Abstract
The visual system represents the most well-developed sensory system in humans, who are highly dependent on vision for organized response to their environment. The region of eye that is responsible for sharp central vision is the fovea. Thus, to see the world, images of objects of interest should fall on fovea. This is achieved through various sets of eye movements, all of which work together to keep the image of the target object on the fovea. It is therefore not surprising that a large part of the human brain is devoted to eye movements (e.g., several cortical and subcortical areas, including the brainstem, cerebellum and basal ganglia). Given that a large area of brain is devoted to eye movements, it is not surprising to find eye movement abnormalities in various brain disorders, including movement disorders. In fact, many of the movement disorders commonly encountered in clinical practice are associated with characteristic eye movement abnormalities that not only help in specific diagnosis, but also contribute to morbidity associated with these disorders. In this article, we review the pathophysiology, clinical characteristics, and significance of various eye movement abnormalities in patients with various movement disorders.
Keywords: Eye movements, Parkinsonism, Parkinson's disease, Progressive supranuclear palsy, Multiple system atrophy, Cortico-basal-ganglionic degeneration, Huntington disease, Movement disorders
Highlights
-
•
Abnormalities of eye movements (EMs) are common in patients of movement disorders (MD).
-
•
Pattern of EM abnormalities may help in identifying correct etiology of MDs.
-
•
EM abnormalities contribute to morbidity in MDs and should be addressed appropriately.
1. Introduction
Human beings are adapted to receive and respond to their world through vision. In fact, a large part of our brain is dedicated to sense of vision [1]. It is therefore not surprising that a schematic examination of the visual system often provides clues to the presence and diagnosis of various neurological disorders. In this review, we will study various eye movement abnormalities in patients with movement disorders (MDs) and learn how a schematic examination of the efferent visual system helps in reaching a correct diagnosis in patients with various MDs.
2. Extra ocular movements (EOMs): Basics
The primary aim of EOMs is to focus an image of an object of interest on the fovea, the region responsible for sharp central vision, and keep it steady. This is accomplished through two distinct types of eye movements (EMs), namely gaze holding (involuntary) and gaze shifting (voluntary) EMs. The former system includes five types of EMs (smooth pursuit, vestibular, optokinetic, fixation and vergence), all of which need some kind of stimulus and are involuntary. The latter includes only one type of EMs, namely saccadic eye movements, which are voluntary [2]. While the vergence system keeps targets moving in an anteroposterior direction on the fovea, the smooth pursuit system achieves the same objective for horizontally moving targets. Vestibular and optokinetic systems keep the target on the fovea when the head is in motion. The fixation system helps in suppressing active eye movements during sustained gaze, and the saccadic system helps in focusing a target on the fovea by generating saccades (i.e., sudden jerky eye movements) [1].
The final common system for EMs includes two sets of three cranial nerves (oculomotor, trochlear and abducens), their motor neurons and six extraocular muscles (superior, inferior, medial and lateral recti; superior and inferior obliques), one for each eye. The different pathways for various EM systems finally converge on motor neurons of cranial nerves for producing various EMs [1].
2.1. Human saccadic eye movements
The saccadic EMs help us in exploring our world. These are brief, rapid (400–900°/s), conjugate EMs of ballistic nature that help us focus on objects of interest. Their purpose is to move the eyes as quickly as possible. All voluntary EMs (in the absence of a moving stimulus) occur in form of saccades. They help in redirecting our gaze to an object of interest in response to various stimuli, such as during reading a book. These can be voluntary (e.g., looking intentionally at a photograph on entering a room), reflexive (e.g. looking at a new person entering a room, looking in the direction of a sound, or looking at the site of a tactile stimulus), memory guided (e.g., visual explorations based on memory of locations in space), executed in response to a command (e.g., “look to the left”) or executed as antisaccades (e.g., intentionally looking to the right side when asked to look to left side) [1,2].
Neural pathways for saccades start in the cerebral cortex. Voluntary saccades are generated in the frontal eye fields, while reflexive saccades are generated in the interparietal sulcus. Other cortical areas also play an important role in generating different types of saccades (i.e., supplementary eye fields generate voluntary saccades in the setting of conflicting motor programs; the dorsolateral prefrontal cortex generates predictive saccades and inhibits unwanted reflexive saccades). All these cortical areas project to the superior colliculus (SC) both directly and indirectly (only frontal eye fields) through the basal ganglia. Both SC and frontal eye fields (FEFs) project to the contralateral parapontine reticular formation (PPRF) and the rostral interstitial nucleus of medial longitudinal fasciculus (riMLF). While horizontal saccades are associated with activity in the contralateral FEF and SC, vertical saccades are associated with activity in FEFs and the superior colliculi on both sides [[3], [4], [5]].
Signals for voluntary saccades originate in the above-mentioned higher cortical centers and are relayed to interneurons in the brainstem reticular formation (PPRF: horizontal saccades; riMLF: vertical saccades), which convert these signals into necessary velocity and position signals to motor neurons of cranial nerves. PPRF projects to the ipsilateral abducens nuclear complex (consisting of the abducens nerve nucleus and interneurons), which projects to the ipsilateral six cranial nerve nucleus and via the medial longitudinal fasciculus (MLF) to the contralateral medial rectus. riMLF, on the other hand, projects to motor neurons of cranial nerves destined to supply oblique, superior, and inferior recti on both sides [2,3].
2.1.1. Actual generation of saccades: pulse, step and neural integrators
At the beginning of a saccade, the firing rate of motor neurons of extraocular muscle increases very rapidly. This is called a pulse of activity. A pulse is required to overcome the viscous drag of eyes and move the eyes quickly to the desired position. Once the eyes reach the desired position, the rate of firing of motor neurons decreases to a level sufficient to maintain a steady-state contraction of extraocular muscles so that the eyes are kept steadily in the new position. The difference between initial and final discharge is called step of activity. Thus, discharge of a motor neuron during voluntary saccade is of a pulse-step type. The height of the pulse determines the velocity of saccade, while height of the step determines the amplitude of the saccade. These pulse and step activities are controlled by different neural pathways. The neurons responsible for pulse (velocity command) type of activity are the burst neurons in the brainstem RF (PPRF and riMLF), while step (position command) is mediated though the neural integrator, which includes the cerebellar flocculus, medial vestibular nuclei and nucleus prepositus hypoglossi. Neurons in these regions are tonic neurons and fire steadily to keep the eye in a desired position. To maintain the desired position, neurons of medial vestibular nuclei and nucleus prepositus hypoglossi need to be activated on both sides. A simple lesion in the midline may be enough to disrupt eye position [2,6]. The burst neurons responsible for initiating a saccade are of three types: (i) long lead burst neurons, which receive excitatory inputs from higher cortical areas and transmit signals to (ii) medium lead burst neurons which excite a) motor neurons and interneurons in abducens nuclear complex and b) inhibitory burst neurons and (iii) inhibitory burst neurons (rostral medulla), which inhibit contralateral abducens neurons and excitatory burst neurons [7]. Another class of pontine neurons called omnipause neurons, lying in the dorsal raphe nucleus on the midline, also play an important role in generating a saccade. These are GABA-ergic neurons which fire continuously and project to and continuously inhibit contralateral pontine and mesencephalic burst neurons. The firing in these neurons stops just before initiation of a saccade. Disorders of omnipause cells result in continuous unwanted saccades manifesting as saccadic intrusions, ocular flutter and opsoclonus, etc. [8].
2.1.1.1. Bedside testing of saccades
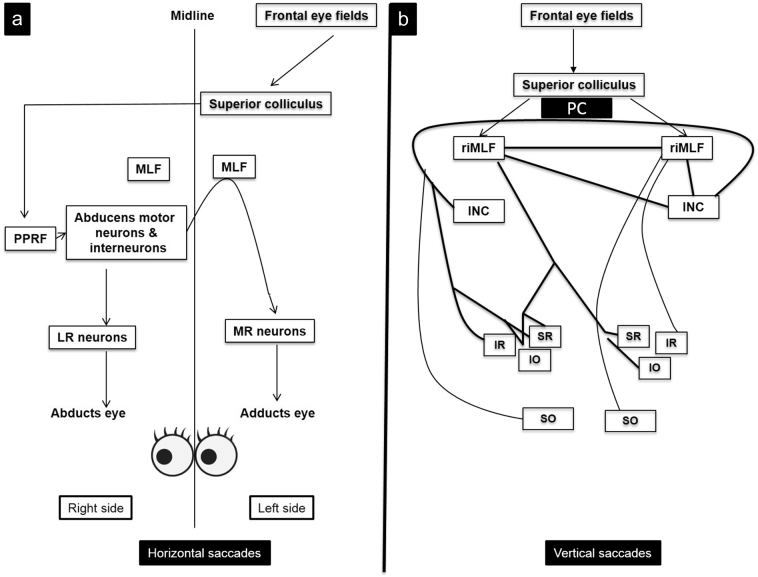
Saccades are tested at bedside by asking a patient to fix alternately on two targets (usually the examiner's finger and a pen) kept 30–40° apart about 40 cm in front of the patient's eyes or by using an optokinetic drum or tape. The saccades are typically examined for latency (i.e., the time between the command and actual initiation of the saccade), velocity (speed of a saccade), antisaccades, accuracy (hypo- or hypermetric) and presence of abnormal, unwanted saccades. Some of the commonly observed abnormalities of saccadic eye movements include micro and macro square wave jerks, opsoclonus, ocular flutter, impaired initiation of saccade, hypometric or hypermetric saccades and abnormal velocity of saccades [9]. The pathways for horizontal and vertical saccades are illustrated in Fig. 1.
Fig. 1.
Pathways for horizontal and vertical saccades. a) Horizontal saccades. To look to the right, a command is sent by frontal eye fields to the contralateral PPRF (parapontine reticular formation) via the superior colliculus. Burst neurons in the PPRF send an excitatory signal to the lateral rectus (LR) motor neurons in the abducens nuclear complex and to medial rectus (MR) motor neurons in the 3rd nerve nuclear complex via the contralateral medial longitudinal fasciculus (MLF). These signals result in simultaneous abduction of the right eye and adduction of the left eye. b) Vertical saccades. Pathways for vertical saccades are bit more complex. Each superior colliculus projects to both rostral interstitial nuclei of MLF (riMLF), which contain burst neurons for generating vertical and torsional saccades. Each riMLF projects to motor neurons of elevator muscles (SR: superior rectus; IO: inferior oblique) bilaterally, but only ipsilaterally to motor neurons of depressor muscles (SO: superior oblique; IR: inferior rectus). INC (interstitial nucleus of Cajal) projects to motor neurons of the contralateral cranial nerves and contralateral INC via posterior commissure (PC).
2.2. Smooth pursuit eye movements
Pursuit EMs are used to keep an image of a moving object on the fovea, such as while visualizing a moving airplane in sky. This system can follow objects moving at a speed of up to 40°/s. Objects moving at higher speeds elicit saccades [1].
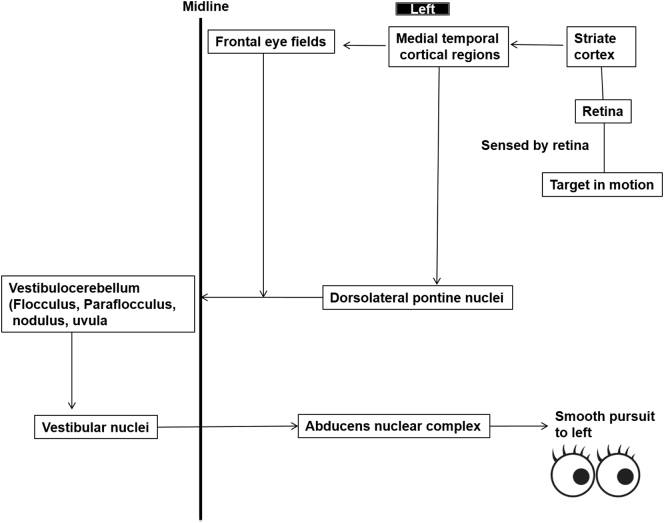
The primary aim of smooth pursuit ocular movements is to match the velocity of eye movement to that of target. Visual motion is first sensed by the medial temporal, medial superior temporal and superior temporal sulcal cortical areas. It is in these areas that velocity of moving targets is assessed. However, medial temporal areas cannot initiate smooth pursuit by themselves and pass this information to FEFs. Both medial temporal areas and FEFs relay this information to the dorsolateral nuclei of the pons, which in turn pass information to the vestibulocerebellum (vermis and flocculus) after decussation. The vestibulocerebellum calculates required eye movement velocity and relays this information to vestibular nuclei, which pass it to ocular motor nuclei after another decussation (double decussation). Thus, cortical areas control smooth pursuit movement to the ipsilateral side. While flocculus helps to track objects moving with constant velocity, vermis helps to track objects whose velocity is changing. Fibers for vertical pursuit follow the same course until vestibular nuclei from where these project to riMLF. Fibers for upward pursuit decussate in the posterior commissure, while those for downward pursuit pass ventral to the cerebral aqueduct. Thus, posterior commissure lesions selectively impair upward gaze [[10], [11], [12]]. Pathways for horizontal smooth pursuit movement to the left side are shown in Fig. 2.
Fig. 2.
Schematic representation of pathways of smooth pursuit eye movements to left side.
(Adapted and modified from Vinny and Lal [1].)
2.2.1. Testing for smooth pursuit movements at bedside
Smooth pursuit movements are tested at bedside by asking the patient to follow the examiner's finger or a pencil which is held at a distance of approximately 1 m. The patient should be able to fixate on the target when instructed not to move his or her head. Smooth pursuit movements are observed for velocity, direction, amplitude and smoothness [1].
2.3. Vestibular and optokinetic systems: Control of gaze during combined head and eye movements
Most of the time, while looking around, our head also moves along with eyes. To keep the target on the fovea, head and eye movements should be coordinated. Owing to the high inertia of the head, head movements lag behind those of the eyes and disrupt the ocular fixation unless a corrective mechanism exists. This correction is achieved through compensatory eye movements (i.e., the vestibule-ocular reflex) that are equal in amplitude but opposite in direction (horizontal, vertical or torsional) to the head movements. The vestibule-ocular reflex (VOR) is mediated through the labyrinth, vestibular nuclei and cerebellar flocculonodular lobe [1].
VOR plays an important role in small amplitude saccades. During small saccades, the eyes move first and fixate on the target while the head moves later (owing to its large inertia) and thus compensation must be provided by VOR to keep the object on the fovea. However, for larger saccades, the head and eyes move simultaneously in the same direction and VOR needs to be suppressed.
2.3.1. Testing for VOR
VOR is tested using the head impulse test. For this test, the patient's head is held by the examiner while the patient is asked to fixate on a target held in front of the patient's eyes. Then the patient's head is rotated horizontally by 20–30°. In healthy subjects, you can observe conjugate eye movements that are opposite in direction to the head movement. Any abnormality in conjugate eye movements, including refixation saccades, suggests abnormality in VOR.
The ability to suppress VOR is tested by asking a patient who is seated in a wheelchair to look at the extended thumbs of their outstretched arms while the wheelchair is being rotated. Any refixation saccades suggest impaired ability to suppress VOR [13].
2.4. Optokinetic reflex (OKR)
OKR, defined as a rhythmic oculomotor response to consistently moving visual scenes, complements VOR at low frequencies, where VOR is less efficient. Also, while VOR is stimulated by acceleration, OKR is stimulated by constant velocity. OKR requires intact smooth pursuit as well as saccadic systems and is thus quite sensitive to abnormalities of the ocular motor system, observed in the form of optokinetic nystagmus (OKN).
OKN is a normal response and is stimulated by constantly moving visual scenes, such as experienced during looking at trees while sitting in a moving train. As one looks at a tree, the eyes follow the tree though smooth pursuit movement until it disappears out of gaze. Then a corrective saccade directs the gaze to the next tree, which is followed by smooth pursuit until this tree also disappears out of gaze and a volitional saccade follows. The cycle repeats again and again, giving rise to a nystagmus (OKN) that is directed way from the direction of movement of the trees.
Pathways for OKN involve both direct retinal and indirect cortical (visual striate cortex and medial superior temporal cortex) inputs to the accessory optic tract, which has several nuclei in the pretectal region. One of these, the nucleus of optic tract (NOT), mediates horizontal OKR while another nucleus, called the lateral terminal nucleus, mediates vertical OKR. NOT projects to vestibular nuclei via the inferior olive and to the flocculus of cerebellum.
2.4.1. Testing for OKR
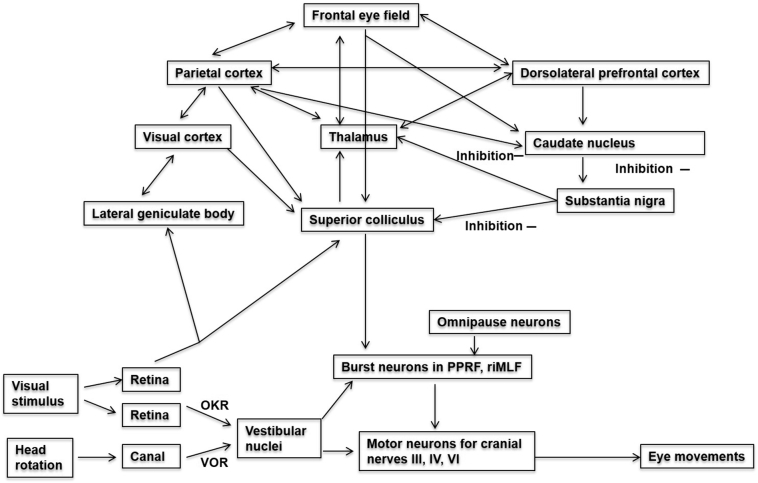
This is tested at bedside using an optokinetic drum or tape with alternating white and black strips (Fig. 3). The patient is instructed to look at the white strips while the drum is rotated horizontally or vertically. While the drum is being rotated (say, horizontally clockwise as seen by the examiner), the patient's eyes will follow the white strip to the left till it disappears, followed by a corrective saccade to the right to refixate on the new strip. This results in nystagmus with a fast component to the right. The test should be repeated by moving the drum vertically (both upwards and downwards) and horizontally in an anticlockwise direction. Observe the patient for direction and symmetry of the response [1,14].
Fig. 3.
Schematic representation of various eye movements. Adapted and modified from Hikosaka et al. [50] (Abbreviations: OKR: optokinetic reflex; VOR: vestibule-ocular reflex; PPRF: parapontine reticular formation; riMLF: rostral interstitial nucleus of medial longitudinal fasciculus).
2.5. Fixation system
This system is used to keep the gaze fixed on the object of interest. Rostral SC plays an important role in fixation of gaze. Its neurons receive signals from the retina as well as from occipital lobes and discharge strongly during visual fixation. These neurons inhibit movement-related neurons in the caudal SC and excite omnipause neurons in the nucleus of dorsal raphe. The result of this is inhibition of unwanted saccades, which may interrupt active visual fixation.
2.5.1. Testing for fixation
Here the patient is asked to fixate on an object kept at 1 m in front of the eyes. The eyes are observed carefully for abnormal ocular movements such as square wave jerks (i.e., small amplitude saccades with an intersaccadic interval), ocular flutter (i.e., horizontal saccades without intersaccadic interval) and opsoclonus (i.e., chaotic saccades in all directions without intersaccadic intervals), as well as other ocular oscillations including nystagmus [1,12].
2.6. Vergence
This system is used during depth tracking of an object, such as when a football player watches a ball coming toward him. It originates in the parietal, frontal and occipital regions.
2.6.1. Testing for vergence
Vergence is tested at bedside by asking the patient to fixate on a pencil held at 1 m which is then slowly moved toward his or her nose. The point of maximum convergence is where both eyes lose convergence and move outwards. This distance is usually 8–10 cm [1,3].
The pathways for various eye movements are illustrated in detail in Fig. 4.
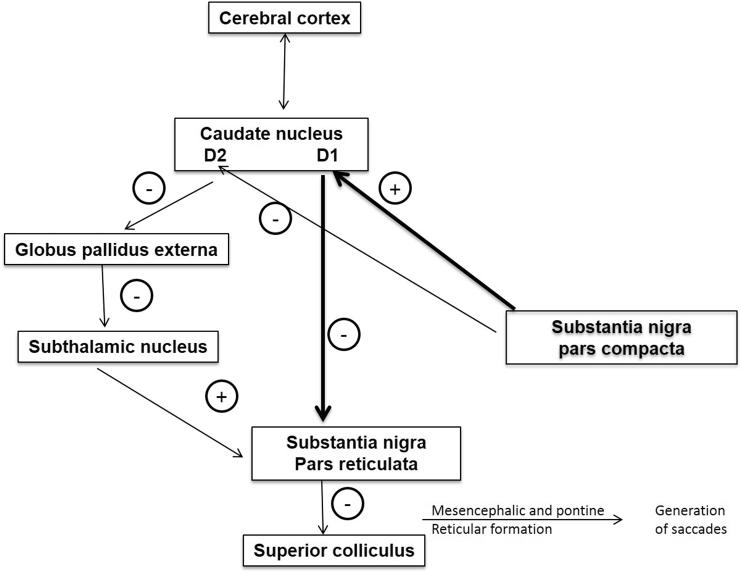
Fig. 4.
Pathophysiological basis of eye movement abnormalities in Parkinson's disease. In direct pathway (thick black arrows), SNpc (substantia nigra pars compacta) activates D1 neurons in striatum that have an inhibitory influence on the substantia nigra pars reticulata (SNpr). Inhibition of SNpr disinhibits superior colliculus, which is then able to generate saccades. In the indirect pathway (thin black arrows), SNpc inhibits D2 receptors in the caudate, which in turn disinhibits globus pallidus extra leading to inhibition of subthalamic nucleus. Inhibition of subthalamic nucleus leads to decreased activity in SNpr with resultant disinhibition of superior colliculus, allowing generation of saccadic eye movements. Dopaminergic depletion in SNpc in Parkinson's disease leads to decreased direct pathway inhibition and increased indirect pathway excitation of SNpr, with resultant inhibition of saccade generation in the superior colliculus resulting in poor generation of saccades.
(Adapted and modified from Hikosaka et al. [50].)
3. Role of superior colliculus, cortex, cerebellum and basal ganglia in eye movements
3.1. Superior colliculus
The SC represents the main station for generation of saccades. While its caudal regions play an important role in generating saccades, its rostral regions are important for visual fixation. The SC integrates visual (from the retina, striate and peri-striate cortices) and motor information (from FEFs) into signals for ocular motor neurons. Individual movement related neurons in SC discharge before saccades of specific amplitudes and direction [[15], [16], [17]]. Thus, SC is the principal structure sending commands to the brainstem saccade generator.
3.2. Cortical control of eye movements
Many different cortical areas are involved in control of EMs. Each area controls several different types of EMs. FEFs, for example, not only control saccades but also play an important role in fixation, pursuit and vergence EMs. While FEFs control visually guided saccades, memory guided saccades, vergence and fixation movements, supplementary eye fields mediate learned complex EMs [18]. Dorsolateral prefrontal cortices play a role in planning saccades for remembered targets. Parietal cortices play an important role in visual attention and also contribute to saccade initiation. These also play a role in initiating visually guided reflexive saccades by projecting to FEFs [19]. The primary visual cortex plays an important role in smooth pursuit and optokinetic EMs. Medial superior temporal cortices play an important role in motion perception and tracking of moving target (i.e. smooth pursuit EMs) [20]. As a rule, posterior cortical areas play a more important role in reflexive, stimulus bound EMs, while anterior areas of the cortex play a more important role in voluntary, self-initiated EMs. During horizontal saccades, contralateral cortical areas are activated, while during horizontal pursuit movements, ipsilateral cortical areas are activated. For vertical movements, corresponding cortical areas are activated on both sides.
The cortical areas do not project directly to ocular motor neurons. Rather, they exert their influence on EMs via polysynaptic pathways involving both excitatory and inhibitory transmission (Fig. 4). For example, saccades are controlled by FEFs through parallel excitatory and inhibitory pathways that reach via basal ganglia to the SC and then to the brainstem reticular formation. In addition, the brainstem reticular formation also receives direct fibers from FEFs.
3.3. Role of basal ganglia
The cortex controls saccades though pathways, which project to the SC both directly and indirectly through basal ganglia circuits.
The SC receives excitatory inputs from FEFs and parietal cortices. In addition, the cortex excites the caudate nucleus, which has an inhibitory influence (nondopaminergic) on the substantia nigra (SN) pars reticulata, which in turn has an inhibitory influence on the SC. Thus, cortical stimulation removes the inhibitory effect of SN on SC. Thus, basal ganglia do exert influence on eye movements primarily through the caudate nucleus and substantia nigra (Fig. 5) [[21], [22], [23]].
Fig. 5.

Optokinetic drum and tape.
3.4. Role of cerebellum in eye movements
The cerebellum fine tunes all eye movements (saccades, smooth pursuit, vergence, optokinetic and vestibular reflexive) through i) the vestibulocerebellum (flocculus, paraflocculus, nodule and ventral uvula), which controls eye movements during motion and ii) the dorsal vermis and fastigial nuclei, which control voluntary gaze shifting.
The fastigial nucleus and nucleus interpositus control vergence and accommodation through projections to midbrain near response cells [24] while the flocculus plays an important role in execution of VOR by receiving signals from the neural integrator and projecting these to vestibular nuclei [25]. The dorsal vermis plays an important role in proper execution of contralateral saccades and smooth pursuit movements by receiving signals from ipsilateral structures and sending signals to contralateral fastigial nuclei, which contribute to acceleration of contralateral saccades and pursuit [26,27].
4. Eye movement abnormalities in movement disorders
As mentioned earlier, a large part of the human brain contributes to the visual system, including the visual cortex; the frontal, parietal and medial temporal lobes; basal ganglia circuitry and superior colliculus; as well as the midbrain and pontine centers. It is therefore not surprising to find abnormalities of the ocular motor system in neurodegenerative movement disorders. The ocular motor abnormalities in some common movement disorders are described in the following sections.
4.1. Eye movement abnormalities in Parkinson's disease (PD)
PD, the second most common neurodegenerative disorder, is characterized pathologically by loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). SNpc activates D1 receptors on basal ganglia output nuclei (BGON) through a direct pathway, which in turn inhibits substantia nigra pars reticulata (SNpr). Through an indirect pathway, SNpc inhibits BGON (through D2 receptors), which disinhibits the globus pallidus externa, resulting in inhibition of the subthalamic nucleus which in turn inhibits SNpr. In PD, dopaminergic depletion leads to decreased inhibition of SNpr through a direct pathway and increased excitation of SNpr through an indirect pathway, resulting in disinhibition of SNpr (Fig. 5). Activated SNpr, in turn, inhibits SC, the main center for generation of saccades, resulting in eye movement abnormalities in PD [21,28,29].
The most commonly observed eye movement abnormalities in PD include hypometric volitional saccades, especially memory guided saccades, where saccades are made to a remembered target location [30,31]. This is due to abnormal function of the frontal cortex-BG-SC circuit [32]. On the other hand, reflexive saccades, pathways for which project directly from the parietal cortex to the SC without BG involvement, are relatively spared, at least in early stages of PD [21]. However, they do become hypometric in later stages of PD, owing mainly to excessive disinhibition of SC.
Another common eye movement abnormality observed in PD patients is the presence of abnormally fragmented or staircase saccades wherein a target of interest is reached through a series of abnormally small saccades. These staircase saccades probably represent excessive inhibition of saccade generator (SC), although their exact mechanism continues to be poorly understood [21,32]. The latency of saccades is increased in PD, more so for volitional saccades, which are affected even in early disease stages. Reflexive saccades are spared, at least in early stages (some patients may even show faster-than-normal reflexive saccades), although in advanced stages, these also reveal prolonged latencies [21,32]. In addition, PD is characterized by impaired ability to suppress unwanted saccades, which correspond to depletion of dopamine in the prefrontal cortex. Patients with PD may fail antisaccades tasks, reflecting early abnormalities of executive function in PD [33,34].
Other ocular motor abnormalities described in PD include square wave jerks (attributed to compensatory overactivity of FEFs), poor convergence and abnormal smooth pursuit movements (saccadic pursuit, increased pursuit latency and decreased gain) [[35], [36], [37]]. In one study (n = 27), there were significant reduction in amplitudes of convergence movements when compared with controls (n = 16) [35]. The convergence amplitudes correlated with motor fluctuations in PD being more severely decreased during off stage than on stage [35]. In patients with advanced PD, this abnormality in convergence results in variable amounts of reading difficulty and near vision diplopia. Furthermore due to constant fluctuations in convergence ability it is often difficult to provide exact optic correction. The prism correction should be provided only after measuring optimal optical corrections both during on as well as off stage [35]. Abnormalities of smooth pursuit ocular movements with both increased initiation latency and low gain in PD are well described in literature. Accordingly pursuit movements in patients with PD are often associated with frequent saccades interrupting the pursuit movements (saccadic pursuit). Whether these saccades are actually catch up saccades to keep moving target on fovea or represent a decreased ability to suppress unwanted saccades remains to be determined. Also whether these disturbances in smooth pursuit precede the motor deficits in PD and accordingly help in presymptomatic diagnosis of PD is still not clear. Whatsoever the mechanism the disturbances in smooth pursuit movements do add to visual disability observed in patients with PD [36,37].
The above-mentioned eye movement abnormalities may account for some of the commonly observed behaviors in PD patients. Visual search patterns shown by PD patients may result from deficient generation of volitional saccades, while fewer hypometric saccades may account for visual-spatial neglect observed in PD patients. Inability to generate proper saccades may also affect visual stability and contribute to patients' inability to judge the emotional expressions of others [21].
Although eye movement abnormalities are thought to be related dopamine deficiency in PD, dopaminergic therapy has provided only inconsistent benefits in terms of correcting ocular motor dysfunction. The ocular abnormalities in genetic parkinsonian syndromes are listed in Table 1.
Table 1.
Eye movement abnormalities in spinocerebellar ataxias.
| Type of SCA | SWJs | Gaze evoked nystagmus | Downbeat nystagmus | Hypometric saccades | Supranuclear gaze palsy | Decreased saccade velocity | Hypermetric saccades | Impaired smooth pursuit | Hypoactive VOR |
|---|---|---|---|---|---|---|---|---|---|
| SCA 1 | − | + | − | − | − | ++ | + | − | + |
| SCA 2 | − | − | − | − | − | +++ | − | − | − |
| SCA 3 | + | ++ | − | ++ | ++ | + | − | ++ | ++ |
| SCA 6 | − | +++ | +++ | + | − | − | ++ | +++ | − |
| SCA 7 | − | − | − | − | ++ | +++ | − | − | − |
| SCA17 | − | − | − | ++ | ++ | − | − | ++ | − |
| FA | +++ | − | − | − | − | − | + | ++ | +++ |
Abbreviation: FA: Friedrich's ataxia; SCA: spinocerebellar ataxia; SWJs: Square wave jerks; VOR: vestibule-ocular reflex.
4.2. Progressive supranuclear palsy (PSP)
Ocular motor abnormalities often help in diagnosis of PSP, which in its classic form is characterized by severely reduced velocity of vertical eye movements, especially saccades, owing to involvement of riMLF, which contain burst neurons for generating vertical saccades. Alternative explanations for impaired vertical saccadic movements include decreased inhibition of omnipause cells [29] and involvement of nigrostriatal dopaminergic pathways. The characteristic ocular abnormalities in PSP include saccadic hypometria, prolonged saccade latencies and impaired smooth pursuit preferentially affecting vertical EMs first, followed by horizontal EMs when the pathological process also involves burst neurons for horizontal saccades. However, despite prominent volitional gaze abnormalities, VOR remains preserved even in advanced stages, suggesting a predominant supranuclear component to gaze disorder [38].
Other prominent ocular motor abnormalities in PSP include deficient visual fixation with frequent large square wave jerks (up to 5°). To understand the occurrence of square wave jerks (SWJs) in PSP, one needs to understand their pathophysiology. During attempted visual fixation by healthy individuals, small (<1°), conjugate horizontal saccadic oscillations (micro SWJs) are commonly observed. These small saccadic intrusions allow better visualization of an object than by steady holding of the image on the fovea. This is because the visual system, like other sensory systems, has better motion responsiveness. So, these microsaccades prevent visual fading by shifting the image on the retina by a small amount (0.5° or so). The frequency and amplitude of these SWJs increase with increasing target size and challenge of the visual scene [29]. The explanation for occurrence of large SWJs (up to 5°) in PSP is thus twofold: i) prolonged duration of saccades increases likelihood of visual fading and thus large SWJs in PSP may reflect a compensatory mechanism to overcome visual fading in PSP and ii) the SWJ coupling mechanism may increase frequency of SWJs to enhance vertical saccade burst [29,39]. Furthermore, PSP patients perform poorly on antisaccades tasks owing to associated frontal lobar dysfunction and have a tendency to keep their eyes fixed on the same target with an inability to shift to another target (visual grasping) [40]. Vergence is also affected early in PSP and may account for the occurrence of diplopia in some patients [41]. In addition, PSP patients may exhibit apraxia of eyelid closure and opening as well as reduced blink rate.
4.3. Multisystem atrophy (MSA)
MSA is characterized clinically by varying combinations of parkinsonism, autonomic dysfunction, pyramidal and cerebellar signs, and pathologically by degeneration of nigrostriatal and olivopontocerebellar pathways. MSA is subdivided into two subtypes: MSA-P and MSA-C based on the predominant clinical phenotype (parkinsonian and cerebellar dysfunction, respectively). The eye movement abnormalities in MSA-C reflect cerebellar dysfunction and include abnormalities of smooth pursuit eye movements and downbeat (30%), rebound or gaze-evoked nystagmus [42]. In fact, presence of nystagmus in patients with parkinsonian syndromes suggests a diagnosis of MSA. MSA-P is, however, difficult to distinguish from PD. Large SWJs are seen in many patients with MSA. Other ocular abnormalities in MSA include mildly hypometric saccades. However, as a rule, MSA patients can generate normal saccade amplitudes, and saccadic velocities also are normal. In MSA, the pathological process involves brainstem nuclei involved in smooth pursuit eye movements. While patients with MSA-C present with impaired smooth pursuit and fine, catch up saccades, patients with MSA-P may show anticipatory saccades in addition to fine, catch up saccades [43]. Also, MSA patients show impaired ability to suppress unwanted saccades and VOR as well as poor performance on tasks based on antisaccades.
4.4. Cortico-basal-ganglionic degeneration (CBGD)
The hallmark eye movement abnormality in CBGD is saccadic apraxia with difficulty and delay in saccade initiation toward a target (i.e., increased saccadic latency) and normal saccadic velocity. Other eye movement abnormalities in CBGD include poor performance on tasks involving antisaccades and impaired smooth pursuit, although not as severe as in PSP.
4.5. Huntington's disease (HD)
Ocular motor abnormalities are one of the earliest signs in HD, an autosomal dominant disorder characterized by chorea, behavioral abnormalities, cognitive decline and abnormally increased CAG repeats. Eye movement abnormalities are reported even in presymptomatic gene carriers and include: i) saccadic apraxia and slow, hypometric saccades with both increased latency and decreased amplitude (hallmark ocular findings in HD) [44], ii) mild impairment of smooth pursuit eye movements [45], iii) abnormalities in visual fixation [46], iv) poor performance on antisaccades tasks and v) decreased ability to suppress unwanted saccades [29]. Saccadic apraxia is more marked for volitional saccades (especially memory guided) and saccades made on verbal commands rather than visual cues. There is severe impairment on antisaccades tasks. All these collectively result in some of the characteristics clinical abnormalities seen in HD, namely head thrusting, distractibility and impersistence of gaze. The vertical saccades are affected more than horizontal ones. A direct correlation exists between prolonged saccadic latency, decreased saccadic velocity as well as antisaccades errors and severity of HD [47]. The saccadic apraxia and hypometria are thought to be related to involvement of the frontal-caudate-SNpr-SC circuit and burst neurons in the pons and midbrain, while antisaccades errors are likely related to dysfunction of the premotor and anterior cingulate cortices, both of which play a role in error detection. Involvement of the FEF-caudate-SNpr-SC circuit explains preferential involvement of voluntary saccades in comparison to reflexive saccades [29,48].
4.6. Eye movement abnormalities in cerebellar disease and spinocerebellar ataxias (SCAs) (Table 3, Table 4)
Table 3.
Eye movement abnormalities in various genetic parkinsonism syndromes.
| Type of mutation | Characteristic eye movement abnormalities |
|---|---|
| PARK 1 | Increased latency of horizontal and vertical saccades as well as antisaccades Frequent multiple vertical saccades instead of single one Poor gain of vertical saccades Reduced precision of vertical saccades Poor performance on tasks involving antisaccades |
| PARK 2 | Decreased gain of horizontal and vertical saccades Poor performance on tasks involving antisaccades |
| PARK 6 | Increased latency of horizontal saccades Frequent multistep vertical saccades |
| PARK 9 | Increased latency of horizontal and vertical saccades as well as antisaccades Reduced gain, precision and velocity of horizontal and vertical saccades Frequent multiple vertical saccades instead of single one Poor performance on tasks involving antisaccades |
(Adapted and modified from Pretegiani and Optican [21].)
Table 4.
Characteristic eye movement abnormalities in various movement disorders.
| Name of movement disorder | Characteristic eye movement abnormalities |
|---|---|
| Parkinson's disease | Decreased amplitude of saccades (more for volitional & memory guided saccades than visually guided ones) Increased saccade latency (more for volitional & memory guided saccades than visually guided ones) Abnormality is saccade amplitude occurs earlier and is more severe than abnormality in latency Abnormal convergence movements with correlate with degree of motor abnormality Abnormal smooth pursuit movements with abnormal saccades during pursuit Excessive square wave jerks |
| PDD or DLBD | Greater impairment of visually guided saccades and complex saccades (memory guided, antisaccades) |
| MSA-P | Same as described under Parkinson's disease + Square wave jerks, moderate saccadic hypometria, impaired smooth pursuit, abnormal suppression of VOR |
| MSA-C | Gaze evoked, downbeat and rebound nystagmus Impaired smooth pursuit Square wave jerks |
| PSP | Prominent gaze abnormalities Vertical saccades affected earlier and greater than horizontal saccades Slowing of downward saccades: hallmark and diagnostic criteria Loss of saccades and pursuit in advanced stages Normal eye movements on VOR Excessive small amplitude square wave jerks Errors on tasks involving antisaccades |
| CBGD | Hallmark- Saccadic apraxia (difficulty and delay in initiation of saccades toward target i.e. increased saccadic latency with normal saccade velocity) Errors on tasks involving antisaccades |
| HD | Saccadic apraxia and slow hypometric saccades (abnormal saccade latency and velocity) Verbally guided, memory guided and antisaccades are affected more than visually guided ones Vertical saccades are affected more than horizontal Mild impairment of smooth pursuit Extent of decrease in velocity and increase in latency of saccades as well as errors on antisaccades correlate with disease severity |
Abb: PDD: Parkinson disease with dementia; DLBD: Diffuse Lewy body disease; MSA-P: Multisystem atrophy (parkinsonism type); MSA-C: Multiple system atrophy (cerebellar type); PSP: Progressive supranuclear palsy; CBGD: Cortico-basal-ganglionic degeneration; HD: Huntington disease; VOR: vestibulo-ocular reflex.
Several eye movement abnormalities have been described as a consequence of cerebellar disease. These include i) various forms of nystagmus, including gaze-evoked nystagmus, downbeat nystagmus, periodic alternating nystagmus, etc., ii) micro and macro SWJs, iii) hypo- or hypermetric saccades, iv) impaired smooth pursuit eye movements, v) slowing of saccades, vi) pulse step mismatch resulting in post-saccadic drift [29] (Table 2).
Table 2.
Eye movement abnormalities in cerebellar disease.
| 1. Involvement of Vestibulocerebellum |
| a) Cogwheel saccades |
| b) Impaired pursuit |
| c) Impaired suppression of vestibulo-ocular reflex (VOR): Gaze paretic, rebound, downbeat nystagmus |
| d) Upbeat nystagmus |
| e) Deficient VOR gain |
| f) Opsoclonus |
| 2. Involvement of dorsal cerebellum (controls amplitude of saccades) |
| a) Saccadic dysmetria |
| 3. Involvement of lateral cerebellum |
| a) Saccadic dysmetria |
| b) Impaired smooth pursuit |
| c) Downbeat nystagmus |
| d) Positional nystagmus |
| e) Rebound nystagmus |
| f) Gaze evoked nystagmus |
| g) Abnormality in visual fixation |
| h) Post saccadic drift |
SCAs constitute a heterogeneous group of disorders characterized pathologically by expanded trinucleotide repeat and autosomal dominant inheritance. Many of these are associated with characteristic eye movement abnormalities which may help in diagnosis of the specific SCA subtype. An association has been reported between reduction in velocity of saccades and number of CAG repeats and age of clinical disease in SCA-2 [49].
4.7. Gaucher and Niemann Pick disease
Gaucher disease (GD) is the commonest autosomal recessive lysosomal storage disorder and may present with parkinsonism. Other clinical features include hepatosplenomegaly and pancytopenia. Eye movement abnormalities have been reported as an early and diagnostic feature in GD and involve horizontal eye movements much more than vertical ones. The characteristic ocular findings in GD include saccadic hypometria, increased saccade latency and decreased saccadic velocity preferentially affecting horizontal saccades. Other findings include saccadic pursuit and oculomotor apraxia, again primarily affecting horizontal eye movements [50].
In contrast to GD, Niemann Pick disease, another lysosomal storage disorder, is characterized by selective impairment of vertical gaze with relative or total preservation of horizontal gaze. Presence of supranuclear vertical gaze palsy with near-total sparing of horizontal gaze helps in diagnosis of this rare condition characterized clinically by dystonia, spasticity, progressive cognitive decline, seizures and visceromegaly [51].
5. Conclusions
Abnormalities of eye movements are common in patients of movement disorders. Recognizing these abnormalities may help in arriving at a correct diagnosis of the specific movement disorder. In addition, these abnormalities contribute significantly to morbidity and should be addressed appropriately whenever possible.
Declaration of competing interest
The authors does not have any conflict of interest.
Footnotes
Source of funding: Nil.
Acknowledgements: Nil.
References
- 1.Vinny P.W., Lal V. Gaze disorders: a clinical approach. Neurol. India. 2016;64:121–128. doi: 10.4103/0028-3886.173627. [DOI] [PubMed] [Google Scholar]
- 2.Kennard C. Disorders of higher gaze control. Handb. Clin. Neurol. 2011;102:379–402. doi: 10.1016/B978-0-444-52903-9.00020-0. [DOI] [PubMed] [Google Scholar]
- 3.Walker H.K., Hall W.D., Hurst J.W. third ed. Butterworth; Boston: 1990. Clinical Methods: The History, Physical and Laboratory Examinations. [PubMed] [Google Scholar]
- 4.Nachev P., Kennard C., Husain M. Functional role of supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 5.Pierrot-Deseilligny C., Muri R.M., Ploner C.J., Gaymard B., Rivaud-Pechoux S. Cortical control of ocular saccades in humans: a model for motricity. Prog. Brain Res. 2003;142:3–17. doi: 10.1016/S0079-6123(03)42003-7. [DOI] [PubMed] [Google Scholar]
- 6.Sparks D.L. The brainstem control of saccadic eye movements. Nat. Rev. Neurosci. 2003;9:252–264. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- 7.Walton M.M.G., Freedman E.G. Activity of long lead burst neurons in pontine reticular formation during head- unrestrained gaze shifts. J. Neurophysiol. 2014;111:300–312. doi: 10.1152/jn.00841.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucker J.C., Ying S.H., Moore W., Optican L.M., Buttner-Ennever J., Keller E.L., Shapiro B.E., Leigh R.J. Do brainstem omnipause neurons terminate saccades? Ann. N. Y. Acad. Sci. 2001;1233:48–57. doi: 10.1111/j.1749-6632.2011.06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brazis P.W., Masdeu J.C., Biller J. Wolter Kluwer Health/Lippincott Williams and Wilkins; Philadelphia: 2011. Localization in Clinical Neurology. [Google Scholar]
- 10.Petit L., Haxby J.V. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J. Neurophysiol. 1999;82:463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- 11.Pierrot-Deseilligny C., Gaymard B. Smooth pursuit disorders. Baillieres Clin. Neurol. 1992;1:435–454. [PubMed] [Google Scholar]
- 12.Bender M.B. Brain control of conjugate horizontal and vertical eye movements: a survey of the structural and functional correlates. Brain. 1980;103:23–69. doi: 10.1093/brain/103.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Fernandez N., Gallegos-Constantino V., Barona-Lleo L., Manrique-Huarte R. Clinical and video assisted examination of vestibule-ocular reflex: a comparative study. Acta Otorrinolaringol. Esp. 2012;63:429–435. doi: 10.1016/j.otorri.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Papanagnu E., Brodsky M.C. Is there a role for optokinetic nystagmus testing in contemporary orthoptic practice? Old tricks and new perspectives. Am. Orthopt. J. 2014;64:1–10. doi: 10.3368/aoj.64.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Munoz D.P., Wurtz R.H. Saccade related activity in monkey superior colliculus: I. Characteristics of burst and build up cells. J. Neurophysiol. 1995;73:2313. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- 16.Munoz D.P., Wurtz R.H. Saccade related activity in monkey superior colliculus: II. Spread of activity during saccades. J. Neurophysiol. 1995;73:2334. doi: 10.1152/jn.1995.73.6.2334. [DOI] [PubMed] [Google Scholar]
- 17.Sommer M.A., Wurtz R.H. Frontal eye field neurons orthodromically activated from the superior colliculus. J. Neurophysiol. 1998;80:3331. doi: 10.1152/jn.1998.80.6.3331. [DOI] [PubMed] [Google Scholar]
- 18.Dias E., Kiesau M., Segraves M.A. Acute activation and inactivation of macaque frontal eye fields with GABA related drugs. J. Neurophysiol. 1995;(6):2744. doi: 10.1152/jn.1995.74.6.2744. [DOI] [PubMed] [Google Scholar]
- 19.Segraves M.A., Goldberg M.E., Deng S.-Y. The role of striate cortex in guidance of eye movements in the monkey. J. Neurosci. 1987;7:3040. doi: 10.1523/JNEUROSCI.07-10-03040.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miles F.A. The neural processing of 3-D visual information. Eur. J. Neurosci. 1998;10:811. doi: 10.1046/j.1460-9568.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- 21.Pretegiani E., Optican L.M. Eye movements in Parkinson disease and inherited parkinsonian syndromes. Front. Neurol. 2017;8 doi: 10.3389/fneur.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hikosaka O., Takikawa Y., Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 2000;80(3):953e78. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 23.Eggenberger E.R. Supranuclear eye movement abnormalities. Continuum. (Minneap. Minn.). 2014;20:981–992. doi: 10.1212/01.CON.0000453308.50604.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H., Gamlin P.D. Neurons in the posterior interposed nucleus of cerebellum related to vergence and accommodation: I. Steady state characteristics. J. Neurophysiol. 1998;79:1255. doi: 10.1152/jn.1998.79.3.1255. [DOI] [PubMed] [Google Scholar]
- 25.Lisberger S.G., Miles F.A., Zee D.S. Signals used to compute errors in monkey vestibulo-ocular reflex: possible role of flocculus. J. Neurophysiol. 1984;52:1140. doi: 10.1152/jn.1984.52.6.1140. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs A.F., Robinson F.R., Straube A. Role of caudal fastigial nucleus in saccade generation: I. Neuronal discharge patterns. J. Neurophysiol. 1993;70:1723. doi: 10.1152/jn.1993.70.5.1723. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs A.F., Robinson F.R., Straube A. Participation of caudal fastigial nucleus in smooth pursuit eye movements: I. Neuronal activity. J. Neurophysiol. 1994;72:2714. doi: 10.1152/jn.1994.72.6.2714. [DOI] [PubMed] [Google Scholar]
- 28.DeLong M.R., Wichmann T. Circuits and circuit disorders of basal ganglia. Arch. Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 29.Gorges M., Pinkhardt E.H., Kassubek J. Alterations of eye movement control in neurodegenerative movement disorders. J. Ophthalmol. 2014 doi: 10.1155/2014/658243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terao Y., Fukuda H., Ugawa Y., Hikosaka O. New perspectives on the pathophysiology of Parkinson's disease as assessed by saccade performance: a clinical review. Clin. Neurophysiol. 2013;124:1491–1506. doi: 10.1016/j.clinph.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White O.B., Saint-Cyr J.A., Tomlinson R.D., Sharpe J.A. Ocular motor deficits in Parkinson's disease. II. Control of the saccadic and smooth pursuit systems. Brain. 1983;106(Pt 3):571–587. doi: 10.1093/brain/106.3.571. [DOI] [PubMed] [Google Scholar]
- 32.Terao Y., Fukuda H., Yugeta A., Hikosaka O., Nomura Y., Segawa M. Initiation and inhibitory control of saccades with the progression of Parkinson's disease – changes in three major drives converging on the superior colliculus. Neuropsychologia. 2011;49:1794–1806. doi: 10.1016/j.neuropsychologia.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walton C.C., O'Callaghan C., Hall J.M., Gilat M., Mowszowski L., Naismith S.L. Antisaccade errors reveal cognitive control deficits in Parkinson's disease with freezing of gait. J. Neurol. 2015;262:2745–2754. doi: 10.1007/s00415-015-7910-5. [DOI] [PubMed] [Google Scholar]
- 34.Ewenczyk C., Mesmoudi S., Gallea C., Welter M.L., Gaymard B., Demain A. Antisaccades in Parkinson disease: a new marker of postural control? Neurology. 2017;88:853–861. doi: 10.1212/WNL.0000000000003658. [DOI] [PubMed] [Google Scholar]
- 35.Almer Z., Klein K.S., Marsh L., Gerstenhaber M., Repka M.X. Ocular motor and sensory function in Parkinson's disease. Ophthalmology. 2012;119:178–182. doi: 10.1016/j.ophtha.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibasaki H., Tsuji S., Kuroiwa Y. Oculomotor abnormalities in Parkinson's disease. Arch. Neurol. 1979;36:360–364. doi: 10.1001/archneur.1979.00500420070009. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima K., Fukushima J., Barnes G.R. Clinical application of eye movement tasks as an aid to understanding Parkinson's disease pathophysiology. Exp. Brain Res. 2017;235:1309–1321. doi: 10.1007/s00221-017-4916-5. [DOI] [PubMed] [Google Scholar]
- 38.Anderson T.J., MacAskill M.R. Eye movements in patients with neurodegenerative disorders. Nat. Rev. Neurol. 2013;9:74–85. doi: 10.1038/nrneurol.2012.273. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Conde S., Macknik S.L., Troncoso X.G., Dyar T.A. Microsaccades counteract visual fading during fixation. Neuron. 2006;49:297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 40.Chen A.L., Riley D.E., King S.A. The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front. Neurol. 2010;1 doi: 10.3389/fneur.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardwick A., Rucker J.C., Cohen M.L. Evolution of oculomotor and clinical findings in autopsy-proven Richardson syndrome. Neurology. 2009;73:2122–2124. doi: 10.1212/WNL.0b013e3181c67ba2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson T., Luxon L., Quinn N., Daniel S., Marsden C.D., Bronstein A. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov. Disord. 2008;23:977–984. doi: 10.1002/mds.21999. [DOI] [PubMed] [Google Scholar]
- 43.Pinkhardt E.H., Kassubek J., Sussmuth S., Ludolph A.C., Becker W., Jürgens R. Comparison of smooth pursuit eye movement deficits in multiple system atrophy and Parkinson's disease. J. Neurol. 2009;256:1438–1446. doi: 10.1007/s00415-009-5131-5. [DOI] [PubMed] [Google Scholar]
- 44.Turner T.H., Goldstein J., Hamilton J.M. Behavioral measures of saccade latency and inhibition in manifest and premanifest Huntington's disease. J. Motor Behav. 2011;43:295–302. doi: 10.1080/00222895.2011.580390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fielding J., Georgiou-Karistianis N., Bradshaw J. Impaired modulation of the vestibulo-ocular reflex in Huntington's disease. Mov. Disord. 2004;19:68–75. doi: 10.1002/mds.10658. [DOI] [PubMed] [Google Scholar]
- 46.Becker W., Jurgens R., Kassubek J., Ecker D., Kramer B., Landwehrmeyer B. Eye-head coordination in moderately affected Huntington's disease patients: do head movements facilitate gaze shifts? Exp. Brain Res. 2009;192 doi: 10.1007/s00221-008-1559-6. [DOI] [PubMed] [Google Scholar]
- 47.Golding C.V., Danchaivijitr C., Hodgson T.L., Tabrizi S.J., Kennard C. Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology. 2006;67:485–487. doi: 10.1212/01.wnl.0000218215.43328.88. [DOI] [PubMed] [Google Scholar]
- 48.Fielding J., Georgiou-Karistianis N., White O. The role of the basal ganglia in the control of automatic visuospatial attention. J. Inter. Neuropsych. Soc. 2006;12:657–667. doi: 10.1017/S1355617706060784. [DOI] [PubMed] [Google Scholar]
- 49.Velazquez-Perez L., Seifried C., Abele M. Saccade velocity is reduced in presymptomatic spinocerebellar ataxia type 2. Clin. Neurophysiol. 2009;120:632–635. doi: 10.1016/j.clinph.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 50.Benko W., Ries M., Wiggs E.A., Brady R.O., Schiffmann R., Fitzgibbon E.J. The saccadic and neurological deficits in type 3 Gaucher disease. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta A., Kumar N., Saharan R., Rastogi P., Vishnu V.Y., Lal V. Teaching video neuroimages: vertical supranuclear ophthalmoparesis a diagnostic pearl for Niemann Pick. Neurology. 2016;86:e108. doi: 10.1212/WNL.0000000000002442. [DOI] [PubMed] [Google Scholar]