ABSTRACT
The emergence of more transmissible and/or more virulent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOC) has triggered intensive genomic surveillance, which is costly and difficult to sustain operationally over the long term. To address this problem, we developed a set of four multiplex mutation-specific PCR-based assays with same-day reporting that can detect five VOC and three variants of interest (VOI), as defined in the March 2021 guidelines from the U.S. Centers for Disease Control and Prevention (https://www.cdc.gov/coronavirus/2019-ncov/). The screening results were compared to the whole-genome sequencing (WGS) and showed 100% concordance for strain typing for B.1.1.7 (n = 25) and P.1 (n = 5) variants using spike (S) mutation S-N501Y, S-E484K, and S-H69–V70del assays. The S-L450R assay, designed to detect the B.1.427/429 VOC, also identified multiple isolates of a newly emerging multiply mutated B.1.526.1 variant that is now rapidly increasing in the eastern United States. PCR approaches can be easily adopted in clinical laboratories, providing rapid screening methods to allow early detection of newly emergent variants and to efficiently triage cases for full genomic sequencing.
KEYWORDS: SARS-CoV-2, variant screening, newly emerging SARS-CoV-2 variant
INTRODUCTION
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved considerably in the last 6 months. Most mutations of interest occur in the spike (S) protein, the viral protein that binds to the angiotensin-converting enzyme 2 (ACE2) cell receptor to initiate the attachment of the virus. Beginning in late 2020, several biologically significant S mutations were shown to be associated with increased transmissibility and virulence and diminished protection by antibodies from convalescent or vaccinated antisera, as well as decreased response to monoclonal antibody treatment. Therefore, strategies to cost-effectively monitor for shifts in SARS-CoV-2 variants are needed.
Three variants of concern (VOC) with multiple mutations in the S gene have been identified as particularly concerning. The B.1.1.7 variant (501Y.V1) emerged in England, rapidly became the dominant variant in the United Kingdom, and has now spread to more than 50 countries (1). B.1.1.7 contains 8 to 13 different S mutations, including N501Y in the receptor binding domain (RBD). Studies suggested that it may be associated with higher transmissibility and increased virulence (2, 3). The B.1.3.5.1 variant (510Y.V2) was first reported in late 2020 in South Africa (4), and the P.1 variant (20J/501Y.V3) arose in November 2020 in Brazil. The three spike mutations (K417N/T, E484K, and N501Y) carried by both the Brazil and South African variants are associated with increased binding to the human ACE2 receptor, are more transmissible, and mediate partial escape from the protective humoral immunity resulting from prior infection or vaccination (5–7).
In March 2021, the U.S. Centers for Disease Control and Prevention (CDC) included B.1.427/B.1.429 as an additional VOC due to its rapid rise in incidence, and introduced the “variant of interest” (VOI) category to encompass strain mutations that might override neutralizing antibody responses, principally S-E484K (8, 9). B.1.427/B.1.429 carries the S-L452R mutation, which appears linked to the increased transmissibility of this strain (10). S-L452R may be associated with reduction in neutralization using convalescent, postvaccination sera and resistance to the neutralization by some monoclonal antibodies that have acquired Emergency Use Authorization for treatment (10–12).
The emergence of these VOC highlights that enhanced surveillance is urgently needed. Real-time identification of both VOC and VOI will have a significant impact not only on management of this ongoing pandemic but also patient care (e.g., monoclonal antibody therapy). However, this is not possible without local sequencing capacity, which is not always available or may be limited in throughput capacity; whole-genome sequencing (WGS) is also costly and takes considerable time to complete. An accurate screening strategy would allow the selective use of WGS by targeting samples of interest. This would maximize the use of WGS and broaden the clinical laboratories able to actively participate in identification of VOC.
Here, we developed real-time reverse transcriptase PCR (RT-PCR)-based assays to screen for spike protein deletions 69 to 70 (S-Δ69–70) and 242 to 244 (S-Δ242–244) and mutations S-N501Y, S-E484K, and S-L452R in clinical samples known to be positive for SARS-CoV-2. The pattern of positivity accurately typed each VOC (as confirmed by genomic sequencing), except for B.1.427/429, where the majority of cases detected (in March to April 2021) corresponded to a newly emerging multiply mutated variant (previously typed as B.1.526.1).
MATERIALS AND METHODS
Study samples.
Nasopharyngeal (NP) swab samples were collected from pediatric patients and employees of Nationwide Children’s Hospital, Columbus, Ohio. A flocked NP swab was used and placed into viral transport medium for transport to the laboratory for testing by a SARS-CoV2 PCR assay. This surveillance study utilized residual samples for assay validation and was thus exempt from human subject research. The set comprised 247 samples, including 156 SARS-CoV-2-positive swabs from 2 February to 1 April 2021 that included all adequate samples with threshold cycle (CT) values of less than 35, and 91 samples collected from 1 January to 1 February 2021 with CT values of less than 35 that were randomly selected from adequate residual samples.
Diagnostic SARS-CoV-2 PCR assay.
We utilized a laboratory-developed modification of the CDC SARS-CoV-2 RT-PCR assay, which received Emergency Use Authorization by the FDA on 17 April 2020. (https://www.fda.gov/media/137424/download [accessed 24 March 2021]). Briefly, total nucleic acid was obtained by extraction using the NucliSENS easyMag platform (bioMérieux, Durham, NC), and 5 μl of the eluate was added to a 25-μl total-volume reaction mixture (5 μl of 1× TaqPath 1-Step RT-qPCR Master Mix, CG [Thermo Fisher Scientific, Waltham, MA], 1.5 μl of RT-PCR primer/probe set [N1 or N2, 2019-nCoV kit; Integrated DNA Technologies, Coralville, IA], and 13.5 μl nuclease-free water). The RT-PCR was carried out using the Applied Biosystems QuantStudio 7 Flex Real Time PCR detection system with QuantStudio Real-Time PCR software v.1.3 (Thermo Fisher Scientific, Waltham, MA) under the following running conditions: 25°C for 2 min, 50°C for 15 min, and enzyme activation at 95°C for 2 min, followed by 45 cycles of 95°C for 3 s and 55°C for 30 s.
Mutation screening assays.
SARS-CoV-2-positive samples were screened by four multiplex RT-PCR assays, with results available on the same day.
The sequences for detection of Δ69–70 were adapted from a multiplex real-time RT-PCR assay for detection of SARS-CoV-2 (13). The probe overlaps with the sequences that contain amino acids 69 to 70; therefore, a negative result for this assay predicts the presence of deletion S-Δ69–70 in the sample. Using a similar strategy, a primer/probe set that targets the deletion S-Δ242–244 was designed and was run in the same reaction with S-Δ69–70. In addition, three separate assays were designed to detect spike mutations S-501Y, S-484K, and S-452R and wild-type positions S-501N, S-484E, and S-452L. The sequences for each oligonucleotide are presented in Table 1.
TABLE 1.
Mutation-specific reverse transcriptase PCR primers and probes
| Assay | Oligonucleotide | Sequencea |
|---|---|---|
| S-Δ69–70/Δ242–244 multiplex assayb | S-Δ69–70 F | 5′-TCAACTCAGGACTTGTTCTTAC-3′ |
| S-Δ69–70 R | 5′-TGGTAGGACAGGGTTATCAAAC-3′ | |
| S-Δ69–70 P | 5′-FAM-TGGTCCCAGAGACATGTATAGCAT-BHQ1-3′ | |
| S-Δ242–244 F | 5′-TGGTAGATTTGCCAATAGGTATTAACA-3′ | |
| S-Δ242–244 R | 5′-CTGAAGAAGAATCACCAGGAGTCA-3′ | |
| S-Δ242–244 P | 5′-VIC-ACTTTACTTGCTTTACATAGAAG-MGB-3′ | |
| S-N501Y assayc | S-N501Y F | 5′-TGTTACTTTCCTTTACAATCATATGGTTTC-3′ |
| S-N501Y R | 5′-GAAAGTACTACTACTCTGTATGGTTGGTAACC-3′ | |
| S-501N P1 | 5′-VIC-CAACCCACTaATGGTGTT-MGB-3′ | |
| S-501Y P2 | 5′-FAM-CAACCCACTtATGGTGTT-MGB-3′ | |
| S-E484K assayc | S-E484K F | 5′-CAGGCCGGTAGCACACCTT-3′ |
| S-E484K R | 5′-GTTGGAAACCATATGATTGTAAAGGA-3′ | |
| S-484E P1 | 5′-FAM-TAATGGTGTTgAAGGTTT-MGB-3′ | |
| S-484K P2 | 5′-VIC-TGTAATGGTGTTaAAGGT-MGB-3′ | |
| S-L452R assayc | S-L452R F | 5′-TGATAGATTTCAGTTGAAATATCTCTCTCA-3′ |
| S-L452R R | 5′-AATCTTGATTCTAAGGTTGGTGGTAATTAT-3′ | |
| S-452L P1 | 5′-FAM-CCTAAACAATCTATACaGGTAA-MGB-3′ | |
| S-452R P2 | 5′-VIC-CTAAACAATCTATACcGGTAAT-MGB-3′ | |
FAM, 6-carboxyfluorescein; BHQ1, black hole quencher 1; MGB, minor groove binder; lowercase letters within probes represent the locations of nucleotide mutations.
Described in reference 13.
Primers and probes designed by authors.
Briefly, 5 μl of the total nucleic acid eluate was added to a 20-μl total-volume reaction mixture (1× TaqPath 1-Step RT-qPCR Master Mix, CG [Thermo Fisher Scientific, Waltham, MA], with 0.9 μM each primer and 0.2 μM each probe). The RT-PCR was carried out using the ABI 7500 thermocycler (Life Technologies, Grand Island, NY). The S-N501Y, S-E484K, and S-L452R assays were carried out under the following running conditions: 25°C for 2 min, then 50°C for 15 min, followed by 10 min at 95°C and 45 cycles of 95°C for 15 s and 65°C for 1 min. The Δ69–70/Δ242–244 assay was run under the following conditions: 25°C for 2 min, then 50°C for 15 min, followed by 10 min at 95°C and 45 cycles of 95°C for 15 s and 60°C for 1 min. Samples displaying typical amplification curves above the threshold were considered positive.
Samples that yielded a negative result or results in the S-Δ69–70/Δ242–244 assay or were positive for S-501Y P2, S-484K P2, and S-452R P2 were considered screen positive and assigned to a VOC based on the scoring in Table 2. All screening-positive samples and a similar number of screening-negative samples collected in the same period were sent for WGS.
TABLE 2.
Strain-typing rules for interpretation of screening results
| Strain type | Presence of spike mutationa: |
||||
|---|---|---|---|---|---|
| Δ69–70 | Δ242–244 | N501Y | E484K | L452R | |
| Variants of concern | |||||
| B.1.1.7 | Pos | Neg | Pos | Neg | Neg |
| B.1.351 | Neg | Pos | Pos | Pos | Neg |
| P.1 | Neg | Neg | Pos | Pos | Neg |
| B.1.427/B.1.429 or B.1 80G-157L-4 | Neg | Neg | Neg | Neg | Pos |
| Variants of interest | |||||
| B.1.525 | Pos | Neg | Neg | Pos | Neg |
| R.1, P.2, B.1.525, B.1.526 | Neg | Neg | Neg | Pos | Neg |
| B.1.2/501Y or B.1.1.165 | Neg | Neg | Pos | Neg | Neg |
Pos, positive; Neg, negative.
SARS-CoV-2 whole-genome sequencing.
Previously extracted RNA underwent 1st- and 2nd-strand cDNA synthesis (NEBNext Ultra II Non-Directional RNA Second Strand Synthesis Module; NEB, Ipswich, MA), followed by sequencing using two different clinically validated amplicon-based methods. Most samples were analyzed using the SARS-CoV-2 Research Panel primers (ThermoFisher) on the Ion Chef-S5 sequencer (ThermoFisher) per the manufacturer’s conditions. The remaining sequences were analyzed using the CovidSeq kit (Illumina, San Diego, CA) per the manufacturer’s conditions and sequenced on the NextSeq 550 sequencer (Illumina). Strain typings by the two different assays were cross-validated using a set of 20 SARS-CoV-2-positive samples and 5 SARS-CoV-2-negative samples.
Genomic analyses and strain typing.
Sequence analysis tools include custom pipelines utilizing GATK and Mutect2 (Broad Institute) and Dragen SARS-COVID variant detection (for the CovidSeq assay). Adequate sequencing required coverage of strain-distinguishing areas of the open reading frame 1a (ORF1a), S, N, and ORF8 genes to at least 100× depth. All but two sequences in this series yielded adequate strain-typeable sequences, and no sample contained a mixed population of viruses. Viral sequences were strain typed using Pangolin (https://cov-lineages.org) and NextStrain (https://nextstrain.org/blog/2021-01-06-updated-SARS-CoV-2-clade-naming) criteria. Novel combinations of mutations were investigated for similar viruses using the laboratory internal sequence database (1,110 sequences as of 1 April 2021) and/or the GISAID database (accessed at https://www.gisaid.org).
RESULTS
Correlation of RT-PCR mutation screen results with whole-genome SARS-CoV-2 sequencing.
From January to 1 April 2021, we screened 247 samples for VOC and VOI. Among all screened samples, 186 samples were screen negative and 61 were screen positive, with mean diagnostic PCR CT values of 21.5 and 21.7, respectively. A total of 128 samples were collected from hospital employees, 91 samples were obtained from outpatients, and 28 samples were obtained from hospitalized or emergency room patients. A total of 102 samples were sent for WGS.
There were 61 screen-positive samples and 41 screen-negative samples sent for WGS. The screening assays were 100% concordant with the WGS for identification of the three receptor binding domain (RBD) mutations (Table 3). The screening assays successfully predicted the presence of all 25 B.1.1.7 and 5 P.1 VOC (Table 4). Six samples were positive for only the E484K mutation, with five confirmed to be P.2 and one confirmed to be R.1 by WGS. The mean diagnostic PCR CT values are 22.6 for 25 B.1.1.7-carrying samples, 19.1 for 5 P.1-carrying samples, and 19.6 for the 3 B.1.427/B.1.429-carrying samples. The screening PCR assays had 100% negative agreement compared to WGS for prediction of these VOC and VOI. Sixteen of the samples were positive only for N501Y and were shown by sequencing to be the 20G/501Y strain we have previously described (14) in 14 samples and B.1.1.165 in 2 samples. Eight samples were screen positive for S-L452R, with 3 of them strain typed as B.1.427/B.1.429 and five representing the newly emerging B.1.526.1 variant described in more detail below.
TABLE 3.
Performance of the screening assays for detection of RBD mutations
| Screening assay result | No. of WGS results (n = 102) for spike mutationa: |
|||||
|---|---|---|---|---|---|---|
| L452R |
E484K |
N501Y |
||||
| Pos | Neg | Pos | Neg | Pos | Neg | |
| Pos | 8 | 0 | 12 | 0 | 46 | 0 |
| Neg | 0 | 94 | 0 | 90 | 0 | 56 |
For each comparison of positive (Pos) and negative (Neg) screening assay results and WGS results for each mutation shown, there was 100% positive percentage of agreement (PPA) and 100% negative percentage of agreement (NPA).
TABLE 4.
Performance of the screening assays for detection of variants of concern or interest
| Screening assay result | No. of WGS results (n = 102) fora: |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variants of concern |
Variants of interest |
|||||||||||||
| B.1.1.7 |
B.1.351 |
P.1 |
B.1.427 or B.1.429 |
B.1.525 |
P.2, B.1.526, or R.1 |
20G/501Yb or B.1.1.165 |
||||||||
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | |
| Pos | 25 | 0 | 0 | 0 | 5 | 0 | 3 | 5c | 1 | 0 | 6d | 0 | 16 | 0 |
| Neg | 0 | 77 | 0 | 102 | 0 | 97 | 0 | 94 | 0 | 101 | 0 | 96 | 0 | 86 |
For each comparison of positive (Pos) and negative (Neg) screening assay results and WGS results for each variant shown, there was 100% positive percentage of agreement (PPA) and 100% negative percentage of agreement (NPA), except for B.1.351 (PPA not applicable) and B.1.427 or B.1.429 (PPA of 37.5%).
As in reference 14.
All five samples were identified carrying a newly emerged variant: B.1.526.1.
Five samples were identified as strain P.2, and one sample was strain R.1.
All negative screening samples were negative for VOC or VOI by WGS. Forty-one screen-negative samples were found to be commonly encountered B.1 or B.1.2, with no S mutations of concern. No sample was positive only for S deletion Δ69–70 or Δ242–244. One sample that had suboptimal amplification in the S-Δ69–70 assay was found to have a mutation in the primer binding site.
Timing of emergence of PCR-detected variants of concern matches results.
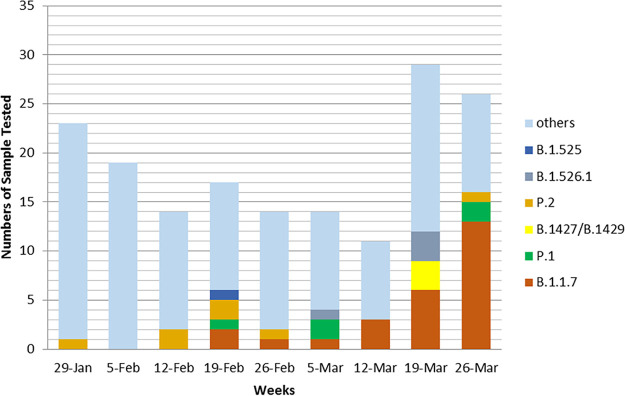
From January to 1 April 2021, we screened 247 SARS-CoV-2 samples. As shown in Fig. 1, the earliest case with B.1.1.7 was identified in mid-February 2021, subsequently, cases of B.1.1.7 were found in every batch of samples tested. The earliest case with P.1 was also collected in mid-February 2021, with a substantial increase in P.1-positive cases over time. The first B.1.429 case in our population appeared in late March 2021.
FIG 1.
Shifts in the proportions of SARS-CoV-2 strains from January to March 2021.
In the third week of February 2021, one sample collected from a patient was found to carry both N501Y and E484K mutations and was sent for WGS. The sequences of this sample are closely related to a Brazilian P.1. VOC, and it was designated into lineage B.1.1.28 and was the first P.1 sample reported in the state of Ohio (EPI_ISL_1164183). In the same week, another sample collected from an employee was found to carry Δ69–70 and the E484K mutation, which represents the first Ohio sample of the emerging B.1.525 lineage (EPI_ISL_1203843).
Three of the L452R-bearing viruses in our screen were documented to be B.1.427/429. This VOC first appeared in California in July 2020 (EPI_ISL_765997) and is characterized by spike mutations S13I and W152C, as well as L452R. In early November 2020, B.1.427/429 became the predominant strain in southern California before spreading throughout the United States in December to January 2020. The observed increase in B.1.427/429 in our study matches that seen in CDC totals from WGS in Ohio.
Emergence of the B.1.526.1 L452R-containing variant.
Unlike the other VOC in our study, we noted that a positive screen for L452R still required sequencing to definitively strain type. This was due to the emergence in late March in our area of another L452R-bearing strain besides the B.1.427/429 VOC. Five of 8 (62.5%) screen-positive L452R-containing viruses from March 2021 represented a newly emerging strain that has been tentatively strain typed as B.1.526.1 (Table 5). The diagnostic PCR CT values are presented in Table 6. In addition to S-L452R, this strain has four additional S mutations—D80G, F157S, T859N, and D950H—which include both N-terminal and stalk variants. It shares with B.1.427/429, the 20C/B.1 backbone as indicated by mutations ORF1-T265I, ORF1a-A1679S, ORF1a-L3201P, ORF1b-P314L, ORF3a-Q57H, and S-D614G. None of these isolates harbors the E484K mutation.
TABLE 5.
Spike mutations associated with a newly emerged S-L452R-containing variant
| Amino acid change(s) | cDNA location | No. (%) of viruses with the indicated spike mutation |
Domainb | Other lineage(s) commonly showing mutation | |
|---|---|---|---|---|---|
| GISAIDa | B.1.526.1 | ||||
| p.Asp80Gly | c.239A>G | 587 | 548 (93.4) | NTD | |
| p.Phe157Ser | c.470T>C | 661 | 588 (89.0) | NTD | |
| p.Leu452Arg | c.1355T>G | 12,997 | 608 (4.7) | RBD | B.1.427/429, A.2.5 |
| p.Thr859Asn | c.2576C>A | 840 | 461 (54.9) | CR | B.1.36.9 |
| p.Asp950His | c.2848G>C | 459 | 419 (91.3) | HR1 | |
| 4 or 5 of these S mutations | 449 | 449 (100) | |||
Data as of 10 April 2021.
Abbreviations: p, protein (HUGO nomenclature); c, coding positions in reference to the Wuhan-Hu-1 strain (MN908947.2) (HUGO nomenclature); NTD, N-terminal domain; RBD, receptor binding domain; CR, connecting region; HR1, heptad repeat 1.
TABLE 6.
CT values of the samples carrying newly emerged S-L452R-containing variant
| Source of B.1.526.1 sample |
CT value |
|
|---|---|---|
| N1 | N2 | |
| Patient 1 | 31.8 | 33.3 |
| Patient 2 | 23.2 | 23.5 |
| Employee 1 | 13.6 | 12.5 |
| Patient 3 | 29.2 | 28.8 |
| Patient 4 | 23.4 | 24.2 |
Based on the frequency of the spike mutations in B.1.526.1 viruses reported in GISAID as of 10 April 2021, the variant likely developed from an L452R-bearing B.1 strain, then acquired F157S, D80G, and Y145del, followed by T859 and D950H. The few GISAID sequences that do not follow this pattern are reported as suboptimal quality studies. A small number (n = 7) of B.1.526.1 viruses contain D80N/N81Y instead of D80G. The earliest GISAID-reported instances of B.1.526.1 were two sequences from New York City collected on 18 December 2020 (EPI_ISL_794288 and EPI_ISL_794289), with all sequences through 5 February 2021 reported from New York City (mostly Bronx County). Spread to 11 other states (including Ohio) occurred in February and then throughout the United States in March 2021. Given that 578 of 2,369 (24.4%) viruses typed as B.1.526.1 lineage in GISAID do not contain any of the spike mutations described above, lineage classification may be provisional.
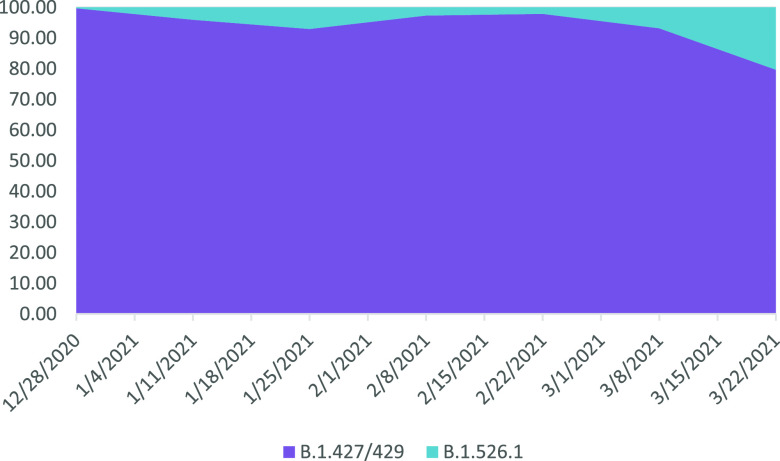
A comparison of the incidence of the B.1.427/429 and B.1.526.1 strains in GISAID during 2021 shows a declining proportion of the forms and increases in the latter in March matching our observations in Columbus, OH (Fig. 2).
FIG 2.
Comparison of incidence in the United States of B.1.427/429 and B.1.526.1 in 2021. Spike L452R-bearing SARS-CoV-2 genomes from North America were downloaded from GISAID, and the strain type was confirmed by mutation pattern. Graphed is the proportion of B.1.427/429 versus B.1.526.1 viruses during each 2-week period in 2021.
DISCUSSION
We describe a multiplex, mutation-specific RT-PCR-based strategy to rapidly and reliably screen for SARS-CoV2 genetic variants in a clinical laboratory setting. These assays detect all three target RBD spike mutations and have 100% concordance with the strains identified by WGS. Using simple rules based on the combination of assay results, the strategy successfully identified all B.1.1.7 and P.1 samples, with the frequencies of each of the VOC detected matching their emergence by the CDC and Department of Health WGS surveillance studies over the last several months. Using this strategy, we successfully identified and reported the first P.1 variant in Ohio from a patient, which suggested that the P.1 variant has already spread in the community—a finding confirmed in subsequent reports.
However, for S-L452R-positive screens, we noted that both B.1.427/429 VOC and a newly emerging B.1.526.1 strain were detected (15). The latter samples were identified only in the latest March 2021 samples and match the observed spread of GISAID-reported B.1.526.1 viruses outside the state of New York. Although both of these strains show multiple mutations in other areas of the spike gene, the L452R mutation is their only RBD mutation. This mutation has been associated with resistance to neutralization by RBD-binding monoclonal antibodies, such as bamlanivimab (12), which has affected the use of these therapies. The single RBD mutation in these two strains is in contrast to other VOC, which besides N501Y and E484K (in two of them, P.1 and B.1.351) show additional mutations known to alter receptor binding affinity (16–18). It will be important to monitor sequential samples from these strains to see if they acquire additional RBD mutations, particularly at antibody escape residues, as B.1.1.7 viruses have done in some cases.
Mutation-specific PCR assays can be run in real time with a turnaround time of several hours, which is significantly faster than WGS and in this configuration can be performed cost-effectively on all PCR-positive samples. This broader approach is clearly superior to the use of other VOC indicators such as S gene dropout in the 3-target diagnostic RT-PCR assay (TaqPath kit, Thermo Fisher Scientific, Waltham, MA) which has been reported by others as a method to detect the H69–V70del associated with B.1.1.7 (9, 19–23). Similarly, PCR-based N501Y and E484K mutation-specific assays are sensitive for detection of P.1 and B.1.351 (24, 25), respectively, but will not definitively distinguish them as an increasing number of VOIs also bear these mutations. In this study, we could not fully evaluate all scoring combinations, particularly the use of copositivity for N501Y and Δ242–244 as a marker for the B.1.351 VOC, as we did not find any examples of this virus in our test population. However, the combination of the assays presented here has more diagnostic utility in distinguishing VOC and VOI than single-mutation-specific PCR approaches. In addition, this approach makes it possible to triage for bamlanivimab therapy through detection of the mutation L452R in a few hours.
There are some limitations to a reflex mutant-PCR panel strategy. First, many diagnostic PCR platforms can deplete swab material, leaving an inadequate volume of residual sample for a multitube mutation screen. These assays also did not perform well when the diagnostic PCR CT value is greater than 35 cycles. These assays were designed for maximal specificity with higher annealing temperature; different conditions (lower annealing temperature and preamplification) can be used for samples with high CT values for higher sensitivity but may compromise specificity. This problem is also shared with amplicon-based WGS (26). As demonstrated by our finding with L452R-positive samples, new variants of SARS-CoV-2, are still emerging, so confirmation of some results by WGS will still be required. Finally, to evaluate for novel mutations, a sampling of screen-negative specimens for WGS will be an important component of a full surveillance strategy as this approach cannot predict new variants that carry mutations other than the ones described in this study.
In summary, the use of a panel of multiplex, mutant-specific RT-PCR assays represents an ideal balance of cost, turnaround time, and accuracy for detection of SARS-CoV-2 VOC and VOI. The technology is amenable for use in a larger number of clinical laboratories than next-generation sequencing. In our current environment requiring rapid strain typing to guide both treatment decisions and public health measures, such a rapid and accessible approach will be essential.
ACKNOWLEDGMENTS
For the GIS sequences in Fig. 2, we acknowledge the originating laboratories responsible for obtaining the specimens and the submitting laboratories where genetic sequence data were generated and shared via the GISAID Initiative.
Contributor Information
Amy L. Leber, Email: Amy.Leber@nationwidechildrens.org.
Michael J. Loeffelholz, Cepheid
REFERENCE
- 1.Alpert T, Lasek-Nesselquist E, Brito AF, Valesano AL, Rothman J, MacKay MJ, Petrone ME, Breban MI, Watkins AE, Vogels CBF, Russell A, Kelly JP, Shudt M, Plitnick J, Schneider E, Fitzsimmons WJ, Khullar G, Metti J, Dudley JT, Nash M, Wang J, Liu C, Hui P, Muyombwe A, Downing R, Razeq J, Bart SM, Murphy S, Neal C, Laszlo E, Landry ML, Cook PW, Fauver JR, Mason CE, Lauring AS, St George K, MacCannell DR, Grubaugh ND. 2021. Early introductions and community transmission of SARS-CoV-2 variant B.1.1.7 in the United States. medRxivhttps://www.medrxiv.org/content/10.1101/2021.02.10.21251540v3. [DOI] [PMC free article] [PubMed]
- 2.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. 2021. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 372:n579. 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH, CMMID COVID-19 Working Group. 2021. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 593:270–274. 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N, Mlisana K, von Gottberg A, Walaza S, Allam M, Ismail A, Mohale T, Glass AJ, Engelbrecht S, Van Zyl G, Preiser W, Petruccione F, Sigal A, Hardie D, Marais G, Hsiao N-y, Korsman S, Davies M-A, Tyers L, Mudau I, York D, Maslo C, Goedhals D, Abrahams S, Laguda-Akingba O, Alisoltani-Dehkordi A, Godzik A, Wibmer CK, Sewell BT, Lourenço J, Alcantara LCJ, Kosakovsky Pond SL, Weaver S, Martin D, Lessells RJ, Bhiman JN, Williamson C, de Oliveira T. 2021. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature 592:438–443. 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 5.Zucman N, Uhel F, Descamps D, Roux D, Ricard J-D. 2021. Severe reinfection with South African SARS-CoV-2 variant 501Y.V2: a case report. Clin Infect Dis 10.1093/cid/ciab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyo-Gwete T, Madzivhandila M, Makhado Z, Ayres F, Mhlanga D, Oosthuysen B, Lambson BE, Kgagudi P, Tegally H, Iranzadeh A, Doolabh D, Tyers L, Chinhoyi LR, Mennen M, Skelem S, Marais G, Wibmer CK, Bhiman JN, Ueckermann V, Rossouw T, Boswell M, de Oliveira T, Williamson C, Burgers WE, Ntusi N, Morris L, Moore PL. 2021. SARS-CoV-2 501Y.V2 (B.1.351) elicits cross-reactive neutralizing antibodies. bioRxivhttps://www.biorxiv.org/content/10.1101/2021.03.06.434193v2. [DOI] [PMC free article] [PubMed]
- 7.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, Sigal A, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB. 2021. Circulating SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. medRxivhttps://www.medrxiv.org/content/10.1101/2021.02.14.21251704v2.
- 8.Centers for Disease Control and Prevention. 2021. COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/.
- 9.Annavajhala MK, Mohri H, Wang P, Zucker JE, Sheng Z, Gomez-Simmonds A, Bedford T, Ho DD, Uhlemann A-C. 2021. A novel SARS-CoV-2 variant of concern, B.1.526, identified in New York. medRxivhttps://www.medrxiv.org/content/10.1101/2021.02.23.21252259v2.
- 10.Zhang W, Davis BD, Chen SS, Sincuir Martinez JM, Plummer JT, Vail E. 2021. Emergence of a novel SARS-CoV-2 variant in southern California. JAMA 325:1324. 10.1001/jama.2021.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, Sotomayor-González A, Glasner DR, Reyes KR, Gliwa AS, Reddy NP, San Martin CS, Federman S, Cheng J, Balcerek J, Taylor J, Streithorst JA, Miller S, Kumar GR, Sreekumar B, Chen P-Y, Schulze-Gahmen U, Taha TY, Hayashi J, Simoneau CR, McMahon S, Lidsky PV, Xiao Y, Hemarajata P, Green NM, Espinosa A, Kath C, Haw M, Bell J, Hacker JK, Hanson C, Wadford DA, Anaya C, Ferguson D, Lareau LF, Frankino PA, Shivram H, Wyman SK, Ott M, Andino R, Chiu CY. 2021. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxivhttps://www.medrxiv.org/content/10.1101/2021.03.07.21252647v1. [DOI] [PMC free article] [PubMed]
- 12.Liu Z, VanBlargan LA, Bloyet L-M, Rothlauf PW, Chen RE, Stumpf S, Zhao H, Errico JM, Theel ES, Liebeskind MJ, Alford B, Buchser WJ, Ellebedy AH, Fremont DH, Diamond MS, Whelan SPJ. 2021. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 29:477–488.e4. 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhen W, Berry GJ. 2020. Development of a new multiplex real-time RT-PCR assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection. J Mol Diagn 22:1367–1372. 10.1016/j.jmoldx.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu HAM, Kubatko L, Hunt M, Pan X, Ru P, Garee J, Thomas K, Mohler P, Pancholi P, Jones D. 2021. Distinct patterns of emergence of SARS-CoV-2 spike variants including N501Y in clinical samples in Columbus Ohio. bioRxivhttps://www.biorxiv.org/content/10.1101/2021.01.12.426407v3.
- 15.Greaney AJ, Starr TN, Barnes CO, Weisblum Y, Schmidt F, Caskey M, Gaebler C, Cho A, Agudelo M, Finkin S, Wang Z, Poston D, Muecksch F, Hatziioannou T, Bieniasz PD, Robbiani DF, Nussenzweig MC, Bjorkman PJ, Bloom JD. 2021. Mutational escape from the polyclonal antibody response to SARS-CoV-2 infection is largely shaped by a single class of antibodies. bioRxivhttps://www.biorxiv.org/content/10.1101/2021.03.17.435863v1.
- 16.Starr TN, Greaney AJ, Addetia A, Hannon WW, Choudhary MC, Dingens AS, Li JZ, Bloom JD. 2021. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 371:850–854. 10.1126/science.abf9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaney AJ, Loes AN, Crawford KHD, Starr TN, Malone KD, Chu HY, Bloom JD. 2021. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe 29:463–476.e6. 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greaney AJ, Starr TN, Gilchuk P, Zost SJ, Binshtein E, Loes AN, Hilton SK, Huddleston J, Eguia R, Crawford KHD, Dingens AS, Nargi RS, Sutton RE, Suryadevara N, Rothlauf PW, Liu Z, Whelan SPJ, Carnahan RH, CroweJE, Jr, Bloom JD. 2021. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe 29:44–57.e9. 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bal A, Destras G, Gaymard A, Stefic K, Marlet J, Eymieux S, Regue H, Semanas Q, d'Aubarede C, Billaud G, Laurent F, Gonzalez C, Mekki Y, Valette M, Bouscambert M, Gaudy-Graffin C, Lina B, Morfin F, Josset L, COVID-Diagnosis HCL Study Group. 2021. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, August to December 2020. Euro Surveill 26:2100008. 10.2807/1560-7917.ES.2021.26.3.2100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibba G, Sau R, Angioj F, Abbondio M, Rubino S, Uzzau S. 2021. A straightforward molecular strategy to retrospectively investigate the spread of SARS-CoV-2 VOC202012/01 B.1.1.7 variant. J Infect Dev Ctries 15:242–246. 10.3855/jidc.14972. [DOI] [PubMed] [Google Scholar]
- 21.Gaymard A, Bosetti P, Feri A, Destras G, Enouf V, Andronico A, Burrel S, Behillil S, Sauvage C, Bal A, Morfin F, Van Der Werf S, Josset L, ANRS MIE AC43 COVID-19, French viro COVID Group, Blanquart F, Coignard B, Cauchemez S, Lina B. 2021. Early assessment of diffusion and possible expansion of SARS-CoV-2 Lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, January to March 2021. Euro Surveill 26:2100133. 10.2807/1560-7917.ES.2021.26.9.2100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges V, Sousa C, Menezes L, Gonçalves AM, Picão M, Almeida JP, Vieita M, Santos R, Silva AR, Costa M, Carneiro L, Casaca P, Pinto-Leite P, Peralta-Santos A, Isidro J, Duarte S, Vieira L, Guiomar R, Silva S, Nunes B, Gomes JP. 2021. Tracking SARS-CoV-2 lineage B.1.1.7 dissemination: insights from nationwide spike gene target failure (SGTF) and spike gene late detection (SGTL) data, Portugal, week 49 2020 to week 3 2021. Euro Surveill 26:2100131. 10.2807/1560-7917.ES.2021.26.10.2100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norz D, Pfefferle S, Grunwald M, Fischer N, Aepfelbacher M, Lütgehetmann M. 2021. Modifying a diagnostic SARS-CoV-2 spike PCR to turn a Del69/70 dropout into a discriminatory on-target assay. J Mol Diagn 10.1016/j.jmoldx.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banada P, Green R, Banik S, Chopoorian A, Streck D, Jones R, Chakravorty S, Alland D. 2021. A simple RT-PCR melting temperature assay to rapidly screen for widely circulating SARS-CoV-2 variants. medRxivhttps://www.medrxiv.org/content/10.1101/2021.03.05.21252709v2. [DOI] [PMC free article] [PubMed]
- 25.Matic N, Lowe CF, Ritchie G, Stefanovic A, Lawson T, Jang W, Young M, Dong W, Brumme ZL, Brumme CJ, Leung V, Romney MG. 2021. Rapid detection of SARS-CoV-2 variants of concern, including B.1.1.28/P.1, in British Columbia, Canada. Emerg Infect Dis 27:1673–1676. 10.3201/eid2706.210532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charre C, Ginevra C, Sabatier M, Regue H, Destras G, Brun S, Burfin G, Scholtes C, Morfin F, Valette M, Lina B, Bal A, Josset L. 2020. Evaluation of NGS-based approaches for SARS-CoV-2 whole genome characterisation. Virus Evol 6:veaa075. 10.1093/ve/veaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]