Abstract
Objective: The majority of individuals with spinal cord injury (SCI) experience chronic pain. Chronic pain can be difficult to manage because of variability in the underlying pain mechanisms. More insight regarding the relationship between pain and physical activity (PA) is necessary to understand pain responses during PA. The objective of this study is to explore possible relationships between PA levels and secondary conditions including pain and fatigue.
Design: Prospective cohort analysis of a pilot study.
Setting: Community.
Participants: Twenty individuals with SCI took part in the study, and sixteen completed the study.
Interventions: Mobile-health (mHealth) based PA intervention for two-months during the three-month study.
Outcome measures: Chronic Pain Grade Scale (CPGS) questionnaire, The Wheelchair User’s Shoulder Pain Index (WUSPI), Fatigue Severity Scale (FSS), and PA levels measured by the mHealth system.
Results: A positive linear relationship was found between light-intensity PA and task-specific pain. However, the relationship between moderate-intensity PA and pain interference was best represented by a curvilinear relationship (polynomial regression of second order). Light-intensity PA showed positive, linear correlation with fatigue at baseline. Moderate-intensity PA was not associated with fatigue during any phase of the study.
Conclusion: Our results indicated that PA was associated with chronic pain, and the relationship differed based on intensity and amount of PA performed. Further research is necessary to refine PA recommendations for individuals with SCI who experience chronic pain.
Trial registration: ClinicalTrials.gov identifier: NCT03773692.
Keywords: Physical activity, Chronic pain, Fatigue, Spinal cord injury
Introduction
Chronic pain affects up to 96% of individuals with spinal cord injury (SCI) and can be difficult to manage due to the variability in types of pain.1 Chronic pain is typically considered to be pain that persists for 12 or more weeks.2 Chronic pain after an injury arises from improper healing that results in increased nociceptor sensitivity and subsequent alteration of pain perception in the absence of sensory input.3,4 Individuals with chronic pain experience behavioral changes as well as changes in perception of the magnitude and impact of pain.5 Pain tends to interfere with lower intensities of physical activity (PA) and activities of daily living (ADLs) in individuals with SCI more than in other populations.6 Increased use of the upper extremities for ADLs, transfers, and wheelchair propulsion following SCI heightens the physical demand on an untrained musculoskeletal system, which can lead to pain. While pain prevalence, pain intensity, and pain interference are not associated with level of SCI, upper extremity pain is experienced most frequently by individuals with higher-level injuries because of the combination of increased physical demand and strength deficits associated with SCI.7 Therefore, the manifestation of chronic pain may be related to levels of PA for individuals with SCI.
Prior research has concluded that exercise is an effective intervention for chronic pain in other populations,8–10 and some research has shown that exercise decreases chronic pain in adults with SCI.11–15 Other studies, however, have found no significant relationship between PA and pain in individuals with SCI.16–18 The amount of PA performed by participants may be a crucial factor in pain response. Some individuals with SCI experienced immediate modification in pain levels after one to two hours of exercise,14 while others reported changes after six months of a daily exercise program.15 The duration of vigorous-intensity PA performed was inversely related to levels of pain; however, light-intensity PA showed no correlation to pain levels,19 suggesting that intensity of PA may be an important consideration as well.20 The relationship between PA and pain may not be unidirectional. Psychosocial aspects, such as negative mood and lack of acceptance of health condition, are associated with higher pain levels and decreased overall leisure time PA levels.21
Fatigue has been identified as another secondary condition associated with SCI.22–24 While previous research has shown a correlation between depression and fatigue,25,26 the relationship between PA and fatigue has not been thoroughly investigated. Tawashy et al.20 found that fatigue levels were inversely proportional to vigorous-intensity PA, and depressive symptoms were inversely proportional to light-intensity PA. These findings suggest that different intensities of PA demonstrate different relationships with fatigue in individuals with SCI. Overall, much remains unknown regarding the association between PA and fatigue in individuals with SCI.
Our primary objective for this study was to establish a relationship between PA levels and chronic pain intensity. By evaluating pain interference separately from task-specific upper extremity pain, this study aimed to determine whether PA impacts generalized disruptive pain differently than upper extremity pain during daily activities in individuals with SCI. We hypothesized that PA levels negatively correlate with chronic pain intensity measured throughout the study using the Chronic Pain Grade Scale (CPGS)27–30 and the Wheelchair User’s Shoulder Pain Index (WUSPI).31–33 A secondary objective was to examine the relationship between PA levels and fatigue. Although fatigue has been less studied than pain in SCI research, Tawashy et al.20 suggested a relationship with PA levels may exist. Knowledge of the relationships between PA levels and secondary conditions, including chronic pain and fatigue, may motivate larger efforts to develop targeted PA recommendations for individuals with SCI. Improved PA recommendations have the potential to advance evidence-based practice and assist with the management of chronic pain and fatigue associated with SCI.
Methods
Participants
Twenty individuals with SCI participated in the study, and sixteen participants completed the study. The research team worked with a physical therapist and a physical medicine and rehabilitation physician from two large, urban rehabilitation hospitals to recruit participants for the study. Individuals included in the study met the following criteria: (1) were 18–65 years old, (2) had a diagnosis of traumatic or non-traumatic SCI, (3) were at least six months post-injury, (4) used a manual wheelchair as their primary means of mobility, (5) self-propelled their wheelchair, (6) were medically stable, and (7) had experience using a smartphone. Individuals were excluded if they: (1) had active pelvic or thigh wounds (pressure injuries), (2) had a history of cardiovascular disease, or (3) were pregnant (based on self-report). This study was approved by our university’s Institutional Review Board (IRB) and the recruitment site rehabilitation hospitals. All participants provided written informed consent prior to participating in this study.
Instrumentation
This study was part of a greater research effort that aimed to deploy a prototype mobile health (mHealth) PA motivation system intended to improve PA levels using a behavior-sensitive, just-in-time-adaptive intervention (JITAI).34 The mHealth-based JITAI system used off-the-shelf hardware components, including an Android-based smartphone (Nexus 5 or 5X, LG Corp., Englewood Cliffs, NJ, USA), a wrist-worn smartwatch (LG Urbane, LG Corp., Englewood Cliffs, NJ, USA), and a Bluetooth-based wheel rotation monitor (PanoBike, Topeak Inc., Taichung, Taiwan) to detect and classify wheelchair-based PA. The mHealth system differentiates between light-intensity and moderate- to vigorous-intensity PA while collecting data. Activity that is less than or equal to 3 METs is classified as light-intensity PA, and activity that is greater than 3 METs is classified as moderate-intensity PA. The wheel rotation monitor and smartwatch streamed data to the smartphone. Self-report assessments of pain and fatigue were collected using ecological momentary assessments (EMAs) on the smartphone along with weekly questionnaires.
Protocol
The study was conducted in three phases, each with a one-month duration. The first phase focused on collecting baseline PA level data (baseline). The second phase provided near-real-time feedback on PA level (PA feedback). The third phase provided PA feedback with a JITAI to the participants. During each phase, PA levels of the participants were collected through the mHealth-based JITAI system. Participants wore the smartwatch for 12 hours per day, and data from the devices automatically synchronized each minute. Software processed the incoming motion data from the devices to infer PA patterns of individuals with SCI as they moved about their homes and the community. In the baseline phase, after completing initial surveys of demographics, wheelchair information, and SCI and health history, participants completed the CPGS,27–30 WUSPI,31–33 and Fatigue Severity Scale (FSS) questionnaires.35
For the seven-item CPGS, characteristic pain intensity was calculated by averaging ratings (on a 0–10 scale) of current pain, worst pain in the past six months, and average pain in the past six months; the average was then multiplied by 10.27 Pain-related disability scores were derived by averaging ratings (on a 0–10 scale) of interference with daily, social, and work activities; then multiplying the average by 10, and finally sorting the multiplied averages into a six-point categorical scale.27 Our analyses did not include the pain disability measure, which did not change by more than one point for any participant throughout the study, and the median pain disability across all participants was zero for each phase of the study.
The WUSPI has been validated for wheelchair users. For the WUSPI, pain was calculated by a 15-item self-report survey that specifically measures upper extremity pain during daily functional activities with a 10-point visual analog scale. The WUSPI score range is 0–150 with an adjusted performance score calculated based on the total number of items completed. The minimal clinical difference and minimal detectable change is 5.1 points.31,32 The nine-item FSS measured the severity of fatigue on a seven-point ordinal scale ranging from one (strongly disagree) to seven (strongly agree); an average of the items higher than four indicates significant fatigue.35 After the initial completion of each questionnaire, participants responded to the CPGS, WUSPI, and FSS every week during the first, second, and third phases of the study.
Statistical analysis
A Kolmogorov–Smirnov normality test determined that the data were not normally distributed; therefore, nonparametric analyses were implemented. Spearman’s rank-order correlation analysis was performed to analyze the relationships between PA levels (light-intensity and moderate-intensity) and secondary conditions (pain: CPGS and WUSPI, and fatigue: FSS) for each of the three phases of the study. In addition, regression analyses were performed to examine the trends between PA levels and pain, as well as between PA levels and fatigue. We conducted linear regression analysis as stated by our hypothesis, as well as curvilinear regression analysis for all data. Based off the coefficient of determination (r2) values, we selected a second-order polynomial regression model to describe the association between moderate-intensity PA and pain. All data analyses were performed using MATLAB (MathWorks Inc, ver. R2017b, Natick, MA) and IBM SPSS Statistics software (ver. 25.0, Armonk, NY), with a statistical significance at an alpha level of 0.05.
Results
Table 1 presents the demographics, pain and fatigue scores, and minutes of PA performed per day for participants who completed the study. Table 2 shows the correlation between PA and secondary conditions including pain and fatigue. Pain interference, measured by the CPGS, was weakly associated with light-intensity PA during the PA feedback phase. Task-specific pain, measured by the WUSPI, had moderate association with light-intensity PA during baseline phase, and strong association with light-intensity PA during PA feedback phase. Fatigue was strongly correlated with light-intensity PA during the baseline phase. No significant correlations were found between pain interference, task-specific pain, or fatigue and moderate-intensity PA.
Table 1. Participant demographics, pain and fatigue scores, and minutes of PA performed per day presented as median (interquartile range) or frequency (%).
| Participant demographics | |||
|---|---|---|---|
| Age | 39.0 (32.5–54.3) years | ||
| Time since injury | 11.0 (6.8–17.3) years | ||
| Sex | 12 (75%) male; 4 (25%) female | ||
| Level of injury | 4 (25%) C5–C8; 8 (50%) T1–T6; 4 (25%) T7–T12 | ||
| Severity of injury |
10 (62.5%) complete; 6 (37.5%) incomplete |
||
| Pain, Fatigue, and PA | |||
| Phase |
Baseline |
PA feedback |
PA feedback with JITAI |
| Pain interference (CPGS) | 55.1 (28.1–68.1) | 49.2 (23.3–71.1) | 51.6 (28.3–66.2) |
| Task-specific pain (WUSPI) | 21.7 (16.4–28.5) | 26.5 (18.0–43.3) | 26.48 (18.0–38.7) |
| Fatigue (FSS) | 26.2 (21.8–38.7) | 23.1 (18.3–31.5) | 25.72 (17.8–32.2) |
| Light-intensity PA | 1083.2 (1002.0–1170.5) | 1178.0 (1044.6–1225.8) | 1143.1 (1121.0–1186.3) |
| Moderate-intensity PA | 57.8 (41.95–9.6) | 61.5 (42.4–75.6) | 54.0 (42.8–75.1) |
Table 2. Correlation between PA and secondary conditions (pain interference: Chronic Pain Grade Scale (CPGS), task-specific pain: Wheelchair User’s Shoulder Pain Index (WUSPI), and fatigue: Fatigue Severity Scale (FSS)) throughout each phase of the study.
| Light-intensity PA (n = 16) | Moderate-intensity PA (n = 16) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Baseline | PA feedback | PA feedback with JITAI | Baseline | PA feedback | PA feedback with JITAI | ||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| CPGS | 0.38 | 0.14 | 0.14* | 0.02 | 0.92 | 0.33 | 0.21 | 0.45 | 0.12 | 0.68 | −0.04 | 0.87 |
| WUSPI | 0.59* | 0.02 | 0.26 | 0.35 | 0.74* | <0.01 | 0.04 | 0.88 | 0.13 | 0.66 | 0.13 | 0.65 |
| FSS | 0.88* | <0.01 | 0.32 | 0.25 | 0.31 | 0.26 | 0.07 | 0.80 | 0.02 | 0.93 | −0.32 | 0.24 |
Note: Significant findings are indicated by an asterisk (*).
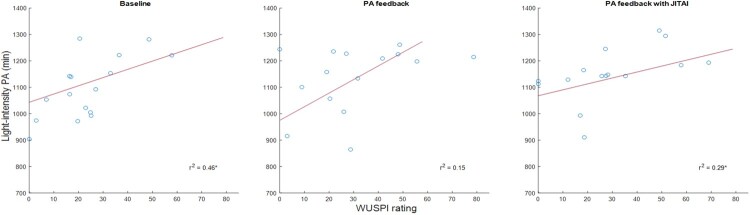
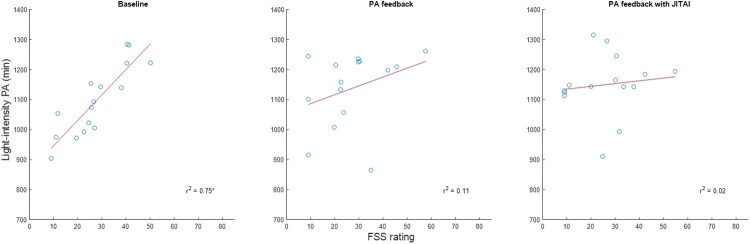
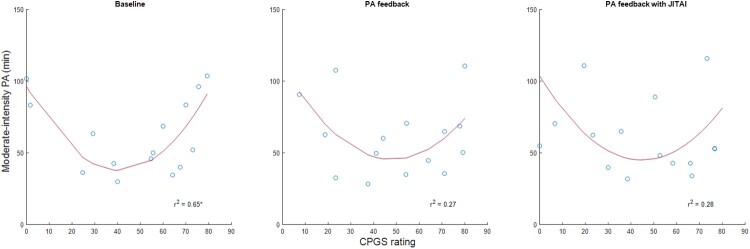
The variance in PA explained by pain and fatigue are displayed in Table 3. While pain interference significantly predicted moderate-intensity PA during the baseline phase, task-specific pain significantly predicted light-intensity PA during the baseline phase and PA feedback with JITAI phase. Fatigue significantly predicted light-intensity PA during the baseline phase. Regression analysis indicated that a significant linear relationship existed between light-intensity PA and task-specific pain (Fig. 1), and light-intensity PA and fatigue (Fig. 2). The relationship between moderate-intensity PA and pain interference was represented by a second-order polynomial (Fig. 3). During baseline, pain interference was significantly associated with moderate-intensity PA levels. The relationship between moderate-intensity PA and pain forms a U-shaped curve, where the highest durations of moderate-intensity PA are observed with both minimal and maximal pain levels. The lowest levels of moderate-intensity PA occurred with a moderate level of pain interference.
Table 3. Coefficient of determination (r2) and adjusted (adj.) r2 values for models of PA and pain, and PA and fatigue.
| Phase | Light-intensity PA (n = 16) | Moderate-intensity PA (n = 16) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | PA feedback | PA feedback with JITAI | Baseline | PA feedback | PA feedback with JITAI | |||||||||||||
| r2 | adj.r2 | P | r2 | adj.r2 | P | r2 | adj.r2 | P | r2 | adj.r2 | P | r2 | adj.r2 | P | r2 | adj.r2 | P | |
| CPGS | 0.25 | 0.19 | 0.05 | 0.02 | −0.06 | 0.61 | 0.02 | −0.05 | 0.58 | 0.65* | 0.59* | <0.01 | 0.27 | 0.15 | 0.15 | 0.28 | 0.16 | 0.14 |
| WUSPI | 0.46* | 0.42* | <0.01 | 0.15 | 0.08 | 0.16 | 0.29* | 0.23* | 0.04 | 0.33 | 0.21 | 0.09 | 0.03 | −0.13 | 0.83 | 0.04 | −0.12 | 0.77 |
| FSS | 0.75* | 0.73* | <0.01 | 0.11 | 0.04 | 0.23 | 0.02 | −0.06 | 0.67 | 0.03 | −0.08 | 0.99 | 0.07 | −0.01 | 0.35 | 0.15 | 0.08 | 0.16 |
Notes: Significant findings are indicated by an asterisk (*). Associations between light-intensity PA and pain interference and task-specific pain (Chronic Pain Grade Scale (CPGS) and Wheelchair User’s Shoulder Pain Index (WUSPI) scores), light-intensity PA and fatigue (Fatigue Severity Scale (FSS) score) are represented by linear regression equations. Associations between moderate-intensity PA and pain interference and task-specific pain (CPGS and WUSPI) are represented by second-order polynomial regression equations. Association between moderate-intensity PA and fatigue (FSS score) is represented by a linear regression equation.
Figure 1.
Association between task-specific pain (Wheelchair User’s Shoulder Pain Index (WUSPI)) and light-intensity PA across the study.
Figure 2.
Association between fatigue (Fatigue Severity Scale (FSS)) and light-intensity PA across the study.
Figure 3.
Association between pain interference (Chronic Pain Grade Scale (CPGS)) and moderate-intensity PA across the study.
Additional analysis (Table 4) indicated that pain interference and task-specific pain were moderately correlated with each other during the baseline phase, and strongly correlated during both the PA feedback and PA feedback with JITAI phases. Pain interference and fatigue were moderately correlated during all three of the baseline, PA feedback, and PA feedback with JITAI phases. Task-specific pain and fatigue were strongly correlated during the baseline phase, moderately correlated during PA feedback phase, and strongly correlated during the PA feedback with JITAI phase.
Table 4. Correlation between secondary conditions (pain interference: Chronic Pain Grade Scale (CPGS), task-specific pain: Wheelchair User’s Shoulder Pain Index (WUSPI), and fatigue: Fatigue Severity Scale (FSS)) throughout each phase of the study.
| Baseline | PA feedback | PA feedback with JITAI | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| CPGS and WUSPI | 0.56* | 0.02 | 0.72* | <0.01 | 0.74* | <0.01 |
| CPGS and FSS | 0.67* | <0.01 | 0.54* | 0.03 | 0.56* | 0.02 |
| WUSPI and FSS | 0.74* | <0.01 | 0.59* | 0.02 | 0.73* | <0.01 |
Note: Significant findings are indicated by an asterisk (*).
Discussion
To our knowledge, this is the first study to analyze the relationships between PA and the secondary conditions chronic pain and fatigue using PA patterns of individuals with SCI measured in the community. We used multiple measures to differentiate between the effects of PA levels on chronic pain and fatigue in individuals with SCI.
PA levels and pain
Pain interference was significantly correlated with light-intensity PA during the PA feedback phase, and task-specific pain was significantly correlated with light-intensity PA during the baseline phase and PA feedback with JITAI phase. Unlike light-intensity PA, chronic pain was not correlated with moderate-intensity PA. This may potentially indicate that engaging in moderate-intensity PA facilitates development of adequate activity tolerance. Both pain interference and task-specific pain demonstrated a curvilinear relationship to moderate-intensity PA. Therefore, higher amounts of moderate-intensity PA were associated with minimal and maximal pain levels. This suggests that pain levels may either be optimized or worsened with higher duration of PA. This novel finding reveals the complexity of the relationship between pain and PA, which is critical to the development of targeted PA interventions. In a qualitative study, patients with SCI conveyed that there is an ideal balance between PA and rest in order to control pain.36 A similar trend has been observed in individuals with low back pain: inactivity and maximal amounts of activity corresponded to more low back pain, whereas moderate amounts of activity corresponded to the least low back pain.37
Perception of pain
Perception of pain is subjective and variable. For example, acute pain thresholds in response to a heat stimulus were elevated in individuals with complete SCI who suffer from chronic pain,38 yet individuals with complete SCI were more likely to experience chronic pain than individuals with incomplete SCI according to a survey.39 Task-specific pain was correlated with pain interference throughout our study, indicating that experiencing task-specific pain overlaps with experiencing pain interference for individuals with chronic pain. Though similar, each type of pain may impact overall pain levels differently across individuals.
PA and fatigue
Light-intensity PA was positively correlated with fatigue, and moderate-intensity PA levels were not associated with fatigue. These results are inconsistent with previous findings by Tawashy et al. that higher-intensity activity was associated with lower levels of fatigue.20 There may be variations in fatigue levels that occurred with PA that were not fully captured by our results due to a relatively small size of change and the limited sample of our participants. Additionally, our study accumulated moderate-intensity PA that was three minutes or longer in duration, which may have distributed fatigue levels throughout the day. Another study found that fatigue levels significantly decreased throughout the course of SCI rehabilitation, however this change was attributed primarily to demographic and psychological factors, and therefore it was suggested that psychological intervention may impact fatigue levels.24 In contrast, other research has found fatigue levels remained constant from rehabilitation admission to discharge.23
Implications for future research
The results of this study should be interpreted with caution, but since exercise has been a promising prospect for pain management in other studies, future randomized control trials could investigate if there is a therapeutic amount of PA ideal for pain management, and delineate type of pain (i.e. neuropathic vs. musculoskeletal) and level of injury (i.e. paraplegia vs. tetraplegia) in order to develop targeted PA interventions. Additional studies could also explore the relationship between fatigue and PA, as it remains unclear. This insight would assist in the advancement of chronic pain and fatigue management interventions for individuals with SCI, which ultimately might improve their quality of life.
Limitations
The primary limitation of this study was the sample size, which is not representative of all individuals with SCI who use a manual wheelchair and does not address individuals with SCI who walk or use other assistive technologies, such as a power wheelchair or cane, for mobility. Because of the limited sample size, we were also unable to delineate between individuals with tetraplegia and individuals with paraplegia. Individuals with tetraplegia are more susceptible to shoulder dysfunction, which can contribute to pain. Although previous studies have found no association between fatigue and level of injury,23,40 we did not account for medications that participants may have been taking, which could have potentially contributed to fatigue levels. Another limitation of this study is the constraints of the assessment tools we used. The CPGS and WUSPI may each encompass an overlapping combination of both pain interference and task-specific pain in some individuals, and they are unable to delineate between types of pain (i.e. neuropathic and musculoskeletal). Furthermore, the CPGS does not indicate location of pain on the body, so location of pain causing pain interference is not specified. Lastly, items on the CPGS regarding pain in the past six months may not have fully captured changes from the PA intervention, as the reported pain could have potentially occurred outside of the enrollment time. Nonetheless, the CPGS data we collected exhibited changes in pain levels that we were able to analyze.
Conclusions
The results of this pilot study indicated that PA is related to chronic pain, and the relationship differs based on the amount and intensity of PA. Appropriate duration and intensity of PA may modulate pain, whereas improper amounts of PA may worsen pain. Therefore, understanding the relationship between pain and PA may guide PA recommendations for management of chronic pain. Further research is necessary in order to refine PA recommendations for chronic pain management in individuals with SCI.
Acknowledgement
We thank the individuals with SCI who took part in our study.
Disclaimer statements
Contributors None.
Funding This work was supported by the Craig H. Neilsen Foundation [Project# 382252]. The mobile phone sensing system was made possible, in part, through funding from the National Institutes of Health [grant number R21 HL108018-01].
Conflict of interest statement The authors declare no conflicts of interest of any material presented within this manuscript.
References
- 1.Dijkers M, Bryce T, Zanca J.. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev 2009;46(1):13–29. PubMed PMID: 19533517. doi: 10.1682/JRRD.2008.04.0053 [DOI] [PubMed] [Google Scholar]
- 2.Leadley RM, Armstrong N, Reid KJ, Allen A, Misso KV, Kleijnen J.. Healthy aging in relation to chronic pain and quality of life in Europe. Pain Pract 2014;14(6):547–58. doi: 10.1111/papr.12125. PubMed PMID: 24138082. doi: 10.1111/papr.12125 [DOI] [PubMed] [Google Scholar]
- 3.May A. Chronic pain may change the structure of the brain. Pain 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. PubMed PMID: 18410991. doi: 10.1016/j.pain.2008.02.034 [DOI] [PubMed] [Google Scholar]
- 4.Helme RD, Gibson S, Khalil Z.. Neural pathways in chronic pain. Med J Aust 1990;153(7):400–6. PubMed PMID: 1977074. doi: 10.5694/j.1326-5377.1990.tb125499.x [DOI] [PubMed] [Google Scholar]
- 5.Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB.. Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch Phys Med Rehabil 2017;98(6):1132–8. doi: 10.1016/j.apmr.2016.09.124. PubMed PMID: 27780743. doi: 10.1016/j.apmr.2016.09.124 [DOI] [PubMed] [Google Scholar]
- 6.Hanley MA, Jensen MP, Ehde DM, Robinson LR, Cardenas DD, Turner JA, et al. Clinically significant change in pain intensity ratings in persons with spinal cord injury or amputation. Clin J Pain 2006;22(1):25–31. Epub 2005/12/13. PubMed PMID: 16340590. doi: 10.1097/01.ajp.0000148628.69627.82 [DOI] [PubMed] [Google Scholar]
- 7.Ullrich PM, Jensen MP, Loeser JD, Cardenas DD.. Pain intensity, pain interference and characteristics of spinal cord injury. Spinal Cord 2008;46(6):451–5. Epub 02/19. doi: 10.1038/sc.2008.5. PubMed PMID: 18283293. doi: 10.1038/sc.2008.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH.. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev 2017;4:CD011279. doi: 10.1002/14651858.CD011279.pub3. PubMed PMID: 28436583; PubMed Central PMCID: PMC5461882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrose KR, Golightly YM.. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol 2015;29(1):120–30. doi: 10.1016/j.berh.2015.04.022. PubMed PMID: 26267006; PubMed Central PMCID: PMC4534717. doi: 10.1016/j.berh.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daenen L, Varkey E, Kellmann M, Nijs J.. Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin J Pain 2015;31(2):108–14. doi: 10.1097/AJP.0000000000000099. PubMed PMID: 24662498. doi: 10.1097/AJP.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 11.Todd KR, Martin Ginis KA.. An examination of diurnal variations in neuropathic pain and affect, on exercise and non-exercise days, in adults with spinal cord injury. Spinal Cord Ser Cases 2018;4:94. doi: 10.1038/s41394-018-0130-3. PubMed PMID: 30393565; PubMed Central PMCID: PMC6204132 of interest. doi: 10.1038/s41394-018-0130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheuren PS, Gagne M, Jutzeler CR, Rosner J, Mercier C, Kramer JLK.. Tracking changes in neuropathic pain after acute spinal cord injury. Front Neurol 2019;10:90. doi: 10.3389/fneur.2019.00090. PubMed PMID: 30837931; PubMed Central PMCID: PMC6382744. doi: 10.3389/fneur.2019.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 2003;41(1):34–43. doi: 10.1038/sj.sc.3101389. PubMed PMID: 12494319. doi: 10.1038/sj.sc.3101389 [DOI] [PubMed] [Google Scholar]
- 14.Mulroy SJ, Thompson L, Kemp B, Hatchett PP, Newsam CJ, Lupold DG, et al. Strengthening and optimal movements for painful shoulders (STOMPS) in chronic spinal cord injury: a randomized controlled trial. Phys Ther 2011;91(3):305–24. doi: 10.2522/ptj.20100182. PubMed PMID: 21292803. doi: 10.2522/ptj.20100182 [DOI] [PubMed] [Google Scholar]
- 15.Curtis KA, Tyner TM, Zachary L, Lentell G, Brink D, Didyk T, et al. Effect of a standard exercise protocol on shoulder pain in long-term wheelchair users. Spinal Cord 1999;37(6):421–9. PubMed PMID: 10432262. doi: 10.1038/sj.sc.3100860 [DOI] [PubMed] [Google Scholar]
- 16.Gant KL, Nagle KG, Cowan RE, Field-Fote EC, Nash MS, Kressler J, et al. Body system effects of a multi-modal training program targeting chronic, motor complete thoracic spinal cord injury. J Neurotrauma 2018;35(3):411–23. doi: 10.1089/neu.2017.5105. PubMed PMID: 28795657. doi: 10.1089/neu.2017.5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan M, Richardson EJ.. Effects of virtual walking treatment on spinal cord injury-related neuropathic pain: pilot results and trends related to location of pain and at-level neuronal hypersensitivity. Am J Phys Med Rehabil 2016;95(5):390–6. doi: 10.1097/PHM.0000000000000417. PubMed PMID: 26544859. doi: 10.1097/PHM.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 18.Ditor DS, Latimer AE, Ginis KA, Arbour KP, McCartney N, Hicks AL.. Maintenance of exercise participation in individuals with spinal cord injury: effects on quality of life, stress and pain. Spinal Cord 2003;41(8):446–50. doi: 10.1038/sj.sc.3101487. PubMed PMID: 12883542. doi: 10.1038/sj.sc.3101487 [DOI] [PubMed] [Google Scholar]
- 19.Summers JD, Rapoff MA, Varghese G, Porter K, Palmer RE.. Psychosocial factors in chronic spinal cord injury pain. Pain 1991;47(2):183–9. Epub 1991/11/01. doi: 10.1016/0304-3959(91)90203-a. PubMed PMID: 1762813. doi: [DOI] [PubMed] [Google Scholar]
- 20.Tawashy AE, Eng JJ, Lin KH, Tang PF, Hung C.. Physical activity is related to lower levels of pain, fatigue and depression in individuals with spinal-cord injury: a correlational study. Spinal Cord 2009;47(4):301–6. doi: 10.1038/sc.2008.120. PubMed PMID: 18936771; PubMed Central PMCID: PMC3095632. doi: 10.1038/sc.2008.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taran S, Conti J, Routhier F, Latimer-Cheung AE, Noreau L, Sweet SN.. Leisure time physical activity, perception of impact of pain and life satisfaction after spinal cord injury. Ann Phys Rehabil Med 2018;61(4):273–5. doi: 10.1016/j.rehab.2018.02.007. PubMed PMID: 29524588. doi: 10.1016/j.rehab.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 22.Nooijen CF, Vogels S, Bongers-Janssen HM, Bergen MP, Stam HJ, van den Berg-Emons HJ.. Fatigue in persons with subacute spinal cord injury who are dependent on a manual wheelchair. Spinal Cord 2015;53(10):758–62. Epub 2015/04/22. doi: 10.1038/sc.2015.66. PubMed PMID: 25896345. doi: 10.1038/sc.2015.66 [DOI] [PubMed] [Google Scholar]
- 23.Anton HA, Miller WC, Townson AF, Imam B, Silverberg N, Forwell S.. The course of fatigue after acute spinal cord injury. Spinal Cord 2017;55(1):94–7. doi: 10.1038/sc.2016.102. PubMed PMID: 27349608. doi: 10.1038/sc.2016.102 [DOI] [PubMed] [Google Scholar]
- 24.van Diemen T, van Lankveld W, van Leeuwen C, Post M, van Nes I.. Multidimensional fatigue during rehabilitation in persons with recently acquired spinal cord injury. J Rehabil Med 2016;48(1):27–32. Epub 2015/10/10. doi: 10.2340/16501977-2018. PubMed PMID: 26449895. doi: 10.2340/16501977-2018 [DOI] [PubMed] [Google Scholar]
- 25.Corfield EC, Martin NG, Nyholt DR.. Co-occurrence and symptomatology of fatigue and depression. Compr Psychiatry 2016;71:1–10. Epub 2016/10/23. doi: 10.1016/j.comppsych.2016.08.004. PubMed PMID: 27567301. doi: 10.1016/j.comppsych.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Larkin D, Martin CR.. The interface between chronic fatigue syndrome and depression: a psychobiological and neurophysiological conundrum. Neurophysiol Clin 2017;47(2):123–9. Epub 2017/03/21. doi: 10.1016/j.neucli.2017.01.012. PubMed PMID: 28314518. doi: 10.1016/j.neucli.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 27.Penny KI, Purves AM, Smith BH, Chambers WA, Smith WC.. Relationship between the chronic pain grade and measures of physical, social and psychological well-being. Pain 1999;79(2–3):275–9. doi: 10.1016/S0304-3959(98)00166-3 [DOI] [PubMed] [Google Scholar]
- 28.Smith BH, Penny KI, Purves AM, Munro C, Wilson B, Grimshaw J, et al. The chronic pain grade questionnaire: validation and reliability in postal research. Pain 1997;71(2):141–7. doi: 10.1016/S0304-3959(97)03347-2 [DOI] [PubMed] [Google Scholar]
- 29.Underwood MR, Barnett AG, Vickers MR.. Evaluation of two time-specific back pain outcome measures. Spine 1999;24(11):1104–12. doi: 10.1097/00007632-199906010-00010 [DOI] [PubMed] [Google Scholar]
- 30.Von Korff M, Ormel J, Keefe FJ, Dworkin SF.. Grading the severity of chronic pain. Pain 1992;50(2):133–49. doi: 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- 31.Curtis K, Roach K, Applegate E, Amar T, Benbow C, Genecco T, et al. Reliability and validity of the wheelchair user’s shoulder pain index (WUSPI). Spinal Cord 1995;33(10):595. doi: 10.1038/sc.1995.126 [DOI] [PubMed] [Google Scholar]
- 32.Curtis K, Roach K, Applegate EB, Amar T, Benbow C, Genecco T, et al. Development of the wheelchair user’s shoulder pain index (WUSPI). Spinal Cord 1995;33(5):290. doi: 10.1038/sc.1995.65 [DOI] [PubMed] [Google Scholar]
- 33.Brose SW, Boninger ML, Fullerton B, McCann T, Collinger JL, Impink BG, et al. Shoulder ultrasound abnormalities, physical examination findings, and pain in manual wheelchair users with spinal cord injury. Arch Phys Med Rehabil 2008;89(11):2086–93. doi: 10.1016/j.apmr.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 34.Hiremath SV, Amiri AM, Thapa-Chhetry B, Snethen G, Schmidt-Read M, Ramos-Lamboy M, et al. Mobile health-based physical activity intervention for individuals with spinal cord injury in the community: a pilot study. PLoS One 2019;14(10):e0223762. doi: 10.1371/journal.pone.0223762 doi: 10.1371/journal.pone.0223762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD.. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–3. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 36.Lofgren M, Norrbrink C.. “But I know what works”–patients’ experience of spinal cord injury neuropathic pain management. Disabil Rehabil 2012;34(25):2139–47. doi: 10.3109/09638288.2012.676146. PubMed PMID: 22512334. doi: 10.3109/09638288.2012.676146 [DOI] [PubMed] [Google Scholar]
- 37.Heneweer H, Vanhees L, Picavet HS.. Physical activity and low back pain: a U-shaped relation? Pain 2009;143(1–2):21–5. Epub 2009/02/17. doi: 10.1016/j.pain.2008.12.033. PubMed PMID: 19217208. doi: 10.1016/j.pain.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 38.Defrin R, Ohry A, Blumen N, Urca G.. Acute pain threshold in subjects with chronic pain following spinal cord injury. Pain 1999;83(2):275–82. doi: 10.1016/S0304-3959(99)00115-3 [DOI] [PubMed] [Google Scholar]
- 39.Ravenscroft A, Ahmed Y, Burnside I.. Chronic pain after SCI. A patient survey. Spinal Cord 2000;38(10):611. doi: 10.1038/sj.sc.3101073 [DOI] [PubMed] [Google Scholar]
- 40.Lidal IB, Jensen AE, Larsen TW, Stanghelle JK.. Fatigue in persons who have lived with spinal cord injury for >20 years. Spinal Cord 2013;51(2):103–8. Epub 2012/10/17. doi: 10.1038/sc.2012.110. PubMed PMID: 23069767. doi: 10.1038/sc.2012.110 [DOI] [PubMed] [Google Scholar]