Maternal sepsis is a leading cause of preventable maternal mortality that requires early recognition, expedient evaluation, and appropriate management.
Abstract
Maternal sepsis is an obstetric emergency and a leading cause of maternal morbidity and mortality. Early recognition in a pregnant or postpartum patient can be a challenge as the normal physiologic changes of pregnancy may mask the signs and symptoms of sepsis. Bedside assessment tools may aid in the detection of maternal sepsis. Timely and targeted antibiotic therapy and fluid resuscitation are critical for survival in patients with suspected sepsis. Once diagnosed, a search for etiologies and early application of source control measures will further reduce harms. If the patient is in septic shock or not responding to initial treatment, multidisciplinary consultation and escalation of care is necessary. Health care professionals should be aware of the unique complications of sepsis in critically ill pregnant and postpartum patients, and measures to prevent poor outcomes in this population. Adverse pregnancy outcomes may occur in association with sepsis, and should be anticipated and prevented when possible, or managed appropriately when they occur. Using a standardized approach to the patient with suspected sepsis may reduce maternal morbidity and mortality.
Sepsis is the leading cause of mortality and critical illness worldwide, with a mortality rate of 28.6% in the nonobstetric population.1 Maternal sepsis, defined as sepsis with onset during pregnancy or postpartum, is responsible for 10.7% of global maternal deaths.2 In the United States, maternal sepsis is the fourth leading cause of maternal mortality, occurring in 0.04% of deliveries, but accounting for 23% of all deaths.3 Contemporary data estimate that 63% of maternal deaths from sepsis may be preventable, and that for each maternal death, there are 50 women who experience life-threatening morbidity from sepsis.4 Therefore, the early recognition, expedient evaluation, and appropriate management of maternal sepsis are necessary to reduce severe morbidity and mortality.
The diagnosis of maternal sepsis remains a challenge as the normal physiologic adaptations of pregnancy can mask the recognition of common signs and symptoms. Moreover, robust data on recognition and treatment of maternal sepsis are lacking, and clinicians must largely rely on extrapolation of sepsis-based guidelines for nonpregnant adults. Nonetheless, clinicians must be aware of best practices to recognize and treat sepsis as decreasing time to intervention improves outcomes. Common etiologies for maternal sepsis differ from that of the general population resulting in differences in initial and subsequent antimicrobial selection. Additionally, optimal management requires clinicians to consider the unique needs of both the patient and fetus. The purpose of this expert review is to provide simple and easy-to-remember pearls for the early recognition, evaluation and management of maternal sepsis (Box 1).
Box 1.
Top 10 Pearls for Managing Maternal Sepsis
Recognition is key
Pearl 1. Always maintain a high index of suspicion for sepsis.
Pearl 2. Implement a rapid bedside tool for detection of maternal deterioration.
Move fast during the golden hour to save lives
Pearl 3. Implement sepsis bundles to facilitate rapid escalation of care.
Pearl 4. Laboratory and radiologic studies are keys to search for etiology and source control.
Pearl 5. Know your “bugs,” their likely origin, and that group A streptococcus can kill quickly.
Pearl 6. Choose antimicrobials tailored to the most likely diagnosis.
Pearl 7. Fluid resuscitation should be initiated rapidly for patients with a blood lactate greater than 4 mmol/L or mean arterial pressure less than 65 mm Hg.
Beyond the golden hour
Pearl 8. Escalation of care is critical to survival.
Pearl 9. Once the patient is stabilized, get to the source of the problem.
Pearl 10. Anticipate and prevent adverse pregnancy outcomes.
Pearl 1. Recognition is key: always maintain a high index of suspicion for sepsis.
Maternal sepsis can present with multiple and varied symptoms such as lethargy, chills and rigors, generalized malaise, rashes, lower abdominal or pelvic pain, foul lochia, contractions, malodorous or discolored leaking of fluid from the vagina, and breast engorgement. Signs of maternal sepsis include fever or hypothermia, tachycardia, hypotension, uterine tenderness, preterm labor or preterm prelabor rupture of membranes, altered mental status, and end-organ dysfunction.
Although there are no standardized criteria to diagnose maternal sepsis, vital signs changes are an early indicator of infection. However, these early vital sign changes may be dismissed as normal physiologic changes of pregnancy such as an increase in heart rate, and decrease in blood pressure.5,6 Additionally, external influences (eg, blood loss during delivery, common infections, fluid administration, medications, and effects of anesthesia) may further confuse the clinical picture.5,6 Often there is no obvious source of infection in maternal sepsis, which makes recognition more challenging and may result in delays in treatment and source control.5–7
Because the symptoms of maternal sepsis are often nonspecific, health care professionals need to maintain a high index of suspicion.8 The Society for Maternal-Fetal Medicine recommends that health care professionals consider the diagnosis of sepsis in pregnant patients with otherwise unexplained end-organ damage in the presence of an infectious process, regardless of the presence of a fever.9 Although every pregnant patient is at risk for sepsis, there are specific patient characteristics that are associated with increased risk for sepsis (Box 2).10–12 Close surveillance of pregnant or postpartum patients with these conditions may aid in the early detection of sepsis.11
Box 2.
Risk Factors Associated With Maternal Sepsis
Patient factors
Obesity
Impaired immunity or immunosuppressant therapy
Anemia
Impaired glucose tolerance
Vaginal discharge
History of pelvic infection
History of group B streptococcal infection
Group A streptococcal infection in close contacts
Age older than 35 y
Disadvantaged socioeconomic background
Congestive heart failure
Chronic renal failure
Chronic liver failure
Systemic lupus erythematous
Obstetric factors
Cesarean delivery
Retained products of conception
Prolonged rupture of membranes
Multiple gestation
Cervical cerclage
Amniocentesis or other invasive procedure
Complex perineal lacerations
Wound hematoma
Adapted by permission from BMJ Publishing Group Limited. Buddeberg BS, Aveling W. Puerperal sepsis in the 21st century: progress, new challenges and the situation worldwide. Postgraduate Medical Journal 2015; 91:572–578. Copyright 2015.
Pearl 2. Recognition is key: implement a rapid bedside tool for detection of maternal deterioration.
Bedside assessment tools, such as qSOFA (quick Sepsis-related Organ Failure Assessment), are available to predict mortality in patients with suspected sepsis, and are now frequently used in nonobstetric patients to identify those who are at greater risk for a poor outcome.13–15 However, these bedside tools, including the qSOFA, have not been validated for use in obstetric patients.
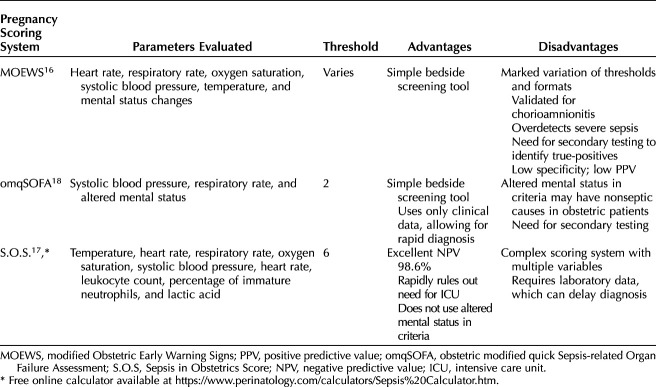
Three pregnancy-specific scoring systems that allow early recognition of maternal deterioration include MOEWS (Modified Obstetric Early Warning System), S.O.S. (Sepsis in Obstetrics Score), and omqSOFA (obstetric modified quick SOFA) (Table 1).16–18 The MOEWS and omqSOFA scoring systems stratify level of risk based on changes in vital signs and mental status, whereas the S.O.S. uses vital sign changes and laboratory values. The MOEWS is a simple bedside tool but it is only validated for the detection of chorioamnionitis and has wide variation in alert thresholds, format, and accuracy.16 The omqSOFA criteria, widely used in Australia, uses clinical data (blood pressure, respiratory rate, and mental status) allowing for rapid diagnosis of sepsis; however, concerns have been raised that altered mental status is not a common presenting symptom for maternal sepsis, potentially making it less useful in the evaluation of suspected sepsis in the obstetric population.18 The S.O.S. scoring system is a validated pregnancy-specific score to predict intensive care unit admission. The S.O.S. modifies parameters from the Rapid Emergency Medicine Score, as well as the sepsis criteria as defined by the Surviving Sepsis Campaign, in accordance with well-known physiologic changes in pregnancy. The internal validation trial of the S.O.S. demonstrated a low positive predictive value of 15%; however, it's excellent negative predictive value of 98.6% effectively rules out the need for intensive care unit admission.19
Table 1.
Characteristics of Common Maternal Early Warning Systems for Sepsis
Although the best tool for identifying infection or predicting mortality in pregnant or postpartum patients remains unknown,20 the authors recommend a step-wise approach, using a simple bedside screening tool such as the MEOWS or omqSOFA, followed by further evaluation for evidence of end-organ damage. A sample flowchart for the diagnosis and treatment of maternal sepsis using a two-step approach was developed by the California Maternal Quality Care Collaborative. The first step involves screening of vital signs parameters (eg, temperature, heart rate and respiratory rate) and the most recent white blood cell count within 24 hours. If any two of these four parameters are positive, source-directed antibiotics and intravenous fluids are administered, and further evaluation is recommended. The second diagnostic step involves an evaluation for end-organ injury screening for sepsis, using the Centers for Medicare & Medicaid Services criteria for end-organ injury modified to account for normal maternal physiologic changes.21 More research to examine the validity of scoring systems for the identification, evaluation and monitoring of pregnant and postpartum patients with sepsis is needed.20
Pearl 3. Move fast during the “golden hour” to save lives: implement sepsis bundles to facilitate rapid escalation of care.
The concept of the golden hour of sepsis highlights the importance of timely initiation of antibiotic treatment to improve outcomes. In the nonobstetric population, each hour delay in antibiotic treatment reduces sepsis survival by 7.6%.22 Conversely, initiation of effective antimicrobial therapy within the first hour of diagnosis was associated with 79.9% survival to hospital discharge.22 Early studies validating the golden hour principle excluded pregnant patients; however, poor outcomes and an increased risk of maternal death have been observed with delays in recognition of sepsis, timely administration of antibiotics and escalation of care in the obstetric population as well.7,23,24 Therefore, the best evidence suggests that the golden hour principle is applicable to obstetric patients, and that maternal sepsis should be classified as an obstetric emergency.
If there is concern for maternal deterioration after the initial evaluation, the nurse or physician must immediately call for help from a rapid response team, and stabilize the patient. More research is needed to evaluate the efficacy of sepsis bundles and clinical care pathways for the rapid diagnosis and treatment of maternal sepsis4; however, in the absence of available trials, the authors recommend implementation of sepsis bundles simply to improve standardization of care.25 Rapid response teams specifically trained in the early recognition, diagnosis, and treatment of patients with suspected or diagnosed sepsis have been found to decrease in-hospital mortality by 2–3%, and decrease length of stay.26,81 An efficient and coordinated response by the “sepsis rapid response team” aids in facilitating the correct treatment measures and resource utilization. The authors recommend that institutions implement a standardized pathway to alert the health care team of maternal deterioration, and that these trained professionals arrive at the bedside within minutes of being called to stabilize the patient and administer antibiotics.
When arriving at the bedside, a primary survey is performed. If the patient shows signs or symptoms of hemodynamic instability or shock (eg, mean arterial pressure less than 65 mm Hg, respiratory rate 25 or greater or shortness of breath, abnormal heart rate, mental status changes, peripheral cyanosis, cold extremities, mottling, oliguria, or chest pain), a rapid response should be called. When the patient is stabilized, a more detailed history and physical examination is performed. History should focus on presenting symptoms or those proximate to maternal deterioration, current or prior infections diagnosed, interventions or procedures performed, current medications including recent antimicrobial exposure and medication allergies. Physical examination should focus on current vital signs (eg, fever, tachycardia, or hypotension); general appearance (eg, lethargy); cardiovascular examination (eg, delayed capillary refill, murmurs); respiratory examination (eg, use of accessory muscles, rales or rhonchi), neurologic examination (eg, mental status changes), skin examination (eg, cool skin, cyanosis, discoloration, pallor or rash), and reproductive system evaluation (eg, breast engorgement, leaking of fluid, preterm contractions, or fetal tachycardia). This initial survey will then guide further management, help determine whether the patient needs to be moved to a higher level of care, and guide immediate therapies.
Pearl 4. Move fast during the golden hour to save lives: laboratory and radiology studies are keys to identifying the etiology and gaining source control.
Initial laboratory assessment of patients with suspected sepsis should prioritize collection of a complete blood count with differential, serum lactate, and cultures from various sources (Table 2). Established institutional protocols may assist with rapid collection of laboratory values before administration of antibiotics.4 However, antibiotic initiation should never be delayed more than 1 hour if cultures cannot be collected in a timely fashion, and collection of cultures is still recommended even after antibiotics have been initiated.27 Additional laboratory values proposed in the evaluation and management of maternal sepsis include a comprehensive metabolic panel that includes hepatic and renal function, coagulation panel with international normalized ratio, arterial blood gas, and peripheral blood smear (Table 2). Rapid molecular testing for viral pathogens is recommended as part of the initial laboratory assessment for pregnant or postpartum patients with suspected sepsis who present with respiratory complaints, flu-like symptoms, rash, or hepatitis. Once the patient is stabilized, radiologic assessment may be performed. Imaging studies should be guided by the bedside clinical assessment. A chest radiograph should be obtained, unless a source of infection is already known (eg, urosepsis with normal respiratory status). A computed tomography scan of the chest, abdomen, and pelvis can be considered to further evaluate for sources of infection if the source remains unknown. In some circumstances, ultrasonography may also be used; for example, in the setting of pyelonephritis, ultrasonography may identify a renal or perirenal abscess.
Table 2.
Common Laboratory Studies for Initial Evaluation of Maternal Sepsis
Pearl 5. Move fast during the golden hour to save lives: know your bugs, their origin, and that group A streptococcus kills quickly!
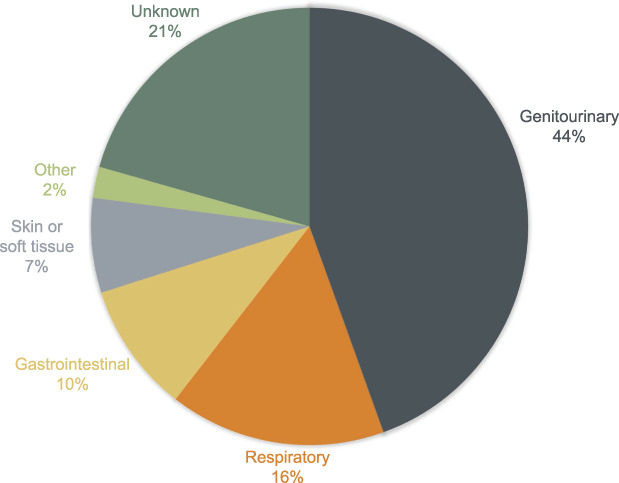
Causes of maternal sepsis differ from those of the nonobstetric population. Based on the National Readmissions Database from 2019, the most frequent sources of infection associated with episodes of sepsis during and after hospitalization for delivery were genitourinary and respiratory (Fig. 1).3 In comparison, the most common sources of infection for nonobstetric patients admitted to the intensive care unit with sepsis were respiratory, abdominal, and bloodstream infections.82
Fig. 1. Most frequent sources of maternal infection associated with episodes of sepsis during and after hospitalization for delivery. Data from Hensley MK, Bauer ME, Admon LK, Prescott HC. Incidence of maternal sepsis and sepsis-related maternal deaths in the United States. JAMA 2019; 322:890‒92. doi: 10.1001/jama.2019.9818.
Shields. Pearls for Managing Maternal Sepsis. Obstet Gynecol 2021.
Causes of maternal sepsis vary based on timing of infection (eg, antenatal, intrapartum or postpartum). Genitourinary infections are the most common source of infection throughout pregnancy and postpartum, and are most commonly diagnosed antenatally.3,11 Pyelonephritis is one of the leading causes of nonobstetric antepartum hospitalization.28,29 Sepsis associated with chorioamnionitis is most likely to present intrapartum. Respiratory infections are equally distributed during pregnancy and postpartum.3 Sepsis from endomyometritis, mastitis, gastrointestinal, and soft tissue sources are more commonly encountered postpartum.3,30
Knowledge of the potential pathogens associated with each sepsis etiology is critical to optimal management and antibiotic stewardship. The major pathogens causing sepsis in the puerperium are Escherichia coli, group B streptococcus, Staphylococcus aureus, anaerobic bacteria, and Listeria monocytogenes.28 Similar to the general population, the most common pathogen identified in positive blood cultures from pregnant and postpartum patients is E coli, which occurs in up to one half of cases.28,31,32 E coli is also the predominant isolate in cases of urosepsis and chorioamnionitis or endometritis.33
The deadliest pathogen in infectious sepsis is invasive group A streptococcus (also known as Streptococcus pyogenes).7 Group A streptococcus is not part of the normal microbiome of the urogenital tract. It is present in only 0.03% of individuals, so routine screening is not recommended.34 Group A streptococcus causes a diverse range of infections including endomyometritis, necrotizing fasciitis, pneumonia, cellulitis, and pharyngitis.35 Patients with group A streptococcus have rapid clinical deterioration; in 75% there are less than 9 hours between the first signs of infection and septic shock, and in 50% of patients this progression occurs in less than 2 hours.36 Due to this rapid clinical deterioration, about 20% of women will die within 7 days of diagnosis.37 Although the incidence of invasive group A streptococcus has been increasing globally over the past 30 years, the incidence has remained stable in the United States at 3.48 per 100,000 persons.35,38
Both viral and fungal pathogens can cause maternal sepsis. Pregnant women are at greater risk of viral sepsis than the general population.39 The most common viral pathogens associated with maternal sepsis are influenza, varicella zoster, and herpes simplex virus.30,39,40 Patients with sepsis from viral infections typically present with pneumonia, but may also present with hepatitis, encephalitis, coagulopathy, acute respiratory distress syndrome, or septic shock.41–43
Several viral infections confer a high risk of mortality in pregnancy. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), may result in end-organ dysfunction and mortality rate on the order of 1.3% during pregnancy.44 A rare but important viral pathogen is varicella zoster, which causes chickenpox, and severe infections are associated with mortality rates of 3–14% in pregnancy.35 The risk for varicella pneumonia increases with advancing gestational age.45,46 Disseminated herpes simplex infection, although uncommon during pregnancy, may occur with a primary mucous membrane infection during the third trimester, and carries a maternal mortality rate of approximately 50%.42
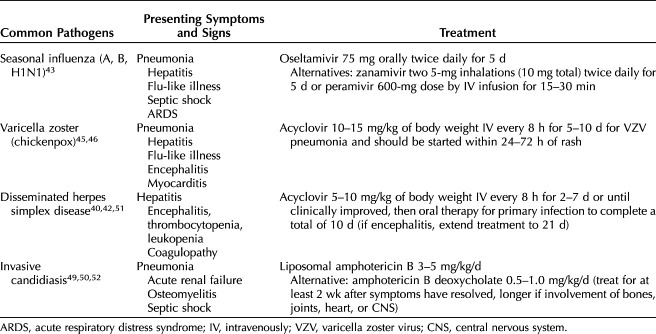
Fungal sepsis accounts for 5% of all sepsis cases in the general population, and is an increasingly frequent cause of sepsis in critically ill patients.47,48 Candida species account for the majority of cases of fungal sepsis. Fortunately, fungal sepsis is extremely rare in pregnancy and postpartum, but is associated with a very high mortality rate.49 Table 3 reviews the presenting symptoms and signs and recommended treatment for common viral and fungal infections that cause maternal sepsis.42,43,45,49–52
Table 3.
Presenting Signs and Symptoms and Recommended Treatment for Common Viral and Fungal Infections in Maternal Sepsis
Pearl 6. Move fast during the golden hour to save lives: choose antimicrobials tailored to the most likely diagnosis.
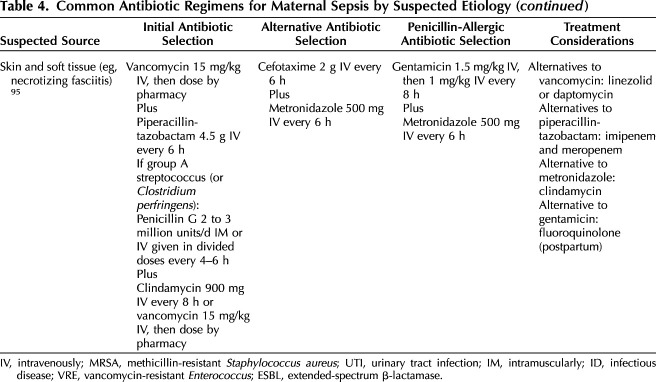
The appropriate initial selection of antibiotics in nonobstetric patients has been shown to decrease mortality.53–55 Therefore, once the diagnosis of sepsis is suspected, broad-spectrum antibiotics tailored to the most likely diagnosis should be initiated. Because most cases of maternal sepsis are due to genitourinary sources, a cost-effective, first-line therapy with intravenous ampicillin, gentamicin, and clindamycin is usually suitable, especially if the source is unknown. However, if a soft tissue infection is present, or if initial evaluation suggests a respiratory cause, first-line antibiotic selection will differ (Table 4). In addition, local resistance patterns as well as emerging strains of resistance and prior antibiotic exposure in the prior 30 days should be considered.56–58 After initiating first-line antibiotics, reviewing the hospital antibiogram of antimicrobial susceptibilities and consulting with an infectious disease specialist may be useful to help tailor antibiotic selection.59 If a viral or fungal etiology is suspected, targeted antimicrobial treatment can be concurrently administered (Table 3).
Table 4.
Common Antibiotic Regimens for Maternal Sepsis by Suspected Etiology
In certain situations, drug monitoring may be considered; this should be performed in patients with 1) septic shock or admitted to the intensive care unit, 2) liver or kidney impairment, or 3) a large volume of distribution.60 The antibiotic regimen should be reassessed daily, with the goal of narrowing the spectrum as soon as possible. Clinical improvement or isolation of a pathogen with susceptibility patterns should prompt de-escalation of antibiotic therapy. This is necessary to prevent the development of antibiotic resistance, and to reduce the risk of superinfection (eg, candida, vancomycin-resistant enterococcus, and clostridium), toxicity, and costs.60
Pearl 7. Move fast during the golden hour to save lives: fluid resuscitation should be initiated rapidly for patients with a blood lactate level greater than 4 mmol/L or mean arterial pressure less than 65 mm Hg.
Adequate tissue perfusion is vital to proper cellular function and fluid resuscitation should be initiated rapidly for patients with a blood lactate greater than 4 mmol/L or the mean arterial pressure less than 65 mm Hg.61,62 Early fluid resuscitation optimizes cardiac preload, afterload, and contractility in pregnant patients.35 Crystalloid fluids (eg, lactated Ringer's solution or normal saline) are the mainstay of therapy. For resuscitation in sepsis, the initial infusion is 30 mL/kg followed by additional fluids as clinically applicable; in a 70 kg patient this would translate to a minimum of 2.1 L of crystalloid fluid as an initial bolus.63 Patients can be assessed quickly for likelihood of fluid responsiveness by undergoing a passive leg raise of approximately 45°. This test results in approximately 300–500 mL autotransfusion.9 Those who are likely to respond to fluids will have an increase in cardiac output with this maneuver, often apparent as an immediate rise in blood pressure or appropriate decrease in heart rate.64 Passive leg raise may be less predictive of fluid responsiveness in the third trimester due to occlusion of the great vessels by the uterus.65
Pearl 8. Beyond the golden hour: escalation of care is critical to survival.
Once sepsis is recognized and initial evaluation and management are underway,66 rapid escalation of care is critical. Early consultation with physicians trained in infectious disease and critical care medicine is advisable to assist with escalation of care and management of the patient. In some cases, prompt transfer of the critically ill pregnant patient to a higher level of care may be necessary as death from septic shock is reported to be as high as 50%.67
Pregnant and postpartum patients with septic shock have significantly higher rates of disseminated intravascular coagulation, altered mental status, total bilirubin greater than 4 mg/dL, failure in three or more organ systems, and maternal death when compared with pregnant and postpartum patients without septic shock.6 Mean arterial pressures (estimated as the sum of the diastolic blood pressure plus one third of the difference from the systolic to the diastolic blood pressure) consistently lower than 65 mm Hg indicate the presence of septic shock. Studies in critically ill patients with sepsis have shown that mean arterial pressure greater than 65 mm Hg is associated with lower morbidity and mortality.68 A mean arterial pressure greater than 65 mm Hg has been put forth as optimal for uterine and fetal perfusion as well.35 Frequent measurement of vital signs, and interventions to maintain a mean arterial pressure of 65 mm Hg or greater, should therefore be a cornerstone of managing maternal sepsis. The goal mean arterial pressure remains the same whether determined by invasive (eg, direct intra-arterial catheter measurement or arterial line) or noninvasive blood pressure devices (eg, a properly sized arm cuff), because evidence-based superiority of one modality over the other has not been demonstrated.69 Although the mean arterial pressure is easily calculable and often automatically reported in electronic medical records, a ready alternative that is associated with tissue hypoperfusion is a systolic blood pressure less than 100 mm Hg; this parameter is also used in the qSOFA scoring system.
If the mean arterial pressure of 65 mm Hg or greater cannot be maintained with adequate fluid resuscitation, vasopressors should be initiated. First-line therapy for refractory hypotension in pregnancy is norepinephrine, though this may not be readily available on all units; less potent though still efficacious alternatives to norepinephrine are phenylephrine and ephedrine, which are often available from our anesthesiology colleagues. Ongoing use of vasopressors requires an appropriate clinical setting with physicians trained in critical care medicine.
Serial assessment of serum lactate can be used as an informative marker of adequate tissue perfusion. Serum lactate should be obtained at least every 6 hours until normalized (less than 2.2 mmol/L). Earlier correction of serum lactate is associated with survival benefit.70 Continuous pulse oximetry should be used along with arterial blood gas assessments to dictate oxygen supplementation and respiratory support.35 If the patient is severely anemic or has acute blood loss, blood transfusion may be used as fluid replacement.
Sepsis and pregnancy are both independent risk factors for thrombus formation.71,72 The incidence of venous thromboembolism in patients with sepsis or septic shock has been reported to be as high as 37.2%.71 Unfractionated heparin and low molecular weight heparin are used extensively in pregnancy and are effective in prevention of thromboembolism; additionally, patients can be reassured that these medications do not cross the placental barrier.73 Prophylactic dosing is appropriate for most patients; full-dose anticoagulation should be reserved for the usual indications, and intermediate dosing may be considered for some patients based on the unique clinical scenario, such as multiple risk factors for venous thromboembolism. Because some of the excess risk in both septic and obstetric patients may be related to immobility, ambulation is also recommended whenever feasible.74 For patients without clinical illness resulting in immobility, the decision to use pharmacologic anticoagulation, compared with ambulation alone, should take into account the respective risks and benefits for each in the specific clinical scenario.
Pearl 9. Beyond the golden hour: once the patient is stabilized, get to the source of the problem!
The concept of “source control” is an important aspect of sepsis therapy whenever feasible, and refers to removing as much of a nidus of infection as possible.75 Source control may be accomplished using surgical or procedural interventions, removal of foreign bodies associated with the infection such as catheters and intravenous access, and optimization of medications that concentrate in the targeted anatomical areas (eg, kidneys, within the blood–brain barrier). Delays in rapid identification and directed therapies for source control are associated with excess mortality.75 As it pertains to maternal care, source control may include targeted antibiotic regimens, surgical debridement, delivery, uterine evacuation or curettage, or even hysterectomy. Utilization of additional collaborative resources, such as expertise from our general surgery or interventional radiology colleagues, should be employed as needed to achieve source control, with subsequent clear documentation of a collaborative plan in the medical record.
If signs and symptoms of ongoing infection persist despite perceived source control, reevaluation of potential etiologies as well as expansion of diagnostic evaluations should be undertaken, including for those that may have some clinical overlap with sepsis, such as diabetic ketoacidosis, pancreatitis, hepatic dysfunction, adrenal insufficiency, and drug or transfusion reactions. In the setting of maternal sepsis without other obvious sources, amniocentesis may be performed to evaluate for intraamniotic infection. Given that culture results may not be available for several days, initial diagnosis of chorioamnionitis. is often made based on the gram stain, cell count and glucose level.33 If sepsis is due to chorioamnionitis, delivery of the pregnancy is a suitable source control measure irrespective of gestational age.
Pearl 10. Beyond the golden hour: anticipate and prevent adverse pregnancy outcomes.
Sepsis as a lone diagnosis is not an indication for delivery unless intraamniotic infection is suspected. However, preterm delivery is common, reported in 29% of cases with bacteremia.76 Additionally, a fetal mortality rate of 10–12% has been reported in cases of maternal sepsis, and fetal deaths appear to be higher in sepsis with a genital tract origin.28,76 Knowles et al28 noted that 78.1% of all fetal and neonatal deaths from maternal bacteremia were due to one of the following organisms: E coli, group B streptococcus, anaerobic bacteria, and Haemophilus influenzae.
Sepsis also results in significant changes in the maternal circulation that may compromise uteroplacental circulation when the mean arterial pressure falls below the premorbid mean arterial pressure.77 A systolic blood pressure of 90 mm Hg or greater and mean arterial pressure of 65 mm Hg or greater will usually maintain uteroplacental perfusion.19,35 A collaborative approach is recommended if the patient is critically ill, and a plan for delivery should be guided by the maternal and fetal status, gestational age, and underlying etiology for sepsis.78 In cases in which preterm delivery is anticipated, neonatology should also be consulted to help determine the most appropriate facility for delivery to optimize care of the neonate. In some cases, delivery will be warranted before maternal transport, and may require transport of the neonate with a neonatal care team.
Antenatal corticosteroids should be considered if the gestational age is less than 34 weeks and may be considered if the gestational age is between 34 0/7 and 36 6/7 weeks in patients who have not received a previous course of antenatal corticosteroids.79 However, delivery should not be delayed for steroid administration if maternal life is at risk.18
In the event of maternal deterioration with a risk of cardiac arrest, preparations for a bedside resuscitative cesarean delivery (eg, scalpel, clamps, sponges, suture, and needle driver) should be made if there is significant aortocaval compression from the pregnant uterus, generally when the fundus is at or above the umbilicus, corresponding to a gestational age of 20 weeks or more.80 We recommend consulting early with the neonatal intensive care unit team in case maternal status deteriorates, requiring emergent preterm delivery.
CONCLUSION
Maternal sepsis is a leading cause of maternal morbidity and mortality. Recognition of maternal sepsis remains a challenge for health care workers as the signs and symptoms of maternal sepsis often overlap with the normal physiologic changes of pregnancy. Implementation of a simple bedside screen with immediate evaluation if the screen is positive may aid in the early diagnosis of maternal sepsis and timely treatment. Once diagnosed, appropriate antibiotics should be initiated within the first hour, and hypoperfusion corrected. Further evaluation for end-organ damage and a search for etiologies of maternal sepsis and application of source control measures may reduce morbidity and mortality. If the patient is in septic shock or not responding to initial treatment, rapid escalation of care with multidisciplinary collaboration is necessary to optimize outcomes. Adverse pregnancy outcomes occur in association with maternal sepsis, and should be anticipated and prevented when possible or managed appropriately when they occur. In summary, early recognition, focused evaluation, and expedient treatment tailored to the most likely etiology of maternal sepsis, including aggressive source control, are necessary steps to reduce maternal morbidity and mortality from sepsis.
CME FOR THE CLINICAL EXPERT SERIES
Learning Objectives for “Top 10 Pearls for the Recognition, Evaluation, and Management of Maternal Sepsis”
After completing this learning experience, the involved learner should be able to:
Describe signs and symptoms associated with early recognition of maternal sepsis;
Conduct an expedient evaluation; and
Institute appropriate management for patients with this life-threatening condition.
Instructions for Obtaining AMA PRA Category 1 Credits™
Continuing Medical Education credit is provided through joint providership with The American College of Obstetricians and Gynecologists.
Obstetrics & Gynecology includes CME-certified content that is designed to meet the educational needs of its readers. This article is certified for 2 AMA PRA Category 1 Credits.™ This activity is available for credit through August 31, 2024.
Accreditation Statement
ACCME Accreditation
The American College of Obstetricians and Gynecologists is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
AMA PRA Category 1 Credit(s)™
The American College of Obstetricians and Gynecologists designates this journal-based CME activity for a maximum of 2 AMA PRA Category 1 Credits.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
College Cognate Credit(s)
The American College of Obstetricians and Gynecologists designates this journal-based CME activity for a maximum of 2 Category 1 College Cognate Credits. The College has a reciprocity agreement with the AMA that allows AMA PRA Category 1 Credits™ to be equivalent to College Cognate Credits.
Disclosure of Faculty and Planning Committee Industry Relationships
In accordance with the College policy, all faculty and planning committee members have signed a conflict of interest statement in which they have disclosed any financial interests or other relationships with industry relative to article topics. Such disclosures allow the participant to evaluate better the objectivity of the information presented in the articles.
How to Earn CME Credit
To earn CME credit, you must read the article in Obstetrics & Gynecology and complete the quiz, answering at least 70 percent of the questions correctly. For more information on this CME educational offering, visit the Lippincott CMEConnection portal at https://cme.lww.com/browse/sources/196 to register and to complete the CME activity online. ACOG Fellows will receive 50% off by using coupon code, ONG50.
Hardware/software requirements are a desktop or laptop computer (Mac or PC) and an Internet browser. This activity is available for credit through August 31, 2024. To receive proper credits for this activity, each participant will need to make sure that the information on their profile for the CME platform (where this activity is located) is updated with 1) their date of birth (month and day only) and 2) their ACOG ID. In addition, participants should select that they are board-certified in obstetrics and gynecology.
The privacy policies for the Obstetrics & Gynecology website and the Lippincott CMEConnection portal are available at http://www.greenjournal. org and https://cme.lww.com/browse/sources/196, respectively.
Contact Information
Questions related to transcripts may be directed to educationcme@acog.org. For other queries, please contact the Obstetrics & Gynecology Editorial Office, 202-314-2317 or obgyn@greenjournal.org. For queries related to the CME test online, please contact ceconnection@wolterskluwer.com or 1-800-787-8985.
Footnotes
Financial Disclosure Dr. Shields is the Principal Investigator of an AHRQ grant for developing a simulation course on maternal cardiac arrest; provided volunteer expert testimony to KJS law; was an examiner for the ABOG specialty certifying exam; is a member of Varda5, LLC, a consulting company for patient safety and quality initiatives; is a member of Body Wisdom S.A., LLC, a wellness company; and is a member of Overlevende, LLC, for personal assets. The other authors did not report any potential conflicts of interest.
The authors thank Lara Ouellette, MLS, from Baylor College of Medicine for assisting our team with a literature review on maternal sepsis.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C363.
Contributor Information
Viviana de Assis, Email: vivianadeassis@live.com.
Torre Halscott, Email: torre.halscott@outlook.com.
Figure.
No available caption
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, Tuncalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 3.Hensley MK, Bauer ME, Admon LK, Prescott HC. Incidence of maternal sepsis and sepsis-related maternal deaths in the United States. JAMA 2019;322:890–92. doi: 10.1001/jama.2019.9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The California Pregnancy-Associated Mortality Review. Report from 2002 and 2003 maternal death reviews. California Department of Public Health, Maternal Child and Adolescent Health Division; 2011. [Google Scholar]
- 5.Sriskandan S. Severe peripartum sepsis. J R Coll Physicians Edinb 2011;41:339–46. doi: 10.4997/JRCPE.2011.411 [DOI] [PubMed] [Google Scholar]
- 6.Snyder CC, Barton JR, Habli M, Sibai BM. Severe sepsis and septic shock in pregnancy: indications for delivery and maternal and perinatal outcomes. J Mat Fetal Neonat Med 2013;26:503–6. doi: 10.3109/14767058.2012.739221 [DOI] [PubMed] [Google Scholar]
- 7.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom [published erratum appears in BJOG 2015;122:e1]. BJOG 2011;118(suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x [DOI] [PubMed] [Google Scholar]
- 8.Anbazhagan H. Postpartum pyrexia. Obstet Gynaecol Reprod Med 2015;25:249–54. doi: 10.1016/j.ogrm.2015.06.004 [DOI] [Google Scholar]
- 9.Plante L, Pacheco L, Louis J. SMFM Consult Series #47: sepsis during pregnancy and the puerperium [published erratum appears in Am J Obstet Gynecol 2021;224:224]. Am J Obstet Gynecol 2019;220:B2–10. doi: 10.1016/j.ajog.2019.01.216 [DOI] [PubMed] [Google Scholar]
- 10.Oud L. Pregnancy-associated severe sepsis. Curr Opin Obstet Gynecol 2016;28:73–8. doi: 10.1097/GCO.0000000000000250 [DOI] [PubMed] [Google Scholar]
- 11.Bauer E, Bateman BT, Bauer ST, Shanks AM, Mhyre JM. Maternal sepsis mortality and morbidity during hospitalization for delivery: temporal trends and independent associations for severe sepsis. Anesth Analg 2013;117:944–50. doi: 10.1213/ANE.0b013e3182a009c3 [DOI] [PubMed] [Google Scholar]
- 12.Buddeberg BS, Aveling W. Puerperal sepsis in the 21st century: progress, new challenges and the situation worldwide. Postgrad Med J 2015;91:572–8. doi: 10.1136/postgradmedj-2015-133475 [DOI] [PubMed] [Google Scholar]
- 13.Shankar-Hari M, Phillips G, Levy M, Seymour C, Liu V, Deutschman C, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:775–87. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brink A, Alsma J, Verdonschot RJCG, et al. Predicting mortality in patients with suspected sepsis at the emergency department; a retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS One 2019;14:e0211133. doi: 10.1371/journal.pone.0211133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707–10. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 16.Edwards SE, Grobman WA, Lappen JR, Winter C, Fox R, Lenguerrand E, et al. Modified obstetric early warning scoring systems (MOEWS): validating the diagnostic performance for severe sepsis in women with chorioamnionitis. Am J Obstet Gynecol 2015;212:536.e1–8. doi: 10.1016/j.ajog.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. The Sepsis in Obstetrics Score: a model to identify risk of morbidity from sepsis in pregnancy. Am J Obstet Gynecol 2014;211:39.e1–8. doi: 10.1016/j.ajog.2014.03.01 [DOI] [PubMed] [Google Scholar]
- 18.Bowyer L, Robinson H, Barrett H, Crozier TM, Giles M, Idel I, et al. SOMANZ guidelines for the investigation and management sepsis in pregnancy. Aust N Z J Obstet Gynaecol 2017;57:540–51. doi: 10.1111/ajo.12646 [DOI] [PubMed] [Google Scholar]
- 19.Albright CM, Has P, Rouse DJ, Hughes BL. Internal validation of the sepsis in obstetrics score to identify risk of morbidity from sepsis in pregnancy. Obstet Gynecol 2017;130:747–55. doi: 10.1097/AOG.0000000000002260 [DOI] [PubMed] [Google Scholar]
- 20.Friedman AM, Campbell ML, Kline CR, Wiesner S, D'Alton ME, Shields LE. Implementing obstetric early warning systems. AJP Rep 2018;8:e79–84. doi: 10.1055/s-0038-1641569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs R, Bauer M, Olvera L, Sakowski C, Cape V, Main E. Improving diagnosis and treatment of maternal sepsis: a quality improvement toolkit. Accessed April 10, 2021. https://www.cmqcc.org/resources-toolkits/toolkits/improving-diagnosis-and-treatment-maternal-sepsis [Google Scholar]
- 22.Kumar A, Roberts D, Wood K, Light B, Parillo J, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9 [DOI] [PubMed] [Google Scholar]
- 23.Bauer ME, Lorenz R, Bauer S, Rao K, Anderson F. Maternal deaths due to sepsis in the state of Michigan. Obstet Gynecol 1999;126:747–52. doi: 10.1097/AOG.0000000000001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawton B, MacDonald EJ, Brown SA, Wilson L, Stanley J, Tait J, et al. Preventability of severe acute maternal morbidity. Am J Obstet Gynecol 2014;210:557.e1–6. doi: 10.1016/j.ajog.2013.12.032 [DOI] [PubMed] [Google Scholar]
- 25.Rhodes A, Phillips G, Beale R, Cecconi M, Chiche JD, De Backer D, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med 2015;41:1620–8. doi: 10.1007/s00134-015-3906-y [DOI] [PubMed] [Google Scholar]
- 26.Ju T, Al-Mashat M, Rivas L, Sarani B. Sepsis rapid response teams. Crit Care Clin 2018;34:253–8. doi: 10.1016/j.ccc.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 27.Scheer CS, Fuchs C, Gründling M, Vollmer M, Bast J, Bohnert JA, et al. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clin Microbiol Infect 2019;25:326–31. doi: 10.1016/j.cmi.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 28.Knowles SJ, O'Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, etiology, and outcome for mother and fetus: a prospective study. BJOG 2015;122:663–71. doi: 10.1111/1471-0528.12892 [DOI] [PubMed] [Google Scholar]
- 29.Dawkins JC, Fletcher HM, Rattray CA, Reid M, Gordon-Strachan G. Acute pyelonephritis in pregnancy: a retrospective descriptive hospital based-study. ISRN Obstet Gynecol 2012;2012:519321. doi: 10.5402/2012/519321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta CD, Harrison DA, Rowan K, Lucas D, Kurinczuk J, Knight M. Maternal morbidity and mortality from severe sepsis: a national cohort study. BMJ Open 2016;6:e012323. doi: 10.1136/bmjopen-2016-012323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng MP, Stenstrom R, Paquette K, Stabler SN, Akhter M, Davidson AC, et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: a diagnostic study. Ann Intern Med 2019;171:547–54. doi: 10.7326/M19-1696 [DOI] [PubMed] [Google Scholar]
- 32.Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol 2015;32:481–6. doi: 10.1055/s-0034-1395477 [DOI] [PubMed] [Google Scholar]
- 33.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sosa MEB. Streptococcal A infection: reemerging and virulent. J Perinat Neonat Nurse 2009;23:141–7. doi: 10.1097/JPN.0b013e3181a2ed26 [DOI] [PubMed] [Google Scholar]
- 35.Barton JR, Sibai BM. Severe sepsis and septic shock in pregnancy. Obstet Gynecol 2012;120:689–706. doi: 10.1097/AOG.0b013e318263a52d [DOI] [PubMed] [Google Scholar]
- 36.Moses AE, Ziv A, Harari M, Rahav G, Shapiro M, Englehard D. Increased incidence and severity of Streptococcus pyogenes bacteremia in young children. Pediatr Infect Dis J 1995;14:767–70. doi: 10.1097/00006454-199509000-00007 [DOI] [PubMed] [Google Scholar]
- 37.Health Protection Agency, Group A Streptococcus Working Group. Interim UK guidelines for management of close community contacts of invasive group A streptococcal disease. Commun Dis Public Health 2004;7:354–61. [PubMed] [Google Scholar]
- 38.Anderson BL. Puerperal group A streptococcal infection: beyond Semmelweis. Obstet Gynecol 2014;123:874–82. doi: 10.1097/AOG.000000000000175 [DOI] [PubMed] [Google Scholar]
- 39.Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol 2018;9:2147. doi: 10.3389/fimmu.2018.02147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauerbrei A, Wutzler P. Herpes simplex and varicella-zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 2: varicella-zoster virus infections. Med Microbiol Immunol 2007;196:95–102. doi: 10.1007/s00430-006-0032-z [DOI] [PubMed] [Google Scholar]
- 41.Goodman ZD, Ishak KG, Sesterhenn IA. Herpes simplex hepatitis in apparently immunocompetent adults. Am J Clin Pathol 1986;85:694–9. doi: 10.1093/ajcp/85.6.694 [DOI] [PubMed] [Google Scholar]
- 42.Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Barucca V, Chiarini F, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J 2009;6:40. doi: 10.1186/1743-422X-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assessment and treatment of pregnant women with suspected or confirmed influenza: correction. ACOG Committee Opinion No. 753 [published erratum appears in Obstet Gynecol 2020;135:734]. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;132:e169–73. doi: 10.1097/AOG.0000000000002872 [DOI] [PubMed] [Google Scholar]
- 44.Delahoy MJ, Whitaker M, O'Halloran A, Chai SJ, Kirley PD, Alden N, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 States, march 1-August 22,2020. MMWR Morb Mortal Wkly Rep 2020;69:1347–54. doi: 10.15585/mmwr.mm6938e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamont RF, Sobel JD, Carrington D, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG 2011;118:1155–62. doi: 10.1111/j.1471-0528.2011.02983.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardella C, Brown ZA. Managing varicella zoster infection in pregnancy. Cleve Clin J Med 2007;74:290–6. doi: 10.3949/ccjm.74.4.290 [DOI] [PubMed] [Google Scholar]
- 47.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014;5:161–9. doi: 10.4161/viru.26187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Treatment for invasive candidiasis. Accessed April 15, 2021. https://www.cdc.gov/fungal/diseases/candidiasis/invasive/treatment.html#:∼:text=For%20candidemia%2C%20treatment%20should%20continue,a%20longer%20period%20of%20time
- 49.Potasman I, Leibovitz Z, Sharf M. Candida sepsis in pregnancy and the postpartum period. Rev Infect Dis 1991;13:146–9. doi: 10.1093/clinids/13.1.146 [DOI] [PubMed] [Google Scholar]
- 50.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503–35. doi: 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammad WAB, Konje JC. Herpes simplex virus infection in pregnancy—an update. Eur J Obstet Gynecol Reprod Biol 2021;259:38–45. doi: 10.1016/j.ejogrb.2021.01.055 [DOI] [PubMed] [Google Scholar]
- 52.Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother 2015;70:14–22. doi: 10.1093/jac/dku355 [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Baño J, Millán AB, Domínguez MA, Borraz C, González MP, Almirante B, et al. Impact of inappropriate empirical therapy for sepsis due to health care-associated methicillin-resistant Staphylococcus aureus. J Infect 2009;58:131–7. doi: 10.1016/j.jinf.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 54.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007;60:913–20. doi: 10.1093/jac/dkm318 [DOI] [PubMed] [Google Scholar]
- 55.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118:146–55. doi: 10.1378/chest.118.1.146 [DOI] [PubMed] [Google Scholar]
- 56.Eppes CS, Clark SC. Extended-spectrum β-lactamase infections during pregnancy: a growing threat. Am J Obstet Gynecol 2015;213:650–2. doi: 10.1016/j.ajog.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 57.Biset S, Moges F, Endalamaw D, Eshetie S. Multi-drug resistant and extended-spectrum β-lactamases producing bacterial uropathogens among pregnant women in Northwest Ethiopia. Ann Clin Microbiol Antimicrob 2020;19:25. doi: 10.1186/s12941-020-00365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G, Xu K, Sun F, Sun Y, Kong Z, Fang B. Risk factors of multi-drug resistant bacteria in lower respiratory infections: a systematic review and meta-analysis. Can J Inf Dis Med Microbiol 2020:7268519. doi: 10.1155/2020/7268519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshi S. Hospital antibiogram: a necessity. Indian J Med Microbiol 2010;28:277–80. doi: 10.4103/0255-0857.71802 [DOI] [PubMed] [Google Scholar]
- 60.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228. doi: 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Royal College of Obstetricians and Gynaecologists. Sepsis in pregnancy, bacterial. Green-top Guideline No. 64a. Accessed April 14, 2021. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg64a/
- 62.Royal College of Obstetricians and Gynaecologists. Sepsis following pregnancy, bacterial. Green-top Guideline No. 64b. Accessed April 14, 2021. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg64b/
- 63.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–77. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 64.Marques NR, Martinello C, Kramer GC, Constantine MM, Vadhera RB, Saade GR, et al. Passive leg raising during pregnancy. Am J Perinatol 2015;32:393–8. doi: 10.1055/s-0034-1389089 [DOI] [PubMed] [Google Scholar]
- 65.Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;42:1935–47. doi: 10.1007/s00134-015-4134-1 [DOI] [PubMed] [Google Scholar]
- 66.Critical care in pregnancy. ACOG Practice Bulletin No. 211. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019;133:e303–19. doi: 10.1097/AOG.0000000000003241 [DOI] [PubMed] [Google Scholar]
- 67.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014;5:4–11. doi: 10.4161/viru.27372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armstrong BA, Betzold RD, May AK. Sepsis and septic shock strategies. Surg Clin North Am 2017;97:1339–79. doi: 10.1016/j.suc.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 69.Lakhal K, Ehrmann S, Boulain T. Noninvasive BP monitoring in the critically ill: time to Abandon the arterial catheter? Chest 2018;153:1023–39. doi: 10.1016/j.chest.2017.10.030 [DOI] [PubMed] [Google Scholar]
- 70.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004;32:1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7 [DOI] [PubMed] [Google Scholar]
- 71.Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW, et al. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest 2015;148:1224–30. doi: 10.1378/chest.15-028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bates SM, Middeldorp S, Rodger M, James AH, Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:92–128. doi: 10.1007/s11239-015-1309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bain E, Wilson A, Tooher R, et al. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. The Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No.: CD 0001689. doi: 10.1002/14651858.CD001689.pub3 [DOI] [PubMed]
- 74.Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer-Westendorf J, Spencer FA, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018;2:3198–225. doi: 10.1182/bloodadvances.2018022954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. doi: 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 76.Surgers L, Valin N, Carbonne B, Bingen E, Lalande V, Pacanowski J, et al. Evolving microbiological epidemiology and high fetal mortality in 135 cases of bacteremia during pregnancy and postpartum. Eur J Clin Microbiol Infect Dis 2013;32:107–13. doi: 10.1007/s10096-012-1724-5 [DOI] [PubMed] [Google Scholar]
- 77.Chau A, Tsen LC. Fetal optimization during maternal sepsis; relevance and response of the obstetric anesthesiologist. Curr Opin Anaesthesiol 2014;27:259–66. doi: 10.1097/ACO.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 78.Simpson KR. Critical illness during pregnancy: considerations for evaluation and treatment of the fetus as the second patient. Crit Care Nurs Q 2006;29:20–31. doi: 10.1097/00002727-200601000-00003 [DOI] [PubMed] [Google Scholar]
- 79.Antenatal corticosteroid therapy for fetal maturation. Committee Opinion No. 713. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;130:e102–9. doi: 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 80.American Red Cross. Focused updates and guidelines 2020. American Red Cross; 2020. [Google Scholar]
- 81.Thompson M, Reeves M, Bogan B, DiGiovine B, Posa PJ, Watson SR. Protocol-based resuscitation bundle to improve outcomes in septic shock patients: evaluation of the Michigan Health and Hospital Association keystone sepsis collaborative. Crit Care Med 2016;44:2123–30. doi: 10.1097/CCM.0000000000001867 [DOI] [PubMed] [Google Scholar]
- 82.Vincent J, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323–9. doi: 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 83.Higgins R, Saade G, Polin R, Grobman W, Buhimschi I, Watterberg K, et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol 2016;127:426–36. doi: 10.1097/AOG.0000000000001246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ward K, Theiler RN. Once-daily dosing of gentamicin in obstetrics and gynecology. Clin Obstet Gynecol 2008;51:498–506. doi: 10.1097/GRF.0b013e31818091cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chebbo A, Tan S, Kassis C, Tamura L, Carlson RW. Maternal sepsis and septic shock. Crit Care Clin 2016;32:119–35. doi: 10.1016/j.ccc.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 86.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller L, et al. International clinical practice guidelines for treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Disease Society of American and the European Society for Microbiology and Infectious Disease. Clin Inf Dis 2011;52:e103–20. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 87.Wagenlehner FM, Lichtenstern C, Rolfes C, Mayer K, Uhle F, Weidner W, et al. Diagnosis and management for urosepsis. Int J Urol 2013;20:963–70. doi: 10.1111/iju.12200 [DOI] [PubMed] [Google Scholar]
- 88.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44(suppl):S27–72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Infect Dis Soc America. Clin Infect Dis. 2000;31:347–82. doi: 10.1086/313954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheffield JS, Cunningham FG. Community-acquired pneumonia in pregnancy. Obstet Gynecol 2009;114:915–22. doi: 10.1097/AOG.0b013e3181b8e76d [DOI] [PubMed] [Google Scholar]
- 91.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. doi: 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 92.Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi Ak, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections [published erratum appears in World J Emerg Surg 2017;12:36]. World J Emerg Surg 2017;12:29. doi: 10.1186/s13017-017-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solomkin JS, Mazuski JE, Bradley JS, Rodvoid KA, Goldstein EJ, Baron E, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America [published erratum appears in Clin Infect Dis 2010;50:1695]. Clin Infect Dis 2010;50:133–64. doi: 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 94.Yilmaz HG, Akgun Y, Bac B, Celik Y. Acute appendicitis in pregnancy-risk factors associated with principal outcomes: a case control study. Int J Surg 2007;5:192–7. doi: 10.1016/j.ijsu.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 95.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Disease Society of America [published erratum appears in Clin Infect Dis 2015;60:1448]. Clin Infect Dis 2014;59:e10–52. doi: 10.1093/cid/ciu444 [DOI] [PubMed] [Google Scholar]