Abstract
Sarcoidosis is a heterogeneous granulomatous disease. Biological markers and clinical features could allow specific phenotypes to be associated with different prognosis, severity and treatment responses. This retrospective multicentre study aims to analyse the clinical and immunological features of sarcoidosis and to identify a routine non-invasive biomarker useful in clinical practice.
Materials and methods:
129 Caucasian patients with sarcoidosis (median age IQR, 56 (47-62)) were enrolled retrospectively in the study. Medical history, routine laboratory findings, lung function results and radiological features from the last examination of October 2019 – February 2020 were gathered from the patients’ clinical records.
Results:
Regardless their clinical status at disease onset, at the last clinical examination we didn’t observe any differences in terms of therapeutic management between symptomatic and asymptomatic patients. Stratifying sarcoidosis population according to therapeutic management, the N/L ratio was higher in the treated group than in the non-treated group (p=0.0034). Receiver operating curve (ROC) analysis distinguished these two groups according to N/L ratio with an area under the curve (AUC) of 65.3% and a best cut-off value of 2.21. Peripheral N/L ratio was significantly higher in radiological stages 2-4 than in stages 0-1 (p=0.0090) distinguishing these two groups with an AUC of 64% and a best cut-off value of 2.13.
Discussion:
In our multicentric cohort study similar periodic follow-up can be suggested for symptomatic and asymptomatic sarcoidosis patients at onset. In the heterogeneous context of this disease, N/L ratio proved to be a useful and simple routine laboratory biomarker related to disease activity and need for treatment.
Keywords: sarcoidosis, biomarkers, phenotyping
Abbreviations
- ILD
Interstitial lung diseases
- Th1
T helper 1
- HRCT
High resolution computed tomography
- FDG-PET CT
fluorodeoxyglucose-PET/computed tomography
- N/L
neutrophil-to-lymphocyte ratio
- FEV1
forced expiratory volume in the first second
- FVC
forced vital capacity
- DLCO
diffusing capacity of the lung for carbon monoxide
Introduction
Sarcoidosis is a chronic multisystemic interstitial lung disease (ILD) characterized by non-necrotizing granulomatous inflammation. It has heterogeneous clinical presentations and its aetiology is unknown (1). Environmental or occupational exposure and infectious origin, like Mycobacteria or Propionibacterium, has been suggested (2–4). The interaction between antigens and antigen-presenting cells polarizes T lymphocytes to the T helper 1 phenotype (Th1), leading to formation of sarcoid granulomas consisting of T cells, macrophages, epithelioid cells and giant cells. The management of sarcoidosis has been debated since the 1980s. Diagnosis is based on histological evidence of chronic non-caseating epithelioid-cell granulomas in a specific clinical and radiological context. The disease mainly affects the lungs and lymph nodes, but any organ or system may be susceptible (5,6).
The clinical presentations and evolution of sarcoidosis are unpredictable: for example, it may take the form of a disabling chronic disease or it may resolve spontaneously; it may be acute (including acute Lofgren syndrome), subacute or chronic; it may be symptomatic or asymptomatic and may be associated with many comorbidities and complications (pulmonary fibrosis, kidney or heart failure, visual loss or osteoporosis). This phenotypic variability may reflect differences in genetic background, environmental exposure profile and socioeconomic status of sarcoidosis patients (7,8).
Phenotyping sarcoidosis by identifying biomarkers with adequate sensitivity and specificity for clinical management of the disease is an important aim of research (9,10). Biological markers, genetic background and clinical features could allow specific phenotypes to be associated with different prognosis, severity and treatment responses (11).
However, the definition of disease activity is still unclear (12) and it is confused with the concept of severity (13). Inflammatory bioindicators may provide useful information for disease management, but no single biomarker has been demonstrated to have unequivocal prognostic value for sarcoidosis activity. High resolution computed tomography (HRCT) of the chest evidence of new lung micronodular lesions, peribronchovascular thickening, consolidation or ground glass abnormalities and fluorodeoxyglucose-PET/computed tomography (FDG-PET CT) evidence of increased uptake are considered to be radiological markers of sarcoidosis activity (14,15). Among routine laboratory findings, lymphocyte count and neutrophil-to-lymphocyte (N/L) ratio have been suggested, controversially, as biomarkers of severity (16–18).
The aim of this retrospective multicentre study was to analyse the clinical and immunological features of sarcoidosis, including the impact of symptoms at onset (presence/absence), in relation to follow-up. Another aim was to identify a non-invasive biomarker that could be useful in clinical practice for management of the disease.
Materials and Methods
Study population
One hundred and five Caucasian patients (median age IQR, 56 (47-63)) monitored at the Siena Interstitial Lung Disease Referral Centre were enrolled retrospectively in the study. A second cohort of twenty-four patients (median age IQR, 56 (46-61)) monitored at the Bari Sarcoidosis Referral Centre was added. The diagnosis of sarcoidosis was confirmed by multidisciplinary discussion according to international guidelines (19). Chest X-ray score was performed according to Scadding criteria: stage 0, normal; stage 1, bilateral hilar adenopathy without parenchymal involvement; stage 2, bilateral adenopathy and parenchymal infiltration; stage 3, parenchymal infiltration and stage 4, pulmonary fibrosis associated with sarcoidosis (20). Radiological classification was formulated at the time of clinical assessment. Organ involvement was assessed and clinical phenotypes of population were classified according to GenPhenReSa (Genotype–Phenotype Relationship in Sarcoidosis) criteria (21).
Medical history, routine laboratory findings, lung function results and chest HRCT from the last examination in the period October 2019 – February 2020 were gathered from the patients’ clinical records. According to therapy status, patients were divided into a treated (T) and a non-treated group (NT).
All patients gave their written informed consent to participation in the study which was approved by the Local Ethics Committee (CEAVSE 180712, OSS_REOS 12908, Markerlung 17431).
Lung function tests
The following lung function parameters were measured according to ATS/ERS guidelines (22), using a Jaeger body plethysmograph with corrections for temperature and barometric pressure: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO). All parameters were expressed as percentages of predicted values.
Statistical analysis
Results were expressed as median and interquartile range. Since the data did not show a normal distribution, a one-way ANOVA non parametric test (Kruskal-Wallis test) and the Dunn test were used for multiple comparisons. The Mann-Whitney test was used for comparison of pairs of variables. The Fischer exact test was used for categorical variables as appropriate. Possible relationships between laboratory findings and clinical data were tested by Spearman correlation and linear regression. A p-value less than 0.05 was considered statistically significant. Statistical analysis and graphic representation of the data was performed with GraphPad Prism 5.0 software.
Results
Study population
The main characteristics of our subacute/chronic sarcoidosis population, including demographic data, LFT parameters and GenPhenReSa phenotyping, are reported in Table 1. As expected, the study population showed a prevalence of non-smoker (77%) females (58%) (17). Seventy-eight patients (60%) had a follow-up of more than 5 years.
Table 1.
Main characteristics of sarcoidosis population including age, gender, smoking habit, GenPhenReSa classification and lung function test parameters.
| Demographic and Clinical Information | |
| Total n. of patients | 129 |
| Age (years) | 56 (47-63) |
| Gender (Male/Female) | 54/75 |
| Smoking habit (Never/Former) | 8 (100/21) |
| Median years to diagnosis | 5 (1-10) |
| Symptomatic/asymptomatic at disease onset | 99/30 |
| GenPhenReSa | |
| Abdominal | 16 |
| Ocular-cardiac-cutaneous-CNS | 17 |
| Musculoskeletal-cutaneous | 10 |
| Pulmonary and intrathoracic lymph node | 86 |
| Extrapulmonary | 0 |
| Lung Function Test parameters (median IQR) | |
| FVC% | 104 (90-115) |
| FEV1% | 98 (83-108) |
| DLCO% | 86 (75-105) |
Clinical findings
At disease onset, 99 patients (77%) were symptomatic and 30 (23%) were asymptomatic. At the last clinical evaluation, 20 (66.6%) patients of the latter group and 62 (62.6%) patients of the former were being treated with pharmacological therapy. There was no significant difference between these percentages (p>0.05).
Chest X-ray showed the following distribution of Scadding stages in our population: stage 0 (n=34, 26%), stage 1 (n=16, 12%), stage 2 (n=58, 45%), stage 3 (n=11, 9%), stage 4 (n=10, 8%).
HCRT scan of the chest revealed 16 cases of isolated pulmonary sarcoidosis (12%), 16 cases of mediastinal lymph node involvement (12%) and 96 cases of combined pulmonary and lymph node involvement (74%); 128/129 patients showed lung and/or lymph nodes involvement. Isolated cutaneous sarcoidosis was diagnosed in one patient. Interestingly, 33/34 patients with Scadding stage 0 showed HRCT evidence of lung or lymphatic alterations. The number of patients with extra-thoracic localizations was 42 (32%). The extra-thoracic localizations involved the heart (n=3), central nervous system (n=2), eyes (n=3), skin (n=19), bone (n=2), kidneys (n=1), liver (n=6) and spleen (n=10).
At the time of data collection, 73 patients (57%) were on pharmacological therapies (T group) including prednisone (n=40, 53%) and disease-modifying anti-rheumatic drugs (DMARDs, such as methotrexate, azathioprine, hydroxychloroquine) (n=33, 43%). In the T group, 47 patients (74%) had maintained stable sarcoidosis therapy for at least a year, while 26 (36%) had started taking a new drug shortly before the last clinical assessment.
Fifty-six patients (43%) were not taking any treatment at the enrolment (NT group). Fifteen of them (27%) interrupted pharmacological therapy within the 12 months prior to the last examination due to clinical, functional and/or radiological improvement, while the remnant 41 (73%) showed a complete disease remission and didn’t require therapy in the 12 months prior to the last examination. Among those on therapy at the last examination, 42 (57%) were symptomatic, their main symptoms being chronic dry cough, asthenia and muscle and joint pain. Comorbidities were reported in 88 patients (68%), including arterial hypertension (n=35; 39%), dyslipidemia (n=12; 9%), diabetes mellitus (n=13; 10%), blood or solid cancers (n=11; 9%), thyroid disorders (n=10; 8%), depression (n=7; 5%), obesity (n=5; 4%), asthma (n=4; 3%), rheumatoid arthritis (n=2; 2%), celiac disease (n=2; 2%), arrhythmias (n=2; 2%), glomerulonephritis (n=2; 2%), polycythaemia (n=1; 1%), vitiligo (n=1; 1%), previous acute pancreatitis (n=1; 1%), previous myocardial infarction (n=4; 3%) and previous stroke (n=1; 1%). The comorbidities were distributed evenly between the T group (n=48; 55%) and NT group (n=40; 45%).
Laboratory findings
Laboratory findings of sarcoidosis patients, stratified according to GenPhenReSa clinical phenotypes, are shown in Table 2. Monocytes (x103/µl) were higher in abdominal group in respect with OCCC group (p=0.0191). Lymphocyte count was not significantly different between patients treated with prednisone and/or DMARDs (median IQR, 1.78 (1.03-2.70) vs 1.46 (1.01-2.19), p=0.2968). When the sarcoidosis population was stratified on the basis of therapeutic management, a higher frequency of lymphopenia (lymphocyte count <1000) was observed in the T than in the NT group (p=0.0140), whereas neutrophil counts were in the normal range in both groups (median IQR, 3.7 (2.9-5.2) vs 3.5 (3.05-4.60)). N/L ratio was significantly higher in the T group than in the NT group (median IQR, 2.79 (1.78-3.84) vs 1.98 (1.51-2.67), p=0.0034) (figure 1a). ROC analysis distinguished these two groups according to N/L ratio with an AUC of 65.3% (95% CI 55.6-74.9) and a best cut-off value of 2.21 (74% specificity, 61% sensitivity) (figure 1b).
Table 2.
the main laboratory findings of sarcodiosis population dividing according to GenPhenReSa classification. All data were expressed as median IQR. (1. P=0.0191)
| Laboratory findings | All patients n=129 | Pulmonary and intrathoracic lymph node n=86 | Ocular-cardiac-cutaneous-CNS n=17 | Abdominal n=16 | Musculoskeletal-cutaneous n=10 |
| ACE (U/l) | 47 (37-65) | 49 (39-70) | 45 (35-50) | 58 (31-65) | 39 (35-46) |
| CRP (mg/dl) | 0.3 (0.16-0.71) | 0.28 (0.14-0.74) | 0.34 (0.23-0.71) | 0.19 (0.13-0.56) | 0.21 (0.08-0.36) |
| Hb (g/dl) | 13.9 (12.9-15.1) | 14 (13.1-15.1) | 13.5 (11.7-15) | 14.1 (12.4-14.6) | 13.7 (12.7-14.9) |
| WBC (x103/µl) | 6.4 (5.2-8.0) | 6.5 (5.5-8.0) | 5.9 (4.9-7.6) | 5.3 (4.8-7.6) | 6.6 (5.7-8.1) |
| Neutrophils (x103/µl) | 3.6 (2.8-5.1) | 3.8 (3.0-5.2) | 3.5 (3.1-4.8) | 3.2 (2.6-4.6) | 4.2 (3.2-4.9) |
| Lymphocytes (x103/µl) | 1.7 (1.1-2.2) | 1.7 (1.2-2.4) | 1.9 (1.4-2.1) | 1.4 (0.9-1.9) | 1.7 (1.1-2.1) |
| Monocytes (x103/µl) | 0.6 (0.5-0.7) | 0.6 (0.5-0.7) | 0.52 (0.46-0.54)1 | 0.63 (0.51-0.72)1 | 0.59 (0.58-0.66) |
| Eosinophils (x103/µl) | 0.15 (0.09-0.21) | 0.15 (0.09-0.21) | 0.14 (0.10-0.18) | 0.23 (0.11-0.72) | 0.15 (0.12-0.21) |
| Neutrophil (%) | 62.2 (53.9-67.4) | 62.5 (54.1-67.4) | 62.4 (53.1-66.8) | 59.4 (54.4-65.2) | 62.9 (55-66.8) |
| Lymphocites (%) | 25.7 (19.3-33.1) | 25.7 (19.5-31) | 27.6 (21.2-34.7) | 24.7 (18.8-29.1) | 25.1 (19-30.3) |
| N/L ratio | 2.4 (1.6-3.4) | 2.4 (1.8-3.4) | 2.3 (1.5-3.1) | 2.4 (1.9-3.6) | 2.45 (1.87-3.53) |
| Monocytes (%) | 9.1 (7.7-10.9) | 9.2 (7.7-10.8) | 8.0 (7.5-9.2) | 11.2 (8.4-12.1) | 10.2 (7.9-11) |
| Eosinophils (%) | 2.3 (1.6-3.4) | 2.3 (1.6-3.3) | 2.1 (1.5-3.2) | 4.1 (2.4-8.1) | 2.5 (1.9-3) |
| PLT (x103/µl) | 240 (210-283) | 242 (212-278) | 258 (222-305) | 208 (191-301) | 217 (212-256) |
| Creatinine (mg/dl) | 0.85 (0.74-0.99) | 0.89 (0.76-1.01) | 0.77 (0.73-0.83) | 0.97 (0.85-1.06) | 0.81 (0.69-0.98) |
| Total Bilirubin (mg/dl) | 0.5 (0.4-0.6) | 0.5 (0.4-0.6) | 0.5 (0.4-0.7) | 0.45 (0.4-0.52) | 0.4 (0.3-0.4) |
| SGOT (U/l) | 19.5 (15-24) | 19 (15-23) | 21 (18-25) | 17.5 (12.5-20.2) | 15 (14-21) |
| SGPT (U/l) | 18 (14-25) | 18 (14-25) | 18 (14-30) | 14.5 (9.2-24.5) | 15 (15-20) |
Figure 1.
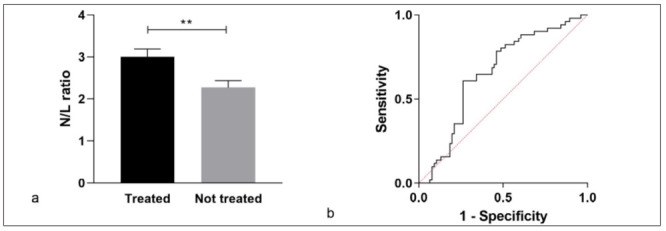
N/L ratio in sarcoidosis patients divided into a treated (T) and a non-treated group (NT).
No statistical differences of laboratory findings were reported in patients with different Scadding stages, as reported in Table 3. As suggested by Sweiss et al. (18), patients in chest X-ray stages 0 and 1 were grouped together, as were patients in stages 2, 3 and 4; the latter shared parenchymal involvement. Peripheral N/L ratio was significantly higher in stages 2, 3 and 4 than in stages 0-1 (median IQR, 1.98 (1.45-3.13) vs 2.6 (1.89-3.86), p=0.0090) (figure 2a). Receiver operating curve (ROC) analysis distinguished these two groups according to N/L ratio with an area under the curve (AUC) of 64% (95% CI 54-74) and a best cut-off value of 2.13 (70.4% sensitivity, 54.3% specificity) (figure 2b).
Table 3.
The main laboratory findings of sarcodiosis population dividing according to Scadding stages. All data were expressed as median IQR.
| Parameters | STAGE 0 | STAGE 1 | STAGE 2 | STAGE 3 | STAGE 4 |
| ACE | 47.0 (36-65) | 41.5 (28-47) | 50.0 (37-72.5) | 44.0 (39-57) | 61.0 (31-75) |
| CRP | 0.2 (0.1-0.7) | 0.21 (0.08-0.6) | 0.37 (0.13-1) | 0.26 (0.16-0.93) | 0.34 (0.24-0.56) |
| Hb | 13.4 (12.3-15.2) | 14.7 (12.6-15.4) | 13.9 (12-15.1) | 14.3 (13-14.6) | 14.1 (13-15.1) |
| WBC | 6.47 (5.8-7.9) | 6.21 (5.1-8.3) | 6.50 (5-7.9) | 7.20 (4.8-8.7) | 5.95 (5.3-7.2) |
| Neutro (%) | 57.70 (50.2-65.3) | 62.70 (54.1-611.6) | 63.30 (55-68) | 63.80 (53.5-63.4) | 62.70 (53.4-66.7) |
| Neutro (na) | 3.819 (2.9-4.7) | 3.672 (2.4-5.8) | 3.616 (3-5.2) | 4.594 (1.9-5.8) | 3.513 (3-4.5) |
| Linfo (%) | 30.30 (23.8-35.6) | 24.00 (18.1-30.8) | 24.20 (17.9-30.8) | 26.10 (20.9-30.3) | 23.25 (19-35) |
| Linfo (na) | 2.052 (1.5-2.9) | 1.540 (1-1.8) | 1.630 (1-2.2) | 1.879 (1.4-2.3) | 1.576 (1-2.3) |
| N/L RATIO | 1.904 (1.4-2.7) | 2.549 (1.8-3.9) | 2.504 (1.8-3.9) | 2.444 (1.7-3.2) | 2.807 (1.6-3.5) |
| Mono (%) | 8.6 (7.7-9.9) | 8.2 (7.2-10.9) | 9.6 (7.7-11.8) | 8.9 (7.8-10.1) | 10.2 (8.2-11.7) |
| Mono (na) | 0.57 (0.4-0.7) | 0.53 (0.44-0.59) | 0.6 (0.51-0.69) | 0.66 (0.44-0.66) | 0.59 (0.55-0.68) |
| Eos (%) | 1.9 (1.1-2.6) | 2.3 (1.9-5.5) | 2.5 (1.5-3.6) | 2.5 (2.2-3.6) | 2.3 (1.3-2.7) |
| Eos (na) | 0.12 (0.07-0.2) | 0.19 (0.10-0.29) | 0.15 (0.11-0.21) | 0.21 (0.13-0.24) | 0.12 0.07-0.16) |
| PLT | 258 (223-313) | 217 (195-219) | 242 (207-285) | 230 (205-274) | 236 (204-274) |
| creatinine | 0.84 (0.74-1) | 0.90 (0.73-0.99) | 0.85 (0.7-1) | 0.90 (0.67-0.97) | 0.86 (0.8-0.96) |
| Total bilirubin | 0.45 (0.3-0.6) | 0.50 (0.4-1) | 0.50 (0.4-0.7) | 0.40 (0.3-0.7) | 0.50 (0.4-0.6) |
| GOT | 19.5 (14.7-24.2) | 19.50 (14-21) | 20.00 (17-25) | 17.50 (15.2-23.7) | 20.50 (15-24) |
| GPT | 17.0 (13-24.5) | 20.00 (15-31) | 20.00 (14.2-26) | 16.00 (11.5-24) | 17.00 (15.5-22) |
Figure 2.
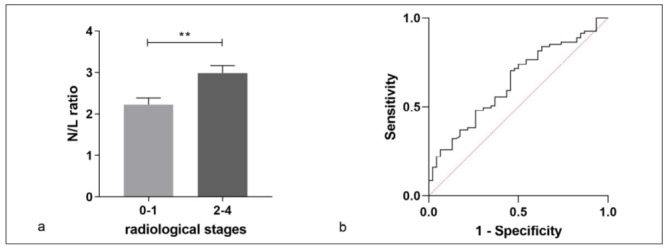
N/L ratio in sarcoidois patients in chest X-ray stages 0 and 1 were grouped together, as were patients in stages 2, 3 and 4
Discussion
Sarcoidosis is a heterogeneous granulomatous disease in which the definition of disease activity is unclear. Reliable biomarkers would be useful for clinical management (23–35). Wells et al. underlined the lack of a uniform treatment approach and suggested treating severe cases, evaluated on the basis of clinical symptoms, imaging and functional parameters (36).
It is difficult to identify the degrees of activity that can lead to organ damage. Here we analysed the clinical and immunological features of sarcoidosis and symptoms at onset in relation to follow-up, in the search for a routine non-invasive biomarker for use in clinical practice.
The demographic and epidemiological data of our population was in line with that of previous studies (37), except for the mean age of patients at diagnosis, which was higher than indicated in the literature (38). This was because in our population we selected chronic non-Lofgren syndrome population, usually younger than chronic patients. Lofgren patients are rarely referred to our Sarcoidosis Referral Centre, because they generally have a rapid evolution with good prognosis. Mostly, chronic and persistent sarcoidosis patients are referred to us.
In line with the literature, asymptomatic cases accounted for less than 30% of our population (38). In these cases, diagnosis is usually incidental, and the lack of symptoms could induce physicians to schedule less frequent follow-up. Our study demonstrated that, irrespective of the presence/absence of symptoms at disease onset, at the last examination in the study period, there were no differences in the therapeutic management between the two subgroups. Thus, our results suggest that clinical disease onset is not a reliable prognostic indicator and may reflect the need for intensive follow-up for all sarcoidosis patients in the first years of follow-up.
In our population we recorded a higher incidence of radiological evidence of lung involvement than in the ACCESS study (66% vs 53%), in line with the predominant percentage of chronic patients. Interestingly, we demonstrate that nearly all patients with Scadding stage 0 had HRCT evidence of lung/lymph-node involvement: our data is in line with recent studies and underlined the importance of HRCT scan for a more precise classification of sarcoid disease in respect with chest X-rays (39).
Moreover, fewer extra-thoracic manifestations were observed in our population than in the ACCESS study (32% vs 60%), but in line with most recent epidemiological studies (40,41). The incidence of comorbidities partially confirmed the results of Nowinski et al. who reported a higher incidence of arterial hypertension and cancer as comorbidities of sarcoidosis (42). Thyroid disorders and cancers are frequent comorbidities of sarcoidosis reported in the literature, as well as in our study, suggesting intriguing pathogenetic links to be further investigated in larger, multicentre prospective studies (43).
Analysis of routine laboratory features in our population allowed us to single out the N/L ratio as a marker with clinical potential for sarcoidosis. This marker was widely discussed in literature (38, 44, 45) and it could be added in a panel of biomarkers (including chitotriosidase, Krebs von den Lungen-6, sIL2R etc) to help physicians in the clinical management of sarcoidosis patients. In a population of 116 patients, Dirican et al. demonstrated the importance of blood parameters in sarcoidosis and reported a N/L ratio significantly higher in stage 2-3 than in stage 0-1 patients and controls (17). Our study confirms these findings, moreover including a subgroup of patients with fibrotic sarcoidosis.
Moreover, when we stratified our cohort according to therapeutic management, the N/L ratio was higher in the treated group than in non-treated patients. If the need for pharmacological therapy is considered an indicator of active sarcoidosis (recently called “burnt on” disease), the N/L ratio could therefore represent a useful disease activity marker. Interestingly, we demonstrated here that an elevated N/L ratio was due more to lymphopenia than to neutrophilia. Considering that lymphopenia did not depend on the drugs used for treatment, as suggested by Sweiss et al. (18) and confirmed by our findings, we believe that N/L ratio could be really helpful in the routinary clinical management of these patients, regardless their therapy status.
In conclusion, in our multicentre cohort study of clinical features and laboratory findings, we phenotyped sarcoidosis patients according to symptoms at onset, radiological stages and treatment requirements. Similar periodic follow-up can be suggested for symptomatic and asymptomatic sarcoidosis patients at onset. In the heterogeneous context of this disease, N/L ratio proved to be a useful and simple routine laboratory biomarker related to disease activity and need for treatment.
Conflict of interest:
The authors declared that they have no conflict of interest.
References
- 1.Baughman RP, Lower EE, Gibson K. Pulmonary manifestations of sarcoidosis. Presse Med. 2012 Jun;41(6 Pt 2):e289–302. doi: 10.1016/j.lpm.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc. 2007 Aug 15;4(5):465–8. doi: 10.1513/pats.200608-155MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake WP, Oswald-Richter K, Richmond BW, Isom J, Burke VE, Algood H, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol. 2013 Sep;149(9):1040–9. doi: 10.1001/jamadermatol.2013.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asakawa N, Uchida K, Sakakibara M, Omote K, Noguchi K, Tokuda Y, et al. Immunohistochemical identification of Propionibacterium acnes in granuloma and inflammatory cells of myocardial tissues obtained from cardiac sarcoidosis patients. PLoS ONE. 2017;12(7):e0179980. doi: 10.1371/journal.pone.0179980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007 Nov 22;357(21):2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 6.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999 Aug;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 7.Müller-Quernheim J, Schürmann M, Hofmann S, Gaede KI, Fischer A, Prasse A, et al. Genetics of sarcoidosis. Clin Chest Med. 2008 Sep;29(3):391–414, viii. doi: 10.1016/j.ccm.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Kreider ME, Christie JD, Thompson B, Newman L, Rose C, Barnard J, et al. Relationship of environmental exposures to the clinical phenotype of sarcoidosis. Chest. 2005 Jul;128(1):207–15. doi: 10.1378/chest.128.1.207. [DOI] [PubMed] [Google Scholar]
- 9.Baughman RP, Scholand MB, Rahaghi FF. Clinical phenotyping: role in treatment decisions in sarcoidosis. Eur Respir Rev. 2020 Mar 31;29(155) doi: 10.1183/16000617.0145-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thillai M, Chang W, Chaudhuri N, Forrest I, Ho L-P, Lines S, et al. Sarcoidosis in the UK: insights from British Thoracic Society registry data. BMJ Open Respir Res. 2019;6(1):e000357. doi: 10.1136/bmjresp-2018-000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Moorsel CHM. To progress understanding of disease triggers and modifiers in sarcoidosis, stratification is the key. Eur Respir J. 2017;50(6) doi: 10.1183/13993003.02002-2017. [DOI] [PubMed] [Google Scholar]
- 12.Consensus conference: activity of sarcoidosis. Third WASOG meeting, Los Angeles, USA, September 8-11, 1993. Eur Respir J. 1994 Mar;7(3):624–7. [PubMed] [Google Scholar]
- 13.Bonham CA, Strek ME, Patterson KC. From granuloma to fibrosis: sarcoidosis associated pulmonary fibrosis. Curr Opin Pulm Med. 2016;22(5):484–91. doi: 10.1097/MCP.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes H, Uzunhan Y, Gille T, Lamberto C, Valeyre D, Brillet P-Y. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur Respir J. 2012 Sep;40(3):750–65. doi: 10.1183/09031936.00025212. [DOI] [PubMed] [Google Scholar]
- 15.Keijsers RGM, Grutters JC. In Which Patients with Sarcoidosis Is FDG PET/CT Indicated. J Clin Med. 2020 Mar 24;9(3) doi: 10.3390/jcm9030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gungor S, Akturk UA, Yalcinsoy M, Kocak ND, Goksenoglu NC, Altunbey SA, et al. What is the neutrophil/lymphocyte ratio in sarcoidosis. Bratisl Lek Listy. 2016;117(3):152–5. doi: 10.4149/bll_2016_030. [DOI] [PubMed] [Google Scholar]
- 17.Dirican N, Anar C, Kaya S, Bircan HA, Colar HH, Cakir M. The clinical significance of hematologic parameters in patients with sarcoidosis. Clin Respir J. 2016;10(1):32–9. doi: 10.1111/crj.12178. [DOI] [PubMed] [Google Scholar]
- 18.Sweiss NJ, Salloum R, Gandhi S, Ghandi S, Alegre M-L, Sawaqed R, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS ONE. 2010 Feb 5;5(2):e9088. doi: 10.1371/journal.pone.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999 Aug;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 20.Keijsers RG, Veltkamp M, Grutters JC. Chest Imaging. Clin Chest Med. 2015 Dec;36(4):603–19. doi: 10.1016/j.ccm.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Schupp JC, Freitag-Wolf S, Bargagli E, Mihailović-Vučinić V, Rottoli P, Grubanovic A, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. 2018;51(1) doi: 10.1183/13993003.00991-2017. [DOI] [PubMed] [Google Scholar]
- 22.Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med. 2017 01;196(11):1463–72. doi: 10.1164/rccm.201710-1981ST. [DOI] [PubMed] [Google Scholar]
- 23.Bargagli E, Olivieri C, Rottoli P. Cytokine modulators in the treatment of sarcoidosis. Rheumatol Int. 2011 Dec;31(12):1539–44. doi: 10.1007/s00296-011-1969-9. [DOI] [PubMed] [Google Scholar]
- 24.Bargagli E, Magi B, Olivieri C, Bianchi N, Landi C, Rottoli P. Analysis of serum amyloid A in sarcoidosis patients. Respir Med. 2011 May;105(5):775–80. doi: 10.1016/j.rmed.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Bargagli E, Mazzi A, Mezzasalma F, Perrone A, Olivieri C, Prasse A, et al. The analysis of tryptase in serum of sarcoidosis patients. Inflammation. 2009 Oct;32(5):310–4. doi: 10.1007/s10753-009-9137-z. [DOI] [PubMed] [Google Scholar]
- 26.Bargagli E, Bianchi N, Margollicci M, Olivieri C, Luddi A, Coviello G, et al. Chitotriosidase and soluble IL-2 receptor: comparison of two markers of sarcoidosis severity. Scand J Clin Lab Invest. 2008;68(6):479–83. doi: 10.1080/00365510701854975. [DOI] [PubMed] [Google Scholar]
- 27.Bergantini L, Cameli P, d’Alessandro M, Vagaggini C, Refini RM, Landi C, et al. NK and NKT-like cells in granulomatous and fibrotic lung diseases. Clin Exp Med. 2019 Nov;19(4):487–94. doi: 10.1007/s10238-019-00578-3. [DOI] [PubMed] [Google Scholar]
- 28.Bergantini L, Bianchi F, Cameli P, Mazzei MA, Fui A, Sestini P, et al. Prognostic Biomarkers of Sarcoidosis: A Comparative Study of Serum Chitotriosidase, ACE, Lysozyme, and KL-6. Dis Markers. 2019. 2019:8565423. doi: 10.1155/2019/8565423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameli P, Gonnelli S, Bargagli E, d’Alessandro M, Bergantini L, Favetta V, et al. The Role of Urinary Calcium and Chitotriosidase in a Cohort of Chronic Sarcoidosis Patients. Respiration. 2020;99(3):207–12. doi: 10.1159/000505653. [DOI] [PubMed] [Google Scholar]
- 30.d’Alessandro M, Carleo A, Cameli P, Bergantini L, Perrone A, Vietri L, et al. BAL biomarkers’ panel for differential diagnosis of interstitial lung diseases. Clin Exp Med. 2020 May;20(2):207–16. doi: 10.1007/s10238-020-00608-5. [DOI] [PubMed] [Google Scholar]
- 31.d’Alessandro M, Bergantini L, Perrone A, Cameli P, Cameli M, Prasse A, et al. Serial investigation of Angiotensin-Converting Enzyme in sarcoidosis patients treated with Angiotensin-Converting Enzyme Inhibitor. Eur J Intern Med. 2020 Apr 16 doi: 10.1016/j.ejim.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 32.d’Alessandro M, De Vita E, Bergantini L, Mazzei MA, di Valvasone S, Bonizzoli M, et al. Galactin-1, 3 and 9: Potential biomarkers in idiopathic pulmonary fibrosis and other interstitial lung diseases. Respir Physiol Neurobiol. 2020 Nov;282:103546. doi: 10.1016/j.resp.2020.103546. [DOI] [PubMed] [Google Scholar]
- 33.Bergantini L, d’Alessandro M, Vietri L, Rana GD, Cameli P, Acerra S, et al. Utility of serological biomarker’ panels for diagnostic accuracy of interstitial lung diseases. Immunol Res. 2020;68(6):414–21. doi: 10.1007/s12026-020-09158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d’Alessandro M, Bergantini L, Cameli P, Vietri L, Lanzarone N, Alonzi V, et al. Krebs von den Lungen-6 as a biomarker for disease severity assessment in interstitial lung disease: a comprehensive review. Biomark Med. 2020 Jun;14(8):665–74. doi: 10.2217/bmm-2019-0545. [DOI] [PubMed] [Google Scholar]
- 35.Cameli P, Caffarelli C, Refini RM, Bergantini L, d’Alessandro M, Armati M, et al. Hypercalciuria in Sarcoidosis: A Specific Biomarker With Clinical Utility. Front Med (Lausanne) 2020;7:568020. doi: 10.3389/fmed.2020.568020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouranos V, Jacob J, Wells AU. Severe Sarcoidosis. Clin Chest Med. 2015 Dec;36(4):715–26. doi: 10.1016/j.ccm.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999 Oct;14(4):735–7. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 38.Valeyre D, Bernaudin J-F, Uzunhan Y, Kambouchner M, Brillet P-Y, Soussan M, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Respir Crit Care Med. 2014 Jun;35(3):336–51. doi: 10.1055/s-0034-1381229. [DOI] [PubMed] [Google Scholar]
- 39.Ocal N, Dogan D, Ocal R, Tozkoparan E, Deniz O, Ucar E, et al. Effects of radiological extent on neutrophil/lymphocyte ratio in pulmonary sarcoidosis. Eur Rev Med Pharmacol Sci. 2016;20(4):709–14. [PubMed] [Google Scholar]
- 40.Zurkova M, Kolek V, Tomankova T, Kriegova E. Extrapulmonary involvement in patients with sarcoidosis and comparison of routine laboratory and clinical data to pulmonary involvement. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014 Dec;158(4):613–20. doi: 10.5507/bp.2014.026. [DOI] [PubMed] [Google Scholar]
- 41.Li C-W, Tao R-J, Zou D-F, Li M-H, Xu X, Cao W-J. Pulmonary sarcoidosis with and without extrapulmonary involvement: a cross-sectional and observational study in China. BMJ Open. 2018 16;8(2):e018865. doi: 10.1136/bmjopen-2017-018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowinski A, Puscinska E, Goljan-Geremek A, Bednarek M, Kaminski D, Gorecka D. Comorbidities associated with sarcoidosis – Results from long-term observational study. European Respiratory Journal [Internet] 2014 Sep 1 [cited 2020 May 22];44(Suppl 58). Available from: https://erj.ersjournals.com/content/44/Suppl_58/P461 . [Google Scholar]
- 43.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007 Jun;25(3):326–33. doi: 10.1016/j.clindermatol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Iliaz S, Iliaz R, Ortakoylu G, Bahadir A, Bagci BA, Caglar E. Value of neutrophil/lymphocyte ratio in the differential diagnosis of sarcoidosis and tuberculosis. Ann Thorac Med. 2014 Oct;9(4):232–5. doi: 10.4103/1817-1737.140135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farkas JD. The complete blood count to diagnose septic shock. J Thorac Dis. 2020 Feb;12(Suppl 1):S16–21. doi: 10.21037/jtd.2019.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]


