Abstract
Objectives:
To evaluate the long-term effects of seven different cleaning methods on light transmittance, surface roughness, and flexural modulus of a polyurethane retainer material.
Materials and Methods:
Polyurethane retainer specimens (Vivera®, Align Technology Inc) (70 specimens, n = 10 per method, 50.8 mm × 12.7 mm × 1.0 mm) were exposed to seven cleaning methods twice a week for 6 months. Before treatment and after 6 months, light transmittance, surface roughness, and flexural modulus of the specimens were quantified. Qualitative assessment of randomly selected specimens from each solution was performed at baseline and after 6 months using a scanning electron microscope. Statistical analyses were performed at the .05 significance level.
Results:
Of the three test variables, light transmittance through the specimens was the only one that changed significantly from baseline to 6 months for all cleaning solutions, with all of them causing a decrease. However, except for 0.6% sodium hypochlorite showing a change in surface roughness values and 2.5% vinegar and toothbrushing showing an increase in flexural modulus, none of the other four cleaning methods resulted in significant changes in surface roughness or flexural modulus values for the polyurethane specimens between baseline and after 6 months.
Conclusions:
Of the seven cleaning methods, Invisalign® cleaning crystals, Polident®, and Listerine® showed the least amount of change in light transmittance values for the polyurethane specimens over 6 months, and they had no effect on surface roughness and flexural modulus values.
Keywords: Polyurethane, Retainer, Cleaning method, Transmittance, Roughness, Flexural
INTRODUCTION
Orthodontic retention, one of the most important aspects of orthodontic treatment, is planned to prevent relapse.1 Recently, more options for orthodontic retention have been introduced aside from the traditional Hawley retainer. Thermoplastic clear retainers have increased in popularity as a result of their esthetic and translucent properties.2–4 Though preferred because of their translucent nature, it has been reported5 that the physical properties of thermoplastic retainers can be changed as a result of intraoral temperature fluctuations, as well as the retainers being subjected to cyclic deflections. Previous studies have reported poor wear resistance and durability of orthodontic appliances after “short service periods”; furthermore, similar studies on mouth guards, made from comparable thermoplastic orthodontic materials, have demonstrated various dimensional changes induced by aging, again depending on the particular material and processing techniques.6 Common polymers used to fabricate thermoplastic retainers are polyester, polypropylene, and polyurethane.7
Because retainers are essential in preventing orthodontic relapse, it is crucial to have an effective cleaning technique to facilitate long-term use of the retainers. There are, however, some problems associated with long-term use of clear retainers, including loss of translucency and material integrity, discoloration, and plaque and calculus retention.8,9 Although it has been shown to be biocompatible, polyurethane thermoplastic retainer material, such as Vivera® (Align Technology Inc, San Jose, Calif), is known to show sensitivity to heat, humidity, and salivary enzymes.10 In addition, Ryokawa et al.11 have determined that significant environmental factors may influence the mechanical and physical properties of dental thermoplastics.
Translucency and color stability of clear retainers remain critical considerations for both patients and clinicians. Because patients' desire for clear retainers is due to the near-invisible nature of them, maintaining translucency of the retainers is a key concern.8 Proper maintenance of this type of retainer is difficult, and mechanical and chemical cleaning methods are currently the two main cleaning methods available.12 In addition, it is important to understand that the integrity of the thermoplastic material can be compromised from repetitive use and cleaning cycles. Therefore, effective cleaning methods can increase the life span of the retainers and promote overall better retainer compliance.
The aim of this preliminary study was to evaluate the long-term light transmittance, surface roughness, and flexural modulus of Vivera®, a polyurethane retainer material, using seven different cleaning methods over a 6-month period: Invisalign® cleaning crystals (Align Technology Inc), Polident® (GlaxoSmithKline®, Brentford, UK), Listerine® mouthwash (Johnson and Johnson®, New Brunswick, NJ, USA), 2.5% vinegar, 0.6% sodium hypochlorite, 3% hydrogen peroxide, and toothbrushing with distilled water over the course of 6 months. For the purpose of this report, “mean variation” refers to mean differences from baseline to 6 months and was calculated by subtracting baseline values from 6-month values.
MATERIALS AND METHODS
A polyurethane blend of methylene diphenyl diisocyanate and 1,6-hexanediol13 was prepared by Align Technology Inc, at the standard dimensions of 50.8 mm × 12.7 mm × 1.0 mm. These dimensions are recommended by “Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials,” (ASTM D790)14 which provides for alternative test specimen sizes for materials that are less than 1.6 mm thick. This ASTM standard was used instead of ANSI/ADA Standard No 139 (“Dental Base Polymers”) because the sheets used to prepare the specimens were less than the standard thickness specified in Standard No 139.15
Seventy specimens of the prepared material were randomly placed into seven groups (with 10 specimens in each of the cleaning solutions and the toothbrushing group). For all groups, five of the specimens were tested for flexural modulus, and the other five were tested for light transmittance and surface roughness. One specimen from each cleaning group was randomly selected from the specimens used for light transmittance and surface roughness tests for scanning electron microscopy (SEM) analysis. Each specimen was labeled to designate material, number, and cleaning method.
Twice a week, specimens were either immersed in 600 mL of each cleaning solution or brushed with a toothbrushing machine. The cleaning solutions were prepared according to the manufacturers' instructions. Specimens were wrapped in 100% cotton cheesecloth, separated from one another by glass beads, and suspended from glass rods atop beakers filled with each of the six solutions for 15 minutes, with the exception of Polident®, which was used for 3 minutes, per the manufacturer's recommendation, and the solutions were stirred on a magnetic stirrer (Figure 1).
Figure 1.
Specimens submerged in a cleaning solution on a magnetic stir plate.
For the toothbrushing method, specimens were brushed with a custom-fabricated toothbrushing machine (Figure 2) using double-distilled water for 2 minutes, twice weekly, over the course of 6 months. The speed control on the toothbrushing machine was set at 300 strokes/min, and the load was set at 50 g. Specimens were brushed parallel to the long axis (Figure 2, arrow). Following the cleaning sessions, specimens were kept in a fresh batch of artificial saliva16 at 37°C.
Figure 2.
Standardized toothbrushing machine. The arrow represents the direction of brushing stroke.
Light transmittance was determined according to a method published for measuring translucency of dental ceramics.17 This method quantifies the percent light transmittance through the retainer material into a spectrometer/integrating sphere system consisting of the following components: a miniature spectrometer (Flame-S-VIS-NIR, Ocean Optics, Largo, FL, USA); a tungsten halogen lamp (Nikon MK II illuminator, Tokyo, Japan) with a flexible light guide (0.25 inches × 0.312 inches × 72 inches; Dolan-Jenner, Boxborough, MA, USA) integrating sphere (Labsphere, North Sutton, NH), fiber optic cable (QP100-2-UV-VIS, Ocean Optics), and a custom-designed specimen holder (Figure 3). During the procedure, a light energy reading is taken with the tungsten halogen light source connected to the spectrometer/integrating sphere system through a custom-fabricated specimen holder attached to a port in the integrating sphere. Next, the specimen was positioned in the holder in the path of the light source and the light was transmitted again through the specimen. From the two light energy measurements, the percent of light transmittance through the specimen was calculated for wavelengths between 380 nm and 740 nm (Oceanview software, version 1.5, Ocean Optics).
Figure 3.
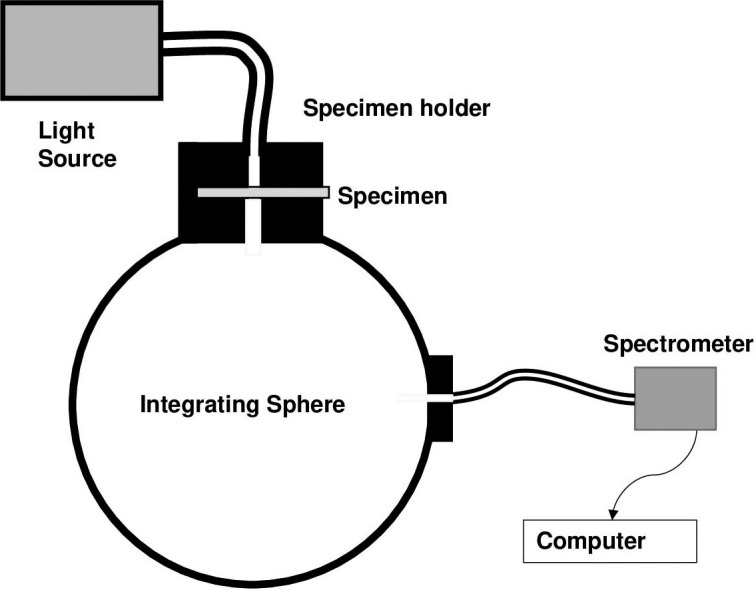
Diagram of light transmission measurement system.
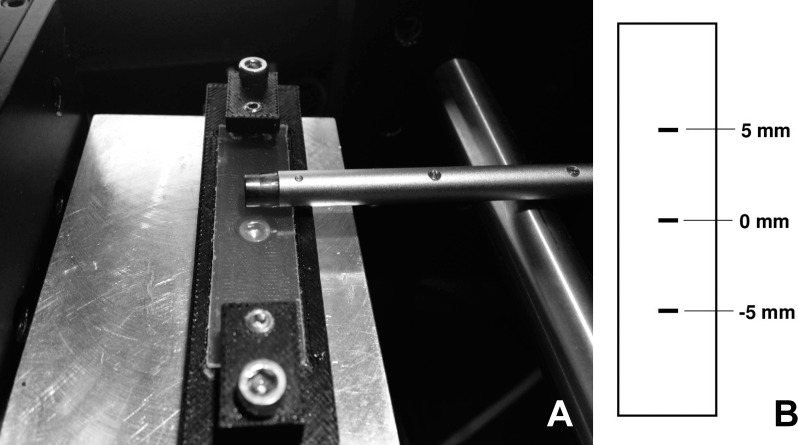
Surface roughness was measured using a Surtronic 3+ profilometer (Taylor Hobson, Leicester, UK) placed on a Thorlabs motorized X-Y-Z stage controlled by Thorlabs APT software (Thorlabs, Newton, NJ, USA), as shown in Figure 4A. The resolution of measurements was set to 0.02 μm, with the other profilometer settings as follows: 2.5-mm traverse length, cut-off value of 0.25 mm, and traverse speed of 1 mm/s. Surface roughness values were measured at three locations centered across the center of the specimen (Figure 4B). The resulting output was electronically transferred to the HyperTerminal application for Microsoft Windows XP (Hilgraeve, Monroe, Mich, USA).
Figure 4.
(A) Photo showing profilometer stylus and specimen holder. (B) Diagram showing specimen measurement locations.
A mechanical testing machine (Instron 5582, Norwood, Mass, USA) was used to conduct three-point bend testing of the specimens to measure flexural modulus. Using the calibrated mechanical test machine, each specimen was loaded at a cross-head speed of 1 mm/min. The specimen was loaded in the linear-elastic region of its stress/strain curve below the yield strength of the material. Pilot testing was performed to determine the ultimate flexural strength of the Vivera® specimens, and then the specimens were loaded to approximately half of the mean ultimate flexural strength of the specimens in the pilot tests. The data were collected and processed using a custom-program in Testworks (MTS Systems, Eden Prairie, Minn, USA).
The JCM-6000 Neoscope II Benchtop SEM (JEOL, Tokyo, Japan) was used to obtain qualitative SEM image data to supplement the quantitative findings of the three previously described tests. SEM images at baseline and the end of the 6-month period were collected. The specimens were gold plated, and images were collected at 10 kV and 500× magnification.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed to compare the mean variations among the cleaning methods on the three properties (light transmittance, surface roughness, and flexural modulus) of the Vivera® specimens. Post hoc Bonferroni multiple-comparison tests were used when needed. For the mean difference between baseline and 6 months for each solution tested in this study, a Student's t-test was performed. Data analyses (SPSS Statistics, v22.0, IBM Corp, Armonk, NY, USA) were performed at the .05 significance level.
RESULTS
At baseline, there was no significance mean difference of the tested properties among the specimens (P > .05). One-way ANOVA showed a statistically significant mean variation only in light transmittance among the cleaning method groups [F(6,28) = 5.368; P = .001]. Multiple comparison indicated that the toothbrushing cleaning group had the greatest decrease in light transmittance, while specimens treated with Invisalign® cleaning crystals, Listerine®, and Polident® showed the least amount of change in the light transmittance values over 6 months, with P-values ranging from .005 to .001 (Table 1). There was no statistically significant mean variation among the cleaning methods for surface roughness and flexural modulus [F(6,28) = 0.789 and F(6,27) = 0.929, with P-values = .586 and .490, respectively].
Table 1.
Descriptive Statistics (%) and Pair-wise (P-Value) Mean Differences of Light Transmittance from Baseline to 6 Months Among the Cleaning Methodsa
|
Cleaning Method |
Mean ± SD |
Invisalign Cleaning Crystals |
Polident |
Listerine |
2.5% Vinegar |
0.6% Sodium Hypochlorite |
3% Hydrogen Peroxide |
Toothbrushing |
| Invisalign® cleaning crystals | −3.24 ± 0.80 | – | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | .003 |
| Polident® | −3.40 ± 1.33 | 1.000 | – | 1.000 | 1.000 | 1.000 | 1.000 | .005 |
| Listerine® | −2.72 ± 1.00 | 1.000 | 1.000 | – | 1.000 | 1.000 | .461 | .001 |
| 2.5% Vinegar | −4.24 ± 0.96 | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | .072 |
| 0.6% Sodium hypochlorite | −4.22 ± 1.29 | 1.000 | 1.000 | 1.000 | 1.000 | – | 1.000 | .068 |
| 3% Hydrogen peroxide | −4.80 ± 1.02 | 1.000 | 1.000 | .461 | 1.000 | 1.000 | – | .353 |
| Toothbrushing | −6.98 ± 2.42 | .003 | .005 | .001 | .072 | .068 | .353 | – |
SD indicates standard deviation.
Student's t-test indicated a consistent loss of light transmittance in the polyurethane specimens when they were immersed in all cleaning materials at 6 months compared to baseline (Figure 5). All methods produced similar roughness values when comparing baseline and 6-month values, except for specimens cleaned in 0.6% sodium hypochlorite (Figure 6A). However, qualitative analysis with the SEM of specimens cleaned in the sodium hypochlorite solution showed no mean difference in surface texture when comparing micrographs at 500× magnification between baseline (Figure 6B) and 6 months (Figure 6C). All cleaning methods showed no mean difference in flexural modulus values when comparing baseline and 6-month time point values, except for specimens cleaned in the 2.5% vinegar solution and those specimens in the toothbrushing groups, with both cleaning methods resulting in increases in the flexural modulus of the specimens (Figure 7).
Figure 5.
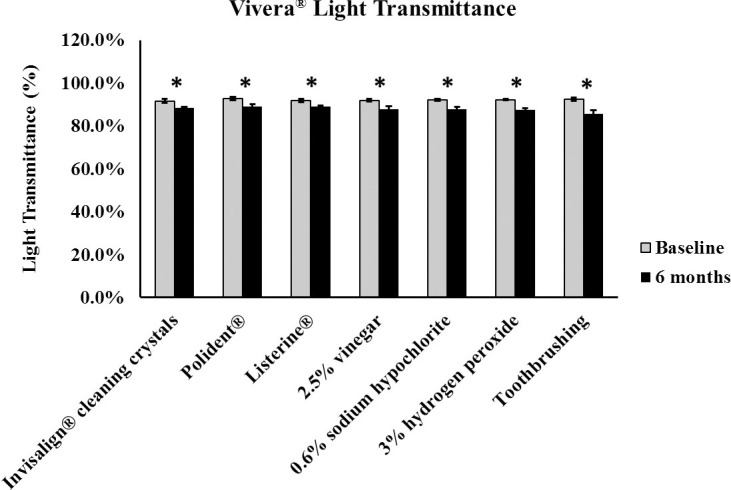
Bar graph of polyurethane light transmittance between baseline and 6 months (* P < .05).
Figure 6.
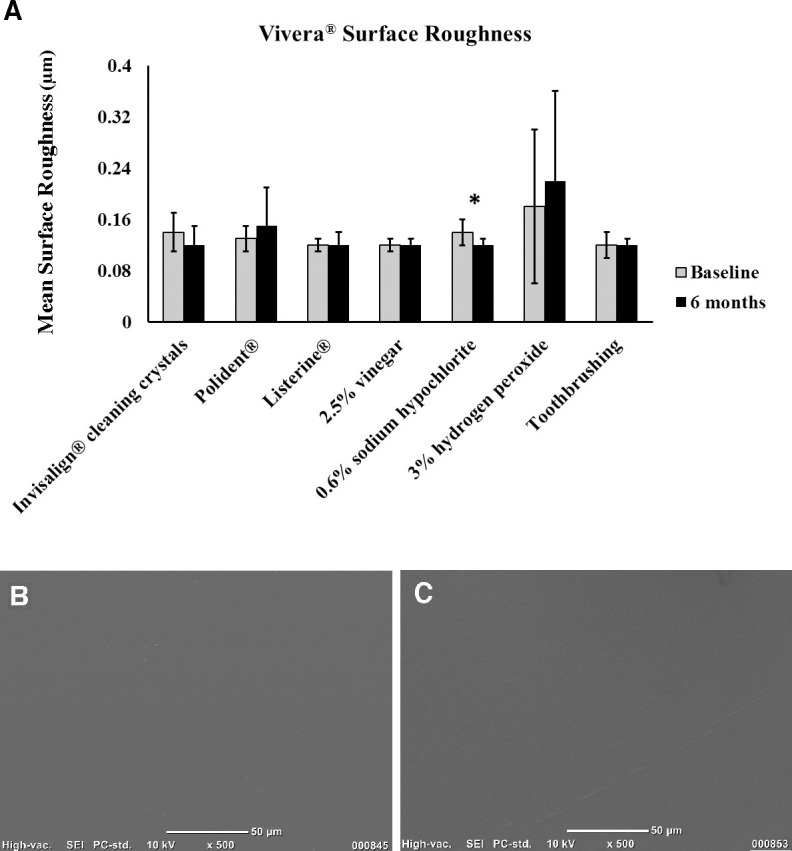
(A) Bar graph of polyurethane surface roughness between baseline and 6 months (* P < .05). (B, C) Scanning electron microscope images of polyurethane specimens from the sodium hypochlorite group at (B) baseline and (C) 6 months.
Figure 7.
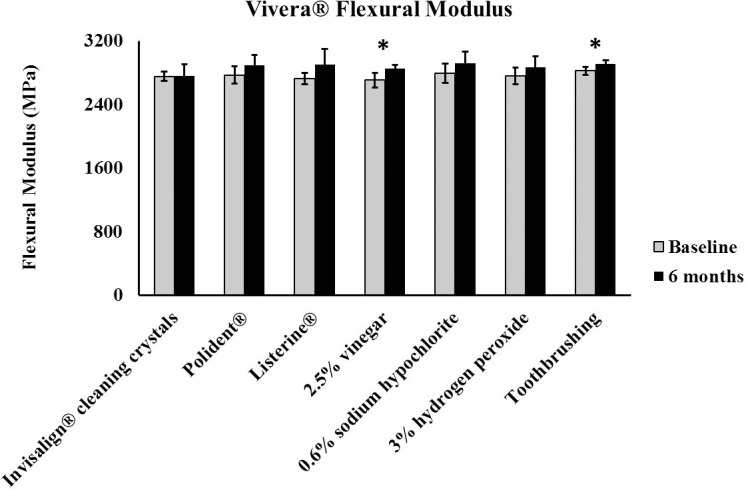
Bar graph of polyurethane flexural modulus between baseline and 6 months (* P < .05).
DISCUSSION
With the increasing popularity of clear retainer use after orthodontic treatment, proper maintenance and cleaning of the retainers will continue to be critical issues. The cleaning methods in this study were chosen based on the information gathered from orthodontic practitioners. Thermoplastic retainer materials are susceptible to changes when exposed to humidity, warmth, and salivary enzymes.18 Currently, very few studies are available addressing the effective cleaning methods for these retainers. This study was the first to evaluate the long-term effects of cleaning methods on physical properties of polyurethane retainer material.
Polyurethanes are extensively used in medical devices and are known to have superb mechanical properties.19 These properties include abrasion resistance, chemical resistance, ease of processing, and both high tensile strength and melting points.19 However, despite good physical properties, polyurethanes are susceptible to degradation by light over time, and they are also known to be affected by heat, moisture, and enzymes.19–21 Furthermore, they are susceptible to water hydrolysis and oxidative degradation, ultimately leading to cracking when left in vivo for extended periods of time.22
The results of our in vitro 6-month study were comparable to the results of a previous in vivo 14-day study10 examining the deterioration of properties of polyurethane appliances over time. The major ingredients of the polymers used in both studies were the same; however, the thicknesses of the specimens used in the in vivo study were thinner than those of the ones in this study.
Based on the results of our study, it is evident that there is a consistent decrease in light transmittance in polyurethane retainer materials due to immersion in cleaning solutions. Polyurethane, the primary component of the aligners, has been known to have loss of transmittance inherent in its physical properties, which could have attributed to the decrease in transmittance.10 Furthermore, artificial saliva will accelerate discoloration of polyurethane material despite daily toothbrushing.10 The current study utilized toothbrushing two times per week over a 6-month period as opposed to the previous study, in which the retainers were brushed every day for 14 days. In contrast to the current study, Gracco et al.10 found surface changes in the intraorally aged and artificially aged aligners. Previous studies8 have found that polyurethanes are particularly vulnerable to pigment adsorption and have poor color stability, supporting a decrease in translucency.
After exposure to seven different cleaning methods for 6 months, the polyurethane specimens were not significantly changed in surface roughness compared to their appearance at baseline, except for specimens subjected to a 0.6% sodium hypochlorite solution. Though the sodium hypochlorite solution did cause a statistically significant increase in surface roughness, qualitative assessment of SEM images comparing baseline images to those taken at 6 months showed no appreciable differences, indicating that the results may not be clinically significant. Further suggesting the lack of clinical significance in the surface roughness results of the specimens subjected to the sodium hypochlorite cleaning solution is the fact that the surface roughness values were all well below 0.5 μm, and it has been shown23 that 0.5 μm approximates the minimum surface roughness that can be detected by a patient's tongue.
Both vinegar and toothbrushing increased the flexural modulus of the polyurethane specimens, thereby decreasing their flexibility. All other cleaning methods had no significant effect on the flexural modulus of the specimens. The attack of polymers by oxygen, or oxidation, is a principal process in the degradation of these materials, and the effects of oxidation on the structure of a polymer may be inferred from changes in physical properties, such as a modulus.5 For instance, elevated temperatures can hasten the oxidation of polymers, resulting in a measurable increase in their modulus, or stiffness.24,25 Furthermore, polyurethanes have been observed to undergo physiochemical changes due to water hydrolysis, leading to swelling and irreversible degradation, which may support the results in the current study of increased stiffness observed with the cleaning methods of 2.5% vinegar solution and toothbrushing.9
Toothbrushing of the polyurethane specimens with double-distilled water elicited the greatest change in light transmittance. The scratches that toothbrushing elicited in the specimens may have contributed to the decrease in transmittance over time. In terms of light transmittance, the results indicated that specimens treated with Invisalign® cleaning crystals, Listerine®, and Polident® showed the least amount of change in light transmittance, and all three treatment methods were significantly better than toothbrushing at retaining the light transmittance values recorded at baseline.
A limitation of this study was that the specimens that were used were flat and did not reflect the real shape of thermoplastic retainers. Thermoplastic retainers are fabricated over a model of a patient's teeth and, therefore, take on a much less uniform shape. However, for the purpose of this study, flat standard specimens with uniform cross-sectional areas were necessary for the flexural modulus and light transmittance measurements, and they provided standard results, which can be used in future studies. Though the specimens were flat, they were processed (heat-vacuum formed) similarly to other orthodontic retainers, which eliminated the processing step as a variable in the study.
CONCLUSIONS
After exposure to seven different cleaning methods for 6 months, polyurethane retainer specimens demonstrated that light transmittance was the only tested property of the specimens that significantly changed from baseline to the 6-month time point.
All of the cleaning methods caused a significant decrease in light transmittance through the polyurethane specimens over 6 months.
No statistically significant mean difference among the cleaning methods was found for surface roughness and flexural modulus.
In this study, of seven tested cleaning methods, Invisalign® cleaning crystals, Polident®, and Listerine® showed the least amount of change in light transmittance values for the polyurethane specimens over 6 months, and they had no effect on surface roughness and flexural modulus values.
ACKNOWLEDGMENTS
This study was funded by Align Technology Inc (2015 North American Align Research Award) and partly supported by NIDCR DE024531.
REFERENCES
- 1.Thilander B. Biological basis for orthodontic relapse. Semin Orthod. 2000;6:195–205. [Google Scholar]
- 2.Mai W, He J, Meng H, et al. Comparison of vacuum-formed and Hawley retainers: a systematic review. Am J Orthod Dentofacial Orthop. 2014;145:720–727. doi: 10.1016/j.ajodo.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Singh P, Grammati S, Kirschen R. Orthodontic retention patterns in the United Kingdom. J Orthod. 2009;36:115–121. doi: 10.1179/14653120723040. [DOI] [PubMed] [Google Scholar]
- 4.Hichens L, Rowland H, Williams A, Hollinghurst A, Ewings P. Cost-effectiveness and patient satisfaction: Hawley and vacuum-formed retainers. Eur J Orthod. 2007;29:372–378. doi: 10.1093/ejo/cjm039. [DOI] [PubMed] [Google Scholar]
- 5.Rodriquez F. Principles of Polymer Systems, 3rd ed. Abingdon, UK: Taylor & Francis; 1995. [Google Scholar]
- 6.Kwon JS, Lee YK, Lim BS, Lim YK. Force delivery properties of thermoplastic orthodontic materials. Am J Orthod Dentofacial Orthop. 2008;133:228–234. doi: 10.1016/j.ajodo.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Bai Y, Ding X, Zhang Y. Preparation and characterization of thermoplastic materials for invisible orthodontics. Dent Mater J. 2011;30:954–959. doi: 10.4012/dmj.2011-120. [DOI] [PubMed] [Google Scholar]
- 8.Zafeiriadis AA, Karamouzos A, Athanasiou AE, Eliades T, Palaghias G. In vitro spectrophotometric evaluation of Vivera clear thermoplastic retainer discolouration. Aust Orthod J. 2014;30:192–200. [PubMed] [Google Scholar]
- 9.Gardner GD, Dunn WJ, Taloumis L. Wear comparison of thermoplastic materials used for orthodontic retainers. Am J Orthod Dentofacial Orthop. 2003;124:294–297. doi: 10.1016/s0889-5406(03)00502-x. [DOI] [PubMed] [Google Scholar]
- 10.Gracco A, Mazzoli A, Favoni O, et al. Short-term chemical and physical changes in Invisalign appliances. Aust Orthod. 2009;25:34–40. [PubMed] [Google Scholar]
- 11.Ryokawa H, Miyazaki Y, Akihiro F, Takashi M, Koutaro M. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod Waves. 2006;65:64–72. [Google Scholar]
- 12.Chang CS, Al-Awadi A, Ready D, Noar J. An assessment of the effectiveness of mechanical and chemical cleaning of Essix orthodontic retainer. J Orthod. 2014;41:110–117. doi: 10.1179/1465313313Y.0000000088. [DOI] [PubMed] [Google Scholar]
- 13.MSDS No. 2088. Align Technology; San Jose, CA: 2013. Invisalign Appliance Materials—EX15, EX30, and EX40. January 7. https//d1um97i1emgxdo.cloudfront.net/files/7098/D16907_Rev_B_Safety_Data_Sheet_EX15_EX30_EX40-US-English.pdf. [Google Scholar]
- 14.ASTM D790-17, Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM; ASTM International; West Conshohocken, Pa: ASTM International; 2017. [Google Scholar]
- 15.ANSI/ADA. ANSI/ADA Standard No 139. Dental Base Polymers. 2012. Available at: http://www.ada.org.
- 16.Nakagawa M, Matsuya S, Shiraishi T, Ohta M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J Dent Res. 1999;78:1568–1572. doi: 10.1177/00220345990780091201. [DOI] [PubMed] [Google Scholar]
- 17.Spink LS, Rungruanganut P, Megremis S, Kelly JR. Comparison of an absolute and surrogate measure of relative translucency in dental ceramics. Dent Mater. 2013;29:702–707. doi: 10.1016/j.dental.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Eliades T, Eliades G, Watts DC. Structural conformation of in vitro and in vivo aged orthodontic elastomeric modules. Eur J Orthod. 1999;21:649–658. doi: 10.1093/ejo/21.6.649. [DOI] [PubMed] [Google Scholar]
- 19.Szycher M. Szycher 's Handbook of Polyurethanes, 2nd ed. Boca Raton, Fla: CRC Press;; 2012. [Google Scholar]
- 20.Stokes K, McVenes R, Anderson JM. Polyurethane elastomer biostability. J Biomater Appl. 1995;9:321–354. doi: 10.1177/088532829500900402. [DOI] [PubMed] [Google Scholar]
- 21.Schuster S, Eliades G, Zinelis S, Eliades T, Bradley TG. Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am J Orthod Dentofacial Orthop. 2004;126:725–728. doi: 10.1016/j.ajodo.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Stachelek SJ, Alferjey I, Ueda M, Eckels EC, Gleason KT, Levy RJ. Prevention of polyurethane oxidative degradation with phenolic-antioxidants covalently attached to the hard segments: structure and function relationships. J Biomed Mater Res A. 2010;94:751–759. doi: 10.1002/jbm.a.32755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarrett DC. Polishing systems. ADA Prof Product Rev. 2010;5((1)):1–16. ed. [Google Scholar]
- 24.Yeganeh H, Shamekhi MA. Poly(urethane-imide-imide), a new generation of thermoplastic polyurethane elastomers with enhanced thermal stability. Polymer. 2004;45:359–365. [Google Scholar]
- 25.Struik LCE. Physical aging in plastics and other glassy materials. Polym Eng Sci. 1977;17:165–173. [Google Scholar]