Abstract
Behçet disease (BD) is a systemic disorder caused by underlying vasculitis of unknown origin. In this paper we present a case of a 26-year-old male patient who was admitted at our Emergency Department with massive haemoptysis due to pulmonary arterial involvement in BD. The discussion of this case helps to remember that BD is the main cause of aneurysm of the pulmonary arteries and a cause of haemoptysis in young patients. Therefore, the radiologist plays a key role in the identification of intrathoracic alterations with chest computed tomography. The knowledge of clinical manifestations and distinctive elements of BD allow an accurate diagnosis and let the patient to be directed towards an appropriate treatment, in order to avoid the onset of life-threatening complications.
Keywords: Systemic vasculitis, Computed tomography, Behçet disease, Emergency
Behçet disease (BD) is a rare systemic inflammatory vasculitis of unknown origin, even if is thought to be an auto-immune process triggered by an infectious or environmental agent in a genetically predisposed individual. Human leukocyte antigen (HLA)-B51 is recognized as the principal genetic predisposing factor. This disease mostly affects middle Asian and Mediterranean populations with a various incidence, between 1:1000 in Turkey to 1:10.000 in Eastern Asia. During the past decades due to immigrations, the prevalence in Europe and North America has gradually increased (1–3). It can affect both genders, although in male the alterations tend to be more severe, and the mean age at the onset is the third decade (4, 5).
As a multisystem pathology, BD alterations can involve nearly all systems of the body. Actually, the most frequently BD manifestations are dermatological (20-50%) and neurological (with ocular) (5-30%) while pulmonary vascular involvement is rare and often fatal (6, 7). BD manifestations are self-limiting, but recurrent. Clinical manifestations of BD can involve nearly all systems of the body, but the major manifestations are, respectively, oral and genital ulcers, skin manifestations as pseudo-folliculitis (also called Behçet’s pustulosis) and erythema nodosum, and ophthalmological involvement (as anterior or posterior uveitis and retinal vasculitis). Minor manifestations include joint involvement (which varies from arthralgia to arthritis/spondylo-arthritis in more than 50% of patients), vascular, neurological and gastrointestinal manifestations. Pleuropulmonary and cardiac manifestations are rarer (1.8%) (7). BD diagnosis is usually based on clinical criteria. The International Study Group (ISG) criteria were the most used in the diagnosis of BD since 1990s but were found to have low sensibility and specificity. National Criteria for Behçet disease (ICBD) was developed in 2006 and revised in 2010 in order to improve the sensitivity of ISG ones (8, 9) (Table 1).
Table 1.
International Criteria for Behçet disease (ICBD 2006) and International Study Group criteria (ISG 1990).
| ICBD (2006) | ISG (1990) | |
| Sign/Symptoms | Points | Recurrent oral ulceration Plus 2 of the following:
|
| Ocular injuries | 2 | |
| Genital ulceration (recurrent) | 2 | |
| Oral ulceration (recurrent) | 2 | |
| Skin injuries | 1 | |
| Neurological manifestations | 1 | |
| Vascular manifestations | 1 | |
| Pathergy test | 1 | |
| Behçet disease à 3 or more points (sensitivity 96%; specificity 88%; accuracy 93.8%) | ||
Diagnosis is clinical, based on the association of certain signs and symptoms, but an evaluation through imaging can improve the diagnostic accuracy and speed up the diagnostic process.
Case Report
A 26-year-old Peruvian man was admitted to the Emergency Department (ED) of our Institution because of the rapid onset of cough and haemoptysis. He had a history of chronic bilateral femoral deep vein thrombosis, for which he was actually treated with anticoagulant therapy. Previous genetic tests had shown the absence of Factor II and Factor V Leiden mutations. On the day of the admission he was afebrile, and his systemic examination revealed aphthous genital ulcerations. An echocardiography showed a right atrial thrombus. A computed tomography (CT) angiography of the pulmonary arterial vessels and thoracic aorta, performed in order to reveal the cause of haemoptysis, didn’t find any bronchial vessels alterations but confirmed the presence of an atrial thrombus and revealed huge bilateral pulmonary artery aneurysms filled with coarse thrombotic formations (Figs. 1-3).
Figure 1.
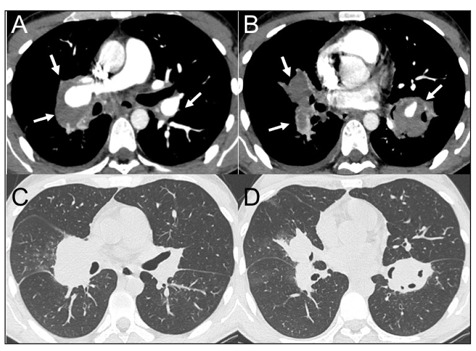
Massive thrombi in pulmonary arteries. Figures in A – B shows CT scans of huge parietal thrombi within pulmonary artery aneurysms (arrows). Figures in C – D are parenchymal axial reconstructions of the same slices.
Figure 3.
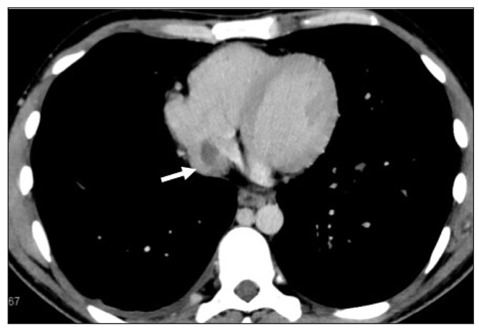
Cardiac thrombus. Axial (A) and coronal (B) CT scans show the presence of an atrial thrombus (arrows).
Figure 2.
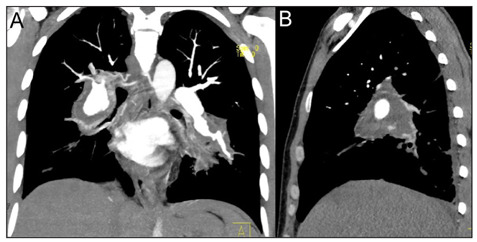
Coronal (A) and sagittal (B) CT reconstruction of the macroscopic pulmonary artery aneurysms.
The irregular massive dilatations involved both the main pulmonary arteries and their major branches, especially the ones for lower lobes, and reached a maximum axial diameter of 35 mm. The multiple thrombi obstructed mostly of the vessels lumen which were rarely and only in part rehabilitated. No other chest alterations were found except for a limited right pleural effusion. Unfortunately, the massive haemoptysis drove quickly the patient into cardiorespiratory failure, and he died soon after his admission in ED. An autopsy was performed, and it confirmed the presence of histopathological alterations plausible for Behçet disease (Figs. 4, 5).
Figure 4.
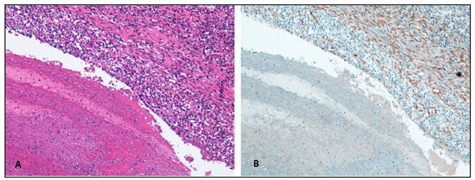
Histopathological findings of Behçet Syndrome’s pulmonary manifestations. (A) Aneurismatic dilatation of a large size branch of pulmonary artery with a thrombotic formation and a dense parietal inflammatory infiltrate (100x Hematoxilin-Eosin). (B) Irregularity and fragmentation of the arterial elastic tissue (100x Smooth Muscle Actin stain, 1A4 clone ©CELL MARQUE).
Figure 5.
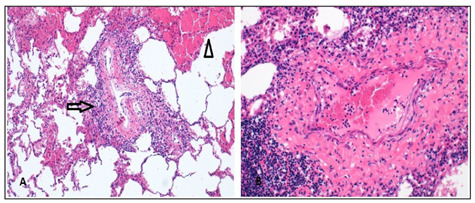
Histopathological findings of Behçet Syndrome’s pulmonary manifestations. (A) A medium size branch of pulmonary artery with inflammatory infiltrate (arrow) nearby an area of acute infarct (arrow-head) (B) The vasculitis is constituted by a dense lymphocytic infiltrate (200x Hematoxilin-Eosin).
Discussion
When approaching massive hemoptysis, especially in young patients, it has to be remembered that more than 90% of cases the source of bleeding is the bronchial artery and the etiology can be various: trauma (lung contusion, airway trauma), infections (tuberculosis, aspergilloma), bronchiectasis (cystic fibrosis), primary or secondary malignant lung alterations (bronchogenic carcinoma, metastasis). Other rarer causes could be Goodpasture syndrome, BD, granulomatosis with polyangiitis or arteriovenous malformations (10–12). After having excluded a non-pulmonary cause of bleeding (such as upper airways or gastrointestinal tract), a bronchoscopy is needed to confirm and localize the site of bleeding. CT imaging can help a characterization of lesion and CT angiography of the thoracic aorta provides definitions of the vessels, especially bronchial ones (8). Of course, CT angiography helps also in the identification of pulmonary embolism, that is one of the main causes of hemoptysis. Instead, BD is one of the rarer causes of hemoptysis. In particular, vascular alterations manifest in about 18% of BD patients but they are the most common cause of mortality: they include inflammation of the veins that leads to deep thrombosis, inflammation of the arteries that causes arterial narrowing and aneurysmal dilatation (6, 7). BD is the most common cause of pulmonary artery aneurysm as the chronic inflammation of the vasa vasorum of the tunica media causes destruction of the elastic structures and parietal dilatation (6). Pulmonary artery thrombosis in BD is usually in situ and pulmonary embolism is rare because, though the presence of deep vein thrombosis, the thrombi in the inflamed veins of the legs are strongly adherent (11, 13). Pulmonary arterial involvement usually manifests as haemoptysis caused by rupture of an aneurysm or by thrombosis of pulmonary vessels (1). Usually this manifestation occurs during the ongoing evolution of the disease and rarely are the first ones. Unfortunately, aneurysms of the pulmonary arteries are a clear sign of poor prognosis and about 30% of these patients die within two years (14). There are similarities between vascular manifestations of BD and those of another disease, Hughes-Stovin syndrome (HSS). It is a rare disorder of unknown aetiology characterized by the association of deep venous thrombosis and pulmonary artery aneurysms (with mural thrombosis) without any other manifestations of BD (15–17). HSS is also known as “incomplete Behçet disease” and its radiologic findings are indistinguishable from those of BD. Some authors have suggested that HSS could be a partial manifestation of a real BD (18). Cardiac involvement is seen in 1-16% (2, 19). Its manifestations include endomyocardial fibrosis, myocarditis, endocarditis, pericarditis and intracardiac thrombosis where right heart is the most common site of involvement (2, 20–25). Pulmonary parenchymal involvement is very rare, while pleural effusion may be attributed to vasculitis of the pleura or pulmonary infarction or superior vena cava thrombosis (1, 2, 6, 7). Chest radiography alterations in thoracic involvement in BD are unspecific: focal or diffuse lung opacities, hilar and mediastinal enlargement (6). CT angiography is the gold standard for diagnosis and evaluation of patients with BD thoracic involvement as it provides excellent delineation of the vessel’s lumen as well as detailed information about mediastinal structures and lung parenchyma (6, 26). Our patient, according to the history and to the physical examination, fitted the diagnostic criteria for BD, and he was promptly suspected as having this disease. The echocardiography and thoracic CT angiography performed soon after, helped to confirm the diagnosis in the shortest time. Unfortunately, the presentation of BD at our ED was massive also in relation to the pre-existing anticoagulant therapy and although a prompt diagnosis, death occurs.
At our advice this case needs to be mentioned as it emphasizes the necessity of taking BD into account in the differential diagnosis of haemoptysis in young patients, as a prompt immune-depressing therapy can save the life in the acute setting in more than 60% of these cases.
Conflict of Interest
The authors declare that they have no conflict of interest related to the publication of this article.
Ethical Approval
All procedures in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Contribution Statement
Authors DC, CM, EC, AB, MCB, CF, VM: equal contribution to manuscript editing, writing, data collection.
References
- 1.Erkan F, Gul A, Tasali E. Pulmonary manifestations of Behçet disease. Thorax. 2001;56:572–578. doi: 10.1136/thorax.56.7.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae EJ, Do KH, Seo JB, Park SH, Kang JW, Jang YM, et al. Radiological and clinical findings of Behçet disease: comprehensive review of multisystemic involvement. Radiographics. 2008 doi: 10.1148/rg.e31. 10.1148/rg.e31. [DOI] [PubMed] [Google Scholar]
- 3.Verity DH, Wallace GR, Vaughan RW, Stanford MR. Behçet disease: from Hippocrates to the third millennium. Br J Ophtalmol. 2003;87:1175–83. doi: 10.1136/bjo.87.9.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, et al. Behçet disease: from east to west. Clin Rheumatol. 2010;29:823–833. doi: 10.1007/s10067-010-1430-6. [DOI] [PubMed] [Google Scholar]
- 5.Davatchi F. Behçet disease. Int J Rheum Dis. 2014;17(4):355–357. doi: 10.1111/1756-185X.12378. doi: 10.1111/1756-185X.12378. [DOI] [PubMed] [Google Scholar]
- 6.Hiller N, Lieberman S, Chajek-Shaul T, et al. Thoracic manifestations of Behçet disease at CT. Radiographics. 2004;24:801–8. doi: 10.1148/rg.243035091. doi: 10.1148/rg.243035091. [DOI] [PubMed] [Google Scholar]
- 7.Tunaci A, Berkmen YM, Gokemen E. Thoracic involvement in Behçet disease: pathologic, clinical and imaging features. AJR Am J Roentgenol. 1995;164:51–56. doi: 10.2214/ajr.164.1.7998568. [DOI] [PubMed] [Google Scholar]
- 8.Criteria for diagnosis of Behçet disease. International Study Group for Behçet disease. Lancet. 1990;335(8697):1078–1080. [PubMed] [Google Scholar]
- 9.International Team for the Revision of the Internationa Criteria for Behçet Disease (ITR-ICBD) The International Criteria for Behçet Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–47. doi: 10.1111/jdv.12107. doi: 10.1111/jdv.12107. [DOI] [PubMed] [Google Scholar]
- 10.Bruzzi JF, Rémy-Jardin M, Delhaye D, et al. Multi-detector row CT of hemoptysis. Radiographics. 2006;26(1):3–22. doi: 10.1148/rg.261045726. doi: 10.1148/rg.261045726. [DOI] [PubMed] [Google Scholar]
- 11.Ong ZY, Chai HZ, How CH, Koh J, Low TB. A simplified approach to hemoptysis. Singapore Med J. 2016;57(8):415–8. doi: 10.11622/smedj.2016130. doi: 10.11622/smedj.2016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borowiec A, Hadzik-Blaszczyk M, Kowalik I, et al. High incidence of venous thromboembolism but not of coronary artery disease in granulomatosis with polyangiitis in first years after diagnosis. Sarcoidosis Diff Lung Dis. 2019;36(3):202–208. doi: 10.36141/svdld.v36i3.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamuryudan V, Yurdakul S, Moral F, et al. Pulmonary artery aneurysms in Behçet syndrome: report of 24 cases. Br J Rheumathol. 1994;33:48–51. doi: 10.1093/rheumatology/33.1.48. [DOI] [PubMed] [Google Scholar]
- 14.Xing W, Swaminathan G, Raj Appadorai D, Sule AA. A rare case of Behçet disease presenting with pyrexia of unknown origin, pulmonary embolism and right ventricular thrombus. Int J Angiol. 2013;22:193–8. doi: 10.1055/s-0033-1347906. doi: 10.1055/s-0033-1347906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erkan F. Pulmonary involvement in Behçet disease. Curr Opin Pulm Med. 1999;5:314–318. doi: 10.1097/00063198-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Durieux P, Bletry O, Huchon G, et al. Multiple pulmonary arterial aneurysms in Behçet disease and Hughes-Stovin syndrome. Am J Med. 1981;71:736–41. doi: 10.1016/0002-9343(81)90245-x. [DOI] [PubMed] [Google Scholar]
- 17.Eliker BM, Webb WR. Wolters & Kluwer – Lippincott Williams & Wilkins; 2013. Fundamentals of high-resolution lung CT; p. 114. Section 2, chapter 7. [Google Scholar]
- 18.Erkan F, Yazici Y, Sanders A, et al. Is Huges-Stovin syndrome Behçet disease. Clin Exp Rheumthol. 2004;22:S64–S68. [PubMed] [Google Scholar]
- 19.Huong DL, Wechsler B, Papo T, et al. Endomyocardial fibrosis in Behçet Disease. Ann Rheum Dis. 1997;56:205–208. doi: 10.1136/ard.56.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atzeni F, Sarzi-Puttini P, Doria A, et al. Behçet Disease and cardiovascular involvement. Lupus. 2005;14:723–726. doi: 10.1191/0961203305lu2208oa. [DOI] [PubMed] [Google Scholar]
- 21.Cozzi D, Dini C, Mungai F, Puccini B, Rigacci L, Miele V. Primary pulmonary lymphoma: imaging findings in 30 cases. Radiol Med. 2019;124(12):1262–1269. doi: 10.1007/s11547-019-01091-z. [DOI] [PubMed] [Google Scholar]
- 22.Houman M, Ksontini I, Ben Ghorbel I, et al. Association of right heart thrombosis, endomyocardial fibrosis, and pulmonary artery aneurysm in Behçet disease. Eur J Intern Med. 2002;13:455. doi: 10.1016/s0953-6205(02)00134-6. [DOI] [PubMed] [Google Scholar]
- 23.Cozzi D, Moroni C, Addeo G, Danti G, Lanzetta MM, Cavigli E, et al. Radiological patterns of lung involvement in inflammatory bowel disease. Gastroenterol Res Pract. 2018; Aug 12:5697846. doi: 10.1155/2018/5697846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marc K, Iraqui G, Jniene A, et al. Intracardiac thrombus and pulmonary artery aneurysms in Behçet disease. Rev Mal Respir. 2008;25(1):69–72. doi: 10.1016/s0761-8425(08)70469-8. [DOI] [PubMed] [Google Scholar]
- 25.Alexandre AT, Vale A, Gomes T. Diffuse alveolar hemorrhage: how relevant is etiology. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36(1):47–52. doi: 10.36141/svdld.v36i1.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emad Y, Abdel-Razek N, Gheita T, et al. Multislice CT pulmonary findings in Behçet Disease (report of 16 cases) Clin Rheumatol. 2007;26(6):879–884. doi: 10.1007/s10067-006-0408-x. [DOI] [PubMed] [Google Scholar]


