Abstract
The effect of different annealing temperatures on structural, optical and magnetic properties of ZnFe2O4 nanoparticles prepared using the coprecipitation technique has been investigated. With the increase in annealing temperature, crystallinity and average crystallite size of nanoparticles increased. The average crystallite size was found to be 5.55 nm, 6.62 nm and 32.9 nm for the samples annealed at 300 °C, 500 °C and 700 °C, respectively. The X-ray diffraction and Fourier-transform infrared spectroscopy revealed the formation of a cubic spinel structure. The optical direct and indirect bandgap energy decreased with an increase in annealing temperature. The saturation magnetization increased from 16.38 emu/g to 25.91 emu/g. The M–H curves depicted the magnetic phase transition from superparamagnetic to ferrimagnetic. The electrical properties were investigated using an impedance analyzer in the frequency range of 300 Hz to 1 MHz. The conduction properties showed enhancement with increased annealing. The humidity sensing properties were investigated in the range of 15–90% RH and revealed a strong dependence of adsorption capacity on the annealing temperature. Electrical conductivity improved with increased humidity. Excellent humidity sensitivity was observed for ferrites annealed at 700 °C attributed to increased crystallinity and reduced lattice strain making them a potential candidate for use in humidity sensors.
Keywords: Ferrites, AC conductivity, Magnetism, Optical properties, Humidity, Sensors
Introduction
The unique combination of structural, magnetic and dielectric properties of spinel ferrites makes them the most studied ceramic materials in recent years. These properties promote the use of ferrites in various fields like high-frequency applications, electronic devices and biomedical field as catalysts, inductors, sensors, transformer cores, choke coils, filters, for drug delivery and nonreciprocal devices, etc. [1–11]. Great attention is focused nowadays on sensors due to increased environmental concerns. Precise humidity measurement is an important aspect in areas like the agricultural sector, manufacturing industries, food storage applications as well as indoor and outdoor air quality [12–17]. Optimal humidity level holds utmost importance in industries for increasing production efficiency [18, 19]. The increased relative humidity is associated with decreased SARS-CoV-2 transmission, the first pandemic of the twenty-first century [20–23]. Detrimental effects are observed on plant growth with imbalanced humidity levels [24–26]. A good humidity sensor possesses several characteristics like high sensitivity, chemical and thermal stability, reversibility and fast response time. Spinel magnetic oxides hold a great advantage for potential use in humidity sensors due to their porous structure, large surface-to-volume ratio, humidity varying resistivity, low cost, ease of synthesis and suitability in a diverse operating environment [27–31]. Humidity, in general, is specified by ‘relative humidity (RH),’ which represents the amount of water vapors in the air at a given temperature. Zinc ferrite, a class of normal spinel ferrite, is reported to be highly stable with good response, high sensitivity, magnificent magnetic properties, tunable optical properties and excellent electrical properties [32–35]. An extensive literature survey reveals a vast majority of sensors are based on ZnFe2O4 nanoparticles [36–40]. Also, ZnFe2O4 shows the highest humidity sensitivity among other ferrite nanoparticles [41]. The literature lacks the study of humidity response of annealed ZnFe2O4 nanoparticles. The sensing properties of a spinel ferrite are characterized by their magnetic and electrical properties which depend on structure, crystallinity, size and composition. Heat treatment improves the crystallinity of materials by enhancing chemical ordering which influences the physicochemical properties of prepared nanocrystalline materials [42–44].
In the present study structural, magnetic, optical and electric properties of ZnFe2O4 nanoparticles prepared by coprecipitation technique and annealed at different temperatures were investigated. The effect of varying relative humidity on the electrical response of annealed samples is studied for potential use in humidity sensors.
Experimental
Materials and preparation
The chemicals used during the preparation were of analytical grade and used without further purification. The process involved the preparation of separate homogenous solutions of Zn(NO3)2.6H2O and Fe(NO3)3.9H2O in distilled water according to the stoichiometric ratio. The solutions were mixed properly using a magnetic stirrer maintained at a temperature of 65 °C. Oleic acid was used as a surfactant to avoid the agglomeration and oxidation of the particles. Ammonia was added dropwise to the solution till pH 10 was attained to obtain precipitates. The precipitates so formed were subjected to increased heat (80 °C) to transform precipitates into hydroxides. The obtained product was washed with distilled water to remove unwanted salt residues. The dried sample was then powdered using a pestle to get ZnFe2O4 magnetic nanoparticles (MNPs). The prepared ferrite powder was annealed at 300 °C, 500 °C and 700 °C to improve crystalline properties. Cylindrical pellets of diameter 10 mm and thickness 3 mm were made from annealed samples under a force of 10 ton/cm2.
Characterization
The crystallinity, structure and phase purity of the annealed samples were identified using X-ray diffraction (Rigaku Ultima-IV powder X-ray diffractometer) employing Cu-Kα radiation over the range of 20–70 °C and Fourier-transform infrared (FTIR) spectra recorded using a PerkinElmer Frontier FTIR spectrophotometer in the range 4000–450 cm−1. The thermal stability of MNPs was studied using thermogravimetric analysis (TGA) in the temperature range of room temperature–800 °C at a heating rate of 10 °C/min. The optical parameters were measured using an ultraviolet–visible spectrophotometer (PerkinElmer Lambda 950 UV–VIS) in the wavelength range of 200–1600 nm. For magnetic measurements, a vibrating-sample magnetometer (Lakeshore model 7400 VSM) is used within the range of ± 1 T. Prepared pellets were exposed to different humidity values in the range of 15 to 90% RH in a closed chamber for a constant time. Dielectric properties of pellets before and after humidity exposure were analyzed using an impedance analyzer in the frequency range of 300 Hz–1 MHz.
Results and discussion
X-ray diffraction (XRD) study
Figure 1 shows the XRD pattern of ZnFe2O4 nanoparticles annealed at 300 °C, 500 °C and 700 °C indexed as Z3, Z5 and Z7, respectively. Broad peaks with less intensity representing crystalline structure with dominant amorphous phase are observed for Z3 and Z5, whereas Z7 showed high-intensity peaks depicting enhanced crystalline structure. All the diffraction peaks (220), (311), (222), (400), (422), (511) and (440) observed match well with the standard diffraction data (JCPDS card no. 22–1012) and confirm the formation of cubic spinel structure with Fd3m space group. The peak corresponding to the (311) plane, demonstrating the highest intensity, was employed to calculate average crystallite size (D) using the Debye–Scherrer formula [45].
| 1 |
Fig. 1.
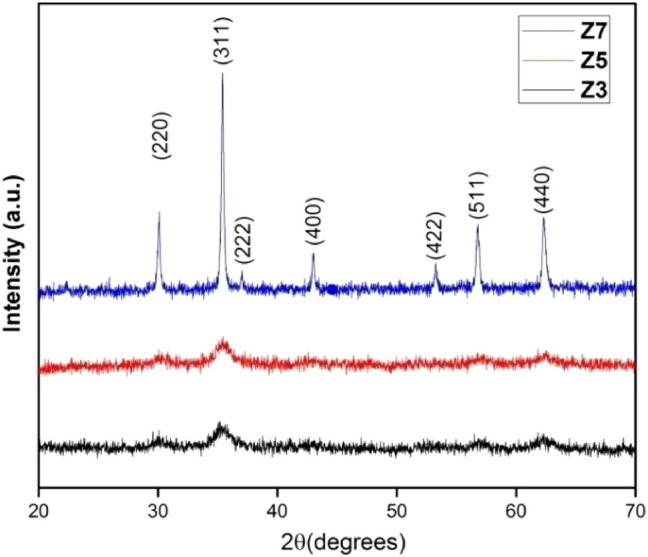
XRD spectra of Z3, Z5 and Z7
In the above equation, k, λ, β and θ denote the shape factor (0.9), X-ray wavelength (1.5406 Å), full width at half maximum of diffraction peak and Bragg’s diffraction angle, respectively. The lattice constant (a), X-ray density (ρx), specific surface area (S), bulk density (ρ) and porosity (P) are calculated using Eqs. 2–6 [46, 47].
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
In the above equations, d is the interplanar spacing and h, k and l are Miller indices of the plane. The results obtained are shown in Table 1.
Table 1.
Structural parameters of Z3, Z5 and Z7
| Sample | D (nm) | a (Å) | ρX(g/cc) | S(m2/g) | ρ(g/cm2) | P | Direct bandgap (eV) | Indirect bandgap (eV) |
|---|---|---|---|---|---|---|---|---|
| Z3 | 5.6 | 8.389 | 5.42 | 199.30 | 1.17 | 0.78 | 2.83 | 0.31 |
| Z5 | 6.6 | 8.395 | 5.41 | 167.44 | 1.66 | 0.69 | 2.71 | 0.22 |
| Z7 | 32.9 | 8.402 | 5.40 | 94.17 | 2.01 | 0.63 | – | – |
The average crystallite size of Z3 was found to be 5.55 nm and showed a minor increase for Z5. A significant increase in average crystallite size was observed for Z7. The increase in thermal energy of grains due to annealing promotes atomic diffusion resulting in increasingly larger grain size [48].
The increase in lattice constant with annealing temperature is attributed to the redistribution of cations among tetrahedral sites (A-sites) and octahedral sites (B-sites) [49]. Annealing the prepared samples also reduces the lattice defects and strains, thereby causing lattice expansion. X-ray density, specific surface area and porosity decrease with annealing. Humidity sensing being a surface phenomenon significantly depends on the porosity and grain size. A higher surface area with a suitable pore size leads to enhanced humidity adsorption [50].
Infrared spectral analysis
The Fourier-transform infrared spectra for Z3, Z5 and Z7 are shown in Fig. 2. It gives an insight into atomic bond vibrations in the infrared region. The perceptible bands in the spectra can be identified based on Waldron’s theory [51]. The intensity variation in the observed peaks signifies the amount of the corresponding functional group. The bands at ~ 3400 cm−1 and 1600 cm−1 depict the presence of adsorbed water on the NPs surface. These significant absorption bands indicate the hygroscopic nature of the prepared ferrites and their suitability in humidity sensing applications. The intensity of these peaks represents the amount of residual water in the sample. The peaks at ~ 2900 cm−1 and 1300 cm−1 confirm the presence of organic compounds used during the synthesis process. The decreased intensity of these peaks demonstrates the removal of water and surfactant on annealing. A characteristic peak at ~ 550 cm−1 relates to the intrinsic vibration of metal–oxygen (M–O) bonds at A-sites. The peak shift toward lower wave number with increased intensity revealing the reduced tetrahedral bond strength with an increased amount of the functional group. The observation suggests the increased occupancy of Zn2+ ions at A-sites with increased annealing temperature, thereby elongating the tetrahedral bond. It eventually leads to lattice expansion. The results are in agreement with XRD data analysis.
Fig. 2.
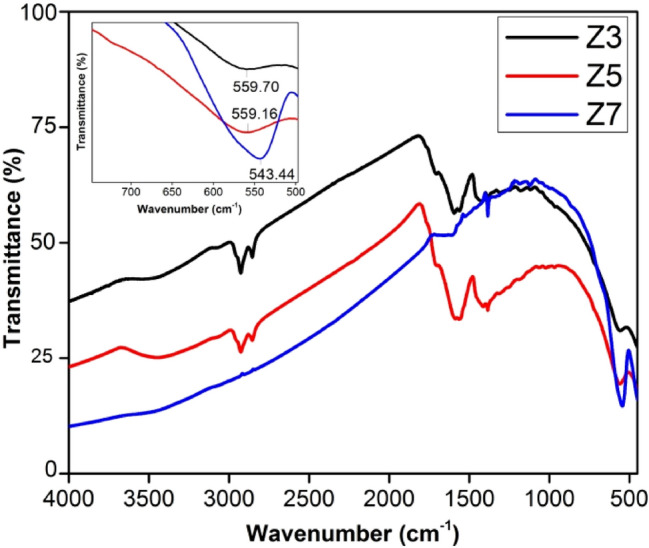
FTIR spectra of Z3, Z5 and Z7. (Inset- Peak corresponding to tetrahedral metal–oxygen bond)
Thermal analysis
The thermogravimetric analysis gives useful information about the decomposition behavior of spinel ferrite NPs with temperature. The TGA and its derivative (DTG) curve for as-prepared ZnFe2O4 NPs are shown in Fig. 3. The curve shows a two-step decomposition process. The first weight loss (2.79%) at ~ 100 °C is due to the removal of adsorbed water during the synthesis. The second major weight loss (56.3%) between 250 and 450 °C accounts for the removal of oleic acid used as a surfactant to bind the NPs [52]. At this stage, the amorphous dominating crystalline state is completely transformed to a regular nanocrystalline structure. For a temperature higher than 450 °C, the sample becomes thermally stable. Thus, it can be inferred that heat treatment above 450 °C removes all organic compounds to form pure phase spinel ferrite NPs.
Fig. 3.
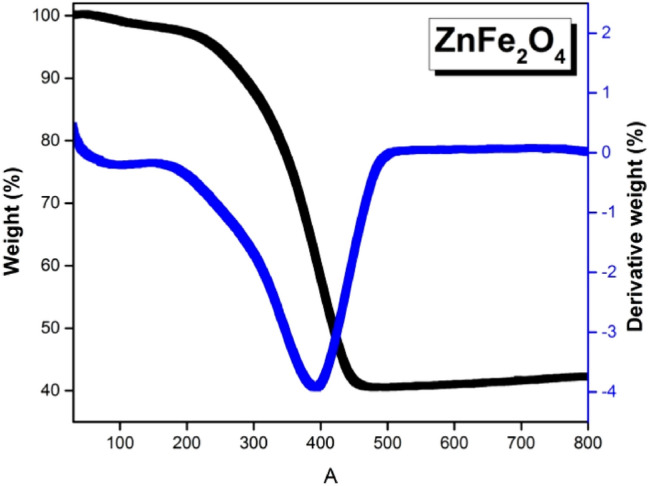
TGA-DTG curve for as-prepared ZnFe2O4 nanoparticles
Optical studies
Figure 4 shows the absorption spectra of samples annealed at 300 °C and 500 °C to study the effect of annealing on the optical properties of ZnFe2O4 nanoparticles. Both FTIR and UV–VIS spectroscopy is useful to investigate the vibrational energy levels and optical energy levels, respectively. The energy in FTIR does not cause electron excitation but it is sufficient to affect the chemical bonds, thereby causing the stretching and bending of molecules. The UV–VIS absorption intensity increases with an increase in annealing temperature. The electrons absorb the light energy and get excited. Due to the instability of higher energy states, they return to the ground state by emitting energy equivalent to the absorbed energy in the form of heat or light. Quantitatively, the intensity of absorption reflects the presence of chromophores in the molecules. The absorption peak at ~ 300 nm corresponds to the inter-sublattice charge transfer transition (ISCT) from 3d5 to 3d44s1 in Fe3+ ions. The absorption band due to inter-valence charge transfer transition (IVCT) among Fe3+ ions at B-sites is noted at ~ 1400 nm [53, 54].
Fig. 4.

UV–VIS spectra for Z3 and Z5
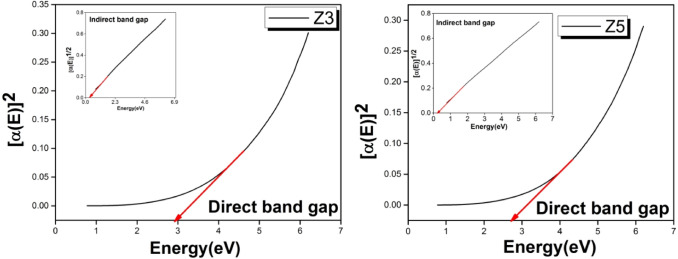
The optical direct and indirect bandgap of the samples can be estimated using Tauc’s relation [55].
| 7 |
In the above equation, α, hν, Eg and A are the absorption coefficient, photon energy, bandgap and proportionality constant, respectively. The value of n defines the type of electronic transition, i.e., n = 1/2 for direct bandgap transition and n = 2 for indirect bandgap transition.
The extrapolation of the linear part of plots of (αhν)2 versus hν and (αhν)1/2 versus hν (shown in Fig. 5) to intersect x-axis gives the value of bandgap energy which is tabulated in Table 2. The decrease in optical bandgap with an increase in annealing is attributed to the electron confinement at the nanoscale. The phenomenon can be explained using the model of a potential well with infinite walls. The confinement of electrons in the conduction band and holes in the valence band can be altered by varying the lattice parameter. The charge carriers are weakly confined in crystals with larger lattice parameter and require less energy for electron excitation [56]. The results validate Brass’s effective mass model [57].
Fig. 5.
Tauc plot for direct and optical bandgap of Z3 and Z5
Table 2.
Magnetic parameters of Z3, Z5 and Z7
| Sample | Saturation magnetization (emu/g) | Magnetic coercivity (Oe) | Retentivity (emu/g) | Anisotropy constant (erg/Oe) | Squareness ratio | ηB (μB) |
|---|---|---|---|---|---|---|
| Z3 | 16.38 | 34.50 | 0.29 | 576.64 | 0.017 | 0.70 |
| Z5 | 18.60 | 36.82 | 0.30 | 698.94 | 0.016 | 0.80 |
| Z7 | 25.91 | 94.74 | 1.31 | 2504.81 | 0.050 | 1.11 |
Magnetic properties
Figure 6 shows the hysteresis curve of the samples recorded at room temperature. The VSM data are used to extract the magnetic parameters such as saturation magnetization MS, retentivity Mr and coercivity HC. These values are further used to calculate the squareness ratio S, anisotropy constant K and magnetic moment ηB [58]. The obtained values are presented in Table 2.
Fig. 6.
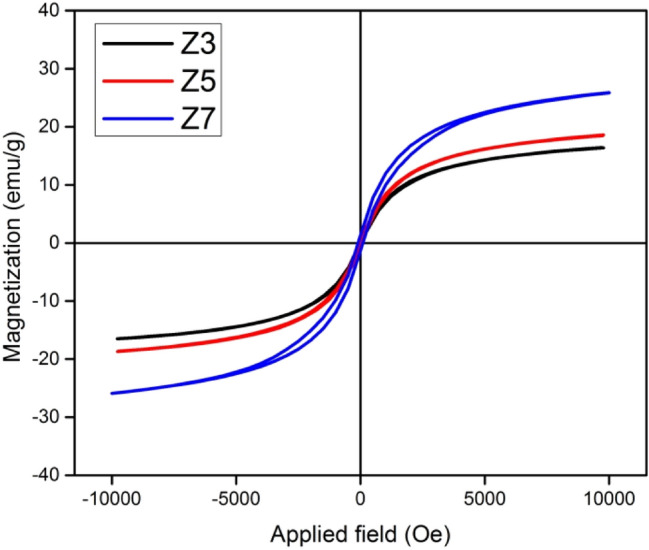
M-H curves for Z3, Z5 and Z7
Magnetic parameters strongly depend on average crystallite size. The observed magnetic behavior reflects modification in the structure of NPs. The hysteresis-absent curves for Z3 and Z5 show superparamagnetic nature with negligible retentivity and small values of coercivity. The M-H curve for Z7 depicts a ferrimagnetic behavior. The saturation magnetization increases with an increase in annealing temperature attributable to the increased average crystallite size. According to the dead layer theory, as smaller crystallites possess a larger surface-to-volume ratio, more atoms are captured by the surface relative to the core. This induces surface disorder which reduces the saturation magnetization. The reduction in surface disorder enhances magnetization [59]. The increase in coercivity with annealing demonstrates the single domain nature of MNPs [60]. The behavior of superparamagnetism for ZnFe2O4 nanoparticles with average crystallite size ~ 5 nm is reported previously [61, 62]. The values of squareness ratio lie below 0.5 depicting magnetostatic particle interactions. The magnetic moment increases with annealing due to the movement of Zn2+ ions from B-sites to A-sites. It is inferred that annealing above 500 °C results in the transformation of superparamagnetic NPs with dominating amorphous phase to ferrimagnetic NPs with enhanced crystalline structure simultaneously maintaining the single-domain particles.
Electrical properties
The variation in AC electrical conductivity of prepared ferrites with frequency at room temperature is shown in Fig. 7. The effect of annealing on the electrical properties of prepared ferrites is depicted in the figure. The conductivity initially increases slowly at lower frequencies followed by a steep increase at higher frequencies. The relation between AC conductivity and frequency is given by Eq. 8 [63].
| 8 |
Fig. 7.
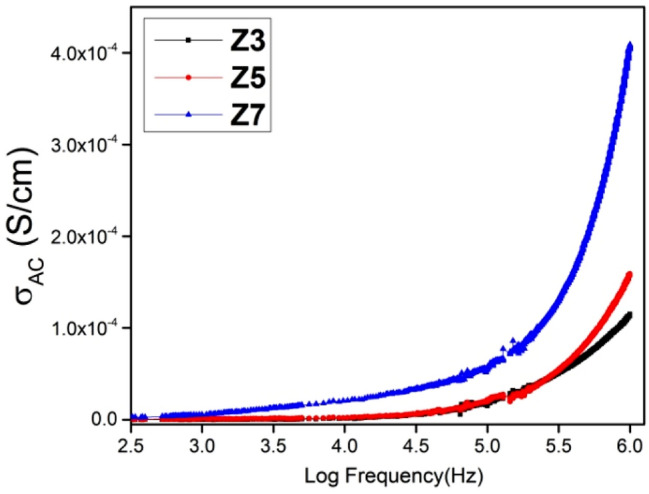
Frequency-dependent AC conductivity variation of Z3, Z5 and Z7
In the above equation, B and n are temperature-dependent constants. The increase in the conductivity values can be explained using Maxwell–Wagner polarization theory and Koop’s two-layer model [64]. The nonconducting grain boundaries are dominant at low frequencies. These insulating grain boundaries trap the electrons, resulting in low AC conductivity values. In the high-frequency region, the dominant nature of conducting grains facilitates the electron-hopping mechanism which is depicted by the sharp increase in AC conductivity values. The value of n belonging to the range 0–1 depicts short-range transitions of frequency-dependent AC conductivity [65].
The AC conductivity values increase with an increase in annealing temperature. The observation can be attributed to the increased crystallite size, cation redistribution and reduced defects [66].
Humidity sensing study
The variation in AC conductivity with relative humidity at a constant high frequency is shown in Fig. 8. The AC conductivity increases with an increase in relative humidity. The conduction mechanism significantly depends on water adsorption and proton conduction on the surface of ferrite. The adsorption of water vapors results in the dissociation of hydrogen ions which form hydroxyl groups by bonding with lattice Fe ions. This leads to free electron liberation which facilitates the conduction mechanism [67, 68]. The phenomenon is represented in (Eqs. 9 and 10).
| 9 |
| 10 |
Fig. 8.
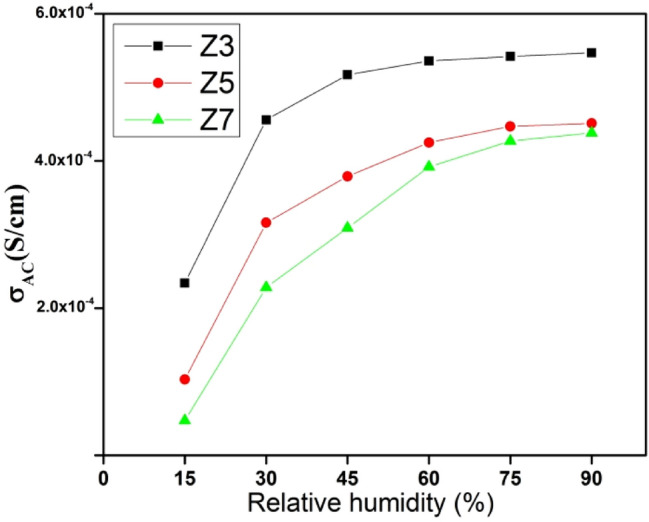
Variation in AC conductivity with relative humidity for Z3, Z5 and Z7
In Eq. 11, OO is the oxygen present at the lattice site. The plot can be explained by a two-stage process attributed to a low humidity region and a high humidity region. In the first stage, two surface hydroxyls per molecule are formed by surface chemisorptions of water molecules. The conductivity takes place due to the hopping mechanism of electrons in the chemisorbed layer. In the second stage corresponding to the high humidity region, capillary condensation of vapors takes place in the pores. As a consequence both the Grotthuss mechanism and hydronium diffusion occurs [69, 70]. The humidity sensitivity coefficient is calculated using Eq. 11.
| 11 |
In the above equation, ΔσAC is the increase in conductivity at x% RH and σAC is the conductivity at lower RH. The calculated sensitivity coefficients are tabulated in Table 3.
Table 3.
Humidity sensitivity for different humidity range for Z3, Z5 and Z7
| Humidity range (% RH) | Humidity sensitivity (%) | ||
|---|---|---|---|
| Z3 | Z5 | Z7 | |
| 15–30 | 35 | 40.3 | 42.3 |
| 30–45 | 7.1 | 8.79 | 12.89 |
| 45–60 | 2.07 | 5.9 | 11.7 |
| 60–75 | 0.6 | 2.6 | 4.4 |
| 75–90 | 0.53 | 0.4 | 1.3 |
The table reveals that sensitivity decreases as we move from the low RH region to the high RH region. Also, the sensitivity is highest for Z7 despite possessing the largest crystallite size, lowest surface area and porosity. Similar unanticipated observations have been reported in the previous study where annealing has a significant impact on the sensitivity of materials [50, 71–75]. The inverse relation between the lattice strain and sensitivity has been proposed in the literature [72]. The highest sensitivity of Z7 can be attributed to its ordered structure, enhanced crystallinity, greater stability and reduced lattice strain.
Conclusion
In this article, we have synthesized ZnFe2O4 nanoparticles using the chemical coprecipitation technique. The prepared NPs were annealed at 300 °C, 500 °C and 700 °C to improve the structural, magnetic, optical and electrical properties. X-ray diffraction revealed the formation of the amorphous-phase dominant crystalline NPs annealed at 300 °C and 500 °C although further annealing helped to enhance crystallinity to a maximum extent. The average crystallite size of the samples belongs to the range of 5–33 nm. FTIR confirmed the spinel ferrite structure of the samples. The thermal analysis demonstrated a two-step weight loss approach to attain a stable form with the complete removal of organic compounds adsorbed during the synthesis process. Absorption spectra in the UV–VIS region depict the enhancement of optical properties with annealing. The magnetic measurements exhibited superparamagnetic to ferrimagnetic phase transition on annealing. The AC conductivity of prepared ferrites in the frequency range of 300 Hz–1 MHz increased with annealing temperature. The humidity sensing properties were examined in a broad relative humidity range of 15–90%. AC conductivity showed a substantial increase with relative humidity. A significant impact of annealing on humidity sensitivity properties of ZnFe2O4 nanoparticles is observed indicating their suitability for efficient humidity sensors operating at room temperature.
Author Contributions
Anu Rana contributed to supervision, software, validation, writing-reviewing and editing; Nitika contributed to data curation, writing-original draft preparation, investigation and formal analysis; Vinod Kumar contributed to conceptualization, methodology, visualization and resources.
Funding
None to declare.
Availability of data and material
The authors declare that all the data supporting the findings of this study are available within the article.
Compliance with Ethical Standards
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thota S, Kashyap SC, Sharma SK, Reddy VR. Micro Raman, Mossbauer and magnetic studies of manganese substituted zinc ferrite nanoparticles: role of Mn. J. Phys. Chem. Solids. 2016;91:136–144. [Google Scholar]
- 2.Lian W, Xuan Y, Li Q. Design method of automatic energy transport devices based on the thermomagnetic effect of magnetic fluids. Int. J. Heat Mass Transf. 2009;52(23–24):5451–5458. [Google Scholar]
- 3.Xi G, Yang L, Lu M. Study on preparation of nanocrystalline ferrites using spent alkaline Zn–Mn batteries. Mater. Lett. 2006;60(29–30):3582–3585. [Google Scholar]
- 4.Nitika RA, Kumar V. Tailoring the structural, magnetic, mechanical, and thermal properties of CoFe2O4 by varying annealing temperature for high-density storage devices. ECS J. Solid State Sci. Technol. 2021;10(3):031005. [Google Scholar]
- 5.Gadkari AB, Shinde TJ, Vasambekar PN. Ferrite gas sensors. IEEE Sensors journal. 2010;11(4):849–861. [Google Scholar]
- 6.Nitika RA, Kumar V, Awasthi AM. Effect of dopant concentration and annealing temperature on electric and magnetic properties of lanthanum substituted CoFe2O4 nanoparticles for potential use in 5G wireless communication systems. Ceram. Int. 2021;47(14):20669–20677. [Google Scholar]
- 7.Raúl V. Novel applications of ferrites. Phys. Res. Int. 2012;2012:9. doi: 10.1155/2012/591839. [DOI] [Google Scholar]
- 8.Tudorache F, Popa PD, Dobromir M, Iacomi F. Studies on the structure and gas sensing properties of nickel–cobalt ferrite thin films prepared by spin coating. Mater. Sci. Eng., B. 2013;178(19):1334–1338. [Google Scholar]
- 9.Amiri M, Salavati-Niasari M, Akbari A. Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv. Coll. Interface. Sci. 2019;265:29–44. doi: 10.1016/j.cis.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 10.T. Tatarchuk, M. Bououdina, J.J. Vijaya, L.J. Kennedy, Spinel ferrite nanoparticles: synthesis, crystal structure, properties, and perspective applications. in International Conference on Nanotechnology and Nanomaterials, (Springer, Cham, 2016), pp. 305–325
- 11.Aisida SO, Ali A, Oyewande OE, Ahmad I, Ul-Hamid A, Zhao TK, Ezema FI. Biogenic synthesis enhanced structural, morphological, magnetic and optical properties of zinc ferrite nanoparticles for moderate hyperthermia applications. J. Nanopart. Res. 2021;23(2):1–14. [Google Scholar]
- 12.Farahani H, Wagiran R, Hamidon MN. Humidity sensors principle, mechanism and fabrication technologies: a comprehensive review. Sensors. 2014;14(5):7881–7939. doi: 10.3390/s140507881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y, Wang F, Carpenter M, Weller RB, Tabor D, Payne SR. The effect of indoor thermal and humidity condition on the oldest-old people's comfort and skin condition in winter. Building Environ. 2020;174:106790. [Google Scholar]
- 14.J. Khatri, N. Sharma, P. Dahlander, L. Koopmans, Effect of relative humidity on water injection technique in downsized spark ignition engines. Int. J. Engine Res. 22(7), 2119–2130. 10.1177/1468087420940854
- 15.B. Alfaresi, Z. Nawawi, R.F. Malik, K. Anwar, L.O. Nur, Humidity Effect to 5G Performance under Palembang Channel Model at 28 GHz. Sinergi 24(1), 49–56 (2020)
- 16.F.B. Albuquerque, H. Shea, Effect of humidity, temperature and elastomer material on the lifetime of silicone-based dielectric elastomer actuators under a constant DC electric field (Conference Presentation). in Electroactive Polymer Actuators and Devices (EAPAD) XXII, Vol. 11375. International Society for Optics and Photonics, pp. 113751E (2020)
- 17.C. Amitrano, C. Arena, S. De Pascale, V. De Micco, Light and low relative humidity increase antioxidants content in mung bean (Vigna radiata L.) sprouts. Plants 9(9), 1093 (2020) [DOI] [PMC free article] [PubMed]
- 18.G.M. Patel, V.R. Shah, G.J. Bhatt, P.T. Deota, Humidity nanosensors for smart manufacturing. Nanosensors for Smart Manufacturing, 555–580 (2021)
- 19.Wernecke R, Wernecke J. Industrial moisture and humidity measurement: a practical guide. John Wiley & Sons; 2013. [Google Scholar]
- 20.Ward MP, Xiao S, Zhang Z. Humidity is a consistent climatic factor contributing to SARS-CoV-2 transmission. Transbound. Emerg. Dis. 2020;67(6):3069–3074. doi: 10.1111/tbed.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biryukov J, Boydston JA, Dunning RA, Yeager JJ, Wood S, Reese AL, Altamura LA. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. MSphere. 2020;5(4):e00441–e520. doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smither SJ, Eastaugh LS, Findlay JS, Lever MS. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerging microbes & infections. 2020;9(1):1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlawat A, Wiedensohler A, Mishra SK. An Overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol and Air Quality Research. 2020;20(9):1856–1861. [Google Scholar]
- 24.A. Rojano Aguilar, R. Salazar Moreno, I. López, W. Ojeda, U. Schmidt, C. Huber, Temperature and humidity as physical limiting factors for controlled agriculture. in International Symposium on High Technology for Greenhouse Systems: GreenSys2009 893. pp. 503–507 (2009)
- 25.Jaba J, Mishra SP, Arora N, Munghate R. Impact of Variegated Temperature, CO2 and Relative Humidity on Survival and Development of Beet Armyworm Spodoptera exigua (Hubner) under Controlled Growth Chamber. Am. J. Clim. Chang. 2020;9(04):357–370. [Google Scholar]
- 26.A. Tullus, K. Rosenvald, R. Lutter, A. Kaasik, P. Kupper, A. Sellin, Coppicing improves the growth response of short-rotation hybrid aspen to elevated atmospheric humidity. Forest Ecol. Manag. 459, 117825 (2020)
- 27.Rana A, Thakur OP, Kumar V, Pant RP, Singh B. Preparation and Characterization of Gd3+ Substituted Nano-Structured Cobalt Ferrites for Humidity Sensor. Sens. Lett. 2014;12(9):1378–1382. [Google Scholar]
- 28.I.C. Sathisha, K. Manjunatha, A. Bajorek, B.R. Babu, B. Chethan, T.R.K. Reddy, V.J. Angadi, Enhanced humidity sensing and magnetic properties of bismuth doped copper ferrites for humidity sensor applications. J. Alloy. Compd. 848, 156577 (2020)
- 29.Priya RS, Chaudhary P, Kumar ER, Balamurugan A, Srinivas C, Prasad G, Sastry DL. Evaluation of structural, dielectric and electrical humidity sensor behaviour of MgFe2O4 ferrite nanoparticles. Ceram. Int. 2021;47(11):15995–16008. [Google Scholar]
- 30.H.R. Lakshmiprasanna, K. Manjunatha, J. Husain, Effect of cerium on structural, microstructural, magnetic and humidity sensing properties of Mn–Bi ferrites. Nano-Struct. Nano-Objects 24, 100608 (2020)
- 31.Şaşmaz Kuru T. Synthesis and investigation of structural, dielectric, impedance, conductivity and humidity sensing properties of Cr 3+-substituted Mg–Zn ferrite nanoparticle. Appl. Phys. A. 2020;126:1–10. [Google Scholar]
- 32.S. Kalunge, A.V. Humbe, M.V. Khedkar, S.D. More, A.P. Keche, A.A. Pandit, Investigation on synthesis, structural and electrical properties of zinc ferrite on gamma irradiation. in Journal of Physics: Conference Series, Vol. 1644, No. 1. (IOP Publishing, 2020), p. 012017
- 33.S.O. Aisida, I. Ahmad, F.I. Ezema, Effect of calcination on the microstructural and magnetic properties of PVA, PVP and PEG assisted zinc ferrite nanoparticles. Phys. B Condens. Matter 579, 411907 (2020)
- 34.Aisida SO, Akpa PA, Ahmad I, Maaza M, Ezema FI. Influence of PVA, PVP and PEG doping on the optical, structural, morphological and magnetic properties of zinc ferrite nanoparticles produced by thermal method. Physica B. 2019;571:130–136. [Google Scholar]
- 35.M. Abdellatif-Youssef, M. Etter, P. Fromme, M. Salerno, Extended properties of magnetic spins of zinc ferrite nanoparticles in the THz frequency range. J. Magn. Magn. Mater. 525, 167574 (2021)
- 36.Miura N, Zhuiykov S, Ono T, Hasei M, Yamazoe N. Mixed potential type sensor using stabilized zirconia and ZnFe2O4 sensing electrode for NOx detection at high temperature. Sens. Actuators, B Chem. 2002;83(1–3):222–229. [Google Scholar]
- 37.Niu X, Du W, Du W. Preparation and gas sensing properties of ZnM2O4 (M= Fe Co, Cr) Sens. Actuators, B Chem. 2004;99(2–3):405–409. [Google Scholar]
- 38.L. Lv, Y. Wang, P. Cheng, B. Zhang, F. Dang, L. Xu, Ultrasonic spray pyrolysis synthesis of three-dimensional ZnFe2O4-based macroporous spheres for excellent sensitive acetone gas sensor. Sens. Actuators B Chem. 297, 126755 (2019)
- 39.M.I. Nemufulwi, H.C. Swart, W.B. Mdlalose, G.H. Mhlongo, Size-tunable ferromagnetic ZnFe2O4 nanoparticles and their ethanol detection capabilities. Appl Surf Sci. 508, 144863 (2020)
- 40.Yang H, Bai X, Hao P, Tian J, Bo Y, Wang X, Liu H. A simple gas sensor based on zinc ferrite hollow spheres: Highly sensitivity, excellent selectivity and long-term stability. Sens. Actuators, B Chem. 2019;280:34–40. [Google Scholar]
- 41.Jeseentharani V, George M, Jeyaraj B, Dayalan A, Nagaraja KS. Synthesis of metal ferrite (MFe2O4, M= Co, Cu, Mg, Ni, Zn) nanoparticles as humidity sensor materials. J. Exp. Nanosci. 2013;8(3):358–370. [Google Scholar]
- 42.Aisida SO, Ahmad I, Zhao TK, Maaza M, Ezema FI. Calcination effect on the photoluminescence, optical, structural and magnetic properties of polyvinyl alcohol doped ZnFe2O4 nanoparticles. Journal of Macromolecular Science, Part B. 2020;59(5):295–308. [Google Scholar]
- 43.Okoroh DO, Ozuomba JO, Aisida SO, Asogwa PU. Properties of zinc ferrite nanoparticles due to PVP mediation and annealing at 500 C. Advances in nanoparticles. 2019;8(02):36. [Google Scholar]
- 44.V. Kumar, N. Kumar, S.B. Das, R.K. Singh, K. Sarkar, M. Kumar, Sol-gel assisted synthesis and tuning of structural, photoluminescence, magnetic and multiferroic properties by annealing temperature in nanostructured zinc ferrite. Materials Today: Proceedings (2021)
- 45.B.D. Cullity, Elements of X-ray Diffraction. (Addison-Wesley Publishing, 1956)
- 46.L. Phor, V. Kumar, Self-cooling device based on thermomagnetic effect of MnxZn1−xFe2O4 (x = 0.3, 0.4, 0.5, 0.6, 0.7)/ferrofluid. J Mater Sci: Mater. Electron. 30, 9322–9333 (2019). 10.1007/s10854-019-01262-8
- 47.T. Theivasanthi, M. Alagar, Titanium dioxide (TiO2) nanoparticles XRD analyses: an insight. arXiv preprint arXiv:1307.1091 (2013)
- 48.Wagner C. Theory of precipitation aging through dissolution (Ostwald ripening) Journal of Electrochemistry, Reports of the Bunsen Society for Physical Chemistry. 1961;65(7–8):581–591. [Google Scholar]
- 49.E. Suharyadi, S.H. Pratiwi, I.P.T. Indrayana, T. Kato, S. Iwata, K. Ohto, Effects of annealing temperature on microstructural, magnetic properties, and specific absorption rate of Zn-Ni ferrite nanoparticles. Mater. Res. Express 8(3), 036101 (2021)
- 50.Virlan C, Tudorache F, Pui A. Increased sensibility of mixed ferrite humidity sensors by subsequent heat treatment. Int. J. Appl. Ceram. Technol. 2017;14(6):1174–1182. [Google Scholar]
- 51.Waldron RD. Infrared spectra of ferrites. Physical review. 1955;99(6):1727. [Google Scholar]
- 52.Nitika R, A., & Kumar, V. Influence of temperature on structural, magnetic and thermal properties of superparamagnetic MnFe2O4 nanoparticles. Materials Today: Proceedings. 2021;45:4774–4776. [Google Scholar]
- 53.Manohar A, Krishnamoorthi C. Photocatalytic study and superparamagnetic nature of Zn-doped MgFe2O4 colloidal size nanocrystals prepared by solvothermal reflux method. J. Photochem. Photobiol., B. 2017;173:456–465. doi: 10.1016/j.jphotobiol.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 54.J.P. Singh, R.C. Srivastava, H.M. Agrawal, Optical behaviour of zinc ferrite nanoparticles. in AIP Conference Proceedings, Vol. 1276, No. 1. (American Institute of Physics, 2010), pp. 137–143
- 55.J. Tauc, (Ed.). Amorphous and liquid semiconductors. (Springer Science & Business Media, 2012)
- 56.Zarandi AA, Alvani AAS, Salimi R, Sameie H, Moosakhani S, Poelman D, Rosei F. Self-organization of an optomagnetic CoFe 2 O 4–ZnS nanocomposite: preparation and characterization. Journal of Materials Chemistry C. 2015;3(16):3935–3945. [Google Scholar]
- 57.S. Jauhar, J. Singh, K. Chandra, S. Bansal, S. Singhal, Structural, morphological, magnetic and optical properties of chromium substituted strontium ferrites, SrCrxFe12− xO19 (x= 0.5, 1.0, 1.5, 2.0 and 2.5) annealed with potassium halides. Powder Technol. 212(1), 193–197 (2011)
- 58.Kumari N, Kumar V, Khasa S, Singh SK. Chemical synthesis and magnetic investigations on Cr3+ substituted Zn-ferrite superparamagnetic nano-particles. Ceram. Int. 2015;41(1):1907–1911. [Google Scholar]
- 59.Budhiraja N, Kumar V, Singh SK. Tailoring the Structural, Optical and Magnetic Properties of NiFe 2 O 4 by Varying Annealing Temperature. J. Supercond. Novel Magn. 2018;31(8):2647–2654. [Google Scholar]
- 60.Massoudi J, Smari M, Nouri K, Dhahri E, Khirouni K, Bertaina S, Bessais L. Magnetic and spectroscopic properties of Ni–Zn–Al ferrite spinel: from the nanoscale to microscale. RSC Adv. 2020;10(57):34556–34580. doi: 10.1039/d0ra05522k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shahraki RR, Ebrahimi M, Ebrahimi SS, Masoudpanah SM. Structural characterization and magnetic properties of superparamagnetic zinc ferrite nanoparticles synthesized by the coprecipitation method. J. Magn. Magn. Mater. 2012;324(22):3762–3765. [Google Scholar]
- 62.Dhiman M, Sharma R, Kumar V, Singhal S. Morphology controlled hydrothermal synthesis and photocatalytic properties of ZnFe2O4 nanostructures. Ceram. Int. 2016;42(11):12594–12605. [Google Scholar]
- 63.Pattanayak S, Choudhary RNP, Pattanayak D. A comparative study of structural, electrical and magnetic properties rare-earth (Dy and Nd)-modified BiFeO 3. J. Mater. Sci.: Mater. Electron. 2014;25(9):3854–3861. [Google Scholar]
- 64.Wagner KW. On the theory of imperfect dielectrics. Ann. Phys. 1913;345(5):817–855. [Google Scholar]
- 65.Darwish MA, Trukhanov AV, Senatov OS, Morchenko AT, Saafan SA, Astapovich KA, Singh C. Investigation of AC-measurements of epoxy/ferrite composites. Nanomaterials. 2020;10(3):492. doi: 10.3390/nano10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yadav RS, Havlica J, Masilko J, Tkacz J, Kuřitka I, Vilcakova J. Anneal-tuned structural, dielectric and electrical properties of ZnFe 2 O 4 nanoparticles synthesized by starch-assisted sol–gel auto-combustion method. J. Mater. Sci.: Mater. Electron. 2016;27(6):5992–6002. [Google Scholar]
- 67.Chou KS, Lee TK, Liu FJ. Sensing mechanism of a porous ceramic as humidity sensor. Sens. Actuators, B Chem. 1999;56(1–2):106–111. [Google Scholar]
- 68.Muthurani S, Balaji M, Gautam S, Chae KH, Song JH, Padiyan DP, Asokan K. Magnetic and Humidity-Sensing Properties of Nanostructured Cu x Co1− x Fe2O4 Synthesized via Autocombustion. J. Nanosci. Nanotechnol. 2011;11(7):5850–5855. doi: 10.1166/jnn.2011.4455. [DOI] [PubMed] [Google Scholar]
- 69.Agmon N. The grotthuss mechanism. Chem. Phys. Lett. 1995;244(5–6):456–462. [Google Scholar]
- 70.Usman M, Rasool K, Batool SS, Imran Z, Ahmad M, Jamil H, Hasan MM. Humidity effect on transport properties of titanium dioxide nanoparticles. J. Mater. Sci. Technol. 2014;30(8):748–752. [Google Scholar]
- 71.Afzal A, Mujahid A, Iqbal N, Javaid R, Qazi UY. Enhanced High-Temperature (600° C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors. Nanomaterials. 2020;10(11):2133. doi: 10.3390/nano10112133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma A, Kumar Y, Shirage PM. Structural, optical and excellent humidity sensing behaviour of ZnSnO 3 nanoparticles: effect of annealing. J. Mater. Sci.: Mater. Electron. 2018;29(13):10769–10783. [Google Scholar]
- 73.Parimon N, Mamat MH, Banu IS, Vasimalai N, Ahmad MK, Suriani AB, Rusop M. Annealing temperature dependency of structural, optical and electrical characteristics of manganese-doped nickel oxide nanosheet array films for humidity sensing applications. Nanomaterials and Nanotechnology. 2021;11:1847980420982788. [Google Scholar]
- 74.Ahmad WRW, Mamat MH, Khusaimi Z, Ismail AS, Rusop M. Impact of annealing temperature to the performance of hematite-based humidity sensor. Indonesian Journal of Electrical Engineering and Computer Science. 2019;13(3):1079–1086. [Google Scholar]
- 75.Virlan C, Tudorache F, Pui A. Tertiary NiCuZn ferrites for improved humidity sensors: a systematic study. Arab. J. Chem. 2020;13(1):2066–2075. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the article.