Abstract
Objective: The correlation between the progression of spinal cord lesions using spinal cord evoked potentials (SCEPs) and neurological findings are unclear. The purpose is to electrophysiologically evaluate relative vulnerability of spinal cord in patients with compressive cervical myelopathy (CCM) at C4–C5 intervertebral level using SCEPs and correlate the progression of spinal cord lesions with neurological findings.
Design: Retrospective study.
Setting: Yamaguchi University Hospital.
Participants: 36 patients.
Methods: SCEPs following median nerve stimulation (MN-SCEPs), ulnar nerve stimulation (UN-SCEPs), transcranial electric stimulation (TCE-SCEPs), and spinal cord stimulation (SC-SCEPs) were intraoperatively recorded. MN-SCEPs are mediated by posterior horns (4, 5 layers), UN-SCEPs by the Burdach tract, TCE-SCEPs by the lateral corticospinal tract, and SC-SCEPs by the Goll tract. We evaluated the neurological findings (numbness, tactile sense and pain sense in the C6 area, tactile sense in the lower extremities, and triceps tendon reflex [TTR]).
Results: The incidence of electrophysiological and clinical abnormalities decreased in the order of UN-SCEPs (100%), TCE-SCEPs (94.4%), MN-SCEPs (77.8%), and SC-SCEPs (69.4%), and in the order of numbness (100%), pain sense (97.2%), TTR (91.7%), tactile sense in the C6 area (83.3%), and tactile sense in the lower extremities (70.0%), respectively.
Conclusions: The relative vulnerability of spinal cord occurred in the order of the Burdach tract, the lateral corticospinal tract, posterior horns (4, 5 layers), and the Goll tract in most patients with CCM at the C4–C5 intervertebral level.
Keywords: Neurological disorders, Disease, Spinal tracts, Electrophysiological findings, Clinical application
Introduction
Degenerative diseases of the spine make up 59% of nontraumatic spinal cord injury (ntSCI) in Japan, 54% in the United States, 31% in Europe, 22% in Australia, between 4% and 30% in Africa.1 The regional incidence of ntSCI in North America, Europe, and Australia was 76, 26, and 6 per million, respectively, and that the prevalence is 1120 per million in Canada and 2310 per million in Kashmir region.2 There are many patients with compressive cervical myelopathy (CCM) around the world. It is important to recognize the common patterns of neurological findings associated with CCM in order to avoid misdiagnosis. Amongst the various neurological tests, several have been chosen for diagnosis because they have proven useful for these patients. These include deep tendon reflexes, pinprick sensation, manual muscle testing, and patient-perceived area of numbness.3,4 Hyperreflexia of triceps tendon reflex (TTR), areas of numbness and sensory disturbance (tactile sense and pain sense) at the C6 spinal cord segment, and biceps brachii weakness are also useful factors for longitudinal level diagnosis of CCM at the C4–C5 intervertebral level.3–5 Several factors can influence the neurologic level diagnosis in CCM, including neuroanatomical variations between patients and differences in the extent and morphology of the affected spinal cord lesions resulting from compression.
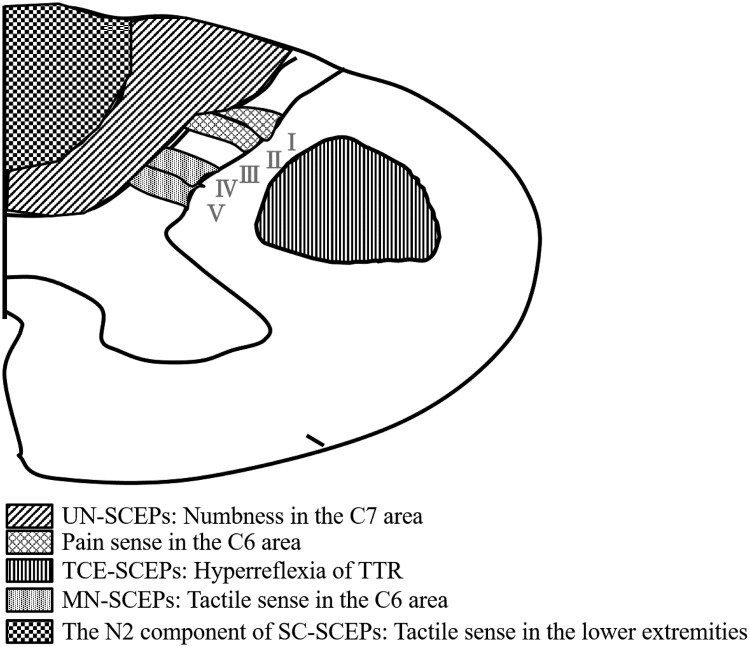
We previously electrophysiologically evaluated the functional integrity of three spinal tracts (lateral corticospinal tract and the lateral parts [Burdach tract] and medial parts [Goll tract] of the posterior column).6 We reported on the correlation between the progression of spinal tract lesions in cervical spondylotic myelopathy (CSM) at the C3–C4 intervertebral level, as estimated by multi-modal spinal cord evoked potentials (SCEPs) and neurological findings.6 Multi-modal SCEPs were SCEPs following median nerve stimulation (MN-SCEPs), transcranial electric stimulation (TCE-SCEPs), and spinal cord stimulation (SC-SCEPs). At the C3–C4 intervertebral level, we found that MN-SCEPs are mediated by Burdach tract, TCE-SCEPs by lateral corticospinal tract, and the N2 component of SC-SCEPs by Goll tract. According to these results, the involvement of long tracts in CSM start in the Burdach tract, followed by the lateral corticospinal tract, and eventually, the Goll tracts. Hattori et al. reported that the posterior horn was involved with mild stage CCM.7 However, it is unclear when the posterior horn is electrophysiologically involved or how the spinal cord lesions including the posterior horn progress from a mild to severe stage. The median and ulnar nerves are formed by the union of the C6–T1 nerve roots and C8/T1 nerve roots, respectively.8 Therefore, MN-SCEPs and UN-SCEPs at the C4–C5 intervertebral level are mediated by the posterior horn (laminae IV–V) and the ascending tract, respectively.9,10 Ueta et al. reported the origin of the UN-SCEPs was mainly the posterior funiculus (the Burdach tract).10 Table 1 shows the relationships between type of SCEPs, area of spinal cord, and neurological findings (Fig. 1). Here, we aimed to electrophysiologically evaluate the function of three spinal tracts and the posterior horn in patients with CCM at the C4–C5 intervertebral level in addition to SCEPs which stimulated the ulnar nerve. Moreover, we aimed to correlate the progression of spinal cord lesions with neurological findings.
Table 1. The relation among SCEPs, area of spinal cord, and neurological findings.
| Type of SCEPs | Area of spinal cord | Neurological findings |
|---|---|---|
| UN-SCEPs | Burdach tract | Numbness in the C7 area |
| TCE-SCEPs | Lateral corticospinal tract | Hyperreflexia of TTR |
| MN-SCEPs | Posterior horn (laminae IV, V) |
Tactile sense in the C6 area |
| The N2 component of SC-SCEPs | Goll tract | Tactile sense in the lower extremities |
| – | Posterior horn (laminae I, ∐) |
Pain sense in the C6 area |
SCEPs: spinal cord evoked potentials, UN: ulnar nerve stimulation, TCE: transcranial electric stimulation, MN: median nerve stimulation, SC: spinal cord stimulation, TTR: triceps tendon reflex.
Figure 1.
The relationship between abnormalities of SCEPs and neurological findings. SCEPs: spinal cord evoked potentials, TTR: triceps tendon reflex, UN: ulnar nerve stimulation, TCE: transcranial electric stimulation, MN: median nerve stimulation, SC: spinal cord stimulation.
Materials and methods
We conducted a retrospective study of 223 patients with CCM who underwent surgery as well as SCEP studies between October 2011 and August 2018. The Human Experimentation Ethics Committee of Yamaguchi University Graduate School of Medicine approved the preoperative magnetic resonance imaging (MRI) investigation, computed tomography after myelography (CTM), and electrophysiological studies, and written consent was obtained from all patients. Those who fulfilled the following criteria were enrolled: diagnosis of myelopathy based on hyperreflexia presence, including a positive Hoffmann’s sign, upper extremity sensory disturbance, and obvious MRI- or CTM-documented cervical spinal cord compression.
Of the 223 patients, 36 patients (23 men and 13 women) exhibited SCEPs abnormalities and spinal cord compression at the C4–C5 intervertebral level only. The mean age was 68.7 years (range 43–86 years); our clinical findings are summarized in Table 2. According to the dermatome proposed by Brain, we classified clinical abnormality as when the patients experienced numbness in the area of middle finger (the C7area) and sensory disturbance in the area from the radial side of the forearm to the index finger (the C6 area).11 Radiographic examinations included preoperative MRI and CTM. The spinal cord morphology as observed in the axial view at the C4–C5 intervertebral level using MRI and CTM was classified into four types: central, lateral, diffuse, and unclassified.12 The median and ulnar nerves were stimulated (square wave pulse, duration: 0.2 ms, rate: 3 Hz) at the wrist. The stimulus intensity was set at 1.5 times that required to produce a thumb twitch and little finger twitch in the awake condition, respectively.13 TCE was delivered as square wave pulses (duration: 0.2 ms, intensity: 100 mA) through the stimulator placed on the skull. The anode was placed 70 mm lateral to the right of the Cz position (10–20 International System) on a line joining the external auditory meatus. The cathode was placed on the opposite side. SC-SCEPs were delivered by an epidural catheter electrode inserted into the dorsal epidural space from the T11–T12 and T12–L1 interlaminar spaces. Square wave pulses (duration: 0.2 ms, rate: 3 Hz) were delivered at 3–10 mA intensity. All SCEPs were intraoperatively recorded with recording electrodes inserted in the ligamentum flavum in each interlaminar space before laminoplasty. A reference electrode was inserted into the subcutaneous tissue in the posterior aspect of the neck to record MN-SCEPs, UN-SCEPs, and SC-SCEPs. A bipolar recording method was used (active proximal and reference distal) to record TCE-SCEPs.
Table 2. Clinical findings.
| Case | Disease | Age/Sex | Sensory disturbance | TTR | |||
|---|---|---|---|---|---|---|---|
| UE (C7area) UE (C6 area) UE (C6 area) | LE | ||||||
| Numbness | Pain sense | Tactile sense | Tactile sense | ||||
| 1 | CSA | 43/F | + | N | N | N | ↑ |
| 2 | OPLL | 43/M | + | + | N | N | N |
| 3 | CSA | 51/M | + | + | N | N | N |
| 4 | CSM | 51/M | + | + | N | +* | ↑ |
| 5 | CSM | 84/M | + | + | + | +* | ↑ |
| 6 | CSM | 76/M | + | + | N | +* | ↑ |
| 7 | CSM | 68/F | + | + | + | N | ↑ |
| 8 | OPLL | 76/M | + | + | + | N | ↑ |
| 9 | CSM | 61/F | + | + | + | N | ↑ |
| 10 | CSM | 70/M | + | + | + | N | ↑ |
| 11 | CLF | 80/F | + | + | + | N | ↑ |
| 12 | CDH | 63/M | + | + | N | + | ↑ |
| 13 | CDH | 63/F | + | + | + | + | ↑ |
| 14 | CSM | 74/M | + | + | + | + | ↑ |
| 15 | CSA | 62/M | + | + | + | N | ↑ |
| 16 | CSM | 68/F | + | + | + | + | ↑ |
| 17 | OPLL | 79/M | + | + | + | + | ↑ |
| 18 | CSM | 74/F | + | + | + | + | ↑ |
| 19 | CSA | 74/M | + | + | + | + | ↑ |
| 20 | CSM | 72/M | + | + | + | + | ↑ |
| 21 | CSM | 75/M | + | + | + | + | ↑ |
| 22 | OPLL | 67/M | + | + | + | + | ↑ |
| 23 | CDH | 67/M | + | + | + | + | ↑ |
| 24 | OPLL | 54/M | + | + | + | + | ↑ |
| 25 | OPLL | 83/M | + | + | + | + | ↑ |
| 26 | CSM | 86/M | + | + | + | + | ↑ |
| 27 | OPLL | 49/F | + | + | + | +* | ↑ |
| 28 | CSM | 74/M | + | + | + | +* | ↑ |
| 29 | CSM | 73/M | + | + | + | + | ↑ |
| 30 | CSM | 85/M | + | + | + | +* | ↑ |
| 31 | OPLL | 81/M | + | + | + | + | N |
| 32 | OPLL | 59/F | + | + | + | + | ↑ |
| 33 | CSM | 67/F | + | + | + | + | ↑ |
| 34 | CSM | 71/F | + | + | + | + | ↑ |
| 35 | CSM | 71/F | + | + | + | + | ↑ |
| 36 | CDH | 80/F | + | + | + | + | ↑ |
Area of numbness and sensory disturbance: according to the dermatome proposed by Brain. CSA: cervical spondylotic amyotrophy, CSM: cervical spondylotic myelopathy, OPLL: ossification of posterior longitudinal ligament, CDH: cervical disc herniation, CLF: calcification of ligamentum flavum, M: male, F: female, UE: upper extremities, LE: lower extremities, TTR: triceps tendon reflex, ↑: hyper-reflex, N: normal.
*Six patients (case 4, 5, 6, 27, 28, and 30) with ossification of the posterior longitudinal ligament (OPLL) in the thoracic spine and/or postoperative history in the lumbar spine.
All SCEPs signals were amplified and filtered with a bandpass of 20–3000 Hz using a standard evoked potential/electromyography instrument (Nihon Kohden, Tokyo, Japan). The means from 100 to 200 MN-SCEPs, UN-SCEPs, and TCE-SCEPs, and 50–100 SC-SCEPs responses were obtained.
MN-SCEPs abnormalities were determined from the amplitude ratio of the spinal responses at each intervertebral level to that recorded at the C6–C7 intervertebral level as reported previously.13 The lower limit of the amplitude ratio was 0.4 for C4–C5 intervertebral level.13 For ascending UN-SCEPs and for the N2 component of SC-SCEPs, C4–C5 intervertebral levels with a marked reduction in the size of the negative peak (reduction of > 50%) compared to the C5–C6 intervertebral level were considered as significant.14,15 For descending TCE-SCEPs, C4–C5 intervertebral levels with marked reduction in the size of the negative peak (reduction of > 50%) compared to the C3–C4 intervertebral level were considered as significant.14,15
The SCEPs findings were compared with clinical signs and symptoms (Table 1). Statistical analysis was performed using the Mann–Whitney U test. P < 0.05 was considered statistically significant.
Results
Spinal cord morphology
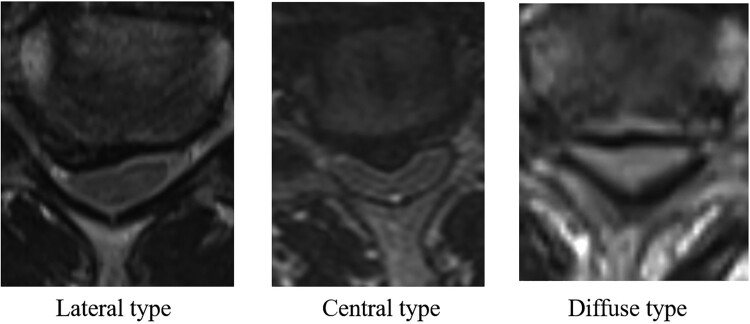
Patients spinal cord morphology was classified into four types according to the shape of the anterior compression: lateral (n = 2), central (n = 6), diffuse (n = 26), or unclassified (n = 2). The lateral type refers to unilateral compression of the cord, the central type to central compression of the cord, and the diffuse type to compression of almost the entire width of the cord (Fig. 2).
Figure 2.
The shapes for anterior compression of the spinal cord were lateral type, central type and diffuse type. When the morphology of spinal cord was central type, it was boomerang shape. When the morphology of spinal cord was diffuse type, it was triangular shape.
SCEP findings
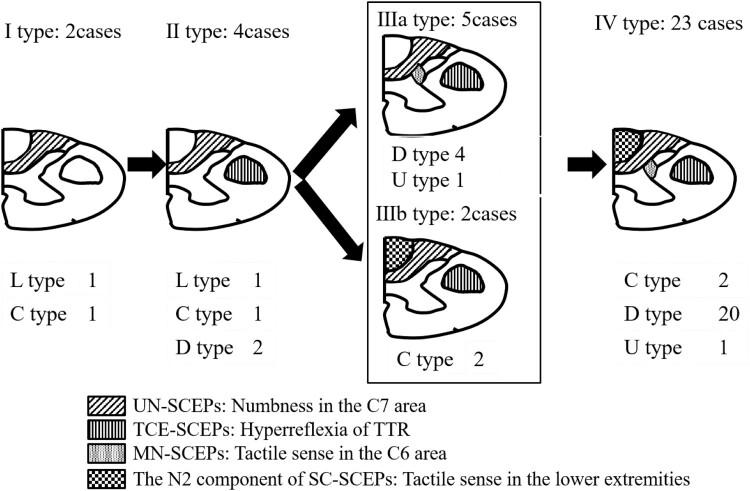
As shown in Table 3, the abnormality incidence decreased in the order of UN-SCEPs (100%), TCE-SCEPs (94.4%), MN-SCEPs (77.8%), and SC-SCEPs (69.4%). Patients were classified into five types according to their SCEP results (types I, II, IIIa, IIIb, and IV). Type I (cases 1 and 2; two patients, 5.6%) refers to abnormalities in UN-SCEPs, type II (cases 3–6; four patients, 11.1%) in UN-SCEPs and in TCE-SCEPs, type IIIa (cases 7–11; five patients, 13.9%) in UN-SCEPs, TCE-SCEPs, and MN-SCEPs, type IIIb (cases 12 and 13; two patients, 5.6%) in UN-SCEPs, TCE-SCEPs, and the N2 component of SC-SCEPs, and finally type IV (cases 14–36; 23 patients, 63.8%) refers to abnormalities in all SCEPs (Fig. 3).
Table 3. SCEPs and incidence of abnormalities.
| Types of SCEPs | Incidence |
|---|---|
| UN-SCEPs | 100% |
| TCE-SCEPs | 94.4% |
| MN-SCEPs | 77.8% |
| SC-SCEPs | 69.4% |
SCEPs: spinal cord evoked potentials, UN: ulnar nerve stimulation, TCE: transcranial electric stimulation, MN: median nerve stimulation, SC: spinal cord stimulation.
Figure 3.
The relationship between classification based on abnormalities of SCEPs and the morphology of spinal cord. SCEPs: spinal cord evoked potentials, L type: lateral type, C type: central type, D type: diffuse type, U type: unclassified type, TTR: triceps tendon reflex.
When the spinal cord morphology was the central type, the incidence of abnormalities decreased in the following order: types I, II, IIIb, and IV. When it was the diffuse type, the abnormality incidence decreased in the following order: types II, IIIa, and IV. Lastly, when the spinal cord morphology was the lateral type, the abnormality incidence decreased in the order of type I and II.
Neurological findings
Table 4 shows the abnormality incidence regarding the neurological findings.
Table 4. The neurological findings and incidence of abnormalities.
| Neurological findings | Incidence |
|---|---|
| Numbness in the C7 area | 100% |
| Pain sense in the C6 area | 97.2% |
| Hyperreflexia of TTR | 91.7% |
| Tactile sense in the C6 area | 83.3% |
| Tactile sense in the lower extremities | 70.0% |
TTR: triceps tendon reflex.
The abnormality incidence decreased in the following order: numbness (100%: 36/36), pain sense in the C6 area (97.2%: 35/36), TTR (91.7%: 33/36), tactile sense in the C6 area (83.3%: 30/36), and tactile sense in the lower extremities (70.0%: 21/30). When we evaluated tactile sense in the lower extremities, we excluded six patients (three type II and type IV patients, respectively) with ossification of the posterior longitudinal ligament (OPLL) in the thoracic spine and/or postoperative history in the lumbar spine.
Table 5 shows the relationship between the type of SCEP and the neurological findings. The mean preoperative Japanese Orthopedic Association (JOA) score was 9.5 points. The score worsened in ascending order of the SCEP type (type I: 13.3, type II: 12.5, type IIIa: 10.7, type IIIb: 9.3, type IV: 8.6). The preoperative JOA scores revealed significant differences between types I and IV, types II and IV, and types IIIa and IV (P < 0.05). According to the neurological findings, the abnormality incidence decreased in following order: the Burdach tract, posterior horn associated with pain sense in the C6 area, corticospinal tract, posterior horn associated with tactile sense in the C6 area, and the Goll tract.
Table 5. The relationship between type of SCEPs and neurological findings.
| Area of lesion | Type of SCEPs | I | ∐ | IIIa | IIIb | IV |
|---|---|---|---|---|---|---|
| Preoperative JOA score | 13.3 | 12.5 | 10.7 | 9.3 | 8.6 | |
| Burdach tract | The incidence of numbness in the C7 area | 100% (2/2) |
100% (4/4) |
100% (5/5) |
100% (2/2) |
100% (23/23) |
| Lateral corticospinal tract | Hyperreflexia of TTR | 50% (1/2) |
75% (3/4) |
100% (5/5) |
100% (2/2) |
95.7% (22/23) |
| Posterior horn | Hypesthesia of pain sense in the C6 area | 50% (1/2) |
100% (4/4) |
100% (5/5) |
100% (2/2) |
100% (23/23) |
| Hypesthesia of tactile sense in the C6 area | 0% (0/2) |
25% (1/4) |
100% (5/5) |
50% (1/2) |
100% (23/23) |
|
| Goll tract | Hypesthesia of tactile sense in the lower extremities | 0% (0/2) |
0% (0/1) |
0% (0/5) |
100% (2/2) |
95.0% (19/20) |
SCEPs: spinal cord evoked potentials, JOA: Japanese orthopaedic association, TTR: triceps tendon reflex.
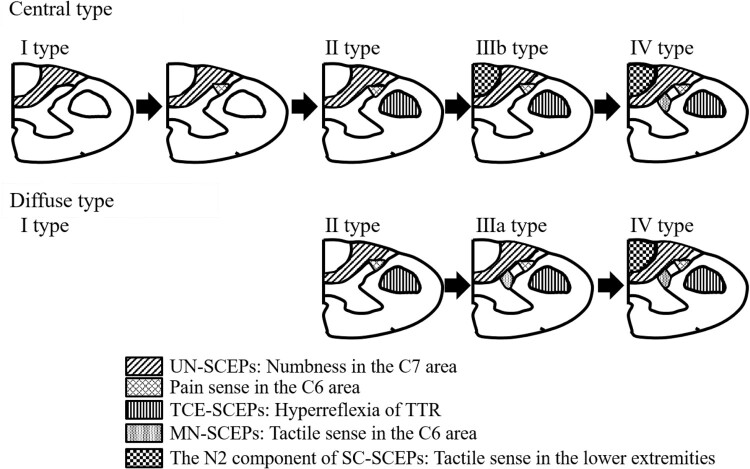
When spinal cord morphology was the central type, the abnormality incidence decreased in the following order: numbness in the C7 area, pain sense in the C6 area, hyperreflexia of TTR, hypesthesia of tactile sense in the lower extremities, and hypesthesia of tactile sense in the C6 area (Fig. 4). Conversely, when it was the diffuse type, the abnormality incidence decreased in the following order: numbness in the C7 area, pain sense in the C6 area, hyperreflexia of TTR, hypesthesia of tactile sense in the C6 area, and hypesthesia of tactile sense in the lower extremities (Fig. 4).
Figure 4.
The relationship between spinal cord morphology and abnormality incidence for neurological findings.
Discussion
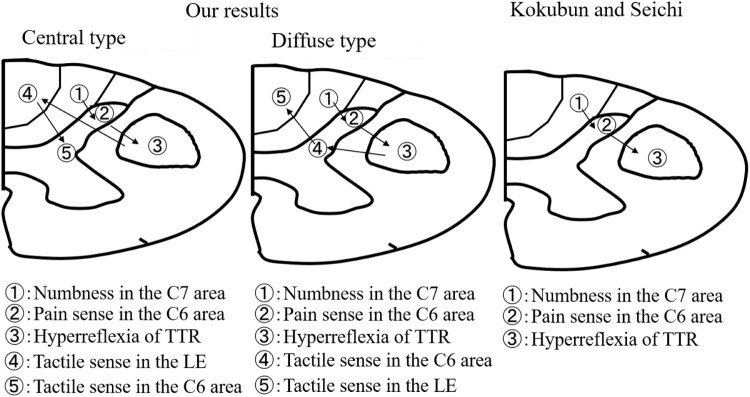
In the present study the relative vulnerability in C4–C5 compressive cervical myelopathy was assessed using multi-modal spinal cord evoked potentials and neurological findings. Differences between the central and diffuse types in terms of SCEPs results and neurological findings were found. Matsumoto et al. reported on the value of neurological examination of the affected level in patients with CCM.4 The correlation between overall neurological and radiological diagnoses was highest at the C3–C4 level (75%) and lowest at the C4–C5 level (56%).4 Patients with CCM at the C4–C5 level are difficult to diagnose using only neurological findings and the reported incidence of abnormality for each neurological parameter varies between studies (Table 6).3,16 The clinical symptoms of CCM differed according to the extent and area of spinal cord compression. We also found differences between the central and diffuse types in terms of SCEP results and neurological findings. The frequency of abnormality for the neurological parameters decreased in the order of numbness, pain sense in the C6 area, and deep tendon reflex.3 Our results were consistent with those of Kokubun, despite the study of different central and diffuse types (Fig. 5).3 In addition to the neurological observations of Kokubun, we also evaluated tactile sense in the C6 area and in the lower extremities. Considering that the intensity of stimulation for median nerves, ulnar nerves, and spinal cord, our data suggest that MN-SCEPs are related with the tactile sense in the C6 area, UN-SCEPs with the area of numbness in the C7 area, the N2 component of SC-SCEPs with the tactile sense in the lower extremities. We could not evaluate SCEPs associated with pain sense. Aδ and C fibers are believed to respond to pain sensation. The diameters of Aδ and C fibers are 1–6 μm and 0.2–1.5 μm, respectively.17 The conduction velocity of Aδ and C fibers is 4–36 m/s and 0.4–−2.0 m/s, respectively.17 Therefore, we did not use a sufficient intensity of stimulation to activate Aδ and C fibers. Aδ and C interneurons are located in the most superficial laminae: laminae I and II.18 Patients experienced pain disturbance before tactile disturbance in this study. According to finite element method analysis, the stress distribution is higher in laminae I and II than in laminae IV–V,19 which is consistent with our clinical findings.
Table 6. Neurological findings at the C4–C5 intervertebral level.
| Neurological findings | Our data | Seichi et al.16 | Kokubun et al.3 |
|---|---|---|---|
| Numbness in the C7 area | 100% | – | 100% |
| Sensory disturbance | 97.2% | 59% | |
| (Pain sense in the C6 area) | (97.2%) | 74% | |
| (Tactile sense in the C6 area) | (83.3%) | ||
| (Tactile sense in the lower extremities) | (70.0%) | ||
| Deep tendon reflex | Hyperreflexia of TTR 91.7% | Hyperreflexia of TTR 73% | Hyporeflexia of BTR 64% |
–: not described, TTR: triceps tendon reflex, BTR: biceps tendon reflex.
Figure 5.
Comparison between results from this study and other studies with regard to the progression of spinal cord lesions and correlation with neurological findings. TTR: triceps tendon reflex, LE: lower extremities.
Relationship between clinical findings and spinal cord morphology
Kameyama et al. reported the relationship between the morphology and pathology in nine patients with OPLL in the cervical spine.20 When there was mild compression upon spinal cords with a boomerang shape (central type), only flattening of the anterior horn and loss of the anterior horn cells were observed, without white matter lesions in three patients. Even in severe compression cases, major pathological changes were limited to the gray matter together with the ventrolateral part of the posterior column (Burdach tract) in two patients. There was no descending degeneration in the lateral corticospinal tract or the Goll tract; there were no clinical findings in one patient with mild compression and one with severe compression. Two of three patients with clinical findings showed sensory loss in the lower extremity; one patient showed spastic tetraparesis and urinary disturbance. Urinary disturbance is caused by the involvement of the anterior funiculus (reticulospinal tract and spinoreticular tract).21,22 Generally, the anterior funiculus is finally disturbed in patients with CSM.23 Therefore, we considered that this patient with urinary disturbance already had sensory disturbance in the lower extremities. The Goll tract was involved in all these patients, although there was only mild compression on the spinal cord. Therefore, the clinical findings were not consistent with the pathological findings. Cord damage is considered to be mediated by ischemia as a result of the mechanical disturbance of the microcirculation.20 It is difficult to assess how spinal tract lesions involving the posterior horn progress from mild to severe using pathological findings. Conversely, in four patients with severe compression on the spinal cord with a triangular shape (diffuse type), both gray and white matters showed severe damage, including necrosis. Only the anterior columns were free of pathological changes. Ascending degeneration of the posterior column, including the Goll tract, as well as descending degeneration of the lateral corticospinal tract were present above and below the compression level.20 According to the pathological findings, the involvement of spinal cords with a triangular shape (diffuse type) was more severe than boomerang shape (central type).
Study limitations
This study has certain limitations. A major limitation was the small number of subjects. Further, the median and ulnar nerves were stimulated at 1.5 times the intensity of the motor threshold. As mentioned, this intensity was insufficient to active the Aδ and C fibers and thus SCEPs could not be recorded for pain sensation.
Conclusions
When the spinal cord morphology was the central type, the incidence of electrophysiological and clinical abnormalities decreased in the order of types I, II, IIIb, and IV, and in the order of numbness in the C7 area, pain sense in the C6 area, hyperreflexia of TTR, hypesthesia of tactile sense in the lower extremities, and hypesthesia of tactile sense in the C6 area, respectively. When the morphology was the diffuse type, the incidence of electrophysiological and clinical abnormalities decreased in the order of types II, IIIa, and IV, and in the order of numbness in the C7 area, pain sense in the C6 area, hyperreflexia of TTR, hypesthesia of tactile sense in the C6 area and hypesthesia of tactile sense in the lower extremities, respectively. Differences between the central and diffuse types in terms of SCEPs results and neurological findings were found. These new information may be very useful for clinicians in diagnosing CCM at the C4–C5 intervertebral level.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of Interest None.
References
- 1.New PW, Cripps RA, Bonne Lee B.. Global maps of non-traumatic spinal cord injury epidemiology: towards a living data repository. Spinal Cord. 2014;52:97–109. doi: 10.1038/sc.2012.165 [DOI] [PubMed] [Google Scholar]
- 2.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG.. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40:E675–93. doi: 10.1097/BRS.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 3.Kokubun S. Neurological localization of the symptomatic level of lesion on cervical spondylotic myelopathy. Rinsho Seikei Geka. 1984;19:417–24. [Google Scholar]
- 4.Matsumoto M, Ishikawa M, Ishii K, Nishizawa T, Maruiwa H, Nakamura M, Chiba K, Toyama Y.. Usefulness of neurological examination for diagnosis of the affected level in patients with cervical compressive myelopathy: prospective comparative study with radiological evaluation. J Neurosurg Spine. 2005;2:535–9. doi: 10.3171/spi.2005.2.5.0535 [DOI] [PubMed] [Google Scholar]
- 5.Hirabayashi K, Satomi K, Wakano K.. Level diagnosis neurology of cervical spondylotic myelopathy-retrospective observation in cases treated by snterior spinal fusion at a single level. Rinsho Seikei Geka. 1984;19:409–15. [Google Scholar]
- 6.Imajo Y, Kato Y, Yonemura H, Kanchiku T, Suzuki H, Taguchi T.. Relative vulnerability of various spinal tracts in C3-4 cervical spondylotic myelopathy: multi-modal spinal cord evoked potentials. Spinal Cord. 2011;49:1128–33. doi: 10.1038/sc.2011.68 [DOI] [PubMed] [Google Scholar]
- 7.Hattori S, Kawai S.. Clinical diagnosis of cervical spondylosis. Mook Orthopaedics. 1979;6:13–40. [Google Scholar]
- 8.Chiba T, Monoeda F, Hogashihara M, Kamiya H, Oishi C, Hatanaka Y, et al. C8 and T1 innervation of forearm muscles. Clin Neurophysiol. 2015;126:837–42. doi: 10.1016/j.clinph.2014.07.031 [DOI] [PubMed] [Google Scholar]
- 9.Jeanmonod D, Shindou M, Mauguiere F.. The human cervical and lumbo-sacral evoked electrospinogram. Data from intra-operative spinal cord surface recordings. Electroencephalogr Clin Neurophysiol. 1991;80:477–89. doi: 10.1016/0168-5597(91)90129-L [DOI] [PubMed] [Google Scholar]
- 10.Ueta E, Tani T, Taniguchi S, Ishida K, Ushida T, Yamamoto H.. Diagnostic value of cervical somatosensory evoked potentials recorded from the intervertebral discs after median and ulnar nerve stimulation in cervical spondylotic myelopathy. J Spinal Disord. 1998;11(6):514–20. doi: 10.1097/00002517-199812000-00011 [DOI] [PubMed] [Google Scholar]
- 11.Brain L, Walton J.. Brain’s disease of the neurous system. 7th ed. London: Oxford University Press; 1969. p. 40–43. [Google Scholar]
- 12.Nishida N, Kato Y, Imajo Y, Kawano S, Taguchi T.. Biomechanical analysis of cervical spondylotic myelopathy: the influence of dynamic factors and morphometry of the spinal cord. J Spinal Cord Med. 2012;35(4):256–61. doi: 10.1179/2045772312Y.0000000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko K, Kawai S, Taguchi T, Fuchigami Y, Iti T, Morita H.. Correlation between spinal cord compression and abnormal patterns of median nerve somatosensory evoked potentials in compressive cervical myelopathy: comparison of surface and epidurally recorded responses. J Neurol Sci. 1998;158:193–202. doi: 10.1016/S0022-510X(98)00119-1 [DOI] [PubMed] [Google Scholar]
- 14.Kanchiku T, Taguchi T, Kaneko K, Fuchigami Y, Yonemura H, Kawai S.. A correlation between magnetic resonance imaging and electrophysiological findings in cervical spondylotic myelopathy. Spine. 2001;26:E294–9. doi: 10.1097/00007632-200107010-00014 [DOI] [PubMed] [Google Scholar]
- 15.Tani T, Ishida K, Ushida T, Yamamoto H.. Intraoperative electroneurography in the assessment of the level of operation for cervical spondylotic myelopathy in the elderly. J Bone Joint Surg Br. 2000;82:269–74. doi: 10.1302/0301-620X.82B2.10257 [DOI] [PubMed] [Google Scholar]
- 16.Seichi A, Takeshita K, Kawaguchi H, Matsudaira K, Higashikawa A, Ogata N, et al. Neurologic level diagnosis of cervical stenotic myelopathy. Spine. 2006;31:1338–43. doi: 10.1097/01.brs.0000219475.21126.6b [DOI] [PubMed] [Google Scholar]
- 17.Gilman S. Joint position sense and vibration sense: anatomical organization and assessment. J Neurol Neurosurg Psychiatry. 2002;73:473–7. doi: 10.1136/jnnp.73.5.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Mello R, Dickenson AH.. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088 [DOI] [PubMed] [Google Scholar]
- 19.Kato Y, Kanchiku T, Imajo Y, Kimura K, Ichihara K, Kawano S, et al. Biomechanical study of the effect of degree of static compression of the spinal cord in ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2010;12:301–5. doi: 10.3171/2009.9.SPINE09314 [DOI] [PubMed] [Google Scholar]
- 20.Kameyama T, Hashizume Y, Ando T, Takahashi A, Yanagi T, Mizuno J.. Spinal cord morphology and pathology in ossification of the posterior longitudinal ligament. Brain. 1995;118:263–78. doi: 10.1093/brain/118.1.263 [DOI] [PubMed] [Google Scholar]
- 21.Ando M. Neurogenic bladder in patients with cervical cord compression disorders. Nihon Hinyokika Gakkai Zasshi 1990;81:243–50. [DOI] [PubMed] [Google Scholar]
- 22.Fowler CJ, Griffiths DJ.. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740 [DOI] [PubMed] [Google Scholar]
- 23.Ito T, Oyanagi K, Takahashi H, Takahashi HE, Ikuta F.. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine. 1996;21:827–33. doi: 10.1097/00007632-199604010-00010 [DOI] [PubMed] [Google Scholar]