Abstract
Objective:
Verbal memory deficits are linked to cannabis use. However, self-reported episodic use does not allow for assessment of variance from other factors (e.g., cannabis potency, route of consumption) that are important for assessing brain-behavior relationships. Further, co-occurring nicotine use may moderate the influence of cannabis on cognition. Here we utilized objective urinary measurements to assess the relationship between metabolites of cannabis, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH), and nicotine (cotinine) on verbal memory in young adults.
Method:
Adolescents and young adults (N=103) aged 16–22 completed urinary drug testing and verbal memory assessment (RAVLT). Linear regressions examined the influence of THCCOOH and cotinine quantitative concentrations, and their interaction, on RAVLT scores, controlling for demographics and alcohol. Cannabis intake frequency was also investigated. Secondary analyses examined whether past month or recency of use related to performance, while controlling for THCCOOH and cotinine concentrations.
Results:
THCCOOH concentration related to both poorer total learning and long delay recall. Cotinine concentration related to poorer short delay recall. Higher frequency cannabis use status was associated with poorer initial learning and poorer short delay. When comparing to self-report, THCCOOH and cotinine concentrations were negatively related to learning and memory performance, while self-report was not.
Conclusions:
Results confirm the negative relationship between verbal memory and cannabis use, extending findings with objective urinary THCCOOH and cotinine concentration measurements. No moderating relationship with nicotine was found, though cotinine concentration independently associated with negative short delay performance. Findings support the use of both urinary and self-report metrics as complementary methods in substance use research.
Keywords: Cannabis, Creatine-normalized THCCOOH concentrations, THCCOOH, Cotinine, Verbal memory, Urinary analysis
Introduction
Levels of daily cannabis use among 12th graders are nearing the highest recorded since 1991 (6.4%; (Johnston et al., 2020)), and 42% of college students and young adults report cannabis use in the last year (Schulenberg et al., 2019). This increase in the frequency of use may be due in part to the emergence of vaping devices, which are exposing teens to unprecedented high potency concentrations of Δ−9-tetrahydrocannabinol (THC, the primary psychoactive constituent of cannabis) in a more accessible fashion. Vaping devices are also crushing the decades of progress in tobacco and nicotine prevention efforts. Johnston et al. (2020) report that an alarming 35% of high school seniors had vaped nicotine in the last year.
As the brain undergoes dynamic developmental changes throughout adolescence and into the mid-20’s (Giedd et al., 2015; Gogtay et al., 2004), the cognitive implications of high potency cannabis and nicotine use are concerning. THC binds to cannabinoid 1 receptors (CB1) (Schneider, 2008) and nicotine activates nicotinic acetylcholine receptors (nAChRs) (Yuan, Cross, Loughlin, & Leslie, 2015) in the central nervous system. CB1 and nAChRs are both found in cortico-limbic brain regions and modulate processes related to neural circuit development, particularly dopamine pathways. Regular activation of these endogenous neural systems due to frequent use of cannabis and nicotine may alter neurodevelopmental trajectories and behavioral outcomes (Hurd et al., 2019; Yuan et al., 2015). Yet most research aiming to delineate the unique effects of substances on neurodevelopment rely on participant self-report. Evidence suggests that up to 13% of adolescents reported use is discordant with urinalysis results (Akinci, Tarter, & Kirisci, 2001), with recommendations to corroborate results with more objective measurements (Harris, Griffin, McCaffrey, & Morral, 2008; Williams & Nowatzki, 2009).
Though cannabis use is associated with a wide range of cognitive deficits in adolescents and young adults (Lisdahl, Wright, Kirchner-Medina, Maple, & Shollenbarger, 2014), one of the most common findings is in relation to verbal memory (Blest-Hopley, Giampietro, & Bhattacharyya, 2020). Acutely, THC has a direct impact on verbal learning and memory (Bhattacharyya et al., 2010; Morgan et al., 2012; Morgan, Schafer, Freeman, & Curran, 2010). Preliminary longitudinal studies also suggested that cannabis onset is related to poorer verbal memory performance over time (Hanson, Medina, Padula, Tapert, & Brown, 2011; Jacobus et al., 2015; Nguyen-Louie et al., 2015). Yet deficits may not be permanent; some studies find improved memory in those who remain abstinent from cannabis for up to a month (Hanson et al., 2010; Schuster et al., 2018), while others do not (Wallace, Wade, & Lisdahl, In Press). A meta-analysis found cannabis to have a small effect on learning and delayed memory performance (d=−.33 and d=−.26, respectively), while no difference in any other cognitive domain was detected following short term abstinence (Scott et al., 2018). However, methodological limitations in cannabis research (e.g., self-report bias, varying consumption pattern and product types) may be contributing to variability in study results and therefore, the inclusion of more objective markers of cannabis exposure may help disentangle discrepancies in the research literature (Huestis, 2007; Smith et al., 2018).
Measured cannabinoid concentrations are influenced by type of product (flower vs. concentrate), product potency (Fabritius et al., 2013; Greene, Wiley, Yu, Clowers, & Craft, 2018), drug administration route (smoked, vaporized, eaten, dabbed) (Newmeyer et al., 2017), frequency of use, and individual genetics (Hryhorowicz, Walczak, Zakerska-Banaszak, Slomski, & Skrzypczak-Zielinska, 2018; Stout & Cimino, 2014). Thus, differences in cannabis products used and consumption rate may influence cognitive outcomes due to changes in THC bioavailability and pharmacokinetics (Sharma, Murthy, & Bharath, 2012; Spindle et al., 2018, 2019). To illustrate, a within-subject design found significant variability of cannabinoid analyte concentrations in healthy individuals who consumed the same three edible products, each a week apart (Schlienz et al., 2018). Utilizing biosamples to measure the THC metabolite (11-nor-9-carboxy-THC or THCCOOH) may bypass this variability to predict more reliable cognitive results. Further, it was suggested (Karschner et al., 2009) that measurable THC concentrations in blood after cessation of cannabis use may explain why some studies find more persistent cognitive decrements, while others do not (e.g., (Scott et al., 2018)). Relatedly, more recent best practice recommendations suggest that studies ideally utilize both self-report and THCCOOH concentrations to best understand patterns and profiles of cannabis use (Smith et al., 2018). Objective quantified urinary concentrations may yield more interpretative results beyond qualitative urinalysis or self-report, yet the potential utility of this approach for assessing neuropsychological outcomes in cannabis research has been sparingly investigated.
Another complicating factor is the co-use of other substances, particularly nicotine (Ramo, Liu, & Prochaska, 2012). Acute nicotine smoking was shown to increase verbal memory performance in young adults (Potter, Hammond, Tuffnell, Walker, & Di Forti, 2018) with decrements in performance occurring during withdrawal (Jacobsen et al., 2005). A recent study suggested chronic nicotine use during young adulthood was positively associated with verbal memory performance in females, but not males (Kangiser, Lochner, Thomas, & Lisdahl, 2019), and some find poorer verbal recall associated with greater intensity (cigarettes per day and duration) of nicotine intake (Vajravelu, Gnanadurai, Krishnan, & Ayyavoo, 2015). Adding to the complexity, some also found that nicotine may mask memory deficits in young adult cannabis users (Hindocha, Freeman, Xia, Shaban, & Curran, 2017; Schuster, Crane, Mermelstein, & Gonzalez, 2015), suggesting interaction between the two substances wherein memory deficits may be most evident among low (or no) levels of nicotine use. In addition, no known studies investigated the relationship between cotinine and verbal memory. Taken together, this establishes a strong need to better assess the influence of nicotine and tobacco product (NTP) use and cotinine concentrations on verbal memory, particularly in young adult cannabis co-users.
For the present study, we aimed to determine if objective urinary markers of cannabis (THCCOOH) and NTP (cotinine) use predict verbal learning and memory performance. Therefore, we hypothesized that current cannabis users would have poorer verbal learning and memory than demographically matched non-users and former users, and that higher urinary THCCOOH and cotinine concentrations would independently associate with poorer performance on learning and memory. Second, THCCOOH has a long urinary detection window in frequent cannabis users following initiation of abstinence (Lowe et al., 2009; Schuster et al., 2020). Therefore, we tested if the cannabis-memory relationship was dependent on cannabis intake frequency (frequent vs. occasional group status). Finally, we explored if urine THCCOOH concentrations provided additional information beyond self-reported recency and past-month cumulative cannabis and nicotine use in relation to potential memory deficits.
Methods
Participants.
One hundred and five participants ages 16–22 were included from an ongoing study in San Diego County, California. Two participants were excluded for having incomplete data due to scheduling conflicts and being unable to complete the full study protocol, resulting in a final sample of 103 participants. Participants were recruited via flyers posted physically and electronically at local high schools, community colleges, and four-year universities. The advertisements described a research opportunity for a study on cannabis, nicotine, and brain development. Interested individuals called in to the laboratory phone. They provided verbal informed consent prior to assessing eligibility (or if <18 years-old, consent from a parent and verbal assent for the participant; parental participation in the study extended only so far as consenting to their child’s participation). Once consented/assented, participants completed a semi-structured brief interview that took approximately 10 minutes. Screening questions ensured participants met inclusion/exclusion criteria as listed below, briefly covering prenatal, medical, mental health, and substance use history.
Inclusion Criteria.
To ensure inclusion of both cannabis users and controls, recruitment and study procedures included participants who had either used cannabis in the past month or, for controls, not used cannabis in the past month. Sixty-six participants had used cannabis in the past month, and 37 had not used cannabis in the past month. In order to test whether the metabolite-performance relationship was dependent on cannabis user type and consistent with prior definitions from the cannabis toxicology literature (Desrosiers, Lee, et al., 2014; Huestis & Smith, 2018), cannabis users were divided into a frequent intake group (more days smoking than not, defined as >15 use episodes in the past month, n = 37) and occasional users (>1 and ≤15 use episodes in the past month, n = 29). Participants reported a wide range of past year episodic cannabis use (0–4,015 cannabis use episodes), suggesting some participants were using cannabis multiple times a day in separate use occasions. NTP use was assessed across both cannabis users and controls.
Exclusion Criteria.
Exclusion criteria for all participants included: excessive prenatal alcohol (maternal use of >2 drinks per occasion, >4 drinks in a week), tobacco, or drug exposure; premature birth (<34 weeks gestation); other gestational or perinatal complications, including low birth weight (<5 lbs); history of serious medical or neurological problems; head trauma with loss of consciousness >2 minutes; current or past DSM-5 diagnoses other than cannabis or nicotine use disorder; learning disability; current use of psychotropic medications; non-correctable vision/hearing difficulties; not fluent in English; pregnancy; use of alcohol or cannabis within 12 hours of study visit which would indicate potential current intoxication (see toxicology section below) (Dahlgren et al., 2020; Hindocha et al., 2017; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001).
In addition, all other substance use history was collected. Minimal use of other substances was observed in this sample. Participants reported individual past and recent episodic use of spice, opiates, amphetamines (other than as prescribed), barbiturates, hallucinogens, cocaine, inhalants, benzodiazepines, ecstasy, ketamine, GHB, and PCP. Participants reported an average of 4.4 other drug use episodes in their lifetime (SD=18.8, range=0–183) and average of 288.4 days of abstinence from other drug use (SD=425.4, range = 2–2,555). Drugs used in the past month include cocaine, ADHD medications (not as prescribed), ecstasy, and hallucinogens; no participants were positive for any of these substances on toxicological analysis. Given some participants had used other drugs than cannabis, NTP, and alcohol within the month leading up to study participation, analyses were run both with and without those who had used other drugs in the past month. Findings remained largely consistent in either case; results presented here include all participants, regardless of past month other substance use.
Procedures.
After confirming eligibility through screening, participants were brought into the laboratory and completed a 4-hour session consisting of cognitive assessment, toxicological analysis, and magnetic resonance imaging (MRI) scan (MRI data to be presented elsewhere). They were asked to remain abstinent from all drug use (other than nicotine) on the day of study participation (see Toxicological section). All participants underwent written informed consent (or consent from a parent if <18 years-old and assent from the participant) in accordance with the University of California, San Diego Human Research Protections Program.
Measures
Verbal Learning and Memory.
Participants were given the Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, 1996). Participants were read a list of 15 words over five trials and asked to repeat back as many of the words as they could remember after each reading. They were then read a second (distractor) list, with an immediate recall trial. After the distractor list, participants were asked to recall the first list again. Finally, after a 30-minute delay, participants were again asked to recall the original list. Variables of interest included raw scores for: initial learning (first trial recall), total learning (sum of trials 1–5 recall), short delay recall, and long delay recall. Each of these variables were included due to the unique aspect of learning and memory that they represent (Strauss, Sherman, & Spreen, 2006). Trial 1 performance indicates initial learning, a measurement of working memory. Trial 1–5 indicates total learning, revealing initial acquisition of learning. Short delay recall (Trial 6) demonstrates initial retention and consolidation, while long delay recall shows encoding and retrieval.
Substance Use History.
Participants completed a modified version of the original Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998; Jacobus et al., 2018; Karoly, Schacht, Jacobus, et al., 2019; Karoly, Schacht, Meredith, et al., 2019) to assess past year and lifetime substance use history. Given the complexity of cannabis use and the rise in vaping products, participants were additionally queried on most common forms of substance use (e.g., flower, concentrate, vaping, dabs) and potency, as well as personal history of use (e.g., age of first use, age of onset of regular use). Cannabis and NTP use were measured episodically, allowing for participants to report multiple use episodes in a single day if they were fully separate instances (e.g., right after waking up; after lunch).
Toxicological Assessment.
Urine, oral fluid, and breathalyzer samples for alcohol were collected to corroborate self-reported substance use. Oral fluid samples examined THC and other substance use using the Draeger DrugTest® 5000 (cutoff = 5 ng/mL THC). The Draeger DrugTest is one of the most sensitive and effective methods for detecting cannabis and/or other substance use within the past 12 hours (Desrosiers, Milman, et al., 2014; Wille SM, Samyn N, Ramírez-Fernández Mdel M, & G., 2010), ensuring participants are not acutely intoxicated (Desrosiers & Huestis, 2019; Desrosiers, Milman, et al., 2014). Redwood Toxicology Laboratory, Santa Rosa, CA, quantified urinary THCCOOH concentrations and normalized these to urinary creatinine, and quantified cotinine concentrations to confirm nicotine use. THCCOOH was confirmed at 5ng/mL (Redwood Laboratory, 2020), making it even more sensitive than federal workplace guidelines for cannabinoid urine testing (Kulig, 2017). Creatinine-normalized THCCOOH accounted for the individual’s state of hydration and reduced variability (Huestis et al., 2019; Huestis & Cone, 1998). THCCOOH, rather than THC, was measured due to the rapid metabolization of THC into THCCOOH once THC is ingested. THCCOOH is also the primary drug analyte tested in urinalysis for cannabinoids (Kulig, 2017). A breathalyzer was used to confirm abstinence from alcohol. Participants were allowed to use NTP ad libitum so as to prevent withdrawal effects; tobacco use recency ranged from 3 minutes to 3,650 days.
Demographics and Verbal Learning Performance.
Demographic characteristics (sex, race, ethnicity, education, maternal education) were considered for inclusion in all analyses. However, as only age differed by group status and no demographic covariates related to RAVLT performance, only age was included as a covariate in analyses. For all group-based analyses, ANOVAs and ANCOVAs examined demographic covariates (i.e., age) and cognitive differences by cannabis group status for comparisons on cognitive measures.
Primary Analyses.
Linear regression models examined the influence of quantitative THCCOOH and quantitative cotinine on four subtest measures of RAVLT performance: initial learning (first trial recall), total learning (sum of trials 1–5 recall), short delay recall, and long delay recall subtests. No participants with substance use and RAVLT data were excluded from analyses. Four hierarchical models were run in total, with THCCOOH, cotinine and covariates (i.e., age and past month alcohol use) included in the first step and a product term between urinary THCCOOH and cotinine entered in the second step for all four models to test the interaction between these variables. Data from significant regressions were assessed for outliers using DFBetas with cut-off value size 0.19, and no outliers were found to influence parameter estimates. Primary regressions were further confirmed through use of weight least squares regressions and iteratively reweighted least squares regressions to ensure sensitivity and robustness of results; results were largely invariant. Additional regressions examined whether cannabis user group type (i.e., frequent vs. occasional; frequent = more days using cannabis than not (Desrosiers, Lee, et al., 2014)) moderated the relation between metabolite concentrations and cognitive performance, given cannabis concentrations can have long detection windows for more frequent users despite abstinence (Huestis & Smith, 2018). For these models, group status (frequent user, occasional user, and control) was included in the first step of the regression with THCCOOH and covariates; two THCCOOH*group interaction terms were included in the second step to reflect dummy coding of frequent v. control and occasional v. control interactions. All individuals who were negative on drug screens had 0 imputed for their quantitative THCCOOH and/or cotinine concentrations. This included controls and some self-reported cannabis (n = 14) and NTP users (n = 24) who had metabolite concentrations that were undetectable in urine drug screening.
Secondary Analyses.
Exploratory analyses were conducted in the full sample on cognitive variables that demonstrated significant relationships with THCCOOH and/or cotinine concentrations in the primary analysis; only the recency analyses were reduced due to excluding participants who had never used cannabis. Hierarchical regressions were run to test if metabolites predicted cognitive performance above and beyond self-reported use over the past month or self-reported recency of substance use, as studies thus far have not directly compared the predictive utility of objective biological markers of cannabis and nicotine as compared to subjective self-reported use of cannabis and nicotine. Separate models tested self-reported recency and self-reported cumulative intake over the past 30 days, in the event one self-report measurement was a more robust predictor. Covariates (age) and recency or cumulative intake, for each respective model, was entered on step 1 and the corresponding metabolite on step 2 for each verbal learning subtest. Separate models were run to investigate self-reported cannabis and THCCOOH concentrations, and self-reported NTP and cotinine concentrations.
Results
Demographics and Verbal Learning Performance.
Mean age of participants was 19 years (SD = 1.6; see Table 1). Cannabis users were significantly older than Controls (F(1,102) = 5.48, p = .02). Cannabis users and Controls did not differ by gender, race, ethnicity, education, maternal education, or by past month NTP or alcohol use episodes (p > .05). Of participants who used cannabis in the past month, relative to low (around <5% THC) or medium (10% THC) flower intake, 46% reported intake of high THC (15%) flower and 20% reported very high THC (>20%) flower intake. Relative to low (around 20% THC) or medium (around 40% THC) concentrate use, 26% reported high potency (60% THC) concentrate intake, while another 33% reported very high (>80% THC) concentrate intake. Participants further reported that 51% mostly to always used high potency flower, while 41% report mostly to always used high potency concentrate.
Table 1.
Demographics and Substance Use Characteristics
| Cannabis Users (n=66) M/% (SD) range |
Controls (n=37) M/% (SD) range |
Whole Sample (n=103) M/% (SD) range |
|
|---|---|---|---|
| Age | 19.3 (1.5) | 18.6 (1.6) | 19.0 |
| Education | 12.8 (1.4) | 12.3 (1.6) | 12.6 (1.5) |
| % Female | 39% | 49% | 43% |
| % Hispanic | 53% | 35% | 47% |
| % Caucasian | 53% | 46% | 50% |
| Days since last cannabis use | 3.73 (5.6) | 255.5 (506.4) | 50.4 (234) |
| Past month cannabis use (episodes) | 37.88 (97.7) | 0 (0) | 24.5 (80.5) |
| Lifetime quantity of cannabis use (episodes) | 868.1 (1883.6) | 32.6 (129.3) | 573 (1565) |
| Age of first cannabis use | 16 (2) | 16.6 (1.5) | 16.1 (2) |
| Age of first regular cannabis use | 17.5 (1.7) | 16.3 (.6) | 17.5 (1.7) |
| Urinary THCCOOH concentration (ng/mL) | 140.8 (209.8) | 0 (0) | 178 (222) |
| Age of first NTP use | 16.4 (2) | 16.1 (1.3) | 16.4 (1.9) |
| Age of first regular NTP use | 17.9 (1.8) | 17 (1.3) | 17.7 (1.8) |
| # Past-Month NTP Users | n=32 | n=11 | n=43 |
| Past month NTP use (episodes) | 71.1 (297.5) | 20.1 (65.8) | 52.7 (234) |
| Lifetime nicotine use (episodes) | 1142.5 | 1120.6 | 1135 |
| Urinary Cotinine concentration (ng/mL) | 20.9 (78.6) | 30.2 (80.6) | 89.3 (133) |
| Age of first alcohol use | 16 (2) | 15.6 (2.7) | 15.8 (2.2) |
| Past month alcohol use (episodes) | 4.9 (5.1) | 3.2 (4.9) | 4.3 (5.1) |
| Lifetime alcohol use (episodes) | 158.5 (225.1) | 78.3 (173.0) | 130 (211) |
| Days since last alcohol use | 19.7 (46.6) | 46.7 (146.7) | 27.26 (87) |
| Lifetime other drug use (episodes) | 6.5 (23.1) | .8 (2.8) | 4.4 (18.8) |
| Days since last other drug use | 284 (441.7) | 309.2 (359.2) | 288.4 (425.5) |
| # used other drug in past month | n=8 | n=1 | n=9 |
Notes: sample size (n) indicated for number of participants who endorsed a response when it was not endorsed by all participants with one or both groups (e.g., not all Controls had used cannabis and so did not endorse an age of first use, never having used previously).
As anticipated, tests of initial (Trial 1) recall (F(1,100) = 8.615, p = 0.004), total learning (F(1,100) = 8.059, p = 0.005), short delay recall (F(1,100) = 4.985, p = 0.03) and long delay recall (F(1,98) = 4.308, p = 0.04) had significantly poorer performance across metrics in all cannabis users as compared to controls (see Table 2 for cognitive performance information). Age was not significantly related to any outcome.
Table 2.
Rey Auditory Verbal Learning Test (RAVLT) Performance
| Controls (n=37) M (SD) range |
Occasional Users (n=29) M (SD) range |
Frequent Users (n=37) M (SD) range |
|
|---|---|---|---|
| Initial Recall (Trial 1) | 6.4 (2.2) | 5.3 (1.5) | 5.3 (1.4) |
| Learning (Trials 1–5) | 53.8 (8.8) | 49.55 (7.7) | 48.8 (6.4) |
| Short Delay Recall | 12.2 (2.6) | 11.4 (2.3) | 10.8 (2.3) |
| Long Delay Recall | 11.8 (2.3) | 10.9 (2.7) | 10.5 (2.7) |
Primary Analyses.
Metabolites in relation to verbal learning.
Regressions including all participants assessed the predictive utility of urinary THCCOOH and cotinine concentrations on verbal memory performance, controlling for age and past-month alcohol use. Higher concentration of creatinine-normalized THCCOOH was significantly related to lower scores on total learning (ß = −0.196, t = −2.055, p = 0.043) and long delay recall (ß = −0.232, t = −2.413, p = 0.018), and is reflected in Figure 1. Higher cotinine concentration was similarly related to fewer recalled words after a short (ß = −0.218, t = −2.251, p = 0.027) and long delay (ß = −0.195, t = −2.032, p = 0.045), and is reflected in Figure 2. More past month self-reported alcohol use also was related to poorer total learning (ß = −0.219, t = −2.224, p = 0.028). The THCCOOH*cotinine interaction term was not significant (ps > 0.450) in the regression models and, therefore, this term was not retained in the final models in favor of parsimony.
Figure 1. RAVLT performance and Creatinine-Normalized THCCOOH Concentration.
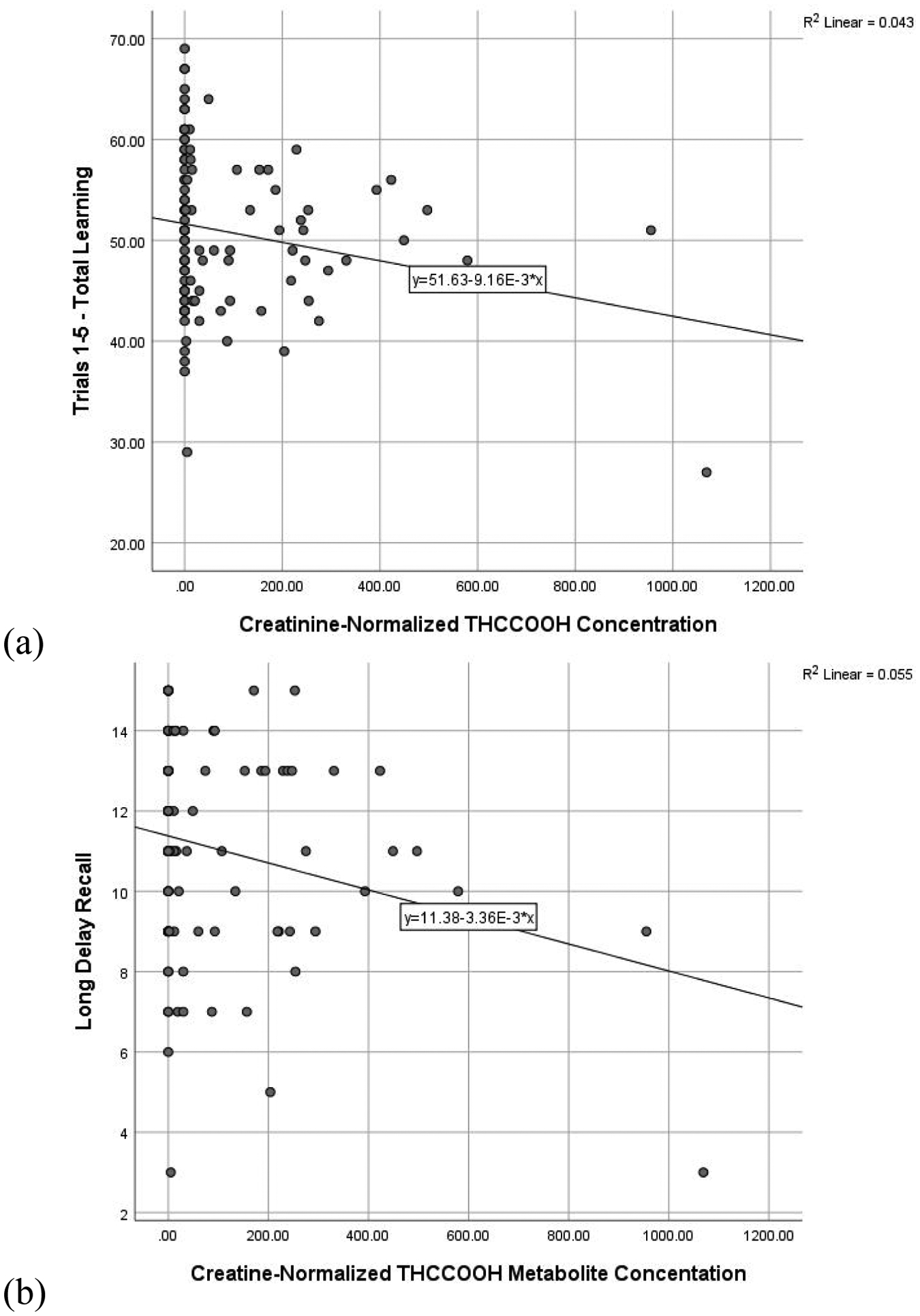
Scatterplot of significant relationships between creatinine-normalized THCCOOH concentration and (a) total learning and (b) long delay recall. Figures presented represent bivariate relationships, which reflect but do not directly correspond to model results summarized within the text.
Figure 2. RAVLT performance and Cotinine Concentration.
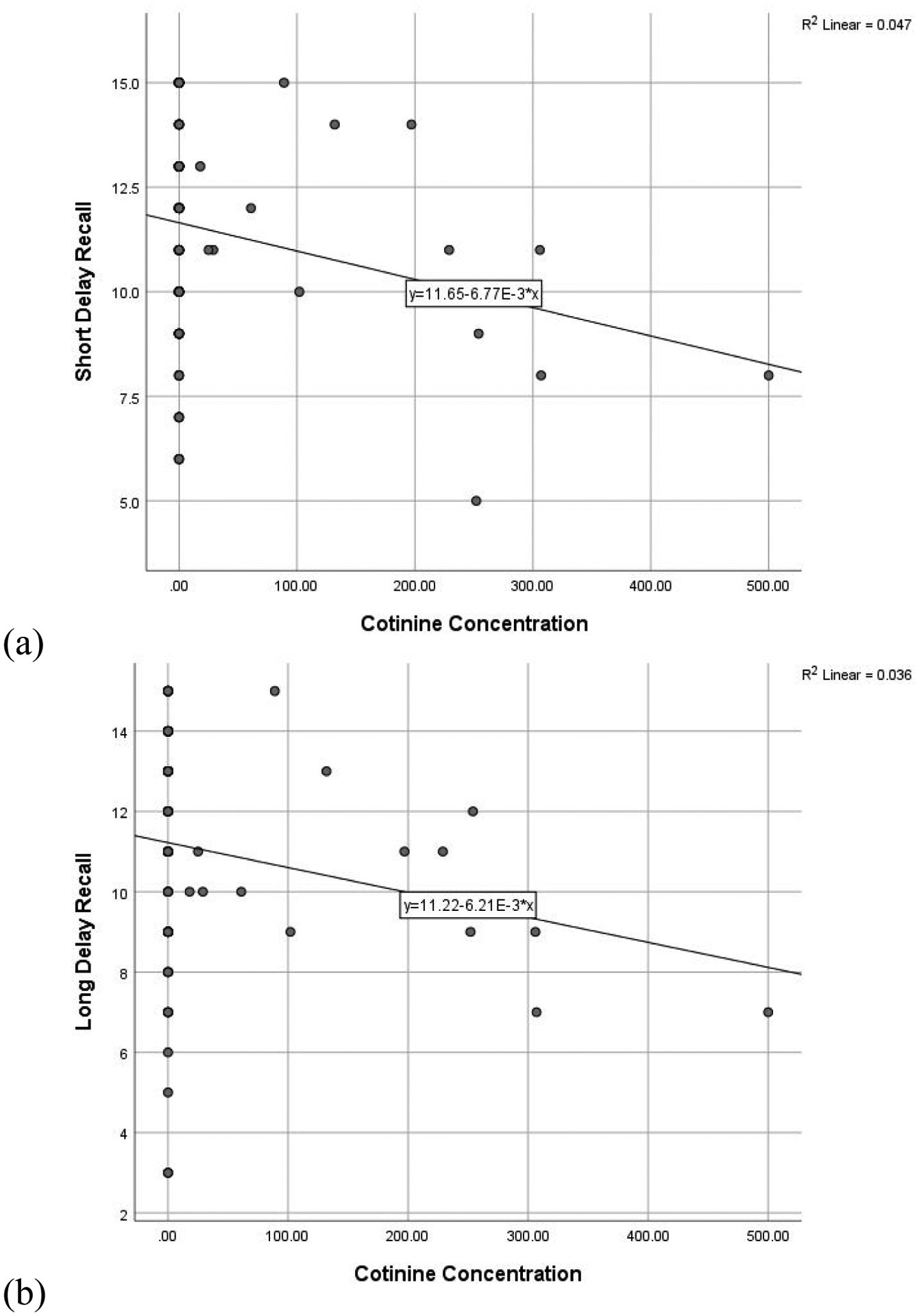
Scatterplot of significant relationships between cotinine concentration and (a) short delay recall and (b) long delay recall. Figures presented represent bivariate relationships, which reflect but do not directly correspond to model results summarized within the text.
Cannabis use frequency.
Regressions assessed verbal learning and memory performance in relation to THCCOOH concentration and group status (controls, occasional users, or frequent users), and the THCCOOH*group interaction, controlling for age. As there was no THCCOOH*group interaction, the interaction term was not included in the final models for parsimony. Group status was significantly related to initial (Trial 1) learning (ß = −0.259, t = −2.252, p = 0.027). Follow-up analyses to determine significant differences by group revealed frequent users exhibited significantly lower performance than controls (p = 0.03) and marginally lower performance than occasional users (p = 0.055). There was no difference between occasional users and controls.
Secondary Analyses.
Recency and cumulative cannabis use.
In assessing the utility of THCCOOH concentrations relative to self-reported length of abstinence, self-reported recency since last use of cannabis did not relate to total learning and/or delayed memory. THCCOOH concentrations continued to relate to long delay recall (ß = −0.226, t = −2.016, p = 0.047, ΔR2 = .053), controlling for self-reported recency (ß = −0.009, p = 0.939) and age (ß = −0.059, p= 0.604). Similarly, in assessing the utility of THCCOOH concentrations relative to self-reported cumulative cannabis use in the past month, cumulative use did not relate to total learning and/or delayed recall. Higher THCCOOH concentrations continued to relate to poorer long delay recall (ß = −0.248, t = −2.338, p = 0.021, ΔR2 = 0.054), controlling for self-reported past month use (ß = 0.018, p = 0.862) and age (ß = −0.026, p = 0.793).
Recency and cumulative nicotine use.
In assessing the utility of nicotine relative to self-reported length of abstinence, self-reported recency of last NTP use was unrelated to short and long delay recall. Cotinine was negatively associated with short delay performance (ß = −0.255, t = −2.089, p = 0.041, ΔR2 = 0.064) controlling for self-reported recency (ß = −0.038, p = 0.759) and age (ß = −0.090, p = 0.466). Similarly, past month cumulative NTP use did not predict short and long delay recall. However, in the second step, cotinine concentration was negatively associated with short delay performance (ß = −.224, t = −2.298, p = .024, ΔR2 = .049), controlling for cumulative NTP use (ß = 0.131, p = .183) and age (ß = −0.90, p = 0.356).
Discussion
Here we assessed the relation between urinary THCCOOH concentrations, cannabis intake frequency, and memory performance. Consistent with prior results (Blest-Hopley et al., 2020), cannabis use was related to poorer verbal learning and memory. Specifically, greater urinary THCCOOH concentrations were related to poorer learning and delayed recall performance across both occasional and frequent users of cannabis; and frequent cannabis users in particular demonstrated poorer performance on initial learning and short delay recall. Cotinine, a metabolite of nicotine, was also significantly related to poorer short delay performance. Finally, comparison of self-report metrics (recency of use and cumulative use) and urinary samples on significant cognitive outcomes found that THCCOOH and cotinine concentrations continued to relate to poorer performance, while self-report variables were unrelated.
Results suggest that cannabis metabolite concentrations in urine predict level of performance on a verbal learning and memory task. Others previously showed urinary THCCOOH concentration to be predictive of learning and memory in adults (Owens et al., 2019; Pope et al., 2001). In addition, having high THC concentrations (versus a median-split low concentration) in hair was associated with decrements in memory performance (Morgan et al., 2012), and higher THC serum concentration was associated with motor impairment (Bonnet, Borda, Scherbaum, & Specka, 2015). Though disparate in biological matrices, the cumulative message of these various studies combined with the present results suggest that reliable, objective biological samples may be better predictors of certain cannabis-related cognitive outcomes. This may be due to numerous factors that influence how an individual processes THC, such as dosing, frequency of use, and product used (Musshoff & Madea, 2006; Sharma et al., 2012).
Implications of cannabinoid metabolite concentrations are interesting to consider. THCCOOH in urine is considered a marker of cannabis use, with the known limitation that it can be detected even after a month of monitored abstinence (Goodwin et al., 2008; Schuster et al., 2020). Notably, the highest concentration of creatinine-normalized THCCOOH is detected usually within a couple days of last use (Goodwin et al., 2008). As the small effect of cannabis on learning and memory may only last for the first initial days of abstinence (Scott et al., 2018), it may be that cognition improves as markers of cannabinoid use decrease (e.g., lower THCCOOH levels, perhaps in conjunction with other factors such as upregulation of cannabinoid activity with recent abstinence (Hirvonen et al., 2012)). Interestingly, self-reported abstinence and recency since last use itself was not related to memory performance. However, this may be due to other important factors that cannot be detected in a broad self-reported variable, such as potency of product used (Fabritius et al., 2013; Greene et al., 2018) or genetics (Hryhorowicz et al., 2018), which may contribute to biometric data. Clinically, then, measuring objective levels of THCCOOH may be a useful indicator of how much cannabis is potentially influencing memory performance; high levels of THCCOOH would make memory performance suspect. More broadly, results speak to the importance of several days of abstinence from cannabis prior to neuropsychological assessment, to ensure results reflect more enduring brain-behavior relationships during neuropsychological testing, rather than acute cannabinoid-related deficits.
Importantly, a near ubiquitous issue in the substance use literature is reliance on self-report. Adolescent substance use misreporting is associated with factors such as age of onset and mental health (Harris et al., 2008), and one study suggests misreporting is leading to a gross underestimate of how many adolescents actually use cannabis (Murphy & Rosenman, 2019). Accordingly, a family substance use study found urine drug screen results were discordant with 13% of male adolescents substance use self-report (Williams & Nowatzki, 2009). Yet, self-report may still provide some meaningful information. As exhibited in the present results, we did not observe a group (e.g., occasional cannabis use, frequent cannabis use) by THCCOOH interaction on verbal memory performance. Yet, groups representing self-reported frequency of use predicted initial learning and short delay performance—two variables that were not predicted by THCCOOH concentrations across all groups of cannabis users. This may suggest self-reported use and self-reported use patterns also capture other cannabis-related factors (e.g., trait characteristics with similar neurobiological etiology or premorbid functioning) that obscure some direct relationships between cannabis and cognitive outcomes. Importantly, the use of creatinine-normalized analytes is thought to be highly reliable and suggested as appropriate for interpretation of objective cannabis measurement (Huestis et al., 2019). Further, use of urinary cannabinoid levels is significantly related to carefully quantified (gram-based) measurement of cannabis use (Tomko et al., 2018), suggesting convergent validity of methodologies. Thus we agree with others who suggested biological samples, in addition to self-report, should be sought in all cannabis research (Smith et al., 2018).
We found one relation between NTP measures and verbal memory, as a higher cotinine concentration was associated with poorer verbal memory. This is in contrast to other cross-sectional studies of young adult nicotine users (Kangiser et al., 2019). We allowed smoking up to an hour before testing and, therefore, do not believe this to be due to withdrawal effects (Jacobsen et al., 2005). Others found intensity of smoking determines cognitive outcomes in young adults (Vajravelu et al., 2015), suggesting disparate results may be due to young adults smoking at low levels of use, which do not correlate to deficits in cognitive functioning. In addition, users here were not necessarily pure NTP users, but often used alcohol if not cannabis as well. However, follow-up analysis that assessed for interactive relationships between THCCOOH and cotinine concentrations on memory performance revealed no significant results, despite prior work suggesting a moderating influence of NTP on cannabis (Schuster et al., 2015). Therefore, more careful teasing apart of co-use of NTP with other substances by including an NTP-only group is in process for future analyses.
Interestingly, all assessed substances were associated with decrements in learning and/or memory. This is consistent with cannabis (Jacobus et al., 2015; Solowij et al., 2011) and alcohol (Spear, 2018) literature suggesting memory deficits in regular adolescent and young adult users. Preliminary evidence also suggests greater frequency of nicotine use is associated with cognitive deficits (Vajravelu et al., 2015), and sex may moderate the influence of nicotine on memory (Kangiser et al., 2019). While nicotine may acutely improve cognition (Campos, Serebrisky, & Castaldelli-Maia, 2016), and attenuate cannabis-related cognitive decline (Schuster et al., 2015), more recent and chronic use (such as would be measured in cotinine) may be associated with unique cognitive decline in consolidation and retention. Thus, our null interaction results may be unsurprising given there is likely a nuanced relationship between frequency of nicotine use, recency of cannabis use, and learning and memory performance. More prospective research is needed on larger samples to fully delineate patterns of use and co-use in relation to memory function.
This study replicates numerous prior reports of emerging adult cannabis users demonstrating deficits in verbal memory relative to controls, while importantly revealing novel relationships with more reliable, objective measurements of cannabis use through urine toxicology. At the same time, there are limitations to be considered. Due to financial limitations, we did not send the urine sample for quantitative confirmation if the on-site screening was negative, therefore, we used 0 for these samples for their creatinine-normalized THCCOOH concentrations. As aims of this project included increased generalizability of cannabis research, and examine cumulative and recency of use, we chose to enroll controls who had recent and remote use of cannabis as well as other substance use. Future studies may benefit from excluding participants with other substance use so as to more directly isolate the effects of cannabis and NTP use. As a cross-sectional study, we are unable to determine directionality between cannabis use and cognitive functioning. Further, while we carefully selected a relatively small group of theoretically-driven verbal memory subtests a priori, results are preliminary in nature and did not include correction for multiple comparisons, and so replication is important. Longitudinal studies which follow youth before the onset of use will be important for inferring causality in the future (e.g., the Adolescent Brain Cognitive Development Study, abcdstudy.org). Here we focused on a neuropsychological test of one of the most commonly cited cognitive correlates of cannabis use, verbal learning and memory; however, the assessment of use of biometrics across additional cognitive domains is warranted.
In summary, results suggest that young adult cannabis users demonstrate poorer verbal learning and memory versus non-users, and this relationship is well captured through use of urinary creatinine-normalized THCCOOH concentrations and through self-reported frequent cannabis use. Urinary cotinine concentrations similarly related to short delay memory performance. Use of biometrics to test for cannabis exposure may be a means of reliably assessing cannabis-related cognitive decrements, given the vast array of cannabis products and individual use characteristics that influence the pharmacokinetic profile (Huestis, 2007).
Acknowledgements:
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism grant T32 AA13525 (PI: Riley/Tapert to Wade) and National Institute on Drug Abuse grants U01 DA041089 and R21 DA047953, and the California Tobacco-Related Disease Research Grants Program Office of the University of California Grant 580264 (PI: Jacobus)
Footnotes
The Authors declare that there is no conflict of interest.
References
- Akinci IH, Tarter RE, & Kirisci L (2001). Concordance between verbal report and urine screen of recent marijuana use in adolescents. Addictive Behaviors, 26, 613–619. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, … McGuire PK (2010). Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology, 35(3), 764–774. doi: 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blest-Hopley G, Giampietro V, & Bhattacharyya S (2020). A Systematic Review of Human Neuroimaging Evidence of Memory-Related Functional Alterations Associated with Cannabis Use Complemented with Preclinical and Human Evidence of Memory Performance Alterations. Brain Sci, 10(2). doi: 10.3390/brainsci10020102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet U, Borda T, Scherbaum N, & Specka M (2015). Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification (Part II): Psychiatric complaints and their relation to delta-9-tetrahydrocannabinol and its metabolites in serum. Drug Alcohol Depend, 155, 302–306. doi: 10.1016/j.drugalcdep.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Brown, Myers MG, Lippke K, Tapert SF, Stewart DG, & Vik PW (1998). Psychometric evaluation of the customary drinking and durg use record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol, 59(4), 427–438. [DOI] [PubMed] [Google Scholar]
- Campos MW, Serebrisky D, & Castaldelli-Maia JM (2016). Smoking and Cognition. Curr Drug Abuse Rev, 9(2), 76–79. doi: 10.2174/1874473709666160803101633 [DOI] [PubMed] [Google Scholar]
- Dahlgren MK, Sagar KA, Smith RT, Lambros AM, Kuppe MK, & Gruber SA (2020). Recreational cannabis use impairs driving performance in the absence of acute intoxication. Drug Alcohol Depend, 208, 107771. doi: 10.1016/j.drugalcdep.2019.107771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers NA, & Huestis MA (2019). Oral Fluid Drug Testing: Analytical Approaches, Issues and Interpretation of Results. J Anal Toxicol, 43(6), 415–443. doi: 10.1093/jat/bkz048 [DOI] [PubMed] [Google Scholar]
- Desrosiers NA, Lee D, Concheiro-Guisan M, Scheidweiler KB, Gorelick DA, & Huestis MA (2014). Urinary cannabinoid disposition in occasional and frequent smokers: is THC-glucuronide in sequential urine samples a marker of recent use in frequent smokers? Clin Chem, 60(2), 361–372. doi: 10.1373/clinchem.2013.214106 [DOI] [PubMed] [Google Scholar]
- Desrosiers NA, Milman G, Mendu DR, Lee D, Barnes AJ, Gorelick DA, & Huestis MA (2014). Cannabinoids in oral fluid by on-site immunoassay and by GC-MS using two different oral fluid collection devices. Anal Bioanal Chem, 406(17), 4117–4128. doi: 10.1007/s00216-014-7813-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabritius M, Chtioui H, Battistella G, Annoni JM, Dao K, Favrat B, … Giroud C (2013). Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal Bioannal Chem, 405, 9791–9803. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, & Rapoport JL (2015). Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology, 40(1), 43–49. doi: 10.1038/npp.2014.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. PNAS, 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RS, Darwin WD, Chiang CN, Shih M, Li S-H, & Huestis MA (2008). Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol, 32(8), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NZ, Wiley JL, Yu Z, Clowers BH, & Craft RM (2018). Cannabidiol modulation of antinociceptive tolerance to Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl), 235(11), 3289–3302. doi: 10.1007/s00213-018-5036-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, & Brown SA (2011). Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst Abuse, 20(2), 135–154. doi: 10.1080/1067828X.2011.555272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, & Tapert SF (2010). Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav, 35(11), 970–976. doi: 10.1016/j.addbeh.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Griffin BA, McCaffrey DF, & Morral AR (2008). Inconsistencies in self-reported drug use by adolescents in substance abuse treatment: implications for outcome and performance measurements. J Subst Abuse Treat, 34(3), 347–355. doi: 10.1016/j.jsat.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Xia JX, Shaban NDC, & Curran HV (2017). Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: a placebo-controlled trial. Psychol Med, 47(15), 2708–2719. doi: 10.1017/S0033291717001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, … Innis RB (2012). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry, 17(6), 642–649. doi: 10.1038/mp.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryhorowicz S, Walczak M, Zakerska-Banaszak O, Slomski R, & Skrzypczak-Zielinska M (2018). Pharmacogenetics of Cannabinoids. Eur J Drug Metab Pharmacokinet, 43(1), 1–12. doi: 10.1007/s13318-017-0416-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA (2007). Human cannabinoid pharmacokinetics. Chem Biodivers, 4(8), 1770–1804. doi: 10.1002/cbdv.200790152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Blount BC, Milan DF, Newmeyer MN, Schroeder J, & Smith ML (2019). Correlation of creatinine- and specific gravity-normalized free and glucuronidated urine cannabinoid concentrations following smoked, vaporized, and oral cannabis in frequent and occasional cannabis users. Drug Test Anal, 11(7), 968–975. doi: 10.1002/dta.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, & Cone EJ (1998). Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol, 22, 445–454. [DOI] [PubMed] [Google Scholar]
- Huestis MA, & Smith ML (2018). Cannabinoid Markers in Biological Fluids and Tissues: Revealing Intake. Trends Mol Med, 24(2), 156–172. doi: 10.1016/j.molmed.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Manzoni OJ, Pletnikov MV, Lee FS, Bhattacharyya S, & Melis M (2019). Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J Neurosci, 39(42), 8250–8258. doi: 10.1523/JNEUROSCI.1165-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, & Pugh KR (2005). Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry, 57(1), 56–66. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, & Tapert SF (2015). Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology, 29(6), 829–843. doi: 10.1037/neu0000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Taylor CT, Gray KM, Meredith LR, Porter AM, Li I, … Squeglia LM (2018). A multi-site proof-of-concept investigation of computerized approach-avoidance training in adolescent cannabis users. Drug Alcohol Depend, 187, 195–204. doi: 10.1016/j.drugalcdep.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2020). Monitoring the Future national survey results on drug use, 1975–2019: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research. [Google Scholar]
- Kangiser MM, Lochner AM, Thomas AM, & Lisdahl KM (2019). Gender Moderates Chronic Nicotine Cigarette Effects on Verbal Memory in Young Adults. Subst Use Misuse, 54(11), 1812–1824. doi: 10.1080/10826084.2019.1613432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Schacht JP, Jacobus J, Meredith LR, Taylor CT, Tapert SF, … Squeglia LM (2019). Preliminary evidence that computerized approach avoidance training is not associated with changes in fMRI cannabis cue reactivity in non-treatment-seeking adolescent cannabis users. Drug Alcohol Depend, 200, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Schacht JP, Meredith LR, Jacobus J, Tapert SF, Gray KM, & Squeglia LM (2019). Investigating a novel fMRI cannabis cue reactivity task in youth. Addict Behav, 89, 20–28. doi: 10.1016/j.addbeh.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, & Huestis MA (2009). Implications of Plasma Δ9 -Tetrahydrocannabinol, 11-HydroxyTHC, and 11-nor-9-Carboxy-THC Concentrations in Chronic Cannabis Smokers. J Anal Toxicol, 33(8), 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulig K (2017). Interpretation of Workplace Tests for Cannabinoids. J Med Toxicol, 13(1), 106–110. doi: 10.1007/s13181-016-0587-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laboratory RT (2020). Laboratory testing cutoffs & methods.
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, & Shollenbarger S (2014). Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Curr Addict Rep, 1(2), 144–156. doi: 10.1007/s40429-014-0019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, & Huestis MA (2009). Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend, 105(1–2), 24–32. doi: 10.1016/j.drugalcdep.2009.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, … Curran HV (2012). Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med, 42(2), 391–400. doi: 10.1017/S0033291711001322 [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, & Curran HV (2010). Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry, 197(4), 285–290. doi: 10.1192/bjp.bp.110.077503 [DOI] [PubMed] [Google Scholar]
- Murphy SM, & Rosenman R (2019). The “Real” Number of Washington State Adolescents Using Marijuana, and Why: A Misclassification Analysis. Subst Use Misuse, 54(1), 89–96. doi: 10.1080/10826084.2018.1496454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musshoff F, & Madea B (2006). Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit, 28(2), 155–163. [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, & Tapert SF (2015). Effects of emerging alcohol and marijuana use behaviors on adolescents’ neuropsychological functioning over four years. J. Stud. Alcohol Drugs, 76, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, McNally S, Petker T, Amlung MT, Balodis IM, Sweet LH, & MacKillop J (2019). Urinary tetrahydrocannabinol is associated with poorer working memory performance and alterations in associated brain activity. Neuropsychopharmacology, 44(3), 613–619. doi: 10.1038/s41386-018-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, & Yurgelun-Todd D (2001). Neuropsychological Performance in Long-term Cannabis Users. Arch Gen Psychiatry, 58, 909–915. [DOI] [PubMed] [Google Scholar]
- Potter DJ, Hammond K, Tuffnell S, Walker C, & Di Forti M (2018). Potency of Delta(9) - tetrahydrocannabinol and other cannabinoids in cannabis in England in 2016: Implications for public health and pharmacology. Drug Test Anal, 10(4), 628–635. doi: 10.1002/dta.2368 [DOI] [PubMed] [Google Scholar]
- Ramo DE, Liu H, & Prochaska JJ (2012). Tobacco and marijuana use among adolescents and young adults: a systematic review of their co-use. Clin Psychol Rev, 32(2), 105–121. doi: 10.1016/j.cpr.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienz NJ, Cone EJ, Herrmann ES, Lembeck NA, Mitchell JM, Bigelow GE, … Vandrey R (2018). Pharmacokinetic Characterization of 11-nor-9-carboxy-Delta9-tetrahydrocannabinol in Urine Following Acute Oral Cannabis Ingestion in Healthy Adults. J Anal Toxicol, 42(4), 232–247. doi: 10.1093/jat/bkx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M (1996). Rey Auditory Verbal Learning Test: A handbook. Los Angeles, CA: Western Psychological Service. [Google Scholar]
- Schneider M (2008). Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol, 13(2), 253–263. doi: 10.1111/j.1369-1600.2008.00110.x [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, & Patrick ME (2019). Monitoring the Future national survey results on drug use, 1975–2018: Volume II, College students and adults ages 19–60. Retrieved from Ann Arbor, MI: [Google Scholar]
- Schuster RM, Crane NA, Mermelstein R, & Gonzalez R (2015). Tobacco may mask poorer episodic memory among young adult cannabis users. Neuropsychology, 29(5), 759–766. doi: 10.1037/neu0000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster RM, Gilman J, Schoenfeld D, Evenden J, Hareli M, Ulysse C, … Evins AE (2018). One Month of Cannabis Abstinence in Adolescents and Young Adults Is Associated With Improved Memory. J Clin Psychiatry, 79(6). doi: 10.4088/JCP.17m11977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster RM, Potter K, Vandrey R, Hareli M, Gilman J, Schoenfeld D, & Evins AE (2020). Urinary 11-nor-9-carboxy-tetrahydrocannabinol elimination in adolescent and young adult cannabis users during one month of sustained and biochemically-verified abstinence. J Psychopharmacol, 34(2), 197–210. doi: 10.1177/0269881119872206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, & Gur RC (2018). Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry, 75(6), 585–595. doi: 10.1001/jamapsychiatry.2018.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Murthy P, & Bharath MMS (2012). Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran J Psychiatry, 7(4), 149–156. [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Alden EC, Herrold AD, Roberts A, Stern D, Jones J, … Breiter HC (2018). Recent self-reported cannabis use is associated iwth the biometrics of delta-9-tetrahydrocannabinol. J. Stud. Alcohol Drugs, 79, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, … Yucel M (2011). Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl), 216(1), 131–144. doi: 10.1007/s00213-011-2203-x [DOI] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci, 19(4), 197–214. doi: 10.1038/nrn.2018.10 [DOI] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, … Vandrey R (2018). Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw Open, 1(7), e184841. doi: 10.1001/jamanetworkopen.2018.4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, … Vandrey R (2019). Acute Pharmacokinetic Profile of Smoked and Vaporized Cannabis in Human Blood and Oral Fluid. J Anal Toxicol, 43(4), 233–258. doi: 10.1093/jat/bky104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SM, & Cimino NM (2014). Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev, 46(1), 86–95. doi: 10.3109/03602532.2013.849268 [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, & Spreen O (2006). A Compendium of Neuropsychological Tests (3rd ed. ed.). New York, New York: Oxford University Press. [Google Scholar]
- Tomko RL, Baker NL, McClure EA, Sonne SC, McRae-Clark AL, Sherman BJ, & Gray KM (2018). Incremental validity of estimated cannabis grams as a predictor of problems and cannabinoid biomarkers: Evidence from a clinical trial. Drug Alcohol Depend, 182, 1–7. doi: 10.1016/j.drugalcdep.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajravelu HR, Gnanadurai TK, Krishnan P, & Ayyavoo S (2015). Impact of Quantified Smoking Status on Cognition in Young Adults. J Clin Diagn Res, 9(12), CC01–03. doi: 10.7860/JCDR/2015/16444.6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AL, Wade NE, & Lisdahl KM (In Press). Impact of Two-Weeks of Monitored Abstinence on Cognition in Adolescent and Young Adult Cannabis Users. Journal of the International Neuropsychological Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille SM, Samyn N, Ramírez-Fernández Mdel M, & G. DB (2010). Evaluation of on-site oral fluid screening using Drugwipe-5(+), RapidSTAT and Drug Test 5000 for the detection of drugs of abuse in drivers. Forensic Sci Int, 198, 2–6. [DOI] [PubMed] [Google Scholar]
- Williams RJ, & Nowatzki N (2009). Validity of Adolescent Self-Report of Substance Use. Substance Use & Misuse, 40(3), 299–311. doi: 10.1081/ja-200049327 [DOI] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, & Leslie FM (2015). Nicotine and the adolescent brain. J Physiol, 593(16), 3397–3412. doi: 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]


