Clinical Implications.
Screening algorithms employed for vaccine allergy risk assessment before administration of the first dose of the Pfizer-BioNTech COVID-19 mRNA vaccines were integral to rollout and can now be revised based on insights gained on epidemiology and mechanisms.
Higher than expected rates of anaphylaxis have been reported with the Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines when compared with historical vaccine anaphylaxis trends.1 Although the mechanism for such reactions remains unclear, and current anaphylaxis classification schemes may be overly sensitive, early interest has been placed on polyethylene glycol (PEG) 2000 present in the lipid nanoparticle carrier system of these mRNA COVID-19 vaccines.2 In order to attempt to preempt allergic reactions, use of mRNA COVID-19 vaccines has been contraindicated in patients with known allergy to PEG and previously contraindicated in those with known allergy to PEG derivatives such as polysorbate where small studies have suggested cross-reactivity with PEG.3 , 4 The Centers for Disease Control (CDC) have recommended caution and avoidance of vaccination with these mRNA vaccines in those with a history of injectable medication or vaccine anaphylaxis where PEG and polysorbate may be present as excipients, but these allergies are thought to be quite rare overall.2, 3, 4 However, the effectiveness of risk stratification by allergy history is unknown and may be stymied by creating unnecessary perception of mRNA COVID-19 vaccine risk and hesitancy in patients, increased complexity for vaccination sites, and a referral burden to allergists for pre-vaccination risk assessment. Therefore, we examined the outcomes of screening mRNA COVID-19 vaccine risk using patient-reported anaphylaxis history at a single academic hospital employee vaccination program.
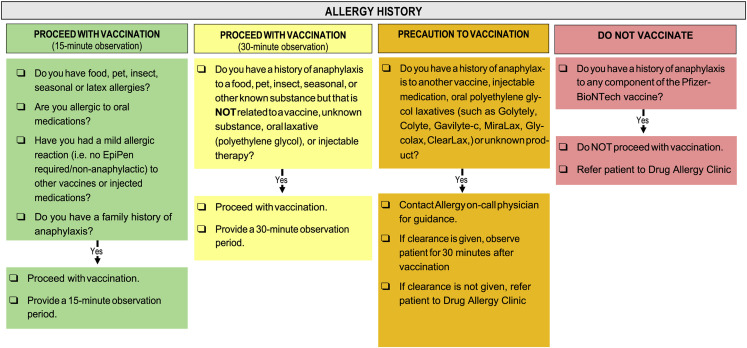
Our study presents a retrospective cohort study performed under institutional review board (IRB) approved protocols from Vanderbilt University Medical Center (VUMC), IRB #210328. Between December 17, 2020, and March 17, 2021, 23,035 sequential health care workers and other affiliates who, at the time of vaccination, met criteria based on the Tennessee Department of Health COVID-19 Vaccination Plan5 received the first dose of the Pfizer-BioNTech mRNA COVID-19 at VUMC Occupational Health sites. The Pfizer-BioNTech was the only formulation of an mRNA COVID-19 vaccine available at VUMC at the time. In collaboration between the Division of Allergy and the Occupational Health Clinic at VUMC, a screening algorithm for stratification of mRNA COVID-19 vaccine risk was developed using CDC and expert guidance6 , 7 (Figure 1 ) and employed at the time of Occupational Health vaccination appointments. Individuals with a history of anaphylaxis to an injectable medication, vaccine, oral PEG3350, or unknown cause (idiopathic) triggered a focused review of the implicated cause by the Allergist On-Call by telephone. If the implicated drug in a previous potentially allergic reaction did not contain PEG or polysorbate, individuals were recommended to proceed to vaccination with a 30-minute observation. If the implicated drug likely contained PEG or polysorbate or the cause unknown, subsequent tolerance of PEG or polysorbate containing medications was questioned. If subsequent tolerance of PEG or polysorbate was known, individuals were recommended to proceed to vaccination with a 30-minute observation. If subsequent tolerance of PEG or polysorbate was not known, individuals did not proceed to vaccination and were referred to the Drug Allergy Clinic for assessment before immunization. Calls were logged in REDCap (Research Electronic Database Capture) hosted at VUMC at the time of the focused review by the Allergist On-Call.8 Demographics (age, sex), anaphylaxis history, subsequent tolerance of PEG and polysorbate if applicable, and dose 1 recommendation were recorded. In addition, the Occupational Health Clinic used a REDCap project to capture individuals who reported an adverse reaction to the Pfizer-BioNTech mRNA COVID-19 vaccine. Those who reported a suspected allergy-related adverse reaction to dose 1 administration were referred by Occupational Health to the Drug Allergy Clinic. All charts were subsequently reviewed for the outcomes of dose 1 administration, including if a referral was placed before receipt of dose 1 or after dose 1 for an adverse reaction. In those who were referred to the Drug Allergy Clinic, PEG and polysorbate skin testing results, by previously reported protocols,3 if applicable, and recommendation on proceeding to dose 1 were also reviewed.
Figure 1.
Screening algorithm for patients with low- (green), moderate- (yellow), and high-risk (orange) allergy history and contraindication to vaccination (red).
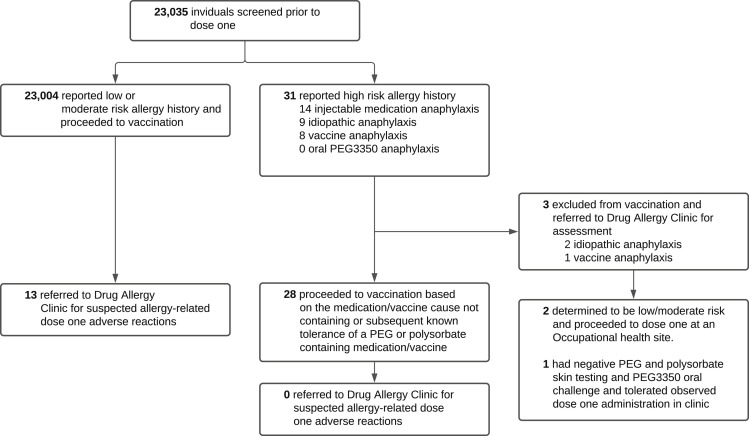
Of 23,035 individuals screened before dose 1 of the Pfizer-BioNTech mRNA COVID-19 vaccine, 31 reported a high-risk allergy history that triggered a focused review by the Allergist On-Call (Figure 2 ). The implicated causes included 14 injectable medication anaphylaxis, 9 idiopathic anaphylaxis, 8 vaccine anaphylaxis, and 0 oral PEG3350 anaphylaxis. For those reporting a high-risk allergy history, the median age was 40 years (interquartile range, 30-53 years) and 71% were female (3 of 31 individuals were missing age and sex data) (Table I ). Of the 31 individuals with a high-risk allergy history, 28 (90%) were recommended to proceed to dose 1 of the Pfizer-BioNTech mRNA COVID-19 vaccine based on implicated cause known to not contain PEG or polysorbate or known subsequent tolerance of a PEG or polysorbate containing medication or vaccine (Table I). Of the 3 individuals recommended to not proceed to dose 1 and referred to the Drug Allergy Clinic for assessment, 1 referral was for vaccine anaphylaxis possibly containing polysorbate and unknown subsequent tolerance of PEG and polysorbate, and 2 referrals were for idiopathic anaphylaxis with unknown subsequent tolerance of PEG or polysorbate. Of the 28 high-risk allergy history workforce members who proceeded to the first dose of the Pfizer-BioNTech COVID-19 mRNA vaccine, none were later referred by Occupational Health for evaluation of a dose 1 adverse reaction (Figure 2). Of the 3 individuals referred to the Drug Allergy Clinic before receipt of dose 1, the 2 patients with a history of idiopathic anaphylaxis were determined not to be high-risk on formal consultation (1 had actually tolerated oral PEG3350 and 1 had an attributable cause of medication-induced anaphylaxis). Neither individual underwent PEG and polysorbate skin testing, and both proceeded to tolerate dose 1 at an Occupational Health site. The third individual referred to the Drug Allergy Clinic before receipt of dose 1 had a history of vaccine anaphylaxis and underwent PEG and polysorbate skin testing and oral PEG3350 challenge, both of which were negative. This individual then went on to receive and tolerate the first dose in the Drug Allergy Clinic. Of the 23,004 workforce members with a low- or moderate-risk allergy history who proceeded to dose 1 vaccination, 13 were referred to the Drug Allergy Clinic for evaluation of dose 1 adverse reactions (Figure 2), none of whom had a previous history of reaction to a PEG or polysorbate containing drug.
Figure 2.
Flowchart of the study. PEG, Polyethylene glycol.
Table I.
Demographics, anaphylaxis history, and outcomes for patients with a high-risk allergy history
| Characteristic | Total N (% total) or median [IQR] |
|---|---|
| Total no. of patients | 31 |
| Age (n = 28, with n = 3 missing) | 40 [30, 53] |
| Sex | |
| Female | 22 (71) |
| Male | 6 (19) |
| Unknown | 3 (10) |
| Anaphylaxis history | |
| Injectable medication | 14 |
| PEG or PS containing | 1 |
| Subsequent PEG or PS tolerance | 1 |
| Vaccine | 8 |
| PEG or PS containing | 7 |
| Subsequent PEG or PS tolerance | 6 |
| Oral PEG3350 | 0 |
| Subsequent PEG or PS tolerance | N/A |
| Unknown cause (idiopathic) | 9 |
| Subsequent PEG or PS tolerance | 7 |
| On-Call Allergy recommendation | |
| Proceed with vaccination | 28 (90) |
| Tolerated vaccination | 28/28 |
| Do not vaccinate and refer to Allergy Clinic | 3 (10) |
| Drug Allergy Clinic assessment outcome | |
| Proceed with vaccination after consultation | 3 |
| Vaccine administered at Allergy Clinic | 1 |
| Tolerated vaccination | 1/1 |
| Vaccine administered at Occupational Health site | 2 |
| Tolerated vaccination | 2/2 |
IQR, Interquartile range; N/A, not available; PEG, polyethylene glycol; PS, polysorbate.
In the experience of a single, large academic hospital employee vaccination program, 0.1% (31 of 23,035) of workforce members reported a high-risk allergy history that triggered a focused review by the Allergist On-Call (Figure 2). Of those with a high-risk allergy history, 90% (28 of 31) were able to proceed to dose 1 due to lack of PEG or polysorbate in the implicated cause and/or known subsequent tolerance of PEG or polysorbate containing medications or vaccines. None of the 28 individuals with a high-risk allergy history who proceeded to dose 1 experienced a dose 1 adverse reaction that necessitated referral to the Drug Allergy Clinic by Occupational Health. Of the 3 high-risk individuals referred to the Drug Allergy Clinic for further risk assessment before dose 1, all 3 ultimately received and tolerated dose 1 uneventfully. Of the 13 referrals to the Drug Allergy Clinic for evaluation of dose 1 adverse reactions, all were from individuals with a low- or moderate-risk allergy history. The overall referral rate to the Drug Allergy Clinic for dose 1 adverse reactions was 0.06% (13 of 23,035) of workforce members. Of particular note, there were no new diagnoses of PEG allergy during this study period.
A limitation of our study is that it is retrospective and may underrepresent the number of individuals with a high-risk allergy history due to complexity of history reporting and vaccination sites interpreting possible history of anaphylaxis in real time. It is also possible that those with highest risk allergy histories were more hesitant and declined COVID-19 vaccination during this initial rollout period. Further, referrals to the Drug Allergy Clinic by Occupational Health may underrepresent the number of individuals who experienced dose 1 adverse reactions, as some may choose to defer second dose vaccination themselves without reporting their reaction to Occupational Health for further assessment. Prospective studies of potentially allergic reactions to the first dose of mRNA COVID-19 vaccines where there has been full ascertainment of symptoms showed rates of approximately 2% in healthcare workers,9 which is significantly higher than our 0.06% of individuals later referred to the Drug Allergy Clinic after dose 1 adverse reactions.
This is the first study that reports on the outcomes and utility of screening mRNA COVID-19 vaccine risk by allergy history. We show that only 0.1% of individuals report a high-risk allergy and none of the high-risk allergy workforce members required referral to the Drug Allergy Clinic for first dose adverse reactions. Rather, all referrals to the Drug Allergy Clinic were from individuals with a low- or moderate-risk allergy history. Overall, only 3 of 23,035 (0.01%) individuals were excluded from vaccination based on prior allergy history at the time of presentation to the Occupational Health vaccine clinics. There are no other reports on exclusion rates at the time of presentation for COVID-19 vaccination based on allergy history and likely are to be higher where Allergy Specialists On-Call are not available. The complexity of current screening algorithms may be causing unwarranted perception of risk and hesitancy toward mRNA COVID-19 vaccination without effectiveness in promoting their safety. Therefore, it may now be possible to revise mRNA COVID-19 vaccination to more targeted criteria, similar to other vaccines, such as: (1) history of anaphylaxis to a prior mRNA COVID-19 vaccine and (2) history of known prior PEG allergy?
Our study demonstrates that a pre-existing PEG/polysorbate allergy is likely to be encountered rarely, and given that there is lack of knowledge regarding mechanisms of COVID-19 mRNA vaccine anaphylaxis and other immediate reactions, there is a potential lack of utility for vaccine risk-stratification algorithms that focus on histories of reactions to drugs or other vaccines in identifying those who react to the first dose of an mRNA COVID-19 vaccine. Importantly, this suggests the likely rarity of PEG IgE-mediated reactions in this group and suggests that for the commonly experienced reactions other mechanisms are at play.
Footnotes
C. A. Stone receives funding from AHRQ/PCORI 1K12HS026395-01. E. J. Phillips reports grants from National Institutes of Health (P50GM115305, R01HG010863, R01AI152183, R21AI139021, U01AI154659) and from the National Health and Medical Research Council of Australia.
Conflicts of interest: E. J. Phillips receives royalties from Uptodate and consulting fees from Janssen, Vertex, Biocryst, and Regeneron. She is co-director of IIID Pty Ltd that holds a patent for HLA-B∗57:01 testing for abacavir hypersensitivity, and has a patent pending for Detection of Human Leukocyte Antigen-A∗32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work. Funders played no role in any aspect of this manuscript. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabanillas B., Akdis C., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76:1617–1618. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 3.Stone C.A., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7 doi: 10.1016/j.jaip.2018.12.003. 1533-40.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z.-H., Stone C.A., Jakubovic B., Phillips E.J., Sussman G., Park J. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2020.11.011. 1731-33.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tennessee Department of Health COVID-19 vaccination plan; March 8, 2021. https://www.tn.gov/content/dam/tn/health/documents/cedep/novel-coronavirus/COVID-19_Vaccination_Plan.pdf Available from:
- 6.Centers for Disease Control and Prevention Interim clinical considerations for use of mRNA COVID-19 vaccines. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html Available from:
- 7.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal K.G., Robinson L.B., Camargo C.A., Shenoy E.S., Banerji A., Landman A.B. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]