Supplemental Digital Content is available in the text.
Keywords: cardiovascular disease, hospitalization, secondary prevention, smartphone
Abstract
Background:
Thirty-day readmissions among patients with acute myocardial infarction (AMI) contribute to the US health care burden of preventable complications and costs. Digital health interventions (DHIs) may improve patient health care self-management and outcomes. We aimed to determine if patients with AMI using a DHI have lower 30-day unplanned all-cause readmissions than a historical control.
Methods:
This nonrandomized controlled trial with a historical control, conducted at 4 US hospitals from 2015 to 2019, included 1064 patients with AMI (DHI n=200, control n=864). The DHI integrated a smartphone application, smartwatch, and blood pressure monitor to support guideline-directed care during hospitalization and through 30-days post-discharge via (1) medication reminders, (2) vital sign and activity tracking, (3) education, and (4) outpatient care coordination. The Patient Activation Measure assessed patient knowledge, skills, and confidence for health care self-management. All-cause 30-day readmissions were measured through administrative databases. Propensity score–adjusted Cox proportional hazard models estimated hazard ratios of readmission for the DHI group relative to the control group.
Results:
Following propensity score adjustment, baseline characteristics were well-balanced between the DHI versus control patients (standardized differences <0.07), including a mean age of 59.3 versus 60.1 years, 30% versus 29% Women, 70% versus 70% White, 54% versus 54% with private insurance, 61% versus 60% patients with a non ST-elevation myocardial infarction, and 15% versus 15% with high comorbidity burden. DHI patients were predominantly in the highest levels of patient activation for health care self-management (mean score 71.7±16.6 at 30 days). The DHI group had fewer all-cause 30-day readmissions than the control group (6.5% versus 16.8%, respectively). Adjusting for hospital site and a propensity score inclusive of age, sex, race, AMI type, comorbidities, and 6 additional confounding factors, the DHI group had a 52% lower risk for all-cause 30-day readmissions (hazard ratio, 0.48 [95% CI, 0.26–0.88]). Similar results were obtained in a sensitivity analysis employing propensity matching.
Conclusions:
Our results suggest that in patients with AMI, the DHI may be associated with high patient activation for health care self-management and lower risk of all-cause unplanned 30-day readmissions.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03760796.
WHAT IS KNOWN
Digital health has been shown to improve secondary prevention for cardiovascular disease in stable outpatient settings, but there has been limited evaluation among patients during the critical post-discharge period following acute myocardial infarction.
Prior digital health studies have raised concerns for a lack of representation of underserved populations at greater risk of poor health outcomes.
WHAT THE STUDY ADDS
Use of a digital health intervention to support acute myocardial infarction recovery, from early during the hospitalization to home, may be associated with high patient activation and lower risk of 30-day all-cause unplanned readmission.
This study supports the feasibility of delivering digital health interventions to diverse patients in the acute care setting following acute myocardial infarction.
Supporting low-health literacy with written content at a sixth to seventh grade reading level, animated videos, and a novel technology loaner program for patients postacute myocardial infarction, this study demonstrated how digital health can be a promising modality toward health equity.
Among the 1 million patients in the United States hospitalized yearly with acute myocardial infarction (AMI), nearly 1 in 6 experiences a 30-day hospital readmission.1–3 Approximately 76% of these readmissions are potentially preventable4 by utilizing best practices5 including predischarge planning, education, and adherence support.6 The Hospital Readmissions Reduction Program penalizes hospitals with excess 30-day unplanned readmissions.7
Digital health interventions (DHIs) are of interest to improve quality of care delivery, especially considering the rapid uptake of telemedicine during the coronavirus disease 2019 (COVID-19) pandemic. A systematic review and meta-analysis of 27 randomized controlled trials (RCTs) demonstrated most DHI studies for secondary prevention of cardiovascular disease (CVD) assessed the impact of text messaging on adherence to guideline-directed recommendations.8 These DHIs increased adherence to pharmacologic and nonpharmacologic therapies.8 Only one study evaluated the impact of a smartphone application (app) on readmissions, finding lower rates among the 19 patients with the DHI in combination with cardiac rehabilitation (CR).8,9
The MiCORE (Myocardial infarction, COmbined-device, Recovery Enhancement) Study10 aimed to advance prior evidence by (1) intervening with a comprehensive DHI, (2) deploying the DHI in the high-acuity setting including intensive care units (ICUs) and ICU step-down units, (3) evaluating readmission outcomes, and (4) enrolling a diverse and larger patient population. The primary objective was to determine if patients with AMI who use a guideline-based self-management DHI have lower rates of all-cause 30-day readmissions compared with a historical control group. We used a multiphase, multicenter, nonrandomized controlled trial study design10 as a resource-efficient and time-sensitive approach for initial technology evaluation.
Methods
Requests for sharing de-identified data will be considered on a case-by-case basis for compliance with the participating sites’ institutional policies. The statistical code that supports the findings of this study is available at https://github.com/jieding-epistat/dhi_ami. MiCORE study methods have been detailed previously10 and are summarized below.
Study Design
MiCORE is a multiphase, multicenter, nonrandomized controlled trial. We decided to use a historical control, which carried a disadvantage with respect to causal inference, while bringing 3 major advantages: (1) more efficient use of resources to complete the study (sufficient resources were not available to conduct a RCT); (2) faster study completion; and (3) from a research ethics standpoint, the ability to offer the DHI to all patients. As compared with an investigational drug or device of uncertain benefit and risk, our intervention targeted delivery of American Heart Association/American College of Cardiology evidence-based guidelines that have been well-established in previous RCT studies. The study was divided into 3 phases: (1) DHI development with patient input, (2) pilot, and (3) prospective study. In phase 1, a team of patients, nurses, physicians, engineers, and Apple designers (Cupertino, CA) developed the Corrie Health digital platform comprised of a smartphone app, smartwatch app, wireless blood pressure (BP) monitor, and backend data system (see Figure 1). Phase 1 aimed to create a DHI to engage patients during hospitalization and postacute care in guideline-based secondary prevention by combining patient experience, clinical and technical expertise, and health behavior change constructs. Patients admitted to Johns Hopkins Hospital (JHH), Johns Hopkins Bayview Medical Center (JHBMC), Massachusetts General Hospital (MGH), and Reading Hospital (RH) for a Type 1 AMI from October 1, 2016 to April 14, 2019 were enrolled into the prospective DHI group as early as possible during their hospitalization, and followed for 30 days post-discharge. In phase 2 (October 1, 2016 to September 30, 2017; n=60), we aimed to assess (1) the feasibility of deploying the DHI to patients in the cardiac ICUs and ICU stepdown units and (2) the usability of the intervention. In phase 3 (October 1, 2017 to April 14, 2019; n=140), we aimed to scale to more patients. Then across phases 2 and 3 (n=200), we assessed the primary objective of readmission outcomes as well as the secondary objectives.
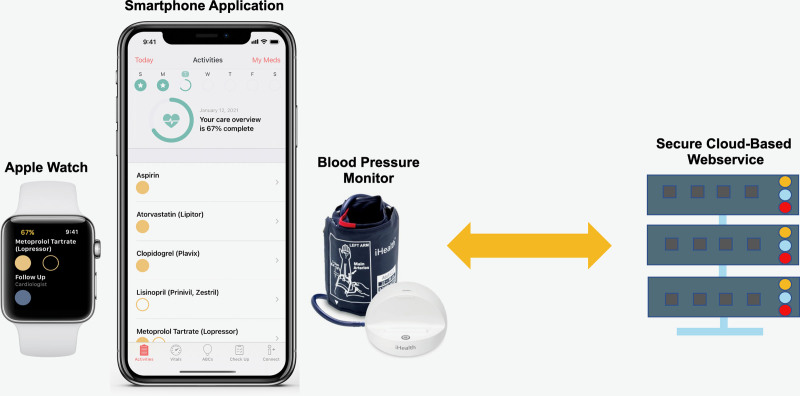
Figure 1.
MiCORE (Myocardial infarction, COmbined-device, Recovery Enhancement) digital health intervention (DHI): Corrie Health Digital Platform. This figure shows the main components of the DHI including a smartphone application, smartwatch, wireless blood pressure monitor, and cloud-based backend data collection system.
The secondary objectives were to evaluate (1) emergency department (ED) visits at 30 days post-discharge; (2) the cost-effectiveness of the DHI to reduce 30-day readmission rates in post-AMI patients; (3) in-hospital satisfaction, perceived app usability, and patient activation among post-AMI patients with the DHI 3 days post-discharge; and (4) perceived app usability, patient activation, user engagement, medication adherence, and attendance of follow-up appointments and CR among post-AMI patients with the DHI 30 days post-discharge. Exploratory objectives were to assess: (1) trends in the physiological data (weight, mood, heart rate, BP, physical activity) and (2) whether abnormal physiological data precedes hospital readmissions.
The historical control group consisted of patients admitted to the 4 hospitals with an ST-elevation myocardial infarction or non ST-elevation myocardial infarction from September 27, 2015 to October 1, 2016, prior to prospective enrollment into the DHI group. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board (IRB00099938) and other participating sites. Prespecified end points were registered on ClinicalTrials.gov. Our primary end point of unplanned all-cause 30-day readmissions, as well as secondary end points including ED visits, attendance of follow-up appointments and CR, perceived app usability, and patient activation are reported here.
Participants
DHI participants were identified by: (1) chart review, (2) inpatient clinician referral, or (3) an automatic electronic medical record trigger alerting the study team of potentially eligible patients. ST-elevation myocardial infarction or type 1 non ST-elevation myocardial infarction patients were eligible to participate if they (1) were ≥18 years old, (2) owned a smartphone, and (3) were approved to participate by their inpatient team. Patients were ineligible if they were (1) non-English speaking, (2) hemodynamically unstable, and/or (3) had severe sensory or motor impairment.
Patients with AMI were eligible for the control group if they were ≥18 years old and English speaking. They were excluded if they had in-hospital death or were transferred to another hospital at the time of discharge. To achieve equal proportions of patients by hospital site in the control group relative to the DHI group (ie, ratio of 5:1), patients were randomly selected from the eligible control group for MGH and RH. The entire eligible sample from JHH and JHBMC was included in the control group.
Intervention and Enrollment Process
Apple ResearchKit, CareKit, and HealthKit provide a digital health framework for patient empowerment. Our interdisciplinary team including clinicians, engineers, and patients developed the multimodal Corrie Health digital platform (Figure 1) with Apple designers. In addition to standard of care, the patients in the DHI group received the Corrie app, Apple Watch, and an iHealth wireless BP monitor, for use during their hospitalization and 30 days post-discharge. The DHI integrated constructs from the widely-accepted Health Belief Model and Social Cognitive Theory to promote health behavior change.12–14 In Table I in the Data Supplement, we include components of these theories, an explanation of each, and how they were integrated into the DHI to promote health behavior change.10
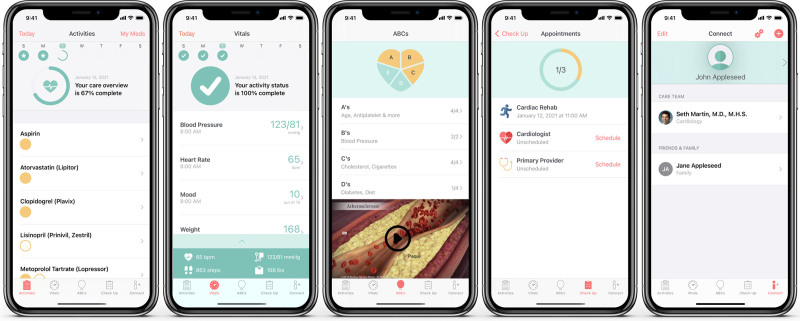
The Corrie app (Figure 2) allowed participants to (1) manage their medications (track daily adherence, indication, and side effects), (2) monitor their vital signs (heart rate, BP, weight, mood, and steps), (3) learn about the risk factors for CVD and lifestyle modification through educational articles (all at a sixth or seventh grade reading level as determined by the Flesch-Kincaid Readability Test Tool) and animated videos (Nucleus Media), (4) schedule and track follow-up appointments, (5) connect with their care team, and (6) store health information such as stent and insurance cards.10 Apple Watch integration allowed participants to monitor their heart rate and physical activity, receive reminders on medications and appointments (also delivered on the iPhone home screen), and log medications directly on the watch.10 The wireless BP monitor integration allowed participants to track and review BP recordings within the DHI.10 There was no monitoring of real-time data by the study or patient’s clinical team.10 At this stage, the DHI was exclusively a self-management tool for patients.10
Figure 2.
MiCORE (Myocardial infarction, COmbined-device, Recovery Enhancement) digital health intervention screenshots and video tour. This figure and corresponding video (link: https://www.youtube.com/watch?v=kZdbK47a48Q&feature=youtu.be) show the application components, including medication tracking, vital signs monitoring, educational articles and animated videos, scheduling follow-up appointments, and connecting with clinicians.
Study team members at each site, coordinated by ES, enrolled participants, consecutively assigned participants to the intervention, and delivered the intervention to the participants in the hospital. The study member used an IRB-approved electronic informed consent Apple ResearchKit app to conduct the informed consent process. The informed consent app enabled a team member to guide patients through the informed consent process and to explain the risks and benefits of participating, all in an easy-to-understand visual format. A copy of the informed consent was automatically emailed to both the participant and the team.
The Corrie app was then downloaded onto the participant’s smartphone. If the participant had an Android or older generation iPhone, they were given a loaner smartphone called an “iShare” preloaded with Corrie and a subscriber identity module card for cellular data access.15 Intervention components were equivalent for those using Corrie on a personal iPhone and for those using Corrie on an iShare.
Further details on the enrollment process, described previously,10 are also included in the Methods in the Data Supplement.
Measures and Data Collection
Baseline Characteristics
Demographic, clinical, and hospitalization characteristics were collected on each patient, for both groups, at the time of admission from the hospitals’ administrative databases. Demographic data included age, sex, race, insurance status, and marital status. Clinical characteristics included body mass index, smoking status, and comorbidity burden defined by the Agency for Healthcare Research and Quality Elixhauser Comorbidity Index.16 The Agency for Healthcare Research and Quality Elixhauser Comorbidity Index measure consists of 29 comorbid conditions (eg, congestive heart failure, pulmonary disease, peripheral vascular disease, and renal failure) based on International Classification of Diseases-Tenth Revision diagnosis codes. Hospitalization characteristics included admitting hospital, type of AMI, length of stay, type of revascularization during admission, and discharge disposition. Years of formal education and income were not available in the hospitals’ administrative databases. Thus, these data were only obtained for participants in the DHI group via self-report during enrollment.
Outcome Measures
The primary outcome was unplanned all-cause readmission within 30 days post-discharge. Unplanned all-cause readmission was chosen as the primary outcome to be consistent with the value-based measure defined by the Center for Medicare and Medicaid Services Hospital Readmission Reduction Program of 30-day unplanned readmission. Cardiovascular-related readmissions, defined as readmissions with a principal diagnosis of coronary artery disease, chest pain, congestive heart failure, arrhythmias, valvular heart disease, pericardial disease, or stroke, were also evaluated in the DHI group.17 In the DHI and control group, readmissions were ascertained from administrative databases in the following instances: (1) enrolled at JHH and JHBMC and readmitted to any hospital in Maryland, (2) enrolled at MGH and readmitted to any hospital participating in their centralized clinical data registry, and (3) enrolled at and readmitted to RH.
For secondary outcomes, we examined ED visits at JHH and JHBMC, where data were available, from the same claims data/hospital administrative databases as readmissions at 30 days post-discharge. Perceived app usability and patient activation were assessed at 3 and 30 days post-discharge via the Systems Usability Scale18 and the Patient Activation Measure,19 respectively, and collected through a Research Electronic Data Capture survey. Attendance at primary care and cardiology appointments, and CR sessions, were assessed at 30 days post-discharge via self-report collected through a Research Electronic Data Capture survey.
Statistical Analysis
After conducting a power analysis, we determined that a sample size of 200 participants in the DHI group and 1000 in the control group would provide ≈90% power at the 2-sided 5% level of significance to detect a hazard ratio (HR) of 0.5, assuming around 85% of patients in the control group survived and were not readmitted by the end of 30-days (Stata stpower) and allowing for 10% dropout.10
The χ2 or Fisher exact test for binary and categorical variables and independent t test or Mann-Whitney U test for continuous variables were performed to compare demographic, clinical, and hospitalization characteristics between DHI and control patients. Cox proportional hazards models were fitted to obtain the estimated HRs and 95% CIs for the association of DHI use with risk of 30-day readmission. Each patient was followed up from the date of hospital discharge to the first readmission or the administrative censoring date. Participants in the DHI group who withdrew from the study were not included in the analyses.
To balance distribution of measured confounding variables at baseline between the DHI and the control group, we used a logistic regression model to compute propensity scores for the conditional probability of being a DHI user, and then categorized the propensity scores into quintiles to include in the Cox model. The propensity score included age, sex, race, insurance status (private insurance/health maintenance organization/preferred provider organization versus Medicare/Medicaid/self-pay), marital status, discharge disposition (home versus not home), length of index hospital stay (natural log transformed), smoking status (current versus never/former), AMI type, revascularization during admission (percutaneous coronary intervention and/or coronary artery bypass grafting surgery versus neither), and tertiles of comorbidity burden (as above, based on 29 comorbidities such as congestive heart failure). Because there have been no established or clinically meaningful cut-offs, we categorized the comorbidity burden scale into tertiles. In a post hoc sensitivity analysis, we reran the analysis by using quartiles of comorbidity burden distribution, and the results were largely unaltered. All baseline covariates were chosen if they were associated with DHI group status and readmission risk. When data for a covariate were missing (17% of patients had at least 1 variable with missing data), values were imputed with ten imputed datasets using the fully conditional specification method (Stata multiple imputation procedures) that imputes on a variable-by-variable basis and does not assume a joint multivariate normal distribution of data.20
The full Cox model was adjusted for hospital sites and quintiles of the propensity scores. The proportionality assumption was tested by using Schoenfeld residuals and was met for all variables. To test the robustness of results in the main analysis, several sensitivity analyses were performed. The first consisted of using a propensity score matching technique on each of the 10 imputed datasets to form matched sets of DHI and control patients who shared a similar propensity score. Specifically, 2:1 risk set matching (aiming to match 2 control patients to each DHI patient) on the logit of propensity score was performed using a greedy (nearest neighbor)–matching algorithm with a maximum caliper equal to 0.2 times the pooled SD of the logit of propensity score.21 After completing many-to-one matching ratio scenarios (2:1, 3:1, 4:1), a matching ratio of 2:1 was used in the main sensitivity analysis to maximize the number of DHI patients being matched while attempting to increase statistical power (refer to Table II in the Data Supplement). The performance of the matching was assessed by comparing the baseline characteristics and a standardized difference <0.1 was generally considered negligible.22,23 Using the matched cohort, we applied marginal Cox models24,25 including intervention group status to assess the association with risk of readmission while accounting for the dependence between patients within a cluster on each dataset and then combined the results using Stata mi estimate.26 For this analysis, we accounted for the matching and not hospital-level clustering. The second and third sensitivity analyses consisted of a complete case analysis of all patients and of those patients who underwent coronary revascularization. A post hoc sensitivity analysis assessing the potential impact of an unmeasured confounder was performed using both the E-value measurement27 and the analytical approach as described by Lin et al28 (refer to the Methods in the Data Supplement). All statistical analyses were performed using Stata (StataCorp LP, College Station, TX) software version 15.1.
Results
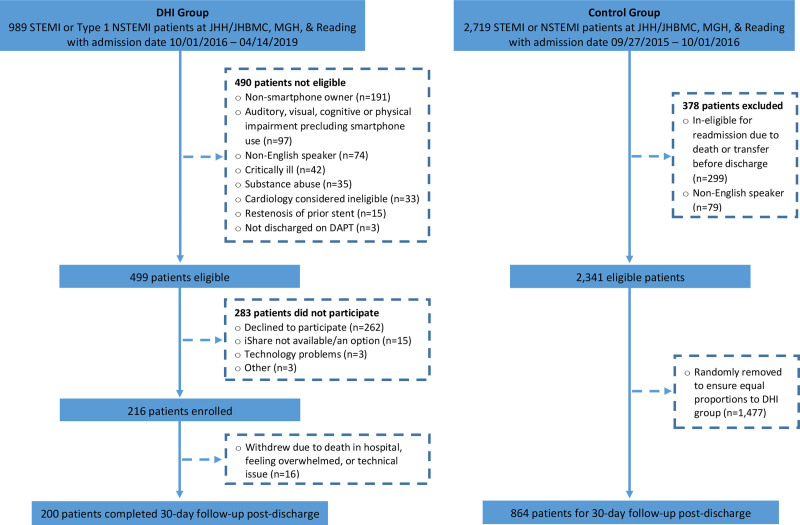
For the DHI group, 989 patients with Type 1 AMI were identified as potentially eligible, of which 490 were subsequently classified as not eligible. Among the 499 eligible patients, 216 enrolled, and then 16 participants withdrew, resulting in 200 participants who completed the study (Figure 3). Recruitment was then stopped as the recruitment goal was met. While not all patients provided a reason for declining participation, the most commonly provided reasons were that (1) they felt too overwhelmed to participate in a research study, (2) patients who owned an Android were not interested in the iShare program/having to carry another phone, (3) patients owned a smartphone but they felt they were too inexperienced to use smartphone technology beyond making phone calls, and (4) they already felt they had a system in place to manage their health care.
Figure 3.
MiCORE (Myocardial infarction, COmbined-device, Recovery Enhancement) study participant flow diagram. This figure shows the number of potentially eligible patients for the digital health intervention (DHI) and historical control group, reasons for exclusion for both groups, and number of patients enrolled/included in both groups. DAPT indicates dual antiplatelet therapy; DHI, digital health intervention; JHBMC, Johns Hopkins Bayview Medical Center; JHH, Johns Hopkins Hospital; MGH, Massachusetts General Hospital; NSTEMI, non-ST-segment-elevation myocardial infarction; and STEMI, ST-segment-elevation myocardial infarction.
For the control group, 2719 ST-elevation myocardial infarction and non ST-elevation myocardial infarction patients were identified as potentially eligible, 378 did not meet eligibility criteria, and an additional 1477 eligible patients were removed from the group via random selection to ensure equal proportions by site to the DHI group. Thus, 864 patients in the control group were included in final analyses. Although a priori we aimed to include 1000 patients in the control group, the available sample size at JHH and JHBMC that met the eligibility criteria was smaller than anticipated (n=734). Furthermore, we only included 70 and 60 participants in the control group, respectively, from MGH and RH to have an equal proportion by site in the control group relative to those enrolled from these sites in the DHI group.
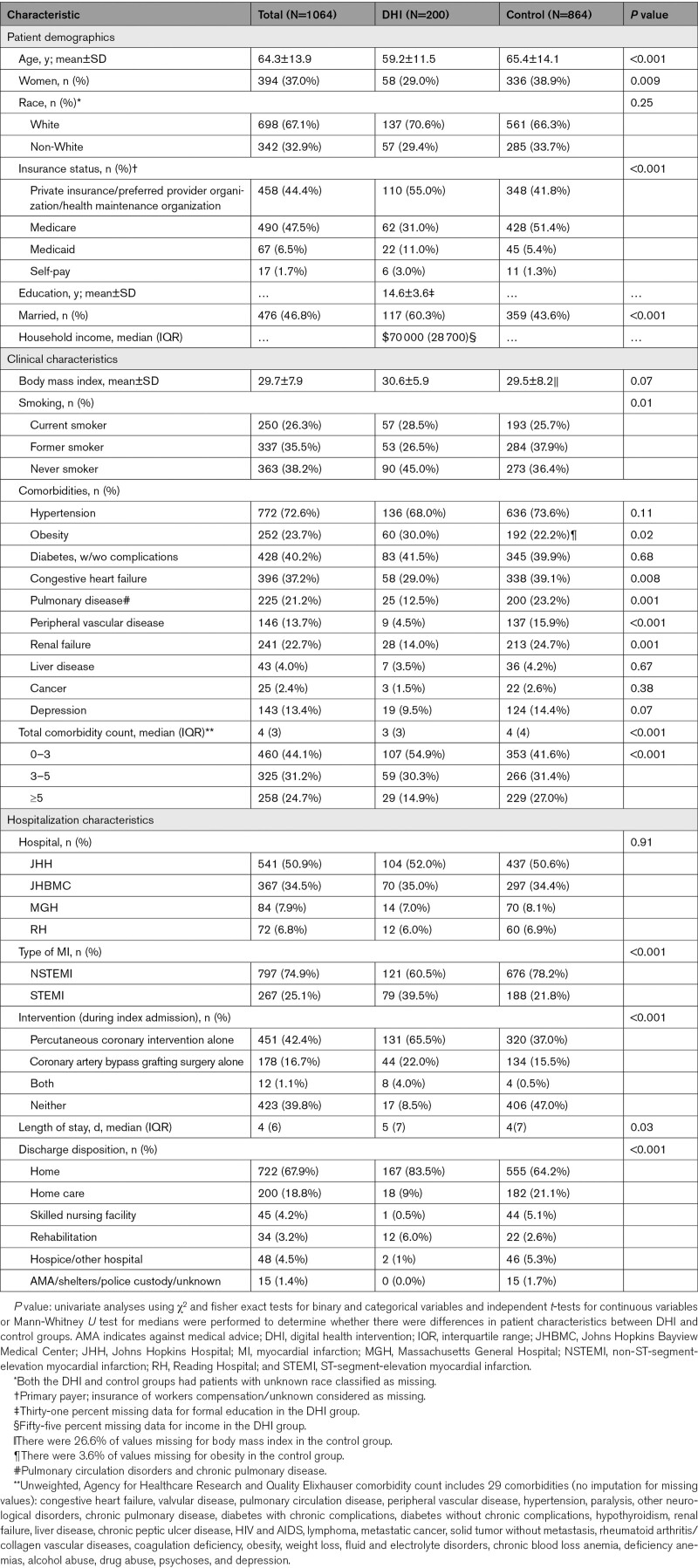
In total, 1064 patients were included in the analysis for the primary end point of 30-day readmission. Before accounting for the propensity score, patients in the DHI group differed significantly from control patients on a number of baseline sociodemographic, clinical, and hospitalization characteristics (Table); they were younger, more likely to be male, more likely to have private insurance, and had a lower comorbidity burden. After accounting for the propensity score, baseline characteristics were well-balanced between the DHI versus control patients (Table III in the Data Supplement): mean age, 59.3 versus 60.1 years, 30% versus 29% Women, 70% versus 70% White, 54% versus 54% with private insurance, and 15% versus 15% with the highest comorbidity burden.
Table.
Baseline Characteristics: DHI and Historical Control Groups
Among DHI participants, 54% (n=108) were enrolled with a native device and 46% (n=92) with an iShare. We found that compared with participants enrolled with a native device, iShare users were slightly younger (mean age, 57.4 [SD 11.5] versus mean age, 60.8 [SD 11.4]; P=0.04), and iShare users were also more likely to be women (33/92, 36% versus 25/108, 23%; P=0.048), of Black race (23/92, 25% versus 15/108, 14%; P=0.046), and insured by Medicaid (18/92, 20% versus 4/108, 4%; P<0.001). On average, DHI participants had access to the DHI for 3.4 inpatient days in addition to the 30 days post-discharge. Following deployment of the DHI backend capabilities part way through phase 1, 83% (138/166) of the DHI users had captured app interactions, meaning they used the DHI at least once during the study and subsequently connected to WiFi or cellular data to connect with the backend. As the backend capabilities were initiated part way through phase 1, the platform did not capture app interactions for the first 34 participants enrolled in the study. Of these 138 participants, there was a median of 213 (interquartile range: 393) app interactions per participant, consisting of number of BP, heart rate, weight, mood, and step count recordings; number of medications tracked; and number of educational articles and videos viewed over the study period. In total, there were 34 997 app interactions over the study period.
Primary Outcome
30-Day All-Cause Hospital Readmissions
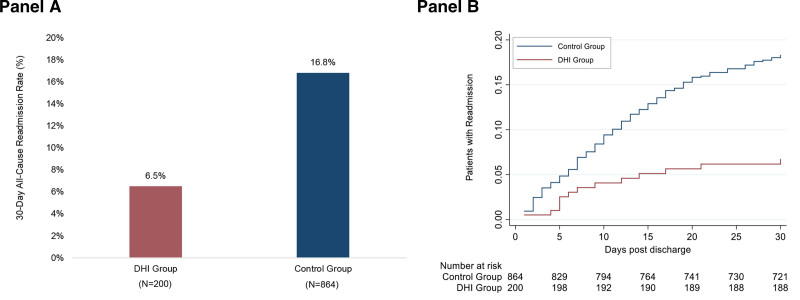
The rate of 30-day all-cause readmission was 6.5% (13/200) for the DHI group and 16.8% (145/864) for the control group (Figure 4A). Adjusting for the propensity score and hospital site, the risk of readmission within 30 days post-discharge was 52% lower in the DHI group as compared with the control group (HR, 0.48 [95% CI, 0.26–0.88]; P=0.018) (Figure 4B). Table IV in the Data Supplement provides the incidence rate of 30-day hospital readmission in the DHI and control groups.
Figure 4.
Thirty-day all-cause readmission by intervention status. A, Shows 30-day all-cause readmission rates for the digital health intervention (DHI) group and the historical control group. B, Shows Nelson-Aalen estimates of the cumulative proportion of patients with 30-day readmission post-discharge. The numbers below the graph are the numbers of patients at risk in each study group at 5, 10, 15, 20, 25, and 30 days, when the last observed readmission event occurred.
Among DHI participants, 38.5% (5/13) of 30-day readmissions were cardiac-related but none were because of a recurrent AMI. Among DHI participants, there was no difference in all-cause readmission within 30 days between those enrolled in phase 2 versus phase 3 of MiCORE (P=0.24, adjusting for age and sex).
Secondary Outcomes
ED Visits
For patients admitted to JHH or JHBMC (n=908), 8.8% (n=80) had an ED visit within 30 days post-discharge that resulted in readmission. Among the remaining 828 patients, 7.9% (13/164) in the DHI group and 5.9% (39/664) in the control group had an ED visit within 30 days post-discharge that did not result in readmission. Adjusting for propensity score and hospital site, there was no difference in hazard (HR, 1.45 [95% CI, 0.69–2.98], P=0.33) for 30-day ED visits not resulting in readmission between the 2 groups.
Attendance of Follow-Up Appointments and CR
Among DHI patients who completed the 30-day survey on attending follow-up appointments (n=104), 18.3% (n=19) reported attending one appointment with a primary care clinician, cardiologist, or CR; 46.2% (n=48) reported attending 2; and 32.7% (n=34) reported attending all 3. Only 2.9% (n=3) reported not attending any appointment. Of the 3 appointments, CR was least frequently reported as attended (n=47, 45.2%).
Patient Activation
DHI patients were predominantly in the highest 2 levels of patient activation for self-management with a mean patient activation total score of 71.9 (SD: 15.7) 3 days post-discharge (n=94) and 71.7 (SD: 16.6) 30 days post-discharge (n=103), with scores ranging from 21.7 to 100.0 (reference range: 0–100, lower scores indicate lower patient activation).
Perceived Application Usability
The mean perceived app usability total score among DHI participants was 58.6 (SD: 11.5) 3 days post-discharge (n=87) and 57.6 (SD: 11.6) 30 days post-discharge (n=104), with scores ranging from 30 to 82.5 (reference range: 0–100, lower scores indicate lower perceived app usability).
Survey Completers
There were no significant differences between completers (n=92) and noncompleters (n=108) of study surveys with respect to major baseline characteristics including age, sex, race, AMI type, and smoking status.
Sensitivity Analyses
When 2:1 matching was used, matched sets with the number ranging from 170 to 174 on each of 10 imputed datasets, were formed. For example, of these 174 matched sets on one imputed dataset, 149 consisted of 2 control patients and 1 DHI patient, while 25 sets consisted of 1 control patient and 1 DHI patient. Thus, a total of 497 patients were included in the matched sample on this imputed data set. Across all imputed datasets, around 85% of DHI patients were matched to at least 1 control patient. The pooled HR estimates for 30-day readmission in the DHI versus control group were 0.49 ([95% CI, 0.26–0.94], P=0.032) from marginal Cox models. In Table III in the Data Supplement, we demonstrate the balance of baseline characteristics between DHI and control patients in the original sample after stratifying into quintiles and in the propensity score matched sample. As shown in this table, the absolute standardized differences between DHI and control patients were all <0.07, suggesting adequate balance on baseline covariates by both propensity score stratification (main analysis) and matching (sensitivity analysis).
When analyzing only patients with complete and valid data, results were similar to those reported from the imputed data set (30-day readmission HR, 0.46 [95% CI, 0.25–0.88], P=0.019; 30-day ED visit HR, 1.49 [95% CI, 0.70–3.18], P=0.303). When analyzing only patients who underwent coronary revascularization during the index admission, results were similar to the main analyses (30-day readmission HR, 0.39 [95% CI, 0.19–0.78], P=0.008; 30-day ED visit HR, 1.59 [95% CI, 0.74–3.43], P=0.24).
Based on results of the post hoc sensitivity analysis estimating the potential impact of an unmeasured confounder, it is unlikely that an unmeasured confounder was masking a null association. The E-values for the point estimate and upper limit of the CI for the DHI were 2.70 and 1.41, respectively, indicating no substantial unmeasured confounding. Furthermore, using the analytical method by Lin et al28 for the point estimate of the adjusted RR to be 1.00, the unmeasured binary confounder would have to be prevalent and strongly associated with the DHI group (eg, a prevalence of 20% in the control group and 80% in the DHI group) and have a very strong association with increased 30-day readmission (RR between 3.5 and 4.0), independent of measured confounders (Figure I in the Data Supplement). None of the measured confounders examined in the present study meet these criteria.
Discussion
The MiCORE multicenter study evaluated a self-management DHI as an adjunct to standard of care for patients with AMI. We found DHI participants had a lower risk of all-cause unplanned 30-day readmissions compared with patients in the control group who received standard of care. Among readmitted DHI participants, less than half were cardiac related and none were because of recurrent AMI. In the context of an exploratory trial, there appears to be a signal that using technology to support AMI care from early during the hospitalization to home, may improve self-management and reduce preventable readmissions by combining constructs from behavior change theories with evidence-based guideline recommendations for AMI care. The effect size for 30-day readmissions was large in both the propensity score stratification and propensity score matching sensitivity analysis. It has been estimated that up to 76% of 30-day readmissions are potentially preventable using proven standards of care.4 Our effect size may be related to the comprehensive nature of the intervention, but this warrants further study.
Delivering the DHI in high acuity settings, including the cardiac ICUs, was feasible. The DHI participants found the application usable and had high patient activation for self-management at 3 and 30 days post-discharge. Self-reported CR attendance at 30 days post-discharge was 45.2% among DHI participants and was 32.3% among US residents who responded to the Behavioral Risk Factor Surveillance System survey in 2015 and who had experienced a prior AMI (representing lifetime participation in CR post-AMI).29 Overall, there is still a major opportunity to improve CR uptake and outpatient care.
Our findings extend prior evidence by evaluating a multimodality DHI for secondary prevention.30 The Tobacco, Exercise and Diet Messages trial, among patients with CVD, demonstrated that a 6-month texting program improved CVD risk factors.31 Studies using smartphone apps in CVD risk management have shown improved medication adherence.32,33 When used in conjunction with CR, 2 earlier studies demonstrated reductions in weight, BP, readmissions, and ED visits,9,34 and a small RCT suggested nonsignificant reductions in readmissions and ED visits.35
This study has potential limitations that need consideration. We noted that 989 patients were identified as potentially eligible for the DHI group over 2.5 years while 2719 patients were identified as potentially eligible for the control group over 1 year. There are a few likely reasons for this difference: (1) the study had limited resources, especially during phase 2 for screening and recruitment, which improved during phase 3 when the goal was to scale to more patients; (2) in the hospital administrative datasets, more patients were identified with a secondary diagnosis of AMI in the control group than in the DHI group; a portion of these patients were later randomly excluded to match the proportion of participants in the DHI group with a secondary diagnosis of AMI; and (3) patients identified as potentially eligible from MGH and RH made up a larger proportion in the control versus DHI group, and ultimately a subset of these patients were randomly excluded to achieve balance with the proportion of DHI participants enrolled at MGH and RH.
Selection bias may hinder generalizability of the results. To mitigate the risk of selection bias for Apple device owners, we provided an iShare to 46% of participants without a DHI compatible device. Additionally, only 22% of eligible inpatients were enrolled in the DHI group after exclusions or declining participation, which is similar to another inpatient DHI study that enrolled 14%.36 In future studies, we will expand eligibility to include patients without a smartphone and who speak non-English languages. Another source of selection bias, which could not be adjusted for, is that DHI participants were likely more engaged in their care than those included in the control group.
As a nonrandomized trial, the DHI and control groups differed on baseline characteristics. To address this, we used a propensity score to balance distribution of measured confounding variables between groups. However, there may have been potential confounders we were unable to include in the propensity score such as medication prescriptions at discharge and socioeconomic predictors such as neighborhood zip code. The median income of DHI participants was similar to national averages according to the US Census 2015 to 2019 American Community Survey, which estimated a median household income of $62 843.37 As this study used a historical control group, an effect in the DHI group may not only be from interacting with the software platform but also from extra attention given to patients in the enrollment/training process, further attention during follow-up through Research Electronic Data Capture surveys, and overall awareness of being monitored that could lead to a Hawthorne effect. As a first evaluation of the DHI, this design was resource-efficient, time-sensitive, and made the DHI available to all eligible patients. This initial evaluation can serve as a foundation for future RCTs.
The follow-up period of 30 days was short in duration. This was based on Centers for Medicare and Medicaid Services timeframe for readmission penalties. A longer follow-up period would strengthen the ability to evaluate persistence in DHI engagement and impact on major adverse CVD events. There is the possibility that some readmissions may not have been captured if patients were readmitted to another hospital/health system. However, this threat to internal validity would have occurred in both groups. Finally, the study design did not allow for differentiation between the effectiveness of DHI components. Future studies could identify components that have a greater impact on engagement and patient outcomes.
This study has important distinguishing strengths. First, the multicenter study pragmatically tested a technology-enabled program focused on self-management to improve guideline-directed AMI care, while paving the way for future approaches incorporating real-time patient generated data in virtual care models harnessing artificial intelligence.38 Second, most DHI studies have enrolled outpatients,31,33,39 whereas this study deployed a DHI to high-acuity cardiovascular patients in intensive care settings. Third, underserved patients were included via our iShare program to improve the generalizability of our findings to vulnerable populations.15,40 Fourth, we employed an interdisciplinary approach to patient-centered technology development and clinical implementation.10,41
Conclusions
Patients with AMI in the DHI group demonstrated a high level of readiness to perform health care self-management activities at home, and they had a lower risk of all-cause unplanned 30-day readmission compared with a historical control. Future RCTs could more rigorously assess the DHI’s impact on 30-day readmissions and determine what components of the intervention are most impactful. This study supports the feasibility of technology usage in acute care settings, emphasizes early patient engagement with guideline-directed medical therapy, and suggests that the DHI may be associated with lower risk of all-cause unplanned 30-day readmissions.
Acknowledgments
We thank our MiCORE participants for their willingness to partake in this study and desire to improve their cardiovascular health. We thank Apple for the donation of Apple Watches and guidance with the digital health intervention (DHI) design. We are especially thankful to Divya Nag, Director of Health at Apple, for inspiring and supporting our team, and Gavi Rawson, the original lead Corrie iOS developer who later joined Apple. We thank Nucleus Medical Media and Ron Collins for providing medical animations and video content for educational purposes for use in the DHI application. We thank Tom Bauer for sharing expertise on patient education and for connecting us with Nucleus. We thank Jeffrey Sham for contributions to Corrie software development. We thank Leah Wang and the team at iHealth for donation of Bluetooth blood pressure monitors. At Johns Hopkins, we thank Sarah Lewis for outstanding administrative support, and Ann Marie Cullen, Grace Nayden, and Allison Olazo for nursing leadership and collaboration on frontline implementation. We thank Roger Blumenthal and the Ciccarone Center for inspirational leadership in preventive cardiology and unwavering support. We thank Thomas Grader-Beck for helping with the automatic electronic medical record trigger for patient identification. We also thank Annelise Ryan, Rebecca Knipp, Nina Allen, and Bao Chau Ly for research assistance.
Sources of Funding
This study received material support from Apple and iHealth and funding from the Maryland Innovation Initiative, Reading Hospital Foundation, Wallace H. Coulter Translational Research Partnership, Louis B. Thalheimer Fund, the Johns Hopkins Individualized Health Initiative, Ciccarone Center, and the Pollin Digital Innovation Fund. Sponsors provided financial support and supplied materials, while the academic investigators had full rights in the design, collection, analysis, or interpretation of the data, and final approval of the article for submission. Dr Spaulding has received the following financial support for the research, authorship, and publication of this article: National Institutes of Health (NIH)/NHLBI T32 HL007024 Post-Doctoral Fellowship in Cardiovascular Epidemiology Institutional Training, NIH/NINR F31 NR017328, Ruth L. Kirschstein National Research Service Award and NIH/NINR T32 NR012704, PreDoctoral Fellowship in Interdisciplinary Cardiovascular Health Research. Drs Carter, Shan, and Huynh have received support from the Aetna Foundation. Dr Wongvibulsin has received support from the Johns Hopkins School of Medicine Medical Scientist Training Program (National Institutes of Health: Institutional Predoctoral Training Grant—T32) and the National Institutes of Health: Ruth L. Kirschstein Individual Predoctoral NRSA for MD/PhD: F30 Training Grant. In addition to the study specific funding above, Dr Martin has received research support from the American Heart Association (20SFRN35380046 and COVID19-811000), PCORI (ME-2019C1-15328), National Institutes of Health (P01 HL108800), the Aetna Foundation, the David and June Trone Family Foundation, the Pollin Digital Innovation Fund, the PJ Schafer Cardiovascular Research Fund, Sandra and Larry Small, CASCADE FH, Apple, Google, and Amgen.
Disclosures
Under a license agreement between Corrie Health and the Johns Hopkins University, the University owns equity in Corrie Health and the University and Drs Marvel, Lee, and Martin are entitled to royalty distributions related to technology described in the study discussed in this publication. Additionally, Drs Marvel, Lee, and Martin are co-founders of and hold equity in Corrie Health. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Dr Martin has served as a consultant to Akcea, Amgen, AstraZeneca, Esperion, Kaneka, Novo Nordisk, Quest Diagnostics, Regeneron, REGENXBIO, Sanofi, and 89bio. He is a co-inventor on a system to estimate LDL cholesterol levels, patent application pending. The other authors report no conflicts.
Supplemental Materials
Methods
Figure I
Tables I–IV
References 42–46
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AMI
- acute myocardial infarction
- App
- application
- BP
- blood pressure
- COVID-19
- coronavirus disease 2019
- CR
- cardiac rehabilitation
- CVD
- cardiovascular disease
- DHI
- digital health intervention
- ED
- emergency department
- HR
- hazard ratio
- ICU
- intensive care unit
- JHBMC
- Johns Hopkins Bayview Medical Center
- JHH
- Johns Hopkins Hospital
- MGH
- Massachusetts General Hospital
- MiCORE
- Myocardial infarction, COmbined-device, Recovery Enhancement
- RCT
- randomized controlled trial
- REDCap
- Research Electronic Data Capture
- RH
- Reading Hospital
F.A. Marvel and E.M. Spaulding contributed equally.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.121.007741.
For Sources of Funding and Disclosures, see page 785.
References
- 1.Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009–2013. HCUP Statistical Brief #196. 2015. Rockville, MD: Agency for Healthcare Research and Quality. Accessed January 7, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.pdf [PubMed] [Google Scholar]
- 2.Yale New Haven Health Services Corporation Center for Outcomes Research and Evaluation. Medicare hospital quality chartbook: variation in 30-day readmission rates across hospitals following hospitalization for acute myocardial infarction. 2015. Accessed 7 May, 2021. https://www.cmshospitalchartbook.com/file/1146/download?token=LuX9PSZr.
- 3.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013; 309:355–363. doi: 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medicare Payment Advisory Commission. Medicare Payment Advisory Commission. Medicare Payment Advisory Commission. Report to the Congress: Promoting Greater Efficiency in Medicare. 2007. Accessed 7 May, 2021. http://www.medpac.gov/docs/default-source/reports/Jun07_EntireReport.pdf. [Google Scholar]
- 5.Brown JR, Chang CH, Zhou W, MacKenzie TA, Malenka DJ, Goodman DC. Health system characteristics and rates of readmission after acute myocardial infarction in the United States. J Am Heart Assoc. 2014; 3:e000714. doi: 10.1161/JAHA.113.000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiggins BS, Rodgers JE, DiDomenico RJ, Cook AM, Page RL, 2nd. Discharge counseling for patients with heart failure or myocardial infarction: a best practices model developed by members of the American College of Clinical Pharmacy’s Cardiology Practice and Research Network based on the Hospital to Home (H2H) Initiative. Pharmacotherapy. 2013; 33:558–580. doi: 10.1002/phar.1231 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. Hospital Readmissions Reduction Program (HRRP). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed 16 January, 2020. Updated 2019. [DOI] [PMC free article] [PubMed]
- 8.Gandhi S, Hong L, Sun K, Schwalm J. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can J Cardiol. 2016; 32:S278. [DOI] [PubMed] [Google Scholar]
- 9.Widmer RJ, Allison T, Lerman L, Lerman A. The augmentation of usual cardiac rehabilitation with an online and smartphone-based program improves cardiovascular risk factors and reduces rehospitalizations. J Am Coll Cardiol. 2014; 63:p.a1296 [Google Scholar]
- 10.Spaulding EM, Marvel FA, Lee MA, Yang WE, Demo R, Wang J, Xun H, Shah L, Weng D, Fashanu OE, et al. Corrie health digital platform for self-management in secondary prevention after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2019; 12:e005509. doi: 10.1161/CIRCOUTCOMES.119.005509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apple. ResearchKit and CareKit. 2017. Accessed 4 April, 2020. https://www.apple.com/researchkit/.
- 12.Painter JE, Borba CP, Hynes M, Mays D, Glanz K. The use of theory in health behavior research from 2000 to 2005: a systematic review. Ann Behav Med. 2008; 35:358–362. doi: 10.1007/s12160-008-9042-y [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974; 2:328–325 [Google Scholar]
- 14.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. 1986, Englewood Cliffs, NJ, US: Prentice-Hall, Inc [Google Scholar]
- 15.Yang WE, Spaulding EM, Lumelsky D, Hung G, Huynh PP, Knowles K, Marvel FA, Vilarino V, Wang J, Shah LM, et al. Strategies for the successful implementation of a novel iPhone loaner system (iShare) in mHealth interventions: prospective study. JMIR Mhealth Uhealth. 2019; 7:e16391. doi: 10.2196/16391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998; 36:8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 17.Southern DA, Ngo J, Martin BJ, Galbraith PD, Knudtson ML, Ghali WA, James MT, Wilton SB. Characterizing types of readmission after acute coronary syndrome hospitalization: implications for quality reporting. J Am Heart Assoc. 2014; 3:e001046. doi: 10.1161/JAHA.114.001046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact. 2008; 24:574–594 [Google Scholar]
- 19.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004; 394 Pt 11005–1026. doi: 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007; 16:219–242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; 10:150–161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiol Drug Saf. 2008; 17:1218–1225. doi: 10.1002/pds.1674 [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014; 33:1242–1258. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016; 22:557–563. doi: 10.1016/j.bbmt.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger E, Sergeant JC, Lunt M. Avoiding pitfalls when combining multiple imputation and propensity scores. Stat Med. 2019; 38:5120–5132. doi: 10.1002/sim.8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017; 167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 28.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998; 54:948–963 [PubMed] [Google Scholar]
- 29.Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the behavioral risk factor surveillance system. J Am Heart Assoc. 2017; 7:e007664. doi: 10.1161/JAHA.117.007664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph J, Velasco A, Hage FG, Reyes E. Guidelines in review: comparison of ESC and ACC/AHA guidelines for the diagnosis and management of patients with stable coronary artery disease. J Nucl Cardiol. 2018; 25:509–515. doi: 10.1007/s12350-017-1055-0 [DOI] [PubMed] [Google Scholar]
- 31.Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, Jan S, Graves N, de Keizer L, Barry T, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015; 314:1255–1263. doi: 10.1001/jama.2015.10945 [DOI] [PubMed] [Google Scholar]
- 32.Johnston N, Bodegard J, Jerström S, Åkesson J, Brorsson H, Alfredsson J, Albertsson PA, Karlsson JE, Varenhorst C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: a randomized study. Am Heart J. 2016; 178:85–94. doi: 10.1016/j.ahj.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 33.Tian M, Ajay VS, Dunzhu D, Hameed SS, Li X, Liu Z, Li C, Chen H, Cho K, Li R, et al. A cluster-randomized, controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard trial) in rural Tibet, China, and Haryana, India. Circulation. 2015; 132:815–824. doi: 10.1161/CIRCULATIONAHA.115.015373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widmer RJ, Allison TG, Lerman LO, Lerman A. Digital health intervention as an adjunct to cardiac rehabilitation reduces cardiovascular risk factors and rehospitalizations. J Cardiovasc Transl Res. 2015; 8:283–292. doi: 10.1007/s12265-015-9629-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widmer RJ, Allison TG, Lennon R, Lopez-Jimenez F, Lerman LO, Lerman A. Digital health intervention during cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2017; 188:65–72. doi: 10.1016/j.ahj.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 36.Patel MS, Polsky D, Kennedy EH, Small DS, Evans CN, Rareshide CAL, Volpp KG. Smartphones vs wearable devices for remotely monitoring physical activity after hospital discharge: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020; 3:e1920677. doi: 10.1001/jamanetworkopen.2019.20677 [DOI] [PubMed] [Google Scholar]
- 37.United States Census Bureau. Median household income. Accessed 29 December, 2020. https://www.census.gov/search-results.html?q=household+income&page=1&stateGeo=none&searchtype=web&cssp=SERP&_charset_=UTF-8.
- 38.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019; 25:44–56. doi: 10.1038/s41591-018-0300-7 [DOI] [PubMed] [Google Scholar]
- 39.Martin SS, Feldman DI, Blumenthal RS, Jones SR, Post WS, McKibben RA, Michos ED, Ndumele CE, Ratchford EV, Coresh J, et al. mActive: a randomized clinical trial of an automated mHealth intervention for physical activity promotion. J Am Heart Assoc. 2015; 4:e002239. doi: 10.1161/JAHA.115.002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung G, Yang WE, Marvel FA, Martin SS. Mobile health application platform ‘Corrie’ personalises and empowers the heart attack recovery patient experience in the hospital and at home for an underserved heart attack survivor. BMJ Case Rep. 2020; 13:e231801. doi: 10.1136/bcr-2019-231801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marvel FA, Wang J, Martin SS. Digital health innovation: a toolkit to navigate from concept to clinical testing. JMIR Cardio. 2018; 2:e2. doi: 10.2196/cardio.7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanderWeele TJ. On a square-root transformation of the odds ratio for a common outcome. Epidemiology. 2017; 28:e58–e60. doi: 10.1097/EDE.0000000000000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linden A, Mathur MB, VanderWeele TJ. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: the evalue package. Stata J. 2020; 20:162–175 [Google Scholar]
- 44.Ding P, VanderWeele TJ. Sensitivity analysis without assumptions. Epidemiology. 2016; 27:368–377. doi: 10.1097/EDE.0000000000000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groenwold RH, Nelson DB, Nichol KL, Hoes AW, Hak E. Sensitivity analyses to estimate the potential impact of unmeasured confounding in causal research. Int J Epidemiol. 2010; 39:107–117. doi: 10.1093/ije/dyp332 [DOI] [PubMed] [Google Scholar]
- 46.Andersen LW, Raymond TT, Berg RA, Nadkarni VM, Grossestreuer AV, Kurth T, Donnino MW; American Heart Association’s Get With The Guidelines–Resuscitation Investigators. Association between tracheal intubation during pediatric in-hospital cardiac arrest and survival. JAMA. 2016; 316:1786–1797. doi: 10.1001/jama.2016.14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.