Abstract
Background:
The outbreak of the coronavirus disease-2019 (COVID-19) has become a global public health challenge. Assessing the effect of COVID-19 on liver injury is of great importance. A systematic review and meta-analysis were conducted to establish the characteristics of liver function tests in COVID-19 patients.
Methods:
A systematic search of publications from December 2019 up to April 2020 in Web of Science, Scopus, and Medline (via PubMed) databases was performed. Both cross-sectional and case series studies reporting an association between liver injury and COVID-19 infection were included. The data were analyzed using the STATA software (version 11.0) and the random-effects model for I2>50% was used to pool the results.
Results:
In this meta-analysis, 42 articles comprising a total of 6,557 COVID-19 patients were studied. The prevalence of increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels was 30% and 21% in non-severe patients and 38% and 48% in severe patients, respectively. Patients with severe COVID-19 infection were 4.22, 4.96, and 4.13 times more likely to have elevated AST, ALT, and lactate dehydrogenase (LDH) levels, respectively.
Conclusion:
Elevation in liver function tests was higher in patients with severe than non-severe COVID-19 infection. Given the widespread use of drugs that increases the risk of hepatotoxicity, healthcare providers should be aware of changes in liver enzymes in COVID-19 patients. The inclusion of other studies from outside China could confirm the pattern of elevation in liver function tests in COVID-19 patients across the globe. Preprint of this article is available on medRxiv, https://www.medrxiv.org/content/10.1101/2020.05.20.20108357v1
Keywords: COVID-19, SARS-CoV-2, Liver function tests
What’s Known
Elevated serum levels of aspartate aminotransferase, alanine aminotransferase, total bilirubin, and low albumin levels are observed in patients with severe COVID-19 infection.
In addition to elevated liver enzymes, patients with liver damage have an increased level of biomarkers such as D-dimer, erythrocyte sedimentation rate, C-reactive protein, and lactate dehydrogenase.
What’s New
Elevation in liver function tests was higher in patients with severe than non-severe COVID-19 infection.
Given the widespread use of drugs that increases the risk of hepatotoxicity, healthcare providers should be aware of changes in liver enzymes in COVID-19 patients.
Introduction
Globally, in February 2021, the cumulative number of coronavirus disease-2019 (COVID-19) infections had reached over 102.1 million reported cases. 1 The incidence and mortality rates are still increasing, especially among older adults and patients with comorbidity. Clinical manifestation of COVID-19 varies from asymptomatic or mild symptomatic symptoms to cough, fever, fatigue, gastrointestinal symptoms; shortness of breath and dyspnea, acute respiratory distress, shock, and even the risk of death. 2 - 6 Infected patients may suffer from liver dysfunction characterized by abnormal liver tests, particularly in severe cases. 7 - 11 Therefore, it is extremely important to assess the effect of COVID-19 infection on liver function. 11 - 13 A recent study on the alteration in liver enzyme levels due to COVID-19 infection has indicated that higher levels of aspartate aminotransferase (AST) and direct bilirubin increase the risk of requiring critical care or admission to an intensive care unit (ICU). Elevated AST, alanine aminotransferase (ALT), total bilirubin (TBIL) levels, and low albumin levels have been reported in severe cases. 14 , 15 It is reported that an AST level of 30.5 (U/L) has a sensitivity of 71.4% and specificity of 68.5% for ICU transfer. 16
Previous studies of patients with liver damage reported increased levels of other biomarkers such as D-dimer, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and lactate dehydrogenase (LDH) in addition to abnormal liver enzymes. 17 , 18 This necessitates an additional focus on the assessment of biomarkers related to liver function in COVID-19 patients with varying degrees of liver damage.
Recent studies on liver damage due to the COVID-19 outbreak have not been comprehensive and lack a comparison between the extent of the damage and the severity of the disease. In the present study, we reviewed both research data and experts’ opinions on the correlation between COVID-19 and liver injury. A systematic review and meta-analysis were conducted to establish the characteristics of liver function tests in COVID 19 patients.
Materials and Methods
Search Strategy
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 19 Our systematic search included publications in Web of Science, Scopus, and Medline (via PubMed) databases from December 2019 up to April 2020. Both cross-sectional and case series studies, even those in preprint state, were included. Important references and related reviews of these articles were examined using the Google Scholar web search engine. The search was performed independently by two researchers using medical keywords “2019 novel coronavirus infection” OR “COVID19 OR COVID-19” OR “Coronavirus disease 2019” OR “Coronavirus disease-19” OR “2019-nCoV disease” OR “2019 novel coronavirus disease” OR “2019-nCoV infection” OR “SARS COV-2” OR “SARS-COV-2” in combination with “Liver function” OR “AST” OR “ALT” OR “Liver toxicity” OR “Bilirubin” OR “SGOT” OR “Aspartate transaminase” OR “Alanine transaminase” OR “SGPT” OR “liver” OR “hepat*”. A combination of keywords and free text was used to broaden the search result.
Inclusion Criteria
Observational studies in the English language, as well as articles in other languages with English abstracts, were assessed. Eligible studies were those that assessed the association between serum levels (ALT, AST, albumin, bilirubin, CRP, ESR, D-dimer, LDH) and severe outcomes of COVID-19 infection as the primary outcomes of main interest. Studies that reported medians and interquartile ranges (IQR) for AST, ALT, albumin, and bilirubin levels in both severe and non-severe COVID-19 infected patients were deemed eligible. Patient age was not considered as an exclusion criterion, however, studies on a particular group of individuals with specific conditions such as cirrhosis and tissue graft were excluded. Other publications such as case reports, articles on experts’ opinions, letters to editors, review articles, books, and animal studies were also excluded. Identification of COVID-19 cases was based on the primary definition of the case study. The severity of COVID-19 infection was defined according to treatment guidelines by the Chinese National Institutes of Health. 20 Increased serum levels from laboratory data were defined as stated by the primary study classification.
Study Selection
Duplicated papers were excluded using the EndNote software X8 (Thomson Reuters, Philadelphia, USA). Two researchers (Malekan and Abounori) independently assessed all potential articles against the inclusion and exclusion criteria of our study. Disagreement between the reviewers was resolved in consultation with a third researcher (Mortazavi). Initially, the titles and abstracts of the articles were screened based on our inclusion criteria. Then, the full text of the selected articles was reviewed based on both our inclusion and exclusion criteria to confirm study eligibility.
Quality Assessment
Two authors independently assessed the quality of the selected articles using the modified version of the Newcastle-Ottawa Scale (NOS). 21 The selection was based on comparability and exposure/outcome criteria and articles with a score of seven or higher were considered high-quality articles. The National Institutes of Health (NIH) quality assessment tool was used for case series studies and the articles were scored as acceptable, fair, or poor quality. 22
Data Extraction
Two researchers (Malekan and Abounori) independently extracted the following information from the selected articles: author’s name, publication year, country, type of publication, study design, sample size, patients’ characteristics, number of patients in severe and non-severe groups, number of discharged and admitted patients, number of deaths, and the mean (standard deviation( of laboratory data in severe and non-severe groups. Laboratory data included serum levels of CRP, D-dimer, ESR, LDH, AST, ALT, albumin, and bilirubin. The reported values for increased/decreased indices (CRP, ALT, AST, LDH, and D-dimer) were used as stated in the reference of the selected articles.
Statistical Analysis
The data were analyzed using the STATA software version 11.0 (Stata Corp LLC- United States). The heterogeneity between the articles was examined using I-square (I2) test. The random-effects model for I2>50% was used to pool the results. To identify the source of heterogeneity between the articles, subgroup analysis was performed based on the severity of the disease. Pooled prevalence and odds ratio (OR) were used to assess the outcomes.
Results
Selection Procedures
The search of Web of Science, Scopus, and Medline (via PubMed) databases identified 351 articles. A manual search of their references, using the Google Scholar search engine, yielded 643 additional articles. After excluding 341 duplicate articles, 653 articles were screened, out of which 92 articles were selected for full-text eligibility assessment. After excluding a further 50 articles, 42 studies were finally included in the meta-analysis. The PRISMA flow diagram of the study selection procedure is presented in figure 1.
Figure 1.
PRISMA flowchart depicts the study selection process.
A total of 6,557 confirmed cases of COVID-19 were reported in the 42 selected articles, and all studies were conducted in China (table 1). The overall quality of the included cross-sectional studies was acceptable and of the case series it was good. The quality assessment score of each study is presented in table 2.
Table 1.
Detailed characteristics of the included articles.
| Author | Country | Type of study | Sample size (male/female) | Mean Age | Number of patients with liver toxicity | CRP (mg/l) | Serum levels (ALT: U/L, AST: U/L, Bilirubin: mg/dL, Albumin: g/L) | LDH (U/L) | D-dimer (μg/mL) | ESR (mm/h) | Diagnosis method | Clinical stage of liver enzymes data | Q/A score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al.2 | China | C/S | 41 (30/11) | 49.0 (41.0-58.0) | - | - | ALT: 32.0 (21.0-50.0) | 286.0 (242.0-408.0) | 0.5 (0.3-1.3) | - | RT-PCR | On admission | 8 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: 31.4 (28.9-36.0) | |||||||||||||
| Guan et al.23 | China | C/S | 1,099 (637/459) | 47.0 (35.0-58.0) | - | - | ALT: - | - | - | - | RT-PCR | On admission | 8 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Fu et al.24 | China | C/S | 52 (28/24) | 44.5 (33.0-56.5) | 10 | 8.8 (3.5-21.4) | ALT: 24.0 (15.3-39.0) | 224.0 (200.0-253.0) | 0.7 (0.5-0.8) | - | RT-PCR | On admission | 7 |
| AST: 27.0 (21.2-34.0) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Zhou et al.25 | China | C/S | 191 (119/72) | 56.0 (46.0-67.0) | - | - | ALT: 30.0 (17.0-46.0) | 300.0 (234.0-407.0) | 0.8 (0.4-3.2) | - | RT-PCR | On admission | 8 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: 32.3 (29.1-35.8) | |||||||||||||
| Chen et al.26 | China | Case series | 9 (0/9) | 29.9 | - | 18.61 | ALT: 253.8 | - | - | - | RT-PCR | On admission | 5 |
| AST: 171.0 | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Chen et al.27 | China | C/S | 291 (145/146) | 46.0 (34.0-59.0) | - | 15.6 (4.4-30.3) | ALT: 20.7 (14.9-28.9) | 172.8 (142.6-220.5) | - | 37.0 (21.0-62.0) | RT-PCR | On admission | 8 |
| AST: 24.7 (19.9-31.4) | |||||||||||||
| Bilirubin: 27.0 | |||||||||||||
| Albumin: 37.3 (34.7-40.3) | |||||||||||||
| Chen et al.28 | China | C/S | 274 (171/103) | 62.0 (44.0-70.0) | 13 | 53.4 (18.6-113.0) | ALT: 23.0 (15.0-38.0) | 321.5 (249.8-510.5) | 1.1 (0.5-3.2) | 32.5 (17.3-53.8) | RT-PCR | On admission | 8 |
| AST: 30.0 (22.0-46.0) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: 33.9 (30.3-37.6) | |||||||||||||
| Chen et al.29 | China | C/S | 99 (67/32) | 55.5 (21.0-82.0) | - | 51.4 | ALT: 39.0 (22.0-53.0) | 336.0 (260.0-447.0) | 0.9 (0.5-2.8) | 49.9 | RT-PCR | On admission | 8 |
| AST: 34.0 (26.0-48.0) | |||||||||||||
| Bilirubin: 15.1 | |||||||||||||
| Albumin: 31.6 | |||||||||||||
| Ai et al.30 | China | C/S | 102 (52/50) | 50.4 (1.5-90.0) | - | 28.2 | ALT: 27.8 | 245.4 | - | 33.3 | RT-PCR and CT-scan | On admission | 8 |
| AST: 30.6 | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Xu et al.31 | China | C/S | 355 (162/193) | - | - | ALT: 35.0 (1.0-414.0) | 296.4 | 2.7 (0.1-382.0) | - | RT-PCR | On admission | 8 | |
| AST: 40.76 (10.0-475.0) | |||||||||||||
| Bilirubin: 14.16 (0.7-511.6) | |||||||||||||
| Albumin: 38.5 (18.2-56.1) | |||||||||||||
| Zhang et al.32 | China | C/S | 95 (42/53) | 49.0 (39.0-58.0) | 50 | 25.0 | ALT: - | - | - | - | RT-PCR and abnormal radiologic findings | During admission | 8 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Zhao et al.5 | China | C/S | 75 (33/42) | 47.0 (34.0-55.0) | - | 13.6 (3.8-48.2) | ALT: 23.0 (14.0-43.0) | 233.0 (176.5-313.0) | - | 30.1 (11.5-69.0) | RT-PCR | On admission | 7 |
| AST: 27.0 (21.0-37.0) | |||||||||||||
| Bilirubin: 14.5 (11.1-18.2) | |||||||||||||
| Albumin: - | |||||||||||||
| Zhao et al.33 | China | C/S | 77 (34/43) | 52.0 | 25 | 17.0 (4.6-51.1) | ALT: 28.0 (20.0-46.0) | - | - | - | RT-PCR | On admission | 8 |
| AST: 29.0 (21.0-42.0) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Tian et al.34 | China | C/S | 37 (17/20) | 44.3 | - | ALT: - | - | - | - | RT-PCR | During admission | 7 | |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Wang et al.35 | China | C/S | 339 (166/173) | 71.0 | 96 | 49.6 (18.5-93.2) | ALT: 27.0 (17.0-44.0) | 301.0 (224.0-429.0) | 1.2 (0.6-3.2) | - | RT-PCR | On admission | 8 |
| AST: 32.0 (23.0-46.0) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Wang et al.36 | China | C/S | 11 (10/1) | 58.0 (49.0-72.0) | 6 | 12.1 (6.2-13.7) | ALT: 24.0 (15.9-27.7) | 396.5 (357.6-529.0) | 1.3 (6.7-4.7) | - | RT-PCR | During admission | 6 |
| AST: - | |||||||||||||
| Bilirubin: 15.1 (11.2-20.4) | |||||||||||||
| Albumin: 33.6 (30.5-37.2) | |||||||||||||
| Huang et al.37 | China | C/S | 36 (25/11) | 69.2 | 22 | 106.2 (60.8-225.3) | ALT: 26.0 (18.0-38.0) | 502.5 (410.0-629.0) | 8.6 (2.4-20.0) | - | RT-PCR | On admission | 5 |
| AST: 43.0 (30.0-51.0) | |||||||||||||
| Bilirubin: 11.2 (7.5-19.2) | |||||||||||||
| Albumin: 30.2 | |||||||||||||
| Zhang et al.38 | China | C/S | 82 (54/28) | 72.5 | 64 | 11.7 (63.3-186.6) | ALT: 26.0 (18.5-47.5) | 515.0 (365.0-755.0) | 5.1 (2.2-21.5) | - | RT-PCR | During admission | 6 |
| AST: 72.0 (30.0-71.0) | |||||||||||||
| Bilirubin: 13.6 (10.0-22.9) | |||||||||||||
| Albumin: 33.1 (30.3-36.9) | |||||||||||||
| Zhang et al.39 | China | C/S | 140 (71/69) | 57.0 | - | 34.2 (12.5-67.4) | ALT: - | - | 0.2 (0.1-0.5) | - | RT-PCR | During admission | 8 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Zhang et al.40 | China | C/S | 28 (17/11) | 65.0 | - | - | ALT: - | 262.9 (168.5-508.0) | - | - | RT-PCR | Medical records, NA | 6 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: 31.1 (28.6-34.8) | |||||||||||||
| Zhao et al.41 | China | C/S | 34 (11/8) | 48.0 | - | 26.5 (10.0-127.1) | ALT: 36.4 (11.8-85.0) | 256.9 (150.0-750.0) | - | - | RT-PCR | After admission | 7 |
| AST: 34.9 (17.6-103.8) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Tang et al.42 | China | C/S | 73 (45/28) | 67.0 | 33 | 87.2 (32.6-104.5) | ALT: 34.5 (24.0-61.0) | 483.0 (351.0-602.0) | 0.6 (0.4-3.4) | - | Clinical presentations, CT-scan | On admission | 7 |
| AST: 25.5 (20.0-42.5) | |||||||||||||
| Bilirubin: 9.8 (8.0-14.5) | |||||||||||||
| Albumin: 33.2 (30.8-36.2) | |||||||||||||
| Tang et al.43 | China | C/S | 26 (17/9) | 6.9 | - | - | ALT: - | - | - | - | RT-PCR | Medical records, NA | 6 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Liao et al.44 | China | C/S | 46 (24/22) | - | - | 2.6 (0.8-9.4) | ALT: 17.9 (11.6-32.5) | 195.5 (145.0-240.0) | 0.3 (0.2- 0.4) | - | RT-PCR assay with a cycle threshold value (Ct-value) of less than 37 was defined as positive | On admission | 8 |
| AST: 18.3 (14.5-26.9) | |||||||||||||
| Bilirubin: 8.7 (5.9-14.6) | |||||||||||||
| Albumin: - | |||||||||||||
| Qian et al.45 | China | C/S | 91 (37/54) | 50.0 (36.5-57.0) | - | 6.8 (1.9-15.3) | ALT: 18.0 (13.0-28.0) | - | 0.3 (0.1-0.4) | - | Real-time reverse transcriptase as a primary method of diagnosis, RT-PCR | On admission | 9 |
| AST: 21.0 (17.0-28.0) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: 40.0 (37.8-42.0) | |||||||||||||
| Qiu et al.46 | China | C/S | 104 (49/55) | 43.0 | 5 | 11.7 (3.5-32.7) | ALT: 20.0 (15.0-34.2) | - | 0.5 (0.2-0.7) | - | RT-PCR | NA | 9 |
| AST: 26.0 (20.8-34.1) | |||||||||||||
| Bilirubin: 10.9 (7.5-16.6) | |||||||||||||
| Albumin: 37.3 | |||||||||||||
| Shi et al.47 | China | C/S | 101 (60/41) | - | 18 | 107.9 | ALT: 56.0 | - | - | - | NA (Medical records) | On admission | 7 |
| AST: 116.8 | |||||||||||||
| Bilirubin: 25.0 | |||||||||||||
| Albumin: - | |||||||||||||
| Wan et al.48 | China | C/S | 135 (72/63) | 47.0 (36.0-55.0) | - | 10.5 (2.7-51.2) | ALT: 26.0 (12.9-33.1) | 320.5 (248.5-385.3) | 0.4 (0.2-0.6) | - | RT-PCR | Medical records, NA | 8 |
| AST: 33.4 (27.8-43.7) | |||||||||||||
| Bilirubin: 8.6 (5.9-13.7) | |||||||||||||
| Albumin: 40.5 (37-43.4) | |||||||||||||
| Xu et al.49 | China | C/S | 55 (22/33) | 49.0 (2.0-69.0) | - | - | ALT: - | - | - | - | RT-PCR | Pathology sample from the liver after death | 5 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Wu et al.50 | China | C/S | 80 (39/41) | 46.1 | 3 | 6.6 (5.3-12.3) | ALT: 24.0 (12.0-38.0) | 226.0 (182.0-308.0) | 0.9 (0.4-2.4) | Epidemiological history, clinical manifestations, RT-PCR | Medical records, NA | 8 | |
| AST: 30.0 (19.0-39.0) | |||||||||||||
| Bilirubin: 6.6 (5.4-12.0) | |||||||||||||
| Albumin: 38.3 (37.0-46.2) | |||||||||||||
| Xie et al.51 | China | C/S | 79 (44/35) | 60.0 (48.0-66.0) | 29 | 13.9 (3.1-51.9) | ALT: 34.0 (18.0-67.0) | - | 0.7 (0.3-1.3) | 39.0 (24.0-58.0) | RT-PCR, clinical data | On admission | 8 |
| AST: 30.0 (23.0-50.0) | |||||||||||||
| Bilirubin: 13.6 (8.8-17.6) | |||||||||||||
| Albumin: - | |||||||||||||
| Xu et al.52 | China | C/S | 45 (29/16) | 56.7 | 17 | ALT: 29.0 (20.1-50.0) | 338.0 (248.0-437.9) | - | - | All patients had positive throat swabs of SARS-CoV-2 | On admission | 8 | |
| AST: 27 (22.0-39.5) | |||||||||||||
| Bilirubin: 15.5 (10.5-21.3) | |||||||||||||
| Albumin: 31.6 (30.2-34.5) | |||||||||||||
| Zhou et al.25 | China | C/S | 191 (119/72) | 56.0 (46.0-67.0) | - | ALT: 30.0 (17.0-46.0) | 300.0 (234.0-407.0) | 0.8 (0.4-3.2) | - | Real-time RT-PCR methods | On admission | 7 | |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: 32.3 (29.1-35.8) | |||||||||||||
| Zhou et al.53 | China | C/S | 197 (99/98) | 55.9 | 8 | 55.0 | ALT: 38.4 | 266.2 | 2.3 | - | RT-PCR, CT-scan | On admission | 7 |
| AST: 38.8 | |||||||||||||
| Bilirubin: 16.3 | |||||||||||||
| Albumin: - | |||||||||||||
| Cai et al.54 | China | C/S | 417 (198/219) | 47.0 (34.0-60.0) | 22 | ALT: 21.0 (15.0-31.0) | - | - | - | RT-PCR | On admission | 8 | |
| AST: 26.5 (21.0-35.0) | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Feng et al.17 | China | C/S | 476 (271/205) | 53.0 (40.0-64.0) | - | 18.8 (5.2-57.0) | ALT: - | 259.0 (202.0-356.0) | 0.58 (0.35-1.48) | 48.0 (30.0-80.0) | Real-time RT-PCR, CT-scan | On admission | 8 |
| AST: - | |||||||||||||
| Bilirubin: 10.1 (7.5-14.0) | |||||||||||||
| Albumin: 37.9 (32.8-41.8) | |||||||||||||
| Feng et al.55 | China | C/S | 564 (284/280) | 47.0 (36.0-58.0) | - | ALT: 20.3 (15.0-30.4) | 189.0 (152.0-244.0) | - | - | RT-PCR assay for nasal and pharyngeal swab specimens, CT-scan | On admission | 8 | |
| AST: 24.3 (19.5-31.5) | |||||||||||||
| Bilirubin: 11.9 (8.7-17.6) | |||||||||||||
| Albumin: 39.0 (35.7-42.4) | |||||||||||||
| Fu et al.18 | China | C/S | 50 (27/23) | - | - | - | ALT: - | - | - | - | RT-PCR, CT-scan | NA | 8 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Ji et al.56 | China | C/S | 208 (117/91) | 44.0 | - | ALT: 24.0 (14.0-37.3) | 234.0 (200.0-283.0) | 0.3 (0.2-0.5) | - | RT-PCR | On admission | 8 | |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Han et al.57 | China | C/S | 25 (12/13) | 44.0 (22.0-70.0) | - | - | ALT: - | - | - | - | NA | On admission | 7 |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
| Jiang et al.58 | China | C/S | 55 (27/28) | 45.0 (27.0-60.0) | - | 8.8 (3.5-21.4) | ALT: 21.0 (16.0-48.0) | - | 0.3 (0.2-0.6) | - | RT-PCR | Medical records, NA | 7 |
| AST: 24.0 (20.0-32.0) | |||||||||||||
| Bilirubin: 7.0 (4.0-10.0) | |||||||||||||
| Albumin: 42.0 (39.0-45.0) | |||||||||||||
| Yang et al.59 | China | C/S | 92 | - | 15 | 15.6 (4.4-30.3) | ALT: - | - | - | RT-PCR | Medical records, NA | 8 | |
| AST: - | |||||||||||||
| Bilirubin: - | |||||||||||||
| Albumin: - | |||||||||||||
CRP: C-reactive protein, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, LDH: Lactate dehydrogenase, ESR: Erythrocyte sedimentation rate, Q/A: Quality assessment, RT-PCR: Reverse transcription polymerase chain reaction, CT-scan: Computed tomography scan, C/S: Cross-sectional
Table 2.
Quality assessment scores for the included cross-sectional studies
| Author | Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure | Comparability | Assessment of the outcome | Statistical test | Total |
|---|---|---|---|---|---|---|---|---|
| Huang et al.2 | + | + | - | ++ | ++ | + | + | 8 |
| Guan et al.23 | + | + | - | ++ | ++ | + | + | 8 |
| Fu et al.24 | - | + | - | ++ | ++ | + | + | 7 |
| Zhou et al.25 | + | + | - | ++ | ++ | + | + | 8 |
| Chen et al.26 | + | + | - | ++ | N/A | + | + | 6 |
| Chen et al.27 | + | + | - | ++ | ++ | + | + | 8 |
| Chen et al.28 | + | + | - | ++ | ++ | + | + | 8 |
| Chen et al.29 | + | + | - | ++ | ++ | + | + | 8 |
| Ai et al.30 | + | + | - | ++ | ++ | + | + | 8 |
| Xu et al.31 | + | + | - | ++ | ++ | + | + | 8 |
| Zhang et al.32 | + | + | - | ++ | ++ | + | + | 8 |
| Zhao et al.5 | - | + | - | ++ | ++ | + | + | 7 |
| Zhao et al.33 | + | + | - | ++ | ++ | + | + | 8 |
| Tian et al.34 | - | + | - | ++ | ++ | + | + | 7 |
| Wang et al.35 | - | + | + | ++ | ++ | + | + | 8 |
| Wang et al.36 | - | + | + | ++ | N/A | + | + | 6 |
| Huang et al.37 | - | + | - | ++ | N/A | + | + | 5 |
| Zhang et al.38 | - | + | + | ++ | N/A | + | + | 6 |
| Zhang et al.39 | + | + | - | ++ | ++ | + | + | 8 |
| Zhang et al.40 | + | + | - | ++ | N/A | + | + | 6 |
| Zhao et al.41 | + | + | + | ++ | ++ | + | + | 7 |
| Tang et al.42 | - | + | - | ++ | ++ | + | + | 7 |
| Tang et al.43 | - | + | + | ++ | N/A | + | + | 6 |
| Liao et al.44 | + | + | - | ++ | ++ | + | + | 8 |
| Qian et al.45 | + | + | + | ++ | ++ | + | + | 9 |
| Qiu et al.46 | + | + | + | ++ | ++ | + | + | 9 |
| Shi et al.47 | - | + | - | ++ | ++ | + | + | 7 |
| Wan et al.48 | - | + | + | ++ | ++ | + | + | 8 |
| Xu et al.49 | - | + | - | ++ | N/A | + | + | 5 |
| Wu et al.50 | + | + | - | ++ | ++ | + | + | 8 |
| Xie et al.51 | + | + | - | ++ | ++ | + | + | 8 |
| Xu et al.52 | + | + | - | ++ | ++ | + | + | 8 |
| Zhou et al.25 | - | + | - | ++ | ++ | + | + | 7 |
| Zhou et al.53 | - | + | - | ++ | ++ | + | + | 7 |
| Cai et al.54 | + | + | - | ++ | ++ | + | + | 8 |
| Feng et al.17 | + | + | - | ++ | ++ | + | + | 8 |
| Feng et al.55 | + | + | - | ++ | ++ | + | + | 8 |
| Fu et al.18 | + | + | - | ++ | ++ | + | + | 8 |
| Ji et al.56 | + | + | - | ++ | ++ | + | + | 8 |
| Han et al.57 | - | + | - | ++ | ++ | + | + | 7 |
| Jiang et al.58 | - | + | - | ++ | ++ | + | + | 7 |
| Yang et al.59 | - | + | - | ++ | ++ | ++ | + | 8 |
N/A: Not applicable
Characteristics of the Patients
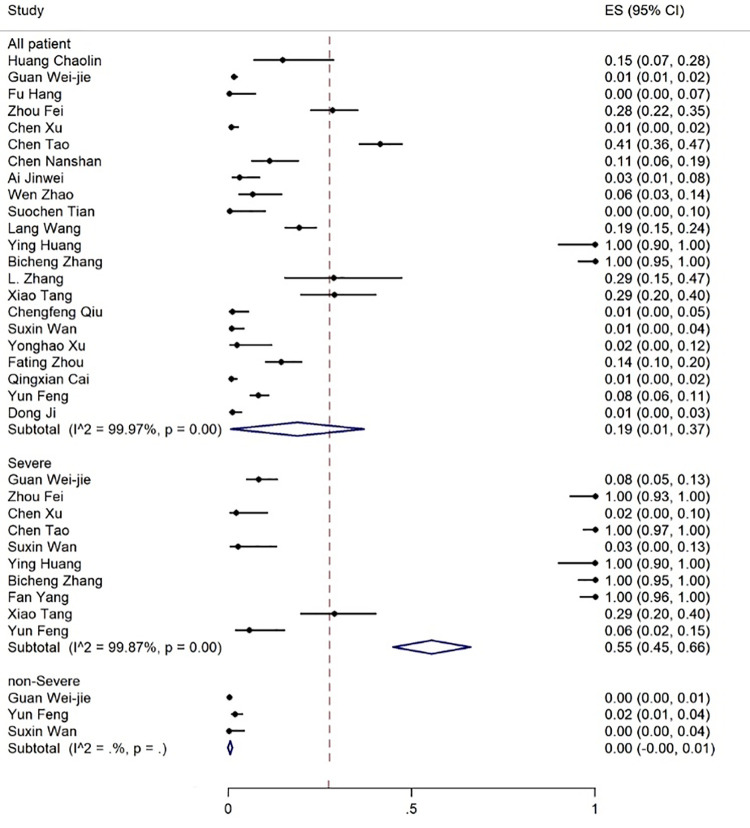
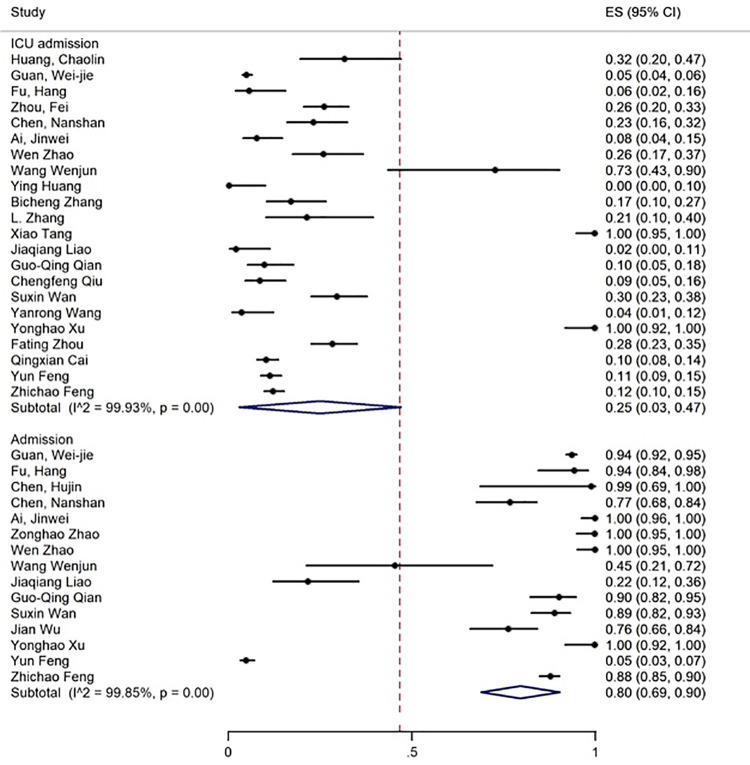
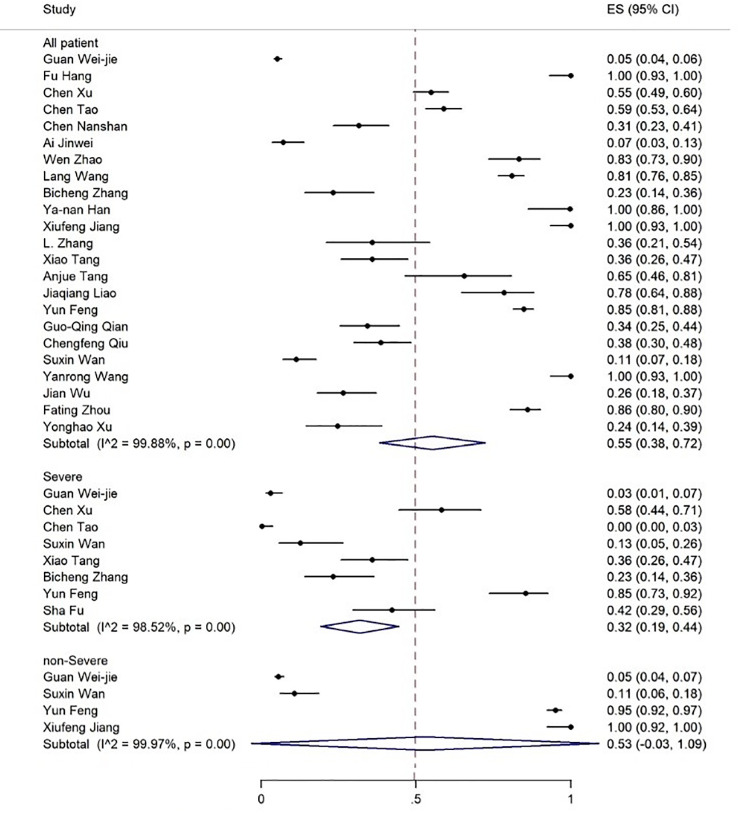
The age range of the patients was 1.5-90 years. The results showed that 19% of the patients had expired (figure 2), 80% required inpatient care services, 25% were admitted to the ICU (figure 3), and about 55% were discharged (figure 4).
Figure 2.
The results of the meta-analysis showing the prevalence of mortality in all patients with COVID-19, categorized by disease severity. ES: Effect size
Figure 3.
The results of meta-analysis showing the prevalence of ICU admission and admission of all patients with COVID-19. ES: Effect size
Figure 4.
The results of meta-analysis showing the prevalence of all discharged patients with COVID-19, categorized by disease severity. ES: Effect size
Laboratory Findings
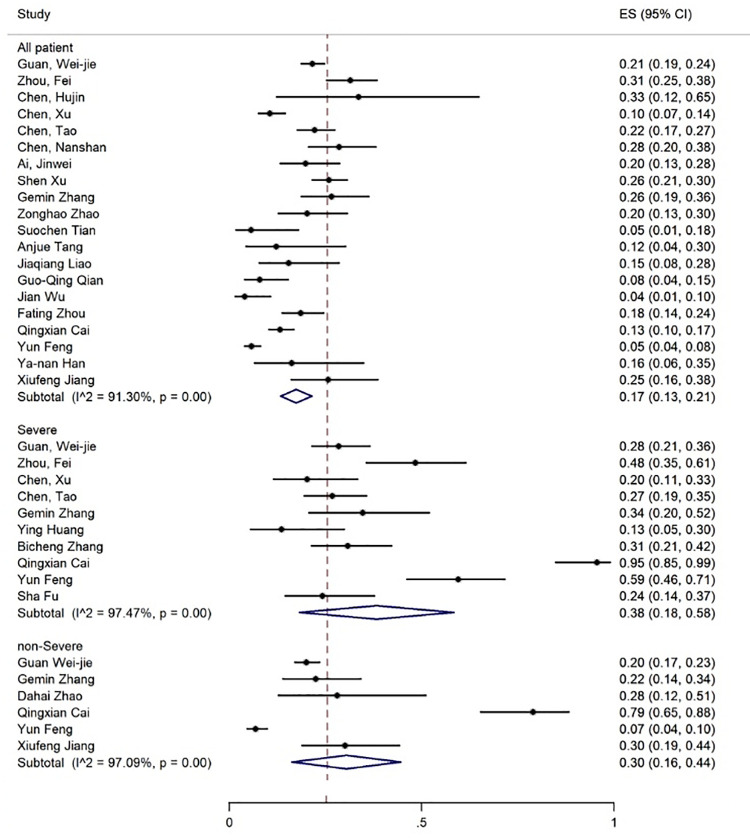
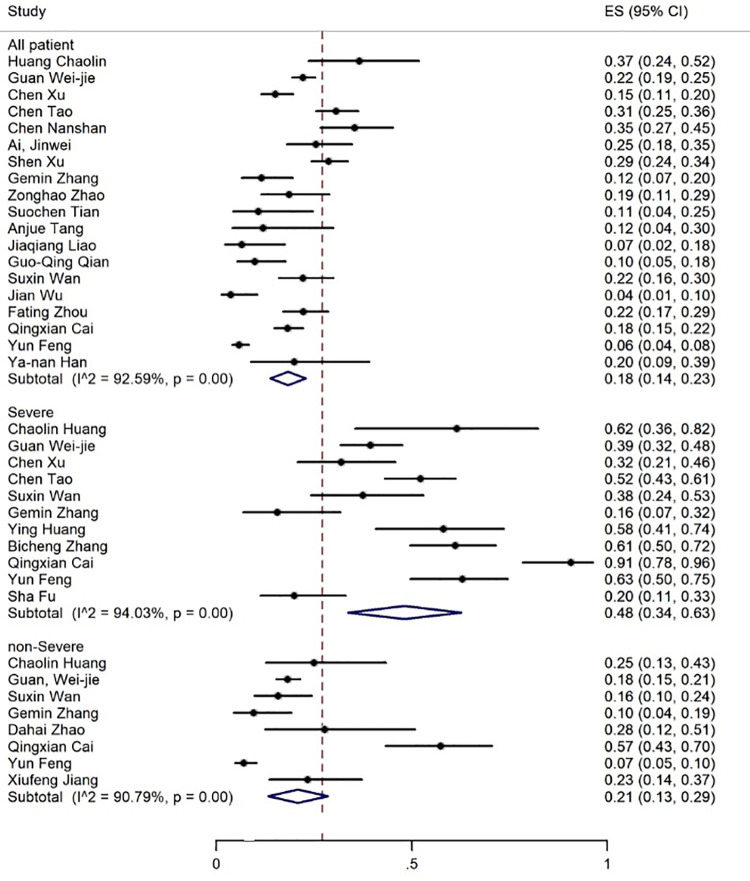
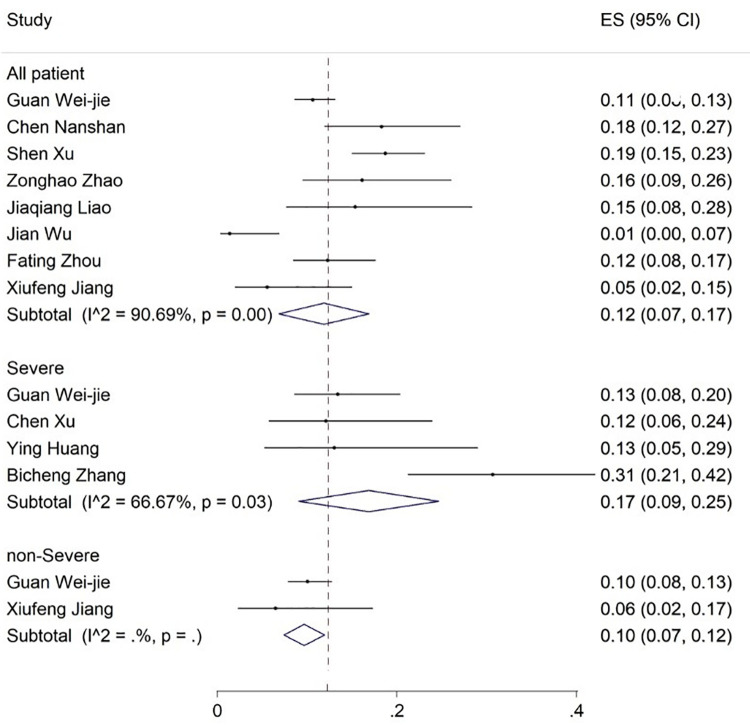
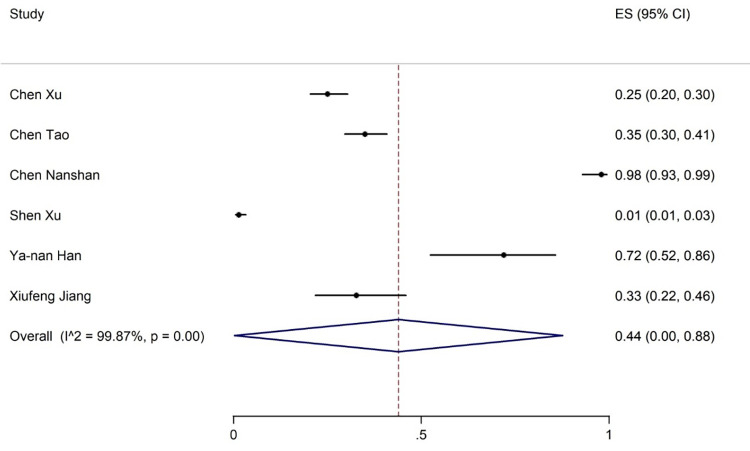
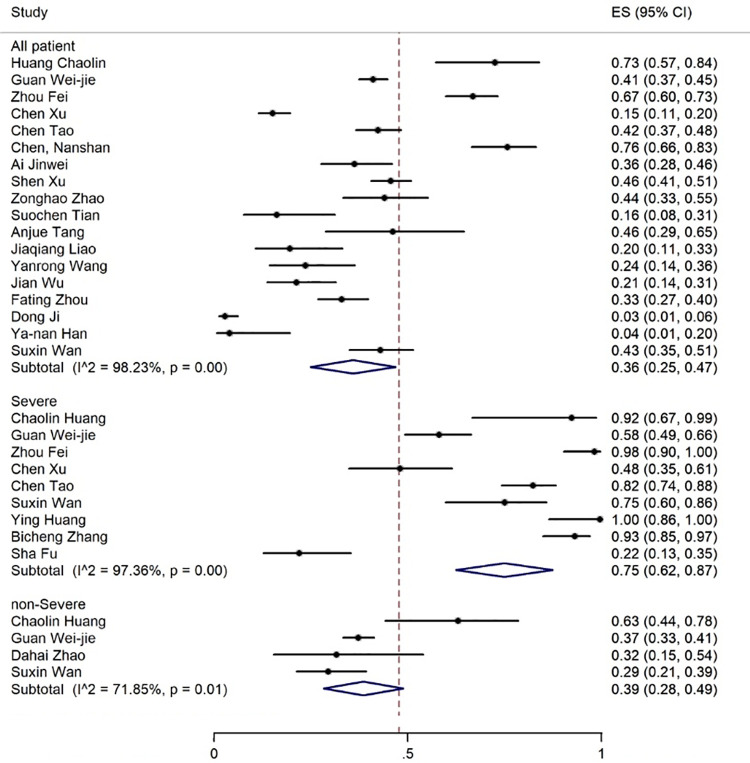
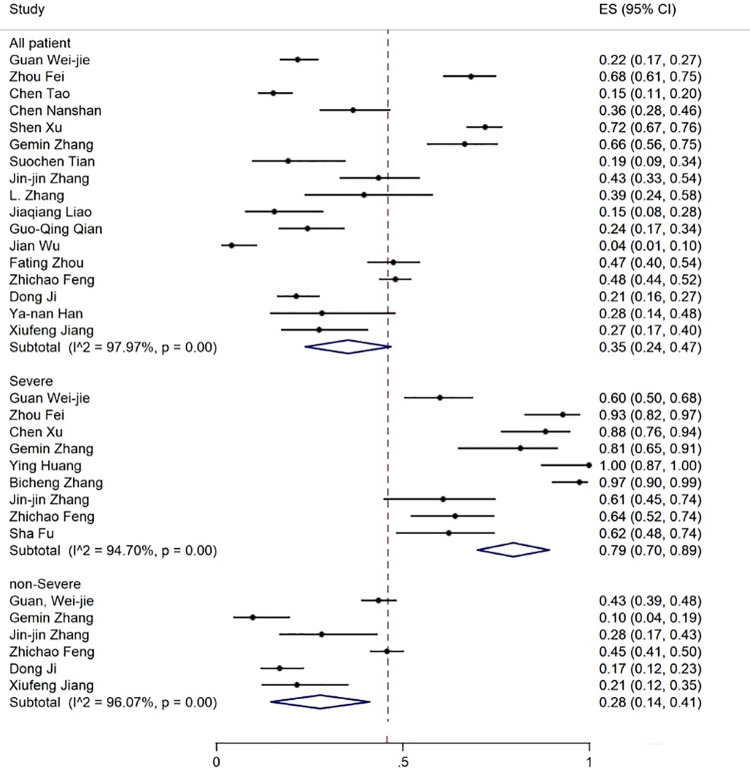
Elevated levels of ALT, AST, and TBIL were reported in 20, 19, and 8 articles with the prevalence rates of 17% (95% CI: 13-21), 18% (95% CI: 14-23), and 12% (95% CI: 7-17), respectively (figures 5-7). Six articles examined reduced albumin levels with a prevalence of 44% (95% CI: 0-88) (figure 8).
Figure 5.
The results of meta-analysis showing the prevalence of elevated ALT levels in all patients with COVID-19, categorized by disease severity. ES: Effect size
Figure 6.
The results of meta-analysis showing the prevalence of elevated AST levels in all patients with COVID-19, categorized by disease severity. ES: Effect size
Figure 7.
The results of meta-analysis showing the prevalence of elevated bilirubin levels in all patients with covid-19, categorized by disease severity. ES: Effect size
Figure 8.
The results of meta-analysis showing the prevalence of decreased albumin levels in all patients with COVID-19. ES: Effect size
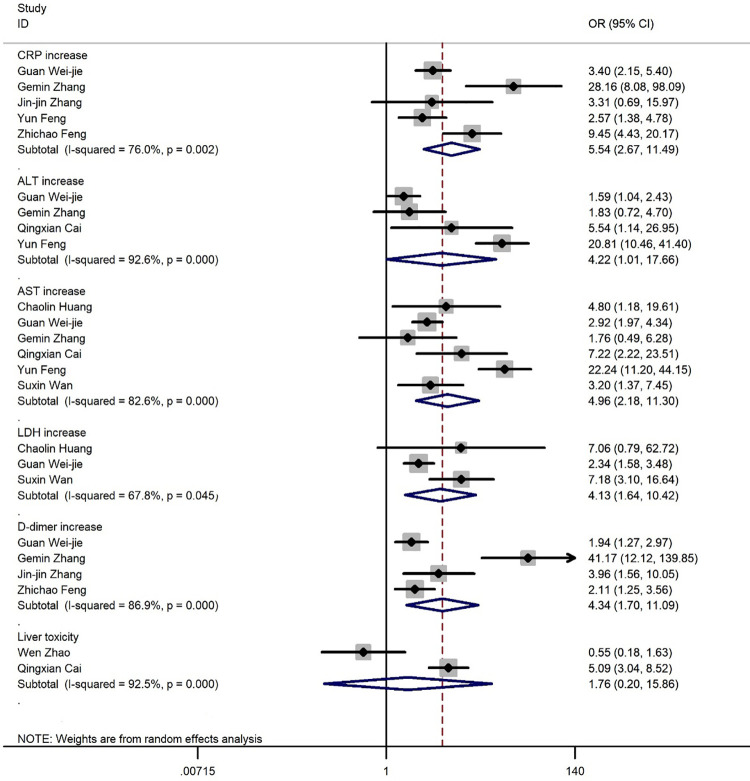
Figure 9 shows the results of risk estimations from the random effects model combining the OR of laboratory findings between severe and non-severe patients. We noted an increase in CRP (OR=5.54, 95% CI: 2.67-11.49, I2=76.0%), ALT (OR=4.22, 95% CI: 1.01-17.66, I2=92.6%), AST (OR=4.96, 95% CI: 2.18-11.3, I2=82.6%), LDH (OR=4.13, 95% CI: 1.64-10.42, I2=67.8%), D-dimer (OR=4.34, 95% CI: 1.7-11.09, I2=86.9%), and Liver toxicity (OR=1.67, 95% CI: 0.20-15.86, I2=92.5%) (figure 10).
Figure 9.
The forest plot depicts the odds ratios of laboratory findings.
Figure 10.
The results of meta-analysis showing the prevalence of liver toxicity levels in all patients with COVID-19, categorized by disease severity. ES: Effect size
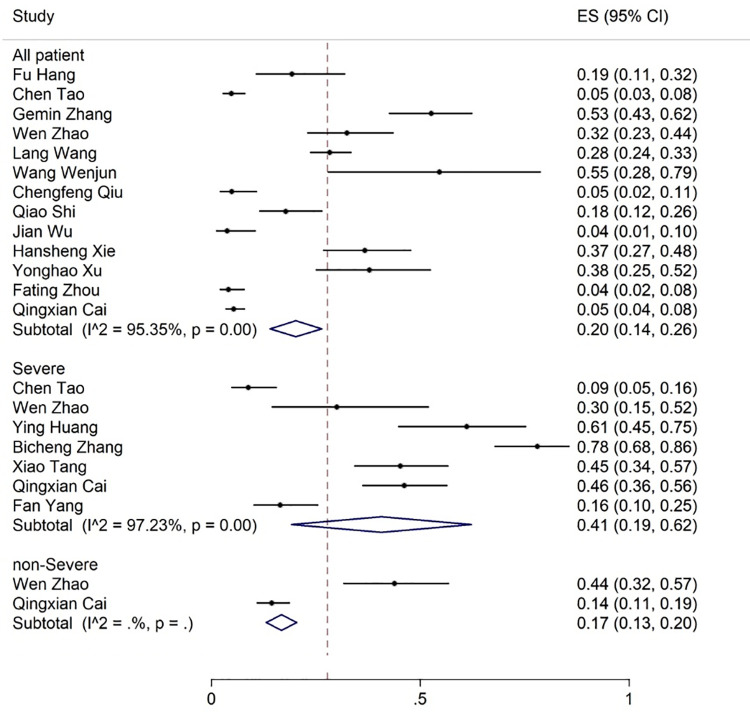
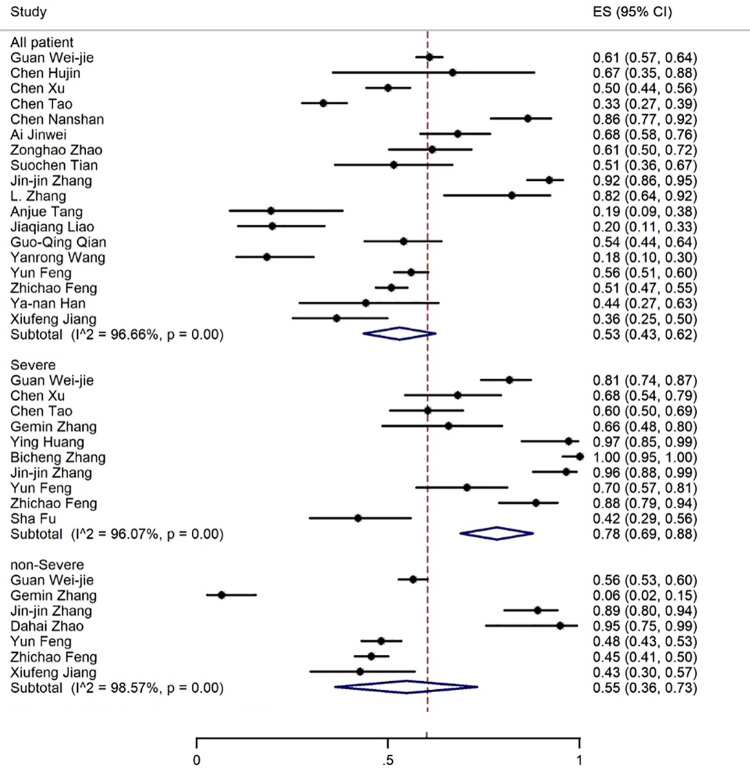
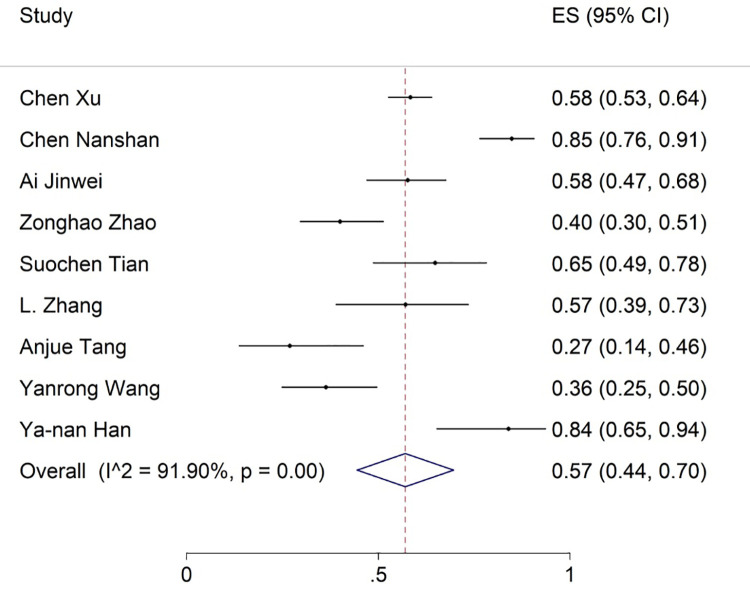
The most common observations from the laboratory findings of COVID-19 patients were increased ESR, CRP, LDH, and D-dimer levels reported in 9, 18, 18, and 17 articles, respectively. The meta-analysis of the results for CRP, LDH, ESR, and D-dimer was 57% (95% CI: 44-70), 53% (95% CI: 43-62), 36% (95% CI: 25-47) and 35% (95% CI: 24-47), respectively (figures 11 -14).
Figure 11.
The results of meta-analysis showing the prevalence of elevated CRP levels in all patients with COVID-19, categorized by disease severity. ES: Effect size
Figure 12.
The results of meta-analysis showing the prevalence of elevated LDH levels in all patients with COVID-19, categorized by disease severity. ES: Effect size
Figure 13.
The results of meta-analysis showing the prevalence of elevated ESR levels in all patients with COVID-19. ES: Effect size
Figure 14.
The results of meta-analysis showing the prevalence of elevated D-dimer levels in all patients with COVID-19, categorized by disease severity. ES: Effect size
Clinical Outcomes based on Disease Severity
The prevalence of mortality in non-severe and severe COVID-19 patients was 0% (95% CI: 0-1) and 55% (95% CI: 45-66), respectively (figure 2, table 3).
Table 3.
A summary of pooled results from the included articles
| Variable | All patients | Severe | Non-severe | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of articles | I- squared | Prevalence % (95% CI) | Number of articles | I- squared | Prevalence % (95% CI) | Number of articles | I- squared | Prevalence % (95% CI) | |
| Clinical outcomes | |||||||||
| Discharged | 23 | 99.88 | 55 (38-72) | 8 | 98.52 | 32 (19-44) | 4 | 99.97 | 53 (-3-109) |
| Death | 22 | 99.97 | 19 (1-37) | 10 | 99.87 | 55 (45-66) | 3 | 43.21 | 0 (0-1) |
| Hospitalization | 15 | 99.85 | 80 (69-90) | - | - | - | - | - | - |
| ICU admission | 22 | 99.93 | 25 (3-47) | - | - | - | - | - | - |
| Laboratory findings | |||||||||
| Increase in ALT | 20 | 91.30 | 17 (13-21) | 10 | 97.47 | 38 (18-58) | 6 | 97.09 | 30 (16-44) |
| Increase in AST | 19 | 92.59 | 18 (14-23) | 11 | 94.03 | 48 (34-63) | 8 | 90.79 | 21 (13-29) |
| Increase in total bilirubin | 8 | 90.69 | 12 (7-17) | 4 | 66.67 | 17 (9-25) | 2 | - | 10 (7-12) |
| Decrease in albumin | 6 | 99.87 | 44 (0-88) | 3 | - | 36 (4-67) | - | - | - |
| Increase in C-Reactive Protein | 18 | 96.66 | 53 (43-62) | 10 | 96.07 | 78 (69-88) | 7 | 98.57 | 55 (36-73) |
| Increase in LDH | 18 | 98.23 | 36 (25-47) | 9 | 97.36 | 75 (62-87) | 4 | 71.85 | 39 (28-49) |
| Increase in ESR | 9 | 91.90 | 57 (44-70) | - | - | - | - | - | - |
| Increase in D-dimer | 17 | 97.97 | 35 (24-47) | 9 | 94.70 | 79 (70-89) | 6 | 96.07 | 28 (14-41) |
| Complication | |||||||||
| Liver toxicity | 13 | 95.35 | 20 (14-26) | 7 | 97.23 | 41 (19-62) | 2 | - | 17 (13-20) |
Laboratory Findings Related to Disease Severity
The prevalence rate of increased ALT and AST levels was 30% and 21% in the non-severe and 38% and 48% in the patients with severe COVID-19 infection, respectively (figures 6 and 7, table 3).
Elevation of CRP, LDH, D-dimer, and bilirubin levels was found in 78%, 75%, 79%, and 17% of the severe cases compared to 55%, 39%, 28%, and 10% of the non-severe cases, respectively. A decrease in albumin levels occurred in 36% of the severe patients (figures 7, 11, 12, 14). Only one article reported a 29% decrease in albumin levels in non-severe patients. 58 Liver toxicity affected 41% of the severe and 17% of the non-severe cases (figure 10, table 3).
Discussion
In a systematic review and meta-analysis, patients with severe and non-severe COVID-19 infection were compared. The results showed elevated ALT, AST, LDH, D-dimer, CRP, and TBIL levels and lower levels of albumin.
Previous studies reported that small amounts of ACE2 receptors are expressed in the human hepatocyte, 60 , 61 indicating an insignificant effect of SARS-CoV-2 infection on liver function in non-severe and mild cases. 17 , 62 Recent studies have reported the incidence of liver damage in severe cases of COVID-19, mostly with elevated levels of ALT, AST, LDH, CRP, D-dimer, TBIL, and low levels of albumin. 8 , 17 , 45 , 62 , 63 It is also reported that higher levels of D-dimer, CRP, and AST are related to the severity of COVID-19 infection. 17 A previous study on deceased cases of COVID-19 with liver abnormalities reported that ALT, AST, and TBIL levels were higher than the normal levels in patients with severe complications. TBIL was also reported to be lower than the upper limit of the normal range. 59 Other studies have reported lower albumin levels in severe cases. 17 , 64 Hypoalbuminemia is mainly due to inadequate nutrition intake and overconsumption of protein during hospitalization. 62 Elevated AST, ALT, and TBIL serum levels and reduced levels of albumin have been observed in severe cases. In a retrospective study, 15 the meta-analysis of AST (95% CI: 5.97 to 11.71, I2=73.4%), ALT (95% CI: 4.77 to 9.93, I2=57.2%), TBIL (95% CI: 1.24 to 3.36, I2=68.8%), and albumin (95% CI: -6.20 to -2.28, I2=95.7%) levels were different compared to our results. In the present study, the meta-analysis of AST (95% CI:14-23, I2=94.03%), ALT (95% CI: 13-21, I2=97.47%), and TBIL (95% CI: 7-17, I2=66.67%), and albumin (95% CI: 0-88, I2=0%) levels in severe cases produced better results (except for albumin) due to the inclusion of a higher number of studies.
Another systematic review and meta-analysis of articles on liver manifestations of COVID-19 reported elevated levels of AST (95% CI: 13.6-16.5) and ALT (95% CI: 13.6-16.4) in 15% of the infected patients. 10 An increase in TBIL levels was also reported in 16.7% of patients (95% CI: 15.0-18.5). Such increases in the upper limit of the normal range were attributed to drug or virus-induced hepatic injury. Moreover, drug-induced hepatotoxicity with remdesivir and favipiravir was also reported. Notably, liver injury due to lopinavir/ritonavir has not been observed in COVID-19 patients. Furthermore, liver injury due to chloroquine and hydroxychloroquine is rarely reported. The main limitation of the above-mentioned study is the absence of a comparison of elevated levels between non-severe and severe cases.
Wu and colleagues reported elevated LDH levels in COVID-19 patients with severe pneumonia. 65 LDH is an essential element in glucose metabolism and its activity is widespread in numerous body tissues, especially in myocardial and liver cells. LDH is released in cells when the cytoplasmic membrane is damaged. 34 In previous studies on SARS and MERS, elevated LDH levels were also observed. Therefore, it can be concluded that LDH can independently act as a risk factor with poor clinical outcomes, which calls for further research. 45 , 49 Increased levels of LDH might be caused by a broader expression of ACE2 receptors in cardiac blood vessels. 60 , 66 It is also attributed to myositis induced by virus infection.(68) Moreover, since ACE2 receptors are present in hepatocytes, LDH levels will increase due to hepatocyte injuries. This clarifies the fact that liver or cardiac damage could occur as a direct effect of SARS-Cov-2 on targeted organs. 34 Therefore, elevated LDH enzymes in severe cases might occur as a result of direct hepatic or extra-hepatic damage.
Our results showed that the likelihood of developing liver toxicity as a complication of COVID-19 was 1.76 times higher in severely infected patients. Liver toxicity (hepatotoxicity) is the leading systemic toxicity of drugs and chemicals that commonly occurs in clinical practice. 67 Many drugs used to treat COVID-19 patients can also damage the liver. For example, lopinavir/ritonavir is associated with a seven-fold increased risk of liver injury and might cause liver damage and adversely affect liver function tests. 63 , 68 Other drugs used in the treatment of COVID-19 patients (e.g., antibiotics, antiviral agents, and steroids) might also potentially result in liver damage. Although these adverse effects require further clarification, 69 more attention should be paid to drug-induced liver damage in hospitalized patients. In general, elevated liver enzymes during hospitalization could be caused by such drugs and the observed abnormalities in liver function tests might be due to sepsis or shock. 54
Like SARS-CoV, SARS-CoV-2 could affect lymphocytes, especially T lymphocytes. 34 Patients with damaged T cells are more vulnerable to infections and are at increased risk for severe illness. We found that CRP levels were higher in severe than non-severe patients and a CRP level >100 mg/dl could be indicative of bacterial infection. CRP level can be used as a prognostic factor, since it may also indicate the risk for other infections (mostly opportunistic infections) that could negatively affect the liver or lead to hepatitis. 69 - 72 On the other hand, cytokine profiles marked by higher concentrations of CRP, ESR, ferritin, and hs-CRP are associated with the severity of COVID-19. As a result, elevated cytokine factors in the blood might suggest pro-inflammatory cytokines (cytokine storm). 18
Sepsis is another severe complication in COVID-19 patients, which is associated with some clinical symptoms and laboratory manifestations. Laboratory data analysis mostly revealed hyperbilirubinemia, acidosis, high lactate, coagulopathy, and thrombocytopenia in COVID-19 patients in the ICU. 73 , 74 As mentioned above, sepsis is one of the causes of liver injury during infection with SARS-Cov-2.
Aberrant coagulation has been suggested in the case of abnormal laboratory findings in severely affected patients. 75 Furthermore, some recent studies have revealed that COVID-19 is associated with disseminated intravascular coagulation (DIC) 76 and subsequent consumption coagulopathy. 77 D-dimer is a fibrin degradation product, which can be significantly elevated in patients with DIC. 78 Significant increase in D-dimer level can also occur in patients with liver cirrhosis and progressively increase as the degree of liver dysfunction becomes more severe. 79 , 80 Overall, higher levels of D-dimer of hepatic or extra-hepatic origin could be used as a prognostic factor for COVID-19. However, it has been reported that individual liver indices such as ALT, AST, TBIL, alkaline phosphatase (ALP), albumin, globulin, international normalized ratio (INR), LDH, and CRP did not have an association with the severity of COVID-19. 62
To sum up, in this systematic review and meta-analysis, we established that there is an interrelationship between the level of abnormality in liver markers and the severity of COVID-19 infection. Elevated levels of ALT, AST, LDH, CRP, D-dimer, TBIL, and lower levels of albumin could be prognostic factors for COVID-19 patients when they occur concomitantly rather than individually.
Although most included articles were of acceptable quality, the main limitation of the present study is the inclusion of studies from China only, which may undermine the generalizability of our findings. It is therefore recommended to include studies from other countries in future research.
Conclusion
Elevation of liver function tests was higher in patients with severe than non-severe cases of COVID-19 infection. Given the widespread use of drugs that increases the risk of hepatotoxicity, healthcare providers should be aware of changes in liver enzymes in COVID-19 patients. The inclusion of other studies from outside China could confirm the pattern of elevated liver function tests in COVID-19 patients across the globe. Further research is recommended to identify the main factors associated with elevated levels of liver enzymes to determine whether the effect is directly or indirectly due to the virus or drug toxicity.
Acknowledgement
The authors would like to express their gratitude for the support from the Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran. The study was approved by the Ethics Committee of the University (code: IR.MAZUMS.REC.1399.049).
Conflict of Interest: None declared.
References
- 1.Organization WH. Coronavirus disease (COVID-2019) situation reports. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhal T. A Review of Coronavirus Disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–6. doi: 10.1007/s12098-020-03263-6. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. 2019-nCoV Pneumonia in a Normal Work Infectious Diseases Hospital Besides Hubei Province, China. SSRN. 2020 doi: 10.2139/ssrn.3542163. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z, Xie J, Yin M, Yang Y, He H, Jin T, et al. Clinical and laboratory profiles of 75 hospitalized patients with novel coronavirus disease 2019 in Hefei, China. MedRxiv. 2020 doi: 10.1101/2020.03.01.20029785. [DOI] [Google Scholar]
- 6.Heydari K, Rismantab S, Shamshirian A, Lotfi P, Shadmehri N, Houshmand P, et al. Clinical and paraclinical characteristics of COVID-19 patients: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.03.26.20044057. [DOI] [Google Scholar]
- 7.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu L, Fei J, Xu S, Xiang H-X, Xiang Y, Tan Z-X, et al. Acute liver injury and its association with death risk of patients with COVID-19: a hospital-based prospective case-cohort study. Medrxiv. 2020 doi: 10.1101/2020.04.02.20050997. [DOI] [Google Scholar]
- 9.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–30. doi: 10.1016/S2468-1253(20)30084-4. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147–R51. doi: 10.1016/j.cub.2017.09.019. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924–35. doi: 10.1111/hepr.13510. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res . 2020;25:30. doi: 10.1186/s40001-020-00432-3. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omrani-Nava V, Maleki I, Ahmadi A, Moosazadeh M, Hedayatizadeh-Omran A, Roozbeh F, et al. Evaluation of hepatic enzymes changes and association with prognosis in COVID-19 patients. Hepatitis Monthly. 2020;20 doi: 10.5812/hepatmon.103179. [DOI] [Google Scholar]
- 17.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med. 2020;201:1380–8. doi: 10.1164/rccm.202002-0445OC. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu S, Fu X, Song Y, Li M, Pan P-h, Tang T, et al. Virologic and clinical characteristics for prognosis of severe COVID-19: a retrospective observational study in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.04.03.20051763. [DOI] [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health NIo. Coronavirus disease 2019 (COVID-19) treatment guidelines. NIH: Bethesda; 2020. [PubMed] [Google Scholar]
- 21.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Newcastle-Ottawa quality assessment scale cohort studies. Ottawa: University of Ottawa; 2014. [Google Scholar]
- 22.Health NIo. Quality Assessment of Case-Control Studies. NIH: Bethesda; 2020. [Google Scholar]
- 23.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu H, Xu H, Zhang N, Xu H, Li Z, Chen H, et al. Association between Clinical, Laboratory and CT Characteristics and RT-PCR Results in the Follow-up of COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.03.19.20038315. [DOI] [Google Scholar]
- 25.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Zheng F, Qing Y, Ding S, Yang D, Lei C, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. MedRxiv. 2020 doi: 10.1101/2020.03.03.20030353. [DOI] [Google Scholar]
- 28.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ai J, Chen J, Wang Y, Liu X, Fan W, Qu G, et al. The cross-sectional study of hospitalized coronavirus disease 2019 patients in Xiangyang, Hubei province. MedRxiv. 2020 doi: 10.1101/2020.02.19.20025023. [DOI] [Google Scholar]
- 31.Xu S, Fu L, Fei J, Xiang H-X, Xiang Y, Tan Z-X, et al. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: a hospital-based retrospective analysis. medRxiv. 2020 doi: 10.1101/2020.03.24.20042408. [DOI] [Google Scholar]
- 32.Zhang F, Weng D, Su Y, Yin C, Shen L, Zhang Y, et al. Therapeutic effect of subcutaneous injection of low dose recombinant human granulocyte-macrophage colony-stimulating factor on pulmonary alveolar proteinosis. Respir Res. 2020;21:1. doi: 10.1186/s12931-019-1261-1. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao W, Yu S, Zha X, Wang N, Pang Q, Li T, et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. MedRxiv. 2020 doi: 10.1101/2020.03.13.20035436. [DOI] [Google Scholar]
- 34.Tian S, Chang Z, Wang Y, Wu M, Zhang W, Zhou G, et al. Clinical Characteristics and Reasons for Differences in Duration From Symptom Onset to Release From Quarantine Among Patients With COVID-19 in Liaocheng, China. Front Med (Lausanne) 2020;7:210. doi: 10.3389/fmed.2020.00210. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–45. doi: 10.1016/j.jinf.2020.03.019. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Liu X, Wu S, Chen S, Li Y, Nong L, et al. Definition and Risks of Cytokine Release Syndrome in 11 Critically Ill COVID-19 Patients With Pneumonia: Analysis of Disease Characteristics. J Infect Dis. 2020;222:1444–51. doi: 10.1093/infdis/jiaa387. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. MedRxiv. 2020 doi: 10.1101/2020.02.27.20029009. [DOI] [Google Scholar]
- 38.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al. A Comparative Study on the Clinical Features of Coronavirus 2019 (COVID-19) Pneumonia With Other Pneumonias. Clin Infect Dis. 2020;71:756–61. doi: 10.1093/cid/ciaa247. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, et al. Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang A, Xu W, Chen P, Li G, Liu Y, Liu L. A retrospective study of the clinical characteristics of COVID-19 infection in 26 children. medRxiv. 2020 doi: 10.1101/2020.03.08.20029710. [DOI] [Google Scholar]
- 44.Liao J, Fan S, Chen J, Wu J, Xu S, Guo Y, et al. Epidemiological and Clinical Characteristics of COVID-19 in Adolescents and Young Adults. Innovation (N Y) 2020;1:100001. doi: 10.1016/j.xinn.2020.04.001. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113:474–81. doi: 10.1093/qjmed/hcaa089. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu C, Deng Z, Xiao Q, Shu Y, Deng Y, Wang H, et al. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. J Med Virol. 2020;92:2027–35. doi: 10.1002/jmv.25975. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Q, Zhao K-L, Yu J, Feng J-R, Zhao K-P, Zhang X-Y, et al. Clinical characteristics of 101 non-surviving hospitalized patients with COVID-19—A single center, retrospective study. MedRxiv. 2020 doi: 10.2139/ssrn.3550014. [DOI] [Google Scholar]
- 48.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71:706–12. doi: 10.1093/cid/ciaa199. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–6. doi: 10.1111/liv.14449. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Xu Z, Liu X, Cai L, Zheng H, Huang Y, et al. Clinical findings in critical ill patients infected with SARS-Cov-2 in Guangdong Province, China: a multi-center, retrospective, observational study. MedRxiv. 2020 doi: 10.1101/2020.03.03.20030668. [DOI] [Google Scholar]
- 53.Zhou F, Yu X, Tong X, Zhang R. Clinical features and outcomes of 197 adult discharged patients with COIVD-19 in Yichang, Hubei. medRxiv. 2020 doi: 10.1101/2020.03.26.20041426. [DOI] [Google Scholar]
- 54.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–74. doi: 10.1016/j.jhep.2020.04.006. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng Z, Li J, Yao S, Yu Q, Zhou W, Mao X, et al. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis. medRxiv. 2020 doi: 10.1101/2020.04.08.20057539. [DOI] [Google Scholar]
- 56.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin Infect Dis. 2020;71:1393–9. doi: 10.1093/cid/ciaa414. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han YN, Feng ZW, Sun LN, Ren XX, Wang H, Xue YM, et al. A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus-infected children and adults. J Med Virol. 2020;92:1596–602. doi: 10.1002/jmv.25835. [DOI] [PubMed] [Google Scholar]
- 58.Jiang X, Tao J, Wu H, Wang Y, Zhao W, Zhou M, et al. Clinical features and management of severe COVID-19: A retrospective study in Wuxi, Jiangsu Province, China. medRxiv. 2020 [Google Scholar]
- 59.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92:2067–73. doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 60.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol . 2004;203:631–7. doi: 10.1002/path.1570. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grace JA, Herath CB, Mak KY, Burrell LM, Angus PW. Update on new aspects of the renin-angiotensin system in liver disease: clinical implications and new therapeutic options. Clin Sci (Lond) 2012;123:225–39. doi: 10.1042/CS20120030. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 63.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. Characteristics of Liver Tests in COVID-19 Patients. Journal of hepatology. 2020 [Google Scholar]
- 64.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, et al. A Tool for Early Prediction of Severe Coronavirus Disease 2019 (COVID-19): A Multicenter Study Using the Risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833–40. doi: 10.1093/cid/ciaa443. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu MY, Yao L, Wang Y, Zhu XY, Wang XF, Tang PJ, et al. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir Res. 2020;21:171. doi: 10.1186/s12931-020-01427-8. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol. 2014;11:413–26. doi: 10.1038/nrcardio.2014.59. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramirez T, Strigun A, Verlohner A, Huener HA, Peter E, Herold M, et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch Toxicol. 2018;92:893–906. doi: 10.1007/s00204-017-2079-6. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meraviglia P, Schiavini M, Castagna A, Vigano P, Bini T, Landonio S, et al. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: evaluation of risk factors for liver enzyme elevation. HIV Med. 2004;5:334–43. doi: 10.1111/j.1468-1293.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu B, Wang Y, Zhao Y, Shi H, Zeng F, Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20:1891–5. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gille-Johnson P, Hansson KE, Gardlund B. Clinical and laboratory variables identifying bacterial infection and bacteraemia in the emergency department. Scand J Infect Dis. 2012;44:745–52. doi: 10.3109/00365548.2012.689846. [DOI] [PubMed] [Google Scholar]
- 71.Cals JW, Schot MJ, de Jong SA, Dinant GJ, Hopstaken RM. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Ann Fam Med. 2010;8:124–33. doi: 10.1370/afm.1090. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khadijah. Analysis of the association opportunistic infections with c-reactive protein focus toxoplasma, cytomegalovirus, rubella, and hepatitis in human immunodeficiency virus. IOP Conference Series: Earth and Environmental Science; 2018 [Google Scholar]
- 73.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321:2003–17. doi: 10.1001/jama.2019.5791. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19) Treasure Island (FL): StatPearls; 2021. [PubMed] [Google Scholar]
- 75.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020;58:1070–6. doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- 77.Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18:167–9. doi: 10.2450/2020.0083-20. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019;94:833–9. doi: 10.1002/ajh.25482. [DOI] [PubMed] [Google Scholar]
- 79.Gram J, Duscha H, Zurborn KH, Bruhn HD. Increased levels of fibrinolysis reaction products (D-dimer) in patients with decompensated alcoholic liver cirrhosis. Scand J Gastroenterol. 1991;26:1173–8. doi: 10.3109/00365529108998610. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Qi X, Li H, Dai J, Deng H, Li J, et al. D-dimer level for predicting the in-hospital mortality in liver cirrhosis: A retrospective study. Exp Ther Med. 2017;13:285–9. doi: 10.3892/etm.2016.3930. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]