Abstract
Background
Limited existing data suggest that the novel COVID-19 may increase risk of VTE, but information from large, ethnically diverse populations with appropriate control participants is lacking.
Research Question
Does the rate of VTE among adults hospitalized with COVID-19 differ from matched hospitalized control participants without COVID-19?
Study Design and Methods
We conducted a retrospective study among hospitalized adults with laboratory-confirmed COVID-19 and hospitalized adults without evidence of COVID-19 matched for age, sex, race or ethnicity, acute illness severity, and month of hospitalization between January 2020 and August 2020 from two integrated health care delivery systems with 36 hospitals. Outcomes included VTE (DVT or pulmonary embolism ascertained using diagnosis codes combined with validated natural language processing algorithms applied to electronic health records) and death resulting from any cause at 30 days. Fine and Gray hazards regression was performed to evaluate the association of COVID-19 with VTE after accounting for competing risk of death and residual differences between groups, as well as to identify predictors of VTE in patients with COVID-19.
Results
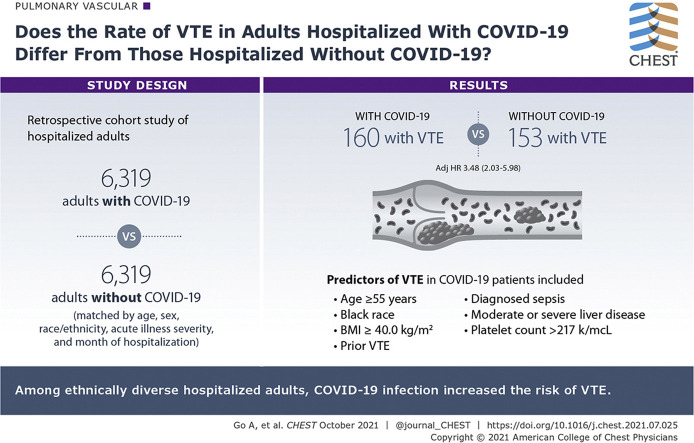
We identified 6,319 adults with COVID-19 and 6,319 matched adults without COVID-19, with mean ± SD age of 60.0 ± 17.2 years, 46% women, 53.1% Hispanic, 14.6% Asian/Pacific Islander, and 10.3% Black. During 30-day follow-up, 313 validated cases of VTE (160 COVID-19, 153 control participants) and 1,172 deaths (817 in patients with COVID-19, 355 in control participants) occurred. Adults with COVID-19 showed a more than threefold adjusted risk of VTE (adjusted hazard ratio, 3.48; 95% CI, 2.03-5.98) compared with matched control participants. Predictors of VTE in patients with COVID-19 included age ≥ 55 years, Black race, prior VTE, diagnosed sepsis, prior moderate or severe liver disease, BMI ≥ 40 kg/m2, and platelet count > 217 k/μL.
Interpretation
Among ethnically diverse hospitalized adults, COVID-19 infection increased the risk of VTE, and selected patient characteristics were associated with higher thromboembolic risk in the setting of COVID-19.
Key Words: covid-19, epidemiology, risk factor, VTE
Abbreviations: aHR, adjusted hazard ratio; EMR, electronic medical record
Graphical Abstract
COVID-19 remains a leading cause of death internationally.1 , 2 In addition to respiratory and multiorgan failure, thrombosis is an increasing concern, particularly among adults hospitalized with COVID-19. Recent studies have reported that COVID-19 is associated with abnormal coagulation profiles,3, 4, 5 and a recent meta-analysis suggested that it may predispose patients to venous thromboembolic events and other types of thromboembolism, as well as worse outcomes after VTE.6 However, few data exist about the incidence of VTE associated with COVID-19 in large, ethnically diverse populations. Existing studies have focused primarily on only critically ill patients with COVID-19, with uncertainty about VTE risk compared with appropriate control participants without COVID-19 and after accounting for the competing risk of non-VTE-related death associated with COVID-19.6 These insights are key to understanding who potentially may benefit from prevention strategies, given that limited evidence exists to help guide which patients with COVID-19 should be offered VTE prophylaxis initially or after hospitalization and at what anticoagulation intensity.7
To address these knowledge gaps, we evaluated the rate of VTE among a large, ethnically diverse cohort of adults hospitalized with COVID-19 compared with matched control participants without COVID-19 and also identified predictors of VTE in patients with COVID-19.
Methods
Source Population
The source population was derived from Kaiser Permanente Northern California and Southern California integrated health care delivery systems, which provide comprehensive care that is captured through electronic medical record (EMR) systems covering inpatient, ED, and outpatient settings. Kaiser Permanente Northern California serves > 4.5 million members at 21 hospital-based medical centers, and Kaiser Permanente Southern California serves > 4.6 million members at 15 hospital-based medical centers in California. Their membership is highly representative of their local surroundings and the statewide population with regard to age, sex, and race and ethnicity.8 , 9
Institutional review boards at Kaiser Permanente Northern California and Southern California approved the study (Identifier: 1279252). A waiver of informed consent was obtained because of the nature of this retrospective, data-only study.
Study Sample
The Kaiser Permanente Virtual Data Warehouse and EMR were the primary data sources for patient identification and characterization at participating sites. The Virtual Data Warehouse comprises EMR-based datasets with linked sociodemographic, administrative, pharmacy, laboratory results, and health care use data.10 , 11
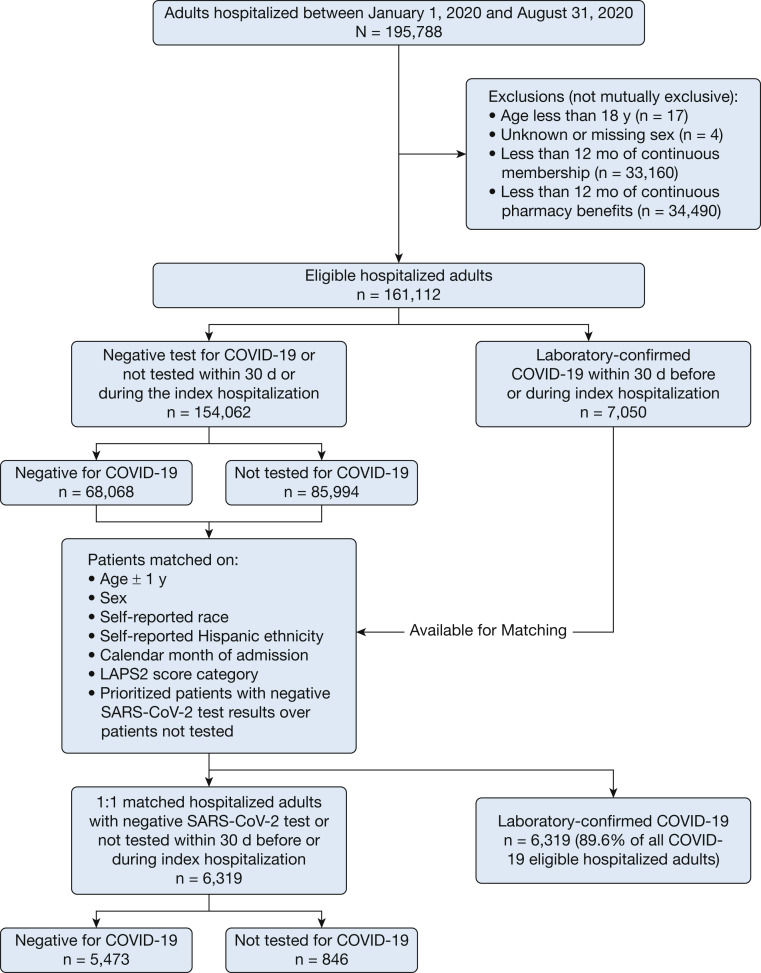
We performed a retrospective cohort study of hospitalized adults with COVID-19 and matched hospitalized control participants without evidence of COVID-19 (Fig 1 ). We first identified adults (age ≥ 18 years) hospitalized at a Kaiser Permanente facility between January 1 and August 31, 2020. We excluded people with missing sex or fewer than 12 months of continuous enrollment and pharmacy benefits before admission to ensure adequate data on covariates.
Figure 1.
Cohort assembly of hospitalized adults with COVID-19 and matched adults without COVID-19. LAPS2 = Laboratory-Based Acute Physiology Score, version 2.
We identified patients with laboratory-confirmed COVID-19 based on polymerase chain reaction testing for SARS-CoV-2 ascertained through Kaiser Permanente laboratory systems, which also includes results for approved assays from outside laboratories. We assigned each patient’s index date as the admission date after the first SARS-CoV-2 test showing positive results within 30 days before or during the index hospitalization.
We next identified a matched cohort of hospitalized adults without evidence of COVID-19 (ie, negative SARS-CoV-2 polymerase chain reaction test results or not having received a test up to 30 days before or during the index hospitalization). If the patient demonstrated negative test results, the hospitalization within 30 days of those results was used as the index hospitalization; if the patient was hospitalized but never underwent SARS-CoV-2 testing, their index date was assigned as the first admission date during the study period. We next performed 1:1 matching between patients with COVID-19 and control participants based on Laboratory-Based Acute Physiology Score, version 2, at admission, age (± 1 year), self-reported sex, self-reported race and Hispanic ethnicity, and calendar month of index hospitalization. The Laboratory-Based Acute Physiology Score, version 2, is a validated, integrated physiology-based score of severity of illness calculated from multiple laboratory results and vital signs that is highly predictive of short-term mortality.12
Outcomes
Censoring occurred at the earliest of a VTE event, death, health plan disenrollment, or completing 30 days of follow-up. The primary outcome was acute VTE (DVT or pulmonary embolism) on or after index date, which could occur in the inpatient, postdischarge outpatient, or ED setting. We initially searched EMR systems for inpatient encounters with primary or secondary International Classification of Disease, Tenth Revision, diagnosis codes for DVT and pulmonary embolism (codes available on request). Postdischarge ED or outpatient encounters also required evidence of a relevant radiology procedure (eg, chest CT angiography or extremity ultrasound) within 14 days of an encounter with an associated VTE diagnosis code. Given that we demonstrated previously the suboptimal accuracy of VTE diagnosis codes in inpatient, ED, and outpatient settings within participating sites,13 we developed and validated a rules-based natural language processing algorithm to identify valid acute VTE events from unstructured EMR data within Kaiser Permanente Northern and Southern California. The algorithm was developed and trained on 479 confirmed VTE events, with a positive predictive value of 95% and a negative predictive value of 97% compared with physician adjudication of medical records using standardized diagnostic criteria.14
We identified deaths using comprehensive data from health plan databases (including inpatient and ED deaths and proxy reports), state death certificate files, and Social Security Administration Death Master File.10 , 15, 16, 17 Information on presumed cause(s) of death was unavailable.
Covariates
Demographic characteristics (age, self-reported sex, and self-reported race and Hispanic ethnicity) were collected from EMR data. We ascertained relevant medical history up to 5 years before index date using previously validated approaches based on International Classification of Disease, Ninth and Tenth Revisions, diagnosis and procedure codes, Current Procedure Terminology procedure codes, laboratory results, pharmacy dispensings, and vital sign data.10 , 11 , 18, 19, 20 This included cardiovascular conditions (coronary heart disease, acute myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass surgery, heart failure, rheumatic heart disease, atrial fibrillation or flutter, mitral or aortic valvular disease, peripheral artery disease, ischemic stroke, or transient ischemic attack), cardiovascular risk factors (diabetes mellitus, hypertension, dyslipidemia, smoking status), and other medical history (coagulopathy, hypercoagulable hematologic conditions, thrombophilia, chronic lung disease, liver disease, peptic ulcer disease, intracranial hemorrhage, hospitalization for GI hemorrhage, hospitalization for other bleeding, hemiplegia or paraplegia, inflammatory bowel disease, rheumatic disease, cancer, prior mechanical fall, dementia, depression, and substance or alcohol misuse). We also included information on diagnosed sepsis during the index hospitalization based on diagnostic codes. We ascertained kidney function from the estimated glomerular filtration rate before the index date using the CKD-EPI equation21 (in milliliters per minute per 1.73 m2) and serum creatinine concentration on or within 2 days of admission. We also collected preadmission BP, BMI, hemoglobin level, and platelet count, if available, or within 2 days of admission.
We characterized preadmission receipt of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, aldosterone receptor antagonists, nonaspirin antiplatelet agents, statins, and other lipid-lowering agents based on data on estimated day supply per dispensed prescription and refill patterns in pharmacy databases using validated methods.22 Baseline oral anticoagulant use was defined as a dispensed prescription covering any part of the 60 days before or on the index date. Parenteral (heparin, fondaparinux, enoxaparin, dalteparin, and tinzaparin) or oral (warfarin, dabigatran, rivaroxaban, apixaban, edoxaban, and betrixaban) anticoagulant use during the index hospitalization was defined as dispensing during the hospital stay, but before a VTE, if one occurred. Anticoagulant exposure at discharge was characterized as dispensing on or within 7 days after discharge, but before a VTE event, if one occurred during this period.
Statistical Analysis
Analyses were performed using SAS version 9.4 software (SAS Inc.). Baseline characteristics were presented as mean ± SD or median and interquartile range for continuous variables and frequencies with percentages for categorical data. Any missing data were assigned to a missing or unknown category. Given the large sample size, we compared characteristics between matched patients using Cohen’s D value23 , 24 for continuous variables and Cramér’s V25 , 26 for categorical variables, with a value of > 0.10 considered potentially significant. We used the Kruskal-Wallis test to compare median values, with P < .05 considered significant.
We calculated rates (per 100 person-years) with associated 95% CIs for VTE and death at 30 days, and we compared the cumulative incidence function of VTE by group using Gray’s test. Given the concern for competing risk of death that may not be the result of VTE associated with COVID-19, we performed multivariate Fine-Gray subdistribution hazard modeling27 to account for competing risk of death and potential confounders, with covariates included that significantly differed between groups. Finally, among the subgroup of patients with COVID-19, we conducted a Fine-Gray subdistribution hazard model to identify multivariate predictors of VTE using patient characteristics in Table 1 as candidate predictors.
Table 1.
Characteristics of Hospitalized Adults With COVID-19 and Matched Hospitalized Adults Without Evidence of COVID-19
| Baseline Characteristic | Overall (N = 12,638) | Patients With COVID-19 (n = 6,319) | Patients Without COVID-19 (n = 6,319) | D or V Value |
|---|---|---|---|---|
| Age, y | ... | ... | ... | ... |
| Mean ± SD | 60.0 ± 17.2 | 60.0 ± 17.2 | 60.0 ± 17.2 | 0.00 |
| Range | 18.2-104.3 | 18.2-103.5 | 18.2-104.3 | ... |
| Age group, y | ... | ... | ... | ... |
| ≤ 54 | 4,747 (37.5) | 2,377 (37.6) | 2,370 (37.5) | |
| 55-64 | 2,855 (22.6) | 1,430 (22.6) | 1,425 (22.6) | |
| 65-74 | 2,402 (19.0) | 1,192 (18.9) | 1,210 (19.1) | 0.00 |
| 75-84 | 1,716 (13.6) | 857 (13.6) | 859 (13.6) | |
| ≥ 85 | 918 (7.3) | 463 (7.3) | 455 (7.2) | |
| Women | 5,802 (45.9) | 2,901 (45.9) | 2,901 (45.9) | 0.00 |
| Race | ... | ... | ... | ... |
| White | 9,340 (73.9) | 4,670 (73.9) | 4,670 (73.9) | |
| Black | 1,298 (10.3) | 649 (10.3) | 649 (10.3) | |
| Asian/Pacific Islander | 1,848 (14.6) | 924 (14.6) | 924 (14.6) | 0.00 |
| Other | 96 (0.8) | 48 (0.8) | 48 (0.8) | |
| Unknown | 56 (0.4) | 28 (0.4) | 28 (0.4) | |
| Hispanic ethnicity | 6,710 (53.1) | 3,355 (53.1) | 3,355 (53.1) | 0.00 |
| Days between COVID-19 test and index hospitalization | ... | ... | ... | ... |
| Mean ± SD | 3.3 ± 5.4 | 3.6 ± 4.8 | 3.0 ± 5.9 | 0.10 |
| Median (IQR) | 1.0 (0.0-4.0) | 2.0 (0.0-6.0) | 1.0 (0.0-3.0) | < .001a |
| Not tested | 846 (6.7) | 0 (0.0) | 846 (13.4) | ... |
| Baseline medical history | ... | ... | ... | ... |
| Cardiovascular diseases | ... | ... | ... | ... |
| Coronary heart disease | 945 (7.5) | 380 (6.0) | 565 (8.9) | 0.06 |
| Acute myocardial infarction | 305 (2.4) | 125 (2.0) | 180 (2.8) | 0.03 |
| Unstable angina | 92 (0.7) | 32 (0.5) | 60 (0.9) | 0.03 |
| Percutaneous coronary intervention | 747 (5.9) | 310 (4.9) | 437 (6.9) | 0.04 |
| Coronary artery bypass surgery | 81 (0.6) | 31 (0.5) | 50 (0.8) | 0.02 |
| Heart failure | 1,326 (10.5) | 492 (7.8) | 834 (13.2) | 0.09 |
| Rheumatic heart disease | 192 (1.5) | 64 (1.0) | 128 (2.0) | 0.04 |
| Atrial fibrillation or flutter | 1,280 (10.1) | 528 (8.4) | 752 (11.9) | 0.06 |
| Mitral or aortic valvular disease | 741 (5.9) | 259 (4.1) | 482 (7.6) | 0.08 |
| Peripheral artery disease | 738 (5.8) | 276 (4.4) | 462 (7.3) | 0.06 |
| Ischemic stroke or transient ischemic attack | 440 (3.5) | 184 (2.9) | 256 (4.1) | 0.03 |
| Cardiovascular risk factors | ... | ... | ... | ... |
| Diabetes mellitus | 4,862 (38.5) | 2,559 (40.5) | 2,303 (36.4) | 0.04 |
| Hypertension | 6,828 (54.0) | 3,315 (52.5) | 3,513 (55.6) | 0.03 |
| Dyslipidemia | 8,100 (64.1) | 4,083 (64.6) | 4,017 (63.6) | 0.01 |
| Former or current smoker | 4,868 (38.5) | 2,201 (34.8) | 2,667 (42.2) | 0.08 |
| Other medical history | ... | ... | ... | ... |
| Coagulopathy | 1,242 (9.8) | 474 (7.5) | 768 (12.2) | 0.08 |
| Hypercoagulable condition | 278 (2.2) | 111 (1.8) | 167 (2.6) | 0.03 |
| Thrombophilia | 99 (0.8) | 50 (0.8) | 49 (0.8) | 0.001 |
| Chronic lung disease | 2,898 (22.9) | 1,404 (22.2) | 1,494 (23.6) | 0.02 |
| Moderate or severe liver disease | 249 (2.0) | 58 (0.9) | 191 (3.0) | 0.08 |
| Mild liver disease | 391 (3.1) | 105 (1.7) | 286 (4.5) | 0.08 |
| Peptic ulcer disease | 182 (1.4) | 53 (0.8) | 129 (2.0) | 0.05 |
| Intracranial hemorrhage, nontraumatic | 172 (1.4) | 60 (0.9) | 112 (1.8) | 0.04 |
| Intracranial hemorrhage, traumatic | 104 (0.8) | 43 (0.7) | 61 (1.0) | 0.02 |
| Hospitalized gastrointestinal hemorrhage | 257 (2.0) | 77 (1.2) | 180 (2.8) | 0.06 |
| Hospitalized other bleeding | 39 (0.3) | 18 (0.3) | 21 (0.3) | 0.004 |
| Hemiplegia or paraplegia | 326 (2.6) | 123 (1.9) | 203 (3.2) | 0.04 |
| Inflammatory bowel disease | 163 (1.3) | 61 (1.0) | 102 (1.6) | 0.03 |
| Rheumatic disease | 367 (2.9) | 161 (2.5) | 206 (3.3) | 0.02 |
| Cancer | 1,132 (9.0) | 348 (5.5) | 784 (12.4) | 0.12 |
| Prior mechanical fall | 243 (1.9) | 108 (1.7) | 135 (2.1) | 0.02 |
| Diagnosed dementia | 709 (5.6) | 403 (6.4) | 306 (4.8) | 0.03 |
| Diagnosed depression | 2,343 (18.5) | 997 (15.8) | 1,346 (21.3) | 0.07 |
| Substance abuse | 445 (3.5) | 130 (2.1) | 315 (5.0) | 0.08 |
| Alcohol abuse | 826 (6.5) | 261 (4.1) | 565 (8.9) | 0.10 |
| Prior VTE | 228 (1.8) | 80 (1.3) | 148 (2.3) | 0.04 |
| Time between prior VTE and index admission, y | ... | ... | ... | ... |
| Mean ± SD | 1.6 ± 1.2 | 1.6 ± 1.2 | 1.5 ± 1.2 | 0.11 |
| Median (IQR) | 1.3 (0.4-2.6) | 1.6 (0.5-2.8) | 1.2 (0.4-2.5) | 0.42a |
| Sepsis diagnosed during index hospitalization | 4,368 (34.6) | 3,047 (48.2) | 1,321 (20.9) | 0.29 |
| Anticoagulant exposure before and during the index hospitalization but before a VTE | ... | ... | ... | ... |
| Baseline oral anticoagulant use | 879 (7.0) | 309 (4.9) | 570 (9.0) | 0.08 |
| Parenteral anticoagulant during index hospitalization | 8,342 (66.0) | 5,269 (83.4) | 3,073 (48.6) | 0.37 |
| Oral anticoagulant during index hospitalization | 852 (6.7) | 389 (6.2) | 463 (7.3) | 0.02 |
| Distribution of anticoagulant use before and after discharge from index hospitalization | ... | ... | ... | ... |
| Oral anticoagulant within 60 d before index date and oral anticoagulant after discharge | 694 (5.5) | 239 (3.8) | 455 (7.2) | |
| Oral anticoagulant within 60 d before index date, but not after discharge | 185 (1.5) | 70 (1.1) | 115 (1.8) | 0.10 |
| Oral anticoagulant after discharge only | 492 (3.9) | 316 (5.0) | 176 (2.8) | |
| No anticoagulant use 60 d before index date or after discharge | 11,267 (89.1) | 5,694 (90.1) | 5,573 (88.2) | |
| Baseline medication use | ... | ... | ... | ... |
| Angiotensin-converting enzyme inhibitor | 2,598 (20.6) | 1,282 (20.3) | 1,316 (20.8) | 0.01 |
| Angiotensin II receptor blocker | 1,679 (13.3) | 883 (14.0) | 796 (12.6) | 0.02 |
| β-blocker | 2,996 (23.7) | 1,289 (20.4) | 1,707 (27.0) | 0.08 |
| Calcium channel blocker | 2,188 (17.3) | 1,043 (16.5) | 1,145 (18.1) | 0.02 |
| Diuretic | 3,052 (24.1) | 1,402 (22.2) | 1,650 (26.1) | 0.05 |
| Aldosterone receptor antagonist | 326 (2.6) | 100 (1.6) | 226 (3.6) | 0.06 |
| Nonaspirin antiplatelet agent | 521 (4.1) | 210 (3.3) | 311 (4.9) | 0.04 |
| Statin | 5,184 (41.0) | 2,602 (41.2) | 2,582 (40.9) | 0.003 |
| Other lipid-lowering agents | 222 (1.8) | 100 (1.6) | 122 (1.9) | 0.01 |
| Vital signs | ... | ... | ... | ... |
| BP, mm Hg | ... | ... | ... | ... |
| Systolic | ||||
| ≥ 180 | 2 (0.0) | 2 (0.0) | 0 (0.0) | |
| 160-179 | 55 (0.4) | 20 (0.3) | 35 (0.6) | |
| 140-159 | 727 (5.8) | 283 (4.5) | 444 (7.0) | |
| 130-139 | 1,202 (9.5) | 538 (8.5) | 664 (10.5) | 0.08 |
| 121-129 | 1,769 (14.0) | 852 (13.5) | 917 (14.5) | |
| ≤ 120 | 8,864 (70.1) | 4,617 (73.1) | 4,247 (67.2) | |
| Missing | 19 (0.2) | 7 (0.1) | 12 (0.2) | |
| Diastolic | ... | ... | ... | ... |
| ≥ 110 | 1 (0.0) | 0 (0.0) | 1 (0.0) | |
| 100-109 | 14 (0.1) | 3 (0.0) | 11 (0.2) | |
| 90-99 | 126 (1.0) | 35 (0.6) | 91 (1.4) | |
| 85-89 | 232 (1.8) | 95 (1.5) | 137 (2.2) | 0.07 |
| 81-84 | 307 (2.4) | 121 (1.9) | 186 (2.9) | |
| ≤ 80 | 11,939 (94.5) | 6,058 (95.9) | 5,881 (93.1) | |
| Missing | 19 (0.2) | 7 (0.1) | 12 (0.2) | |
| BMI, kg/m2 | ... | ... | ... | ... |
| < 18.5 | 321 (2.6) | 107 (1.7) | 214 (3.4) | |
| 18.5-25.0 | 2,614 (20.7) | 1,064 (16.8) | 1,550 (24.5) | |
| 25.0-25.9 | 3,805 (30.1) | 1,833 (29.0) | 1,972 (31.2) | |
| 30.0-39.9 | 4,425 (35.0) | 2,457 (38.9) | 1,968 (31.1) | 0.13 |
| ≥ 40 | 1,382 (10.9) | 809 (12.8) | 573 (9.1) | |
| Unknown | 91 (0.7) | 49 (0.8) | 42 (0.7) | |
| Laboratory values | ... | ... | ... | ... |
| Preadmission estimated glomerular filtration rate, mL/min/1.73 m2 | ... | ... | ... | ... |
| ≥ 90 | 4,511 (35.7) | 2,295 (36.3) | 2,216 (35.1) | |
| 60-89 | 3,962 (31.3) | 2,023 (32.0) | 1,939 (30.7) | |
| 45-59 | 1,160 (9.2) | 550 (8.7) | 610 (9.6) | |
| 30-44 | 677 (5.4) | 318 (5.0) | 359 (5.7) | 0.08 |
| 15-29 | 283 (2.2) | 111 (1.8) | 172 (2.7) | |
| < 15 | 125 (1.0) | 45 (0.7) | 80 (1.3) | |
| Dialysis or transplant | 462 (3.7) | 165 (2.6) | 297 (4.7) | |
| Unknown | 1,458 (11.5) | 812 (12.9) | 646 (10.2) | |
| Serum creatinine on or within 2 d of index hospitalization, mg/dL | ... | ... | ... | ... |
| Mean ± SD | 1.3 ± 1.7 | 1.2 ± 1.6 | 1.4 ± 1.8 | 0.15 |
| Median (IQR) | 0.8 (0.7-1.1) | 0.8 (0.7-1.0) | 0.9 (0.7-1.2) | < .001a |
| Missing | 1,271 (10.1) | 356 (5.6) | 915 (14.5) | ... |
| Hemoglobin, g/dL | ... | ... | ... | ... |
| ≥ 13.0 | 4,630 (36.6) | 2,791 (44.2) | 1,839 (29.1) | |
| 12-12.9 | 2,412 (19.1) | 1,356 (21.5) | 1,056 (16.7) | |
| 11-11.9 | 1,945 (15.4) | 956 (15.1) | 989 (15.7) | |
| 10-10.9 | 1,413 (11.2) | 576 (9.1) | 837 (13.2) | 0.24 |
| 9-9.9 | 990 (7.8) | 331 (5.2) | 659 (10.4) | |
| < 9 | 1,133 (9.0) | 266 (4.2) | 867 (13.7) | |
| Missing | 115 (0.9) | 43 (0.7) | 72 (1.2) | |
| Platelet, K/μL | ... | ... | ... | ... |
| Mean ± SD | 230.9 ± 100.0 | 245.8 ± 104.3 | 216.0 ± 93.1 | 0.30 |
| Median (IQR) | 217.0 (164.0-283.0) | 229.0 (172.0-301.0) | 206.0 (156.5-263.0) | < .001a |
| Missing | 125 (1.0) | 46 (0.7) | 79 (1.3) | ... |
Data are presented as No. (%), unless otherwise indicated. IQR = interquartile range.
P value using Kruskal-Wallis test.
Results
Baseline Characteristics in Matched Patients
Between January 1 and August 31, 2020, we identified 7,050 eligible hospitalized adults with laboratory-confirmed COVID-19. We matched 6,319 patients with COVID-19 (89.6%) with a hospitalized control group without evidence of COVID-19 based on age, sex, race or ethnicity, level of acute illness severity, and calendar month of hospitalization (Fig 1). Of note, 91.4% of matched patients with COVID-19 were considered to have severe disease (ie, oxygen saturation < 94% or receipt of high-flow oxygen or mechanical ventilation).28
Matched patients were similar at baseline, although patients with COVID-19 were less likely to have cancer and more likely to have sepsis diagnosed during the index hospitalization and to have a higher BMI (Table 1). Among tested patients, those with COVID-19 showed slightly lower median serum creatinine concentration, but higher hemoglobin and platelet counts compared with matched control participants.
During the index hospitalization, patients with COVID-19 were more likely to receive parenteral anticoagulant therapy compared with those without evidence of COVID-19, but no difference was found in the receipt of anticoagulants at discharge (Table 1). Compared with matched control participants, patients with COVID-19 had longer median length of stay and were more likely to die during the index hospitalization or be discharged to a skilled nursing facility (Table 2 ).
Table 2.
Hospital Length of Stay and Discharge Status in Hospitalized Adults With COVID-19 and Matched Hospitalized Adults Without Evidence of COVID-19b
| Baseline Characteristic | Overall (N = 12,638) | Patients With COVID-19 (n = 6,319) | Patients Without COVID-19 (n = 6,319) | D or V Value |
|---|---|---|---|---|
| Hospital length of stay, d | ||||
| Mean ± SD | 8.7 ± 10.7 | 11.2 ± 12.8 | 6.2 ± 7.5 | 0.48 |
| Median (IQR) | 5.0 (3.0-9.0) | 7.0 (4.0-13.0) | 4.0 (3.0-7.0) | < .001a |
| Range | 1.0-137.0 | 1.0-137.0 | 1.0-125.0 | |
| Discharge status (listed in descending order by frequency) | 0.22 | |||
| Home | 7,367 (58.3) | 3,641 (57.6) | 3,726 (59.0) | |
| Home health | 2,911 (23.0) | 1,192 (18.9) | 1,719 (27.2) | |
| Died | 1,024 (8.1) | 838 (13.3) | 186 (2.9) | |
| Skilled nursing facility | 752 (6.0) | 432 (6.8) | 320 (5.1) | |
| Hospice | 230 (1.8) | 70 (1.1) | 160 (2.5) | |
| Against medical advice | 62 (0.5) | 23 (0.4) | 39 (0.6) | |
| Transferred to other acute inpatient hospital | 37 (0.3) | 13 (0.2) | 24 (0.4) | |
| Residential facility | 35 (0.3) | 21 (0.3) | 14 (0.2) | |
| Still in Kaiser Permanente hospital | 27 (0.2) | 13 (0.2) | 14 (0.2) | |
| Rehabilitation facility | 14 (0.1) | 4 (0.1) | 10 (0.2) | |
| Nursing home | 2 (0.0) | 1 (0.0) | 1 (0.0) | |
| Other | 9 (0.1) | 5 (0.1) | 4 (0.1) | |
| Unknown | 168 (1.3) | 66 (1.0) | 102 (1.6) |
Data are presented as No. (%), unless otherwise indicated. IQR = interquartile range.
P value using Kruskal-Wallis test.
Data in this table reflects the full index hospitalization period, even if it lasted longer than 30 days.
COVID-19 and Risk of VTE
During the 30-day follow-up, we observed 1,172 deaths from any cause (817 patients with COVID-19, 355 control participants), with a significantly higher crude rate of death in those with COVID-19 compared with matched control participants (163.7 vs 68.6 per 100 person-years, respectively; P < .001).
A total of 313 patients experienced a confirmed VTE during follow-up (160 patients with COVID-19, 153 control participants), with no significant difference in VTE type (Table 3 ) or timing (COVID-19 patients: median, 2 days [interquartile range, 1.0-2.0 days]; matched control participants: median, 1 day [interquartile range, 1.0-2.0 days]; P = .25). In comparing unadjusted cumulative incidence function of VTE, patients with COVID-19 had an unadjusted rate (per 100 person-years) of VTE of 0.025 compared with 0.024 (P = .69) in matched control participants during the 30-day follow-up.
Table 3.
Distribution of VTE Outcome in Hospitalized Adults With COVID-19 and Matched Adults Without Evidence of COVID-19
| VTE Characteristic | Overall (N = 313) | Patients With COVID-19 (n = 160) | Patients Without COVID-19 (n = 153) | P Value |
|---|---|---|---|---|
| Type of VTE | ... | ... | ... | ... |
| Pulmonary embolism | 159 (50.8) | 81 (50.6) | 78 (51.0) | |
| Lower extremity DVT | 90 (28.8) | 51 (31.9) | 39 (25.5) | |
| Upper extremity DVT | 43 (13.7) | 21 (13.1) | 22 (14.4 | .31 |
| Mesenteric VTE | 15 (4.8) | 4 (2.5) | 11 (7.2) | |
| Other or unknown VTE | 6 (1.9) | 3 (1.9) | 3 (1.9) | |
| VTE timing | ... | ... | ... | ... |
| During index hospitalization | 282 (90.1) | 148 (92.5) | 134 (87.6) | .19 |
| After index hospitalization discharge | 31 (9.9) | 12 (7.5) | 19 (12.4) |
Data are presented as No. (%), unless otherwise indicated.
However, in multivariable Fine-Gray regression that accounted for competing risk of death and potential confounders (prior VTE, cancer, prior anticoagulant use, BMI, hemoglobin, platelet count, diagnosed sepsis, and receipt of anticoagulation during index hospitalization or at discharge), patients with COVID-19 showed a more than threefold higher adjusted risk of VTE compared with matched patients without evidence of COVID-19 (adjusted hazard ratio [aHR], 3.48; 95% CI, 2.03-5.98).
Finally, among patients with COVID-19, significant multivariable predictors of VTE included age 55 to 64 years (aHR, 1.90; 95% CI, 1.16-3.10 years vs 18-54 years), age 65 to 74 years (aHR, 2.49; 95% CI, 1.52-4.06 years vs 18-54 years), age ≥ 75 years (aHR, 3.32; 95% CI, 1.86-5.92 years vs 18-54 years), Black race (aHR, 1.92; 95% CI, 1.24-2.97 vs White race), prior VTE (aHR, 3.49; 95% CI, 2.83-6.66), moderate or severe liver disease (aHR, 5.95; 95% CI, 2.52-14.07), BMI of ≥ 40.0 kg/m2 (aHR, 2.02; 95% CI, 1.09-3.74 kg/m2 vs 18.5-25.0 kg/m2), platelet count of 217 to 283 K/μL (aHR, 1.70; 95% CI, 1.05-2.77 K/μL vs < 164.0 K/μL), platelet count of > 283 K/μL (aHR, 1.81; 95% CI, 1.13-2.90 K/μL vs < 164.0 K/μL), and having received a diagnosis of sepsis (aHR, 2.68; 95% CI, 1.89-3.79).
Discussion
Among hospitalized adults, COVID-19 was associated independently with a nearly 3.5-fold higher risk of VTE in multivariable analyses that accounted for competing risk of death, a wide range of confounders, and more frequent use of VTE prophylaxis compared with contemporary matched hospitalized control participants. VTE occurred early, and the excess adjusted risk persisted throughout the 30-day follow-up period. The risk of VTE in adults with COVID-19 also was higher with older age, Black race, prior VTE, moderate to severe liver disease, severe obesity, platelet count of > 217 K/μL, and having received a diagnosis of sepsis.
Previous studies exploring VTE in COVID-19 largely have included case reports, case series, or cohorts of only patients with COVID-19 with limited sample size and diversity.6 In a systematic review of 16 published studies of VTE in patients with COVID-19 from multiple countries, significant heterogeneity was present across studies in terms of sample size, severity of COVID-19, and definition of VTE used, which makes combining results across studies challenging.6 Across these studied patients with COVID-19, a reported composite VTE risk of 5% in patients not in the ICU and 31% in patients in the ICU was found, for an overall summary estimate of 21% (95% CI, 17%-26%).6 Bilaloglu et al29 subsequently examined 3,334 hospitalized patients with COVID-19 in New York and reported 6.2% with clinically recognized VTE. Importantly, however, few published studies have included comparable patients without COVID-19. Helm et al30 examined 77 patients with COVID-19 with acute respiratory distress syndrome admitted to four French hospitals and a historical propensity-matched cohort of 145 patients with ARDS and reported a higher crude risk of pulmonary embolism in patients with COVID-19 (11.7% vs 2.1%; P = .01) with adjusted OR of 9.3 (95% CI, 2.2-40). In contrast, Roberts et al31 studied 1,877 patients with COVID-19 discharged alive and observed nine VTE events within 42 days after discharge (0.48 per 100 discharges), with no significant association of COVID-19 hospital discharge compared with 18,159 hospital discharges for any medical illness in 2019 at two London hospitals (OR, 1.6; 95% CI, 0.8-3.1), but this study had limited precision and did not adjust for potential confounders. Our study materially expanded on prior studies to clarify more precisely the short-term risk of VTE associated with hospitalized COVID-19 compared with matched hospitalized patients without COVID-19 derived from a very large, ethnically diverse population receiving care from 36 hospitals that specifically accounted for the impact of competing risk of death. In addition, we provided information on readily available demographic and clinical risk factors for VTE in COVID-19.
The exact mechanisms for why COVID-19 increases VTE risk are not fully delineated, with existing studies having focused primarily on critically ill patients with COVID-19. In fatal COVID-19, widespread alveolar damage, endothelial injury, and severe microangiopathy have been observed.32 Abnormalities in selected coagulation parameters (eg, D-dimer, fibrin degradation product, fibrinogen, prothrombin time, activated partial thromboplastin time) also seem to be common, with significantly worse abnormalities in these parameters in fatal cases.3 In addition, a high proportion of selected patients with severe COVID-19 had potentially pathogenic acquired antiphospholipid antibodies.33 , 34 Importantly, however, the patterns of derangement in coagulation and inflammatory pathways associated with severe COVID-19 may vary from models described for sepsis-induced coagulopathy or disseminated intravascular coagulation,35 , 36 which could have important management implications given the need for robust randomized trial evidence for effective VTE prevention strategies across the spectrum of COVID-19 severity.37 , 38
We studied a carefully matched, demographically diverse cohort with comprehensive longitudinal data through 30 days to facilitate evaluation of the risk of VTE associated with COVID-19 and its severity that extended beyond the index hospitalization. We used a combination of diagnosis codes and a validated natural language processing algorithm applied to EMR data to identify validated acute VTE. We addressed the competing risk of death to evaluate more accurately the excess risk of VTE with COVID-19. Finally, we identified independent predictors of VTE associated with COVID-19 that are readily available clinically.
Our study also had limitations. To maximize generalizability, we included hospitalized adults as control participants if they met matching criteria and did not have a SARS-CoV-2 test performed, but this only affected 13% of all control participants and if some of these patients had undiagnosed COVID-19, this would bias our results to the null. Given the high rate of anticoagulant use during the index hospitalization in the overall cohort and preferentially higher use in patients with COVID-19, the excess risk associated with COVID-19 may be underestimated. Similarly, our models accounted for the competing risk of death from any cause, and it is possible that there were some deaths resulting from undiagnosed VTE, but this tends to bias results toward the null given the substantially higher rate of death in patients with COVID-19. Given that we studied only hospitalized adults, we were unable to evaluate whether the risk of VTE associated with COVID-19 differs in outpatients with less severe COVID-19. Although our study was performed within integrated health care delivery systems caring for populations that are highly representative of the California-wide population, our results may not completely generalize to all countries and health systems.
Interpretation
In a large, diverse, matched cohort of hospitalized adults, COVID-19 was associated independently with a higher risk of VTE, and several patient characteristics were linked to higher VTE risk in the setting of COVID-19. Randomized trials are needed to identify effective and safe thromboembolism preventive strategies in patients with COVID-19.
Take-home Points.
Study Question: Does the novel COVID-19 independently increase the risk for VTE (DVT or pulmonary embolism)?
Results: Among 12,638 ethnically diverse matched hospitalized adults with and without COVID-19, after accounting for competing risk of death and potential confounders, adults with COVID-19 showed a nearly 3.5-fold higher adjusted risk of experiencing VTE compared with matched adults without evidence of COVID-19 (adjusted hazard ratio, 3.48; 95% CI, 2.03-5.98); older age, Black race, prior VTE, diagnosis of sepsis, pre-existing liver disease, severe obesity, and elevated platelet count were associated with higher risk of VTE in patients with COVID-19.
Interpretation: COVID-19 infection significantly increases the risk of clinically recognized VTE in hospitalized adults, with selected readily available patient characteristics associated with a higher risk of VTE in the setting of COVID-19.
Acknowledgments
Author contributions: A. S. G. and G. H. T. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A. S. G., K. R., and M. C . F. conceived and designed the study. A. S. G., G. H. T., E. G., and D. F. acquired the data. A. S. G., K. R., G. H. T., P. A. P., S. H. S., E. G., C. P., D. F., A. P. P., and M. C. F. analyzed and interpreted the data. A. S. G., G. H. T., S. H. S., and M. C. F. drafted the manuscript. A. S. G., K. R., G. H. T., P. A. P., S. H. S., E. G., C. P., D. F., A. P. P., and M. C. F. critically revised the manuscript for important intellectual content. G. H. T. performed the statistical analysis. A. S. G., G. H. T., P. A. P., S. H. S., E. G., C. P., and D. F. provided administrative, technical, and material support. A. S. G., K. R., and M. C. F. supervised the study.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The funder had no role in the design or analysis, interpretation of decision to publish the study results.
Other contributions: The authors thank Rong Wei, MS, and Chengyi Zheng, PhD, for their technical or administrative assistance.
Footnotes
FUNDING/SUPPORT: The study was supported by a research grant from the Patient-Centered Outcomes Research Institute [Grant NOACs-1510-32651]. A. S. G., K. R., and M. C. F. received support through a research grant from the Patient-Centered Outcomes Research Institute for this work.
References
- 1.National Center for Health Statistics, Centers for Disease Control and Prevention National Excess deaths associated with COVID-19. 2020. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm#dashboard
- 2.Bilinski A., Emanuel E.J. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 2020;324(20):2100–2102. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkoudah E., Piazza G., Hecht T.E.H. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF Anticoagulation Action Initiative. Am J Med. 2020;133(suppl 1):1–27. doi: 10.1016/j.amjmed.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Koebnick C., Langer-Gould A.M., Gould M.K. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go A.S., Magid D.J., Wells B. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1(2):138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 11.Magid D.J., Gurwitz J.H., Rumsfeld J.S., Go A.S. Creating a research data network for cardiovascular disease: the CVRN. Expert Rev Cardiovasc Ther. 2008;6(8):1043–1045. doi: 10.1586/14779072.6.8.1043. [DOI] [PubMed] [Google Scholar]
- 12.Escobar G.J., Gardner M.N., Greene J.D., Draper D., Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 13.Fang M.C., Fan D., Sung S.H. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE Study. Med Care. 2016;12:e137–e143. doi: 10.1097/MLR.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortel T.L., Neumann I., Ageno W. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go A.S., Yang J., Ackerson L.M. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 16.Newman T.B., Brown A.N. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4(3):233–237. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arellano M.G., Petersen G.R., Petitti D.B., Smith R.E. The California Automated Mortality Linkage System (CAMLIS) Am J Public Health. 1984;74(12):1324–1330. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 19.Go A.S., Hsu C.Y., Yang J. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol. 2018;13(6):833–841. doi: 10.2215/CJN.12591117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go A.S., Lee W.Y., Yang J., Lo J.C., Gurwitz J.H. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go A.S., Yang J., Gurwitz J.H., Hsu J., Lane K., Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008;168(22):2415–2421. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 23.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 24.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotrlik J.W., Williams H.A., Jabor M.K. Reporting and interpreting effect size in quantitative agricultural education research. J Agric Educ. 2011;52(1):132–142. [Google Scholar]
- 26.Rea L.M., Parker R.A. Jossey-Bass; 2014. Designing and Conducting Survey Research: A Comprehensive Guide. [Google Scholar]
- 27.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 28.Olender S.A., Perez K.K., Go A.S. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts L.N., Whyte M.B., Georgiou L. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Cao W., Jiang W. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo Y., Estes S.K., Ali R.A. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12(570) doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24(1):360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piazza G., Morrow D.A. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA. 2020;324(24):2548–2549. doi: 10.1001/jama.2020.23422. [DOI] [PubMed] [Google Scholar]
- 37.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moores L.K., Tritschler T., Brosnahan S. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]