Abstract
Ventral striatal dopamine is thought to be important for associative learning. Dopamine exerts its role via activation of dopamine D1 and D2 receptors in the ventral striatum. Upregulation of dopamine D2R in ventral striatopallidal neurons impairs incentive motivation via inhibiting synaptic transmission to the ventral pallidum. Here, we determined whether upregulation of D2Rs and the resulting impairment in ventral striatopallidal pathway function modulates associative learning in an auditory Pavlovian reward learning task as well as Go/No-Go learning in an operant based reward driven Go/No-Go task. We found that upregulation of D2Rs in indirect pathway neurons of the NAc did not affect Pavlovian learning or the extinction of Pavlovian responses, and neither did it alter No-Go learning. A delay in the Go component of the task however could indicate a deficit in learning though it may be attributed to locomotor hyperactivity of the mice. In combination with previously published findings our data suggest that D2Rs in ventral striatopallidal neurons play a specific role in regulating motivation by balancing cost/benefit computations but do not necessarily affect associative learning.
Introduction:
The role of ventral striatal dopamine and its receptors in the regulation motivation and learning has been an intensive area of study for the last decades. Pharmacological studies have uncovered an important role for dopamine receptors in the Nucleus accumbens (NAc) in the regulation of incentive motivation and the willingness to work for reward (Aberman, Ward, & Salamone, 1998; Berridge, 2007; Salamone, Correa, Farrar, & Mingote, 2007). In this context dopamine is thought to regulate effort-related processes that are important to overcome work-related response costs rather than to adapt the animals response to changes in reward value (Filla et al., 2018; Hamid et al., 2016; Kelley, Baldo, Pratt, & Will, 2005; Ostlund, Wassum, Murphy, Balleine, & Maidment, 2011; Phillips, Walton, & Jhou, 2007; Salamone et al., 2007; Wanat, Kuhnen, & Phillips, 2010). Upregulation of dopamine D2 receptors (D2Rs) in the adult NAc core enhances performance in a progressive ratio and a concurrent choice tasks that probe for incentive motivation and effort related decision making (Donthamsetti et al., 2018; Gallo et al., 2018; Trifilieff et al., 2013). Notably, cell specific upregulation of D2Rs in ventral striatopallidal projection neurons (D2R-OENAcInd mice) is sufficient to enhance motivation, whereas upregulation in cholinergic interneurons (D2R-OEChAT mice), which also express D2Rs had no effect on progressive ratio performance (Gallo et al., 2018).
D2Rs in ventral striatopallidal neurons are transported to axonal terminals, where they reduce inhibitory transmission at intra-striatal collaterals and striato-pallidal synapses (Cooper & Stanford, 2001; Dobbs et al., 2016; Floran, Floran, Sierra, & Aceves, 1997; Kohnomi, Koshikawa, & Kobayashi, 2012; Tecuapetla, Koos, Tepper, Kabbani, & Yeckel, 2009). Slice physiological recordings revealed that D2R upregulation in ventral striatopallidal neurons enhances this modulation by dopamine. Thus, D2R-OENAcInd mice display decreased baseline synaptic transmission and an enhanced inhibition of synaptic transmission by D2R activation (Gallo et al., 2018). As you would expect this effect was recorded at intra-striatal collaterals to the direct pathway and the canonical projections to the ventral pallidum (Gallo et al., 2018). A follow up in vivo physiological analysis showed that the effects of disinhibition in D2R-OENAcInd mice are mostly measurable at the level of the striato-pallidal synapse (Gallo et al., 2018). Furthermore, selective inhibition of striato-pallidal synapses in the ventral pallidum is sufficient to enhance progressive ratio performance suggesting that ventral striatopallidal D2Rs promote incentive motivation via enhanced inhibition of striato-pallidal transmission (Gallo et al., 2018).
Ventral striatal dopamine has also been implicated in associative learning. Dopamine neurons have been shown to encode a reward prediction error providing a teaching signal that is required for learning and that is thought to be transmitted to the NAc via the release of dopamine (Day, Roitman, Wightman, & Carelli, 2007; Schultz, Dayan, & Montague, 1997; Steinberg et al., 2013). D2R-OENAcInd mice should be more sensitive to this signal so that dopamine released in response to a reward predicting cue leads to a stronger inhibition of synaptic transmission, which could affect associative learning. To address this hypothesis, we tested D2R-OENAcInd mice in Pavlovian conditioning, an associative learning task. In this task mice learn that an auditory stimulus (conditioned stimulus: CS+) predicts the delivery of a food reward, whereas a different auditory stimulus (CS-) is not reinforced. Importantly, CS+ presentation leads to the release of dopamine when mice are acquiring the task (M. R. Bailey et al., 2018). We then extinguished the importance of the CS+ by adding 5 days of extinction training in which animals were not rewarded.
As inhibition of the ventral striatopallidal pathway has been shown to increase response initiation, we further hypothesized that D2R upregulation impairs learning if actions must be suppressed (Carvalho Poyraz et al., 2016). We thus tested D2R-OENAcInd mice in an instrumental Go/No-Go learning task where in a first step mice learn to press a lever in the presence of a visual stimulus. In a second step they then must learn to withhold from pressing the lever when the stimulus is absent. Last, we measured the activity of D2R-OENAcInd mice in an open field to determine the functionality of the upregulated receptors in these new cohorts of mice.
We replicated previous findings showing hyperactivity in the open field (Donthamsetti et al., 2018; Gallo et al., 2015). In contrast to our expectations, D2R upregulation neither affected Pavlovian, extinction nor Go/No-Go learning suggesting that D2R upregulation in ventral striatopallidal neurons enhance motivation (Donthamsetti et al., 2018; Gallo et al., 2015) but does not affect Pavlovian or No-Go learning.
Methods:
Animals:
Adult male and female Drd2-Cre BAC transgenic mice (ER44; GENSAT) backcrossed onto the C57BL/6J background were group housed under 12-h light/dark cycle. All experimental procedures were conducted following NIH guidelines and were approved by Institutional Animal Care and Use Committees by Columbia University and New York State Psychiatric Institute. We chose Drd2-Cre over A2A-Cre mice to recapitulate the conditions in which we saw enhanced progressive ratio performance. Also, Cre levels are higher in Drd2-Cre mice as they can be visualized with anti-Cre immunohistochemistry (Cazorla et al., 2014; Gallo et al., 2018), whereas we cannot detect Cre expression in A2A-Cre mice using the same anti-serum. In our hands Drd2-Cre mice leads to recombination of AAV expression constructs in about 5% to 10% of ChAT neurons (Carvalho Poyraz et al., 2016; Gallo et al., 2018).
Stereotaxic Surgery:
Mice (≥8 weeks old) were bilaterally injected with 450 nL/hemisphere of a previously characterized Cre-dependent double-inverted open reading frame (DIO) adeno-associated viruses (AAVs) encoding D2R-ires-Venus (5.1 X 1013 GC/mL) or EGFP (6.69 X 1013 GC/mL) (UNC Vector Core, Chapel Hill, NC) into the nucleus accumbens (NAc) using stereotactic Bregma-based coordinates: AP, + 1.70 mm; ML, ± 1.20 mm; DV, −4.1 mm (from dura). Mice were induced with 4% isoflurane anesthetic and maintained at 1–2% throughout the stereotaxic surgery. Following induction, mice were placed on the stereotaxic setup and a midline incision was made using a sterile scalpel. A high-speed rotary micromotor kit (Foredom, Bethel, CT) was used to make holes in the skull and a glass pipette was lowered into the brain to deliver the AAVs. Mice were given 4 weeks to recover from the surgery. Groups of mice used for experiments were first assigned their AAV-genotype in a counterbalanced fashion that accounted for sex, age, home cage origin.
Operant Apparatus:
Eight operant chambers (model Env-307w; Med-Associates, St. Albans, VT) equipped with liquid dippers were used. Each chamber was in a light-and sound-attenuating cabinet equipped with an exhaust fan, which provided 72-dB background white noise in the chamber. The dimensions of the experimental chamber interior were 22 × 18 × 13 cm, with flooring consisting of metal rods placed 0.87 cm apart. A feeder trough was centered on one wall of the chamber. An infrared photocell detector was used to record head entries into the trough. Raising of the dipper inside the trough delivered a drop of evaporated milk reward. A retractable lever was mounted on the same wall as the feeder trough, 5 cm away. A house light located on wall opposite to trough illuminated the chamber throughout all sessions.
Dipper Training:
Four weeks after AAV surgery, mice underwent operant training. Mice were weighed daily and food-restricted to 85–90% of baseline weight; water was available ad libitum. In the first training session, 20 dipper presentations were separated by a variable inter-trial interval (ITI) and ended after 20 rewards were earned or after 30 min had elapsed, whichever occurred first. Criterion consisted of the mouse making head entries during 20 dipper presentations in one session. In the second training session, criterion was achieved when mice made head entries during 30 of 30 dipper presentations.
Pavlovian Conditioning:
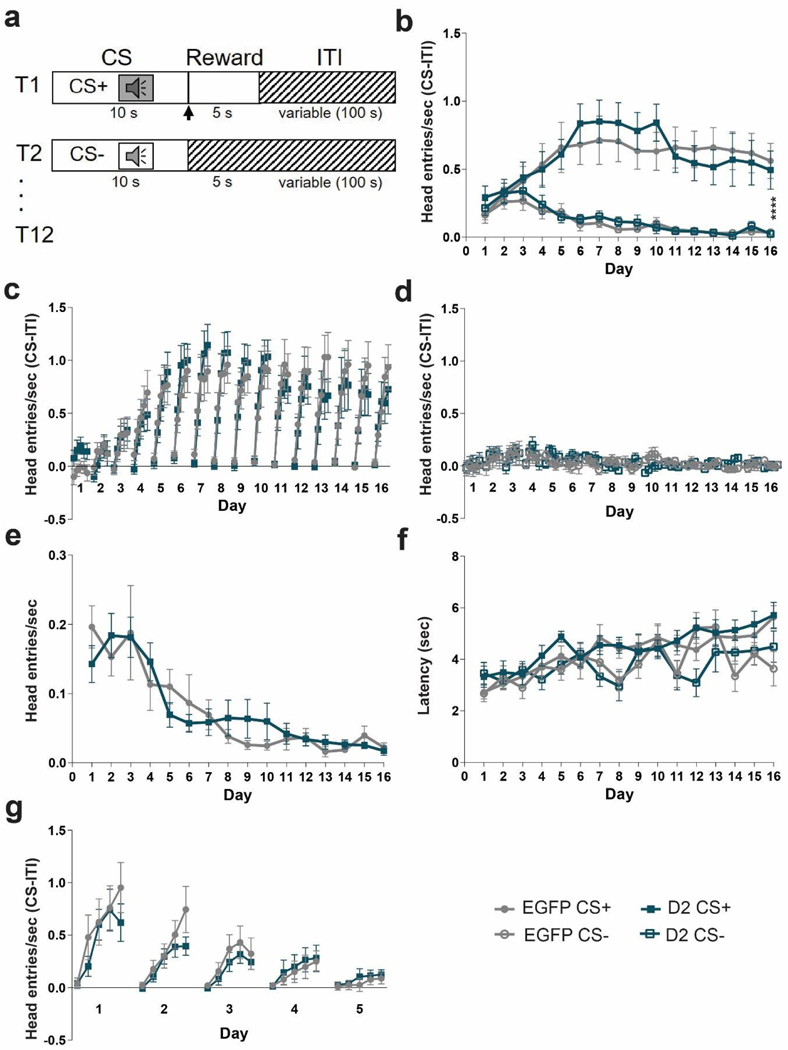
Mice were trained for 16 consecutive days in a Pavlovian conditioning paradigm, which consisted of 12 conditioned stimulus-positive (CS+) trials and 12 CS-trials occurring in a pseudorandom order. Each trial consisted of an 80-dB auditory cue presentation for 10 sec, of either a 3 kHz (Cohort 1) or 8 kHz (Cohort 2) tone or white noise (counterbalanced between mice) and after cue offset a milk reward was delivered only in CS+ trials, whereas no reward was delivered in CS-trials. There was a 100 sec variable intertrial interval, drawn from an exponential distribution of times. Head entries in the food port were recorded throughout the session, and anticipatory head entries during the presentation of the cue were considered the conditioned response. The differential score (Head entries/sec (CS – (ITI))) was calculated using either the 2 sec or 10 sec of ITI proceeding the cue. We used 10 sec of ITI to calculate the differential score during the entire 10 sec cue (Figure 3b) and 2 sec ITI for the 2 sec binning (Figure 3c & d).
Figure 3. D2R overexpression does not impair Pavlovian learning.
(a) Task design. Mice are trained with 24 (12 CS+, 12 CS-) trials /day for 16 days. Each trial starts with a 10 sec tone (CS+ or CS-). At the end of the CS+ a dipper comes up presenting milk as a food reward for 5 sec. There is an intertrial interval (ITI) variable in length (100 sec). (b) To determine learning, anticipatory responses (head entries/sec (CS-ITI)) were measured. Anticipatory head entries during the CS+ increased for both EGFPNAcInd (filled light circles) and D2R-OENAcInd (filled dark squares) mice and decreased during the CS-for both EGFPNAcInd (open light circles) and D2R-OENAcInd (open dark squares) mice. Both groups learned to distinguish the CS+ and CS-(****p< 0.0001). There was no effect of genotype on learning over the 16 days of training (p= 0.9977). (c) Within each day, the 10 sec CS+ was split into 5 two-second time bins to better visualize the anticipatory response. EGFPNAcInd (filled light circles) and D2R-OENAcInd (filled dark squares) sharply increased head entries into the reward port across the 10 sec CS+ (p< 0.0001) and continued this anticipatory behavior across the 16 days of training (p< 0.0001). There was no effect of genotype on learning (p= 0.9375). (d) EGFPNAcInd (open light circles) and D2R-OENAcInd (open dark squares) showed no anticipatory response during the 10 sec CS-. There was no effect of genotype on learning (p= 0.9988). (e) Head entries during the ITI decreased over the 16 days of training comparably for both groups with no difference between groups (p= 0.6173). (f) Latency for the first head entry during the CS+ and CS-increased for EGFPNAcInd (filled light circles/open light circles) and D2R-OENAcInd (filled dark squares/open dark squares). There was no difference between groups. (g) Anticipatory response to the CS+ attenuated over 5 days of extinction learning. Both groups similarly decreased anticipatory head entries during the CS+ (p< 0.0001). There was no difference in extinction learning between the two groups (p= 0.5247). Data from 8 or 16 animals per genotype was used to calculate all statistics reported.
Continuous Reinforcement schedule (CRF):
For lever press training, lever presses were reinforced on a continuous reinforcement (CRF) schedule. Levers were retracted after each reinforcer and were presented again after a variable ITI (average 40 sec). The reward consisted of raising the dipper for 5 sec. The session ended when the mouse earned 60 reinforcements, or one hour elapsed, whichever occurred first. Sessions were repeated daily until mice achieved 60 reinforcements.
Go/No-Go schedule:
Mice were first trained on Go trials in which they were required to press a lever within 5 sec of its presentation to receive a reward. If the 5 sec elapsed with no response, the lever would retract, no reward would be presented, and a new ITI (average 40 sec) would begin. Mice were trained on these 5 sec Go-only trials until they reached 75% accuracy over three consecutive days. Once this criterion was achieved, No-Go trials were added in which the lever was presented simultaneously with two cues (the house lights turning off, and a small LED light above the lever turning on). A lack of any lever press within 5 sec, resulted in a reward. A lever press during this period caused the lever to retract, the house lights to turn on, the LED light to turn off, and a new ITI to begin without any reward for that trial. In each session, 30 Go trials were interspersed with 30 No-Go trials presented pseudo-randomly such that there were an equal number of both kinds of trials in every block of 10 trials. Mice were tested for 35 days, and false alarm rate and hit rate were analyzed.
Locomotor Activity:
D2-Cre mice injected with D2R-Or EGFP-expressing AAVs were tested in open field boxes equipped with infrared photobeams to measure locomotor activity (Med Associates, St. Albans, VT). Data were acquired using Kinder Scientific Motor Monitor software (Poway, CA) and expressed as total distance traveled (cm) over 90 min.
Histology:
Mice were transcardially perfused with ice-cold 4% paraformaldehyde (Sigma, St. Louis, MO) in PBS under deep anesthesia. Brains were harvested, post-fixed overnight and washed in PBS. Free-floating 50-μm coronal sections were obtained using a Leica VT2000 vibratome (Richmond, VA). After incubation in blocking solution (5% horse serum, 0.5% bovine serum albumin in 0.5% PBS-Triton X-100) for 2 hr at room temperature, sections were labeled overnight at 4 °C with primary antibodies against GFP (chicken; 1:1000; AB13970 Abcam, Cambridge, MA). Sections were incubated with fluorescent secondary antibodies (Goat anti-chicken, A488, A11039, ThermoFisher) for 2 hr at RT. Sections were then mounted on slides and cover slipped with Vectashield containing DAPI (Vector, Burlingame, CA). Digital images were acquired using a Zeiss epifluorescence microscope.
Statistical analysis:
Data are expressed as mean ± SEM. Students’ t-tests were used to compare between two groups. Analyses involving multiple conditions were evaluated by one-way, two-way, or three-way repeated measures ANOVA, using GraphPad Prism software. Statistical significance was considered for p <0.05.
Results:
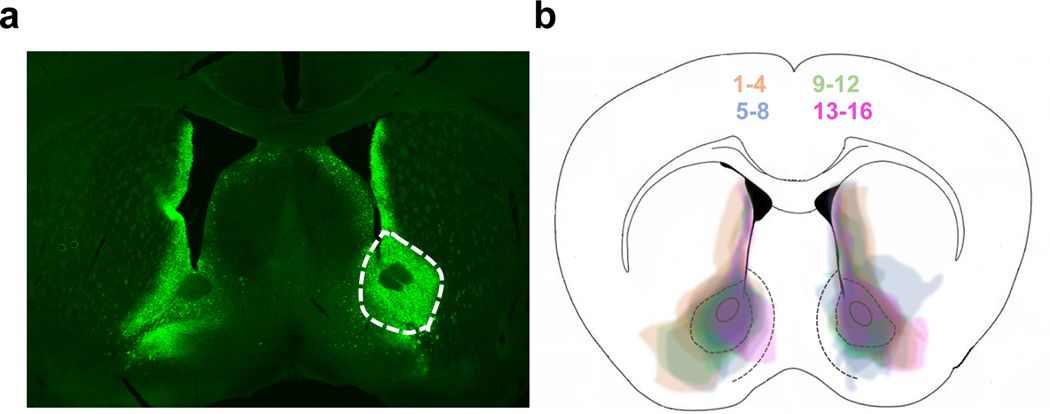
To test the effects of D2R upregulation in ventral striatopallidal neurons on Pavlovian conditioning and Go/No-Go learning we generated two cohorts of mice. Cohort 1 was first tested in the Pavlovian conditioning task followed by the Go/No-Go task. Cohort 2 was first tested in the Pavlovian conditioning task followed by an extinction procedure. At the end of behavioral testing both cohorts were run in the open field as a positive control. Adult D2R-OENAcInd mice were generated by injecting a Cre-depending AAV1 expressing D2R-ires-mVenus or GFP into the NAc core of Drd2-Cre mice that express Cre recombinase in ventral striatopallidal neurons. This leads to a 3-fold increase in D2 receptor levels in the NAc (Gallo et al., 2018). Figure 1a shows a representative image of a coronal section from a mouse with bilateral expression of the D2R-ires-mVenus virus in the NAc. Figure 1b shows the spread of virus-mediated expression with injections from all mice superimposed on each other in different colors for better visualization. We see dense viral expression in the NAc core with some leakage into the lateral NAc shell and dorsal medial striatum (DMS).
Figure 1. Confirmation of viral spread.
(a) Coronal section showing D2R-mVenus expression in the NAc of a Drd2-Cre mouse. (b) Superimposed traces of viral spread from coronal sections at ~1.0 mm anterior to bregma for all 16 D2ROENAcInd mice injected with the AVV1-hSyn-DIO-D2R-mVenus into the NAc. Mice are shown in 4 different colors (4 mice/color) for better visualization of viral spread.
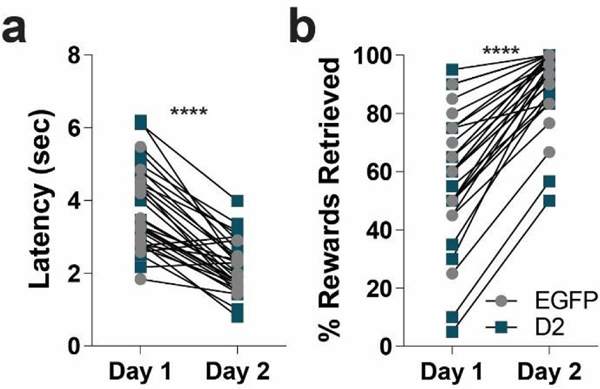
Four weeks after AAV injections mice were tested in the Pavlovian conditioning task. First, mice were trained in an operant box to retrieve a food reward (evaporated milk) from an automatic dipper. Over the two training days, both groups learned that the dipper provided a reward (Figure 2). There was no difference between the two groups, EGFPNAcInd and D2R-OENAcInd mice decreased their latency to retrieve the milk reward (Figure 2a, RM 2-way ANOVA: F(1,15)= 123.1, p< 0.0001, main effect of day) and increased the total number of rewards retrieved (Figure 2b, Mann Whitney, p< 0.0001, main effect of day). These results indicate that D2R upregulation in ventral striatopallidal neurons does not affect reward retrieval. During auditory Pavlovian conditioning, mice were presented with two 10 second auditory cues (tone verses white noise, counterbalanced between viral groups). After offset of the conditioned stimulus (CS+), a milk reward was delivered whereas no reward was delivered after the offset of the unconditioned stimulus (CS-). Figure 3a provides a schematic of the task. Both groups of mice increased their head entries during the CS+ presentation over the 16 days of training (Figure 3b). In contrast, during the CS-, head entries increased slightly during the first 4 days and then went down (Figure 3b). Both groups were able to distinguish between the CS+ and CS-and demonstrated learning across 16 days of training (RM 3-way ANOVA: F(15, 15)= 4.744, p< 0.0001, main effect of conditioned stimuli over 16 days of training). However, there were no significant difference in the interaction between rate of anticipatory head poking during the CS+ or CS-between D2R and EGFP expressing mice over the 16 days of training (Figure 3b, RM 3-way ANOVA: F(15,15)= 0.2672, p= 0.9977, N=16/group).
Figure 2. D2R upregulation does not impair trough training.
(a) Latency to retrieve an unexpected milk reward via head entry into a retractable dipper significantly decreased over 2 days of training for both D2R-OENAcInd (dark squares) and EGFPNAcInd (light circles) mice (**** p< 0.0001). (b) Total number of rewards retrieved significantly increased from Day 1 to Day 2 for both groups (**** p< 0.0001). Data from 16 animals per genotype was used to calculate all statistics reported.
To better visualize the pattern of anticipatory head entries, we plotted head entries during the CS+ and CS-in 2 second bins. As mice learn the fixed duration of the CS+, their anticipatory response sharply increased during the 10 second CS+ (Figure 3c). We found that both D2R-OENAcInd and EGFPNAcInd mice comparably increased their head entries over the duration of the CS+ and continued this pattern of anticipatory behavior across the 16 days of training. However, there was no significant interaction between days, head entries during the CS+ and viral manipulation (Figure 3c, RM 3-way ANOVA:0.5077, p=0.9375). Similarly, we observed no effect of day, head entries during CS-presentation and viral manipulation (Figure 3d, RM 3-way ANOVA: F(15, 15)= 0.2368, p= 0.9988). In addition, we observed no effect of viral manipulation (D2R vs EGFP) on the rate of head entries during the variable intertrial interval (ITI) (Figure 3e, RM 2-way ANOVA: F(15, 450)= 0.8535, p=0.6173). To further quantify their ability to learn the length of both cues, we measured the latency of first head entry with cue onset. For both groups, this latency increased only during the CS+ over the 16 days of conditioning, but no difference between groups (Figure 3f). This result further indicates that the mice have learned both the cue that predicts the reward and the timing of the reward that follows.
To test the effects of D2R upregulation on eliminating a conditioned response, we ran our 2nd cohort of mice through 5 days of Pavlovian extinction. To reinstate the conditioned behavior, the mice received 3 days of Pavlovian conditioning as previously described (Figure 3a). For extinction, the protocol was identical to Pavlovian conditioning except now the mice no longer received a reward following the CS+. Both D2R-OENAcInd and EGFPNAcInd mice attenuated their anticipatory head poking during the CS+ each day with near complete extinction by Day 5 (Figure 3g). Both groups had a comparable decay in their anticipatory head entries during the 10 second CS+ over the 5 days of extinction testing (RM 2-way ANOVA: F(4,4)= 6.536, p<0.0001, main effect of day). However, there was no interaction between day, CS+ anticipatory head entries and viral manipulation (RM 3-way ANOVA: F(4,4)= 0.8083, p= 0.5247), suggesting that D2R upregulation has no effect on extinction learning.
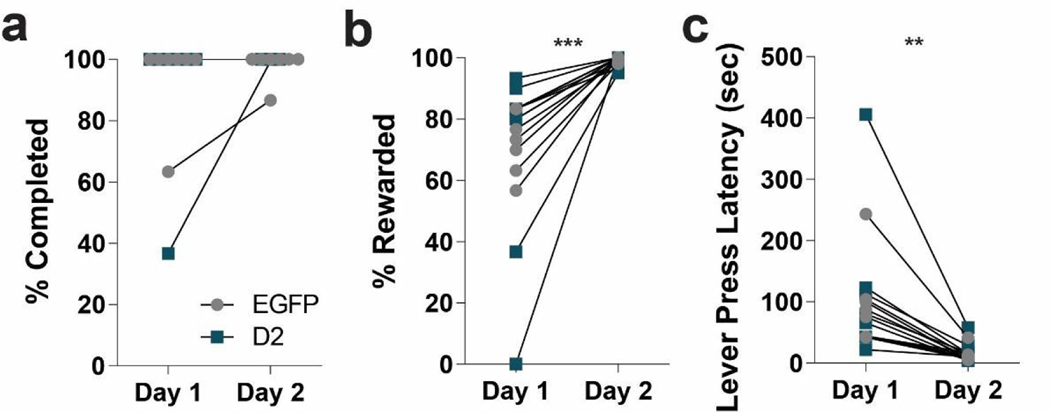
Next, to determine if D2R-OENAcInd mice have issues learning to withhold responses, we first trained mice to press a lever to earn a food reward and then tested them in a Go/No-Go task. Mice learned to press a lever to receive a milk reward using a continuous reinforcement (CRF) schedule. Over the two days of training, both D2R-OENAcInd and EGFPNAcInd mice were able to complete all trials (30 or 60) during the allotted time (60 min) (Figure 4a). Furthermore, both groups improved their performance from Day 1 to Day 2. The number of successful trials (% Rewarded) increased (Figure 4b) for both the EGFPNAcInd and D2R-OENAcInd mice (RM 2-way ANOVA: F(1,14)= 22.12, p= 0.0003, main effect of day) while there was no group effect of the viral manipulation (RM 2-way ANOVA: F(1, 14)= 0.2216, p= 0.6451). Both EGFPNAcInd and D2R-OENAcInd mice got faster at the task as measured by a decrease in lever press latency (Figure 4c, RM 2-way ANOVA: F(1,14)= 15.76, p= 0.0014, main effect of day) with no difference between groups (RM 2-way ANOVA: F(1, 14)= 0.2255, p= 0.6422). We conclude that D2R upregulation has no effect on learning to press a lever for a food reward as we observed no difference in performance between the two groups.
Figure 4. D2R upregulation does not affect reinforcement learning.
Mice were trained to press a lever to obtain a milk reward on a continuous reinforcement (CRF) schedule for two days. (a) EGFPNAcInd (light circles) and D2R-OENAcInd (dark squares) were able to complete all CRF trials on both days of training. (b) The number of rewarded trials (% Rewarded) comparably increased for EGFPNAcInd (light circles) and D2R-OENAcInd (dark squares) mice over the 2 days of training (*** p= 0.0003). There was no difference between groups (p= 0.6451). (c) Lever press latency decreased from Day 1 to Day 2 for both groups (** p= 0.0014). There was no difference between groups (p= 0.6422). Data from 8 animals per genotype was used to calculate all statistics reported.
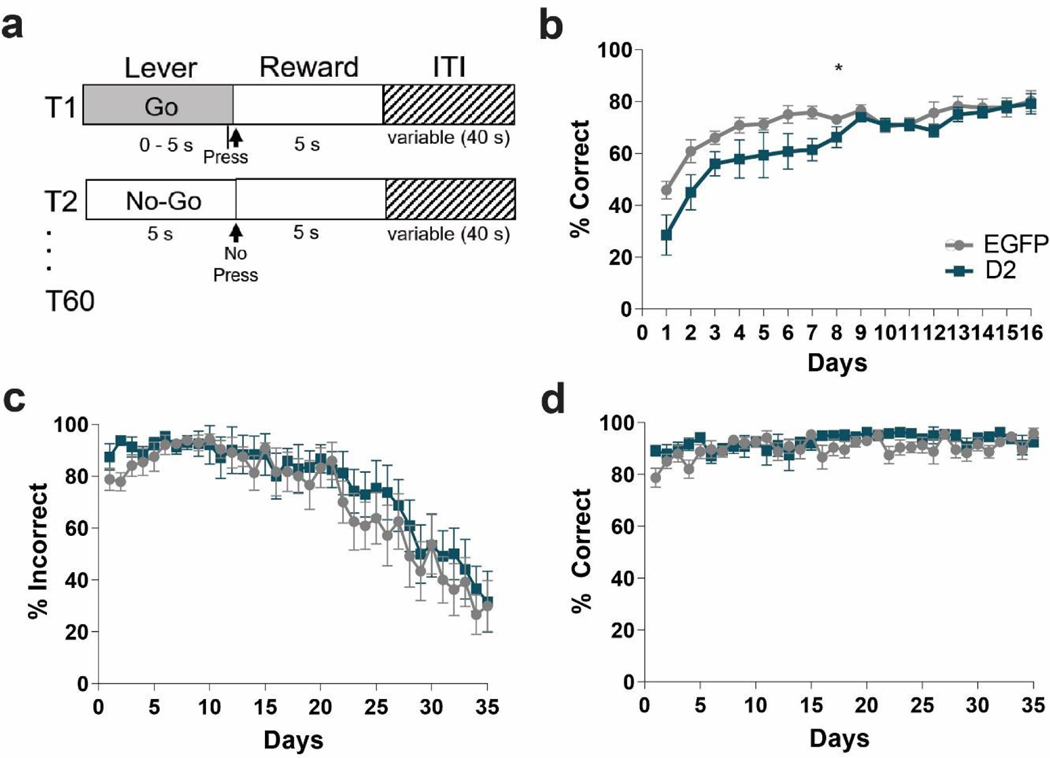
During the Go/No-Go task, mice use distinct visual cues to learn to either press a lever (“Go” trial) or withhold pressing of the same lever (“No-Go” trial) to receive a milk reward. In both trials, the lever is available for 5 seconds in which the animal must decide to press or not. A schematic of the task is shown in Figure 5a. The mice were first trained exclusively on Go trials and learning was established once they reached a criterion of 75% accuracy over three consecutive days. Next, No-Go trials were randomly intermixed with Go trials (60 total trials). Surprisingly, D2R-OENAcInd mice showed a deficit in the acquisition of Go trials compared to the EGFPNAcInd mice (Figure 5b, RM 2-way ANOVA: F(15, 210)= 2.029, p= 0.0148, interaction effect). During the Go/No-Go task, both D2R-OENAcInd and EGFPNAcInd control mice improved on withholding lever pressing during No-Go trials as measured by a decrease in incorrect responses (% incorrect) over the 35 days of testing (Figure 5c, RM 2-way ANOVA: F(34, 476)= 20.66, p<0.0001, main effect of day). However, there was no significant difference between the two groups (RM 2-way ANOVA: F(34, 476)= 0.4606, p=0.9965). Both groups also improved their performance on Go trials as measured by an increase in correct responses (% correct) the 35 days of Go/No-Go testing (Figure 5d, RM 2-way ANOVA: F (34, 476)= 2.076, p=0.0005, main effect of day). Again, there was no difference in correct responses between the two groups during Go\No-Go testing (RM 2-way ANOVA: F(34, 476)= 1.2, p=0.2073).
Figure 5. D2R upregulation does not impair No-Go learning.
(a) Task design. Go trials: mice were trained to press a lever within 5 seconds to obtain a milk reward. No-Go trials: mice learned to withhold pressing the lever for 5 seconds to receive a milk reward. Mice only received a reward if they made a correct choice. Each session consisted of 30 Go and 30 No-Go trials that were randomly mixed. (b) Acquisition of Go trials only. D2R-OENAcInd (dark squares) showed a delay in the acquisition compared to EGFPNAcInd (light circles) (*p= 0.0148). (c) Performance on No-Go trials during Go/No-Go testing. Both EGFPNAcInd (light circles) and D2R-OENAcInd (dark squares) improved on No-Go trials and learned to withhold responding (decrease in % incorrect) over the 35 days of testing (p< 0.0001). There was no difference in performance between the two groups (p= 0.9965). (d) Performance on Go trials during Go/No-Go testing. Both groups performed better on Go trials (increase in % correct) over the 35 days of testing (p=0.0005). There was no difference in performance between groups (p= 0.2073). Data from 8 animals per genotype was used to calculate all statistics reported.
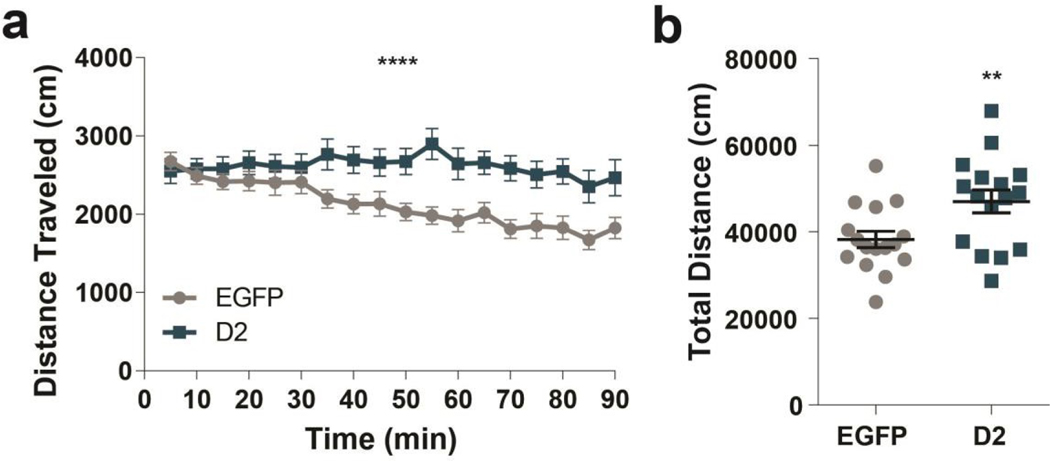
To determine whether D2R upregulation in this two cohorts leads to hyperlocomotion as has been described before (Donthamsetti et al., 2018; Gallo et al., 2018) we tested all mice in the open field. D2R-OENAcInd mice showed an increase in locomotor activity in a standard 90-minute open field session replicating previous findings consistent with functional upregulation of D2Rs in ventral striatopallidal neurons (Figure 6). D2R-OENAcInd mice continued to traverse the enclosure during the entire session while EGFPNAcInd control mice attenuated their locomotion (Figure 6a, RM 2-way ANOVA: F(17, 510)= 5.231, p<0.0001, interaction effect). Furthermore, D2R-OENAcInd mice traveled a significantly greater total distance compared to controls (Figure 6b, 2-way ANOVA: F(1,15)= 8.925, p= 0.0092, main effect of genotype). These results confirmed that our viral manipulation of over-expressing D2Rs in ventral striatopallidal neurons was functional.
Figure 6. D2R upregulation induces hyperlocomotion.
(a) D2R-OENAcInd (dark squares) mice traveled more distance in 5 min bins during a 90 min period than EGFPNAcInd (light circles) mice (p< 0.0001). (b) D2R-OENAcInd mice traveled a greater total distance compared to EGFP mice (p= 0.0092). Data from 16 animals per genotype was used to calculate all statistics reported.
Discussion:
Here, we examined enhanced D2R expression in ventral striatopallidal neurons and determined if the resulting deficit in ventral striatopallidal synaptic transmission is important for associative reward learning and cognitive function in mice. To determine this, we used a viral approach to selectively over-express D2Rs in ventral striatopallidal neurons and tested mice in an auditory Pavlovian conditioning task followed by a Go/No-Go paradigm. We found that upregulation of D2Rs in ventral striatopallidal neurons does not impair associative reward learning nor did it affect the extinction of response behavior when the Pavlovian cue was no longer paired with a reward. In contrast to our initial hypothesis, decreased function of the ventral striatopallidal pathway did not cause deficits in No-Go learning, however, we did observe a slight delay in the acquisition of Go learning. Lastly, we replicated previous findings that D2R upregulation in ventral striatopallidal neurons enhances locomotion. These data suggest that while D2R upregulation in ventral striatopallidal neurons enhances locomotor activity and the motivation to work for food (Donthamsetti et al., 2018; Gallo et al., 2018) it does not affect reward learning, at least under the conditions tested.
D2R upregulation in ventral striatopallidal neurons does not impair Pavlovian conditioning and extinction learning
Accumulating evidence suggests that dopamine in the NAc core is important for associative reward learning. First, dopamine is released in response to reward predicting cues during a Pavlovian conditioning task (M. R. Bailey et al., 2018; Collins, Aitken, Greenfield, Ostlund, & Wassum, 2016). Single-unit recordings from rat spiny projection neurons (SPNs) further showed that 75% of NAc neurons change their activity in response to reward-predicting cues. Of these neurons, half showed an increase in firing and half a decrease indicating a possible cell-type specific regulation consistent with D1R activation enhancing striatomesencephalic activity and D2R activation inhibiting striatopallidal activity (Day, Wheeler, Roitman, & Carelli, 2006). However, ventral striatopallidal neurons are not always inhibited after reward predicting cues. Pathway specific Ca2+ imaging revealed that reward-predicting cues enhanced, ventral striatopallidal activity in the lateral part of the ventral striatum, whereas it decreased ventral striatopallidal activity in the ventro-medial striatum (Tsutsui-Kimura et al., 2017). This suggests that the regulation of ventral striatopallidal and striatomesencephalic activity in response to cue induced dopamine is more complicated than the dichotomous model would suggest.
Second, inhibition of NAc core projecting DA neurons disrupt Pavlovian reward learning (Heymann et al., 2020). Contrasting this systemic administration of a D2R antagonist was found to enhance approach behavior and promoted learning in rats during an auditory Pavlovian conditioning paradigm (Eyny & Horvitz, 2003). However, the latter finding is difficult to interpret due to the systemic actions of the antagonist. Local infusion of dopamine receptor antagonists into the NAc revealed that D1R antagonism impaired memory consolidation during appetitive Pavlovian learning, whereas D2R antagonism had no effect on learning (Dalley et al., 2005). Similarly, local NAc core infusion of a D1R antagonist have been shown to impair Pavlovian Instrumental Transfer, whereas the infusion of a D2R antagonist had only mild effects (Lex & Hauber, 2008).
Third, animals demonstrate a divergence in approach behavior during Pavlovian learning that may be directed either towards the CS itself (sign-tracking) or the location of the reward delivery (goal-tracking). Dopamine is differentially involved in these approach behaviors; it is necessary for the development and expression of sign-tracking behaviors but only required for the expression of goal-tracking behaviors (Flagel et al., 2011). Furthermore, dopamine D1 and D2 receptors (D1Rs and D2Rs) play a differential role in approach behaviors. Both D2Rs and D1Rs play an important role in sign-tracking behaviors while only the activity of D1Rs is necessary for the development of goal-tracking behaviors (Roughley & Killcross, 2019). Here, our behavioral paradigm limited our analysis to only measure goal-tracking behaviors, which may explain why D2R upregulation did not affect Pavlovian learning. Taken together, these results implicate a role for NAc dopamine in associative reward learning using Pavlovian cues, however, the underlying mechanism seems to involve D1Rs rather than D2Rs. In so far, our results that D2R upregulation in ventral striatopallidal neurons does not affect Pavlovian reward learning is not surprising.
Following the Pavlovian task, we tested D2RNAcInd mice in extinction learning. We rationalized that reward omission during extinction learning should result in a dip in dopamine release in accordance with the reward predicting error model (Bromberg-Martin, Matsumoto, & Hikosaka, 2010). This dip in dopamine should decrease D2R mediated inhibition of ventral striatopallidal neurons, thereby facilitating the learning of avoiding the Pavlovian response (Hikida, Kimura, Wada, Funabiki, & Nakanishi, 2010; Kravitz, Tye, & Kreitzer, 2012). Higher levels of D2Rs may make ventral striatopallidal neurons more responsive to this regulation if under wild-type conditions all receptors are occupied by dopamine at baseline, whereas in the condition of D2R upregulation additional receptors are occupied. However, we found that that both D2R-OENAcInd and EGFPNAcInd control mice attenuated their anticipatory head poking during the presentation of the previously reward predicting cue. Thus, D2R upregulation in ventral striatopallidal neurons neither enhances nor impaired extinction learning.
D2R upregulation in ventral striatopallidal neurons does not affect “No-Go” Learning
D2R-expressing ventral striatopallidal neurons have been proposed to suppress movement or action initiation by gating the output of the basal ganglia via connections through the globus pallidus external (GPe) (Albin, Young, & Penney, 1989). Furthermore, inhibition of these neurons using the Gi-coupled designer receptor hM4DGi enhances response initiation in a progressive hold own task, where the mouse as to hold down a lever for an increasing amount of time in order to obtain a food reward (M.R. Bailey et al., 2015; Carvalho Poyraz et al., 2016). Thus, we hypothesized that upregulation of Gi-coupled D2Rs impairs learning if actions need to be suppressed. However, our Go/No-Go data did not reveal any impairment in No-Go learning and performance. During the Go/No-Go paradigm, both D2R-OENAcInd and control EGFPNAcInd mice improved their performance and plateaued at the same level, demonstrating their ability to withhold responding during No-Go trials. This result shows that D2R upregulation in ventral striatopallidal neurons is not sufficient to disrupt learning when actions need to be withheld.
Prior to Go/No-Go testing, the mice were trained exclusively on Go trials. During 16 days of Go training, the D2R-OENAcInd mice showed a mild impairment during the first 9 days. We believe that this impairment was due to a newly implemented time restriction for lever availability during Go training. During the preceding two days of continuous reinforcement (CRF) training, the lever was extended until the animal pressed the lever. However, during Go training the lever was only available for 5 seconds, and if no press was made the lever would retract and a new trial would start. Previous work from our group showed that D2R-OENAcInd mice are hyperactive in the open field (Gallo et al., 2015; Welch et al., 2019), a finding we replicated here. One possibility is that increased exploration in the operant chamber distracts D2R-OENAcInd mice from lever responding within the 5 sec time limit. Alternatively, D2R-OENAcInd mice have a deficit in recognizing and adapting to the changing circumstance of the 5 sec time limit.
D2R upregulation in ventral striatopallidal neurons induces hyperlocomotion
D2R upregulation in ventral striatopallidal neurons increases locomotion (Gallo et al., 2015; Welch et al., 2019). To confirm that our manipulation was functional, we tested activity of D2R-OENAcInd and EGFPNAcInd control mice in a standard open field box. We replicated hyperactivity in D2R-OENAcInd mice. Furthermore, post-hoc immunohistochemistry staining confirmed that our viral expression was targeted to the ventral striatum. Since D2R-OENAcInd mice showed a bimodal distribution in the open field with 5 mice performing similar to EGFPNAcInd control mice, we re-analyzed the data only using the hyperactive D2R-OENAcInd mice. Still no alteration in behavior was observed in the Pavlovian or No-Go learning conditions (data not shown) but the deficit in the acquisition of the Go component became more significant (RM 2-way ANOVA: F(15,180)=2.774, p=0.0008, interaction between genotype (EGFP and D2ROE) and day). Taken together, these results give us confidence that D2R upregulation was functional as in our previous publication where we established with slice and in vivo electrophysiological measures that D2R upregulation impairs synaptic transmission of ventral striatopallidal neurons collaterals within the NAc and the canonical projections to the ventral pallidum.
We set out to determine how altered D2R levels affects associative and Go/No-Go learning in mice. We found that selective D2R upregulation on ventral striatopallidal neurons does not impair associative reward learning or No-Go learning. In our previous published studies, we found that D2R upregulation enhances the willing ness to work for food if response efforts are high (Donthamsetti et al., 2018; Gallo et al., 2018). In contrast, both the Pavlovian conditioning and the Go/No-Go task do not require much effort to perform the task. One explanation is that the behavioral assays we tested are not sensitive enough to detect any changes in learning and that our D2R-OENAcInd mice could have a deficit in Pavlovian learning with probabilistic outcomes, where task difficulty varies with reward predictability. Together with our previous published findings our data suggest that ventral striatopallidal D2Rs play a specific role in regulating motivation by balancing cost/benefit computations but does not affect associative learning (M. R. Bailey, Chun, Schipani, Balsam, & Simpson, 2020; Gallo et al., 2018; Simpson & Kellendonk, 2017; Ward et al., 2012).
Acknowledgements:
This work has been supported by RO1MH093672 (C.K), RO1MH093672S1 (K.M.), and R01 MH068073 (P.B.).
References:
- Aberman JE, Ward SJ, & Salamone JD (1998). Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav, 61(4), 341–348. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, & Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci, 12(10), 366–375. doi: 0166-2236(89)90074-X [pii] [DOI] [PubMed] [Google Scholar]
- Bailey MR, Chun E, Schipani E, Balsam PD, & Simpson EH (2020). Dissociating the effects of dopamine D2 receptors on effort-based versus value-based decision making using a novel behavioral approach. Behav Neurosci, 134(2), 101–118. doi: 10.1037/bne0000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Goldman O, Bello EP, Chohan MO, Jeong N, Winiger V, . . . Simpson EH (2018). An Interaction between Serotonin Receptor Signaling and Dopamine Enhances Goal-Directed Vigor and Persistence in Mice. J Neurosci, 38(9), 2149–2162. doi: 10.1523/JNEUROSCI.2088-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Jensen G, Taylor K, Mezias C, Williamson C, Silver R, . . . Balsam PD. (2015). Dissecting Goal-Directed Action and Arousal Components of Motivated Behavior. Behav Neurosci, 129(3), 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl), 191(3), 391–431. doi: 10.1007/s00213-006-0578-x [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, & Hikosaka O. (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron, 68(5), 815–834. doi: 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Poyraz F, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA, . . . Kellendonk C. (2016). Decreasing Striatopallidal Pathway Function Enhances Motivation by Energizing the Initiation of Goal-Directed Action. J Neurosci, 36(22), 5988–6001. doi: 10.1523/JNEUROSCI.0444-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, . . . Kellendonk C. (2014). Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron, 81(1), 153–164. doi: 10.1016/j.neuron.2013.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Aitken TJ, Greenfield VY, Ostlund SB, & Wassum KM (2016). Nucleus Accumbens Acetylcholine Receptors Modulate Dopamine and Motivation. Neuropsychopharmacology, 41(12), 2830–2838. doi: 10.1038/npp.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, & Stanford IM (2001). Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology, 41(1), 62–71. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, & Robbins TW (2005). Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A, 102(17), 6189–6194. doi: 10.1073/pnas.0502080102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, & Carelli RM (2007). Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci, 10(8), 1020–1028. doi: 10.1038/nn1923 [DOI] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, & Carelli RM (2006). Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci, 23(5), 1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, & Alvarez VA (2016). Dopamine Regulation of Lateral Inhibition between Striatal Neurons Gates the Stimulant Actions of Cocaine. Neuron, 90(5), 1100–1113. doi: 10.1016/j.neuron.2016.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donthamsetti P, Gallo EF, Buck DC, Stahl EL, Zhu Y, Lane JR, . . . Javitch JA (2018). Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol Psychiatry. doi: 10.1038/s41380-018-0212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyny YS, & Horvitz JC (2003). Opposing roles of D1 and D2 receptors in appetitive conditioning. J Neurosci, 23(5), 1584–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla I, Bailey MR, Schipani E, Winiger V, Mezias C, Balsam PD, & Simpson EH (2018). Striatal dopamine D2 receptors regulate effort but not value-based decision making and alter the dopaminergic encoding of cost. Neuropsychopharmacology, 43(11), 2180–2189. doi: 10.1038/s41386-018-0159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, . . . Akil H. (2011). A selective role for dopamine in stimulus-reward learning. Nature, 469(7328), 53–57. doi: 10.1038/nature09588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floran B, Floran L, Sierra A, & Aceves J. (1997). D2 receptor-mediated inhibition of GABA release by endogenous dopamine in the rat globus pallidus. Neurosci Lett, 237(1), 1–4. [DOI] [PubMed] [Google Scholar]
- Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, . . . Kellendonk, C. (2018). Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun, 9(1), 1086. doi: 10.1038/s41467-018-03272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EF, Salling MC, Feng B, Moron JA, Harrison NL, Javitch JA, & Kellendonk C. (2015). Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology, 40(7), 1609–1618. doi: 10.1038/npp.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, . . . Berke JD (2016). Mesolimbic dopamine signals the value of work. Nat Neurosci, 19(1), 117–126. doi: 10.1038/nn.4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann G, Jo YS, Reichard KL, McFarland N, Chavkin C, Palmiter RD, . . . Zweifel LS (2020). Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement. Neuron, 105(5), 909–920 e905. doi: 10.1016/j.neuron.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, & Nakanishi S. (2010). Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron, 66(6), 896–907. doi: S0896-6273(10)00379-X [pii] 10.1016/j.neuron.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, & Will MJ (2005). Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav, 86(5), 773–795. doi: 10.1016/j.physbeh.2005.08.066 [DOI] [PubMed] [Google Scholar]
- Kohnomi S, Koshikawa N, & Kobayashi M. (2012). D(2)-like dopamine receptors differentially regulate unitary IPSCs depending on presynaptic GABAergic neuron subtypes in rat nucleus accumbens shell. J Neurophysiol, 107(2), 692–703. doi: 10.1152/jn.00281.2011 [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, & Kreitzer AC (2012). Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci, 15(6), 816–818. doi: nn.3100 [pii] 10.1038/nn.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex A, & Hauber W. (2008). Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem, 15(7), 483–491. doi: 10.1101/lm.978708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, & Maidment NT (2011). Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J Neurosci, 31(1), 200–207. doi: 10.1523/JNEUROSCI.4759-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, & Jhou TC (2007). Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl), 191(3), 483–495. doi: 10.1007/s00213-006-0626-6 [DOI] [PubMed] [Google Scholar]
- Roughley S, & Killcross S. (2019). Differential involvement of dopamine receptor subtypes in the acquisition of Pavlovian sign-tracking and goal-tracking responses. Psychopharmacology (Berl), 236(6), 1853–1862. doi: 10.1007/s00213-019-5169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, & Mingote SM (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl), 191(3), 461–482. doi: 10.1007/s00213-006-0668-9 [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. doi: 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- Simpson EH, & Kellendonk C. (2017). Insights About Striatal Circuit Function and Schizophrenia From a Mouse Model of Dopamine D2 Receptor Upregulation. Biol Psychiatry, 81(1), 21–30. doi: 10.1016/j.biopsych.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, & Janak PH (2013). A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci, 16(7), 966–973. doi: 10.1038/nn.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Koos T, Tepper JM, Kabbani N, & Yeckel MF (2009). Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci, 29(28), 8977–8990. doi: 10.1523/JNEUROSCI.6145-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, . . . Javitch JA (2013). Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry, 18(9), 1025–1033. doi: 10.1038/mp.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui-Kimura I, Natsubori A, Mori M, Kobayashi K, Drew MR, de Kerchove d’Exaerde A, . . . Tanaka KF (2017). Distinct Roles of Ventromedial versus Ventrolateral Striatal Medium Spiny Neurons in Reward-Oriented Behavior. Curr Biol, 27(19), 3042–3048 e3044. doi: 10.1016/j.cub.2017.08.061 [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Kuhnen CM, & Phillips PE (2010). Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci, 30(36), 12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, . . . Balsam PD (2012). Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology, 37(7), 1699–1707. doi: 10.1038/npp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AC, Zhang J, Lyu J, McMurray MS, Javitch JA, Kellendonk C, & Dulawa SC (2019). Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol Psychiatry. doi: 10.1038/s41380-019-063 [DOI] [PMC free article] [PubMed] [Google Scholar]