Abstract
Receipt of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination was not associated with placental histopathologic lesions.
INTRODUCTION
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been approved for emergency use, but, despite elevated risk of severe disease, pregnant women were excluded from the clinical trials that led to their authorization.1 Placental findings can indicate potential clinical risk and could be an early signal for rare injury seen only after widespread use in the pregnant population.2–6
Maternal SARS-CoV-2 infection has been associated with decidual arteriopathy, fetal vascular malperfusion, and chronic histiocytic intervillositis.7–9 mRNA vaccines induce an immune response through activation of TLR3, which has been linked to decidual arteriopathy, growth restriction, preterm delivery, and fetal loss in mouse models.10–14
Our objective was to evaluate the frequency of these key placental lesions in patients who received SARS-CoV-2 vaccination in pregnancy.
METHODS
The study methods have been described previously and were approved by the Northwestern University institutional review board.7,15 We report results from patients who tested negative for SARS-CoV-2 infection on polymerase chain reaction who received vaccine (delivering between January and April 2021) and unvaccinated women in a control group (negative for SARS-CoV-2 infection on polymerase chain reaction, immunoglobulin G– and immunoglobulin M–negative, delivering between April 2020 and April 2021) from an ongoing coronavirus disease 2019 (COVID-19) cohort study. Antibody testing used the ACCESS SARS-CoV-2 spike protein RBD test.
Statistical testing was performed with unpaired t tests or Fisher exact test for demographics and logistic regression with gestational age as a covariate for placental lesions (Python SciPy 1.6.1). A post hoc power calculation was performed, demonstrating at least 80% power to identify a 2.5-fold or higher increased risk of any lesion with a baseline prevalence of 10% or greater and a threefold or higher increased risk of any lesion with a baseline prevalence of 7% or greater (Stata 15.0).
RESULTS
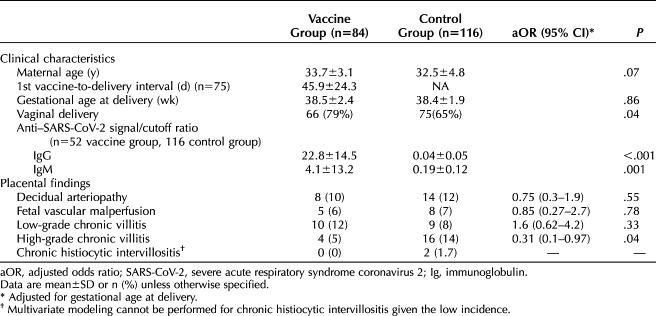
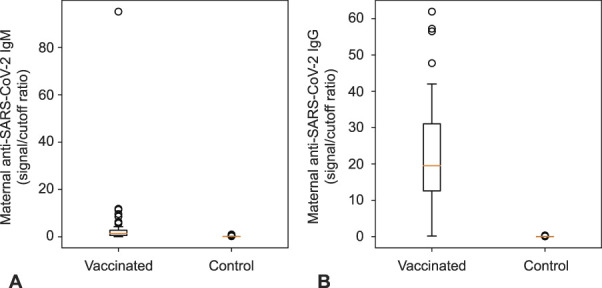
We report findings in 84 women who received a SARS-CoV-2 vaccine during pregnancy and 116 women in a control group who did not receive a vaccine (Table 1). Women with vaccination were more likely to deliver vaginally. The first inoculation was 46±24 days before delivery for the 75 patients with known vaccination timing. Vaccinated women showed robust antibody responses, whereas women in the control group were negative (Fig. 1 and Table 1).
Table 1.
Clinical, Immunologic, and Histologic Findings
Fig. 1. Maternal anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein immunoglobulin (Ig)M (A) and IgG (B) at delivery. Vaccinated patients showed frequent (30/52 over cutoff) IgM and robust (50/52) IgG; women in the control group did not (0/116 and 0/116).
Shanes. Placental Pathology in SARS-CoV-2 Vaccination. Obstet Gynecol 2021.
Placental examination in women with vaccination showed no increased incidence of decidual arteriopathy, fetal vascular malperfusion, low-grade chronic villitis, or chronic histiocytic intervillositis compared with women in the control group (Table 1). Incidence of high-grade chronic villitis was higher in the control group than in the vaccinated group.
DISCUSSION
In our cohort of vaccinated pregnant patients, there was no observed increase in the incidence of findings characteristic of SARS-CoV-2 infection in pregnancy and no evidence of vaccine-triggered breakdown in maternal immunologic tolerance of the fetus.16 Although limited by population differences between vaccinated and unvaccinated patients,17,18 these findings add to the growing literature supporting the safety of SARS-CoV-2 vaccination in pregnancy.
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
Supported by the Friends of Prentice (funder) and NIBIB K08EB030120 (to JAG), Stanley Manne Research Institute and K23AI139337 (to LBM).
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print May 11, 2021.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C331.
REFERENCES
- 1.Kucirka LM, Norton A, Sheffield JS. Severity of COVID-19 in pregnancy: a review of current evidence. Am J Reprod Immunol 2020;84:e13332. doi: 10.1111/aji.13332. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm KM, Heerema-McKenney A. Fetal thrombotic vasculopathy: significance in liveborn children using proposed society for pediatric pathology diagnostic criteria. Am J Surg Pathol 2015;39:274–80. doi: 10.1097/PAS.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 3.Ernst LM, Bit-Ivan EN, Miller ES, Minturn L, Bigio EH, Weese-Mayer DE. Stillbirth: correlations between brain injury and placental pathology. Pediatr Dev Pathol 2016;19:237–43. doi: 10.2350/15-06-1658-OA.1 [DOI] [PubMed] [Google Scholar]
- 4.Redline RW, Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Hum Pathol 1995;26:80–5. doi: 10.1016/0046-8177(95)90118-3 [DOI] [PubMed] [Google Scholar]
- 5.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol 2005;192:452–7. doi: 10.1016/j.ajog.2004.07.030 [DOI] [PubMed] [Google Scholar]
- 6.Redline RW, O'Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med 2000;124:1785–91. doi: [DOI] [PubMed] [Google Scholar]
- 7.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol 2020;154:23–32. doi: 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baergen RN, Heller DS. Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol 2020;23:177–80. doi: 10.1177/1093526620925569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz DA, Baldewijns M, Benachi A, Bugatti M, Collins RRJ, De Luca D, et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med 2020;145:517–28. doi: 10.5858/arpa.2020-0771-SA [DOI] [PubMed] [Google Scholar]
- 10.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov 2018;17:261–79. doi: 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Wei H, Wu D, Tian Z. Toll-like receptor 3 agonist induces impairment of uterine vascular remodeling and fetal losses in CBA×DBA/2 mice. J Reprod Immunol 2007;74:61–7. doi: 10.1016/j.jri.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 12.Baines KJ, Rampersaud AM, Hillier DM, Jeyarajah MJ, Grafham GK, Eastabrook G, et al. Antiviral inflammation during early pregnancy reduces placental and fetal growth trajectories. JI 2020;204:694–706. doi: 10.4049/jimmunol.1900888 [DOI] [PubMed] [Google Scholar]
- 13.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, et al. Original article: activation of TLR3 in the trophoblast is associated with preterm delivery: activation of TLR3 in trophoblast. Am J Reprod Immunol 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. NKG2D blockade inhibits poly(I:C)-Triggered fetal loss in wild type but not in IL-10 −/− mice. JI 2013;190:3639–47. doi: 10.4049/jimmunol.1203488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2021. doi: 10.1016/j.ajog.2021.03.035. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østergaard SD, Schmidt M, Horváth-Puhó E, Thomsen RW, Sørensen HT. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet 2021;397:1441–3. doi: 10.1016/S0140-6736(21)00762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quansah R, Gissler M, Jaakkola JJK. Work as a nurse and a midwife and adverse pregnancy outcomes: a Finnish nationwide population-based study. J Women's Health 2009;18:2071–6. doi: 10.1089/jwh.2008.1062 [DOI] [PubMed] [Google Scholar]
- 18.Park C, Kang MY, Kim D, Park J, Eom H, Kim EA. Adverse pregnancy outcomes in healthcare workers: a Korean nationwide population-based study. Int Arch Occup Environ Health 2017;90:501–6. doi: 10.1007/s00420-017-1213-3 [DOI] [PubMed] [Google Scholar]