment of single-segment thoracic spinal tuberculosis with neurological deficits in adults: a retrospective study of 34 cases. BMC Musculoskelet Disord. 2015;16:186. doi: 10.1186/s12891-015-0640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson SJ, Holst RA, Hemmy DC, Sances A. Jr.. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45(6):628–37. doi: 10.3171/jns.1976.45.6.0628 [DOI] [PubMed] [Google Scholar]
- 20.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH.. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20(17):1910–6. doi: 10.1097/00007632-199509000-00013 [DOI] [PubMed] [Google Scholar]
- 21.Sundararaj GD, Behera S, Ravi V, Venkatesh K, Cherian VM, Lee V.. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85-B(1):100–6. doi: 10.1302/0301-620X.85B1.13300 [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Song Y, Liu L, Gong Q, Zhou C.. One-stage posterior surgical treatment for lumbosacral tuberculosis with major vertebral body loss and kyphosis. Orthopedics. 2013;36(8):e1082–90. doi: 10.3928/01477447-20130724-28 [DOI] [PubMed] [Google Scholar]
- 23.Pang X, Wu P, Shen X, Li D, Luo C, Wang X.. One-stage posterior transforaminal lumbar debridement, 360 degrees interbody fusion, and posterior instrumentation in treating lumbosacral spinal tuberculosis. Arch Orthop Trauma Surg. 2013;133(8):1033–9. doi: 10.1007/s00402-013-1751-4 [DOI] [PubMed] [Google Scholar]
- 24.Maiman DJ, Larson SJ.. Management of odontoid fractures. Neurosurgery. 1982;11(4):471–6. doi: 10.1227/00006123-198210000-00001 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MH, Larson SJ, Maiman DJ.. The lateral extracavitary approach to the thoracic and lumbar spine. Neurosurg Clin N Am. 2004;15(4):437–41. doi: 10.1016/j.nec.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 26.Kemp HB, Jackson JW, Jeremiah JD, Cook J.. Anterior fusion of the spine for infective lesions in adults. J Bone Joint Surg Br. 1973;55-B(4):715–34. doi: 10.1302/0301-620X.55B4.715 [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Lv G, Liu W, Cheng I.. Anterior radical debridement and reconstruction using titanium mesh cage for the surgical treatment of thoracic and thoracolumbar spinal tuberculosis: minimium five-year follow-up. Turk Neurosurg. 2011;21(4):575–81. [PubMed] [Google Scholar]
- 28.Cho DY, Lee WY, Sheu PC.. Treatment of multilevel cervical fusion with cages. Surg Neurol. 2004;62(5):378–85. discussion 385–6. doi: 10.1016/j.surneu.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 29.Moreland DB, Asch HL, Clabeaux DE, Castiglia GJ, Czajka GA, Lewis PJ, et al. . Anterior cervical discectomy and fusion with implantable titanium cage: initial impressions, patient outcomes and comparison to fusion with allograft. Spine J. 2004;4(2):184–91; discussion 191. doi: 10.1016/j.spinee.2003.05.001 [DOI] [PubMed] [Google Scholar]