Abstract
Background
Coronavirus disease 2019 (COVID-19) elicits robust inflammatory reaction that may result in a declining albumin serum level. This meta-analysis aimed to evaluate the prognostic properties of hypoalbuminemia for poor prognosis and factors that may influence the relationship.
Method
A systematic literature search of PubMed was conducted from inception to April 22, 2021. The main exposure was albumin level below normal range–defined by the included studies. The outcome of interest was composite poor outcome that comprises of mortality, severity, and the requirement of mechanical ventilation or intensive care unit.
Results
There were 6200 patients from 19 studies. Meta-analysis showed that hypoalbuminemia was associated with composite poor outcome (OR 6.97 (95% CI 4.20–11.55), p < 0.001; I2 = 91.3%, p < 0.001). Meta-regression analysis showed that age (p = 0.44), gender (p = 0.76), HT (p = 0.97), DM (p = 0.40), CKD (p = 0.65), liver disease (p = 0.72), and malignancy (p = 0.84) did not affect the association. Subgroup analysis showed that hypoalbuminemia increased mortality (OR 6.26 (95% CI 3.26–12.04), p < 0.001; I2 = 69.6%, p < 0.01) and severity of the disease (OR 7.32 (95%CI 3.94–13.59), p < 0.001; I2 = 92.5%, p < 0.01). Pooled diagnostic analysis of hypoalbuminemia yielded a sensitivity of 0.63 (95% CI 0.52–0.72), specificity of 0.81 (95% CI 0.73–0.87), and AUC of 0.77. The probability of poor outcome was 70% in patients with hypoalbuminemia and 24% in patients with normal albumin level.
Conclusion
Hypoalbuminemia was associated with poor prognosis in COVID-19 patients.
Keywords: Albumin, COVID-19, Hypoalbuminemia, Prognosis
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has infected millions of people worldwide. The high prevalence of the disease followed by long incubation periods of the virus yields an unprecedented pandemic with an increasing number of cases across countries making COVID-19 the worst pandemic outbreak in modern history [1,2]. Multidimensional strategies have been taken with various objectives–from prevention of viral transmission to prevent the complication of the disease.
COVID-19 has wide spectrums of severity and clinical manifestation of the illness. Patients are varied from asymptomatic to severely ill that requires hospitalization [1,3]. The progression of the disease is also ranging and confounded by a multitude of factors. Early steps to mitigate disease progression by assessing risk stratification are needed to prevent adverse outcomes. Utilizing biomarkers especially that are regularly measured to the patients is paramount to assess risks and allocate resources efficiently [4,5]. Previous studies have been done to investigate the correlation of blood serum parameters and disease severity and the outcomes. Albumin level has been studied extensively of its association with inflammatory process [6,7]. The level of human serum albumin has been shown to have a negative correlation with disease severity in various chronic illnesses [[7], [8], [9]]. In addition, a previous study showed disease severity and adverse outcomes were significantly associated with lower serum albumin concentration [10]. However, the prognostic value of albumin and whether the value is influenced by various comorbidities remains unknown. This present meta-analysis aimed to evaluate the prognostic properties of hypoalbuminemia in patients with COVID-19.
2. Material and methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRIMSA) [11]. A detailed protocol has been previously registered in PROSPERO (CRD42021238733).
2.1. Eligibility criteria
The PECO (Population, Exposure, Comparison, Outcome) structure design was used to define the inclusion and exclusion criteria of exposure and outcomes. The inclusion criteria were research articles and letters that report COVID-19 patients with information on Albumin serum level (categorical) along with measurable outcomes–mortality/severity/critically ill/ICU admission/invasive mechanical ventilation (IMV).
Following types or article were excluded: case report, non-research letter, editorial, invited commentary, review, abstract-only article, and preprint. Also, studies reporting only continuous variable of albumin serum level were excluded. There was no language restriction applied in this study.
2.2. Search strategy and study selection
A systematic search of PubMed and Cochran Collaboration Central Register of Controlled Clinical Trials were performed from the inception to 22nd of April 2021 by two independent investigators (NNMS and FAD). The following search terms were used (“Albumins"[Mesh] OR “Serum Albumin"[Mesh] OR Hypoalbuminemia[tiab] OR Albumin[tiab]) AND (“COVID-19"[Mesh] OR “SARS-CoV-2"[Mesh] OR COVID-19[tiab] OR COVID19[tiab] OR Coronavirus[tiab]). We used ‘related articles’ feature and hand searched the reference lists of the included articles to expand the search and obtain additional studies. Duplicate results were removed after the initial search.
2.3. Data extraction
Data extraction was conducted independently by two authors (NNMS and FAD). Standardized forms that included author, year, study design, sample size, cut-off value, age, gender, measurable outcome, hypertension (HT), diabetes mellitus (DM), chronic kidney disease (CKD), liver disease, and malignancy. Different perception in extracting data or eliminating possible duplicates were resolved through discussion and consensus.
The primary exposure was hypoalbuminemia as the level of albumin serum was below the specified cut-off defined by each individual study. The measured outcome of interest was composite poor outcome that comprised of mortality, severe COVID-19, critically ill COVID-19, ICU admission, disease progression, and adverse effects from the given nutritional support. Severe COVID-19 was defined as patients who met the criteria of respiratory distress (respiratory rate ≥ 30 breaths/min); pulse oxygen saturation ≤93% on room air; and low arterial oxygenation ratio (PaO2/fraction of inspired oxygen ≤ 300). Subsequently, patients were considered as critically ill if they had respiratory failure requiring a form of mechanical ventilation; shock; or had complications with other organ failure that require monitoring and treatment in the intensive care unit (ICU).
We used Newcastle–Ottawa Scale (NOS) to assess the quality of included studies. Following aspects were taken into consideration in the assessment: cohort selection, the comparability of cohort on the basis of the design or analysis, the way exposure is determined, and the way of outcomes of interest are evaluated. Discrepancies of perception were resolved by discussion.
2.4. Statistical analysis
All statistical analysis was performed using R (version 4.0.4, The R Foundation, Vienna, Austria). The incidence of poor composite outcome and hypoalbuminemia was pooled using meta-analysis of proportion. Also, ORs were calculated using DerSimonian and Laird method random-effects model. The inconsistency index (I 2) and subgroup analysis using Chi-square test were used to explore potential sources of heterogeneity. An I 2 of more than 50% and p value of less than 0.05 were considered as significant for heterogeneity [12]. Random effects mode was used regardless of the heterogeneity value. Meta-regression was also conducted with restricted-maximum likelihood random effects with age, gender, and comorbidities (HT, DM, CKD, Liver disease, and malignancy).
Diagnostic meta-analysis was done through calculating pooled sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR). Pooled sensitivity and specificity were displayed using forest plot and summary receiver operating characteristics (SROC) curve [13]. Fagan's nomogram was also used to present the prediction of post-test probability from pre-test probability. We also performed Deek's funnel plot asymmetry test to identify publication bias [14].
3. Results
3.1. Study selection
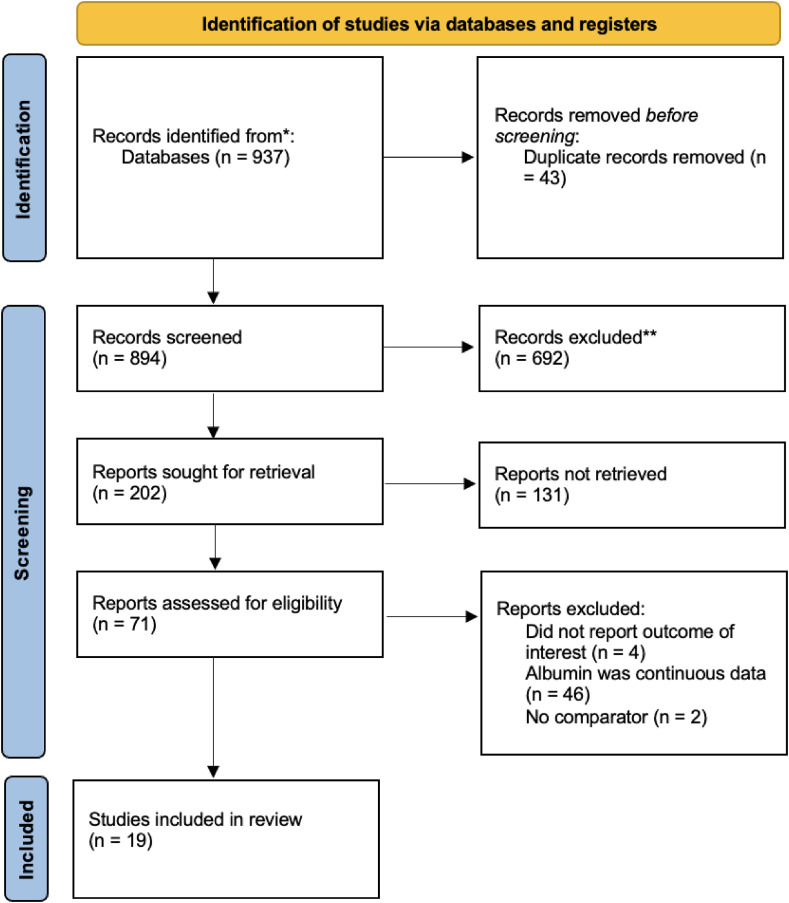
The selection to obtain included studies in the systematic review is shown in (Fig. 1 ). The initial search yielded 937 articles from PubMed and Cochran Collaboration Central Register of Controlled Clinical Trials. We found a total of 894 records remained after duplicates were removed and 202 articles after title and abstract screening. A total of 132 records were excluded after screening the title and abstract. After assessing 71 articles for the eligibility, we excluded 52 articles in which the studies did not report the outcome of interest, the reported albumin value was continuous data, and no comparator was reported. 19 articles met the eligibility criteria and were included in the systematic review and meta-analysis [8,9,[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]].
Fig. 1.
PRISMA flow of study.
3.2. Characteristics of included studies
The characteristics of the included studies are available in Table 1 . There are 6200 patients from 19 studies included in our systematic review and meta-analysis. All patients were adult confirmed COVID-19 cases. All the included studies were observational studies and were from, China, France, and Italy. There was a variability of cut offs used in between studies. However, the amount of study using each of different cut offs was inadequate to perform diagnostic meta-analysis with different cut points to generate optimal cut off value. In addition, we extracted the number of comorbidities reported in the included studies cumulatively.
Table 1.
Characteristics of included studies.
| Author | Design | Samples | Cut-off (g/L) | Age (years) | Male (%) | Outcome | HT (%) | DM (%) | CKD (%) | Liver Disease (%) | Malignancy (%) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bassoli et al., 2021 [15] | Retrospective Cohort | 207 | 30 | 61 | 68.6 | Severity | 34.3 | 13.1 | NA | NA | NA | 7 |
| Chen (1) et al., 2020 [16] | Retrospective Cohort | 274 | 32 | 62 | 62 | Death | 34 | 17 | 1 | 4 | 3 | 8 |
| Liu et al., 2020 [17] | Retrospective Cohort | 12 | 40 | 59 | 67 | Severity | 25 | 16.7 | 16.7 | 0 | 0 | 7 |
| Chen (2) et al., 2020 [18] | Retrospective Cohort | 21 | 32 | 56 | 81 | Severity | 23.1 | 14.1 | NA | NA | NA | 7 |
| Deng et al., 2020 [19] | Retrospective Cohort | 65 | 40 | 35 | 55 | Severity | 4.6 | 3.1 | NA | NA | 1.53 | 8 |
| Gao et al., 2020 [20] | Retrospective Cohort | 210 | 30 | 71 | 48 | Death | 55 | 18 | 9 | 9 | 3 | 8 |
| Hirashima et al., 2020 [21] | Retrospective Cohort | 8 | 32 | 65 | 87.5 | Severity | 62.5 | 62.5 | NA | NA | NA | 7 |
| Hu et al., 2020 [22] | Retrospective Cohort | 40 | 35 | 51 | 60 | Severity | 32.5 | 15 | NA | NA | 2.5 | 8 |
| Huang et al., 2020 [23] | Retrospective Cohort | 299 | 35 | 53 | 53 | Severity & Death | 24.7 | 11.7 | NA | NA | 3 | 7 |
| Hundt et al., 2020 [8] | Retrospective Cohort | 1827 | 35 | 65 | 53 | Severity | NA | 39 | NA | NA | NA | 8 |
| Lei et al., 2020 [24] | Retrospective Cohort | 115 | 35 | 66 | 50 | Severity | NA | NA | NA | NA | NA | 8 |
| Li et al., 2020 [25] | Retrospective Cohort | 523 | 35 | 54 | 48 | Severity & Death | 25. | 18 | NA | NA | NA | 8 |
| Ma et al., 2020 [26] | Retrospective Cohort | 523 | 30 | 44 | 55.26 | Severity & Death | 15.9 | 9.1 | 1.63 | NA | 3.04 | 8 |
| Nakamura et al., 2020 [27] | Retrospective Cohort | 32 | 30 | 75 | 69 | Death | 41 | 22 | NA | NA | 100 | 7 |
| Pan et al., 2020 [28] | Retrospective Cohort | 124 | 28.2 | 68 | 68.5 | Death | 50 | 20.2 | NA | NA | NA | 8 |
| Shi et al., 2020 [29] | Retrospective Cohort | 87 | 33 | 60 | 56.3 | Severity | NA | NA | 1.8 | NA | 2.3 | 7 |
| Wang et al., 2020 [30] | Retrospective Cohort | 275 | 45 | 49 | 46.5 | Severity | 19.6 | 6.2 | 1.5 | 2.2 | 0.7 | 7 |
| Yu et al., 2020 [31] | Retrospective Cohort | 1443 | 35 | 64 | 50.4 | Severity | 20.9 | 14.7 | 1.9 | 2.3 | 1.1 | 7 |
| Zhang et al., 2020 [9] | Retrospective Cohort | 115 | 40 | 50 | 42.6 | Severity | NA | NA | NA | 0 | NA | 7 |
3.3. Hypoalbuminemia and poor outcomes
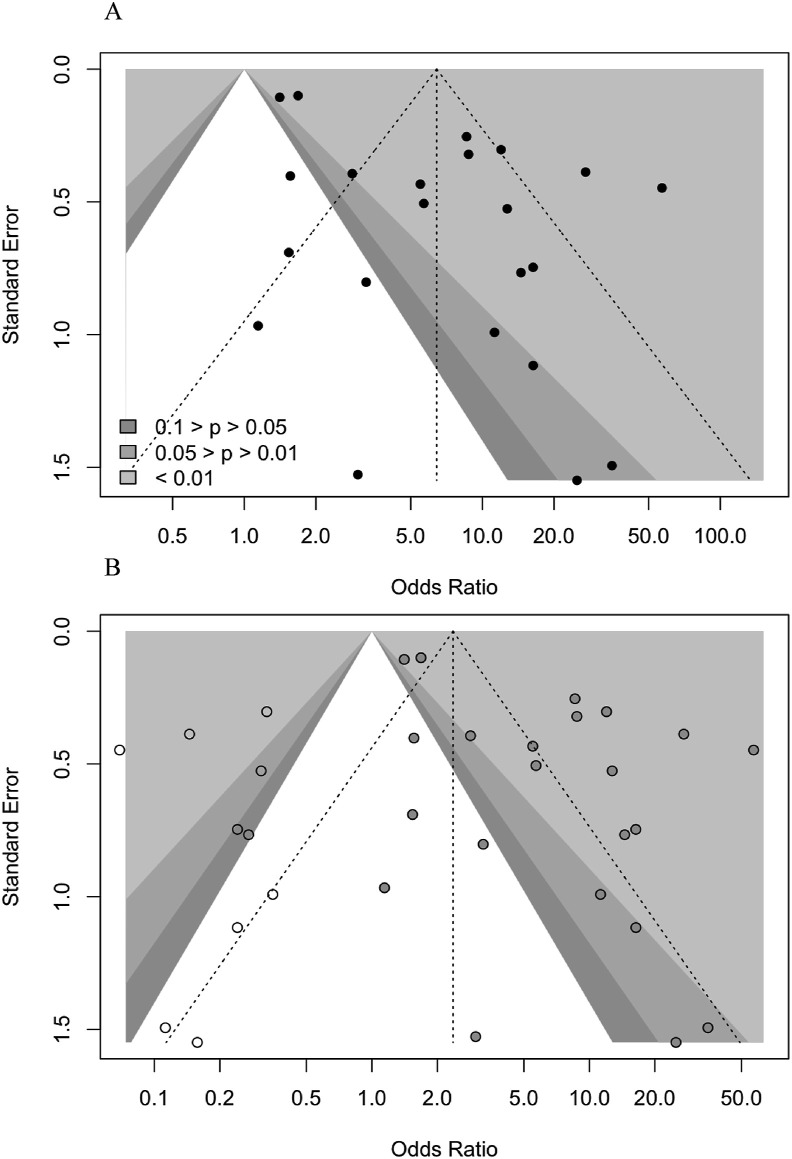
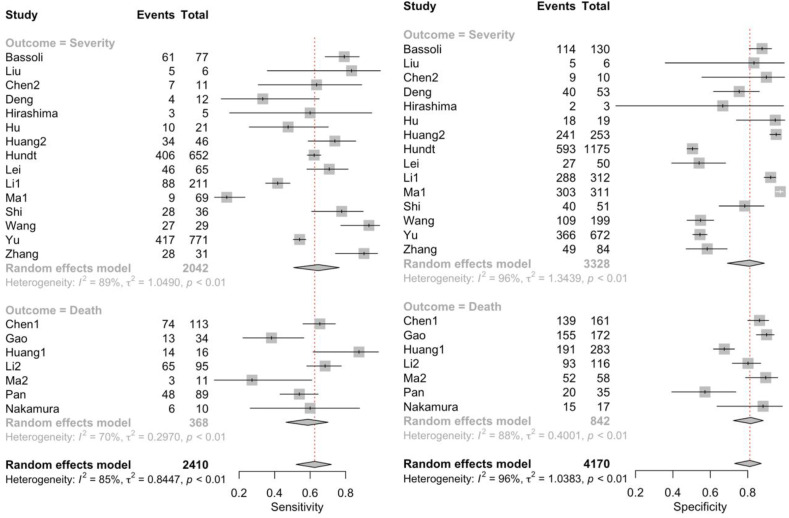
Low serum level of albumin was associated with increased composite poor outcome (OR 6.96 (95% CI 4.20–11.55), p < 0.001; I 2 = 91.3%, p < 0.001). Subgroup analysis shows that hypoalbuminemia was associated with increased mortality (OR 6.26 (95% CI 3.26–12.04), p < 0.001; I 2 = 69.6%, p < 0.01) and severity (OR 7.32 (95%CI 3.94–13.56), p < 0.001; I 2 = 92.5%, p < 0.01) (Fig. 2 ). The funnel-plot analysis was performed, Egger's test indicated a small study effect showed in asymmetrical shape (p = 0.006) (Fig. 3 a). Trim and fill analysis with imputation of 10 studies yielded an OR of 2.38 (95%. CI 1.44–3.93) for poor outcomes (Fig. 3b). Meta regression showed that the incidence was not influenced by age (p = 0.44), gender (p = 0.76), HT (p = 0.97), DM (p = 0.40), CKD (p = 0.65), liver disease (p = 0.72), and malignancy (p = 0.84). The incidence of composite poor outcome was 41%.
Fig. 2.
Forest plot of hypoalbuminemia and composite poor outcome.
Fig. 3.
a) Funnel plot b) Trim and fill analysis.
3.4. Diagnostic meta-analysis
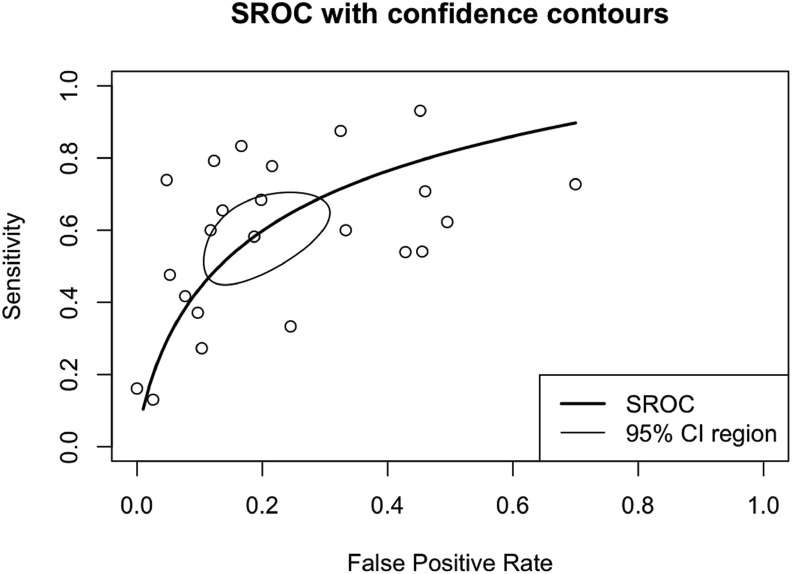
Hypoalbuminemia had a sensitivity of 0.63 (95% CI 0.52–0.72), specificity of 0.81 (95% CI 0.73–0.87), DOR of 6.96 (95% CI 4.20–11.55), and AUC of 0.77 (Fig. 4 ). Pooled sensitivity and specificity to predict poor outcomes showed in Fig. 4. A subgroup analysis of sensitivity and specificity showed sensitivity to predict death was 0.59 (95% CI 0.46–0.70) and severe COVID-19 was 0.65 (95% CI 0.51–0.76), and the specificity on mortality was 0.82 (95%CI 0.72–0.88) and on severity was 0.81 (0.69–0.89). Also, a summary receiver operating characteristics curve to predict composite poor outcome was conducted (Fig. 5 ).
Fig. 4.
Pooled sensitivity and specificity.
Fig. 5.
Summary receiver operating characteristics (SROC).
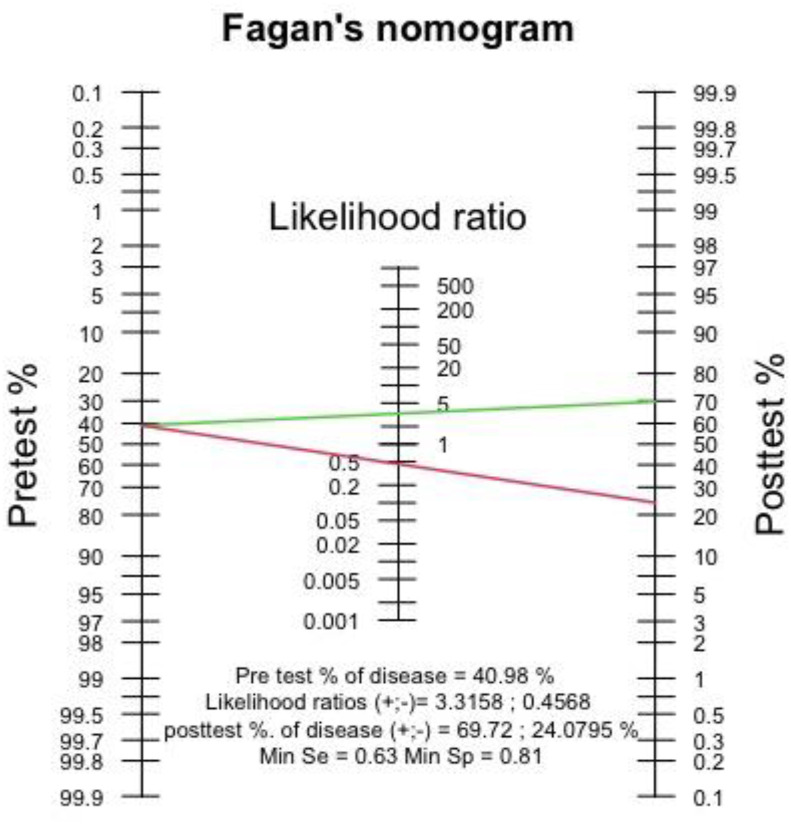
Fagan's nomogram showed that the post-test probability of poor outcome was 70% in hypoalbuminemia and the post-test probability of poor outcome in group with normal albumin was 24% (Fig. 6 ). Deek's funnel plot showed asymmetry with respect to the regression line and asymmetry test showed significant result (p = 0.01).
Fig. 6.
Fagan's nomogram.
4. Discussion
The correlation between albumin serum level and patient's outcome in COVID-19 has been investigated. This study summarizes the diagnostic performance of hypoalbuminemia to predict clinical outcomes in COVID-19 patients. From all of 19 included studies, most studies showed a significant correlation between low albumin levels and poor outcomes. This finding was consistent with the previous meta-analysis in which a lower albumin serum level was associated with adverse outcomes and more severe disease significantly [10,32]. To date, this is the first study to demonstrate a comprehensive prognostic analyses from the existing evidence of hypoalbuminemia to the outcome of COVID-19. A previous study by Aziz et al. showed similar finding–hypoalbuminemia was associated with more severe COVID-19 outcome (OR 12.6, 95% CI 7.5–21.1) [32]. The strength of the association between previous study and this present study is caused by the greater number of included studies in this present meta-analysis.
Low albumin serum level was associated with six-fold increase in poor outcome in COVID-19 patients compared with patients without hypoalbuminemia. Previous studies showed comorbidities like hypertension, diabetes mellitus, chronic kidney disease, liver disease, and malignancy were associated with declined serum albumin levels [[33], [34], [35], [36]]. Also, other studies showed that the relationship between albumin serum levels and COVID-19 outcomes varies with age and gender [10,37]. In the present study, meta-regression analyses indicate that age, gender, hypertension, diabetes, kidney disease, liver disease, and malignancy did not influence the association between low-albumin level and COVID-19 outcomes. These results emphasize that those abovementioned factors did not significantly impact the association of hypoalbuminemia and disease outcomes. In addition, the difference between regression analyses on age may be due to different types of data used in the previous study–continuous variable and the present study–categorical variable. Other important factors such as heart failure and chronic obstructive pulmonary disease were not included due to insufficient number of studies that reported those characteristics [38,39].
Hypoalbuminemia has 81% of specificity and 63% of positive probability of poor outcome with 41% pre-test probability of poor outcome. The sensitivity was low (63%) and therefore, hypoalbuminemia in COVID-19 is better to be implemented to rule in the risk of poor outcomes rather than ruling it out. With the level of heterogeneity in our results, it is likely due to different cut-off points, diagnostic tools, and quantification methods used among studies that explain the high heterogeneity of the results [40]. Subsequently, publication bias was also noted in this study as the funnel plot showed a slightly asymmetrical. Trim-and-fill analysis was performed, and it shows a reduced effect estimate without altering its significance after 10 imputed studies were included. Therefore, the prognostic performance will not be affected by the inclusion of additional studies.
Several studies have investigated hypoalbuminemia in COVID-19 patients. Although the specific mechanism that explains the relationship between albumin level and COVID-19 remains elusive, albumin is notable to play an important role in homeostasis. It functions as molecular transport and binder, free radical scavenger, platelet function inhibitor, and to maintain colloid osmotic pressure and its effects on vascular permeability [7,41,42]. The underlying mechanisms of how hypoalbuminemia correlates with more severe COVID-19 cases was thought to be associated with inflammatory conditions. This will lead to an increased in vascular permeability and shortened the half-life of the albumin. Viral infection stimulates inflammatory cytokines that will result in an increase in vascular permeability. Recent studies suggest that the resulting hypoalbuminemia may also be caused by capillary leak syndrome–which occurs due to epithelial-endothelial barrier injury of the alveolar epithelium as a result of direct viral effects and hypoxemia [[43], [44], [45], [46]]. This shifting–of intravascular fluids to the extravascular space may signify the correlation between albumin level and COVID-19 severity. Additionally, in a hyperinflammatory state, albumin mass will be significantly reduced as a consequence of shorter albumin half-life due to rapid liver and proliferating cells intracellular breakdowns of the albumin [36,43]. Taken together, the more severe the disease, the higher inflammatory reaction robustness yielding to a greater decline in serum albumin level which correlates with poor disease outcome.
The main limitation of this present study is due to retrospective studies that have a higher risk of bias. In addition, the number of studies on each cut-off value was insufficient to implement subgroup analysis based on cut-off value. The variability of the value may also cause high heterogeneity of the results. Other important factors such as heart failure, chronic obstructive pulmonary disease, and patients’ sociodemographic(10) were not included in the regression analysis due to an insufficient amount of reported variables. Further studies using a single cut-off value or analysis with multiple cut-off values are suggested.
5. Conclusion
This study shows that hypoalbuminemia was associated with poor outcomes in patients with COVID-19 and did not vary with age, male gender, hypertension, diabetes, chronic kidney disease, liver disease, and malignancy. Pooled diagnostic analysis indicates sensitivity of 63% and specificity of 81%.
Financial support
None.
Source of financial support
None.
Declaration of competing interest
None.
References
- 1.Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12–16. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021 Jun;26(3):107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaysen G.A., Levin N.W. Why measure serum albumin levels? J Ren Nutr. 2002;12(3):148–150. doi: 10.1053/jren.2002.33509. [DOI] [PubMed] [Google Scholar]
- 7.Don B.R., Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 8.Hundt M.A., Deng Y., Ciarleglio M.M., Nathanson M.H., Lim J.K. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. Hospital network. Hepatology. 2020;72(4):1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 10.Paliogiannis P., Mangoni A.A., Cangemi M., Fois A.G., Carru C., Zinellu A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): a systematic review and meta-analysis. Clin Exp Med. 2021:1–12. doi: 10.1007/s10238-021-00686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moses L.E., Shapiro D., Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12(14):1293–1316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 14.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 15.Bassoli C., Oreni L., Ballone E., Foschi A., Perotti A., Mainini A. Role of serum albumin and proteinuria in patients with SARS-CoV-2 pneumonia. Int J Clin Pract. 2021;75(4) doi: 10.1111/ijcp.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng M., Qi Y., Deng L., Wang H., Xu Y., Li Z. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity. 2020;28(10):1815–1825. doi: 10.1002/oby.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao S., Jiang F., Jin W., Shi Y., Yang L., Xia Y. Risk factors influencing the prognosis of elderly patients infected with COVID-19: a clinical retrospective study in Wuhan, China. Aging. 2020;12(13):12504–12516. doi: 10.18632/aging.103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirashima T., Arai T., Kitajima H., Tamura Y., Yamada T., Hashimoto S. Factors significantly associated with COVID-19 severity in symptomatic patients: a retrospective single-center study. J Infect Chemother. 2021;27(1):76–82. doi: 10.1016/j.jiac.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H., Du H., Li J., Wang Y., Wu X., Wang C. Early prediction and identification for severe patients during the pandemic of COVID-19: a severe COVID-19 risk model constructed by multivariate logistic regression analysis. J Glob Health. 2020;10(2) doi: 10.7189/jogh.10.020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J., Cheng A., Kumar R., Fang Y., Chen G., Zhu Y. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92(10):2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei P., Zhang L., Han P., Zheng C., Tong Q., Shang H. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14(5):733–742. doi: 10.1007/s12072-020-10087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G., Zhou C.L., Ba Y.M., Wang Y.M., Song B., Cheng X.B. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2021;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma X., Li A., Jiao M., Shi Q., An X., Feng Y. Characteristic of 523 COVID-19 in henan province and a death prediction model. Front Public Health. 2020;8:475. doi: 10.3389/fpubh.2020.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura S., Kanemasa Y., Atsuta Y., Fujiwara S., Tanaka M., Fukushima K. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. 2021;26(3):485–493. doi: 10.1007/s10147-020-01837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan F., Yang L., Li Y., Liang B., Li L., Ye T. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17(9):1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S., Liu X., Xiao J., Wang H., Chen L., Li J. Prediction of adverse clinical outcomes in patients with coronavirus disease 2019. J Clin Lab Anal. 2021;35(1) doi: 10.1002/jcla.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Liao B., Guo Y., Li F., Lei C., Zhang F. Clinical characteristics of patients infected with the novel 2019 coronavirus (SARS-Cov-2) in guangzhou, China. Open Forum Infect Dis. 2020;7(6):ofaa187. doi: 10.1093/ofid/ofaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu C., Lei Q., Li W., Wang X., Li W., Liu W. Epidemiological and clinical characteristics of 1663 hospitalized patients infected with COVID-19 in Wuhan, China: a single-center experience. J Infect Public Health. 2020;13(9):1202–1209. doi: 10.1016/j.jiph.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aziz M., Fatima R., Lee-Smith W., Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24(1):255. doi: 10.1186/s13054-020-02995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stehouwer C.D., Gall M.A., Twisk J.W., Knudsen E., Emeis J.J., Parving H.H. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 34.Maahs D.M., Snively B.M., Bell R.A., Dolan L., Hirsch I., Imperatore G. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30(10):2593–2598. doi: 10.2337/dc07-0450. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho J.R., Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol. 2018;17(4):547–560. doi: 10.5604/01.3001.0012.0916. [DOI] [PubMed] [Google Scholar]
- 36.Soeters P.B., Wolfe R.R., Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN - J Parenter Enter Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sikorska D., Olewicz-Gawlik A., Baum E., Pawlaczyk K., Oko A. The importance of hypoalbuminemia in peritoneal dialysis patients: impact of gender. Adv Clin Exp Med. 2019;28(6):729–735. doi: 10.17219/acem/79653. [DOI] [PubMed] [Google Scholar]
- 38.Violi F., Cangemi R., Romiti G.F., Ceccarelli G., Oliva A., Alessandri F. Is albumin predictor of mortality in COVID-19? Antioxidants Redox Signal. 2020 doi: 10.1089/ars.2020.8142. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Stavem K., Dahl F.A., Humerfelt S., Haugen T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD) Int J Chronic Obstr Pulm Dis. 2014;9:99–105. doi: 10.2147/COPD.S51467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Infusino I., Panteghini M. Serum albumin: accuracy and clinical use. Clin Chim Acta. 2013;419:15–18. doi: 10.1016/j.cca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Arroyo V., García-Martinez R., Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan D.H., Johnson L.E., Dennis R.A., Roberson P.K., Heif M., Garner K.K. The Interrelationships among albumin, nutrient intake, and inflammation in elderly recuperative care patients. J Nutr Health Aging. 2011;15(4):311–315. doi: 10.1007/s12603-010-0297-1. [DOI] [PubMed] [Google Scholar]
- 43.Wu M.A., Fossali T., Pandolfi L., Carsana L., Ottolina D., Frangipane V. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J Intern Med. 2021 doi: 10.1111/joim.13208. [DOI] [PubMed] [Google Scholar]
- 44.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]