Abstract
Background:
Breast cancer is the second most common cancer in women worldwide. Developing drugs increase the radiosensitivity effect of tumoral tissue, while protecting normal tissues has gained much attention. Ginsenoside Rg3, one of the active components of ginseng, has been shown to possess various pharmacological effects and antiproliferation activity on cancer cell lines. In this study, we assessed the anti-cancer effect of co-treatment with ginsenoside 20(S)-Rg3 and curcumin on MDA-MB-231 breast cancer cells with and without radiotherapy.
Methods:
MTT assay was applied using different concentrations of ginsenoside 20(S)-Rg3 (0, 10, 80, 150 µmol/l) and curcumin (0, 10, 30, 50, 90 µg/mL). The inhibitory effect of co-treatment with these herbal drugs with and without 4 Gy radiotherapy on the MDA-MB-231 cell line was examined. Flow cytometry was applied to measure the effect of co-treatment of the drugs on radiation-induced apoptosis. The data were analyzed using ANOVA and Kruskal-Wallis tests. P values<0.05 were considered statistically significant.
Results:
The results of the MTT assay showed that ginsenoside 20(S)-Rg3 and curcumin had an inhibitory effect on the MDA-MB-231 cell line in a concentration-dependent manner. Ginsenoside 20(S)-Rg3 and curcumin inhibited tumor cell development and proliferation at concentrations of 80 µmol/L and 30 μg/mL, respectively, with 50% cell viability (P=0.018, P=0.01, respectively) at 48 hour incubation time.
Conclusion:
Ginsenoside 20(S)-Rg3 and curcumin inhibited MDA-MB-231 cell growth in a dose- and time-dependent manner and increased the radiosensitivity of cancer cells. These herbal drugs can be considered as a radiosensitizer in radiotherapy.
Keywords: Radiotherapy, Curcumin, Ginsenoside Rg3
What’s Known
Ginsenoside 20(S)-Rg3 and curcumin have been shown to inhibit tumor growth and induce apoptosis in cancer cells.
What’s New
For the first time, the effect of co-treatment with ginsenoside 20(S)-Rg3 and curcumin together with radiotherapy was evaluated.
Co-treatment with these herbal drugs and radiotherapy had a synergistic and complementary effect on breast cancer cell apoptosis.
Introduction
Breast cancer is the second most common cancer in women and becoming more prevalent among those younger than 40 years old. 1 The standard treatment modalities are surgery, chemotherapy, radiotherapy, and endocrine therapy, out of which, radiotherapy is one of the main treatments. 2 However, in many cases, it becomes less effective due to cellular resistance against radiation. 3 An adverse effect of irradiation is that, in addition to tumor cells, normal healthy cells are also destroyed. For many years, researchers have studied synthetic and herbal drugs to increase the radiosensitivity of cancer cells while protecting normal healthy cells. 4
The main challenge in cancer treatment is the use of therapeutic agents with the least toxic potential, e.g. herbal plants. 5 One such plant is ginseng, as it contains ginsenoside in its root, known for its significant therapeutic effects in cancer therapy. 5 , 6 It has been reported that ginsenoside 20(S)-Rg3 has anti-tumor activity in gastric carcinoma, melanoma, leukemia, breast, liver, ovarian, and colon cancer. 7 Ginsenoside Rg3 has been shown to induce apoptosis in cancer cells by activating the caspase-3 pathway as well as inhibiting cellular metabolism and tumor growth. 8 - 10 Sin and colleagues showed that ginsenoside Rg3 is an active tumor suppressor. 11 Based on the proteomic analysis, Lee and colleagues reported that ginsenoside 20(S)-Rg3 and curcumin have anti-metastatic properties. 12 Curcumin is a natural component of the rhizome of Curcuma longa and one of the most powerful chemopreventive and anticancer agents. 13 A review study reported that curcumin contains biological and pharmacological properties and exhibits anti-oxidant, anti-inflammatory, immunomodulatory, anti-microbial, anti-ischemic, anti-carcinogenic, hepatoprotective, nephroprotective, hypoglycemic, and anti-rheumatic activities. 14
Considering the above, it is important to look for suitable natural compounds that can be used as sensitizers in combination with ionizing radiation. Hence, the present study assessed the anti-cancer effect of co-treatment with ginsenoside 20(S)-Rg3 and curcumin on MDA-MB-231 breast cancer cell line with and without radiation therapy.
Materials and Methods
The present experimental study was conducted at Tehran University of Medical Sciences (Tehran, Iran) in 2019. The study was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1396.2151).
Preparation of Ginsenoside 20(S)-Rg3 and Curcumin
Ginsenoside 20(S)-Rg3 and curcumin were purchased from Sigma-Aldrich (UK). Solutions of ginsenoside 20(S)-Rg3 and curcumin were prepared by dissolving the compounds in powder form in dimethyl sulfoxide (DMSO). The solutions were diluted using a cell culture medium (RPMI-1640 medium).
Cell Culture
The MDA-MB-231 cell line was obtained from the Pasture Institute (Tehran, Iran) and cultured in RPMI-1640 medium with 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin. The cell line was then incubated in a humidified CO2 incubator at 37 °C. 15 All reagents were purchased from Merck (Germany).
Irradiation
Irradiation was performed at a dose of 4 Gy using a 6MV X-ray beam from a medical linear accelerator with 25×25 cm2 field size and 100 cm source-cell distance at 180 degrees gantry angle.
Cell Viability Assay
3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay was used to determine the effect of the treatment on cell viability. MDA-MB-231 cell lines were cultured in 96-well plates. When the cells became adherent after 24 hours, the cell culture medium in the experimental group was replaced with 100 μL medium containing different concentrations of ginsenoside 20(S)-Rg3 (0, 10, 80, 150 µmol/L) and curcumin (0, 10, 30, 50, 90 µg/mL). Cells in the control group were only treated with the same medium. One hour after the treatment, the cells were radiated and then incubated at 37 °C. After 24, 48, and 72 hours of exposure to ginsenoside 20(S)-Rg3, curcumin, and radiation. The MTT solution (0.5 mg/ml final concentration) was added and incubated for an additional four hours at 37 °C. The liquid was removed, and 100 μL of the MTT solvent was added, and then it was shaken for 10 min. Finally, spectrophotometric absorbance was measured at 570 nm using ELISA reader, and the optical density (OD) for each well was determined.
Apoptosis Assay by Flow Cytometry
Cell lines were seeded in 6-well plates, added with 2 mL medium/well, and cultured overnight. Afterwards, they were treated with 30 µg/mL of curcumin and 80 µmol/L ginsenoside 20(S)-Rg3 for one hour and exposed to 4 Gy X-rays. After 48 hours, flow cytometry was performed with Annexin V-FITC/PI kit (BioLegend, USD), according to the manufacturer’s instructions. The treated cells were rinsed twice with PBS and trypsinized for three min to obtain a single-cell suspension. Trypsinization was stopped by adding the medium. Cells were rinsed twice in pre-cooled PBS and then resuspended in Annexin V Binding Buffer at a concentration of 1×106 cells/mL. Initially, 100 µL of cell suspension was transferred in a 5 mL test tube, and then 5 µL of FITC Annexin V and 10 µL of PI solution were added. Cells were vortexed and incubated for 15 min at 25 °C in the dark. Next, 400 µL of Annexin V binding buffer was added to each tube. Finally, the samples were analyzed with BD FACSCaliburTM (Biosciences, San Jose, CA, USA) using flow cytometry. The experiment was repeated three times. 16
Statistical Analysis
Data were analyzed using IBM SPSS software version 19.0 with the ANOVA and Kruskal-Wallis test. P values<0.05 were considered statistically significant. Graphpad prism 8.3.1 Mac (GraphPad Software Inc., USA) was used to plot the charts.
Results
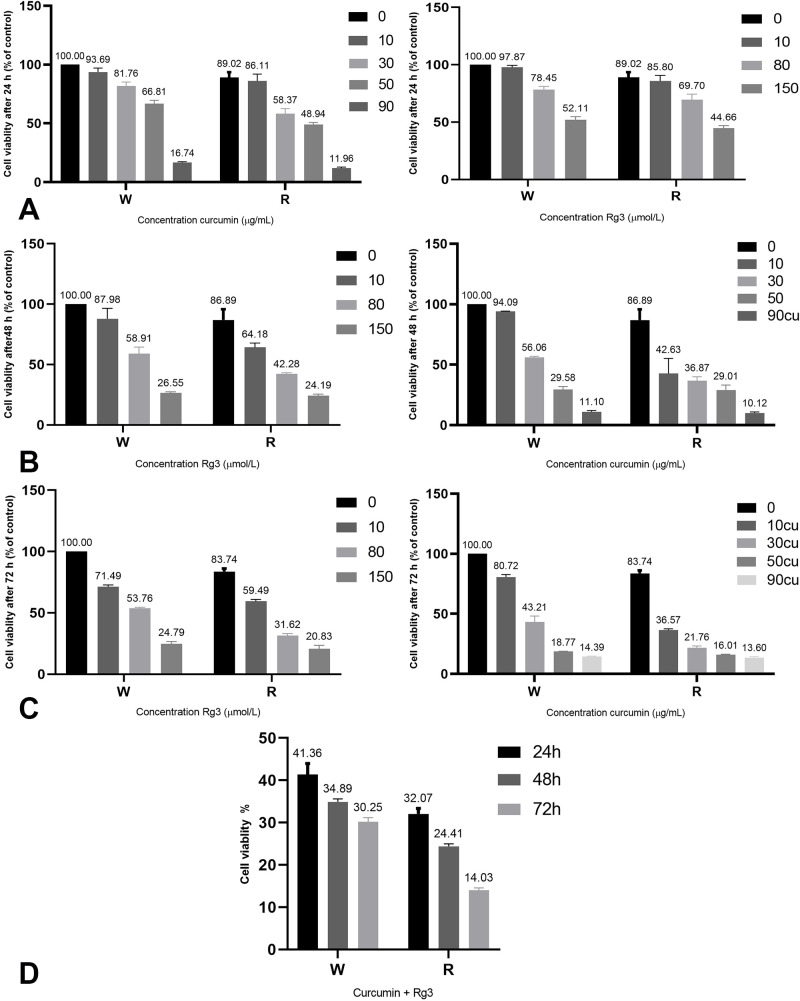
The inhibitory effect of ginsenoside 20(S)-Rg3 and curcumin combined with radiation on cell viability of MDA-MB-231 cells was examined using MTT assay. The results showed that the growth of MDA-MB-231 cells decreased in all groups, however, the decrease in the treated groups was relatively higher (figures 1A, 1B, 1C). The combination of ginsenoside 20(S)-Rg3 and curcumin at concentrations of 80 µmol/L and 30 µg/mL, respectively, resulted in increased cell death (figure 1D). In comparison with the control group, the effect was significant (P<0.05) except for the concentration of 10 µg/mL curcumin without radiation at 24 hours and 48 hours and the concentration of 10 µmol/lLginsenoside 20(S)-Rg3 without radiation at 24 hours (table 1).
Figure 1.
The results of MTT assay showed that various concentrations of ginsenoside 20(S)-Rg3 and curcumin inhibited cell viability of MDA-MB-231 cells in a concentration-dependent manner, with and without 4 Gy radiation, at 24, 48, 72 hour. Effect of ginsenoside 20(S)-Rg3 and curcumin plus radiation on cell death(A): after 24 hour, (B): 48 hour, (C): 72 hour, and (D): combined effect Statistically significant differences were determined using Kruskal-Wallis test. The data were significant with respect to the control group (P<0.05) except for 10 µg/mL curcumin (P=0.520) and 10 µmol/L ginsenoside 20(S)-Rg3 (P=0.513) concentrations. W: Non-irradiated samples, R: Irradiated samples
Table 1.
The effect of different concentrations of ginsenoside 20(S)-Rg3 and curcumin with/without radiation on MDA-MB-231 cell line after 24, 48, and 72 hour
| Treatment duration | Concentration of ginsenoside Rg3 (µmol/L) | P value | Treatment duration | Concentration of ginsenoside Rg3 (µmol/L) | P value |
|---|---|---|---|---|---|
| With radiation | Without radiation | ||||
| 24 hour | 10 | 0.021 | 24 hour | 10 | 0.513 |
| 80 | 0.020 | 80 | 0.030 | ||
| 150 | 0.021 | 150 | 0.040 | ||
| 48 hour | 10 | 0.034 | 48 hour | 10 | 0.038 |
| 80 | 0.021 | 80 | 0.018 | ||
| 150 | 0.021 | 150 | 0.018 | ||
| 72 hour | 10 | 0.020 | 72 hour | 10 | 0.021 |
| 80 | 0.020 | 80 | 0.021 | ||
| 150 | 0.010 | 150 | 0.020 | ||
| Treatment duration | Concentration of curcumin (µg/mL) | P value | Treatment duration | Concentration of curcumin (µg/mL) | P value |
| With radiation | Without radiation | ||||
| 24 hour | 10 | 0.021 | 24 hour | 10 | 0.520 |
| 30 | 0.020 | 30 | 0.040 | ||
| 50 | 0.020 | 50 | 0.030 | ||
| 90 | 0.020 | 90 | 0.030 | ||
| 48 hour | 10 | 0.020 | 48 hour | 10 | 0.237 |
| 30 | 0.021 | 30 | 0.017 | ||
| 50 | 0.020 | 50 | 0.018 | ||
| 90 | 0.030 | 90 | 0.017 | ||
| 72 hour | 10 | 0.019 | 72 hour | 10 | 0.020 |
| 30 | 0.019 | 30 | 0.030 | ||
| 50 | 0.021 | 50 | 0.030 | ||
| 90 | 0.010 | 90 | 0.010 | ||
All the comparisons were made with the control group; Kruskal-Wallis test was done; The significance level was 0.05
The result of flow cytometry showed that ginsenoside 20(S)-Rg3 (80µm/l) plus radiation led to 31.1% apoptosis and curcumin (30µg/mL) plus radiation-induced 51.4% apoptotic cells at 48 hour. Apoptosis in the case of co-treatment with ginsenoside 20(S)-Rg3 and curcumin was 57.8%, while radiation alone induced 12.5% apoptosis (figure 2).
Figure 2.
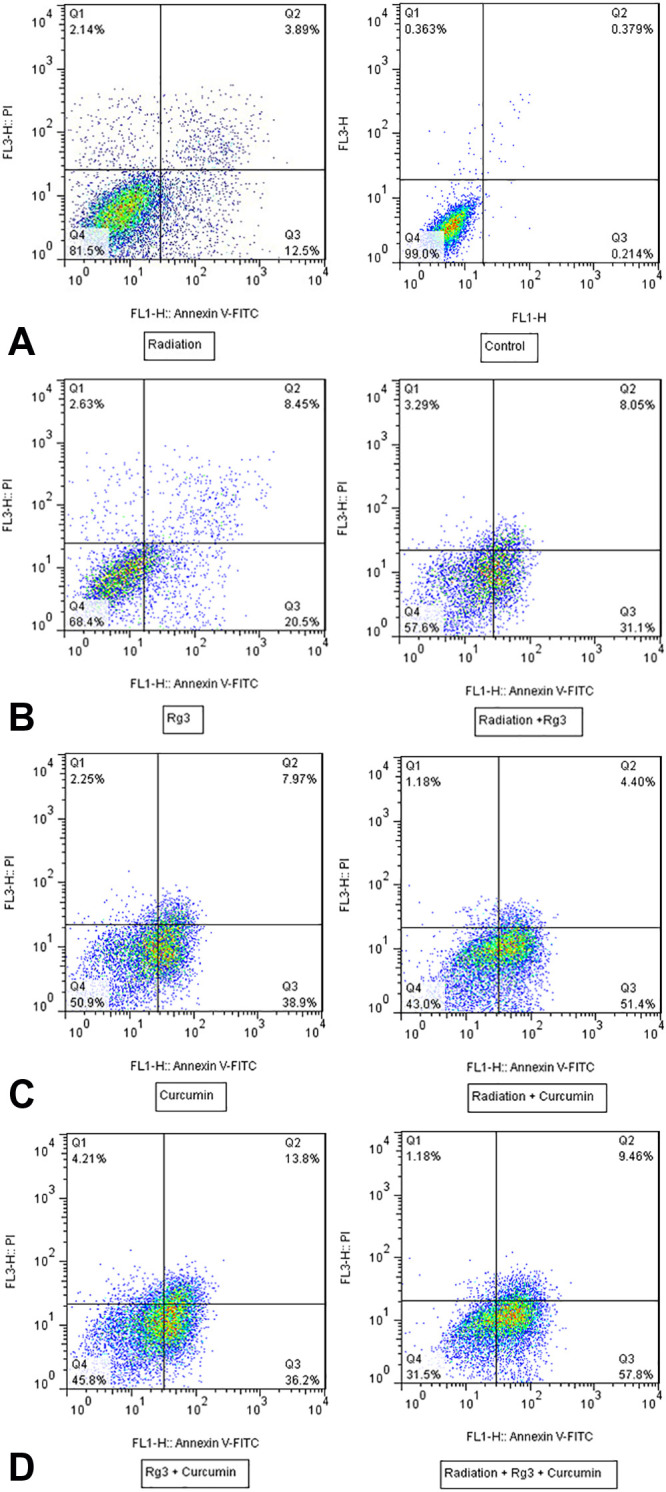
Effects of combined 80 µmol/L ginsenoside 20(S)-Rg3 and 30 µg/mL curcumin with and without radiation (4 Gy) on the apoptosis of MDA-MB 231 cell line. The results showed that ginsenoside 20(S)-Rg3 and curcumin-induced apoptotic cell death in MDA-MB-231 cells. Cells were treated with ginsenoside 20(S)-Rg3 and curcumin plus radiation for 48 hour and apoptosis was determined using Annexin V-FITC flow cytometry assay. Q1, Q2, Q3, and Q4 quadrants represent necrotic, late apoptotic, early apoptotic, and viable cells, respectively.
Discussion
The results of the present study showed that ginsenoside 20(S)-Rg3 and curcumin significantly increased apoptosis in MDA-MB-231 cells exposed to 4 Gy radiation. Various concentrations of ginsenoside 20(S)-Rg3 (0, 10, 80, 150 µmol/L) and curcumin (0, 10, 30, 50, 90 µg/mL) were used in the MTT assay. The results showed that cell viability decreased in a time- and dose-dependent manner. The antiproliferative and inhibitory effects of ginsenoside 20(S)-Rg3 and curcumin against cancer cell lines have been previously assessed and reported in the literature. Peng and colleagues reported that different concentrations of ginsenoside Rg3 (20, 50, 100 µm) had an inhibitory effect against the PC3 cell line through ROS-induced cell cycle arrest. They showed that ginsenoside Rg3 inhibited cell proliferation, and leads to the accumulation of ROS in a dose-dependent manner. 17 The inhibitory effect of ginsenoside Rg3 has been assessed on MDA-MB-231 cell line. Kim and colleagues used different concentrations of ginsenoside Rg3 (10, 20, 30, 50 µm) in the MTT assay, and demonstrated that cell viability decreased in a dose-dependent manner. 18 It has been reported that curcumin at 15 µm concentration induced 65.7% cell viability in MDA-MB-231 cell line after 48 hours incubation. 19
Our results showed that ginsenoside 20(S)-Rg3 and curcumin could induce apoptosis in MDA-MB-231 cancer cell line. Radiation-induced 12.5% apoptosis in MDA-MB-231 cell line, while treatment of cells with 80 µmol/l ginsenoside 20(S)-Rg3, one hour before radiation, increased the rate of apoptosis to 31.1 %. Moreover, treatment of cells with 30 µg/mL curcumin, one hour before radiation, led to 51.4 % apoptosis in MDA-MB-231 cell line. Co-treatment of ginsenoside 20(S)-Rg3 and curcumin caused 57.8% apoptosis in MDA-MB-231 cell line. Previous studies have also confirmed the role of these herbal drugs in enhancing apoptosis in cancer cells. Zhang and colleagues showed that ginsenoside 20(S)-Rg3, the main ingredient of ginsenosides, led to cell apoptosis in gallbladder cancer cells via the p53 pathway. 20 Ginsenoside 20(S)-Rg3 is reported to have strong free radical scavenging properties 21 and has anticancer effects through several different mechanisms. Previous studies have shown that ginsenoside Rg3 induces apoptosis in cancer cells by activating caspase-3 and degrading poly ADP-ribose polymerase (PARP) through the production of reactive oxygen species (ROS). Moreover, ginsenoside 20(S)-Rg3 increases the ratio of pro-apoptotic Bax and anti-apoptotic Bcl-2. It also prevents the binding of NF-κB to DNA. Note that NF-κB is a transcription factor that is constitutively active in breast cancer cells and drives further cell cycle progression, proliferation, and inhibition of apoptosis. 18 , 22 The anti-metastatic effect of ginsenoside 20(S)-Rg3 and curcumin against colon cancer cell lines SW480 and SW620 have been reported in the literature. These components inhibited the migration of cancer cells by suppressing the synthesis of fatty acid and histone H4. 23
In the present study, we demonstrated the anti-tumor effects of ginsenoside 20(S)-Rg3 and curcumin on MDA-MD-231 cell line. Our result showed that these herbal drugs have a radiosensitizer effect on MDA-MB-231 cell line, inhibit cell proliferation, and induce apoptosis. The main limitation of the present study was limited access to components and equipment due to the economic sanctions imposed on Iran.
Conclusion
Ginsenoside 20(S)-Rg3 and curcumin decrease cell viability in MDA-MB-231 cancer cell line. Apoptosis rate significantly increased in cells treated with these herbal drugs compared to radiation alone. Co-treatment with both herbal drugs had better therapeutic outcomes than individual use. These herbal drugs can be considered as a radiosensitizer in radiotherapy. In vivo studies are recommended to examine the simultaneous effect of these herbal drugs in combination with radiation.
Acknowledgement
The present study was supported by Tehran University of Medical Sciences, Tehran, Iran (grant number: 34560).
Conflict of Interest: None declared.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Zhang K, Li Y. Effects of ginsenoside compound K combined with cisplatin on the proliferation, apoptosis and epithelial mesenchymal transition in MCF-7 cells of human breast cancer. Pharm Biol. 2016;54:561–8. doi: 10.3109/13880209.2015.1101142. [DOI] [PubMed] [Google Scholar]
- 3.Bigdeli B, Goliaei B, Masoudi-Khoram N, Jooyan N, Nikoofar A, Rouhani M, et al. Enterolactone: A novel radiosensitizer for human breast cancer cell lines through impaired DNA repair and increased apoptosis. Toxicol Appl Pharmacol. 2016;313:180–94. doi: 10.1016/j.taap.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Kim HG, Jang SS, Lee JS, Kim HS, Son CG. Panax ginseng Meyer prevents radiation-induced liver injury via modulation of oxidative stress and apoptosis. J Ginseng Res. 2017;41:159–68. doi: 10.1016/j.jgr.2016.02.006. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakhjavani M, Hardingham JE, Palethorpe HM, Tomita Y, Smith E, Price TJ, et al. Ginsenoside Rg3: Potential Molecular Targets and Therapeutic Indication in Metastatic Breast Cancer. Medicines (Basel) 2019;6 doi: 10.3390/medicines6010017. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XJ, Zhang XJ, Shui YM, Wan JB, Gao JL. Anticancer Activities of Protopanaxadiol- and Protopanaxatriol-Type Ginsenosides and Their Metabolites. Evid Based Complement Alternat Med. 2016;2016:5738694. doi: 10.1155/2016/5738694. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao Q, Zhang PH, Wang Q, Li SL. Ginsenoside F(2) induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine. 2014;21:515–22. doi: 10.1016/j.phymed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Shim SH, Baek K-H, Kim YS. Inhibition of human 20S proteasome by ginsenosides from Panax ginseng. Bulletin of the Korean Chemical Society. 2009;30:1385–7. doi: 10.5012/bkcs.2009.30.6.1385. [DOI] [Google Scholar]
- 9.Ng WY, Yang MS. Effects of ginsenosides Re and Rg3 on intracellular redox state and cell proliferation in C6 glioma cells. Chin Med. 2008;3:8. doi: 10.1186/1749-8546-3-8. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan X, Aziz F, Tian LL, Wang XQ, Yan Q, Liu JW. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int J Oncol. 2015;46:1667–76. doi: 10.3892/ijo.2015.2886. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sin S, Kim SY, Kim SS. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. Int J Oncol. 2012;41:1669–74. doi: 10.3892/ijo.2012.1604. [DOI] [PubMed] [Google Scholar]
- 12.Lee JG, McKinney KQ, Pavlopoulos AJ, Park JH, Hwang S. Data supporting the identification of anti-metastatic drug and natural compound targets in isogenic colorectal cancer cells. Data Brief. 2014;1:73–5. doi: 10.1016/j.dib.2014.10.005. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng R, Deng Q, Liu Y, Zhao P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/beta-Catenin Signaling Pathway. Med Sci Monit. 2017;23:163–71. doi: 10.12659/msm.902711. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother. 2017;85:102–12. doi: 10.1016/j.biopha.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 15.Wang CZ, Aung HH, Zhang B, Sun S, Li XL, He H, et al. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008;28:2545–51. [ PMC Free Article ] [PMC free article] [PubMed] [Google Scholar]
- 16.Telford W, Tamul K, Bradford J. Measurement and Characterization of Apoptosis by Flow Cytometry. Curr Protoc Cytom. 2016;77:9 49 1–9 28. doi: 10.1002/cpcy.1. [DOI] [PubMed] [Google Scholar]
- 17.Peng Y, Zhang R, Yang X, Zhang Z, Kang N, Bao L, et al. Ginsenoside Rg3 suppresses the proliferation of prostate cancer cell line PC3 through ROS-induced cell cycle arrest. Oncol Lett. 2019;17:1139–45. doi: 10.3892/ol.2018.9691. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BM, Kim DH, Park JH, Na HK, Surh YJ. Ginsenoside Rg3 Induces Apoptosis of Human Breast Cancer (MDA-MB-231) Cells. J Cancer Prev. 2013;18:177–85. doi: 10.15430/jcp.2013.18.2.177. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abuelba H, Cotrutz CE, Stoica BA, Stoica L, Olinici D, Petreus T. In vitro evaluation of curcumin effects on breast adenocarcinoma 2D and 3D cell cultures. Rom J Morphol Embryol. 2015;56:71–6. [PubMed] [Google Scholar]
- 20.Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X, et al. 20(S)-ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Des Devel Ther. 2015;9:3969–87. doi: 10.2147/DDDT.S84527. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YJ, Kim HY, Kang KS, Lee JG, Yokozawa T, Park JH. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg Med Chem Lett. 2008;18:4515–20. doi: 10.1016/j.bmcl.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 22.Kim BM, Kim DH, Park JH, Surh YJ, Na HK. Ginsenoside Rg3 Inhibits Constitutive Activation of NF-kappaB Signaling in Human Breast Cancer (MDA-MB-231) Cells: ERK and Akt as Potential Upstream Targets. J Cancer Prev. 2014;19:23–30. doi: 10.15430/jcp.2014.19.1.23. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JG, McKinney KQ, Pavlopoulos AJ, Park JH, Hwang S. Identification of anti-metastatic drug and natural compound targets in isogenic colorectal cancer cells. J Proteomics. 2015;113:326–36. doi: 10.1016/j.jprot.2014.10.009. [DOI] [PubMed] [Google Scholar]